Abstract
Background and aim:
Skin prick test (SPT) with a wheal diameter of >3 mm, generally accepted as a positive, is most commonly use diagnostic tool for Allergic rhinitis. Aim was to validate wheal size of Skin Prick Test for the Bermuda grass, in desert environment, with positive Bermuda grass Nasal challenge in same environment.
Methods:
In 53 adults, mean age 33.43 ± 9.36 years, both gender (females: 33.96%), SPT positive on Bermuda grass with cut off wheal longest diameter of 3 mm, Bermuda grass nasal challenge test (bgNCT) was carried out. Response was assessed subjectively (scored) and objectively (PNIF). Safety profile was assessed by PEF measurement.
Results:
Mean weal size of SPT (mm) was bigger in bgNCT positive patients (n=47; 88.68%) 8 [4, 15] vs 5 [3, 6] (p<0.0001). ROC analysis showed Bermuda Grass SPT at the threshold of >6.5mm enabled identification of Bermuda challenge with sensitivity of 82.98% and specificity of 100.0% (area under the curve 0.9326, standard error 0.03528; 95% confidence interval (CI): 0.8635 to 1.002; p=0.0006203).
Conclusions:
A SPT wheal size ≥6.5mm might be considered as an appropriate wheal size for confirming Bermuda grass allergy in adults with SAR, avoiding the demanding, time consuming and often unavailable bgNCT, especially in patients eligible for allergen immunotherapy. In these patients, bgNCT is recommended if SPT wheal size is <6.5 mm. (www.actabiomedica.it)
Keywords: Bermuda grass, desert environment, nasal challenge test, skin prick test
Introduction
Despite the fact that Kuwait, as a desert country, has scarce vegetation, seasonal allergic rhinitis (SAR) is one of the most common respiratory allergies (1). Previous studies demonstrated that the prevalence of allergic rhinitis (AR) symptoms ever, current symptoms of allergic rhinitis, and physician-diagnosed allergic rhinitis were 43.9%, 30.7%, and 17.1%, respectively (2). Grass pollens are a major cause of SAR in many parts of the world (3), but in desert environment the rate of sensitization to grass although high, is lower in comparison to weeds (76.7% vs 38.0 - 55.5%) (4,5). From hundreds of types of grasses, only a few are responsible for allergy symptoms. It seems that Cynodon dactylon (Bermuda grass; BG) is the only grass able to survive the harsh desert climate and often triggers allergies in Kuwait (5) and Middle East (6). Although both grasses and weeds pollinate all year round, the pollination peak is identified: April-May for grasses and September-October for weeds (2).
With a little exception, there is a high degree of cross-reactivity among all grass-pollens (7). The concentration of airborne grass pollen influences the degree of symptoms in AR patients while the geographic location and climate may determine which grasses may be responsible for the development of symptoms. Consequently, the identification of clinically relevant allergens is the key step of the diagnosis and depends on the type and characteristics of the different allergens and the country where the patients lives (8).
The most common diagnostic tools in allergen sensitization identification are skin prick test (SPT) and an in vitro test to detect serum specific immunoglobulin E (ssIgE). Serum specific IgE results were usually interpreted as a positive or negative at a threshold of 0.35 kU/L of ssIgE (ImmunoCAP, Phadia, Sweden), regardless of the patients’ characteristics or type of antigen (9). Thus, SPT, as a safe and simple procedure (10,8) remains fundamental in the practice of clinical allergy. Although cut off for a positive immediate skin reaction of a 3 mm wheal diameter is a widely accepted criterion (11) it seems to be no consensus among researchers on the diagnostic accuracy of skin testing for allergies, including allergic rhinitis (12,13). With respect to inhalant allergens, several investigations documented that SPT cut off of the 3 mm criterion is not always sufficient in allergy diagnosis (14). It is suggested to establish more scientific guidelines for interpreting the skin tests and assess what they predict (15,9). However, a little scientific data is available to evaluate the validity of this assumption (15). Among diagnostic methods for assessment of allergic sensitization, Nasal Challenge Test (NCT) is considered to be more specific and sensitive than SPT (16,17).
