Abstract
Septicemia is associated with excessive inflammation, oxidative stress and apoptosis, causing myocardial injury that results in high mortality and disability rates worldwide. The abnormal expression of multiple microRNAs (miRNAs/miRs) is associated with more severe sepsis-induced myocardial injury (SIMI) and miR-335 has been shown to protect cardiomyocytes from oxidative stress. The present study aimed to investigate the role of miR-335 in SIMI. An SIMI model was established by cecal ligation and puncture (CLP) in mice. An miRNA-335 precursor (pre-miR-335) was transfected to accelerate miR-335 expression and an miR-335 inhibitor (anti-miR-335) was used to inhibit miR-335 expression. CLP or sham surgery was performed on pre-miR-335, anti-miR-335 and wild-type mice and miR-335 expression was determined by reverse transcription-quantitative PCR. Inflammatory factors (TNF-α, IL-6 and IL-10) and troponin (cTNI), brain natriuretic peptide (BNP), creatine kinase (CK), lactate dehydrogenase (LDH) and aspartate aminotransferase (AST) were assessed using commercial kits. Apoptosis was detected by flow cytometry and cardiac function was assessed using a Langendorff isolated cardiac perfusion system. miR-335 expression was upregulated and an elevation in inflammatory factors and cTNI, BNP, CK, LDH and AST was observed. Compared with the wild-type control group, pre-miR-335 mice treated with CLP exhibited significantly reduced left ventricular development pressure, maximum pressure increased reduction rates, as well as decreased levels of TNF-α, IL-6 and IL-10, myocardial injury and apoptosis; by contrast, these features were amplified in CLP-treated anti-miR-335 mice. In conclusion, the upregulation of miR-335 exerted ameliorative effects on myocardial injury following sepsis and may indicate a novel therapeutic intervention for SIMI.
Keywords: microRNA-335, sepsis, myocardial injury, inflammatory factors, apoptosis
Introduction
Septicemia remains an all too common pathology that is associated with multiple abnormalities and heterogeneous responses, but particularly with cardiovascular dysfunction (1). Due to a lack of effective interventions, sepsis is one of the primary causes of high morbidity and mortality rates worldwide, with confirmed mortality rates of ≤47% in patients with sepsis (2). Despite efforts to improve diagnosis and treatment, sepsis remains a major medical challenge. Cardiac dysfunction is considered to be a widely accepted manifestation of sepsis, which is closely related to sepsis-associated dilemma. Studies have indicated that 40–50% of patients with sepsis exhibit varying degrees of myocardial dysfunction, with a mortality rate of 70–90% in those with myocardial injury, compared with 20% in those without it (3,4). Therefore, the determination of novel interventions for sepsis-induced myocardial injury (SIMI) is an urgent requirement for reducing the morbidity and mortality rates of individuals with sepsis.
MicroRNAs (miRNAs/miRs) are small endogenous non-coding single-stranded RNAs (~22 nucleotides in length), which are transcribed and transported to the cytoplasm from the nucleus (5). miRNAs negatively regulate gene expression by interacting with the 3′untranslated regions of target mRNAs, resulting in mRNA inhibition or degradation (6). It has been demonstrated that miRNAs may be involved in the regulation of almost all major cellular functions, including development, differentiation, proliferation and apoptosis (7). Previous studies have shown that miRNAs are also associated with a diverse array of disorders, including sepsis, heart disease and diabetes (8–10). Studies have confirmed that the dysregulation of multiple miRNAs during sepsis (including miRNA-146a, miRNA-499-5p, miRNA-297, miRNA-574-5p, miRNA-122 and miRNA-133a) accelerates sepsis-associated disorders (11–15). Studies have also shown that loss of the miRNA-144/451 cluster impairs ischemic preconditioning-mediated cardio-protection (16–18). Furthermore, overexpression of miRNA-494 has a protective effect on ischemia/reperfusion-induced cardiac injuries, while elevation of miRNA-320 has a detrimental effect (19–21). miR-214 overexpression also protects against SIMI (22) and the same results obtained by Ge et al (22) also confirmed that miR-214 overexpression showed ameliorative effect against SIMI (data not published) and the protective effects miRNA-335 on H2O2-induced cardiomyocyte injury have been demonstrated (23). However, whether miRNA-335 expression is also associated with SIMI remains to be elucidated. As myocardial damage is the leading cause of sepsis-related mortality, the present study aimed to determine the association between miRNA-335 upregulation and SIMI and the effect of miRNA-335 on the progression of SIMI.
Materials and methods
Animals
All protocols were approved by the Medical Animal Ethics Committee of Hunan Academy of Chinese Medicine (Xiang 20200015). All animals were handled in strict accordance with good animal practice as defined by the Hunan Academy of Chinese Medicine animal welfare bodies. Healthy male Kunming mice (age 7–8 weeks; weight, 25–30 g; n=100) purchased from the Experimental Animal Center of Central South University were kept under standardized laboratory conditions of a maintained on a 12-h light/dark cycle, temperature-controlled environment. Mice were housed 4–5 per cage in an enriched environment with an ambient temperature of 22±1°C and humidity of 50±5% for with ad libitum access to food and water for 1 week before the experiments started. All of the mice were randomly divided (15 mice per group) into 6 groups: Normal group (NC); Sham group; CLP group (cecal ligation and puncture); pre-miR-335 group; anti-miR-335 group and wild-type group (WT). The experiment continued for one week and all surgical procedures were performed under anesthesia with an initial intraperitoneal injection of sodium pentobarbital (30 mg/kg body weight) to minimize animal suffering until they lost consciousness. All the animals were sacrificed following anesthesia by exsanguination (24,25). During all the experiments, the care and treatment of the animals received prior institutional approval from the Ethical Commission on Animal Research of the Hunan Academy of Chinese Medicine.
Establishment of septicemia
Sepsis was established by cecal ligation and puncture (CLP) as previously described (26) and with slight modifications. Following anesthesia with an intraperitoneal injection of sodium pentobarbital (30 mg/kg) to minimize animal suffering, the cecum was exposed via a 1–2 cm midline incision across the abdomen, the cecum was exposed via a 1–2 cm midline incision across the abdomen. The cecum was ligated with a 5-0 sterile silk thread just below the ileocecal valve. The area between the ligation point and the tip of the cecum was punctured twice with a 18-gauge needle (with a 1-cm drainage tube attached). A small amount of feces was gently squeezed from the cecum, which was then returned to its anatomical position. Warm saline (0.05 ml/g) was then subcutaneously injected into each mouse. Mice in the Sham group underwent cecum exposure only.
Determination of cardiac function by Langendorff perfusion
Mice were anesthetized with an intraperitoneal injection of sodium pentobarbital (30 mg/kg) to minimize animal suffering at 3, 6, 12 and 24 h post-sham or -CLP surgery. A midsternal sternotomy was performed and the heart was mounted on a Langendorff isolated cardiac perfusion system. The aorta was intubated with a 30-gauge cannula and perfusion was initiated by intubating in retrograde mode. The perfusion solution consisted of 7 mmol/l glucose, 0.4 mmol/l oleate, 1% BSA and a low concentration of insulin (10 µU/ml). Pressure was monitored by inserting a water-filled latex balloon into the left ventricle, which was connected to a pressure sensor. The balloon volume was adjusted to a constant baseline left ventricular end-diastolic pressure of 5 mm Hg for 15 min. The signal acquisition system (cat. no. RM6240B; Chengdu Biological Instruments) was used to record the left ventricular development pressure (LVDP), maximum pressure increase rate (+dp/dt), maximum pressure reduction rate (-dp/dt), heart rate, cardiac output and stroke output.
