Abstract
There is a well-known association of traumatic experiences and posttraumatic stress disorder (PTSD) with body size and composition, including consistent differences between sexes. However, the biology underlying these associations is unclear. To understand the genetic underpinnings of this complex relationship, we investigated genome-wide datasets informative of African and European ancestries from the Psychiatric Genomic Consortium, the UK Biobank, the GIANT Consortium, and the Million Veteran Program. We used genome-wide association statistics to estimate sex-specific genetic correlations (rg) of traumatic experiences, social support, and PTSD with multiple anthropometric traits. After multiple testing corrections (false discovery rate, FDR q < 0.05), we observed 58 significant rg relationships in females (e.g., childhood physical abuse and body mass index, BMI rg = 0.245, p = 3.88 × 10−10) and 21 significant rg relationships in males (e.g., been involved in combat or exposed to warzone and leg fat percentage; rg = 0.405, p = 4.42 × 10−10). We performed causal inference analyses of these genetic overlaps using Mendelian randomization and latent causal variable approaches. Multiple female-specific putative causal relationships were observed linking body composition/size with PTSD (e.g., leg fat percentage→PTSD; beta = 0.319, p = 3.13 × 10−9), traumatic experiences (e.g., childhood physical abuse→waist circumference; beta = 0.055, p = 5.07 × 10−4), and childhood neglect (e.g., “someone to take you to doctor when needed as a child”→BMI; beta = −0.594, p = 1.09 × 10−5). In males, we observed putative causal effects linking anthropometric-trait genetic liabilities to traumatic experiences (e.g., BMI→childhood physical abuse; beta = 0.028, p = 8.19 × 10−3). Some of these findings were replicated in individuals of African descent although the limited sample size available did not permit us to conduct a sex-stratified analysis in this ancestry group. In conclusion, our findings provide insights regarding sex-specific causal networks linking anthropometric traits to PTSD, traumatic experiences, and social support.
Keywords: Trauma, Anthropometric traits, Sex, Mendelian randomization, PTSD
Highlights
-
•
Investigate the sex-specific relationship of PTSD with body size and composition
-
•
Sex differences in genetic correlations of PTSD with anthropometric traits
-
•
Sex-specific causal effects among trauma, PTSD, and fat accumulation
-
•
Social support reduces fat accumulation in women
1. Introduction
Traumatic events include any experiences that are deeply distressing or disturbing (e.g., exposure to or threatened with death, actual or threatened serious injury, and/or sexual violence) (Harper, 2014). Trauma exposure can have significant consequences for mental health. Depending on trauma type and pre-trauma risk factors, a portion of trauma-exposed individuals develop posttraumatic stress disorder (PTSD) (Kilpatrick et al., 2013). The lifetime prevalence of PTSD is higher in women (10–12%) than in men (5–6%) (Olff, 2017). It is unclear whether this difference in prevalence reflects a differential vulnerability for the disorder per se, or whether because women are more likely to be exposed to events with a high conditional risk for PTSD (Tolin and Foa, 2006). Twin studies estimated that PTSD heritability ranges from 24% to 72% (Segman and Shalev, 2003; Stein et al., 2002; True et al., 1993; Xian et al., 2000) with females presenting two to three times higher estimates than males (Broekman et al., 2007; Cornelis et al., 2010; Duncan et al., 2015). The interplay between biological mechanisms related to early stressful experiences and changes in stress response systems (e.g., hypothalamus-pituitary-adrenal axis, sympathetic nervous system, and sex hormone regulation) (Feng et al., 2019; McEwen, 1998; Ramikie and Ressler, 2016; Solomon, 1989; Solomon et al., 2017) and environmental factors, such as nature of trauma (Tolin and Foa, 2006) could play an important role in PTSD sex differences.