NCT could be considered the gold standard for detecting true allergies in allergic rhinitis (AR) and should be indicated as a specific diagnostic confirmation of AR when discrepancies arise, or difficulties exist in the assessment of the patient’s medical history and the results of skin and/or serological allergy testing (18). On the other hand, the only potentially disease-modifying treatment for SAR is Allergen Immunotherapy (AIT) (19), and according to the recent EAACI position paper, NCT is considered one of the key diagnostic tools when initiating AIT (20). Its disadvantages include its complexity and lack of reliable performance as well as its frequent unavailability.
To our knowledge, there have been no studies about the optimal cut off value of SPT wheal for BG in desert climate.
The aim of this study was to assess the validity of BG SPT (bgSPT) wheal size in detecting positive bgNCT, to determine true allergy among adult patients with SAR in need of BG AIT.
Subjects, materials and methods
The 53 adults with moderate-severe SAR patients referred to Al Rasheed Allergy Centre in Kuwait (from September 2017 to February 2018) were included in the study. Diagnosis of moderate-severe SAR defined according to ARIA guidelines: sleep disturbance and/or impaired daily activities occurring at least 4 days per week for at least 4 weeks (21). All included patients had a positive clinical history for moderate-severe SAR lasting at least 2 consecutive years and positive SPT (based on threshold of ≥3 mm wheal longest diameter) to multiple inhalant allergens, including Bermuda grass pollens. In all of them nasal challenge test with BG (bgNCT) was performed. Pregnancy, patients with dermographism and those on treatment with antihistamines, have been ruled out. Furthermore, patients with significant comorbidities such as acute upper respiratory infection (confirmed by a C-reactive protein [CRP] value), moderate to severe asthma, severe cardio-vascular and other severe chronic diseases, as well as patients with peak nasal inspiratory flow (PNIF) <60 L/min, Peak expiratory flow (PEF) <350 L/min, choanal atresia, nasal polyp, septal perforation, atrophic rhinitis, adenoids obstructing nasal ventilation, were excluded from challenge procedure.
All patients were informed about the risk and outcomes of the procedure and provided informed consent. Ethical clearance was granted by Ministry of Health Research Ethics Committee (number 2017/669).
Skin prick test
SPT was used as the gold standard to describe atopic status. SPT was performed by single head prick lancets on the volar aspect of the forearm, 2 to 3 cm from the wrist and the antecubital fossa as recommended (10). We used a battery of indoor and outdoor inhalant allergens (Diater, Spain) which included BG, one of the leading pollen allergens in our environment. Histamine (10 mg/mL) and saline were used as positive and negative controls, respectively. Results are read 15-20 minutes following allergen extract application. The longest diameter of skin prick wheal ≥3 mm was considered as a positive.
