Abstract
Background
Longer duration from symptom onset is associated with increased risk of perforation in appendicitis. In previous studies, in-hospital delay to surgery has had conflicting effects on perforation rates. Although preoperative antibiotics have been shown to reduce postoperative infections, there are no data showing that administration of antibiotics while waiting for surgery has any benefits. The aims of this study are to evaluate the role of both in-hospital delay to surgery and antibiotic treatment while waiting for surgery on the rate of appendiceal perforation.
Methods
This prospective, open-label, randomized, controlled non-inferiority trial compares the in-hospital delay to surgery of less than 8 hours versus less than 24 hours in adult patients with predicted uncomplicated acute appendicitis. Additionally, participants are randomized either to receive or not to receive antibiotics while waiting for surgery. The primary study endpoint is the rate of perforated appendicitis discovered during appendicectomy. The aim is to randomize 1800 patients, that is estimated to give a power of 90 per cent (χ2) for the non-inferiority margin of 5 percentage points for both layers (urgency and preoperative antibiotic). Secondary endpoints include length of hospital stay, 30-day complications graded using Clavien–Dindo classification, preoperative pain, conversion rate, histopathological diagnosis and Sunshine Appendicitis Grading System classification.
Discussion
There are no previous randomized controlled studies for either in-hospital delay or preoperative antibiotic treatment. The trial will yield new level 1 evidence.
EU Clinical Trials Register, EudraCT Number: 2019–002348-26; registration number: NCT04378868 (http://www.clinicaltrials.gov)
Study protocol for a randomized non-inferiority trial (PERFECT). The aims of this study are to evaluate the role of in-hospital delay to surgery and antibiotic treatment whilst waiting for surgery on the rate of appendiceal perforation.
Introduction
Worldwide, appendicitis remains one of the most common causes of an acute abdomen in adults and appendicectomy is one of the most frequently performed emergency surgeries1,2. The clinical classification into uncomplicated (non-perforated) and complicated (perforated) appendicitis is used to describe the severity of the infection. For perforated appendicitis, there is a strong agreement on urgent appendicectomy. There is no consensus on the urgency of appendicectomy for uncomplicated or non-perforated appendicitis. In large series, perforation at appendicectomy has been observed in 15–22 per cent of patients who were thought before surgery to have non-perforated appendicitis3–5. Particularly for this group of patients, it is important to determine whether the in-hospital delay to surgery or antibiotic treatment influence perforation rate.
Several studies have investigated the association of in-hospital delay and risk of perforation in these patients, but the results have been conflicting6–13. A large meta-analysis based on retrospective studies demonstrates that delaying appendicectomy for up to 24 hours is safe for patients with no preoperative signs of complicated appendicitis7. On the contrary, lengthened in-hospital delay was associated with elevated risk of perforation in patients with appendicitis assumed before surgery to be uncomplicated in a recent large retrospective series8. It seems that mild appendicitis does not progress to perforation in most patients and even spontaneous resolution with or without antibiotics has been demonstrated3,4,14–16.
The guideline recommendations vary in uncomplicated acute appendicitis. Two guidelines recommend to operate on acute appendicitis as soon as feasible17,18, while World Society of Emergency Surgery (WSES) guidelines recommend to plan laparoscopic appendicectomy for the next available operating list within 24 hours whilst minimizing the delay wherever possible19. Currently the daily clinical practice has huge variation between countries, and even within countries, as some hospitals carry out night-time appendicectomies for predicted uncomplicated appendicitis, while others do not. All retrospective series are biased as sicker patients are more likely to be operated on sooner than patients with mild symptoms. This selection will inevitably lead to results favouring longer delay.
Even though uncomplicated appendicitis can be managed without surgery, the effect of antibiotics on the perforation rate while waiting for appendicectomy has remained unclear. The infection in appendicitis is caused by intestinal bacteria, therefore it is assumed that antibiotics given before surgery could decelerate the disease’s evolution to gangrene and further to perforation. In that case, antibiotic treatment could benefit patients with an increased risk of developing complicated appendicitis. Most guidelines recommend preoperative antibiotics to start as soon as acute appendicitis is diagnosed17,20 while other guidelines do not define the timing of preoperative antibiotics18,21. Recently updated WSES guidelines as well as The Surgical Infection Prevention Guideline Writers Workgroup recommend the administration of a single dose of broad-spectrum antibiotics within 60 minutes before incision19,22. Again, the use of antibiotics while waiting for appendicectomy for uncomplicated appendicitis varies hugely between hospitals due to lack of evidence.