There is growing evidence that body mass index and body composition are associated clinically and genetically with psychiatric traits (Peters et al., 2020), as depression (Luppino et al., 2010; Speed et al., 2019), ADHD (Nigg et al., 2016), and PTSD (Polimanti et al., 2017). The variability across anthropometric traits could reflect the complex interplay among genetic susceptibility, pre-trauma risk factor, response to traumatic experiences (Midei and Matthews, 2011), and PTSD (Hartwig et al., 2017; Kubzansky et al., 2014; Pooley et al., 2018). In particular, traits related to body composition and fat accumulation (e.g., body mass index, BMI; waist circumference, WC; waist-hip ratio, WHR) are known to be associated with psychological stress and certain psychiatric disorders and behavioral traits (Hübel et al., 2019; Polimanti et al., 2017). Additionally, anthropometric measures related to body development and growth (e.g., height) are strongly affected by early traumatic events (Gunstad et al., 2006; Kubzansky et al., 2014). The mechanisms linking trauma exposure or PTSD and body composition are complex. For example, alterations in neurobiological systems might lead to dysregulation in the neuroendocrine regulation (Aliev et al., 2020) and changes in eating (Dallman et al., 2003; Taylor, 2010; Wichers et al., 2009), affecting BMI (Kubzansky et al., 2014; Roenholt et al., 2012) and weight (Mitchell et al., 2016; Wolf et al., 2017).
Since both PTSD and anthropometric traits show major sex differences, understanding the complex network linking PTSD and anthropometric traits could contribute to disentangling the mechanisms responsible for sex differences in the vulnerability to PTSD. In this study, our primary aim was to estimate sex-specific genetic overlap and to infer putative causal associations of anthropometric traits with PTSD and traits related to trauma and social support.
2. Methods
2.1. Study design
This study was designed to estimate differences between sexes for genetic overlap and putative causal influences linking anthropometric measures to traits related to traumatic experiences, social support, and PTSD using large-scale genome-wide data derived from multiple cohorts: the UK Biobank (UKB), Genetic Investigation of Anthropometric Traits (GIANT), the Psychiatric Genomics Consortium for PTSD (PGC-PTSD), and the Million Veteran Program (MVP). Similar to previous studies (Dong et al., 2021; Langdon et al., 2019; Shen et al., 2020; Zheng et al., 2020), we tested a wide range of traits to generate new hypotheses regarding the possible mechanisms linking anthropometric traits to PTSD and related phenotypes. The wide spectrum of our investigation aims to generate novel hypotheses to understand the pathways involved in the associations among body measurements, PTSD, and correlated traits.
We implemented a multi-step analytic design. Firstly, we applied linkage disequilibrium score regression (LDSC) to estimate SNP-heritability and genetic correlation (rg) among anthropometric traits, PTSD, traumatic experiences, and social support. For trait pairs with significant rg, we used the latent causal variables (LCV) method to investigate whether the rg between two traits is mediated by a latent variable with a causal effect on one of the traits tested (O'Connor and Price, 2018). We used LCV results to select trait pairs to be tested with bidirectional polygenic risk scores (PRS). Best-fit PRS was used to determine the genetic instruments to employ for Mendelian randomization (MR) tests. Lastly, we applied MR analyses to investigate the causal relationships among the traits using genetic instruments based on a PRS analysis (Carvalho et al., 2020; Muniz Carvalho et al., 2021; Polimanti et al., 2019b; Wendt et al., 2019) (Supplemental Fig. 1). Across these different analyses, we applied a false discovery rate (FDR) multiple testing correction to account for the correlation among the hypotheses tested. FDR q values are derived from the distribution of the original p-values and a significance threshold of FDR 5% can adequately control for multiple testing independently from the number of tests performed (Chen et al., 2010).
2.2. Data sources
PTSD. We used the genome-wide association statistics from a subsample of the two most recent GWAS of PTSD: the PGC-PTSD freeze 1.5 dataset (Nievergelt et al., 2019) and PGC-MVP meta-analysis for PTSD (Stein et al., 2021). In our analyses, we considered GWAS data generated from participants of European descent from PGC cohorts (12 823 cases and 35 648 controls; 40% female) and African-ancestry PGC-MVP meta-analysis (11 920 PTSD cases and 39 116 controls). Additional information regarding these datasets is available in the Supplemental Methods. We decided to not use the larger PGC-PTSD freeze 2 that includes participants from PGC cohorts and UKB cohort to avoid biases related to the sample overlap between exposure and outcome traits in the PRS and MR analyses. Overlap of participants between the two samples can increase false-positive results in both PRS and MR analyses (Burgess et al., 2016; Choi et al., 2020).