Nasal Challenge Test
Bilateral NCT with BG (bgNCT) allergen (Diater Spain), was carried out in patients polysensitized to inhalant allergens including those positive SPT to BG. The challenge was done at least 4 weeks after pollen season of BG; 4 weeks after respiratory infection; 3 weeks after an acute episode of rhinitis (confirmed by normal CRP value); 1 week after discontinuation of oral antihistamine, nasal corticosteroid, and nasal decongestant; and 2 weeks after antidepressant and oral corticosteroids or the equivalent (≥10 mg/day). Fifteen minutes after accommodation to room temperature and saline nasal challenge to exclude nasal hyper reactivity, progressively increasing concentrations (0.5 and 5 HEP/mL) of freshly reconstituted, commercial freeze-dried allergen solution (5 HEP/mL) were administered intranasally at 20-min intervals using a nasal spray (100 μL/puff) in inferior turbinate. Nasal reaction was assessed following the manufacturer’s recommendations 20 min (pinched nose for 10 min and 10 min un- pinched) after each dose (concentration) of allergen, as follows: sneezing: 0 (0–2 sneezes), 1 (3–4 sneezes), 3 (≥5 sneezes); nasal itching, rhinorrhea, and nasal obstruction: 1 (mild), 2 (moderate), 3 (severe); palate, eyes, and/or ears itching: 0 (absent), 1 (present). In the case of a positive response to any concentration, further provocation was interrupted. The nasal reaction was expressed as total nasal symptoms score (TNSS) considered positive if the score was ≥5 of the maximal 15 points (22). PNIF measures served as objective measurements of bgNCT outcome, while PEF measures as a safety control. Three PNIF measurements were taken: before challenge (basal value), 20 min after placebo (saline), after each given allergen concentration and 8 h after the challenge. The best of the 3 PNIF measurements at each time point was recorded. PEF was measured at the same time points and the best of the 3 measurements was recorded. Reduction in PNIF ≥20% after bgNCT, compared to a baseline value, was an objective measure of nasal patency. A reduction in PEF ≤20% excluded the involvement of the lower airways during the procedure. PNIF and PEF were measured with peak flow meters (Clement-Clark Int. Ltd., Harlow, UK).
Statistics
Accuracy and normality were determined using the Kolmogorov-Smirnov and Shapiro-Wilk tests. Non-parametric and parametric methods were used to calculate statistical significance. Student’s t test, the Mann-Whitney U test, Fisher’s test, and the χ2 test were used to calculate the differences between groups. ANOVA was used to calculate the relative difference distribution variance between variables. Receiver operating characteristics (ROC) analysis was used to determine the optimum value of the SPT wheal size predictive score, and the Hanley and McNeil methods were used to calculate the area under the curve. The statistical hypotheses were tested at the level of α=0.05, and the difference between the groups in the sample was considered significant with two-sided p<0.05. Statistical significance was considered to be achieved at p<0.05, p<0.01, and p<0.001. All data was analyzed using GraphPad Prism 7 (San Diego, CA, USA).
Results
The group of 53 patients included more males (66.04%) than females with similar age distribution among genders (Table 1).
Table 1.
Patients’ baseline and follow up characteristics.
| Patients (number) | 53 | |||
| Females (number, %) | 18 (33.96%) | |||
|
Age (years)
(mean ± standard deviation) |
33.43 ± 9.36 | |||
|
SPT mean wheal size (mm)
(median [minimum, maximum]) |
3 [3, 12] | |||
| bgNCT positive (number, %) | 47 (88.68%) | |||
|
TNSS
(median[minimu, maximum]) |
12 [9, 15] | |||
|
SPT mean wheal size (mm)
(median [minimum, maximum]) |
bgNCT positive |
bgNCT negative |
p value | |
| 8 [3, 15] | 5 [3, 6] | 0.0003* | ||
|
PNIF
(mean ± standard deviation) |
Before bgNCT |
After positive bgNCT |
8 hours after positive bgNCT |
p value |
| 90.85 ± 19.75 | 63.49 ± 24.37 | 93.68 ± 18.69 | <0.0001* | |
|
PNIF fall after bgNCT
(median [minimum, maximum]) |
-30.0 [-100.0, 30.0] | |||
|
PEF
(mean ± standard deviation) |
Before bgNCT |
After bgNCT |
p value | |
| 456.6 ± 70.41 | 442.15 ± 82.84 | 0.3354 | ||
|
PEF fall after bgNCT
(median [minimum, maximum]) |
-0 [-100, 50] | |||
SPT - Skin Prick Test; bgNCT - Bermuda Grass Nasal Challenge Test; TNSS - Total Nasal Symptom Score; PNIF - Peak Nasal Inspiratory Flow; PEF - Peak Expiratory Flow.
