Abstract
Background:
Transcatheter aortic valve replacement (TAVR) for low gradient (LG) severe aortic stenosis (AS) with preserved left ventricular ejection fraction (LVEF) remains an area of clinical uncertainty.
Methods:
Retrospective review identified 422 patients who underwent TAVR between September 4, 2014 and July 1, 2016. Procedural indication other than severe AS (n = 22) or LVEF ≤50% (n = 98) were excluded. Outcomes were defined by valve academic research consortium two criteria when applicable and compared between LG (peak velocity <4.0 m/s and mean gradient <40 mmHg; n = 73) and high gradient (HG) (n = 229) groups. The LG group was further categorized as low stroke volume index (SVI) (n = 41) or normal SVI (n = 32). Median follow-up was 747 days [interquartile range 220–1013].
Results:
Baseline thirty-day mortality risk (LG 6.2% [3.8–8.1] vs HG 5.7% [4.1–7.4], P = 0.43) did not differ between groups. Short-term outcomes, including procedural success rate (86.1% vs 88.8%, P = 0.53), peri-procedural complications (intra-procedural heart block: 6.8% vs 7.9%, P = 0.99; permanent pacemaker placement: 11.0% vs 13.6%, P = 0.69; moderate paravalvular regurgitation: 2.7% vs 1.3%, P = 0.60), and all-cause in-hospital mortality (2.7% vs 0.9%, P = 0.25) did not differ between LG and HG groups. On long-term follow-up, all-cause mortality also did not differ between LG and HG groups (6.8% vs 10.0%, plog-rank = 0.33) or between the LG low SVI (9.8%), LG normal SVI (3.1%), and HG (10.0%) groups (plog-rank = 0.39).
Conclusion:
Patients with preserved LVEF undergoing TAVR for severe AS with LG, including LG with low SVI, have no significant difference in adverse outcomes when compared to patients with HG.
Keywords: aortic stenosis, transcatheter aortic valve replacement
1 |. INTRODUCTION
Severe aortic stenosis (AS) is a growing health problem as the United States population ages.1 Although the majority of patients with severe AS and preserved left ventricular ejection fraction (LVEF) have high gradients (HG) across the aortic valve, up to 35% may have low gradients (LG).2 There is some controversy as to whether or not LG severe AS with preserved LVEF is a true clinical entity given potential sources of error in left ventricular outflow tract diameter measurement and/or Doppler gradients across the aortic valve. Data suggest, however, that LG severe AS carries a poorer prognosis than both HG severe AS and moderate AS despite the setting of preserved LVEF.2–3 Furthermore, up to 55% of patients with LG severe AS with preserved LVEF may have low flow (stage D3) versus normal flow (stage D4).3 Recent studies, overall, suggest that patients in both categories may benefit from aortic valve replacement if the patient is symptomatic.4
LG severe AS with preserved LVEF may represent progression of AS as it is associated with higher left ventricular afterload.2,5 However, although patients with LG severe AS demonstrate a history of a greater increase in afterload over time than those with HG severe AS, mean gradients do not increase as rapidly, suggesting a unique pathophysiology with ongoing remodeling.6 Regardless of the underlying process, patients with LG severe AS have low survival rates when medically managed, and observational data suggest improvement in prognosis with surgery.2,7,8 Given the totality of the evidence to date, aortic valve replacement for LG severe AS with preserved LVEF is only indicated in the presence of symptoms likely attributable to AS.9 There are no indications to consider aortic valve replacement in asymptomatic individuals unless there is a HG or decreased LVEF.
With the evolution of transcatheter aortic valve replacement (TAVR), both in terms of clinical trial data and device modifications, the patient populations considered for a transcatheter versus surgical approach have expanded.10–11 Accordingly, in this retrospective observational study, we sought to determine the characteristics and outcomes of patients with LG versus HG severe AS in the setting of preserved LVEF after TAVR in an all-comers, real-world setting.
2 |. METHODS
2.1 |. Study population
Consecutive patients who underwent TAVR at NYU Langone Medical Center, a tertiary care center in New York City, between September 4, 2014 and July 1, 2016 were identified (n = 422). Patients with a procedural indication other than severe AS (n = 22) or LVEF ≤50% (n = 98) were excluded from the current study. Each patient was evaluated by a multidisciplinary transcatheter valve team, which consists of a cardiothoracic surgeon trained in both cardiac surgery and interventional cardiology, two interventional cardiologists, a cardiologist with a specialized focus on structural heart disease imaging via echocardiography, and a specialist in cardiac computed tomography. Patients either met clinical criteria for TAVR or were enrolled in an institutional review board-approved study and randomized to TAVR. The institutional review board approved this study with a waiver for informed consent.
2.2 |. Variables of interest
Demographics were self-reported and a trained clinician assistant measured body mass index. History of tobacco use was defined as ever use of >100 cigarettes or five cigars or pipes in the patients’ lifetime. History of hypertension and dyslipidemia required treatment with anti-hypertensive and lipid-lowering therapy, respectively. History of diabetes mellitus was defined when there was either a documented history in the electronic medical record (EMR) or HbA1c>6.5%. History of chronic lung disease was defined as presence of chronic obstructive pulmonary disease, chronic bronchitis, or emphysema as documented in the EMR. History of chronic renal insufficiency was defined as an estimated glomerular filtration <60 mL/min/1.73m2.
Severe AS was defined as aortic valve area <1.0 cm2 on transthoracic echocardiogram using the continuity equation, and confirmed by planimetry and/or right heart catheterization data when available. Patients were categorized as either LG (defined as a peak velocity <4.0 m/s and mean gradient <40 mmHg) or HG (defined as peak velocity ≥4.0 m/s or mean gradient ≥40 mmHg) based on the most recent echocardiogram prior to TAVR. Patients with LG were further categorized as low stroke volume index or normal stroke volume index (>35 cc/m2) based on echocardiographic data and corroborated by right heart catheterization data if performed prior to TAVR.
