Abstract
Objective
Patients with coronavirus disease vaccine associated lymphadenopathy are increasingly being referred to healthcare services. This work is the first to report on the incidence, clinical course and imaging features of coronavirus disease vaccine associated cervical lymphadenopathy, with special emphasis on the implications for head and neck cancer services.
Methods
This was a retrospective cohort study of all patients referred to our head and neck cancer clinics between 16 December 2020 and 12 March 2021. The main outcomes measured were the proportion of patients with vaccine-associated cervical lymphadenopathy, and the clinical and imaging characteristics.
Results
The incidence of vaccine-associated cervical lymphadenopathy referrals was 14.8 per cent (n = 13). Five patients (38.5 per cent) had abnormal-looking enlarged and rounded nodes with increased vascularity. Only seven patients (53.9 per cent) reported full resolution within an average of 3.1 ± 2.3 weeks.
Conclusion
Coronavirus disease vaccine associated cervical lymphadenopathy can mimic malignant lymphadenopathy and therefore might prove challenging to diagnose and manage correctly. Healthcare services may encounter a significant increase in referrals.
Key words: COVID-19 Vaccine, Lymphadenopathy, Head And Neck Neoplasms, Neck, Ultrasonography, Head And Neck Cancer
Introduction
The coronavirus disease 2019 (Covid-19) pandemic, caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), has resulted in millions of deaths and strained healthcare systems around the globe. Huge collaborative efforts led to the development and deployment of successful vaccinations that reduce the risk of severe infections and mortality.1–3 In the UK, following a rigorous review of safety and efficacy data, the Pfizer/BioNTech vaccine was the first Covid-19 vaccine to be approved by the Medicines and Healthcare products Regulatory Agency, followed by the Oxford/AstraZeneca and Moderna vaccines.1–3 The rollout of Covid-19 vaccination in the UK, and in many other countries, prioritised those most likely to die from the disease, especially older care home residents and immunocompromised adults, as well as protecting health and social care workers.
Similarly to other vaccines, local adverse drug reactions like shoulder pain and erythema, in addition to mild systemic symptoms like fatigue, myalgia and headache, are commonly reported after Covid-19 vaccination.3–6 However, data from recent clinical trials and early post-marketing clinical experience have suggested a higher incidence of local lymphadenopathy reactions in the axilla and neck.3,5–7 With the widespread rollout of Covid-19 vaccination programmes, lymphadenopathy has created a diagnostic and therapeutic dilemma for cancer screening and diagnosis services.8–10 For this reason, the United States Society of Breast Imaging, the Canadian Society of Breast Imaging, the Canadian Association of Radiologists, and a multidisciplinary team (MDT) of experts from three leading cancer centres in the USA have all recently released emergency recommendations for the management of Covid-19 vaccine associated lymphadenopathy.7,10,11
Cases of ipsilateral lymphadenopathy in the lower neck and supraclavicular region following Covid-19 vaccinations are quickly emerging in the international literature, and are being increasingly referred to healthcare services for advice and management.12–14 As lower neck lymphadenopathy harbours malignancy in around 75 per cent of cases, the UK National Institute for Health and Care Excellence recommended fast-track referral of unexplained or persistent cases through a dedicated pathway for suspected head and neck cancer.15,16 The differential diagnosis of lymphadenopathy in the lower neck is broad, but it is imperative to exclude pathologies like head and neck malignancy, lymphoma, and metastatic lung or cutaneous cancers.10,15 However, as vaccine deployment is still in its early stages, no data are yet available regarding the presentation, clinical course or imaging characteristics of Covid-19 vaccine associated cervical lymphadenopathy to guide the decision-making process in such patients.
This work is the first to report on the characteristics and clinical course of cervical lymphadenopathy following Covid-19 vaccination, with special emphasis on potential implications for head and neck cancer services.
Materials and methods
Study design and setting
We conducted a retrospective cohort study of individuals referred to our fast-track suspected head and neck cancer clinics. Our hospital is a leading National Health Service trust, providing tertiary head and neck cancer services with a dedicated regional MDT. The study period covered 12 weeks between 16 December 2020 and 12 March 2021. Data were collected from fast-track clinic referral forms and electronic records.
