Abstract
First applications of high focused ultrasound as intracranial ablative therapy were firstly described in early 50’. Since then, the technological innovations have shown an increasingly safe and effective face of this technique. And in the last few years, Magnetic Resonance (MR) guided Focused Ultrasound (gFUS) has become a valid minimally invasive technique in the treatment of several diseases, from bone tumors to symptomatic uterine fibroids or essential tremors. MR guidance, through the tomographic view, offers the advantage of an accurate target detection and treatment planning. Moreover, real-time monitoring sequences allow to avoid non-target ablation. An adequate knowledge of FUS is essential to understand its clinical effectiveness. Therefore, this brief review aims to debate the physical characteristics of US and the main fields of clinical application.
Keywords: MRgFUS, HIFU, Magnetic Resonance guided focused ultrasound, clinical application, physics
Introduction
Over the last decades, the technological innovations applied to both diagnostic and interventional radiology has reached important milestones (1-14).
A combination in which high diagnostic effectiveness pairs with early diagnosis, and the development of new treatment modalities leading to the improvement of survival and/or quality of life (15-25).
In this relatively new era of minimal invasiveness, High-Intensity Focused Ultrasound (HIFU) Magnetic Resonance (MR) guided, also known as MRgFUS, has become a valid minimally invasive technique in the treatment of several diseases (26, 27).
Bone tumors, symptomatic uterine fibroids, and essential tremors are examples in which clinical results of MRgFUS have reached high effectiveness.
MRgFUS is a non-invasive technique in which a focus thermal ablation is induced. MR imaging enables identifying the target lesion, allowing accurate planning and focus of the treatment.
Moreover, MR offers the advantage of real-time monitoring, avoiding non-target ablations. This precision, in the order of few millimeters, has made this method fascinating for the treatment of numerous pathologies as an alternative to more invasive surgical procedures or radiation therapy.
However, the fields of application differ not only for the wide range of treatable lesions but also for the different effects deriving from the physical properties of the US itself. Therefore, an adequate knowledge of FUS is essential to understand its clinical effectiveness.
This paper aims to provide a brief review of the physical characteristics of US and the main fields of clinical application.
Physical principle
US waves are mechanical waves that propagate as alternate zones of compression and rarefaction.
US wavefront progression is ensured by the swinging of each single particle included in the longitudinal front of the mechanical wave. Each particle then returns to equilibrium. In this way, the wavefront progress without moving the medium, and energy is transmitted from one particle to the other.
US waves can achieve different diagnostic or therapeutic applications depending mainly on their physical properties, i.e., different wavelength, velocity, pressure, power, intensity, and frequency (27).
Intensity and frequency
The intensity represents the energy of a single wave.
A mathematical correlation links US frequency, penetration depth, sharpness of the lesion, and intensity.
An absorbing medium where the mechanical wave propagates attenuates the wave with an exponential correlation. An increasing frequency determines an increased absorption by the medium. Consequently, high frequency showed a lower penetration. Therefore, high intensity is needed to focus on a target point.
Conversely, the low frequency would increase the wave penetration (28).
Acoustic impedance
Acoustic impedance is essential in wavefront progression.
Acoustic impedance is given by the product of the medium density and the speed of sound into the medium.
The impedance of two different tissues at the interface predicts the capability of a wave to get through the medium itself. The transmission of an acoustic wave is maximized only when the impedance is the same on both sides of the interface.
High transmission of the wavefront is necessary for therapeutic applications of US.
Interaction US-wave/energy
The interaction of single particles within the medium and wavefront depends on the energy given by the wave. Each particle is not capable of dissipating the absorbing energy over a specific threshold. This mechanism results in energy storage and increasing kinetic energy, which results in thermal energy. The transfer of thermal energy is based on two mechanisms:
The first is given by the absorption of the elastic relaxation force, which returns the particles to the equilibrium position after their displacement;
The second one, called classical absorption, is given by the friction between particles which convert the energy into heat (mainly involved in the FUS treatment).
Application
FUS uses high acoustic intensity.
Currently, US therapy exploits two different effects.
US waves could determine both mechanical/cavitation forces or thermal heating.
Cavitation effects result from the capacity of US waves to form microbubbles in tissues that violently collapse, releasing a high amount of energy; this focalized energy fragments the tissue, ultimately creating a hole.
Mechanical and cavitation tissue effects of US waves are used for extracorporeal shock waves lithotripsy.
The thermal heating effect is mainly a consequence of classical absorption, instead.
An increase in energy storage and friction between particles determines an increase in temperature. When a value of 56°C is reached for at least one second, coagulative necrosis occurs because of protein denaturation. Thermal heating effect is used to treat different tumors and treat essential tremors (29).
Bone lesions
MRgFUS can be used to treat painful bone lesions with palliative intentions like for bone metastases or for the treatment of benign lesions (30).
In fact, in addition to traditional approaches such as surgery, radiotherapy, and chemotherapy, MRgFUS treatment is recognized as a non-invasive and radiation-free treatment that, if needed, can also be repeated (31, 32).
Compared to soft tissues, lower acoustic powers are sufficient to cause the periosteal denervation of the bone (27, 33).