2.3 |. Outcomes
The primary outcome measure was all-cause mortality. Secondary outcomes included intra-procedural complications, such as conversion to alternative access, femoral artery rupture, annulus rupture, device migration, ectopic valve deployment, ad-hoc valve-in-valve deployment, valve thrombosis, coronary artery obstruction, cardiac tamponade, and heart block. Variables related to valve performance, including paravalvular aortic regurgitation and procedural success, were also reported. Paravalvular regurgitation was assessed intra-operatively with transthoracic echocardiography when monitored anesthesia care was utilized and transesophageal echocardiography when general anesthesia was utilized. Procedural success was defined as absence of procedural mortality, correct positioning of a single prosthetic valve, and intended performance of the prosthetic valve (a composite of no patient prosthesis mismatch, and mean aortic valve gradient <20 mmHg or peak velocity across the aortic valve <3 m/s, and no moderate or severe aortic valve regurgitation). Other secondary outcomes included in-hospital complications that occurred post-TAVR, such as implantation of a permanent pacemaker, cardiac tamponade, stroke, major vascular complications, acute renal failure, bleeding, and cardiac arrest. All outcomes were defined by the Valve Academic Research Consortium-2 (VARC-2) criteria where applicable.12
2.4 |. Statistical analyses
Categorical variables are presented as frequency (proportions) and compared using tests of proportions, while continuous variables (test for skewness with Kolmogorov-Smirnov and Shapiro-Wilks tests) are presented as median [interquartile range] and compared using Mann Whitney test or Kruskal-Wallis test. The association between LG versus HG preserved LVEF severe AS and mortality was assessed with a cox proportional hazards model. This model included demographics (age, sex, race, ethnicity, body mass index) and baseline variables that differed between the LG and HG groups with a significance level set at ≤0.05 (prior stroke or transient ischemic attack, aortic valve area, transfemoral access, device type, and use of post-dilation). In the evaluation of the association between LG low stroke volume index, LG normal stroke volume index, and HG groups and mortality, the model was additionally adjusted for atrial arrhythmias given the significant difference in this co-morbidity at baseline among the three group comparisons. All-cause mortality on follow-up was assessed with Kaplan-Meier estimates from the date of TAVR and compared between groups using the log-rank test. Level of significance was set at a two-sided alpha of 0.05. Statistical analyses were performed using the IBM SPSS Statistics software, version 23 (IBM Corporation, Armonk, New York) and SAS 9.4 (SAS Institute Inc., Cary, NC).
3 |. RESULTS
3.1 |. Baseline characteristics
Baseline demographic and clinical characteristics of patients in the LG (n = 73) versus HG (n = 230) groups are presented in Table 1. Overall, the groups were well-balanced with a median age of 84 years in both groups, a little less than half of the patients in each group of male sex, and the majority of patients in each group of white race. There was, numerically, a lower proportion of Hispanics in the LG versus HG group. Half of the patients had a history of tobacco use and chronic renal insufficiency, while the majority of patients had a history of hypertension, dyslipidemia, and congestive heart failure. About a quarter of the patients had prior cardiac surgery, but only a fifth of these patients with prior cardiac surgery had prior surgical aortic valve replacement. There were more patients with a history of atrial arrhythmias or a history of cerebrovascular event in the LG versus HG group. Overall, these baseline characteristics translated to a numerically higher but not significantly different 30-day mortality risk predicted by the Society of Thoracic Surgery in the LG versus HG group.
TABLE 1.
Baseline characteristics of patients with low versus high gradient severe aortic stenosis in the setting of normal left ventricular ejection fraction
| Low gradient normal ejection fraction severe aortic stenosis (n = 73) | High gradient normal ejection fraction severe aortic stenosis (n = 229) | P-value | |
|---|---|---|---|
| Age, years | 84 [79–89] | 84 [80–88] | 0.75 |
| Male sex | 32 (43.8%) | 112 (48.9%) | 0.50 |
| Race | 0.34 | ||
| White | 70 (95.9%) | 203 (89.0%) | |
| Black | 1 (1.4%) | 10 (4.4%) | |
| Asian | 0 | 3 (1.3%) | |
| Other | 2 (2.7%) | 12 (5.3%) | |
| Ethnicity | |||
| Hispanic | 2 (2.7%) | 15 (6.6%) | 0.38 |
| Body mass index, kg/m2 | 25.5 [23.3–28.7] | 26.6 [23.6–30.8] | 0.10 |
| History of tobacco use | 36 (50.7%) | 112 (50.0%) | 0.99 |
| Hypertension | 56 (76.7%) | 179 (78.2%) | 0.87 |
| Dyslipidemia | 53 (72.6%) | 174 (76.3%) | 0.53 |
| Diabetes mellitus | 25 (34.2%) | 70 (30.6%) | 0.57 |
| Prior myocardial infarction | 9 (12.5%) | 17 (7.5%) | 0.23 |
| Prior cardiac surgery | 20 (27.4%) | 58 (25.4%) | 0.76 |
| Prior surgical aortic valve replacement | 3 (4.2%) | 14 (6.2%) | 0.77 |
| Congestive heart failure | 63 (86.3%) | 197 (86.4%) | 0.99 |
| Chronic lung disease | 18 (24.7%) | 57 (25.1%) | 0.99 |
| Atrial arrhythmia | 30 (41.1%) | 65 (28.5%) | 0.06 |
| Prior permanent pacemaker placement | 12 (16.4%) | 26 (11.5%) | 0.31 |
| Prior stroke or transient ischemic attack | 16 (21.9%) | 26 (11.4%) | 0.03 |
| Chronic renal insufficiency | 37 (52.9%) | 108 (48.6%) | 0.59 |
| Number of native coronary arteries diseased | 0.55 | ||
| 0 | 36 (52.2%) | 128 (60.4%) | |
| 1 | 18 (26.1%) | 39 (18.4%) | |
| 2 | 6 (8.7%) | 18 (8.5%) | |
| 3 | 9 (13.0%) | 27 (12.7%) | |
| Society of Thoracic Surgery predicted 30-day mortality risk, % | 6.2 [3.8–8.1] | 5.7 [4.1–7.4] | 0.43 |
Continuous data shown as median [interquartile range] and compared using Mann-Whitney test. Categorical data shown as frequency (proportion) and compared using tests of proportions
Baseline echocardiographic data are presented in Table 2 and demonstrate, as expected, a significantly lower median aortic valve peak velocity and mean gradient in the LG compared with the HG group. Median aortic valve area was significantly higher in the LG compared with the HG group.
TABLE 2.