RNA extraction and reverse transcription-quantitative (RT-q)PCR
TRIzol® (Thermo Fisher Scientific, Inc.) was used to isolate total RNA from the heart tissues of mice that underwent sham or CLP surgery at 6, 12 and 24 h. RNA purity was determined using a microplate reader at a wavelength of 260 nm. Cardiac mRNA expression levels were determined by RT-qPCR (Applied Biosystems; Thermo Fisher Scientific, Inc.). First, the extracted total RNA was purified with 75% ethanol and its concentration was determined by spectrophotometry. The RNA (200 ng per sample) was then used as a template for reverse transcription using the PrimeScript® RT reagent kit (cat. no. RP1201, BioTeke Corporation) with U6 as the internal standardized control. In the reaction system with a total amount of 10 µl, 2X SYBR-Green Mixture 5.0, 0.5 µl 5 µm/l positive and reverse primers, 1 µl cDNA and ddH2O replenishment volume to 10.0 µl. The conditions of reverse transcription reaction were: 50°C for 15 min, 85°C for 5 min. Reaction conditions were denaturation at 95°C for 5 min, 95°C for 15 sec, 60°C for 1 min, for 40 cycles and were performed on Bio-Rad CFX96 Real-time Quantitative Fluorescence PCR (Bio-Rad Laboratories, Inc.). The following primers were provided by GeneCopoeia Inc.: miR-335 forward, 5′-GCGGTCAAGAGCAATAACGAA−3′ and reverse, 5′-GTGCAGGGTCCGAGGTATTC-3′; U6 (internal control) forward, 5′-CTCGCTTCGGCAGCACA-3′ and reverse, 5′-AACGCTTCACGAATTTGCGT-3′. To determine a ΔCt value, the Ct value of the target gene was normalized by subtracting the U6 Ct value. The relative expression level between treatments was then calculated using the following equation: relative gene expression=2-(ΔCt sample-ΔCt control) (27).
Transfection and survival
Transfection and survival were performed as previously described (28–31). Mice were intravenously injected with miR-335 precursor (5′-UCAAGAGCAAUAACGAAAAAUGU-3′) or inhibitor (5′-ACAUUUUUCGUUAUUGCUCUUGA-3′; purchased from Guangzhou RiboBio Co., Ltd.) and corresponding controls (negative control: 5′-UUCUCCGAACGUGUCACGUTT−3′; negative control inhibitor 5′-CAGUACUUUUGUGUAGUACAA-3′; Thermo Fisher Scientific, Inc.) using Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) at a final concentration of 50 nM according to the manufacturer's protocols and transfection efficiency was assessed after 12 h. CLP was performed 4 days post-transfection. For the wild-type (WT), pre-miR-335 negative control, anti-miR-335 negative control, pre-miR-335 and anti-miR-335 mice (10 mice per group), survival rates were observed every 2 h for 36 h.
The mice were transfected with miR-335 precursor or anti-miR-335 using adenovirus (2×1011 pfu; GenePharma, Shanghai, China) for 4 days according to the manufacturer's protocol. The mice were anesthetized and 200 µl adenovirus was injected into tail veins.
Detection of inflammatory and diagnostic markers
The mice were anesthetized intraperitoneally with an intraperitoneal injection of sodium pentobarbital (30 mg/kg) to minimize animal suffering. Retro-orbital puncture was performed to take a 100 µl blood sample with a Brand micropipette, where the eye was pulled out to collect at ≥500 µl blood. At 3, 6, 12 and 24 h post-sham or -CLP surgery, the animals were anesthetized with an intraperitoneal injection of sodium pentobarbital (30 mg/kg) till loss of consciousness and sacrificed following anesthesia by exsanguination. The heart tissues were collected and homogenized. The blood samples and tissue homogenates were centrifuged at 500 × g for 10 min at 4°C and the supernatants were collected for storage at −80°C. The levels of TNF-α, IL-6 and IL-10 and troponin (cTNI; cat. no. MAB3152; Sigma-Aldrich; Merck KGaA), brain natriuretic peptide (BNP; cat. no. AF6336; Sigma-Aldrich; Merck KGaA), creatine kinase (CK; cat. no. MAK116; Sigma-Aldrich; Merck KGaA), lactate dehydrogenase (LDH; cat. no. MAK066; Sigma-Aldrich; Merck KGaA) and aspartate aminotransferase (AST; cat. no. MAK055; Sigma-Aldrich: Merck KGaA) were determined using commercially available kits.
Flow cytometric detection of apoptosis
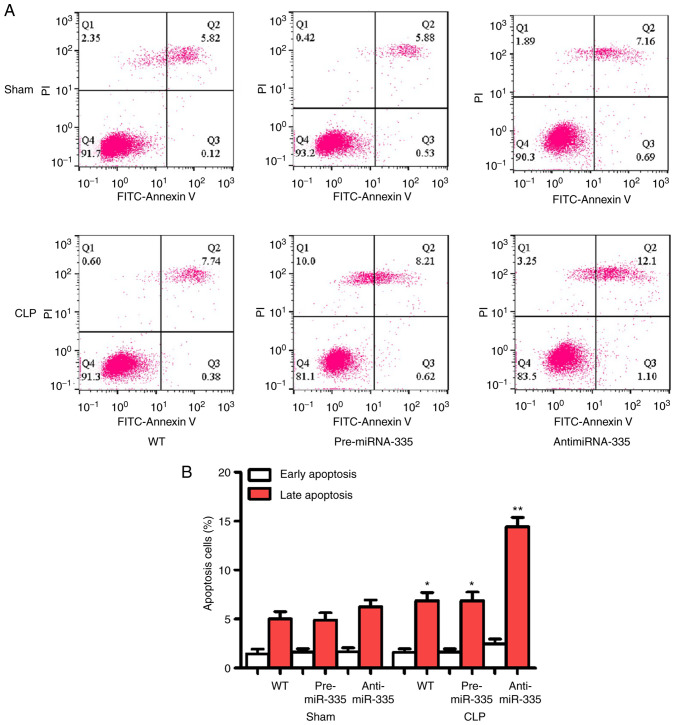
Heart tissues were collected from the WT, pre-miR-335 and anti-miR-335 mice 12 h post-CLP. The animals were anesthetized with an initial intraperitoneal injection of sodium pentobarbital (30 mg/kg) prior to exsanguination till loss of consciousness (24,25). Then the chest cavity was opened and the heart was removed and washed with 0°C calcium solution (140 mM NaCl, 1 mM CaCl2, 1 mM MgCl2, 0.33 mM NaH2PO4, 10 mM glucose, and 10 mM HEPES, pH 7.2). The aorta was isolated and filled with calcium-free solution (140 mM NaCl, 1 mM CaCl2, 0.33 mM NaH2PO4, 10 mM glucose, and 10 mM HEPES, pH 7.2) until the heart stopped beating. The heart was then placed in a pre-prepared solution of collagen and albumin (4°C), after which the tissues were minced and digested in KB solution for 1 h (4°C) (32). A cardiomyocyte sample was collected from one mouse in each group and an elemental suspension was prepared. Following the protocols of Annexin V-fluorescein isothiocyanate (FITC) apoptosis detection kit (cat. no. C1065; Beyotime Institute of Biotechnology), Annexin V-FITC, propidium iodide (PI), hydroxyethyl piperazine ethylsulfonic acid buffer was added to Annexin V-FITC/PI staining solution in the proportion of 1:2:50. Cells at a concentration of 1×106 cells/100 µl of the staining solution were resuspended, incubated at ambient temperature for 15 min in the dark and added with 1 ml of 4-(2-hydroxyethyl)-1-piperazineëthanesulfonic acid buffer and then analyzed using BD Accuri C6 software (version 5.0; BD Biosciences). A flow cytometer (BD Accuri C6; BD Biosciences) was employed to determine the excitation wavelength at 488 nm. The excitation wavelength at 525 or 620 nm was employed to detect FITC or PI fluorescence for cell apoptosis, respectively. The samples (n=10) were randomly selected in each group. The apoptotic rate was calculated as the percentage of early apoptotic cells, or the percentage of late apoptotic cells.