The aims of this randomized controlled trial (PERFECT) are to evaluate the role of both delay to surgery for predicted uncomplicated appendicitis, and antibiotics while waiting for surgery, on the rate of appendiceal perforation.
Methods/design
Hypothesis
This study has two hypotheses: first, that longer in-hospital delay is not inferior to shorter delay, and second, that waiting for appendicectomy without antibiotics is not inferior to waiting with antibiotics in patients with predicted uncomplicated appendicitis.
Patient evaluation and selection
Patients are recruited in two hospitals (Meilahti and Jorvi hospitals) at Helsinki University Hospital District in Finland. Patients who have been diagnosed with acute appendicitis and are aimed to be operated upon, are eligible for the study. Acute appendicitis can be diagnosed clinically or by imaging. Patients with high clinical suspicion of acute appendicitis can be diagnosed without imaging by using Adult Appendicitis Score (AAS) (16 or higher)23. Acute appendicitis can also be diagnosed by using ultrasonography, CT, or MRI. Before randomization, diagnostic imaging (CT/MRI) should be performed on all patients who have had symptoms for over 3 days to rule out complicated appendicitis24. Patients with suspicion of complicated appendicitis based on high plasma C-reactive protein level (CRP) (100 mg/l or greater)8, high body temperature (over 38.5°C), signs of complicated appendicitis on imaging studies, or clinical generalized peritonitis are excluded from the trial.
Inclusion criteria
All patients with clinically diagnosed appendicitis or appendicitis confirmed by imaging (ultrasonography/CT/MRI) that is aimed to be operated on are included.
Exclusion criteria
Patients are excluded if they have imaging-confirmed complicated appendicitis (perforation or abscess). Appendicitis is defined as complicated if any of the following radiological findings is present: extraluminal air or extraluminal appendicolith, fluid collection, abscess or phlegmon near to appendix, or an unenhanced appendix (or part of it). They are also excluded if there is a suspicion of complicated appendicitis based on the preoperative laboratory examinations, defined as plasma CRP 100 mg/l or higher8,25, or clinical generalized peritonitis or other reason requiring an urgent operative intervention.
Other exclusion criteria are pregnancy; age less than 18 years or over 100 years; no written consent; an allergy to cephalosporin or metronidazole or previous anaphylactic reaction to penicillin or other contraindication for metronidazole (only for the antibiotic layer); the patient is a known carrier of multiresistant bacteria species (only for the antibiotic layer); or antibiotic treatment started before randomization (only for the antibiotic layer).
Randomization
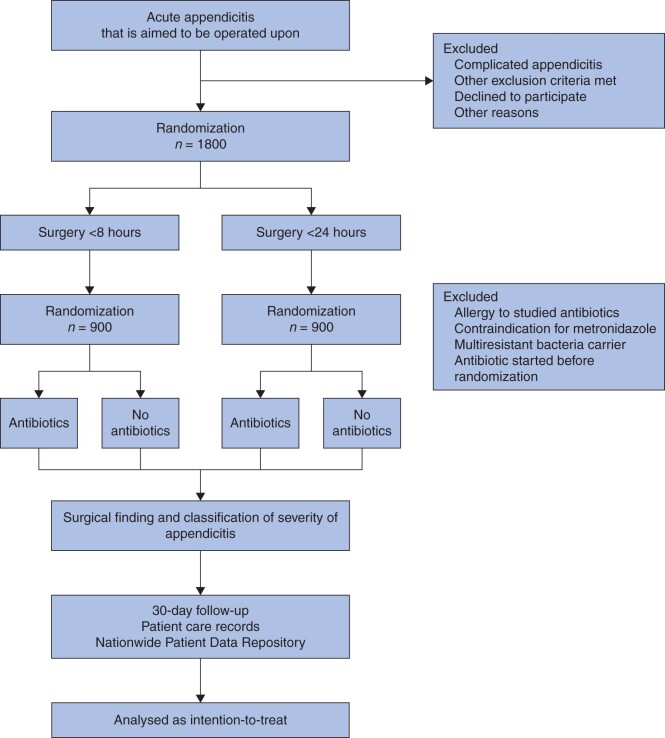
The trial consists of two layers: urgency of surgery and antibiotic treatment while waiting for appendicectomy (Fig. 1). Patients participating in the trial are randomized twice. First, eligible patients are randomly allocated (1 : 1) to appendicectomy either within 8 hours or within 24 hours from randomization. Subsequently, the patients randomized to either arm of urgency will undergo a second randomization (1 : 1) either to receive antibiotics whilst waiting for surgery or not to receive antibiotics during the waiting period. Patients who have exclusion criteria that apply only to the antibiotic layer will not undergo the second randomization but will undergo the first randomization for urgency. These patients that do not participate the antibiotic layer of the trial will not get antibiotics unless started earlier for another indication. In that case the antibiotics continue as prescribed.