Echocardiographic and procedural data of patients with low versus high gradient severe aortic stenosis in the setting of normal left ventricular ejection fraction
| Low gradient normal ejection fraction severe aortic stenosis (n = 73) | High gradient normal ejection fraction severe aortic stenosis (n = 229) | P-value | |
|---|---|---|---|
| Aortic valve peak velocity, m/sec | 3.5 [3.2–3.7] | 4.3 [4.1–4.7] | <0.001 |
| Mean gradient, mmHg | 28 [23–31] | 44 [40–55] | <0.001 |
| Aortic valve area, cm2 | 0.75 [0.68–0.86] | 0.70 [0.57–0.80] | <0.001 |
| Left ventricular ejection fraction, % | 65 [60–70] | 65 [60–70] | 0.77 |
| Left ventricular end-diastolic dimension, cm | 4.1 [3.6–4.7] | 4.2 [3.7–4.7] | 0.67 |
| Left ventricular end-systolic diameter, cm | 2.7 [2.3–3.2] | 2.8 [2.4–3.1] | 0.88 |
| Interventricular septum thickness, cm | 1.3 [1.2–1.5] | 1.3 [1.2–1.5] | 0.84 |
| Posterior/inferolateral wall thickness, cm | 1.2 [1.1–1.3] | 1.2 [1.1–1.3] | 0.10 |
| Aortic regurgitation (moderate or severe), % | 6 (8.2%) | 20 (8.7%) | 0.99 |
| Mitral regurgitation (moderate or severe), % | 16 (21.9%) | 31 (13.5%) | 0.10 |
| Mitral stenosis (severe), % | 0 | 7 (3.3%) | 0.20 |
| Tricuspid regurgitation (severe), % | 2 (2.7%) | 4 (1.7%) | 0.63 |
| Aortic root, cm | 3.1 [2.8–3.4] | 3.1 [2.8–3.4] | 0.48 |
| Procedure status, % | |||
| Elective | 71 (97.3%) | 218 (95.2%) | 0.74 |
| Urgent | 2 (2.7%) | 11 (4.8%) | |
| Emergency/Salvage | 0 | 0 | |
| Access, % | 0.05 | ||
| Transfemoral | 70 (95.9%) | 228 (99.6%) | |
| Transapical | 2 (2.7%) | 1 (0.4%) | |
| Subclavian | 1 (1.4%) | 0 | |
| Anesthesia, % | 0.14 | ||
| Monitored anesthesia care | 61 (83.6%) | 207 (90.4%) | |
| General anesthesia | 12 (16.4%) | 22 (9.6%) | |
| Device, % | 0.02 | ||
| 1st generation self-expanding valve | 21 (28.8%) | 61 (26.6%) | |
| 2nd generation self-expanding valve | 34 (46.6%) | 115 (50.2%) | |
| 1st generation balloon-expandable valve | 9 (12.3%) | 8 (3.5%) | |
| 2nd generation balloon-expandable valve | 9 (12.3%) | 45 (19.7%) | |
| Predilation, % | 16 (22.2%) | 75 (32.8%) | 0.11 |
| Postdilation, % | 20 (27.8%) | 98 (42.8%) | 0.03 |
Continuous data shown as median [interquartile range] and compared using Mann-Whitney test. Categorical data shown as frequency (proportion) and compared using tests of proportions.
3.2 |. Procedural data
Procedural data are shown in Table 2. The majority of TAVR procedures were performed electively and via a transfemoral approach with monitored anesthesia care. There was a numerically lower proportion of transfemoral access in the LG versus HG group. Self-expanding valves were used more frequently than balloon-expanding valves overall; there were more 1st generation valves used in the LG versus HG group, possibly indicating a greater proportion of LG patients earlier in the study period. There was a significantly lower rate of post-dilation in the LG versus HG group.
3.3 |. Outcomes
Intra-procedural complications were infrequent, with the exception of heart block, which occurred in less than 10% of cases and did not differ between the LG and HG groups. The proportion of patients with moderate aortic paravalvular regurgitation post-TAVR was low and did not differ between groups. No patients had severe aortic paravalvular regurgitation post-TAVR (Table 3).
TABLE 3.
Peri-procedural complications and outcomes in patients with low versus high gradient severe aortic stenosis in the setting of normal left ventricular ejection fraction
| Low gradient normal ejection fraction severe aortic stenosis (n = 73) | High gradient normal ejection fraction severe aortic stenosis (n = 229) | P-value | |
|---|---|---|---|
| Intra-procedural complications, % | |||
| Conversion to alternative access | 0 | 0 | - |
| Femoral artery rupture | 0 | 0 | - |
| Annulus rupture | 0 | 0 | - |
| Device migration | 0 | 1 (0.4%) | 0.99 |
| Ectopic valve deployment | 0 | 0 | - |
| Valve-in-valve | 0 | 0 | - |
| Valve thrombosis | 0 | 0 | - |
| Coronary artery obstruction | 0 | 0 | - |
| Cardiac tamponade | 0 | 1 (0.4%) | 0.99 |
| Heart block | 5 (6.8%) | 18 (7.9%) | 0.99 |
| Paravalvular regurgitation | |||
| None | 16 (21.9%) | 48 (21.4%) | 0.99 |
| Trace | 35 (47.9%) | 115 (51.3%) | 0.69 |
| Mild | 20 (27.4%) | 58 (25.9%) | 0.88 |
| Moderate | 2 (2.7%) | 3 (1.3%) | 0.60 |
| Severe | 0 | 0 | - |
| Patient-prosthesis mismatch | 0.52 | ||
| Moderate | 6 (8.6%) | 18 (8.3%) | |
| Severe | 0 | 4 (1.8%) | |
| Procedural success, % | 62 (86.1%) | 199 (88.8%) | 0.53 |
| In-hospital complications, % | |||
| Permanent pacemaker placement | 8 (11.0%) | 31 (13.6%) | 0.69 |
| Cardiac tamponade | 2 (2.7%) | 2 (0.9%) | 0.25 |
| Stroke | 2 (2.7%) | 2 (0.9%) | 0.25 |
| Major vascular complication | 0 | 0 | - |
| Acute renal failure | 0.99 | ||
| Stage 1 | 0 | 3 (1.3%) | |
| Stage 2 or 3 | 0 | 0 | |
| Bleeding | 0.74 | ||
| Life-threatening or disabling | 0 | 2 (0.9%) | |
| Major | 2 (2.7%) | 4 (1.7%) | |
| Minor | 7 (9.6%) | 17 (7.4%) | |
| Cardiac arrest | 1 (1.4%) | 0 | 0.24 |
| Post-procedure length of stay, days | 2 [1–3] | 2 [1–3] | 0.80 |
| Total length of stay, days | 2 [2–5] | 3 [2–4] | 0.68 |
| In-hospital all-cause mortality, % | 2 (2.7%) | 2 (0.9%) | 0.25 |
Continuous data shown as median [interquartile range] and compared using Mann-Whitney test. Categorical data shown as frequency (proportion) and compared using tests of proportions
In-hospital complications were also infrequent, with the exception of permanent pacemaker placement, which did not differ between groups. The majority of bleeding events was defined as minor per the VARC-2 criteria. Length of stay, both post-procedural and total, as well as in-hospital all-cause mortality, were low and did not differ between groups (Table 3).
On a median follow-up of 747 days [interquartile range 220–1013], all-cause mortality was low and did not differ between groups (LG 6.8% vs HG 10.0%, plog-rank = 0.33) (Figure 1). There was no significant association between LG preserved LVEF severe AS and all-cause mortality on follow-up (Unadjusted Hazard Ratio 0.62, 95% confidence interval [0.24–1.6]), even after adjustment for age, sex, race, ethnicity, body mass index, prior stroke or transient ischemic attack, aortic valve area, transfemoral access, type of TAVR device deployed, and use of post-dilation (Adjusted Hazard Ratio 0.48, 95% confidence interval [0.16–1.46]).