When the cortical bone surface is intact, the high absorption of heat causes secondary heating of the adjacent periosteum (34).
As demonstrated by Bitton et al., tissue decay rates and cooling times near the treated bone are 3.5 times longer than in soft tissues (such as in fibroid treatment).
Furthermore, the bone’s density directly affects heating. Whereas sclerotic lesions increase the US attenuation, osteolytic lesions may not lead to sufficient heating (26)
Because of these reasons, to obtain efficacy, an accurate selection of both the patient and the lesion is needed.
The importance of the targeted lesion’s location is also given by the necessity of an adequate acoustic window and the correct identification of highly sensitive structure in its proximities. E.g., an epiphyseal lesion often shows a limited acoustic window due to the complexity of the articular structure. However, different studies have shown the high effectiveness and feasibility in the treatment of superficial epiphyseal bone lesions shielding the cartilaginous or synovial structures and with no signs of the precocious onset of osteoarthritis (35).
Differently, for spinal lesions, HIFU does not find a primary indication considering the nearness of the spinal cord and spinal roots. An abnormal spread of the US beam could cause a severe injury in these structures. Moreover, all highly sensitive structures, such as tendons or nerves, must be protected from the potential damage given by an erroneous US beam spread.
Furthermore, erratic transmission of thermal energy could also result from a contiguous area or the refraction of US waves on perilesional calcifications induced by the intense inflammatory activity of bone lesions.
In this regard, however, MR allows obtaining real-time monitoring on thermal variations of the target area during the sonication (or ablation). The proton resonance frequency (PRF) is a sequence that evaluates the temperature-dependent phase changes in gradient-recalled echo (GRE) sequences. Temperature-dependent phase changes during the sonication are subtracted from those acquired before the ablation, thus providing accurate thermometric maps of the treated region. The only two exceptions are bone and fat tissue, which have poor water content.
Patients with bone metastases often have poor survival due to the primary cancer (36-38).
Since the first study of Catale et al., MRgFUS has achieved critical goals in bone metastases treatment, with a proved efficacy in the pain palliation through both the periosteum denervation and lesion ablation (39).
However, MRgFUS currently finds a limited indication due to the capacity of other “needle” techniques to achieve excellent results with shorter procedure times and the possibility to perform consolidation procedures during the same treatment (40).
Conversely, MRgFUS has gained a critical role in Osteoid Osteoma ablation, and its effectiveness is widely described in literature, despite the gold standard remains RF ablation (41, 42)(figure 1).
Figure 1.

Treatment of osteoid osteoma of the tibia (img c). In images a and b, “mobile” transducer allows easy access to the lesions. Comparison before and after treatment: img d and e show the progressive reduction of bone edema; img f and g show progressive lesion densification after the ablative treatment.
Moreover, promising results were also derived by the treatment of Osteoblastomas, nevertheless the higher biological aggressiveness and a greater tendency to grow compared to Osteoid Osteomas (43)(figure. 2).
Figure 2.

Intra-articular elbow histologically-confirmed osteoblastoma (img c). In image a and b, accurate positioning of patient and PFR monitoring with thermometric map indicating effective temperature reached in the lesion. Comparison before and after treatment: img c and d show the progressive reduction of bone edema and synovitis; img e and f show a progressive resitutio-ad-integrum.
MRgFUS could be helpful also in the treatment of superficial symptomatic “do not touch lesion” such as pseudo-tumoral or inflammatory lesions that do not require surgical treatment (44). Sometimes these lesions could cause severe pain and functional impairments. Undoubtedly, in image-guided therapies, the well-defined visualization of the lesion is considered utmost importance, allowing a complete eradication of the painful bone lesion. Conversely, surgical options are often inadequate due to the higher recurrence ratio (figure 3)
Figure 3.
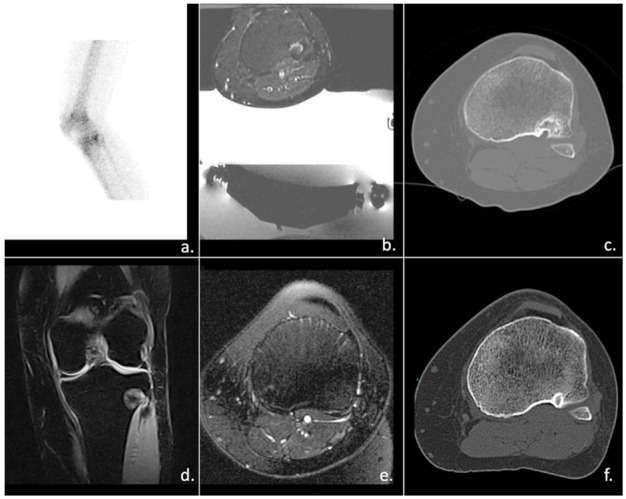
Recurrence of chondroblastoma (img a and c). In image b, MRgFUS approach. 1y after the treatment img e show the progressive reduction of bone edema; img f shows the progressive resitutio-ad-integrum of tibial plate.