Anthropometrics traits. We used GWAS datasets from GIANT and UKB. GIANT is an international consortium investigating genetic variants associated with human body size and shape measures, such as BMI, height, and traits related to WC (see https://portals.broadinstitute.org/collaboration/giant/index.php/Main_Page). We used sex-stratified European-ancestry genetic data from up to 171 977 females and 152 893 males (Locke et al., 2015; Shungin et al., 2015). UKB is an international health resource that provides genetic information and data about a wide range of diseases, lifestyle habits, and behaviors in up to 502 543 individuals (aged 40–69 years) (Allen et al., 2014; Bycroft et al., 2018). Details regarding GWAS quality control criteria and methods are reported in the Supplemental Methods. We investigated sex-stratified GWAS association statistics of anthropometric traits derived from up to 193 000 female and 166 440 male UKB-EUR participants of European descent as defined by principal component analysis of the genetic data. The UKB traits investigated (Supplemental Table 1) are included in two data categories: “body size measures” and “impedance measures”. The “body size measures” category (UKB Field ID: 100010) includes data on body composition measures that were taken manually (i.e., standing/sitting height, waist/hip circumference, weight, and body mass index). The “impedance measures” category (UKB Field ID: 100009) include data on whole-body bio-impedance measures obtained by the Tanita BC418MA body composition analyzer (i.e., basal metabolic rate, body type, weight, body fat mass, body fat percentage, fat-free mass, water mass, predicted muscle mass and impedance for the right arm, right leg, left arm, left leg and trunk). Information regarding GIANT and UKB GWAS of anthropometric traits is reported in Supplemental Table 1. To explore the association identified in other ancestry groups, we investigated genome-wide association statistics generated from the analysis of UKB participants of African descent (N = 6570). Details regarding these datasets are available in the Supplemental Methods.
Traits related to trauma and social support. We examined sex-stratified GWAS data derived from the UKB traits included in the category “traumatic events” (UKB Field ID: 145; Supplemental Table 1). These traits were assessed in up to 65 000 female participants and 51 000 male participants of European descent. These traits were assessed using the previously validated UKB online mental health questionnaire (Davis et al., 2019). These include traits related to traumatic exposure, response to traumatic events, and social support.
2.3. Linkage disequilibrium score regression
Linkage Disequilibrium Score Regression (LDSC) (Bulik-Sullivan et al, 2015a, 2015b) (available at https://github.com/bulik/ldsc) was used to calculate the SNP-heritability estimates and rg using GWAS association statistics. SNP-heritability corresponds to the proportion of phenotypic variance captured by the associated SNPs in the GWAS (Yang et al., 2017). The rg is a measure of the genetic components shared by two phenotypes investigated (van Rheenen et al., 2019). We investigated the SNP-heritability for anthropometric traits (38 traits from UKB-EUR and 7 traits from GIANT consortium), PTSD (PGC Freeze 1.5), and 21 traits related to traumatic experiences and social support. As recommended by LDSC developers (Bulik-Sullivan et al, 2015a, 2015b), we conducted the genetic correlation analysis only with the traits with heritability z-score>4 (Bulik-Sullivan et al, 2015a, 2015b). We performed Z-tests to calculate the sex differences in SNP-heritability based on the estimates and the standard errors derived from the female- and male-specific LDSC analyses. A FDR multiple testing correction (q < 0.05) was applied to determine the statistical significance of the rg calculated.
2.4. Latent causal variable (LCV)
The latent causal variable (LCV) method postulates that the rg between two traits can be mediated by a latent variable that has a causal effect on the traits tested (O'Connor and Price, 2018). LCV estimates the genetic causality proportion (GCP) using z-score converted per-variant effects and regression weights for genome-wide association statistics (O'Connor and Price, 2018). We performed an initial causal inference analysis for significant rg identified via LDSC. This method differs from MR in that it uses genome-wide information rather than a select subset of the most powerful available genetic instruments. A multiple testing FDR correction (q < 0.05) was applied to all GCP estimates.
LCV tests the null hypothesis that GCP = 0 (i.e., there is no latent causal effect between traits). P-values and z-scores indicate the significance of the test where z-scores > 0 indicate GCP≠ 0. GCP estimates range from −1 to 1. The magnitude of GCP indicates the proportion of causality between two traits. The sign of each GCP estimate indicates the causal direction inferred from each test with GCP>0 indicating that trait 1 causes trait 2 and GCP< 0 indicating that trait 2 causes trait 1. GCP estimates were interpreted if the h2 z-score for both traits in a pair were >7. In Results section, we describe all LCV results in their interpreted causal direction reporting only positive GCP estimates. All LCV data and their interpretation are reported as Supplementary Material.