FIGURE 1.
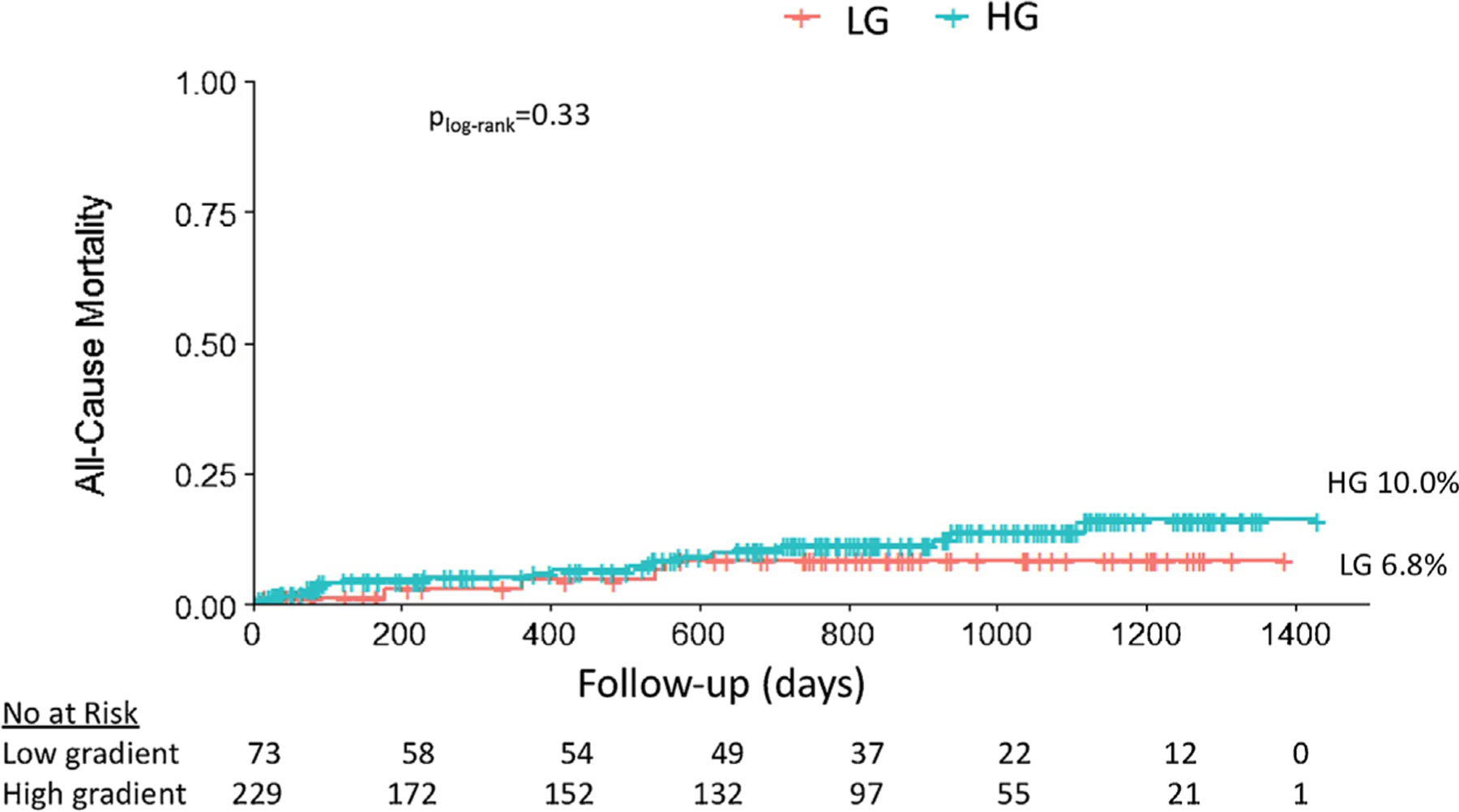
Kaplan-Meier curves of all-cause mortality for patients with low (LG) versus high gradient (HG) severe aortic stenosis in the setting of normal left ventricular ejection fraction
3.4 |. Analysis by stroke volume index
Of the 73 patients with LG severe AS in the setting of normal LVEF, stroke volume index was low in 56% (n = 41). Baseline characteristics of patients in the LG low stroke volume index, LG normal stroke volume index, and HG groups are presented in Table 4. Overall, the groups remain well-balanced. However, there were a significantly higher proportion of patients with atrial arrhythmias and a numerically higher proportion of patients with moderate or severe mitral regurgitation on pre-operative echocardiogram in the LG low stroke volume index group when compared to the other two groups.
TABLE 4.