Histological examination
At 24 h post-CLP, all animals received cardiac perfusion under anesthesia. The heart specimens were then fixed in 4% paraformaldehyde for 24 h at room temperature, dehydrated, cleared and embedded in paraffin. A microtome was used to cut the specimens into 5-µm sections and each section was stained with hematoxylin and eosin at room temperature for 5 min. Changes in the myocardial tissue structure were observed under an light microscope (magnification, ×400).
Statistical analysis
SPSS 21.0 (IBM Corp) was used for statistical analysis. All data are presented as the mean ± standard deviation. Measurements at single time point were performed by the Student's unpaired t-test. One-way analysis of variance was used for comparison between multiple groups, followed by Tukey's post hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
CLP accelerates myocardial injury in mice
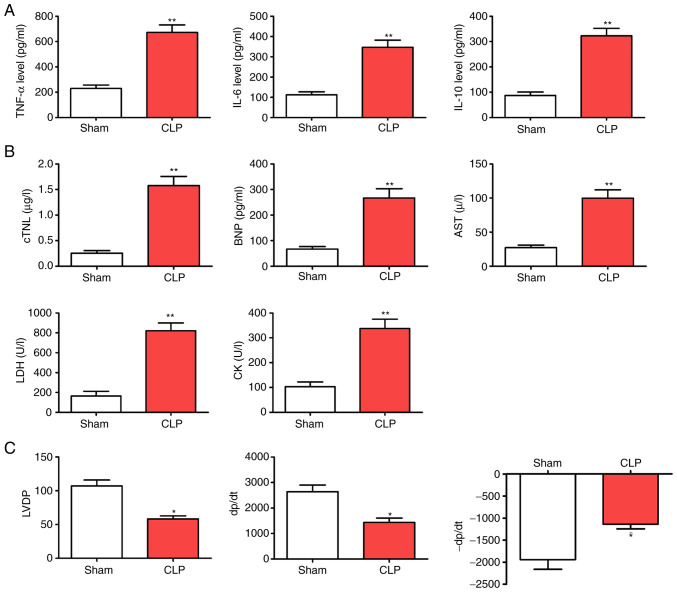
Compared with the Sham group, CLP led to reduced animal activity and slow response to stimuli with increasing time, as well as a decreased appetite. In a pre-study experiment, the mortality rates of the septic mice following CLP were 50% at 48 h, rising to 80% at 72 h and 90% at 96 h. Over time, cardiac functions, including heart rate, cardiac output and stroke output, showed an increase and then decreased. Additionally, cardiac evaluations from the perfused CLP-mice showed that LVDP, +dP/dt and -dP/dt were significantly reduced compared with those of the WT group. By contrast, inflammatory factors (TNF-α, IL-6 and IL-10) and myocardial enzyme indicators (cTNI, BNP, AST, LDH and CK) were elevated in the septic mice at various time points (Fig. 1; P<0.05).
Figure 1.
CLP accelerates myocardial injury following CLP surgery. (A) Levels of inflammatory factors (TNF-α, IL-6 and IL-10). (B) Myocardial enzyme indicators (cTNI, BNP, AST, LDH and CK). (C) Alterations in cardiac function (LVDP, +dP/dt and -dP/dt). *P<0.05 and **P<0.01 vs. the Sham group; n=5. CLP, cecal ligation and puncture; cTNI, troponin; BNP, brain natriuretic peptide; AST, aspartate aminotransferase; LDH, lactate dehydrogenase; CK creatine kinase; LVDP, left ventricular development pressure; +dp/dt, maximum pressure increase rate; -dp/dt maximum pressure reduction rate.
miR-335 is upregulated in myocardial tissues following septic assault
Differential expression of multiple different miRNAs has been reported in diseases of the heart (33). In particular, the expression of miR-335 has been shown to significantly increase, which has been demonstrated to protect cardiomyocytes from oxidative stress (34,35). Therefore, it was hypothesized that miR-335 may also be involved in SIMI. To confirm this hypothesis, RT-qPCR was used to detect the expression of miR-335 at 6, 12 and 24 h post-CLP. The results showed that the expression of miR-335 in the myocardial tissues of septic mice was significantly higher than that of the sham-operated mice at 6, 12 and 24 h (Fig. 2; P<0.05).
Figure 2.
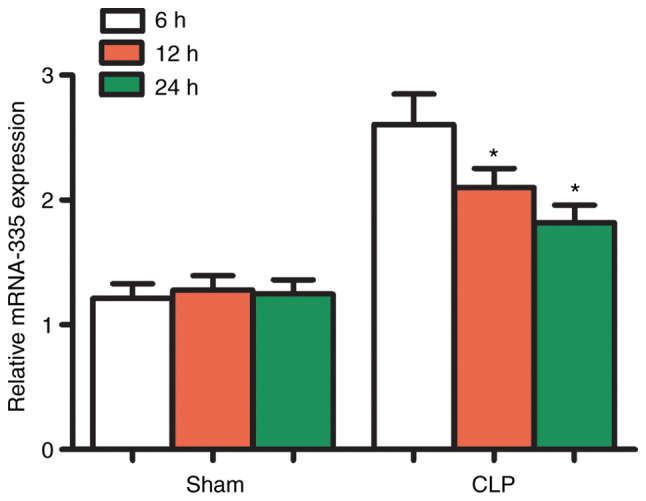
miRNA expression profiles of mouse hearts following CLP. After 6, 12 and 24 h of CLP, miR-335 expression in mouse hearts was significantly upregulated. *P<0.05 vs. the Sham group; n=5. miRNA/miR, micro RNA; CLP, cecal ligation and puncture.
Regulation of miR-335 expression in mouse cardiac tissues
miR overexpression and silencing were performed to further investigate the expression of miR-335 in cardiac tissue. Preliminary experiments showed that miR-335 transfection efficiency is highly effective (Fig. S1). Compared with the CLP group, miR-335 expression was increased in heart tissues after transfection with miR-335 precursors peaked at 12 h and then decreased after 24 h (Figs. 3 and S2). By contrast, the miR-335 inhibitor caused a decrease in miR-335 expression. The effects of the precursors and inhibitor were both specific to miR-335 and no off-target effects on other miRNAs were observed. In addition, all transfected mice were healthy and without significant cardiac dysfunction (Fig. 3).
Figure 3.
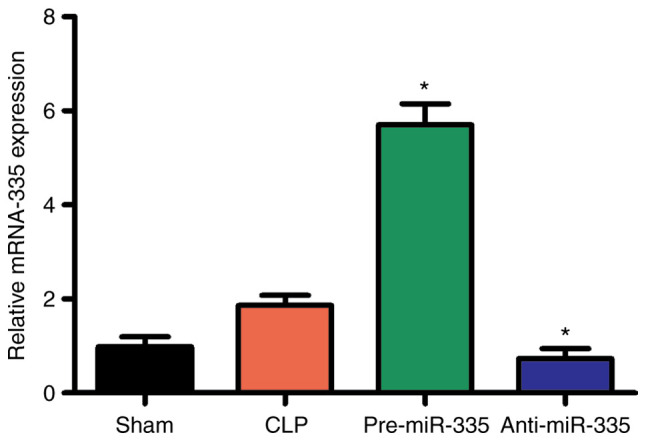
miRNA expression profiles of mouse hearts following transfection. At 12 h, compared with CLP-treated mice, miR-335 expression increased following miR-335 precursor treatment, while miR-335 inhibitors decreased expression. *P<0.05 vs. the CLP group; n=5. miRNA/miR, micro RNA; CLP, cecal ligation and puncture.