The most important selection criteria are:
The quality of the cortical bone’s surface (because of the difference in the heat absorption depending on whether it is intact or sclerotic);
The skeletal location (considering the necessity of an adequate acoustic window and the correct identification of highly sensitive structure in its proximities);
Medium acoustic impedance with an adequate acoustic window.
Main contraindications are:
Relative and absolute contraindications to MR;
Bone fragility;
Skin scars in the area to be treated.
Uterine fibroids
Many studies have confirmed MRgFUS as a safe, organ-preserving treatment for symptomatic uterine fibroids (45-51)(figure 4).
Figure 4.
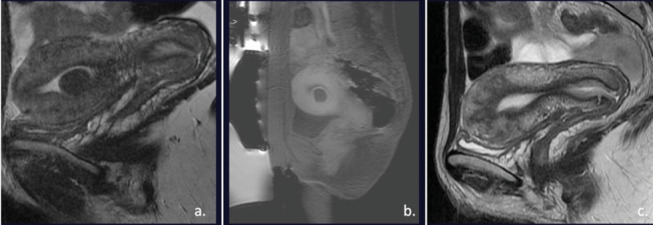
Submucos uterine fibroid (img a). T1 weighted images after the treatment show a complete necrosis of the fibroid. After 1y, MR images showed a complete resolution (img c).
MRgFUS finds indication in the treatment of symptomatic uterine fibroids with an anatomical position that can be safely accessed by the US (without the intestines, the bladder, nerves, or large scars within the beam path). Treatment should be withdrawn if the fibroid is larger than 10cm or more than five fibroids.
Apart from fibroids dimensions and the MR imaging, counseling with the gynecologist considering the patient’s pregnancy desire is mandatory even though different studies have reported natural pregnancy cases after MRgFUS treatment (52).
Evaluation of T2-weighted MR images is fundamental; in fact, the T2 signal intensity of uterine fibroids is a well-known predictor of the immediate therapeutic responses to MR-guided HIFU ablation therapy. Different studies have shown that fibroids with high T2 signal intensity (also known as Type III considering Funaki’s fibroids classification) are more resistant to heating. Therefore they are typically excluded from treatment.
Furthermore, the evaluation of the fibroids’ perfusion by Perfusion MR imaging is mandatory; in fact, degenerated fibroids which do not have any vascularization are excluded.
In a recent meta-analysis including 1725 pts, comparing HIFU to other techniques, MRgFUS showed a pooled efficacy of .94. Despite the overall non-significant higher response rate, MRgFUS resulted superior in safety in terms of pain/discomfort, fever, transfusion, genital tract, gastrointestinal tract, and anesthesia-related complications (53, 54).
The main contraindications for MRgFUS treatment of uterine fibroids are:
Relative and absolute contraindications to MR;
Suspicion of malignancy;
Pedunculated subserosal fibroids due to the risk of detachment.
Brain Disorders
For years, MRgFUS’s application in neurological diseases has been limited by the necessity of a craniotomy to allow the intracranial penetration of the US beam. The introduction of transcranial thalamotomy using MRgFUS (tc-MRgFUS) scattering and refraction corrections of the US penetration at the skull has raised the feasibility of this treatment.
The main neurological indications are medication-refractory Essential Tremor (ET) and Parkinson’s Disease (PD) tremor (55)(figure 5).
Figure 5.

Left thalamotomy in patient with essential tremor. During the procedure, simple neurological motor tests are performed to monitor the effectiveness of the treatment (img a). Img b and d show the high improvement in the ability to write precision the day after the procedure. Img c shows the millimeter effect of ablation using HIFU
Moreover, many clinical and pre-clinical trials are focusing on MRgFUS application also in tumors, stroke, epilepsy, and functional disorders (56).
Even though their pathophysiology is different, the MR precisely targets the high-intensity US towards the ventral intermediate nucleus of the thalamus (the area appointed with movement control) with an immediate and durable reduction of the tremor.
Several Systematic Reviews have compared Deep Brain Stimulation (DBS), which currently is the gold standard for ET treatment, to MRgFUS. Considering unilateral treatments (MRgFUS can only treat one side at a time, typically starting from the dominant hand), the main results were that even if the tremor improvement was greater after DBS, the quality-of-life improvement was significantly greater following MRgFUS thalamotomy (57).
The main advantage of DBS is that this procedure is reversible and can be used bilaterally. On the other hand, with the implant of electrodes in the thalamus and a pulse generator, DBS includes surgery’s risks like bleeding, infection, seizure, and the misplacement of the lead (58).
MRgFUS requires an intensity greater than 1000 W/cm2 to induce thermal ablation of the tissue of interest. Furthermore, different studies have shown that around 300W/cm2 can also rattle the synaptic contacts, directly suppressing the neuronal activity.
The hyperthermia obtained by reaching temperatures above 45°C causes the coagulative tissue’s necrosis.
Meanwhile, intervening tissues such as the scalp, skull, cerebrospinal fluid, and superficial structures receive unfocused ultrasonic waves with intensities lower than the heating threshold of tissues (59-61).
Future FUS applications for motor and non-motor Parkinson’s disease symptoms include the treatment of different areas apart from the ventral intermediate nucleus of the thalamus, for example the treatment of the ventral posterior nucleus which receives cerebellar afferents. Furthermore, several studies are evaluating the unilateral pallidotomy in patients with dyskinesia.