Characteristics of patients with low gradient low stroke volume index, low gradient normal stroke volume index, and high gradient severe aortic stenosis in the setting of normal left ventricular ejection fraction
| Low gradient low stroke volume index (n = 41) | Low gradient normal stroke volume index (n = 32) | High gradient stenosis (n = 229) | P-value | |
|---|---|---|---|---|
| Age, years | 84 [80–90] | 84 [78–89] | 84 [80–88] | 0.75 |
| Male sex | 22 (53.7%) | 19 (59.4%) | 117 (51.1%) | 0.67 |
| Race | 0.69 | |||
| White | 40 (97.6%) | 30 (93.8%) | 203 (89.0%) | |
| Black | 0 | 1 (3.1%) | 10 (4.4%) | |
| Asian | 0 | 0 | 3 (1.3%) | |
| Other | 1 (2.4%) | 1 (3.1%) | 12 (5.3%) | |
| Ethnicity | ||||
| Hispanic | 1 (2.4%) | 1 (3.1%) | 15 (6.6%) | 0.46 |
| Body mass index, kg/m2 | 26.8 [24.6–29.8] | 24.4 [22.2–27.3] | 26.6 [23.6–30.8] | 0.03 |
| History of tobacco use | 22 (56.4%) | 14 (43.8%) | 112 (50.0%) | 0.57 |
| Hypertension | 33 (80.5%) | 23 (71.9%) | 179 (78.2%) | 0.66 |
| Dyslipidemia | 32 (78.0%) | 21 (65.6%) | 174 (76.3%) | 0.39 |
| Diabetes mellitus | 18 (43.9%) | 7 (21.9%) | 70 (30.6%) | 0.11 |
| Prior myocardial infarction | 6 (15.0%) | 3 (9.4%) | 17 (7.5%) | 0.29 |
| Prior cardiac surgery | 12 (29.3%) | 8 (25.0%) | 58 (25.4%) | 0.87 |
| Prior surgical aortic valve replacement | 1 (2.5%) | 2 (6.3%) | 14 (6.2%) | 0.64 |
| Congestive heart failure | 35 (85.4%) | 28 (87.5%) | 197 (86.4%) | 0.97 |
| Chronic lung disease | 10 (24.4%) | 8 (25.0%) | 57 (25.1%) | 0.99 |
| Atrial arrhythmia | 22 (53.7%) | 8 (25.0%) | 65 (28.5%) | 0.004 |
| Prior permanent pacemaker placement | 7 (17.1%) | 5 (15.6%) | 26 (11.5%) | 0.53 |
| Prior stroke or transient ischemic attack | 9 (22.0%) | 7 (21.9%) | 26 (11.4%) | 0.08 |
| Chronic renal insufficiency | 24 (61.5%) | 13 (41.9%) | 108 (48.6%) | 0.22 |
| Number of native coronary arteries diseased | 0.64 | |||
| 0 | 17 (45.9%) | 19 (59.4%) | 128 (60.4%) | |
| 1 | 12 (32.4%) | 6 (18.8%) | 39 (18.4%) | |
| 2 | 3 (8.1%) | 3 (9.4%) | 18 (8.5%) | |
| 3 | 5 (13.5%) | 4 (12.5%) | 27 (12.7%) | |
| Society of Thoracic Surgery predicted 30-day mortality risk, % | 6.3 [3.8–8.1] | 6.0 [4.3–8.1] | 5.7 [4.1–7.4] | 0.74 |
| Aortic valve peak velocity, m/sec | 3.5 [3.2–3.7] | 3.6 [3.4–3.8] | 4.3 [4.1–4.7] | <0.001 |
| Mean gradient, mmHg | 26 [22–31] | 30 [25–31] | 44 [40–55] | <0.001 |
| Aortic valve area, cm2 | 0.70 [0.66–0.80] | 0.83 [0.75–0.91] | 0.70 [0.57–0.80] | <0.001 |
| Left ventricular ejection fraction, % | 65 [60–70] | 65 [60–70] | 65 [60–70] | 0.92 |
| Left ventricular end-diastolic dimension, cm | 4.1 [3.9–4.8] | 4.2 [3.4–4.6] | 4.2 [3.7–4.7] | 0.69 |
| Left ventricular end-systolic diameter, cm | 2.9 [2.4–3.4] | 2.6 [2.2–3.0] | 2.8 [2.4–3.1] | 0.09 |
| Interventricular septum thickness, cm | 1.3 [1.1–1.5] | 1.4 [1.2–1.5] | 1.3 [1.2–1.5] | 0.86 |
| Posterior/inferolateral wall thickness, cm | 1.2 [1.0–1.3] | 1.2 [1.1–1.3] | 1.2 [1.1–1.3] | 0.26 |
| Aortic regurgitation (moderate or severe), % | 2 (4.9%) | 4 (12.5%) | 20 (8.7%) | 0.51 |
| Mitral regurgitation (moderate or severe), % | 11 (26.8%) | 5 (15.6%) | 31 (13.5%) | 0.10 |
| Mitral stenosis (severe), % | 0 | 0 | 7 (3.3%) | 0.33 |
| Tricuspid regurgitation (severe), % | 2 (4.9%) | 0 | 4 (1.7%) | 0.29 |
| Aortic root, cm | 3.1 [2.9–3.3] | 3.0 [2.8–3.4] | 3.1 [2.8–3.4] | 0.70 |
| Procedure status, % | ||||
| Elective | 40 (97.6%) | 31 (96.9%) | 218 (95.2%) | 0.74 |
| Urgent | 1 (2.4%) | 1 (3.1%) | 11 (4.8%) | |
| Emergency/Salvage | 0 | 0 | 0 | |
| Access, % | 0.02 | |||
| Transfemoral | 40 (97.6%) | 30 (93.8%) | 228 (99.6%) | |
| Transapical | 1 (2.4%) | 1 (3.1%) | 1 (0.4%) | |
| Subclavian | 0 | 1 (3.1%) | 0 | |
| Anesthesia, % | 0.12 | |||
| Monitored anesthesia care | 36 (87.8%) | 25 (78.1%) | 207 (90.4%) | |
| General anesthesia | 5 (12.2%) | 7 (21.9%) | 22 (9.6%) | |
| Device, % | 0.06 | |||
| 1st generation self-expanding valve | 13 (31.7%) | 8 (25.0%) | 61 (26.6%) | |
| 2nd generation self-expanding valve | 16 (39.0%) | 18 (56.3%) | 115 (50.2%) | |
| 1st generation balloon-expandable valve | 5 (12.2%) | 4 (12.5%) | 8 (3.5%) | |
| 2nd generation balloon—expandable valve | 7 (17.1%) | 2 (6.3%) | 45 (19.7%) | |
| Predilation, % | 11 (26.8%) | 5 (16.1%) | 75 (32.8%) | 0.15 |
| Postdilation, % | 9 (22.0%) | 11 (35.5%) | 98 (42.8%) | 0.04 |
Continuous data shown as median [interquartile range] and compared using Kruskal-Wallis test. Categorical data shown as frequency (proportion) and compared using tests of proportions
Intra-procedural and in-hospital complications in the LG low stroke volume index, LG normal stroke volume index, and HG groups are presented in Table 5. Cardiac arrest and in-hospital mortality were significantly higher in the LG normal stroke volume index group when compared to the other two groups, while outcomes between the LG low stroke volume index group and HG group did not differ.
TABLE 5.