BG NCT (bgNCT) was positive in 88.68% patients with median TNSS 12/15. The mean wheal size was significantly bigger in bgNCT positive patients when compared with challenge negative patients (p<0.0003) (Table 1).
A significant reduction in PNIF after positive bgNCT was detected, with its recovering to the baseline value 8 hours after challenge. In contrast, PEF was similar before and after bgNCT (Table 1).
The optimal SPT wheal cut off for BG was determined using ROC curves, constructed by plotting sensitivity vs specificity at various skin prick wheal diameters for BG challenge positive and negative patients (Figure 1).
Figure 1.
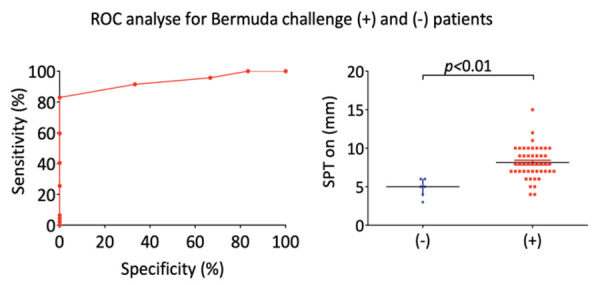
ROC analysis for Bermuda grass positive (+) and negative (-) results of Bermuda Grass Nasal Challenge Test to Skin Prick Test wheal size (mm).
SPT on BG at the threshold of >6.5 mm enabled identification of BG challenge with sensitivity of 82.98% and specificity of 100.0% (area under the curve 0.9326, standard error 0.03528; 95% CI: 0.8635-1.002; p=0.0006203) (Table 2).
Table 2.
Sensitivity and specificity at different cut off of bgSPT in regard of bgNCT positivity
|
Cut off of bgSPT
mean wheal size (mm) |
Sensitivity (%) | 95% CI | Specificity (%) | 95% CI |
Likelihood
Ratio |
| > 3.500 | 100.0 | 92.45% to 100.0% | 16.67 | 0.4211% to 64.12% | > 3.500 |
| > 4.500 | 95.74 | 85.46% to 99.48% | 33.33 | 4.327% to 77.72% | > 4.500 |
| > 5.500 | 91.49 | 79.62% to 97.63% | 66.67 | 22.28% to 95.67% | > 5.500 |
| > 6.500 | 82.98 | 69.19% to 92.35% | 100.0 | 54.07% to 100.0% | > 6.500 |
| > 7.500 | 59.57 | 44.27% to 73.63% | 100.0 | 54.07% to 100.0% | > 7.500 |
| > 8.500 | 40.43 | 26.37% to 55.73% | 100.0 | 54.07% to 100.0% | > 8.500 |
bgSPT - Bermuda Grass Skin Prick Test; bgNCT - Bermuda Grass Nasal Challenge Test; CI - Confidence Interval.