2.5. Polygenic risk score (PRS)
Based on the significant rg and LCV results, PRS was computed to define the genetic instruments for MR tests. To increase the variance explained by genetic instruments, we tested PRS instrument considering multiple p-value thresholds. PRS was calculated using the gtx R package embedded in PRSice v1.25 software. PRS calculates an approximate estimate of the variance explained from a multivariate regression model, and its results can identify evidence for shared genetic etiology between trait pairs. PRS was calculated using the following parameters: clumping with an LD cutoff of R2 = 0.001 in 10 000-kb windows excluding the major histocompatibility complex region of the genome because of its complex LD structure. The LD reference panels for European and African ancestries were derived from the reference populations available from the 1000 Genomes Project. To avoid overlapping individuals, we compared the GWAS datasets available depending on the cohorts: i) anthropometric traits from GIANT vs. UKB-EUR traits related to traumatic experiences and social support; ii) anthropometric traits from UKB-EUR and GIANT vs. PGC-PTSD Freeze 1.5; iii) anthropometric traits from UKB-AFR vs. AFR MVP-PGC PTSD. We applied FDR multiple testing corrections to correct the p-value for the number of PRS p-value thresholds tested, considering q < 0.05 as the significance threshold. The PRS association identified in European-ancestry and African-ancestry datasets and surviving multiple testing correction were tested further in the subsequent MR analyses.
2.6. Mendelian randomization (MR)
MR uses genetic variants to infer causal relationships between an exposure and an outcome of interest (Zheng et al., 2017). MR relies on genetic variants satisfying three assumptions: (i) genetic variants are associated with an exposure in a specific way, (ii) the genetic variants are not associated with confounding factors linking exposure and outcome, and (iii) the genetic variants are associated with the outcome only through their association with the exposure (Davies et al., 2018). Similar to previous studies (Carvalho et al., 2020; Muniz Carvalho et al., 2021; Polimanti et al, 2017, 2019a, 2019c; Ravera et al., 2018; Tamman et al., 2021; Wendt et al., 2019), to maximize our statistical power, we defined the genetic instrument on the basis of the best-fit PRS estimates surviving multiple testing correction. This permitted us to verify that our instrumental variables do not violate the first MR assumption. To confirm the reliability of the results obtained, we used different MR methods: random-effect inverse variance weighted (IVW) (Hemani et al., 2018), MR-Egger (Hartwig et al., 2017), weighted median (Bowden et al., 2016), simple mode (Bowden et al., 2016), and weighted mode (Bowden et al., 2016). These different approaches have different sensitivities with respect to different causal scenarios that may be presented. We also conducted MR sensitivity tests to detect the presence of confounding horizontal pleiotropic effects in genetic variants (MR-Egger regression intercept and MR-PRESSO (Pleiotropy RESidual Sum and Outlier) global test) (Verbanck et al., 2018) and to investigate the heterogeneity of variants (IVW heterogeneity test) [32]. A leave-one-out analysis was also conducted to identify potential outliers among the variants. These sensitivity analyses permitted us to verify possible biases introduced by the presence of horizontal pleiotropy and heterogeneity violating the second and third MR assumptions. FDR multiple testing correction was applied to account for the number of MR tests performed. The MR analysis was conducted in accordance with the STROBE (STrenghtening the Reporting of Observational studies in Epidemiology) - MR reporting guidelines (Davey Smith et al., 2019).
3. Results
3.1. SNP-heritability and genetic correlation
The SNP-heritability was calculated for PTSD, anthropometric traits, and outcomes related to traumatic experiences and social support for both sexes, observing four traits with significant sex differences (FDR q < 0.05; Supplemental Table 2). Consistently with the estimates reported in the PGC-PTSD GWAS (Nievergelt et al., 2019), female SNP-heritability of PTSD was much higher than that observed in males, 15.4% vs. 1.03% (psex-difference = 1.43 × 10−3). Among anthropometric traits, waist circumference adjusted for BMI (WCadjBMI) showed higher heritability in males than in females (17.7% vs. 11.4%, psex-difference = 4.91 × 10−5). Among traumatic experiences, physical violence and belittlement by partner or ex-partner as an adult showed higher SNP-heritability in females than in males (UKB-EUR Field ID 20523: 4.9% vs. 0.7%, psex-difference = 3.21 × 10−4; UKB-EUR Field ID 20521: 6.5% vs. 2.5%, psex-difference = 9.72 × 10−4). Conversely, no sex differences were observed with respect to the SNP-heritability of traits related to social support (Supplemental Table 2).