Peri-procedural complications and outcomes in patients with low gradient low stroke volume index, low gradient normal stroke volume index, and high gradient severe aortic stenosis in the setting of normal left ventricular ejection fraction
| Low gradient low stroke volume index (n = 41) | Low gradient normal stroke volume index (n = 32) | High gradient stenosis (n = 229) | P-value | |
|---|---|---|---|---|
| Intra-procedural complications, % | ||||
| Conversion to alternative access | 0 | 0 | 0 | - |
| Femoral artery rupture | 0 | 0 | 0 | - |
| Annulus rupture | 0 | 0 | 0 | - |
| Device migration | 0 | 1 (0.4%) | 0.85 | |
| Ectopic valve deployment | 0 | 0 | 0 | - |
| Valve-in-valve | 0 | 0 | 0 | - |
| Valve thrombosis | 0 | 0 | 0 | - |
| Coronary artery obstruction | 0 | 0 | 0 | - |
| Cardiac tamponade | 0 | 0 | 1 (0.4%) | 0.85 |
| Heart block | 3 (7.3%) | 2 (6.3%) | 18 (7.9%) | 0.95 |
| Paravalvular regurgitation | ||||
| None | 7 (17.1%) | 9 (28.1%) | 48 (21.4%) | 0.52 |
| Trace | 21 (51.2%) | 14 (43.8%) | 115 (51.3%) | 0.72 |
| Mild | 11 (26.8%) | 9 (28.1%) | 58 (25.9%) | 0.96 |
| Moderate | 2 (4.9%) | 0 | 3 (1.3%) | 0.20 |
| Severe | 0 | 0 | 0 | - |
| Patient-Prosthesis Mismatch | 0.80 | |||
| Moderate | 4 (10.3%) | 2 (6.5%) | 18 (8.3%) | |
| Severe | 0 | 0 | 4 (1.8%) | |
| Procedural success, % | 35 (85.4%) | 27 (87.1%) | 199 (88.8%) | 0.80 |
| In-hospital complications, % | ||||
| Permanent pacemaker placement | 3 (7.3%) | 5 (15.6%) | 31 (13.6%) | 0.49 |
| Cardiac tamponade | 1 (2.4%) | 1 (3.1%) | 2 (0.9%) | 0.46 |
| Stroke | 1 (2.4%) | 1 (3.1%) | 2 (0.9%) | 0.46 |
| Major vascular complication | 0 | 0 | 0 | - |
| Acute renal failure | 0.62 | |||
| Stage 1 | 0 | 0 | 3 (1.3%) | |
| Stage 2 or 3 | 0 | 0 | 0 | |
| Bleeding | 0.25 | |||
| Life-threatening or disabling | 0 | 0 | 2 (0.9%) | |
| Major | 1 (2.4%) | 1 (3.1%) | 4 (1.7%) | |
| Minor | 1 (2.4%) | 6 (18.8%) | 17 (7.4%) | |
| Cardiac arrest | 0 | 1 (3.1%) | 0 | 0.02 |
| Post-procedure length of stay, days | 2 [1–3] | 2 [1–2] | 2 [1–3] | 0.60 |
| Total length of stay, days | 3 [2–4] | 2 [2–5] | 3 [2–4] | 0.63 |
| In-hospital all-cause mortality, % | 0 | 2 (6.3%) | 2 (0.9%) | 0.03 |
Continuous data shown as median [interquartile range] and compared using Mann-Whitney test. Categorical data shown as frequency (proportion) and compared using tests of proportions.
On a median follow-up of 747 days [interquartile range 220–1013], all-cause mortality was low and did not differ between groups (LG low stroke volume index 9.8%, LG normal stroke volume index 3.1%, HG 10.0%, plog-rank = 0.39) (Figure 2). There was no significant association between LG low stroke volume index preserved LVEF severe AS and all-cause mortality on follow-up (Unadjusted Hazard Ratio 0.92, 95% confidence interval [0.32–2.7]), even after adjustment for age, sex, race, ethnicity, body mass index, atrial arrhythmias, prior stroke or transient ischemic attack, aortic valve area, transfemoral access, type of TAVR device deployed, and use of post-dilation (Adjusted Hazard Ratio 0.49, 95% confidence interval [0.15–1.64]).
FIGURE 2.
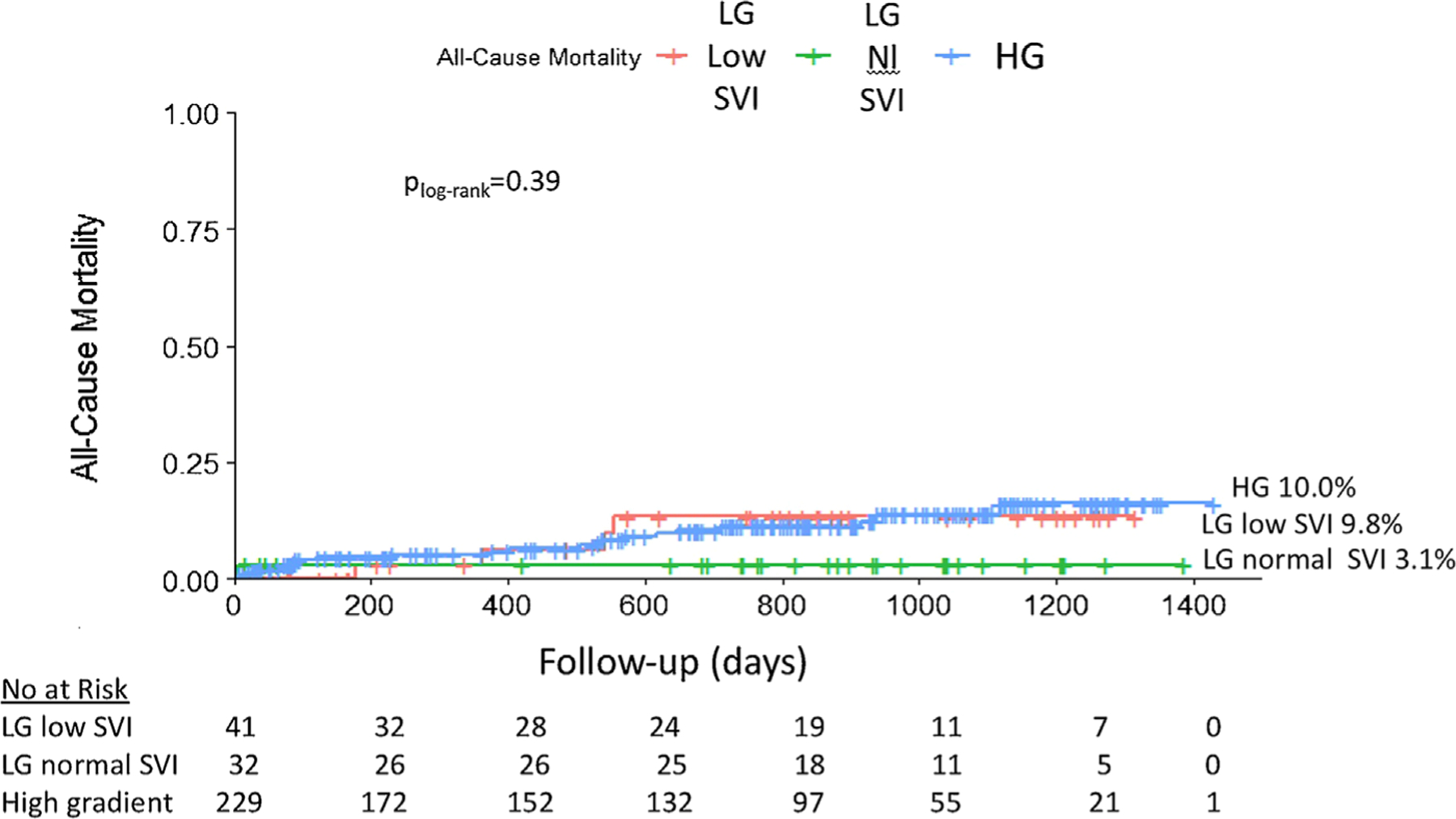
Kaplan-Meier curves of all-cause mortality for patients with low gradient (LG) low stroke volume index (SVI), LG normal SVI, and high gradient (HG) severe aortic stenosis in the setting of normal left ventricular ejection fraction
4 |. DISCUSSION
This is one of the few studies to date to evaluate outcomes in severe AS patients with preserved LVEF undergoing contemporary TAVR in the United States by degree of transvalvular gradients. In this all-comers study, 24% of the defined cohort had a mean gradient <40 mmHg and a peak velocity <4 m/s, and 14% of the patients with severe AS and preserved LVEF had both low gradient and low flow. There were no significant differences in peri-procedural outcomes or all-cause mortality on long-term follow-up post-TAVR between the LG group, including the LG low SVI subset, and HG group.