Main contraindications are:
MR contraindication;
Brain tumors;
Cranial implants;
Hemorrhagic risk factors.
Other application
Considering the advantage of being non-invasive and thanks to its low complications rate, MRgFUS treatment has been clinically approved for several solid malign and benign tumors. The use in Hepatocellular carcinoma (HCC) has proven effective in survival rate and quality of life. Different studies have shown an efficient reduction of the tumor by causing coagulative necrosis and damaging the blood vessels. It is often used in association with other treatments such as chemotherapy and transcatheter arterial chemoembolization (TACE) (62, 63).
Regarding breast tumors, the most crucial downside of MRgFUS is the inability to obtain the histological specimen and assess the lesion’s margins. Although this treatment has first been approved for fibroadenomas, several studies have demonstrated its effectiveness in grating a breast-sparing therapy for early-stage localized breast cancer. This evidence has an essential recognition in patients with a high surgical risk (64-67).
The focal treatment of prostate cancer with MRgFUS has proven effective in significantly reducing the prostate-specific antigen (PSA), increasing the survival rate in patients who are not suitable for surgery (68-70).
Considering the typical late detection of pancreatic cancer and its low surgical indication when diagnosed, MRgFUS is emerging to reduce pain and as a potential treatment. Pancreatic cancer patients often show weight loss, fatigue, jaundice, abdominal pain and nausea. Moreover, a marked peripheral insulin-resistance could be highlighted and several complications included heart failure can derived by many of chemotherapy (71-74).
In this regard, in addition to chemo- and radiotherapy, high-intensity ultrasound can help control the local growth of the tumor, improving the quality of life (75, 76).
Furthermore, although the acoustic beam path is complicated by respiratory and bowel movements, several clinical trials have used MRgFUS to treat renal tumors and esophagus cancer with promising results (77, 78).
Conclusions
HIFU treatment offers a completely non-invasive approach.
Imaging guidance provides a new interventional point of view thanks to both temperature monitoring and lesion visualization. Therefore, MRgFUS allows the visualization of the real-time effectiveness of the treatment and all potential adverse reactions, including the most critical damage to sensitive organs.
Indeed, the desirability of this procedure is proportionate to its complexity. To ensure an efficient and safe treatment, the careful evaluation of the lesion and the region to be treated is mandatory. Nonetheless, specific knowledge and competencies are needed.
Conflicts of interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article.
References
- Barile A, Quarchioni S, Bruno F, et al. Interventional radiology of the thyroid gland: critical review and state of the art. Gland Surg. 2018;7:132–46. doi: 10.21037/gs.2017.11.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barile A, Regis G, Masi R, et al. Musculoskeletal tumours: preliminary experience with perfusion MRI. Radiol Med. 2007;112:550–61. doi: 10.1007/s11547-007-0161-5. [DOI] [PubMed] [Google Scholar]
- Bruno F, Arrigoni F, Palumbo P, et al. The Acutely Injured Wrist. Radiol Clin North Am. 2019;57:943–55. doi: 10.1016/j.rcl.2019.05.003. [DOI] [PubMed] [Google Scholar]
- Bruno F, Arrigoni F, Palumbo P, et al. New advances in MRI diagnosis of degenerative osteoarthropathy of the peripheral joints. Radiol Med. 2019;124:1121–27. doi: 10.1007/s11547-019-01003-1. [DOI] [PubMed] [Google Scholar]
- Bruno F, Barile A, Arrigoni F, et al. Weight-bearing MRI of the knee: a review of advantages and limits. Acta Biomed. 2018;89:78–88. doi: 10.23750/abm.v89i1-S.7011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masciocchi C, Lanni G, Conti L, et al. Soft-tissue inflammatory myofibroblastic tumors (IMTs) of the limbs: potential and limits of diagnostic imaging. Skeletal Radiol. 2012;41:643–9. doi: 10.1007/s00256-011-1263-7. [DOI] [PubMed] [Google Scholar]
- Scialpi M, Cappabianca S, Rotondo A, et al. Pulmonary congenital cystic disease in adults. Spiral computed tomography findings with pathologic correlation and management. Radiol Med. 2010;115:539–50. doi: 10.1007/s11547-010-0467-6. [DOI] [PubMed] [Google Scholar]