Fig. 1.
Flow chart of patients in the trial
The randomization sequence is generated using a R statistical software with Blockrand 1.3 package using block randomization with randomly varying block size from 4 to 6 without stratification. The recruitment and allocation are done by the surgeon responsible for the decision to proceed to appendicectomy using a web-based service. After inputting inclusion and exclusion criteria the service will give the eligible patients’ allocation group for both layers. The allocated intervention is open-label, and the patient, surgeon, other healthcare personnel, data collectors and data analysts are not blinded. The responsible surgeon will schedule an appendicectomy with allocated urgency26 and either commences antibiotics or not based on the allocation.
Intervention
Preoperative treatment
Analgesia is given in accordance to normal clinical practice. All patients, regardless of their allocation group, will receive preoperative prophylactic antibiotics (intravenous cefuroxime 1500 mg and metronidazole 500 mg or a suitable alternative in case of allergy or other contraindication) within 30 minutes before the appendicectomy during induction of anaesthesia.
Preoperative antibiotic treatment
In the antibiotic group, the antibiotic treatment (cefuroxime 1500 mg and metronidazole 500 mg every 8 hours) is started immediately after the randomization and will continue until the induction of anaesthesia. Patients randomized to the no antibiotics group will receive preoperative antibiotics only at the induction of anaesthesia.
Surgery
All randomized patients are approached primarily laparoscopically unless there is a specific contraindication not to do so, and laparoscopic surgery may be converted to open surgery at the decision of the operating surgeon. Appendicitis is classified intraoperatively according to American Association for the Surgery of Trauma (AAST) Grading Scale27 (Table 1) and Sunshine Appendicitis Grading System (SAGS)28 by the operating surgeon. The removed appendix is always sent for histopathological examination.
Table 1.
American Association for the Surgery of Trauma grading scale for the severity of appendicitis
| Grade | Operative criteria |
|---|---|
| 0 | Normal appendix |
| I | Acutely inflamed appendix, intact |
| II | Gangrenous appendix, intact |
| III | Perforated appendix with local contamination |
| IV | Perforated appendix with periappendiceal phlegmon or abscess |
| V | Perforated appendix with generalized peritonitis |
Postoperative treatment
Postoperative antibiotic treatment is similar in all study groups. When AAST grade is I–II, the appendicitis is regarded as uncomplicated and no further antibiotic treatment is needed. If the AAST grade is III–V, the appendicitis is categorized as complicated, and the antibiotic treatment will be continued at least 4 days postoperatively, of which at least 72 hours of treatment will be administered intravenously. The total duration of antibiotic treatment will be assigned at the discretion of the surgeon. Iatrogenic appendiceal perforation or other contamination during surgery does not change the aforementioned postoperative antibiotic policy.
Criteria for discharge
To allow discharge, there must be no further need for intravenous antibiotics, well controlled postoperative pain using normal analgesia (paracetamol, non-steroidal anti-inflammatory drug and/or weak opioid) and no fever (temperature less than 38.5°C).
Follow-up
No routine follow-up is scheduled. Patients are recommended to contact the hospital where the surgery was performed in case of any problems within 30 days after the surgery. Further, all patient visits and (re)admissions in Finland are recorded in the Nationwide Patient Data Repository (NPDR). The NPDR consists of all healthcare units’ patient medical records from public or private medical clinics that are enrolled in Finland. All possible visits to a healthcare unit within 30 days of surgery will be checked and recorded from the NPDR.