LG severe AS in the setting of preserved LVEF was first comprehensively defined in 2007, where 512 subjects with an indexed aortic valve area ≤0.6 cm2/m2 and an LVEF ≥50% were identified retrospectively at a single center in Canada.2 Of these subjects, 35% had a low transvalvular gradient and stroke volume index and aortic valve area was calculated to be smaller in the LG group. The authors demonstrated that the LG group had a significantly lower survival rate compared to the HG group, and this difference improved with surgical aortic valve replacement. These results were reproduced by subsequent studies, including a retrospective study of severe AS patients from the Mayo Clinic that also demonstrated poorer rates of survival in those with low transvalvular gradient and stroke volume index.8 In contrast, the current study demonstrates a lower proportion of LG preserved LVEF severe AS in patients undergoing TAVR in the real-world setting, which may reflect a different selection bias in the setting of TAVR versus surgical aortic valve replacement.
However, not all the data on prognosis associated with LG severe AS have been consistent. A single-center study of patients with AS included 776 patients with LG severe AS in the setting of preserved LVEF and 500 patients with HG severe AS.7 Of the patients that were medically managed, all-cause mortality was significantly lower in the LG preserved LVEF group compared to the HG group, but worse than patients with moderate AS. Another group in France retrospectively identified 809 patients with AS and demonstrated that patients with LG in the setting of preserved LVEF had a similar rate of survival when compared to those with mild to moderate AS.13 Finally, in the randomized, multicenter Simvastatin, and Ezetimibe in Aortic Stenosis (SEAS) trial of asymptomatic AS, LG severe AS and preserved LVEF was present in 29% of the cohort and had adverse outcomes at a rate not different from patients with moderate AS.14 Together, the data emphasize the importance of a nuanced approach to LG severe AS in the setting of preserved LVEF, particularly in the era of TAVR when patients are more likely to be more advanced in age and with a greater burden of co-morbidities compared to their surgical counterparts.
It is also important to differentiate patients with LG low SVI and LG normal SVI. The proportion of patients with LG severe AS with preserved LVEF who have low SVI (56%) in the current study is similar to the proportion reported in the literature.3 There are several technical issues that may account for overestimation of AS severity, including an underestimation of the left ventricular outflow tract diameter measurement and subsequent aortic valve area calculation by the continuity equation, and, therefore, corroboration is suggested with other methods, such as planimetry, dobutamine stress echocardiography, multi-detector computed tomography, and cardiac catheterization procedures.4 The heart team approach used for TAVR programs, such as the one used in the current study, therefore, should include dedicated cardiac imaging specialists and an extensive pre-procedural work-up when indicated. Regardless, patients with low SVI (stage D3) have a better prognosis if treated with aortic valve replacement, although the majority of these data are from the surgical population.15 In the current study, patients in the LG low SVI group had no difference in long-term mortality when compared to the HG group despite a higher rate of several medical comorbidities. With surgical aortic valve replacement, there may be a theoretical elevated operative risk of patient-prosthesis mismatch in patients with LG low SVI due to a small left ventricular cavity.15 However, this risk may be mitigated with TAVR with the use of lower profile valves. Patients with LG low SVI may also be at higher operative risk due to a decrease in left ventricular compliance from more pronounced concentric remodeling and myocardial fibrosis.15 This may not play as much of a role in TAVR given that the majority of the patients in the current study underwent transfemoral access under monitored anesthesia care, rather than general anesthesia, and there are no significant fluid shifts associated with TAVR. Of patients who present with normal SVI (stage D4), up to 50% have severe AS after further evaluation, and, therefore, if symptomatic, may still benefit from aortic valve replacement.4 In the current study, the LG normal SVI group had the highest AVA and the lowest numerical rate of mortality on long-term follow-up despite no difference in baseline STS 30-day mortality risk, which may suggest a lower risk AS cohort overall.
There remains a paucity of data in this cohort undergoing TAVR. Of the 196 patients in cohort A (high surgical risk group) of the large, randomized, multi-center, Placement of Aortic Transcatheter Valves (PARTNER) trial, 93 patients underwent TAVR and 103 patients underwent surgical aortic valve replacement.16 Patients with LG severe AS and preserved LVEF made up 31% of the enrolled cohort and had a significantly lower all-cause mortality with TAVR when compared to medical therapy and no significant difference in all-cause mortality when compared with surgical aortic valve replacement at 2 years follow-up. In the more contemporary setting, a report from France compared post-TAVR outcomes of 31 patients with LG severe AS and preserved LVEF to 172 patients with HG severe AS and 59 patients with LG severe AS in the setting of reduced LVEF.17 The authors demonstrated a higher 30-day mortality in the LG versus HG group but similar all-cause mortality at a median of 13.2 months follow-up. Another study from Israel reported similar outcomes after TAVR in the 30 patients with LG severe AS and preserved LVEF and the 82 patients with HG severe AS at both 1-month and 2-year follow-up.18 Furthermore, an analysis of the Transcatheter Valve Therapies Registry evaluated outcomes in 11 292 patients undergoing TAVR stratified by LVEF and mean aortic valve gradient, separately.19 The authors concluded that low mean gradient, but not LVEF, was significantly associated with a higher rate of 1-year mortality and recurrent heart failure. Although the particular subgroups of LG versus HG severe AS in the setting of preserved LVEF were not evaluated, there was no effect modification between mean gradient and LVEF on outcomes as evidenced by a non-significant interaction P-value.