Discussion
Epidemiological studies have revealed that allergies to BG (Cynodon dactylon; subfamily Chloridoideae) mainly affect people in warm tropical and sub-tropical areas of the world (23, 24). It is documented that BG-pollen allergens differed significantly from allergens of the common grasses of temperate zones (25). Clinical evidence of cross-reactivity among grass pollens has suggested that diagnosis and effective immunotherapy can be achieved with a limited number of grasses, based on regional prevalence (23). On the other side, in the real life the amount of pollen each subject gets exposed to, depends on several uncontrollable factors like climate, lifestyle and the actual pollen load in the air (26). Furthermore, polysensitization and cross-reactivity among different pollen species (27) comorbidity or insufficient clinical history might lead to wrong assessment regarding culprit allergen responsible for specific allergic disease and miscalculation in AIT design as well (28). Although advanced diagnostic tools in allergy might improve the selection of patients for immunotherapy (29), SPT as highly specific (79-86%) and sensitive (85-87%) method (30) remains the technique of choice in an allergy practice for identification of causative allergens in patients with AR. The validity of SPT depends on the skill of the tester, the test instrument (16), potency and stability of test reagents and skin color and patient’s age, as well (10). Those factors and the lack of ssIgE cut offs based on the SPT as the clinical criterion standard for various inhalant allergens, might influence on the interpretation of the SPT, as well (31). A positive immediate skin reaction at the threshold of a 3 mm wheal of the longest (32) or mean diameter (10) is widely accepted criterion. However, 3 mm wheal threshold might lead to the overestimation of allergic disease and increase a risk using inadequate AIT (28). Therefore, patients eligible for AIT with Bermuda grass who have small SPT should be taken into consideration to carry out additional testing to verify a clinically relevant allergen before the start of AIT (33). To improve clinical interpretation of SPT results, in terms of its clinical relevance, Haxel et al. calculated quantitative decision points for 18 inhalant allergens and found that the risk of allergic symptoms to particular allergen increased significantly with larger wheal sizes for 17 of the 18 allergens tested (the 80% PPV varied from 3 to 10 mm depending on the allergen) (34). Similar observation was documented in the present study. In our study, the median of the wheal longest diameter was 7 mm (minimum 3 mm and maximum 14 mm) (Table I). However, 11.32% of our SPT positive patients on threshold of 3 mm wheal longest diameter have not reacted on nasal challenge. Furthermore, we observed that all bgNCT positive patients had significantly bigger wheal diameter (mm) in comparison with those who reacted negatively. That observation support results obtained by others (35) that larger skin reactions predict higher likelihood of positive nasal response and better correlate with clinical allergen reactivity with inhalant allergens, as well. In 2004, Zarei et al. found that a 6 mm wheal appears to distinguish those individuals who are cat allergic from those who are not (15). The authors concluded that instead of taking skin prick wheal cutoffs of 3 mm as standard criterion, the prick wheal size cutoff for each allergen should be determined. Similar results are documented by others (36, 37).
NCT as highly specific (83.7%) and sensitive (100%) (38) is considered as the best “gold standard” (20) in diagnosis of SAR. It is a valuable method in cutoff value determination of the SPT wheal which may be used as the clinical criterion standard (9) if culprit allergen is elusive. On the other hand, NCT is a safe procedure in terms of affecting lower airways (19, 37). The present study also supported safety of bgNCT through PEF measurements, which remained stable during and 8 hours after procedure in all subjects (Table 1). Furthermore, fall in PNIF in bgNCT responders showed recovering trend (Table 1). However, NCT is not popular in clinical practice and is limited to tertiary care centers due to its complexity and inability to test more than one allergen at once (22).
Receiver operating characteristic (ROC) curves was used to determine optimal cutoff values by plotting sensitivity vs specificity at various skin prick wheal diameters for Bermuda grass challenge positive and negative patients. Results are shown on Figure 1 and Table 2. For Bermuda grass the threshold of >6.5 mm enabled identification of BG challenge with sensitivity of 82.98% and specificity of 100.0% (area under the curve 0.9326, standard error 0.03528; 95% CI: 0.8635-1.002; p=0.0006203) (Table 2).
Possible limitation of our study includes small cohort of patients and a single center design. In addition, serum sIgE is not analyzed and compared to wheal cut offs because in vitro test is not routinely carried out in majority of included patients. Finally, there is possible influence of patients’ age on the SPT cut offs for different inhalant allergen (9). As our group of patients has been relatively homogenous in regard of age, we have not focused on that issue. More studies with higher number of patients sensitized to allergen typical for desert climate are necessary.
Conclusion
A SPT wheal size ≥6.5 mm for BG might be considered as an appropriate wheal size for confirming BG allergy in adult patients with SAR, avoiding the demanding, time consuming and often unavailable bgNCT. In these patients, bgNCT is recommended if bgSPT wheal size is <6.5 mm.