Study population
All patients referred to our fast-track head and neck cancer clinics during the study period were initially screened for their Covid-19 vaccination status and the reason for referral. All patients with Covid-19 vaccine associated cervical lymphadenopathy were included. We defined Covid-19 vaccine associated cervical lymphadenopathy as any unilateral and lower cervical lymphadenopathy (level IV or V) first noticed within two weeks of Covid-19 vaccine injection in the ipsilateral deltoid muscle. All patients with bilateral, upper cervical or a known previous history of cervical lymphadenopathy were excluded. We also excluded patients if they had received any other injection in the ipsilateral deltoid muscle within four weeks before the onset of lymphadenopathy.
Main outcome measures
Demographic data regarding age and gender of the included patients were collected. The main outcome measure collected was the number of patients referred with Covid-19 vaccine associated cervical lymphadenopathy. Secondary outcomes included the clinical and imaging characteristics, and follow-up measures.
Reporting guidelines and ethical considerations
Our study design and reporting adhered to the Strengthening the Reporting of Observational Studies in Epidemiology (‘STROBE’) guidelines for cohort studies.17 Because of the retrospective nature of the study, ethical approval was waived by our institution.
Statistical techniques
Continuous variables with normal distribution were summarised using means and standard deviations; otherwise, we used medians and interquartile ranges. The independent samples t-test was used to evaluate associations between groups, and a p-value of 0.05 or less was considered statistically significant. All statistical analyses were performed using SPSS software, version 26.0 (IBM, Armonk, New York, USA).
Results
Patient characteristics
Of 404 patients referred to our fast-track head and neck clinics during the study period, 88 had cervical lymphadenopathy. A total of 13 patients (14.8 per cent) had Covid-19 vaccine associated cervical lymphadenopathy and were consecutively included in the study (Figure 1).
Fig. 1.
Diagram showing the proportion of referrals with coronavirus disease 2019 vaccine associated lymphadenopathy, and a timeline of the clinical course. Covid-19 = coronavirus disease 2019; d = days; w = weeks
The patients’ mean age was 54.8 ± 16.1 years. Interestingly, most of the patients were female (n = 11, 84.6 per cent) (Table 1). All patients had received the Pfizer/BioNTech vaccine, and the majority had been injected in the left deltoid (n = 12, 92.3 per cent). The study period mostly covered the early phases of vaccination rollout in the UK, and most patients (n = 12) had received only one dose by the time of presentation.
Table 1.
Characteristics of patients with Covid-19 vaccine associated lymphadenopathy*
| Clinical parameter | Values |
|---|---|
| Age at diagnosis (mean (SD); days) | 54.8 (16.1); range, 27–81 |
| Gender (n (%)) | |
| – Female | 11 (84.6) |
| – Male | 2 (15.4) |
| Injection site (n (%)) | |
| – Left deltoid | 12 (92.3) |
| – Right deltoid | 1 (7.7) |
| First dose (n (%)) | 12 (92.3) |
| Pfizer/BioNTech vaccine (n (%)) | 13 (100) |
| Symptoms (n (%)) | |
| – Neck lump or fullness | 13 (100) |
| – Tenderness | 6 (46.2) |
| Ultrasound scan (n (%)) | 13 (100) |
| Outcome (n (%)) | |
| – Fully resolved | 7 (53.9) |
| – Partially reduced | 5 (38.5) |
| – Unknown | 1 (7.7) |
| Time for clinical resolution (mean (SD); weeks) | |
| – Fully resolved | 3.1 (2.3); range, 1–8 |
| – Partially reduced | 8.4 (3.1); range, 5–12 |
n = 13. Covid-19 = coronavirus disease 2019; SD = standard deviation
All patients experienced the feeling of a lump in the ipsilateral neck, but pain was only reported in six patients (46.2 per cent) (Tables 1 and 2). The swelling was first noticed by patients within a median of 4 days (interquartile range, 2–7 days) from vaccination. Seven patients (53.9 per cent) were referred to the fast-track clinic within three weeks of symptom onset (Table 2).
Table 2.