- Scialpi M, Palumbo B, Pierotti L, et al. Detection and characterization of focal liver lesions by split-bolus multidetector-row CT: diagnostic accuracy and radiation dose in oncologic patients. Anticancer Res. 2014;34:4335–44. [PubMed] [Google Scholar]
- Zappia M, Reginelli A, Russo A, et al. Long head of the biceps tendon and rotator interval. Musculoskelet Surg. 2013;97(Suppl 2):S99–108. doi: 10.1007/s12306-013-0290-z. [DOI] [PubMed] [Google Scholar]
- Muccio CF, Di Blasi A, Esposito G, Brunese L, D’Arco F, Caranci F. Perfusion and spectroscopy magnetic resonance imaging in a case of lymphocytic vasculitis mimicking brain tumor. Pol J Radiol. 2013;78:66–9. doi: 10.12659/PJR.884011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consoli A, Cuccuini M, Lorenzini F, et al. Anatomical variations of the posterior circulation: case reports and a review of literature. Ital J Anat Embryol. 2012;117:13–22. [PubMed] [Google Scholar]
- Consoli A, Grazzini G, Renieri L, et al. Effects of hyper-early (<12 hours) endovascular treatment of ruptured intracranial aneurysms on clinical outcome. Interv Neuroradiol. 2013;19:195–202. doi: 10.1177/159101991301900208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consoli A, Renieri L, Nappini S, et al. Endovascular treatment with ‘kissing’ flow diverter stents of two unruptured aneurysms at a fenestrated vertebrobasilar junction. J Neurointerv Surg. 2013;5:e9. doi: 10.1136/neurintsurg-2011-010188. [DOI] [PubMed] [Google Scholar]
- Renieri L, Consoli A, Scarpini G, Grazzini G, Nappini S, Mangiafico S. Double arterial catheterization technique for embolization of brain arteriovenous malformations with onyx. Neurosurgery. 2013;72:92–8. doi: 10.1227/NEU.0b013e318276b2c0. discussion 98. [DOI] [PubMed] [Google Scholar]
- Giordano AV, Arrigoni F, Bruno F, et al. Interventional Radiology Management of a Ruptured Lumbar Artery Pseudoaneurysm after Cryoablation and Vertebroplasty of a Lumbar Metastasis. Cardiovasc Intervent Radiol. 2017;40:776–79. doi: 10.1007/s00270-016-1551-7. [DOI] [PubMed] [Google Scholar]
- Manetta R, Palumbo P, Gianneramo C, et al. Correlation between ADC values and Gleason score in evaluation of prostate cancer: multicentre experience and review of the literature. Gland Surg. 2019;8:S216–S22. doi: 10.21037/gs.2019.05.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi A, Bustini M, Prosperini P, et al. Neuromorphological abnormalities in schizophrenic patients with good and poor outcome. Acta Psychiatr Scand. 2000;101:161–6. doi: 10.1034/j.1600-0447.2000.900666.x. [DOI] [PubMed] [Google Scholar]
- Ruscitti P, Bruno F, Berardicurti O, et al. Lung involvement in macrophage activation syndrome and severe COVID-19: results from a cross-sectional study to assess clinical, laboratory and artificial intelligence-radiological differences. Ann Rheum Dis. 2020;79:1152–55. doi: 10.1136/annrheumdis-2020-218048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpino M, Lolli F, Lanzo G, et al. Neurophysiology and neuroimaging accurately predict poor neurological outcome within 24 hours after cardiac arrest: The ProNeCA prospective multicentre prognostication study. Resuscitation. 2019;143:115–23. doi: 10.1016/j.resuscitation.2019.07.032. [DOI] [PubMed] [Google Scholar]
- Silvestri E, Barile A, Albano D, et al. Interventional therapeutic procedures in the musculoskeletal system: an Italian Survey by the Italian College of Musculoskeletal Radiology. Radiol Med. 2018;123:314–21. doi: 10.1007/s11547-017-0842-7. [DOI] [PubMed] [Google Scholar]
- Zoccali C, Rossi B, Zoccali G, et al. A new technique for biopsy of soft tissue neoplasms: a preliminary experience using MRI to evaluate bleeding. Minerva Med. 2015;106:117–20. [PubMed] [Google Scholar]
- Chianca V, Albano D, Messina C, et al. Rotator cuff calcific tendinopathy: from diagnosis to treatment. Acta Biomed. 2018;89:186–96. doi: 10.23750/abm.v89i1-S.7022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Orazio F, Splendiani A, Gallucci M. 320-Row Detector Dynamic 4D-CTA for the Assessment of Brain and Spinal Cord Vascular Shunting Malformations. A Technical Note. Neuroradiol J. 2014;27:710–7. doi: 10.15274/NRJ-2014-10096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pietto F, Chianca V, de Ritis R, et al. Postoperative imaging in arthroscopic hip surgery. Musculoskelet Surg. 2017;101:43–49. doi: 10.1007/s12306-017-0459-y. [DOI] [PubMed] [Google Scholar]
- Gravina GL, Marampon F, Muzi P, et al. PXD101 potentiates hormonal therapy and prevents the onset of castration-resistant phenotype modulating androgen receptor, HSP90, and CRM1 in preclinical models of prostate cancer. Endocr Relat Cancer. 2013;20:321–37. doi: 10.1530/ERC-12-0240. [DOI] [PubMed] [Google Scholar]