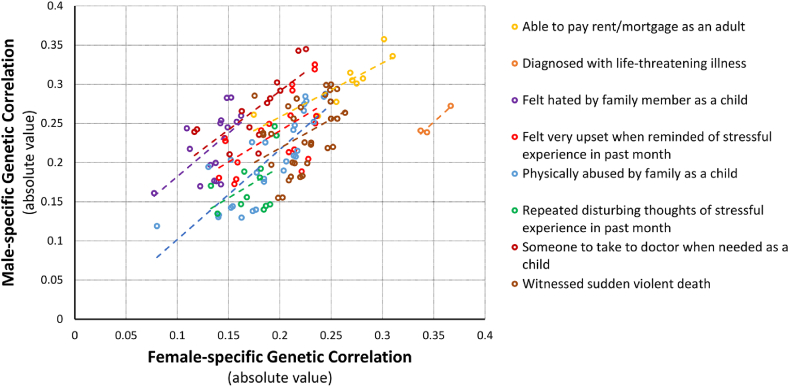
In the sex-stratified rg analysis, the number of significant observations was more than twice significant rg in the female analysis than in the male analysis (Nfemales = 472 vs. Nmales = 189; Fig. 1, Supplemental Table 3). Considering the top rg results for traumatic experiences, the female-specific analysis identified highly significant genetic overlap between childhood traumatic experiences (i.e., UKB-EUR Field ID: 20488 – “physically abused by a family member as a child”) and BMI (UKB-EUR Field ID: 21001; rg = 0.245, p = 3.88 × 10−10) while war-related trauma (i.e., UKB-EUR Field ID: 20527 – “been involved in combat or exposed to warzone”) was genetically correlated with peripheral fat distribution (UKB-EUR Field ID: 23111 - leg fat percentage; rg = 0.405, p = 4.42 × 10−10) in males. With respect to social support, both female and male analyses showed genetic correlation with peripheral fat distribution as the strongest result (female – UKB-EUR Field ID: 20525 – “able to pay rent/mortgage as an adult” vs. UKB-EUR Field ID: 23111 - leg fat percentage, rg = −0.31, p = 3.95 × 10−7; male – UKB-EUR Field ID: 20491 – “Someone to take to doctor when needed as a child” vs. UKB-EUR Field ID: 23111 - leg fat percentage, rg = −0.34, p = 3 × 10−4). Considering PTSD rg, we observed 19 genetically correlated traits in females while no result survived multiple testing correction in males. Similarly, to what was observed with respect to traumatic experiences and social support, the strongest finding was related to peripheral fat distribution (UKB-EUR Field ID: 23111 - Leg fat percentage; rg = 0.224, p = 4.74 × 10−5; Fig. 1, Supplemental Table 3).
Fig. 1.
Sex-stratified genetic correlations of traits related to traumatic events and anthropometric traits surviving FDR multiple testing correction in both sexes. Linear regression (dashed line) is included in relation to the genetic correlation of each traumatic-event trait with the anthropometric traits reported in the figure.
3.2. Latent causal variable analysis
To test whether the significant rg results were due to causal effects rather than shared genetic mechanisms, we applied the LCV method and observed that 39 (females) and 16 (males) combinations tested present significant putative causal effects (FDR q<5%; Fig. 2, Supplemental Table 4).
Fig. 2.
Latent causal variable (LCV) network among traumatic experiences, social support, and anthropometric traits surviving multiple testing correction. Panels A and B show male and female findings, respectively.
In females, we found that two trauma traits have large causal effects on traits linked to body composition with the strongest result being “Witnessed sudden violent death” (UKB-EUR field ID: 20530) → Leg predicted mass (UKB-EUR field ID: 23114, GCP = 0.23, p = 3.45 × 10−47). Also, the genetic liability to a social support trait (UKB-EUR field ID: 20491 – “Someone to take to doctor when needed as a child”) showed a putative effect on peripheral fat distribution (UKB-EUR field ID: 23111 - Leg fat percentage; GCP = 0.76, p = 1.72 × 10−8). In addition, the genetic liability to certain anthropometric traits (Supplemental Table 4) showed an association with a small but significant effect to witnessing sudden violent death (top-result: UKB-EUR field ID 23105 “Basal metabolic rate” → UKB-EUR field ID 20530 “Witnessed sudden violent death”; GCP = 0.094, p = 6.45 × 10−12).