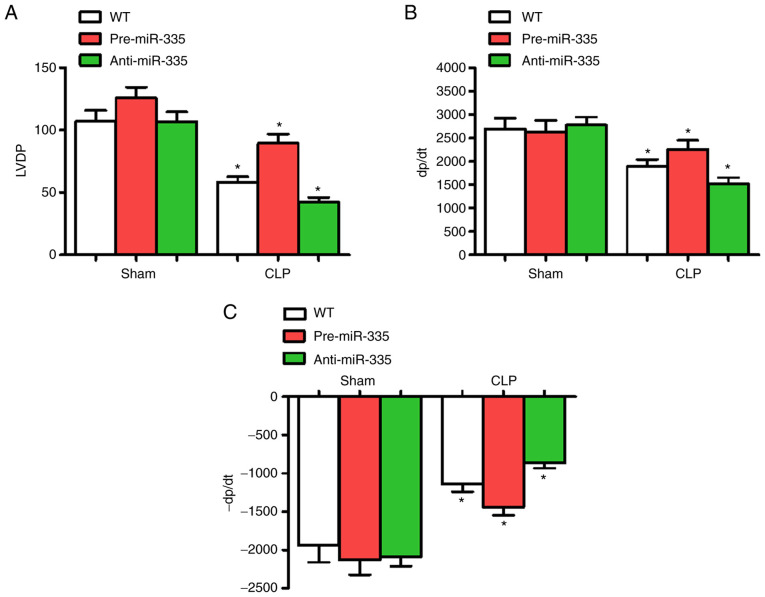
Effect of miR-335 on CLP-induced myocardial dysfunction
At 6 h post-CLP, LVDP, +dP/dt and -dP/dt were significantly altered, thus cardiac functions at 6 h were determined in each group. Compared with the WT group, the administration of miRNA-335 precursors 6 h post-CLP significantly improved cardiac LVDP and ±dP/dt (n=6; P<0.05), while anti-miR-335-pretreated mice experienced a decline in these parameters (n=6; P<0.05). Compared with the WT group, miR-335 expression level had no significant effect on cardiac contractile function (Fig. 4).
Figure 4.
miR-335 upregulation improves sepsis-induced cardiac function. Changes in (A) LVDP, (B) +dP/dt and (C) -dP/dt in Langendorff-perfused mice 6 h post-CLP. *P<0.05 vs. WT. n=5. miRNA/miR, micro RNA; LVDP, left ventricular development pressure; +dp/dt, maximum pressure increase rate; -dp/dt maximum pressure reduction rate; CLP, cecal ligation and puncture; WT, wild-type.
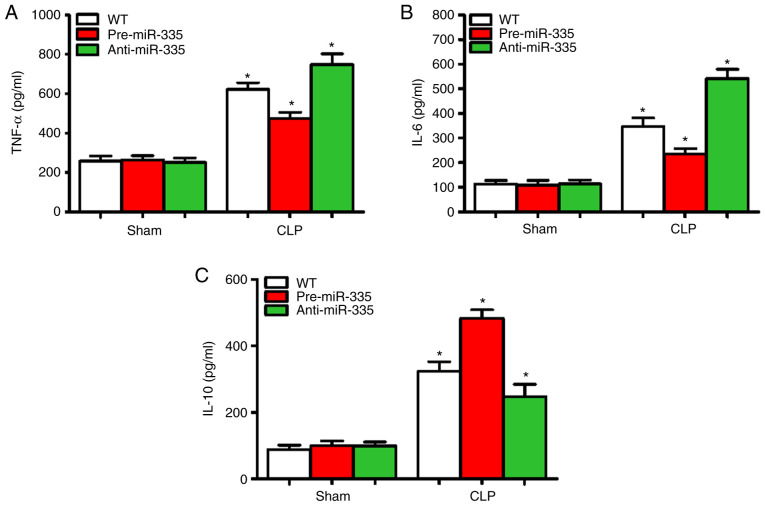
miR-335 inhibits myocardial inflammation in septic mice
Sepsis-induced myocardial suppression may be associated with increased cardiac inflammation (36). Therefore, the expression levels of inflammatory cytokines (TNF-α, IL-6 and IL-10) were detected in the myocardial tissues of mice 12 h after CLP. Compared with the WT group, no changes in myocardial inflammatory cytokine levels (TNF-α, IL-6 and IL-10) were observed in the pre-miR-335 and anti-miR-335 mice (Fig. 5A-C). Compared with the CLP-treated WT mice, pre-miR-335 caused a decrease in TNF-α and IL-6 expression, while inflammation was significantly aggravated in anti-miR-335 mice (Fig. 5A and B; n=6; P<0.05). Changes in IL-10 showed the opposite trend (Fig. 5C). Compared with the WT Sham group, the levels of TNF-α in the CLP-treated group were increased by 1.33 times in the pre-miR-335 group and 2.69 times in the anti-miR-335 group (Fig. 5A; n=6; P<0.05). It is worth noting that compared with the sham-operated mice, CLP caused an increase in IL-6 levels in the WT and pre-miR-335 mice, but particularly in the anti-miR-335-treated mice (Fig. 5B; n=6; P<0.05). By contrast, compared with the sham-operated WT group, IL-10 levels increased by 5.93 times in the pre-miRNA-335 mice post-CLP and were 2.58-fold higher in anti-miRNA-335 than in pre-miRNA-335 mice (Fig. 5C; n=6; P<0.05).
Figure 5.
Upregulation of miR-335 attenuates sepsis-induced inflammation and enhances the anti-inflammatory response. Levels of (A) TNF-α, (B) IL-6 and (C) IL-10 in cardiac homogenates 12 h post-CLP. *P<0.05 vs. WT. n=5. miRNA/miR, micro RNA; CLP, cecal ligation and puncture; WT, wild-type.
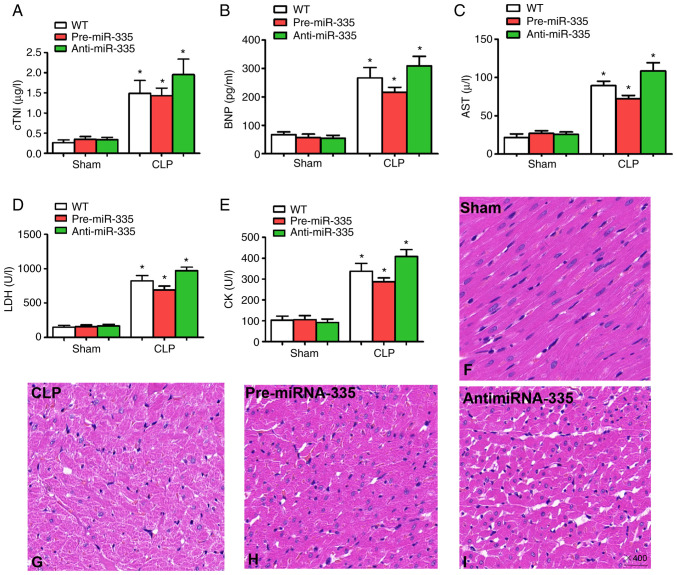
Upregulation of miR-335 attenuates SIMI
Detection of myocardial enzymes is critical for the assessment of myocardial injury (22,37,38). Therefore, in the present study, changes in myocardial enzyme levels were examined 12 h after CLP treatment. Compared with the WT sham-operated mice, no significant differences were observed in cTNI, BNP, AST, LDH or CK in the sham-operated mice treated with miRNA-335 precursors or miRNA-335 inhibitors (Fig. 6A-E; n=6; P>0.05). By contrast, the levels of myocardial enzymes increased significantly in WT, pre-miRNA-335 and anti-miRNA-335 mice following CLP. miRNA-335 precursors were then used to treat septic mice 4 days before surgery. Compared with WT mice receiving CLP, significantly reduced levels of serum cTNI, BNP, AST, LDH and CK were observed, while the miRNA-335 inhibitor increased these levels (Fig. 6A-E; n=6; P<0.05).