- Masciocchi C, Conchiglia A, Gregori LM, Arrigoni F, Zugaro L, Barile A. Critical role of HIFU in musculoskeletal interventions. Radiol Med. 2014;119:470–5. doi: 10.1007/s11547-014-0414-z. [DOI] [PubMed] [Google Scholar]
- Siedek F, Yeo SY, Heijman E, et al. Magnetic Resonance-Guided High-Intensity Focused Ultrasound (MR-HIFU): Technical Background and Overview of Current Clinical Applications (Part 1) Rofo. 2019;191:522–30. doi: 10.1055/a-0817-5645. [DOI] [PubMed] [Google Scholar]
- Schlesinger D, Benedict S, Diederich C, Gedroyc W, Klibanov A, Larner J. MR-guided focused ultrasound surgery, present and future. Med Phys. 2013;40:080901. doi: 10.1118/1.4811136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts WW, Hall TL, Ives K, Wolf JS, Jr, Fowlkes JB, Cain CA. Pulsed cavitational ultrasound: a noninvasive technology for controlled tissue ablation (histotripsy) in the rabbit kidney. J Urol. 2006;175:734–8. doi: 10.1016/S0022-5347(05)00141-2. [DOI] [PubMed] [Google Scholar]
- Masciocchi C, Arrigoni F, La Marra A, Mariani S, Zugaro L, Barile A. Treatment of focal benign lesions of the bone: MRgFUS and RFA. Br J Radiol. 2016;89:20150356. doi: 10.1259/bjr.20150356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrigoni F, Bruno F, Zugaro L, et al. Developments in the management of bone metastases with interventional radiology. Acta Biomed. 2018;89:166–74. doi: 10.23750/abm.v89i1-S.7020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrigoni F, Gregori L, Zugaro L, Barile A, Masciocchi C. MRgFUS in the treatment of MSK lesions: A review based on the experience of the University of L’Aquila, Italy. Translational Cancer Research. 2014;3:442–48. [Google Scholar]
- ten Eikelder HM, Bosnacki D, Elevelt A, et al. Modelling the temperature evolution of bone under high intensity focused ultrasound. Phys Med Biol. 2016;61:1810–28. doi: 10.1088/0031-9155/61/4/1810. [DOI] [PubMed] [Google Scholar]
- Bucknor MD, Ozhinsky E, Shah R, Krug R, Rieke V. Effect of Sonication Duration and Power on Ablation Depth During MR-Guided Focused Ultrasound of Bone. J Magn Reson Imaging. 2017;46:1418–22. doi: 10.1002/jmri.25676. [DOI] [PubMed] [Google Scholar]
- Arrigoni F, Barile A, Zugaro L, et al. Intra-articular benign bone lesions treated with Magnetic Resonance-guided Focused Ultrasound (MRgFUS): imaging follow-up and clinical results. Med Oncol. 2017;34:55. doi: 10.1007/s12032-017-0904-7. [DOI] [PubMed] [Google Scholar]
- Belfiore G, Belfiore MP, Reginelli A, et al. Concurrent chemotherapy alone versus irreversible electroporation followed by chemotherapy on survival in patients with locally advanced pancreatic cancer. Med Oncol. 2017;34:38. doi: 10.1007/s12032-017-0887-4. [DOI] [PubMed] [Google Scholar]
- Cappabianca S, Porto A, Petrillo M, et al. Preliminary study on the correlation between grading and histology of solitary pulmonary nodules and contrast enhancement and [18F]fluorodeoxyglucose standardised uptake value after evaluation by dynamic multiphase CT and PET/CT. J Clin Pathol. 2011;64:114–9. doi: 10.1136/jcp.2010.076562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caranci F, Brunese L, Reginelli A, Napoli M, Fonio P, Briganti F. Neck neoplastic conditions in the emergency setting: role of multidetector computed tomography. Semin Ultrasound CT MR. 2012;33:443–8. doi: 10.1053/j.sult.2012.06.011. [DOI] [PubMed] [Google Scholar]
- Catane R, Beck A, Inbar Y, et al. MR-guided focused ultrasound surgery (MRgFUS) for the palliation of pain in patients with bone metastases-preliminary clinical experience. Ann Oncol. 2007;18:163–67. doi: 10.1093/annonc/mdl335. [DOI] [PubMed] [Google Scholar]
- Cazzato RL, Arrigoni F, Boatta E, et al. Percutaneous management of bone metastases: state of the art, interventional strategies and joint position statement of the Italian College of MSK Radiology (ICoMSKR) and the Italian College of Interventional Radiology (ICIR) Radiol Med. 2019;124:34–49. doi: 10.1007/s11547-018-0938-8. [DOI] [PubMed] [Google Scholar]
- Arrigoni F, Napoli A, Bazzocchi A, et al. Magnetic-resonance-guided focused ultrasound treatment of non-spinal osteoid osteoma in children: multicentre experience. Pediatr Radiol. 2019;49:1209–16. doi: 10.1007/s00247-019-04426-0. [DOI] [PubMed] [Google Scholar]