The limited available evidence on post-TAVR outcomes in LG severe AS patients who have a preserved LVEF makes this an important area for active clinical inquiry. In the current all-comers study, investigations were limited to the preserved LVEF cohort in a relatively contemporary era, but the final population is still relatively large when compared to the published literature. Furthermore, the LG and HG groups in this study were well-matched by surgical risk score and presence of concomitant valvular abnormalities. Nonetheless, there remain several limitations. First, there are limitations that are inherent to a retrospective study design, including unmeasured confounders. However, each patient was evaluated in clinic by a small group of clinicians dedicated to transcatheter valve therapies and the evaluation and documentation processes are protocolized by the group to ensure consistent collection of data. Second, the outcomes data are largely from the peri-procedural period, with only all-cause mortality assessed on long-term follow-up. Data on recurrent hospitalizations due to heart failure was not captured, and data on cardiac versus non-cardiac mortality were not available. Third, a right heart catheterization for invasive data on flow across the aortic valve was not performed in 40% of patients. Finally, the patients in the current study underwent TAVR by a group that does >200 TAVRs a year, and the clinical team evaluating these patients, therefore, has considerable experience. As a result, the outcomes outlined here may not be generalizable to lower volume settings.
5 |. CONCLUSION
In conclusion, this study demonstrates no significant differences in peri-procedural, short-term, and long-term outcomes after TAVR for severe AS between patients who have LG, including the LG low SVI subset, versus HG in the setting of a preserved LVEF. These findings are independent of differences in baseline characteristics. Larger studies with longer follow-up are warranted.
ACKNOWLEDGMENTS
We acknowledge the contributions of Rahul Thakker, BS of New York University School of Medicine in data collection for this manuscript. New York University School of Medicine Cardiovascular Outcomes Group provided part of data analysis and statistical support. Dr. Shah was supported in part by the Biomedical Laboratory Research & Development Service of the VA Office of Research and Development (iK2CX001074).
DISCLOSURES
BS and MW receive research grant support from Siemens. BS is on an advisory board for Philips Volcano. CS, MS, and MW are on the Speakers’ bureau for Medtronic. MS is also on an advisory board for Siemens and the Speakers’ bureau for Phillips. The authors have no conflicts of interest in relation to this manuscript.
REFERENCES
- 1.Otto CM, Prendergast B. Aortic-valve stenosis—from patients at risk to severe valve obstruction. N Engl J Med. 2014;371:744–756. [DOI] [PubMed] [Google Scholar]
- 2.Hachicha Z, Dumesnil JG, Bogaty P, Pibarot P. Paradoxical low-flow, low-gradient severe aortic stenosis despite preserved ejection fraction is associated with higher afterload and reduced survival. Circulation. 2007;115:2856–2864. [DOI] [PubMed] [Google Scholar]
- 3.Clavel MA, Dumesnil JG, Capoulade R, Mathieu P, Senechai M, Pibarot P. Outcome of patients with aortic stenosis, small valve area, and low-flow, low-gradient despite preserved left ventricular ejection fraction. J Am Coll Cardiol. 2012;60:1259–1267. [DOI] [PubMed] [Google Scholar]
- 4.Mohty D, Magne J, Deltreuil M, et al. Outcome and impact of surgery in paradoxical low-flow, low-gradient severe aortic stenosis and preserved left ventricular ejection fraction: a cardiac catheterization study. Circulation. 2013;128:S235–S242. [DOI] [PubMed] [Google Scholar]
- 5.Clavel MA, Burwash IG, Pibarot P. Cardiac imaging for assessing low-gradient severe aortic stenosis. JACC: Cardiovascular Imaging. 2017;10:185–202. [DOI] [PubMed] [Google Scholar]
- 6.Dahl JS, Eleid MF, Pislaru SV, Scott CG, Connolly HM, Pellikka PA. Development of paradoxical low-flow, low-gradient severe aortic stenosis. Heart. 2015;101:1015–1023. [DOI] [PubMed] [Google Scholar]
- 7.Romero J, Chavez P, Goodman-Meza D, et al. Outcomes in patients with various forms of aortic stenosis including those with low-flow low-gradient normal and low ejection fraction. Am J Cardiol. 2014;114:1069–1074. [DOI] [PubMed] [Google Scholar]
- 8.Eleid MF, Sorajja P, Michelena HI, Malouf JF, Scott CG, Pellikka PA. Flow-gradient patterns in severe aortic stenosis with preserved ejection fraction: clinical characteristics and predictors of survival. Circulation. 2013;128:1781–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishimura RA, Otto CM, Bonow RO, et al. AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the american college of Cardiology/American heart association task force on clinical practice guidelines. Circulation. 2017;135:e1159–e1195. [DOI] [PubMed] [Google Scholar]
- 10.Reardon MJ, Van Mieghem NM, Popma JJ, et al. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2017;376:1321–1331. [DOI] [PubMed] [Google Scholar]
- 11.Leon MB, Smith CR, Mack MJ, et al. Transcatheter or surgical aortic valve replacement in intermediate risk patients. N Engl J Med. 2016;374:1609–1620. [DOI] [PubMed] [Google Scholar]
- 12.Kappetein AP, Head SJ, Généreux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. J Am Coll Cardiol. 2012;60:1438–1454. [DOI] [PubMed] [Google Scholar]
- 13.Tribouilloy C, Rusinaru D, Marechaux S, et al. Low-gradient, low-flow severe aortic stenosis with preserved left ventricular ejection fraction: characteristics, outcome, and implications for surgery. J Am Coll Cardiol. 2015;65:55–66. [DOI] [PubMed] [Google Scholar]
- 14.Jander N, Minners J, Holme I, et al. Outcome of patients with low-gradient “severe” aortic stenosis and preserved ejection fraction. Circulation. 2011;123:887–895. [DOI] [PubMed] [Google Scholar]
- 15.Pibarot P, Dumesnil JG. Low-flow, low-gradient aortic stenosis with normal and depressed left ventricular ejection fraction. J Am Coll Cardiol. 2012;60:1845–1853. [DOI] [PubMed] [Google Scholar]
- 16.Herrmann HC, Pibarot P, Hueter I, et al. Predictors of mortality and outcomes of therapy in low-flow severe aortic stenosis: a placement of aortic transcatheter valves (PARTNER) trial analysis. Circulation. 2013;127:2316–2326. [DOI] [PubMed] [Google Scholar]
- 17.Debry N, Sudre A, Amr G, et al. Transcatheter aortic valve implantation for paradoxical low-flow low-gradient aortic stenosis patients. Catheter Cardiovasc Interv. 2016;87:797–804. [DOI] [PubMed] [Google Scholar]
- 18.Biner S, Birati EY, Topilsky Y, et al. Outcome of transcatheter aortic valve implantation in patients with low-gradient severe aortic stenosis and preserved left ventricular ejection fraction. Am J Cardiol. 2014;113:348–354. [DOI] [PubMed] [Google Scholar]
- 19.Baron SJ, Arnold SV, Herrmann HC, et al. Impact of ejection fraction and aortic valve gradient on outcomes of transcatheter aortic valve replacement. J Am Coll Cardiol. 2016;67:2349–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]