Differently from females where multiple traumatic experiences showed putative causal effects on anthropometric traits, we observed that only three traits related to trauma have causal effects on body size and composition (Supplemental Table 4) with the strongest effect observed for “Been in serious accident/believed to be life-threatening” (UKB-EUR Field ID: 20526) with respect to anthropometric traits → waist circumference (UKB-EUR Field ID: 48; GCP = 0.729, p = 3.11 × 10−14). However, genetic liabilities to certain anthropometric traits (Supplemental Table 4) were associated with risk of childhood physical abuse in males (UKB-EUR field ID: 20488) with the strongest effect observed for waist circumference (UKB-EUR Field ID: 48) → childhood physical abuse (GCP = 0.318, p = 1.69 × 10−4). In relation to social support, we observed that the genetic liability to “been in a confiding relationship as an adult” (UKB-EUR field ID: 20522) had a moderate effect on standing and sitting height (UKB-EUR field ID: 50 and 20015, respectively; GCP = 0.44, standing height, p = 2.47 × 10−20; GCP = 0.623, p = 7.82 × 10−20), suggesting a possible nurture effect where parental genotypes affect height development. However, the genetic liability to trunk fat-free mass and trunk predicted mass (UKB-EUR field IDs: 23129 and 23130, respectively) also showed small but significant effects on been in a confiding relationship as an adult (gcp.pm = 0.011, p = 1.33 × 10−6; gcp.pm = 0.015, p = 1.17 × 10−4; respectively).
3.3. Mendelian randomization (MR)
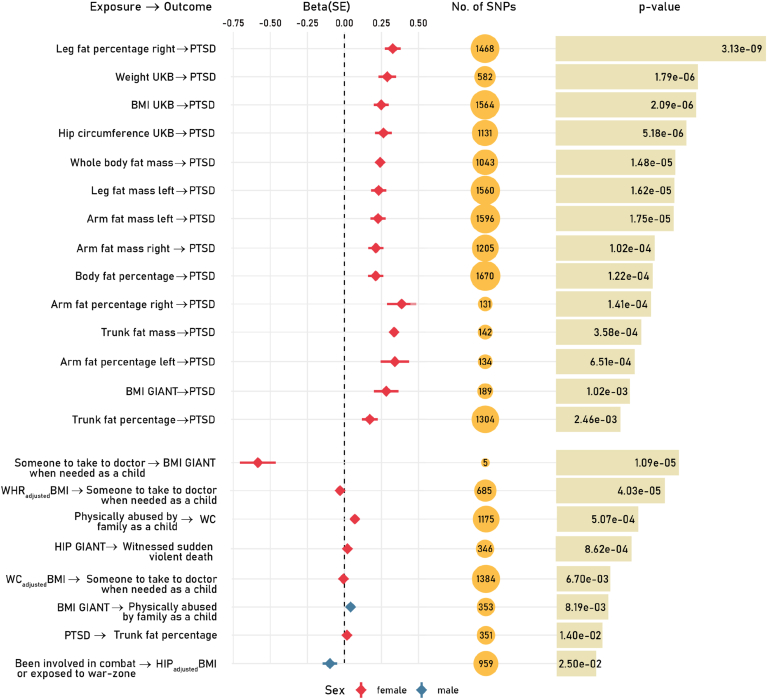
Similar to the strategy used in previous studies (Carvalho et al., 2020; Muniz Carvalho et al., 2021; Polimanti et al., 2019b; Wendt et al., 2019), we used PRS results to define genetic instruments for MR causal inference among the traits that showed significant genetic correlations and LCV. Accounting for the number of PRS thresholds tested, we identified 42 and six genetic instruments (i.e., sets of SNPs) significant after FDR multiple testing correction (q < 0.05) in females and males, respectively (Supplemental Table 5). These were applied with respect to different MR approaches and further tested for potential biases using appropriate sensitivity analyses. We applied FDR multiple testing correction (q < 0.05) to account for the number of MR tests performed. Fig. 3 and Supplemental Table 6 describe the significant MR findings.