Clinical features and timeline of presentation of patients with Covid-19 vaccine associated lymphadenopathy
| Pt no. | Age (years) | Sex | 1st or 2nd dose | Injection site | Lymph node side | Symptom | Vaccination to lymphadenopathy* interval (days) | Lymphadenopathy to referral interval (days) | Referral to USS interval (days) | Outcome† | Time for outcome (weeks) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 54 | F | 1st | Left | Ipsilateral | Tenderness | 11 | 16 | 9 | Fully resolved | 8 |
| 2 | 27 | F | 1st | Left | Ipsilateral | Tenderness | 5 | 7 | 3 | Fully resolved | 3 |
| 3 | 60 | F | 1st | Left | Ipsilateral | None | 3 | 2 | 6 | Fully resolved | 2 |
| 4 | 40 | M | 1st | Left | Ipsilateral | None | 1 | 44 | 8 | Not documented | Not documented |
| 5 | 76 | F | 1st | Left | Ipsilateral | None | 4 | 17 | 7 | Partially reduced | 5 |
| 6 | 41 | F | 1st | Left | Ipsilateral | None | 7 | 26 | 10 | Fully resolved | 2 |
| 7 | 73 | F | 1st | Left | Ipsilateral | Tenderness | 7 | 7 | 5 | Fully resolved | 3 |
| 8 | 55 | F | 1st | Left | Ipsilateral | None | 14 | 41 | 12 | Partially reduced | 12 |
| 9 | 57 | F | 1st | Left | Ipsilateral | None | 2 | 37 | 12 | Partially reduced | 11 |
| 10 | 56 | F | 2nd | Left | Ipsilateral | Tenderness | 1 | 45 | 12 | Partially reduced | 8 |
| 11 | 81 | F | 1st | Right | Ipsilateral | Tenderness | 10 | 38 | 10 | Partially reduced | 6 |
| 12 | 34 | M | 1st | Left | Ipsilateral | None | 2 | 3 | 4 | Fully resolved | 3 |
| 13 | 58 | F | 1st | Left | Ipsilateral | None | 2 | 2 | 7 | Fully resolved | 1 |
*Corresponding to subjective palpable swelling by patients. †Reported by patients during telephone follow up. Covid-19 = coronavirus disease 2019; Pt no. = patient number; USS = ultrasound scan; F = female; M = male
Neck ultrasound lymphadenopathy features
All patients had an ultrasound scan. The median interval between the swelling onset and the scan was 25 days (interquartile range, 10–49 days). Table 3 summarises the ultrasound scan characteristics of the examined lymph nodes.
Table 3.
Ultrasound findings in patients with Covid-19 vaccine associated cervical lymphadenopathy
| Pt no. | Level of LN | Short axis diameter (mm)* | S:L ratio | Vascularity | Fatty hilum | Echogenicity | Overall impression | FNAB |
|---|---|---|---|---|---|---|---|---|
| 1 | IV | 4.9 | 0.5 | Normal | Preserved | Normal | Reactive LN | Not done |
| 2 | V | 7.7 | 0.5 | Normal | Preserved | Normal | Reactive LN | Not done |
| 3 | V | Not done | Not done | Not done | Not done | Not done | Reactive LN | Not done |
| 4 | V | 5.2 | 0.6 | Increased | Preserved | Normal | Reactive LN | Not done |
| 5 | IV | 4.7 | 0.7 | Normal | Preserved | Slightly hypoechoic | Reactive LN | Benign reactive LN |
| 6 | V | 5.2 | 0.7 | Normal | Preserved | Normal | Reactive LN | Not done |
| 7 | V | 6.6 | 0.5 | Normal | Preserved | Normal | Reactive LN | Not done |
| 8 | IV/V | 5.7 | 0.6 | Normal | Preserved | Normal | Reactive LN | Not done |
| 9 | V | 3.4 | 0.5 | Normal | Preserved | Normal | Reactive LN | Not done |
| 10 | V | 5 | 0.4 | Normal | Preserved | Normal | Reactive LN | Not done |
| 11 | IV | 4.2 | 0.5 | Normal | Preserved | Normal | Reactive LN | Not done |
| 12 | V | 8.2 | 0.6 | Increased | Preserved | Slightly hypoechoic | Reactive LN | Benign reactive LN |
| 13 | V | 7 | 0.5 | Normal | Preserved | Normal | Reactive LN | Not done |
Measured in the biggest node. Covid-19 = coronavirus disease 2019; Pt no. = patient number; LN = lymph node; S:L ratio = short axis diameter:long axis diameter ratio; FNAB = fine-needle aspiration biopsy
Targeted ultrasound scans confirmed the presence of one or more lymph nodes, all in level IV or V of the neck. The average short axis diameter of the most prominent nodes was 5.5 ± 1.4 mm, but five patients (38.5 per cent) had more rounded nodes with a short axis:long axis ratio greater than 0.5. Scans performed at or within four weeks of swelling onset showed significantly larger nodes (6.5 ± 1.4 mm) compared with scans performed after four weeks (4.8 ± 0.8 mm) (p = 0.03).