- De Filippo M, Russo U, Papapietro VR, et al. Radiofrequency ablation of osteoid osteoma. Acta Biomed. 2018;89:175–85. doi: 10.23750/abm.v89i1-S.7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrigoni F, Barile A, Zugaro L, et al. CT-guided radiofrequency ablation of spinal osteoblastoma: treatment and long-term follow-up. Int J Hyperthermia. 2018;34:321–27. doi: 10.1080/02656736.2017.1334168. [DOI] [PubMed] [Google Scholar]
- Zoccali C, Arrigoni F, Mariani S, Bruno F, Barile A, Masciocchi C. An unusual localization of chondroblastoma: The triradiate cartilage; from a case report a reconstructive technique proposal with imaging evolution. J Clin Orthop Trauma. 2017;8:S48–S52. doi: 10.1016/j.jcot.2017.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masciocchi C, Arrigoni F, Ferrari F, et al. Uterine fibroid therapy using interventional radiology mini-invasive treatments: current perspective. Med Oncol. 2017;34:52. doi: 10.1007/s12032-017-0906-5. [DOI] [PubMed] [Google Scholar]
- Dobrotwir A, Pun E. Clinical 24 month experience of the first MRgFUS unit for treatment of uterine fibroids in Australia. J Med Imaging Radiat Oncol. 2012;56:409–16. doi: 10.1111/j.1754-9485.2012.02376.x. [DOI] [PubMed] [Google Scholar]
- Froling V, Kroncke TJ, Schreiter NF, et al. Technical eligibility for treatment of magnetic resonance-guided focused ultrasound surgery. Cardiovasc Intervent Radiol. 2014;37:445–50. doi: 10.1007/s00270-013-0678-z. [DOI] [PubMed] [Google Scholar]
- Quinn SD, Gedroyc WM. Thermal ablative treatment of uterine fibroids. Int J Hyperthermia. 2015;31:272–9. doi: 10.3109/02656736.2015.1010608. [DOI] [PubMed] [Google Scholar]
- Ruhnke H, Eckey T, Bohlmann MK, et al. MR-guided HIFU treatment of symptomatic uterine fibroids using novel feedback-regulated volumetric ablation: effectiveness and clinical practice. Rofo. 2013;184:983–91. doi: 10.1055/s-0033-1335289. [DOI] [PubMed] [Google Scholar]
- Leslie TA, Kennedy JE. High intensity focused ultrasound in the treatment of abdominal and gynaecological diseases. Int J Hyperthermia. 2007;23:173–82. doi: 10.1080/02656730601150514. [DOI] [PubMed] [Google Scholar]
- Giurazza F, Corvino F, Silvestre M, et al. Role of interventional radiology in obstetric and gynecological diseases. J Radiol Rev. 2020;7:26–38. [Google Scholar]
- Clark NA, Mumford SL, Segars JH. Reproductive impact of MRI-guided focused ultrasound surgery for fibroids: a systematic review of the evidence. Curr Opin Obstet Gynecol. 2014;26:151–61. doi: 10.1097/GCO.0000000000000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y, Hu K, Zhang Y, et al. High-intensity focused ultrasound (HIFU) treatment for uterine fibroids: a meta-analysis. Arch Gynecol Obstet. 2017;296:1181–88. doi: 10.1007/s00404-017-4548-9. [DOI] [PubMed] [Google Scholar]
- Pieri S, Di Felice M, Moreschi E, et al. Transbrachial approach to the treatment of uterine leiomyomas with embolization of the uterine arteries: a preliminary technical experience. Radiol Med. 2015;120:759–66. doi: 10.1007/s11547-015-0498-0. [DOI] [PubMed] [Google Scholar]
- Bruno F, Catalucci A, Arrigoni F, et al. An experience-based review of HIFU in functional interventional neuroradiology: transcranial MRgFUS thalamotomy for treatment of tremor. Radiol Med. 2020;125:877–86. doi: 10.1007/s11547-020-01186-y. [DOI] [PubMed] [Google Scholar]
- Quadri SA, Waqas M, Khan I, et al. High-intensity focused ultrasound: past, present, and future in neurosurgery. Neurosurg Focus. 2018;44:E16. doi: 10.3171/2017.11.FOCUS17610. [DOI] [PubMed] [Google Scholar]
- Giordano M, Caccavella VM, Zaed I, et al. Comparison between deep brain stimulation and magnetic resonance-guided focused ultrasound in the treatment of essential tremor: a systematic review and pooled analysis of functional outcomes. J Neurol Neurosurg Psychiatry. 2020;91:1270–78. doi: 10.1136/jnnp-2020-323216. [DOI] [PubMed] [Google Scholar]
- Harary M, Segar DJ, Hayes MT, Cosgrove GR. Unilateral Thalamic Deep Brain Stimulation Versus Focused Ultrasound Thalamotomy for Essential Tremor. World Neurosurg. 2019;126:e144–e52. doi: 10.1016/j.wneu.2019.01.281. [DOI] [PubMed] [Google Scholar]
- Fishman PS, Frenkel V. Focused Ultrasound: An Emerging Therapeutic Modality for Neurologic Disease. Neurotherapeutics. 2017;14:393–404. doi: 10.1007/s13311-017-0515-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisman M, van den Bosch MA. MR-guided high-intensity focused ultrasound for noninvasive cancer treatment. Cancer Imaging. 2011;11:S161–6. doi: 10.1102/1470-7330.2011.9041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy JE. High-intensity focused ultrasound in the treatment of solid tumours. Nat Rev Cancer. 2005;5:321–7. doi: 10.1038/nrc1591. [DOI] [PubMed] [Google Scholar]