Endpoints
The primary endpoint is complicated appendicitis AAST grade III–V27 assessed by surgeon intraoperatively.
Secondary endpoints are duration of hospital stay after randomization (hours); complications within 30 days after surgery (Clavien–Dindo classification)29; preoperative pain assessed hourly by the patient using the numeric rating scale (NRS) whilst waiting for surgery (area under NRS curve); surgical-site infections within 30 days after randomization as defined by Centers for Disease Control (superficial, deep incisional, organ/space)30; positive blood culture within 30 days after randomization; rate of conversion to open surgery; histopathological diagnosis: gangrene or perforation; SAGS classification28.
Data collection and analysis
Patients are requested to evaluate the pain they feel hourly whilst waiting for surgery by filling out the NRS form.
Data will be collected using an electronical case report form and from the patient care records. Interim analyses will be made when 300 and 900 patients have been randomized and operated on. Both layers (delay and antibiotics) undergo the same interim analyses. The Peto approach is used in planned interim analyses31. If there is a statistically significant difference in the main outcome with P < 0.001 (χ2 test) between the study arms, the corresponding study layer is terminated. In the final analysis the difference of proportions of main outcome between the study arms with 95 per cent confidence intervals is calculated. Non-inferiority margin is set to 5 percentage points in both layers. Results from both study layers will be published as separate manuscripts in international peer-reviewed journals.
Sample size calculation
Twenty-two per cent of patients with uncomplicated appendicitis on CT were found to be perforated at operation in adults when median in-hospital waiting time for the surgery was over 8 hours5. In the same study, it was estimated that the risk of perforation increases by 2 per cent every hour. On the other hand, in patients with CRP less than 100 mg/l on admission, the rate of perforated appendicitis was 14 per cent8.
The aim is to show that in patients with presumed uncomplicated appendicitis, scheduling appendicectomy within 24 hours does not lead to absolute increase in the rate of perforated appendicitis (AAST grade III–V) compared with scheduling the appendicectomy within 8 hours. It is assumed that in this patient population the rate of perforated appendicitis would be 15 per cent. The non-inferiority margin was set at 5 percentage points in difference of proportions of perforated appendicitis between the arms. To declare the non-inferiority, 1748 patients are needed to achieve a power of 90 per cent (χ2) with 0.05 alpha. To take into account an assumed 2 to 3 per cent drop off rate, sample size is set to 1800 patients.
In the antibiotic layer, an identical (5 percentage points) non-inferiority margin in difference of proportions of perforated appendicitis was set with identical sample size calculation.
Patient recruiting started in May 2020 and the recruitment is estimated to take 2 years.
Ethics and permissions
Participation in this study is voluntary. All participants will give a written informed consent after they have had written information regarding the trial and a possibility to discuss the trial details. This study is conducted in accordance with the principles of the Declaration of Helsinki and ‘good clinical practice’ guidelines. The study has been approved by the Helsinki University Hospital ethics committee, Finnish Medicines Agency (Fimea), and the institutional review board of participating hospitals.
Discussion
Although there are numerous observational studies, both retrospective and prospective, regarding the delay to surgery in acute appendicitis with contradictory results, no prospective randomized controlled study has been published. As appendicectomy remains the standard for treatment of appendicitis with high efficiency and low complication rates32 and since appendicitis is the most common emergency surgery, organizing urgent appendicectomies for vast numbers of patients remains a great burden for healthcare systems worldwide. Approximately 330 000, 75 000 and 6000 appendectomies take place in the USA, UK and Finland, respectively, every year33. Nearly one in 10 people will have appendicitis during their lifetime34. Consequently, the level of urgency of appendicectomy is of great importance. If it is safe to perform appendicectomy for presumed uncomplicated appendicitis within 24 hours, it will allow the surgery to be performed within office hours with enormous implications. For example, it allows treating these patients as day-case surgery, reduces costly and harmful night-time work and frees resources to other urgent emergency surgery.
On the other hand, it is of paramount importance to assess vigorously the safety of longer waiting for appendicectomy, since perforated appendicitis leads to higher morbidity rates11 and longer hospital stay10. The selected primary endpoint, AAST grade III–V, is clinically relevant for patients and also for society because complications increase treatment costs. This study is the first to assess the role of appendicectomy delay in a prospective randomized study yielding level 1 evidence.