The overall scan impression was recorded as benign reactive lymphadenopathy in all patients, but two cases had increased vascularity on colour Doppler ultrasound scans (Figure 2, Table 3). Moreover, two patients had mildly hypoechoic lymph nodes, but none had a fatty hilar abnormality.
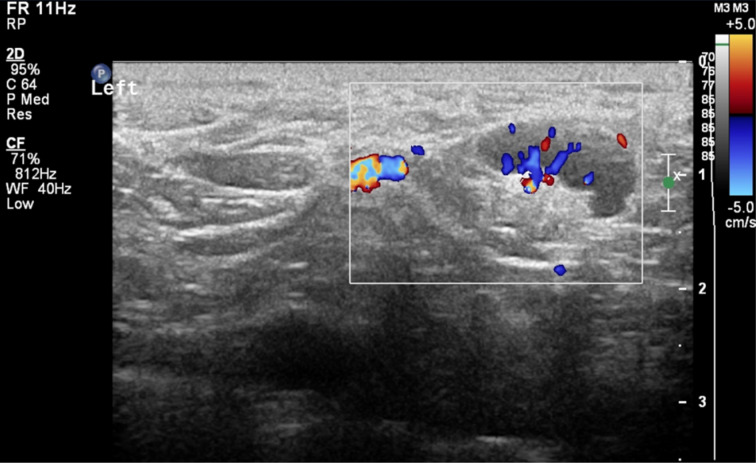
Fig. 2.
Colour Doppler ultrasound scan of patient number 12, showing an 8.2 mm, slightly hypoechoic lymph node in the left supraclavicular region, with increased vascularity.
Two patients underwent fine-needle aspiration biopsy, but cytology demonstrated features of benign reactive lymphadenopathy. Moreover, two patients had a follow-up ultrasound scan that showed a reduction in Covid-19 vaccine associated lymphadenopathy size.
Lymphadenopathy outcomes
The outcomes of lymphadenopathy were subjectively reported by patients during virtual follow up (Table 2). Seven patients (53.9 per cent) reported full resolution of their palpable swelling within an average of 3.1 ± 2.3 weeks from symptom onset. Five patients (38.5 per cent) reported a partial reduction in the size of their palpable lumps over an average period of 8.4 ± 3.1 weeks (Figure 1).
When compared with the full resolution group, the partial reduction group interestingly presented to their general practitioner significantly later (35.6 vs 9.0 days, p = 0.001) and had significantly smaller lymph nodes on the ultrasound scan (4.6 vs 6.4 mm, p = 0.024) (Table 4). However, neither the patients’ age nor the interval between vaccination and lymphadenopathy was found to have a significant impact on the clinical outcome.
Table 4.