Figure 6.
Upregulation of miR-335 attenuates sepsis-induced myocardial injury and histological changes. Levels of (A) cTNI, (B) BNP, (C) AST, (D) LDH and (E) CK 12 h after CLP. Pathological morphology was assessed by hematoxylin and eosin staining; (F) Hearts from the Sham surgery group. (G) CLP mouse heart. (H) Pre-miR-335 and (I) anti-miR-335 mouse heart (magnification, ×400). *P<0.05 vs. WT. n=5. miRNA/miR, micro RNA; cTNI, troponin; BNP, brain natriuretic peptide; AST, aspartate aminotransferase; LDH, lactate dehydrogenase; CK, creatine kinase; CLP, cecal ligation and puncture; WT, wild-type.
In addition, the results of histological analysis revealed myocardial degeneration, myocardial fibrillation and interstitial edema in CLP-treated mice compared with the Sham group (Fig. 6F and G; n=6). Pre-miR-335 mice showed reduced myocardial injury following CLP and accelerated myocardial injury was observed in anti-miRNA-335 mice after CLP (Fig. 6H and I; n=6). Therefore, these findings indicated that treatment with miRNA-335 precursors ameliorates SIMI in septic mice.
miR-335 inhibits apoptosis in SIMI
Apoptosis is considered an important aggravator of myocardial injury and may be an indicator for evaluating this pathology. As shown in Fig. 7, no significant differences in apoptosis were observed between the WT, pre-miR-335 and anti-miR-335 groups following surgery. However, CLP promoted significant apoptosis, which was reversed by pre-miRNA-335 treatment. Anti-miRNA-335-pretreated mice showed stable myocardial apoptosis following CLP.
Figure 7.
miR-335 upregulates cardiomyocyte apoptosis. (A) Flow cells of ardiomyocytes. (B) Statistical results of flow cytometry of ardiomyocytes 24 h after CLP. *P<0.05, **P<0.01 vs. WT. n=5. miRNA/miR, micro RNA; CLP, cecal ligation and puncture; PI, propidium iodide; WT, wild-type.
Discussion
The aim of the present study was to determine the effects and underlying molecular mechanisms of miRNA-335 in SIMI. The results suggested that pre-miRNA-335 reduced cardiac function, the inflammatory response, myocardial damage and myocardial apoptosis. These findings provided insights into the mechanisms underlying miRNA-335 function and suggest that it may be a novel target for attenuating SIMI.
Patients with SIMI exhibit higher rates of mortality and disability worldwide than those without cardiovascular dysfunction (39). SIMI is considered to be difficult to treat. Although there are various available treatments, including early target-oriented remedies, timely appropriate antibiotic management, boosters and positive inotropic interventions and mechanical ventilation (40), the development of effective interventions to decrease mortality rates remains clinically challenging. Clinical trials have widely focused on regulating uncontrolled immune responses to suppress SIMI (41). miRNAs are extensively implicated in biological and pathophysiological processes, including sepsis-associated dysfunction (42–44). Studies have shown that miRNA-335 is markedly upregulated in the infarcted and bordered areas of the heart following acute myocardial infarction in rats (42). Upregulation of miR-335 also ameliorates myocardial ischemia/reperfusion injury by inhibiting hypoxia-inducible factor 1α (45). Furthermore, miR-335 protects the mouse heart from ischemic injury by inhibiting Ca2+ overload and apoptosis (46). These studies indicate that miR-335 exerts protective effects in SIMI. In the present study, miR-335 expression was found to increase after CLP in mice, which proved beneficial to sepsis-associated cardiac dysfunction. The results confirmed that miR-335 protects against SIMI and further demonstrated that miR-335 expression/reactivity is first increased in sepsis, which is a compensatory protective mechanism, followed by a gradual decrease. Further research confirmed that the decrease in miR-335 expression was time-dependent. In order to investigate the potential role of miR-335 in SIMI, miR-335 precursors and inhibitors were then used to regulate miR-335 expression. The results indicated that miR-335 preconditioning improves myocardial dysfunction in septic mice.
miRNAs are a class of small non-coding RNA molecules which are involved in various biological functions, such as cellular differentiation, proliferation and transformation. Studies have demonstrated that the aberrant expression of specific miRNAs is associated with the occurrence and development of numerous pathologies, including tumors and cardiovascular diseases (47–49). A previous study demonstrated that miRNA-335 is involved in the deterioration of a variety of tumors, as well as Alzheimer's disease (50). However, to the best of the authors' knowledge, there have been no reports on the effects of miRNA-335 expression in SIMI. By establishing a sepsis model and detecting the miRNA expression profiles of myocardial tissues, the results of the present study illustrated that miRNA-335 expression in myocardial tissue was significantly upregulated following CLP in mice. However, the trend in miRNA-335 expression gradually decreased, which indicated that miRNA-335 downregulation is time-dependent. An miRNA-335 precursor or inhibitor was then employed to investigate the potential role of miRNA-335 in SIMI. The results revealed that the levels of cTNI, BNP, CK, LDH, AST and BNP decreased in pre-miRNA-335 mice, while the opposite effect was observed in anti-miRNA-335 mice. These results suggested that miRNA-335 was involved in ameliorating sepsis-induced myocardial dysfunction.
The inflammatory response is closely associated with various cardiovascular diseases (51,52). There is evidence to suggest that the accumulation of different subsets of inflammatory cytokines occurs in the ischemic heart (53). As important pro-inflammatory cytokines, TNF-α and IL-6 are regarded as critical contributors to the initiation and regulation of inflammatory responses and their expression is elevated in the majority of cardiovascular diseases (54). For instance, TNF-α is a known potent inducer of cardiovascular disease which accelerates myocardial injury. TNF-α also induces chemokine expression in cardiac tissues, thus promoting uncontrolled inflammation via NF-κB (55). Further evidence supports that TNF-α impairs systolic and diastolic function following electrical stimulation. In addition to TNF-α, IL-6 is also involved in pro-inflammatory processes and may contribute to increases in myocardial injury by promoting neutrophil influx into the injured cardiac tissue (56). IL-6-deficiency reduces acute myocardial I/R injury and there is mounting evidence to demonstrate that the critical role of IL-6 in sepsis-induced cardiac dysfunction is associated with the PI3K/Akt signaling pathway (57). These data suggest that the presence of myocardial inhibitory factors (TNF-α and IL-6) in the serum, or incubation of cardiomyocytes in vitro may promote contractile dysfunction in both animals and patients with sepsis (46). Inhibiting TNF-α and IL-6 with neutralizing antibodies has proven beneficial for treating sepsis (58). Therefore, the significant reduction in inflammatory factors following septic challenge may be one of the most important protective mechanisms of pre-miR-335-mediated myocardial injury. The present results confirmed that the upregulation of miR-335 inhibited pro-inflammatory cytokine release and that its downregulation exacerbated the release of pro-inflammatory cytokines. In addition, anti-miR-335-treated CLP mice produced higher levels of the anti-inflammatory cytokine IL-10, compared with the WT control group, which may lead to an enlarged myocardial inflammatory response.