Fig. 3.
Significant Mendelian randomization (MR) tests based on the inverse variance weighted method (IVW; FDR q < 0.05). Effect size (beta) and 95% confidence interval are reported for each MR test. We reported sex-specific estimates only when the initial PRS analysis to define the genetic instruments survived multiple testing correction (See Study Design and Supplemental Fig. 1).
In the female-specific analysis, we identified evidence of putative causal effects of multiple traits related to body fat distribution on PTSD. The strongest effect on PTSD was observed for peripheral fat distribution (UKB-EUR Field ID: 23111 – Leg fat percentage; IVW beta = 0.319, p = 3.13 × 10−9). We also observed a nominally-significant reverse effect of PTSD on “trunk fat percentage” (IVW beta = 0.0042, p = 0.014). With respect to traumatic experiences, childhood physical abuse (UKB-EUR Field ID: 20488) has a potential causal effect on WC (IVW beta = 0.055, p = 5.07 × 10−4). Conversely, the genetic liability to hip circumference was associated with a higher risk of having witnessed sudden violent death (UKB-EUR Field ID: 20530; IVW beta = 0.0134, p = 8.62 × 10−4). Additionally, the genetic liability to body shape traits (WC and BMI-adjusted WHR) showed a negative effect on childhood social support (UKB-EUR Field ID: 20491 “Someone to take to doctor when needed as a child”, WCadjBMI - IVW beta = −0.020, p = 6.7 × 10−3; WHRadjBMI - IVW beta = −0.032, p = 4.03 × 10−5). Conversely, childhood social support (UKB Field ID: 20491) is associated with reduced BMI (IVW beta = −0.594, p = 1.09 × 10−5). Because the genetic instruments of childhood social support included only five variants, we verified the estimate using the MR-RAPS (Robust Adjusted Profile Score) method that is designed to address possible biases induced by weak genetic instrument (Zhao et al., 2020). MR-RAPS approach confirmed the negative effect of childhood social support (UKB Field ID: 20491) on BMI (beta = −0.449, p-value: 0.001).
In the male-specific analysis, genetic liability to BMI showed a putative effect on childhood physical abuse (UKB-EUR Field ID: 20488; IVW beta = 0.0287, p = 8.19 × 10−3). An inverse effect direction was observed with respect to experiencing war-related events (UKB-EUR Field ID: 20527) with hip circumference adjusted for BMI (HIPadjBMI; IVW beta = −0.11, p = 2.5 × 10−2).
3.4. PRS and MR analyses in individuals of African descent
Leveraging informative datasets for anthropometric traits (UKB) and PTSD (MVP-PGC), we tested the genetic overlap and causal effect in individuals of African descent. Due to the limited sample size available, we cannot stratify these datasets by sex and cannot perform LDSC and LCV analyses. Our PRS analyses (Supplemental Table 7) showed bidirectional relationships of PTSD with several anthropometric measurements of the whole body (fat-free mass, water mass, and fat mass), arm (fat-free mass), and leg (fat-free mass). Conversely, we observed an unidirectional effect of PTSD PRS on BMI and standing height. An opposite direction was observed for trunk measures (fat-free mass and predicted mass) and hip circumference where their PRS was associated with PTSD. Following up these significant PRS associations with a MR analysis, we observed only few putative causal effects surviving multiple testing correction (FDR q < 0.05; Supplemental Table 8). These were mostly the association of fat measures to PTSD: arm fat mass (IVW beta = 0.03, p = 2.3 × 10−5), leg fat mass, (IVW beta = 0.04, p = 2.85 × 10−6), and trunk fat percentage (IVW beta = 0.024, p = 1.91 × 10−5).