Comparison between patients with fully resolved versus partially reduced lymphadenopathy
| Variable | Outcome* | Mean (SD) | P-value |
|---|---|---|---|
| Short axis diameter (mm) | Fully resolved nodes | 6.4 (1.3) | 0.024† |
| Partially reduced nodes | 4.6 (0.9) | ||
| Vaccination to lymphadenopathy interval (days)‡ | Fully resolved nodes | 5.3 (3.3) | 0.728 |
| Partially reduced nodes | 6.2 (5.6) | ||
| Lymphadenopathy to referral interval (days) | Fully resolved nodes | 9 (8.9) | 0.001† |
| Partially reduced nodes | 35.6 (10.9) | ||
| Age (years) | Fully resolved nodes | 49.6 (16.2) | 0.106 |
| Partially reduced nodes | 65 (12.5) |
*Corresponding to subjective palpable swelling by patients. †Indicates statistical significance. ‡Reported by patients during telephone follow up. SD = standard deviation
Discussion
Post-vaccination lymphadenitis is an uncommon phenomenon that can be triggered by intramuscular vaccine injections in the deltoid muscle. It has been previously reported in adults following many viral vaccinations, especially for human papillomavirus18 and H1N1 influenza.19 More recently, cases with Covid-19 vaccine associated lymphadenopathy have been described in reports from the USA, Spain, Israel and the UK.8,12–14,20–22
The available evidence suggests that messenger RNA (mRNA)-based vaccines are likely more immunogenic than standard vaccines, and hence show a higher incidence of Covid-19 vaccine associated lymphadenopathy.7,21,22 In clinical trials on the Pfizer/BioNTech mRNA vaccine, lymphadenopathy was only reported as an unsolicited adverse drug reaction, with incidence in the vaccine group as high as 10 times that in the placebo group (0.3 per cent and 0.03 per cent, respectively).1 As a solicited adverse drug reaction, axillary lymphadenopathy was the second most frequently reported reaction in the Moderna vaccine trials, with an incidence of 10.2 per cent and 14 per cent after the first and second dose, respectively.3 Reporting lymphadenopathy in these trials was based only on physical examination, and the true incidence rate was likely much higher.1,3,7 Interestingly, clinical trials on the Oxford/AstraZeneca adenovirus-vectored vaccine reported a lower incidence of lymphadenopathy in the vaccination group (0.3 per cent) compared with the placebo group (0.4 per cent).2 Our results show that all patients referred to us with Covid-19 vaccine associated cervical lymphadenopathy had the Pfizer/BioNTech vaccine, and none had the Oxford/AstraZeneca vaccine, despite the latter accounting for almost 53 per cent of the total UK vaccine doses given during our study period.4 Moreover, real-life data from the Medicines and Healthcare products Regulatory Agency (reported using the yellow card scheme for adverse drug reactions) have shown that the Pfizer/BioNTech vaccine had almost double the number of reports for lymphatic system disorders compared with the Oxford/AstraZeneca vaccine (22.4 vs 11.7 per 100 000 doses given, respectively).4–6
Most of the Covid-19 vaccinations administered during our study period were nationally prioritised to people aged 65 years or older. The mean age of patients with Covid-19 vaccine associated cervical lymphadenopathy in our study was 54.8 years; only three patients were aged 65 years or older. Data from the Pfizer/BioNTech trials also demonstrated a higher incidence and severity of adverse drug reactions in younger participants, with lymphadenopathy reported five times more commonly in the 16–55 years age group (0.5 per cent) compared with the over 55 years’ age group (0.1 per cent).1 Similarly, Covid-19 vaccine associated lymphadenopathy was more frequently reported in younger individuals (aged 18–64 years) following the first and second doses of the Moderna vaccine (11.6 per cent and 16 per cent, respectively), compared with individuals aged 65 years or more (6.1 per cent and 8.4 per cent, respectively).3 In a case series of 20 female healthcare workers with Covid-19 vaccine associated cervical lymphadenopathy, Fernández-Prada et al.12 reported that 75 per cent of patients (n = 15) had full resolution within 16 days of symptom onset. In our study, full clinical resolution was reported within an average of 3.1 weeks from symptom onset in more than half of the cohort, and partial improvement was reported within an average of 8.4 weeks in 40 per cent of patients. Interestingly, half of our patients were directly referred to our fast-track head and neck cancer clinics within three weeks of symptom onset.
Ultrasound scan features
The role of ultrasonography in the assessment of supraclavicular (level IV/V) lymphadenopathy is well established. All our Covid-19 vaccine associated lymphadenopathy patients had an ultrasound scan within a median of 8 days from referral and a median of 25 days from onset of symptoms. There was a significant inverse association between the timing of the scan and the size of the imaged nodes, possibly highlighting a time-dependent reactive nature of the nodes.