Conflict of Interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article
References
- Goronfolah L. Aeroallergens, atopy and allergic rhinitis in the Middle East. Eur Ann Allergy Clin Immunol. 2016;48(1):5–21. [PubMed] [Google Scholar]
- Behbehani N, Arifhodzic N, Al-Mousawi M, Marafie S, Ashkanani L, Moussa M, et al. The seasonal variation in allergic rhinitis and its correlation with outdoor allergens in Kuwait. Int Arch Allergy Immunol. 2004;133(2):164–7. doi: 10.1159/000076622. [DOI] [PubMed] [Google Scholar]
- D’Amato G, Cecchi L, Bonini S, Nunes C, Annesi-Maesano I, Behrend H, et al. Allergenic pollen and pollen allergy in Europe. Allergy. 2007;62(9):976–90. doi: 10.1111/j.1398-9995.2007.01393.x. [DOI] [PubMed] [Google Scholar]
- Al-Dowaisan A, Fakim N, Khan MR, Arifhodzic N, Panicker R, Hanoon A, et al. Salsola pollen as a predominant cause of respiratory allergies in Kuwait. Ann Allergy Asthma Immunol. 2004;92(2):262–7. doi: 10.1016/S1081-1206(10)61558-X. [DOI] [PubMed] [Google Scholar]
- Dowaisan A, Al-Ali S, Khan M, Hijazi Z, Thomson MS, Ezeamuzie CI. Sensitization to aeroallergens among patients with allergic rhinitis in a desert environment. Ann Allergy Asthma Immunol. 2000;84(4):433–8. doi: 10.1016/s1081-1206(10)62277-6. [DOI] [PubMed] [Google Scholar]
- Almogren A. Airway allergy and skin reactivity to aeroallergens in Riyadh. Saudi Med J. 2009;30(3):392–6. [PubMed] [Google Scholar]
- Alberse RC. Clinically significant cross-reactivities among allergens. Int Arch Allergy Immuno. 1992;99:261–4. doi: 10.1159/000236261. [DOI] [PubMed] [Google Scholar]
- Heinzerling L, Mari A, Bergmann KC, Bresciani M, Burbach G, Darsow U, et al. The skin prick test - European standards. Clin Transl Allergy. 2013;3(1):3. doi: 10.1186/2045-7022-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SD, Ryu G, Seo MY, Jeong JI, Kim HY, Chung SK, et al. Optimal cutoff values of allergen-specific immunoglobulin E to house dust mites and animal dander based on skin-prick test results: Analysis in 16,209 patients with allergic rhinitis. Am J Rhinol Allergy. 2018;32(1):23–6. doi: 10.2500/ajra.2018.32.4483. [DOI] [PubMed] [Google Scholar]
- Bernstein IL, Li JT, Bernstein DI, Hamilton R, Spector SL, Tan R, et al. American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology. Allergy Diagnostic Testing: An Updated Practice Parameter. Ann Allergy Asthma Immunol. 2008;100(3 Suppl 3):S1–148. doi: 10.1016/s1081-1206(10)60305-5. [DOI] [PubMed] [Google Scholar]
- Bousquet PJ, Chatzi L, Jarvis D, Burney P. Assessing skin prick tests reliability in ECRHS-I. Allergy. 2008;63(3):341–6. doi: 10.1111/j.1398-9995.2007.01581.x. [DOI] [PubMed] [Google Scholar]
- Gungor A, Houser SM, Aquino BF, Akbar I, Moinuddin R, Mamikoglu B, et al. A comparison of skin endpoint titration and skin-prick testing in the diagnosis of allergic rhinitis. Ear Nose Throat J. 2004;83(1):54–60. [PubMed] [Google Scholar]