The second layer of the trial, antibiotic treatment before appendicectomy, is also of great importance. The increase of antimicrobial resistance is a major global threat, thus unnecessary antibiotic treatment should be avoided35. Prolonged antimicrobial treatment is associated with increased adverse effects, for example the occurrence of Clostridiumdifficile diarrhoea36. Excessive pharmacological treatment also increases the total medical costs. Antibiotic administration may not be a significant risk for an individual patient but treating large numbers of patients with increased overall use of antibiotics can affect the development of antibiotic resistance. However, if antibiotic treatment while waiting for appendicectomy reduces perforation rate, it could be worthwhile. There are several studies on the efficiency of preoperative antibiotic prophylaxis to prevent postoperative complications19,21,37, but the impact on appendiceal perforation rate is not known.
In 2005, a large Cochrane meta-analysis agreed that broad-spectrum antibiotics given before surgery are effective in decreasing postoperative wound infections and intra-abdominal abscesses, and the impact remains the same in single and multiple doses. No apparent difference in the nature of the removed appendix was determined37. Like the urgency layer, this trial is the first to assess the role of antibiotic treatment while waiting for appendicectomy in a prospective randomized trial.
In non-randomized studies, the delay to appendicectomy is affected by several variables, not least the decisions by the on-call surgeon. Most surgeons tend to operate on clinically sicker patients earlier, leading to longer delays in patients with milder symptoms, who at the same time have lower risk of perforation. This phenomenon leads to a possible false finding that the delay has no role or that it would be safe to delay the surgery. As such, this bias is very difficult to control in any other type of study than a randomized one. Further, many studies have not excluded the patients with a pre-hospital perforation and the in-hospital delay in these patients will not affect the perforation rate6,7,11–13. One study attempted to take this into account by excluding patients with presumed pre-hospital perforation from the analyses. In that study, the increment of in-hospital delay from less than 6 hours to more than 12 hours doubled the proportion of complicated appendicitis from 9.5 per cent to 18.9 per cent8. In this study the usage of antibiotics while waiting for the surgery was not uniform.
Preoperative distinction between uncomplicated and complicated forms of appendicitis is challenging5,8,25,38. There is no impeccable diagnostic method that would identify all complicated forms of acute appendicitis before surgery. This is the main limitation of this trial. If the trial used narrower inclusion criteria, complicated appendicitis could be excluded more accurately, but the results would not be generalized easily. The method to predict complicated appendicitis is pragmatic and easy to generalize because of its simplicity. The large number of patients needed is also a challenge. The recruitment takes place in the surgical emergency room often out of normal working hours.
Funding
Governmental competitive research funds (EVO). V.S. has received funding from University Hospital research grants.
Disclosure. The authors declare no conflict of interest.
Acknowledgements
K.J. drafted the manuscript. All authors participated in the design of the trial and/or are the main organizing local investigators at the participating hospitals. All authors have read, revised and approved the final manuscript.
References
- 1. Stewart B, Khanduri P, McCord C, Ohene-Yeboah M, Uranues S, Vega Rivera F. et al. Global disease burden of conditions requiring emergency surgery. Br J Surg 2014;101:e9–22. [DOI] [PubMed] [Google Scholar]
- 2. Bhangu A, Søreide K, Di Saverio S, Assarsson JH, Drake FT.. Acute appendicitis: modern understanding of pathogenesis, diagnosis, and management. Lancet 2015;386:1278–1287. [DOI] [PubMed] [Google Scholar]