While the overall impression was of benign nature, some nodes in our cohort showed abnormal features. Large nodes with increased vascularity and a high short axis:long axis ratio (more rounded) usually indicate abnormality.23 Ying and Ahuja recommended that the optimum short axis diameter cut-off value in the lower neck should be 3–5 mm (short axis:long axis ratio of 0.4–0.5), with high specificity and moderate sensitivity.23 Our data demonstrated that around half of our patients had nodes larger than 5 mm, and 40 per cent had a short axis:long axis ratio of more than 0.5. These findings are in line with previous reports demonstrating that Covid-19 vaccine associated lymphadenopathy may show abnormal morphology and can appear enlarged, rounded, hypoechoic and with loss of echogenic fatty hila.8,9,13,20
Implications for head and neck cancer services
The impact of Covid-19 vaccine associated lymphadenopathy on clinical services and patients should not be underestimated. In our 12-week study period, Covid-19 vaccine associated cervical lymphadenopathy cases accounted for around 15 per cent of all fast-tracked lymphadenopathy referrals. With the ongoing expansion of the vaccination programme in the UK to cover younger individuals and more second-dose vaccinations, as well as the upcoming rollout of the Moderna mRNA vaccine, we predict that the number of referrals will increase exponentially.14,21
Awareness of the clinical features and course of Covid-19 vaccine associated lymphadenopathy is crucial for radiologists involved in cancer diagnosis and follow up, and it should nowadays be recognised in the differential diagnosis of cervical or axillary lymphadenopathy.9,10 Lymphadenopathy detected clinically or in routine surveillance scans might create a diagnostic and management dilemma for oncology patients.10,21 Not only can Covid-19 vaccine associated lymphadenopathy exhibit abnormal features on ultrasound scans, but it has also been shown to be metabolically active on positron emission tomographic (PET) images, with intensities similar to those of malignant lymphadenopathy.8–10,21 A recent study by Cohen et al. investigated PET-positive supraclavicular and axillary lymphadenopathy in 728 oncology patients following the Pfizer/BioNTech vaccine; the incidence of overall and supraclavicular Covid-19 vaccine associated lymphadenopathy was 36.5 per cent and 8 per cent, respectively, with supraclavicular Covid-19 vaccine associated lymphadenopathy being more commonly encountered after the second dose (9.1 per cent) than the first dose (5.5 per cent).21
Recommendations for appropriate management of Covid-19 vaccine associated lymphadenopathy should aim to strike a balance between avoiding delayed cancer diagnoses and minimising patient harm from invasive biopsies, unnecessary scans and heightened anxiety.8,10,22,24 All requests for imaging in the head and neck and breast regions, and referrals to the fast-track cancer services, should include full information about Covid-19 vaccine status, especially the dates, the site, the side and the vaccine type. For patients with pre-existing history of head and neck malignancy, vaccine administration on the contralateral side is recommended.
Recently, published recommendations from institutions in North America have advised timing head and neck and breast imaging for before, or 4–6 weeks after, Covid-19 vaccination, and recommended considering a follow-up scan 4–12 weeks after the second dose of the vaccine.7,10,11,24 Moreover, evidence from our current study and previously published data have demonstrated that a good proportion of Covid-19 vaccine associated cervical lymphadenopathy fully resolves within three to six weeks.12,13,21 Therefore, it is not unreasonable for primary care physicians to rationalise referrals to specialist cancer clinics, and to prioritise cases with persistent lymphadenopathy beyond three to six weeks or patients with other concerning features of malignancy.10 Until more data become available, head and neck cancer MDTs should carefully advise against delaying vaccine administration, and should weigh the risks and benefits of timing any head and neck imaging to before or to four to six weeks after the Covid-19 vaccination.
Study limitations and strengths
Our results are limited by the inherent weakness in retrospectively collected data. We adapted a strict definition for Covid-19 vaccine associated cervical lymphadenopathy, and excluded cases with upper or bilateral neck nodes, and cases with nodes first noticed after more than 14 days following vaccination. We believe that this definition increased our specificity and confidence in our diagnosis, but it may possibly have reduced the sensitivity and missed some cases that presented in atypical ways. In our cohort, lymphadenopathy was spatially and temporally associated with Covid-19 vaccinations; however, it was difficult to ascertain a causal link. Our results could be useful and universally generalisable to members of cancer MDTs, including surgeons, radiologists, oncologists and haematologists, in addition to primary care physicians, vaccinators and the general public.