Another cardioprotective mechanism of miR-335 during sepsis may lie in its ability to reduce apoptosis (59). A large number of studies has demonstrated that myocardial infarction, myocardial hypertrophy and heart failure induce excessive apoptosis and that myocardial apoptosis is involved in SIMI (37,60–62). Sepsis-associated inflammatory factors induce cardiomyocyte apoptosis by activating the caspase pathway (63). Additionally, sepsis promotes tissue hypoxia/ischemia, resulting in a decline in the accumulation and clearance of oxygen free radicals and an aggravation in contractility and apoptosis in myocardial cells (64). In the present study, pre-miR-335 treatment inhibited myocardial apoptosis in septic mice. By contrast, anti-miR-335-treated CLP mice exhibited accelerated myocardial apoptosis. These results indicated that miRNA-335 reduced myocardial injury by inhibiting apoptosis. Moreover, in vitro experiments have confirmed that miRNA-335 inhibits myocardial cell apoptosis by inhibiting the PTEN/PI3K/Akt pathway (22). These results suggest that PTEN is a pro-apoptotic protein that negatively regulates the primary phosphatase of the PI3K/Akt signaling (65). Studies have demonstrated that miR-335 maintains cell survival by targeting the 3′untranslated region of PTEN, which leads to the downregulation of PTEN and activation of the Akt pathway in ovarian cancer and nasopharyngeal carcinoma (66,67). These studies demonstrate that miR-335 may relieve SIMI. However, whether miR-335 suppresses apoptosis in SIMI-induced myocardial injury, which involves the PTEN/PI3K/Akt pathway, warrants further investigation.
In conclusion, the results of the present study indicated that miR-335 expression is upregulated in SIMI in CLP mice, which is associated with myocardial protection during sepsis. miR-335 reduced the inflammatory response associated with myocardial injury in sepsis, by inhibiting pro-inflammatory cytokine release and myocardial apoptosis. However, the absence of overall inflammation levels of the mice might be a limitation of the present study. Until now, to the best of the authors' knowledge, the present study is the first to suggest that the cardio-protection rendered by miR-335 may represent an effective intervention for SIMI treatment.
Supplementary Material
Acknowledgements
Not applicable.
Funding Statement
This study was supported by Hunan Natural Science Foundation (grant no. 2020JJ7090 to Yongpan Huang).
Funding
This study was supported by Hunan Natural Science Foundation (grant no. 2020JJ7090 to Yongpan Huang).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
XL, YH, JH, XZ, YZ, YW, YT and LL designed and performed experiments, and analyzed, interpreted and presented results for group discussions. XL, YH, JH, XZ, YZ, YW, YT and LL performed some of the experiments and confirm the authenticity of all the raw data. JH and LL made substantial contributions to conception and design. All authors reviewed and approved the final manuscript.
Ethics approval and consent to participate
All protocols were approved by the Medical Animal Ethics Committee of Hunan Academy of Chinese Medicine (Xiang 20200015). All animals were handled in strict accordance with good animal practice as defined by the Hunan Academy of Chinese Medicine animal welfare bodies.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Gomez E, Vercauteren M, Kurtz B, Ouvrard-Pascaud A, Mulder P, Henry JP, Besnier M, Waget A, Hooft Van Huijsduijnen R, Tremblay ML, et al. Reduction of heart failure by pharmacological inhibition or gene deletion of protein tyrosine phosphatase 1B. J Mol Cell Cardiol. 2012;52:1257–1264. doi: 10.1016/j.yjmcc.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Romero-Bermejo FJ, Ruiz-Bailen M, Gil-Cebrian J, Huertos-Ranchal MJ. Sepsis-induced cardiomyopathy. Curr Cardiol Rev. 2011;7:163–183. doi: 10.2174/157340311798220494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hochstadt A, Meroz Y, Landesberg G. Myocardial dysfunction in severe sepsis and septic shock: More questions than answers? J Cardiothorac Vasc Anesth. 2011;25:526–535. doi: 10.1053/j.jvca.2010.11.026. [DOI] [PubMed] [Google Scholar]
- 4.Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism and function. Cell. 2004;116:281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 5.Patop IL, Wüst S, Kadener S. Past, present and future of circRNAs. EMBO J. 2019;38:e100836. doi: 10.15252/embj.2018100836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meng S, Zhou H, Feng Z, Xu Z, Tang Y, Li P, Wu M. CircRNA: Functions and properties of a novel potential biomarker for cancer. Mol Cancer. 2017;16:94. doi: 10.1186/s12943-017-0663-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhelankin AV, Vasiliev SV, Stonogina DA, Babalyan KA, Sharova EI, Doludin YV, Shchekochikhin DY, Generozov EV, Akselrod AS. Elevated Plasma levels of circulating extracellular miR-320a-3p in patients with paroxysmal atrial fibrillation. Int J Mol Sci. 2020;21:3485. doi: 10.3390/ijms21103485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang L, Wang HC, Chen C, Zeng JM, Wang Q, Zheng L, Yu HD. Differential expression of plasma miR-146a in sepsis patients compared with non-sepsis-SIRS patients. Exp Ther Med. 2013;5:1101–1104. doi: 10.3892/etm.2013.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ailawadi S, Wang X, Gu H, Fan GC. Pathologic function and therapeutic potential of exosomes in cardiovascular disease. Biochim Biophys Acta. 2015;1852:1–11. doi: 10.1016/j.bbadis.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halushka PV, Goodwin AJ, Halushka MK. Opportunities for microRNAs in the crowded field of cardiovascular biomarkers. Annu Rev Pathol. 2019;14:211–238. doi: 10.1146/annurev-pathmechdis-012418-012827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang HJ, Zhang PJ, Chen WJ, Feng D, Jia YH, Xie LX. Four serum microRNAs identified as diagnostic biomarkers of sepsis. J Trauma. 2012;73:850–854. doi: 10.1097/TA.0b013e31825a7560. [DOI] [PubMed] [Google Scholar]
- 12.Wang HJ. Serum miRNA-574-5p: A prognostic predictor of sepsis patients. Shock. 2012;37:263–267. doi: 10.1097/SHK.0b013e318241baf8. [DOI] [PubMed] [Google Scholar]
- 13.Wang HJ, Deng J, Wang JY, Zhang PJ, Xin Z, Xiao K, Feng D, Jia YH, Liu YN, Xie LX. Serum miRNA-122 levels are related to coagulation disorders in sepsis patients. Clin Chem Lad Med. 2014;52:927–933. doi: 10.1515/cclm-2013-0899. [DOI] [PubMed] [Google Scholar]
- 14.Tacke F, Roderburg C, Benz F, Cardenas DV, Luedde M, Hippe HJ, Frey N, Vucur M, Gautheron J, Koch A, et al. Levels of circulating miR-133a are elevated in sepsis and predict mortality in critically ill patients. Crit Care Med. 2014;42:1096–104. doi: 10.1097/CCM.0000000000000131. [DOI] [PubMed] [Google Scholar]