- 3. The CODA Collaborative. A randomized trial comparing antibiotics with appendectomy for appendicitis. N Engl J Med 2020;383:1907–1919. [DOI] [PubMed] [Google Scholar]
- 4. Vons C, Barry C, Maitre S, Pautrat K, Leconte M, Costaglioli B. et al. Amoxicillin plus clavulanic acid versus appendicectomy for treatment of acute uncomplicated appendicitis: an open-label, non-inferiority, randomised controlled trial. Lancet 2011;377:1573–1579. [DOI] [PubMed] [Google Scholar]
- 5. Lastunen K, Leppäniemi A, Mentula P.. Perforation rate after a diagnosis of uncomplicated appendicitis on CT scan. BJS Open 2021;5:zraa034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li J, Xu R, Hu D-M, Zhang Y, Gong T-P, Wu X-L.. Effect of delay to operation on outcomes in patients with acute appendicitis: a systematic review and meta-analysis. J Gastrointest Surg 2019;23:210–223. [DOI] [PubMed] [Google Scholar]
- 7. van Dijk ST, van Dijk AH, Dijkgraaf MG, Boermeester MA.. Meta-analysis of in-hospital delay before surgery as a risk factor for complications in patients with acute appendicitis: in-hospital delay before surgery and complications after appendicectomy. Br J Surg 2018;105:933–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sammalkorpi HE, Leppäniemi A, Mentula P.. High admission C-reactive protein level and longer in-hospital delay to surgery are associated with increased risk of complicated appendicitis. Langenbecks Arch Surg 2015;400:221–228. [DOI] [PubMed] [Google Scholar]
- 9. Papandria D, Goldstein SD, Rhee D, Salazar JH, Arlikar J, Gorgy A. et al. Risk of perforation increases with delay in recognition and surgery for acute appendicitis. J Surg Res 2013;184:723–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Busch M, Gutzwiller FS, Aellig S, Kuettel R, Metzger U, Zingg U.. In-hospital delay increases the risk of perforation in adults with appendicitis. World J Surg 2011;35:1626–1633. [DOI] [PubMed] [Google Scholar]
- 11. Schnüriger B, Laue J, Kröll D, Inderbitzin D, Seiler CA, Candinas D.. Introduction of a new policy of no nighttime appendectomies: impact on appendiceal perforation rates and postoperative morbidity. World J Surg 2014;38:18–24. [DOI] [PubMed] [Google Scholar]
- 12. Ingraham AM. Effect of delay to operation on outcomes in adults with acute appendicitis. Arch Surg 2010;145:886. [DOI] [PubMed] [Google Scholar]
- 13. Teixeira PG, Sivrikoz E, Inaba K, Talving P, Lam L, Demetriades D.. Appendectomy timing: waiting until the next morning increases the risk of surgical site infections. Ann Surg 2012;256:538–543. [DOI] [PubMed] [Google Scholar]
- 14. Sallinen V, Akl EA, You JJ, Agarwal A, Shoucair S, Vandvik PO. et al. Meta-analysis of antibiotics versus appendicectomy for non-perforated acute appendicitis. Br J Surg 2016;103:656–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Park HC, Kim MJ, Lee BH.. Randomized clinical trial of antibiotic therapy for uncomplicated appendicitis: antibiotic therapy for uncomplicated appendicitis. Br J Surg 2017;104:1785–1790. [DOI] [PubMed] [Google Scholar]
- 16. Salminen P, Paajanen H, Rautio T, Nordström P, Aarnio M, Rantanen T. et al. Antibiotic therapy vs appendectomy for treatment of uncomplicated acute appendicitis: the APPAC randomized clinical trial. JAMA 2015;313:2340–2348. [DOI] [PubMed] [Google Scholar]
- 17. Gorter RR, Eker HH, Gorter-Stam MAW, Abis GSA, Acharya A, Ankersmit M. et al. Diagnosis and management of acute appendicitis. EAES consensus development conference 2015. Surg Endosc 2016;30:4668–4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fugazzola P, Ceresoli M, Agnoletti V, Agresta F, Amato B, Carcoforo P. et al. The SIFIPAC/WSES/SICG/SIMEU guidelines for diagnosis and treatment of acute appendicitis in the elderly (2019 edition). World J Emerg Surg 2020;15:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Di Saverio S, Podda M, De Simone B, Ceresoli M, Augustin G, Gori A. et al. Diagnosis and treatment of acute appendicitis: 2020 update of the WSES Jerusalem guidelines. World J Emerg Surg 2020;15:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schuster KM, Holena DN, Salim A, Savage S, Crandall M.. American Association for the Surgery of Trauma emergency general surgery guideline summaries 2018: acute appendicitis, acute cholecystitis, acute diverticulitis, acute pancreatitis, and small bowel obstruction. Trauma Surg Acute Care Open 2019;4:e000281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bratzler DW, Dellinger EP, Olsen KM, Perl TM, Auwaerter PG, Bolon MK. et al. ; Society for Healthcare Epidemiology of America (SHEA). Clinical practice guidelines for antimicrobial prophylaxis in surgery. Surg Infect (Larchmt) 2013;14:73–156. [DOI] [PubMed] [Google Scholar]