Reactive cervical lymphadenopathy can be encountered following coronavirus disease 2019 (Covid-19) vaccination
This vaccine-associated cervical lymphadenopathy can mimic malignant lymphadenopathy, with abnormal features on imaging
Vaccine-associated cervical lymphadenopathy might create a diagnostic and management dilemma for head and neck cancer services
Lymphadenopathy might fully resolve in three to six weeks in over half of patients; referrals should therefore be rationalised
In the next few months, primary care and head and neck cancer services may encounter a rise in vaccine-related reactive lymphadenopathy referrals
The Covid-19 vaccination history must be included in all referrals with head and neck lymphadenopathy
Conclusion
The widespread rollout of Covid-19 vaccination has important implications for clinicians and patients. Over the next few months, primary care and head and neck cancer services will potentially encounter a rise in vaccine-related reactive lymphadenopathy referrals. Therefore, Covid-19 vaccination history must be included in all referrals. Reactive Covid-19 vaccine associated cervical lymphadenopathy can mimic malignant lymphadenopathy, and therefore might become challenging to correctly diagnose and manage. Furthermore, consideration should be given to alternative strategies and referral pathways for low-risk patients presenting with lymphadenopathy for which Covid-19 vaccination is the most likely cause.
Competing interests
APG reports being the managing director for Endoscope-I company. There were no other relationships or activities that could appear to have influenced the submitted work.
Data availability statement
The data that support the findings of this study are available on request from the corresponding author.
References
- 1.Medicines and Healthcare products Regulatory Agency. Public Assessment Report. Authorisation for Temporary Supply, COVID-19 mRNA Vaccine BNT162b2 (BNT162b2 RNA) concentrate for solution for injection. In: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/944544/COVID-19_mRNA_Vaccine_BNT162b2__UKPAR___PFIZER_BIONTECH__15Dec2020.pdf [19 April 2021]
- 2.Medicines and Healthcare products Regulatory Agency. Public Assessment Report. Authorisation for Temporary Supply, COVID-19 Vaccine AstraZeneca, solution for injection in multidose container COVID-19 Vaccine (ChAdOx1-S [recombinant]). In: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/963928/UKPAR_COVID_19_Vaccine_AstraZeneca_23.02.2021.pdf [19 April 2021]
- 3.Medicines and Healthcare products Regulatory Agency. Public Assessment Report. Authorisation for Temporary Supply, COVID-19 Vaccine Moderna, 0.20 mg/mL dispersion for injection (COVID-19 mRNA Vaccine [nucleoside modified]). In: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/962920/Public_Assessment_Report_for_Moderna_COVID-19_vaccine.pdf [19 April 2021]
- 4.Medicines and Healthcare products Regulatory Agency. Coronavirus vaccine - weekly summary of Yellow Card reporting (09/12/2020 to 14/03/2021). In: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/972844/Coronavirus_vaccine_-_summary_of_Yellow_Card_reporting_14.03.21.pdf [19 April 2021]
- 5.Medicines and Healthcare products Regulatory Agency. COVID-19 mRNA Pfizer- BioNTech vaccine analysis print (09/12/2020 to 14/03/2021). In: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/972832/COVID-19_mRNA_Pfizer-_BioNTech_Vaccine_Analysis_Print.pdf [28 March 2021]
- 6.Medicines and Healthcare products Regulatory Agency. COVID-19 vaccine AstraZeneca analysis print (04/01/2021 to 14/03/2021). In: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/975786/COVID-19_AstraZeneca_Vaccine_Analysis_Print.pdf [28 March 2021]
- 7.SBI Recommendations for the Management of Axillary Adenopathy in Patients with Recent COVID-19 Vaccination: Society of Breast Imaging Patient Care and Delivery Committee. In: https://www.sbi-online.org/Portals/0/Position%20Statements/2021/SBI-recommendations-for-managing-axillary-adenopathy-post-COVID-vaccination.pdf [19 April 2021]