- 15.Dong S, Cheng Y, Yang J, Li J, Liu X, Wang X, Wang D, Krall TJ, Delphin ES, Zhang C. MicroRNA expression signature and the role of microRNA-21 in the early phase of acute myocardial infarction. J Biol Chem. 2009;284:29514–29525. doi: 10.1074/jbc.M109.027896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang X, Zhu H, Zhang X, Liu Y, Chen J, Medvedovic M, Li H, Weiss MJ, Ren X, Fan GC. Loss of the miR-144/451 cluster impairs ischaemic preconditioning-mediated cardioprotection by targeting Rac-1. Cardiovasc Res. 2012;94:379–390. doi: 10.1093/cvr/cvs096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li W, Dong M, Chu L, Feng L, Sun X. MicroRNA-451 relieves inflammation in cerebral ischemia-reperfusion via the Toll-like receptor 4/MyD88/NF-κB signaling pathway. Mol Med Rep. 2019;20:3043–3054. doi: 10.3892/mmr.2019.10587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kura B, Szeiffova Bacova B, Kalocayova B, Sykora M, Slezak J. Oxidative stress-responsive MicroRNAs in heart injury. Int J Mol Sci. 2020;21:358. doi: 10.3390/ijms21010358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang X, Wang X, Zhu H, Zhu C, Wang Y, Pu WT, Jegga AG, Fan GC. Synergistic effects of the GATA-4-mediated miR-144/451 cluster in protection against simulated ischemia/reperfusion-induced cardiomyocyte death. J Mol Cell Cardiol. 2010;49:841–850. doi: 10.1016/j.yjmcc.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X, Zhang X, Ren XP, Chen J, Liu H, Yang J, Medvedovic M, Hu Z, Fan GC. MicroRNA-494 targeting both proapoptotic and antiapoptotic proteins protects against ischemia/reperfusion induced cardiac injury. Circulation. 2010;122:1308–1318. doi: 10.1161/CIRCULATIONAHA.110.964684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu Y, Gao R, Kaul Z, Li L, Kato Y, Zhang Z, Groden J, Kaul SC, Wadhwa R. Loss-of-function screening to identify miRNAs involved in senescence: Tumor suppressor activity of miRNA-335 and its new arget CARF. Sci Rep. 2016;6:30185. doi: 10.1038/srep30185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ge C, Liu J, Dong S. miRNA-214 protects sepsis-induced myocardial injury. Shock. 2018;50:112–118. doi: 10.1097/SHK.0000000000000978. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Wang H, Ding Y, Li Y, Chen S, Zhang L, Wu H, Zhou J, Duan K, Wang W, et al. N-peptide of vMIP-II reverses paclitaxel-resistance by regulating miRNA-335 in breast cancer. Int J Oncol. 2017;51:918–930. doi: 10.3892/ijo.2017.4076. [DOI] [PubMed] [Google Scholar]
- 24.O'Shea KM, Ananthakrishnan R, Li Q, Quadri N, Thiagarajan D, Sreejit G, Wang L, Zirpoli H, Aranda JF, Alberts AS, et al. The formin, DIAPH1, is a key modulator of myocardial ischemia/reperfusion injury. EBioMedicine. 2017;26:165–174. doi: 10.1016/j.ebiom.2017.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Togashi Y, Shirakawa J, Okuyama T, Yamazaki S, Kyohara M, Miyazawa A, Suzuki T, Hamada M, Terauchi Y. Evaluation of the appropriateness of using glucometers for measuring the blood glucose levels in mice. Sci Rep. 2016;6:25465. doi: 10.1038/srep25465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haak BW, Prescott HC, Wiersinga WJ. Therapeutic potential of the gut microbiota in the prevention and treatment of sepsis. Front Immunol. 2018;9:2042. doi: 10.3389/fimmu.2018.02042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 28.Zhang SB, Lin SY, Liu M, Liu CC, Ding HH, Sun Y, Ma C, Guo RX, Lv YY, Wu SL, et al. CircAnks1a in the spinal cord regulates hypersensitivity in a rodent model of neuropathic pain. Nat Commun. 2019;10:4119. doi: 10.1038/s41467-019-12049-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liang L, Fu J, Wang S, Cen H, Zhang L, Mandukhail SR, Du L, Wu Q, Zhang P, Yu X. MiR-142-3p enhances chemosensitivity of breast cancer cells and inhibits autophagy by targeting HMGB1. Acta Pharm Sin B. 2020;10:1036–1046. doi: 10.1016/j.apsb.2019.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao Y, Zhao H, Li Y. LncRNA MCM3AP-AS1 regulates miR-142-3p/HMGB1 to promote LPS-induced chondrocyte apoptosis. BMC Musculoskelet Disord. 2019;20:605. doi: 10.1186/s12891-019-2967-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liang H, Su X, Wu Q, Shan H, Lv L, Yu T, Zhao X, Sun J, Yang R, Zhang L, et al. LncRNA 2810403D21Rik/Mirf promotes ischemic myocardial injury by regulating autophagy through targeting Mir26a. Autophagy. 2020;16:1077–1091. doi: 10.1080/15548627.2019.1659610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang YP, Gao FF, Wang B, Zheng FC, Zhang YM, Chen YC, Huang ZQ, Zheng YS, Zhong SP, Shi GG. N-n-butyl haloperidol iodide inhibits H2O2-induced Na+/Ca2+-exchanger activation via the Na+/H+ exchanger in rat ventricular myocytes. Drug Des Devel Ther. 2014;8:1257–1267. doi: 10.2147/DDDT.S63163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chistiakov DA, Orekhov AN, Bobryshev YV. Cardiac-specific miRNA in cardiogenesis, heart function and cardiac pathology (with focus on myocardial infarction) J Mol Cell Cardiol. 2016;94:107–121. doi: 10.1016/j.yjmcc.2016.03.015. [DOI] [PubMed] [Google Scholar]
- 34.Bai XY, Ma Y, Ding R, Fu B, Shi S, Chen XM. miR-335 and miR-34a Promote renal senescence by suppressing mitochondrial antioxidative enzymes. J Am Soc Nephrol. 2011;22:1252–1261. doi: 10.1681/ASN.2010040367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Y, Lai P, Deng J, Hao Q, Li X, Yang M, Wang H, Dong B. Micro-RNA335-5p targeted inhibition of sKlotho and promoted oxidativestress-mediated aging of endothelial cells. Biomark Med. 2019;13:457–466. doi: 10.2217/bmm-2018-0430. [DOI] [PubMed] [Google Scholar]
- 36.Wu ZJ, Chen YF, Wang HD, Gao FH. Expression of plasma miRNA-497 in children with sepsis-induced myocardial injury and its clinical significance. Zhongguo Dang Dai Er Ke Za Zhi. 2018;20:32–36. doi: 10.7499/j.issn.1008-8830.2018.01.007. (In Chinese) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang SM, Liu GQ, Xian HB, Si JL, Qi SX, Yu YP. LcRNA NEAT1 alleviates sepsis-induced myocardial injury by regulating the TLR2/NF-κB signaling pathway. Eur Rev Med Pharmacol Sci. 2019;23:4898–4907. doi: 10.26355/eurrev_201906_18078. [DOI] [PubMed] [Google Scholar]
- 38.An R, Feng J, Xi C, Xu J, Sun L. miR-146a attenuates sepsis-induced myocardial dysfunction by suppressing IRAK1 and TRAF6 via Targeting ErbB4 Expression. Oxid Med Cell Longev. 2018;2018:7163057. doi: 10.1155/2018/7163057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shang X, Li J, Yu R, Zhu P, Zhang Y, Xu J, Chen K, Li M. Sepsis-related myocardial injury is associated with mst1 upregulation, mitochondrial dysfunction and the Drp1/F-actin signaling pathway. J Mol Histol. 2019;50:91–103. doi: 10.1007/s10735-018-09809-5. [DOI] [PubMed] [Google Scholar]
- 40.Rudiger A, Singer M. The heart in sepsis: From basic mechanisms to clinical management. Curr Vasc Pharmacol. 2013;11:187–195. doi: 10.2174/157016113805290227. [DOI] [PubMed] [Google Scholar]