- Wu F, Wang ZB, Chen WZ, Bai J, Zhu H, Qiao TY. Preliminary experience using high intensity focused ultrasound for the treatment of patients with advanced stage renal malignancy. J Urol. 2003;170:2237–40. doi: 10.1097/01.ju.0000097123.34790.70. [DOI] [PubMed] [Google Scholar]
- Wu F, Wang ZB, Chen WZ, et al. Extracorporeal high intensity focused ultrasound ablation in the treatment of 1038 patients with solid carcinomas in China: an overview. Ultrason Sonochem. 2004;11:149–54. doi: 10.1016/j.ultsonch.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Wu F, Wang ZB, Cao YD, et al. “Wide local ablation” of localized breast cancer using high intensity focused ultrasound. J Surg Oncol. 2007;96:130–6. doi: 10.1002/jso.20769. [DOI] [PubMed] [Google Scholar]
- Furusawa H, Namba K, Thomsen S, et al. Magnetic resonance-guided focused ultrasound surgery of breast cancer: reliability and effectiveness. J Am Coll Surg. 2006;203:54–63. doi: 10.1016/j.jamcollsurg.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Zippel DB, Papa MZ. The use of MR imaging guided focused ultrasound in breast cancer patients; a preliminary phase one study and review. Breast Cancer. 2005;12:32–8. doi: 10.2325/jbcs.12.32. [DOI] [PubMed] [Google Scholar]
- Valdora F, Cortese K, Calabrese M, et al. Novel imaging techniques and biological methods for breast cancer evaluation: overview and update. J Radiol Rev. 2020;7:105–16. [Google Scholar]
- Cornford P, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part II: Treatment of Relapsing, Metastatic, and Castration-Resistant Prostate Cancer. Eur Urol. 2017;71:630–42. doi: 10.1016/j.eururo.2016.08.002. [DOI] [PubMed] [Google Scholar]
- Rischmann P, Gelet A, Riche B, et al. Focal High Intensity Focused Ultrasound of Unilateral Localized Prostate Cancer: A Prospective Multicentric Hemiablation Study of 111 Patients. Eur Urol. 2017;71:267–73. doi: 10.1016/j.eururo.2016.09.039. [DOI] [PubMed] [Google Scholar]
- Chaussy CG, Thuroff S. High-Intensity Focused Ultrasound for the Treatment of Prostate Cancer: A Review. J Endourol. 2017;31:S30–S37. doi: 10.1089/end.2016.0548. [DOI] [PubMed] [Google Scholar]
- Gargiulo P, Paolillo S, Ferrazzano F, et al. Prognostic Value of Hormonal Abnormalities in Heart Failure Patients. Heart Fail Clin. 2019;15:371–75. doi: 10.1016/j.hfc.2019.02.006. [DOI] [PubMed] [Google Scholar]
- John P, Butler H, Saif MW. Congestive heart failure secondary to gemcitabine nab-paclitaxel in patients with pancreatic cancer. Anticancer Res. 2014;34:7267–70. [PubMed] [Google Scholar]
- Marsico F, Paolillo S, Gargiulo P, et al. Renal function and cardiac adrenergic impairment in patients affected by heart failure. J Nucl Cardiol. 2019 doi: 10.1007/s12350-019-01975-7. [DOI] [PubMed] [Google Scholar]
- Gargiulo PPS, Bruzzese D, Poccia A, Di Napoli P, Dell’Aversana S, Esposito I, Bardi Luca, Diana Gaetano, Ambrosio Antonio, Prastaro Maria, Asile Gaetano, Marciano Caterina, Dellegrotttaglie Santo, Perrone-Filardi Pasquale. Effects of Sodium-Glucose Co-Transporter-2 (Sglt2) Inhibitors on Major Cardiovascular Events in Type 2 Diabetic Patients a Meta-Analysis of Randomized Controlled Trials. Research Square [Google Scholar]
- Marinova M, Rauch M, Mucke M, et al. High-intensity focused ultrasound (HIFU) for pancreatic carcinoma: evaluation of feasibility, reduction of tumour volume and pain intensity. Eur Radiol. 2016;26:4047–56. doi: 10.1007/s00330-016-4239-0. [DOI] [PubMed] [Google Scholar]
- Anzidei M, Marincola BC, Bezzi M, et al. Magnetic resonance-guided high-intensity focused ultrasound treatment of locally advanced pancreatic adenocarcinoma: preliminary experience for pain palliation and local tumor control. Invest Radiol. 2014;49:759–65. doi: 10.1097/RLI.0000000000000080. [DOI] [PubMed] [Google Scholar]
- Vidal-Jove J, Perich E, Del Castillo MA. Ultrasound Guided High Intensity Focused Ultrasound for malignant tumors: The Spanish experience of survival advantage in stage III and IV pancreatic cancer. Ultrason Sonochem. 2015;27:703–06. doi: 10.1016/j.ultsonch.2015.05.026. [DOI] [PubMed] [Google Scholar]
- Illing RO, Kennedy JE, Wu F, et al. The safety and feasibility of extracorporeal high-intensity focused ultrasound (HIFU) for the treatment of liver and kidney tumours in a Western population. Br J Cancer. 2005;93:890–5. doi: 10.1038/sj.bjc.6602803. [DOI] [PMC free article] [PubMed] [Google Scholar]