- 8.Özütemiz C, Krystosek LA, Church AL, Chauhan A, Ellermann JM, Domingo-Musibay Eet al. Lymphadenopathy in COVID-19 vaccine recipients: diagnostic dilemma in oncology patients. Radiology 2021;300:E296–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McIntosh LJ, Bankier AA, Vijayaraghavan GR, Licho R, Rosen MP. COVID-19 vaccination-related uptake on FDG PET/CT: an emerging dilemma and suggestions for management. AJR Am J Roentgenol 2021. Epub 2021 Aug 4 [DOI] [PubMed] [Google Scholar]
- 10.Becker AS, Perez-Johnston R, Chikarmane SA, Chen MM, El Homsi M, Feigin KNet al. Multidisciplinary recommendations regarding post-vaccine adenopathy and radiologic imaging: Radiology Scientific Expert Panel. Radiology 2021;300:E323–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seely JM, Barry MH. The Canadian Society of Breast Imaging recommendations for the management of axillary adenopathy in patients with recent COVID-19 vaccination - update. Can Assoc Radiol J 2021. Epub 2021 Feb 23 [DOI] [PubMed] [Google Scholar]
- 12.Fernández-Prada M, Rivero-Calle I, Calvache-González A, Martinón-Torres F. Acute onset supraclavicular lymphadenopathy coinciding with intramuscular mRNA vaccination against COVID-19 may be related to vaccine injection technique, Spain, January and February 2021. Euro Surveill 2021;26:2100193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hiller N, Goldberg SN, Cohen-Cymberknoh M, Vainstein V, Simanovsky N. Lymphadenopathy associated with the COVID-19 vaccine. Cureus 2021;13:e13524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitchell OR, Dave R, Bekker J, Brennan PA. Supraclavicular lymphadenopathy following COVID-19 vaccination: an increasing presentation to the two-week wait neck lump clinic? Br J Oral Maxillofac Surg 2021;59:384–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cunnane M, Cheung L, Moore A, di Palma S, McCombe A, Pitkin L. Level 5 lymphadenopathy warrants heightened suspicion for clinically significant pathology. Head Neck Pathol 2016;10:509–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Institute for Health and Care Excellence. Suspected cancer: recognition and referral. NICE guideline [NG12]. In: https://www.nice.org.uk/guidance/ng12/ [12 April 2021] [PubMed]
- 17.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JPet al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007;335:806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coates EE, Costner PJ, Nason MC, Herrin DM, Conant S, Herscovitch Pet al. ; VRC 900 Study Team. Lymph node activation by PET/CT following vaccination with licensed vaccines for human papillomaviruses. Clin Nucl Med 2017;42:329–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burger IA, Husmann L, Hany TF, Schmid DT, Schaefer NG. Incidence and intensity of F-18 FDG uptake after vaccination with H1N1 vaccine. Clin Nucl Med 2011;36:848–53 [DOI] [PubMed] [Google Scholar]
- 20.Mortazavi S. Coronavirus disease (COVID-19) vaccination associated axillary adenopathy: imaging findings and follow-up recommendations in 23 women. AJR Am J Roentgenol 2021. Epub 2021 Aug 11 [DOI] [PubMed] [Google Scholar]
- 21.Cohen D, Krauthammer SH, Wolf I, Even-Sapir E. Hypermetabolic lymphadenopathy following administration of BNT162b2 mRNA Covid-19 vaccine: incidence assessed by [18F]FDG PET-CT and relevance to study interpretation. Eur J Nucl Med Mol Imaging 2021;48:1854–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mehta N, Sales RM, Babagbemi K, Levy AD, McGrath AL, Drotman Met al. Unilateral axillary adenopathy in the setting of COVID-19 vaccine. Clin Imaging 2021;75:12–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ying M, Ahuja A. Sonography of neck lymph nodes. Part I: normal lymph nodes. Clin Radiol 2003;58:351–8 [DOI] [PubMed] [Google Scholar]
- 24.Lehman CD, D'Alessandro HA, Mendoza DP, Succi MD, Kambadakone A, Lamb LR. Unilateral lymphadenopathy after COVID-19 vaccination: a practical management plan for radiologists across specialties. J Am Coll Radiol 2021;18:843–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.