- 22. Bratzler DW, Houck PM; Surgical Infection Society. Antimicrobial prophylaxis for surgery: an advisory statement from the National Surgical Infection Prevention Project. Clin Infect Dis 2004;38:1706–1715. [DOI] [PubMed] [Google Scholar]
- 23. Sammalkorpi HE, Mentula P, Leppäniemi A.. A new adult appendicitis score improves diagnostic accuracy of acute appendicitis – a prospective study. BMC Gastroenterol 2014;14:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Elniel M, Grainger J, Nevins EJ, Misra N, Skaife P.. 72 h is the time critical point to operate in acute appendicitis. J Gastrointest Surg 2018;22:310–315. [DOI] [PubMed] [Google Scholar]
- 25. McGowan DR, Sims HM, Zia K, Uheba M, Shaikh IA.. The value of biochemical markers in predicting a perforation in acute appendicitis. ANZ J Surg 2013;83:79–83. [DOI] [PubMed] [Google Scholar]
- 26. Leppäniemi A, Jousela I.. A traffic-light coding system to organize emergency surgery across surgical disciplines: coding system for emergency surgery. Br J Surg 2014;101:e134–e140. [DOI] [PubMed] [Google Scholar]
- 27. Vasileiou G, Ray-Zack M, Zielinski M, Qian S, Yeh DD, Crandall M.. Validation of the American Association for the Surgery of Trauma emergency general surgery score for acute appendicitis—an EAST multicenter study. J Trauma Acute Care Surg 2019;87:134–139. [DOI] [PubMed] [Google Scholar]
- 28. Reid F, Choi J, Williams M, Chan S.. Prospective evaluation of the Sunshine Appendicitis Grading System score. ANZ J Surg 2017;87:368–371. [DOI] [PubMed] [Google Scholar]
- 29. Dindo D, Demartines N, Clavien P-A.. Classification of Surgical Complications. Annals of Surgery 2004;240:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR.. Guideline for Prevention of Surgical Site Infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control 1999;27:97. [PubMed] [Google Scholar]
- 31. Schulz KF, Grimes DA.. Multiplicity in randomised trials II: subgroup and interim analyses. Lancet 2005;365:1657–1661. [DOI] [PubMed] [Google Scholar]
- 32. Podda M, Cillara N, Di Saverio S, Lai A, Feroci F, Luridiana G. et al. Antibiotics-first strategy for uncomplicated acute appendicitis in adults is associated with increased rates of peritonitis at surgery. A systematic review with meta-analysis of randomized controlled trials comparing appendectomy and non-operative management with antibiotics. Surgeon 2017;15:303–314. [DOI] [PubMed] [Google Scholar]
- 33. Ferris M, Quan S, Kaplan BS, Molodecky N, Ball CG, Chernoff GW. et al. The global incidence of appendicitis: a systematic review of population-based studies. Ann Surg 2017;266:237–241. [DOI] [PubMed] [Google Scholar]
- 34. Anderson JE, Bickler SW, Chang DC, Talamini MA.. Examining a common disease with unknown etiology: trends in epidemiology and surgical management of appendicitis in California, 1995–2009. World J Surg 2012;36:2787–2794. [DOI] [PubMed] [Google Scholar]
- 35. Truskett PG. Antimicrobial resistance and antibiotic usage: what can surgeons do? ANZ J Surg 2020;90:198–199. [DOI] [PubMed] [Google Scholar]
- 36. Hensgens MPM, Goorhuis A, Dekkers OM, Kuijper EJ.. Time interval of increased risk for Clostridium difficile infection after exposure to antibiotics. J Antimicrob Chemother 2012;67:742–748. [DOI] [PubMed] [Google Scholar]
- 37. Andersen BR, Kallehave FL, Andersen HK.. Antibiotics versus placebo for prevention of postoperative infection after appendicectomy. Cochrane Database Syst Rev 2005;(2):CD001439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Atema JJ, van Rossem CC, Leeuwenburgh MM, Stoker J, Boermeester MA.. Scoring system to distinguish uncomplicated from complicated acute appendicitis. Br J Surg 2015;102:979–990. [DOI] [PubMed] [Google Scholar]