- 41.Reinhart K, Bauer M, Riedemann NC, Hartog CS. New approaches to sepsis: Molecular diagnostics and biomarkers. Clin Microbiol Rev. 2012;25:609–634. doi: 10.1128/CMR.00016-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Y, Liu L, Zhang J. Protective role of matrine in sepsis-associated cardiac dysfunction through regulating the lncRNA PTENP1/miR-106b-5p axis. Biomed Pharmacother. 2021;134:111112. doi: 10.1016/j.biopha.2020.111112. [DOI] [PubMed] [Google Scholar]
- 43.Sluijter JP, Doevendans PA. Sepsis-associated cardiac dysfunction is controlled by small RNA molecules. J Mol Cell Cardiol. 2016;97:67–69. doi: 10.1016/j.yjmcc.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 44.Wang H, Bei Y, Shen S, Huang P, Shi J, Zhang J, Sun Q, Chen Y, Yang Y, Xu T, et al. miR-21-3p controls sepsis-associated cardiac dysfunction via regulating SORBS2. J Mol Cell Cardiol. 2016;94:43–53. doi: 10.1016/j.yjmcc.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 45.Wu N, Zhang X, Du S, Chen D, Che R. Upregulation of miR-335 ameliorates myocardial ischemia reperfusion injury via targeting hypoxia inducible factor 1-alpha subunit inhibitor. Am J Transl Res. 2018;10:4082–4094. [PMC free article] [PubMed] [Google Scholar]
- 46.Joulin O, Petillot P, Labalette M, Lancel S, Neviere R. Cytokine profile of human septic shock serum inducing cardiomyocyte contractile dysfunction. Physiol Res. 2007;56:291–297. doi: 10.33549/physiolres.930946. [DOI] [PubMed] [Google Scholar]
- 47.Correia de Sousa M, Gjorgjieva M, Dolicka D, Sobolewski C, Foti M. Deciphering miRNAs' Action through miRNA Editing. Int J Mol Sci. 2019;20:6249. doi: 10.3390/ijms20246249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Agnelli L, Bisognin A, Todoerti K, Manzoni M, Taiana E, Galletti S, Cutrona G, Gaffo E, Bortoluzzi S, Neri A. Expanding the repertoire of miRNAs and miRNA-offset RNAs expressed in multiple myeloma by small RNA deep sequencing. Blood Cancer J. 2019;9:21. doi: 10.1038/s41408-019-0184-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang W. MicroRNAs: Biomarkers, diagnostics, and therapeutics. Methods Mol Biol. 2017;1617:57–67. doi: 10.1007/978-1-4939-7046-9_4. [DOI] [PubMed] [Google Scholar]
- 50.Moradifard S, Hoseinbeyki M, Ganji SM, Minuchehr Z. Analysis of microRNA and gene expression profiles in Alzheimer's disease: A Meta-analysis approach. Sci Rep. 2018;8:4767. doi: 10.1038/s41598-018-20959-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Papaioannou V, Pneumatikos I, Maglaveras N. Association of heart rate variability and inflammatory response in patients with cardiovascular diseases: Current strengths and limitations. Front Physiol. 2013;4:174. doi: 10.3389/fphys.2013.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ruiz-Ortega M, Esteban V, Egido J. The regulation of the inflammatory response through nuclear factor-kappab pathway by angiotensin IV extends the role of the renin angiotensin system in cardiovascular diseases. Trends Cardiovasc Med. 2007;17:19–25. doi: 10.1016/j.tcm.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 53.Frangogiannis NG. Pathophysiology of myocardial infarction. Compr Physiol. 2015;5:1841–1875. doi: 10.1002/cphy.c150006. [DOI] [PubMed] [Google Scholar]
- 54.Hanna A, Frangogiannis NG. Inflammatory cytokines and chemokines as therapeutic targets in heart failure. Cardiovasc Drugs Ther. 2020;34:849–863. doi: 10.1007/s10557-020-07071-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bartekova M, Radosinska J, Jelemensky M, Dhalla NS. Role of cytokines and inflammation in heart function during health and disease. Heart Fail Rev. 2018;23:733–758. doi: 10.1007/s10741-018-9716-x. [DOI] [PubMed] [Google Scholar]
- 56.Bai R, Yin X, Feng X, Cao Y, Wu Y, Zhu Z, Li C, Tu P, Chai X. Corydalis hendersonii Hemsl. Protects against myocardial injury by attenuating inflammation and fibrosis via NF-κB and JAK2-STAT3 signaling pathways. J Ethnopharmacol. 2017;207:174–183. doi: 10.1016/j.jep.2017.06.020. [DOI] [PubMed] [Google Scholar]
- 57.Qiu Z, He Y, Ming H, Lei S, Leng Y, Xia ZY. Lipopolysaccharide (LPS) aggravates high glucose- and hypoxia/reoxygenation-induced injury through activating ROS-dependent NLRP3 inflammasome-mediated pyroptosis in H9C2 cardiomyocytes. J Diabetes Res. 2019;2019:8151836. doi: 10.1155/2019/8151836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Beutler B, Milsark IW, Cerami AC. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. J Immunol. 2008;181:7–9. [PubMed] [Google Scholar]
- 59.Gao XL, Li JQ, Dong YT, Cheng EJ, Gong JN, Qin YL, Huang YQ, Yang JJ, Wang SJ, An DD. Upregulation of microRNA-335-5p reduces inflammatory responses by inhibiting FASN through the activation of AMPK/ULK1 signaling pathway in a septic mouse model. Cytokine. 2018;110:466–478. doi: 10.1016/j.cyto.2018.05.016. [DOI] [PubMed] [Google Scholar]
- 60.Fadeel B, Orrenius S, Zhivotovsky B. The potential role of apoptosis in human disease. Med Princ Pract. 2000;9:151–163. doi: 10.1159/000054240. [DOI] [Google Scholar]
- 61.Kong W, Kang K, Gao Y, Liu H, Meng X, Cao Y, Yang S, Liu W, Zhang J, Yu K, Zhao M. GTS-21 protected against LPS-induced sepsis myocardial injury in mice through α7nAChR. Inflammation. 2018;41:1073–1083. doi: 10.1007/s10753-018-0759-x. [DOI] [PubMed] [Google Scholar]
- 62.Wang X, Yu Y. miR-146b protect against sepsis induced mice myocardial injury through inhibition of Notch1. J Mol Histol. 2018;49:411–417. doi: 10.1007/s10735-018-9781-4. [DOI] [PubMed] [Google Scholar]
- 63.Meng YY, Liu Y, Hu ZF, Zhang Y, Ni J, Ma ZG, Liao HH, Wu QQ, Tang QZ. Sanguinarine attenuates lipopolysaccharide-induced inflammation and apoptosis by inhibiting the TLR4/NF-κB pathway in H9c2 cardiomyocytes. Curr Med Sci. 2018;38:204–211. doi: 10.1007/s11596-018-1867-4. [DOI] [PubMed] [Google Scholar]
- 64.Falck M, Osredkar D, Wood TR, Maes E, Flatebø T, Sabir H, Thoresen M. Neonatal systemic inflammation induces inflammatory reactions and brain apoptosis in a pathogen-specific manner. Neonatology. 2018;113:212–220. doi: 10.1159/000481980. [DOI] [PubMed] [Google Scholar]
- 65.Mocanu MM, Yellon DM. PTEN, the Achilles' heel of myocardial ischaemia/reperfusion injury? Br J Pharmacol. 2007;150:833–838. doi: 10.1038/sj.bjp.0707155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou XM, Sun R, Luo DH, Sun J, Zhang MY, Wang MH, Yang Y, Wang HY, Mai SJ. Upregulated TRIM29 promotes proliferation and metastasis of nasopharyngeal carcinoma via PTEN/AKT/mTOR signal pathway. Oncotarget. 2016;7:13634–13650. doi: 10.18632/oncotarget.7215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang H, Kong W, He L, Zhao JJ, O'Donnell JD, Wang J, Wenham RM, Coppola D, Kruk PA, Nicosia SV, Cheng JQ. MicroRNA expression profiling in human ovarian cancer: miR-335 induces cell survival and cisplatin resistance by targeting PTEN MicroRNA expression profiling in human ovarian cancer: miR-335 induces cell survival and cisplatin resistance by targeting PTEN. Cancer Res. 2008;68:425–433. doi: 10.1158/0008-5472.CAN-07-6426. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.