- Nevis IF, Binkley K, Kabali C. Diagnostic accuracy of skin-prick testing for allergic rhinitis: a systematic review and meta-analysis. Allergy Asthma Clin Immunol. 2016;12:20. doi: 10.1186/s13223-016-0126-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sin AZ, Ersoy R, Gulbahar O, Ardeniz O, Gokmen NM, Kokuludag A. Prevalence of Cypress Pollen Sensitization and Its Clinical Importance in Izmir, Turkey, With Cypress Allergy Assessed by Nasal Provocation. J Investig Allergol Clin Immunol. 2008;18(1):46–51. [PubMed] [Google Scholar]
- Zarei M, Remer CF, Kaplan MS, Staveren AM, Lin CK, Razo E, et al. Optimal skin prick wheal size for diagnosis of cat allergy. Ann Allergy Asthma Immunol. 2004;92(6):604–10. doi: 10.1016/S1081-1206(10)61425-1. [DOI] [PubMed] [Google Scholar]
- Carr WW, Martin B, Howard RS, Cox L, Borish L. Immunotherapy Committee of the American Academy of Allergy, Asthma and Immunology. Comparison of test devices for skin prick testing. J Allergy Clin Immunol. 2005;116(2):341–6. doi: 10.1016/j.jaci.2005.03.035. [DOI] [PubMed] [Google Scholar]
- Choi IS, Kim SJ, Won JM, Park MS. Usefulness of House Dust Mite Nasal Provocation Test in Asthma. Allergy Asthma Immunol Res. 2017;9(2):152–7. doi: 10.4168/aair.2017.9.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondon C, Canto G, Blanca M. Local allergic rhinitis: a new entity, characterization and further studies. Curr Opin Allergy Clin Immunol. 2010;10:1–7. doi: 10.1097/ACI.0b013e328334f5fb. [DOI] [PubMed] [Google Scholar]
- Scadding GW, Eifan A, Penagos M, Dumitru A, Switzer A, McMahon O, et al. Local and systemic effects of cat allergen nasal provocation. Clin Exp Allergy. 2015;45(3):613–23. doi: 10.1111/cea.12434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augé J, Vent J, Agache I, Airaksinen L, Campo Mozo P, Chaker A, et al. EAACI Position paper on the standardization of nasal allergen challenges. Allergy. 2018;73(8):1597–608. doi: 10.1111/all.13416. [DOI] [PubMed] [Google Scholar]
- Brożek JL, Bousquet J, Agache I, Agarwal A, Bachert C, Bosnic-Anticevich S, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines-2016 revision. J Allergy Clin Im-munol. 2017;140(4):950–8. doi: 10.1016/j.jaci.2017.03.050. [DOI] [PubMed] [Google Scholar]
- Tantilipikorn P, Vichyanond P, Lacroix JS. Nasal provocation test: how to maximize its clinical use? Asian Pac J Allergy Immunol. 2010 Dec;28(4):225–31. [PubMed] [Google Scholar]
- Tiwari R, Bhalla PL, Singh MB. Mapping of IgE-binding regions on recombinant Cyn d 1, a major allergen from Bermuda Grass Pollen (BGP) Clin Mol Allergy. 2009;7:3. doi: 10.1186/1476-7961-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Nesf MA, Gharbi D, Mobayed HM, Dason BR, Ali RM, Taha S, et al. The association between airborne pollen monitoring and sensitization in the hot desert climate. Clin Transl Allergy. 2020;10:35. doi: 10.1186/s13601-020-00339-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin BG, Mansfield LE, Nelson HS. Cross-allergenicity among the grasses. Ann Allergy. 1985;54(2):99–104. [PubMed] [Google Scholar]
- Boelke G, Berger U, Bergmann KC, Bindslev-Jensen C, Bousquet J, Gildemeister J, et al. Peak nasal inspiratory flow as outcome for provocation studies in allergen exposure chambers: a GA2LEN study. Clin Transl Allergy. 2017;7:33. doi: 10.1186/s13601-017-0169-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tehrani M, Sankian M, Assarehzadegan MA, Falak R, Jabbari F, Varasteh A. Immunochemical characterization of Amaranthus retroflexus pollen extract: extensive cross-reactive allergenic components among the four species of Amaranthaceae/Chenopodiaceae. Iran J Allergy Asthma Immunol. 2010;9(29):87–95. [PubMed] [Google Scholar]
- Til-Pérez G, Carnevale C, Sarría-Echegaray PL, Arancibia-Tagle D, Chugo-Gordillo S, Tomás-Barberán MD. Sensitization profile in patients with respiratory allergic diseases: differences between conventional and molecular diagnosis (a cross-sectional study) Clin Mol Allergy. 2019;17:8. doi: 10.1186/s12948-019-0112-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Du K, She W, Ouyang Y, Sima Y, Liu C, et al. Recent advances in the diagnosis of allergic rhinitis. Expert Rev Clin Immunol. 2018;14(11):957–64. doi: 10.1080/1744666X.2018.1530113. [DOI] [PubMed] [Google Scholar]
- Demoly P, Bousquet J, Romano A. Adkinson NF Jr, Bochner BS, Busse WW, Holgate ST, Lemanske RF Jr, editors. In vivo methods for the study of allergy. doi: 10.1186/s12948-019-0112-4. eCollection 2019. [Google Scholar]
- Zhang H, Karmaus W, Gan J, Bao W, Zhao YD, Rahardja D, et al. Adjusting wheal size measures to correct atopy misclassification. Int J Gen Med. 2011;4:597–606. doi: 10.2147/IJGM.S22193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstantinou GN, Bousquet PJ, Zuberbier T, Papadopoulos NG. The Longest Wheal Diameter Is the Optimal Measurement for the Evaluation of Skin Prick Tests. Int Arch Allergy Immunol. 2010;151(4):343–5. doi: 10.1159/000250443. [DOI] [PubMed] [Google Scholar]
- Haxel BR, Huppertz T, Boessert P, Bast F, Fruth K. Correlation of skin test results and specific immunoglobulin E blood levels with nasalprovocation testing for house-dust mite allergies. Am J Rhinol Allergy. 2016;30(1):60–4. doi: 10.2500/ajra.2016.30.4262. [DOI] [PubMed] [Google Scholar]
- Haxel BR, Huppertz T, Boessert P, Bast F, Fruth K. Correlation of skin test results and specific immunoglobulin E blood levels with nasalprovocation testing for house-dust mite allergies. Am J Rhinol Allergy. 2016;30(1):60–4. doi: 10.2500/ajra.2016.30.4262. [DOI] [PubMed] [Google Scholar]
- Chusakul S, Phannaso C, Sangsarsri S, Aeumjaturapat S, Snidvongs K. House-dust mite nasal provocation: a diagnostic tool in perennial rhinitis. Am J Rhinol Allergy. 2010;24(2):133–6. doi: 10.2500/ajra.2010.24.3441. [DOI] [PubMed] [Google Scholar]
- Karakaya G, Ozturk ab, Kalyoncu AF. Prediction of atopy by skin prick tests in patients with asthma and/or persistent rhinitis. Allergol Immunopathol (Madr) Allergol Immunopathol (Madr) 2012;40(1):37–40. doi: 10.1016/j.aller.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Al-Ahmad M, Jusufovic E, Arifhodzic N, Nurkic J, Hanoun AL. Sensitization to Cat: When Is Nasal Challenge Needed? Int Arch Allergy Immunol. 2019;179(2):108–13. doi: 10.1159/000496835. [DOI] [PubMed] [Google Scholar]
- de Blay F, Doyen V, Lutz C, Godet J, Barnig C, Qi S, Braun JJ. A new, faster, and safe nasal provocation test method for diagnosing mite allergic rhinitis. Ann Allergy Asthma Immunol. 2015 Nov;115(5):385–390.e1. doi: 10.1016/j.anai.2015.07.014. [DOI] [PubMed] [Google Scholar]