4. Discussion
PTSD is a multifactorial condition affecting both mental and physical health (Boscarino, 2004). PTSD and traumatic experiences have been associated with higher BMI and obesity (Mamun et al., 2007; Perkonigg et al., 2009; Vieweg et al., 2006) with sex differences in the specific relationships among PTSD, body composition, and other domains of human health (Duncan et al., 2015; Suliman et al., 2016). Clinical studies reported that women with PTSD and a history of childhood trauma have increased BMI, adiposity, and WHR (Buta et al., 2018; Dedert et al., 2010; Dobie et al., 2004; Duncan et al., 2015; Kubzansky et al., 2014; Masodkar et al., 2016; Roenholt et al., 2012). In the present study, we used large-scale genomic datasets to understand the sex-specific associations linking PTSD, traumatic experiences, and social support with body size and composition. Our analyses highlighted that there are many more genetic correlations and putative causal effects among these traits in females than in men. This may be due to the higher vulnerability to PTSD in women than in men (Olff, 2017; Olff et al., 2007) and/or to the higher heritability of PTSD in women than in men (Koenen et al., 2008; Sartor et al., 2011; Tambs et al., 2009; True et al., 1993; Xian et al., 2000). Additionally, similarly to PTSD-related traits, body size and composition displayed more pronounced genetic effects in women than in men (Randall et al., 2013). With respect to the effect direction linking PTSD-related traits to body size and composition, we found a complex network of putative causal effects. In some cases, PTSD-related traits showed putative causal effects on body size and composition, while in other circumstances the effects were reversed. This suggests that the sex differences observed are not due to a single widespread mechanism linking PTSD, traumatic experiences, and social support with body size and composition. Conversely, we hypothesize that the putative causal effects observed reflect a diverse array of overlapping mechanisms linking PTSD-related traits to multiple biological systems reflected by the individual-variability of body size and composition between sexes. Indeed, anthropometric traits can reflect multiple pathways related to steroid hormone regulation, adipogenesis, lipid storage, muscle metabolism, composition, and contractile speed, skeletal growth and maturation, and lipolysis (Polimanti et al., 2016; Randall et al., 2013). Under this scenario, the effects of PTSD, traumatic experiences, and social support on body size and composition may be due to the pathogenic changes induced on the regulation of these biological pathways. However, we also observed instances where genetic liabilities to certain anthropometric traits are associated with an increased risk of PTSD, traumatic experiences, and low social support.
With respect to PTSD, genetic associations with peripheral fat distribution indicate possible causal effects on PTSD risk in females. This putative effect direction appears to be female-specific (i.e., no effect was observed in males). Conversely, both female and male analyses showed the genetic liability to certain anthropometric traits to be associated with the risk of traumatic experiences. In particular, we observed putative causal effects on childhood physical abuse in both sexes. This reverse direction may reflect the complex relationship of anthropometric traits with other factors, including socioeconomic status (Tyrrell et al., 2016), parental effects on childhood environment (Turner et al., 2012), and propensity to report traumatic events (Gavranidou and Rosner, 2003). Previous studies showed that socioeconomic status is a key mediator in the association of PTSD-related traits with inflammatory biomarkers and behavioral traits (Muniz Carvalho et al., 2021; Polimanti et al., 2019c). A recent brain-wide analysis showed that socioeconomic status has a pervasive effect on the genetics of psychiatric disorders, behavioral traits, and brain imaging phenotypes (Wendt et al., 2020). Based on these previous findings, the putative causal effect of anthropometric traits on PTSD and traumatic experience observed in our study may be affected by the pervasive association of socioeconomic status on the genetics of traits investigated. As mentioned, other factors could also play an important role. For example, the relationship could also reflect that in certain cultures people with high BMI may be more likely to be bullied or victimized and therefore traumatized.
To summarize our results, in females of European descendent, we found that fourteen anthropometric traits related to BMI, weight, leg and arm fat, trunk have a potential causal small effect on PTSD. We observed, in females, that trunk fat percentage genetically predicted has bidirectional causal effects on PTSD. In addition, we did not observe causality of anthropometric traits genetically determined on PTSD, in male individuals of European ancestry. However, interesting results suggest that severe trauma, as war zone exposure, in males was negatively linked to body circumference, but this association was not observed in females. Further, SNPs associated with social support may cause lower BMI, and the reverse causality of this social support was observed in association with body circumference. Lastly, physical abuse in childhood is the unique trait that overlapped in females and males, it was linked with BMI and WC. In the analysis conducted in individuals of African ancestry, we observed that arm and leg fat mass, and trunk fat percentage genetically determined appear to have a small causal effect on PTSD, consistent with the associations observed in females of European descendent, suggesting that the genetic overlap and putative causal effects linking anthropometric traits to PTSD are shared across different populations.
Although our results contribute to a better understanding of sex-specific mechanisms linking PTSD to the inter-individual variability of body size and composition, we also acknowledge several limitations. There are well-known differences in anthropometric traits (e.g., height, BMI, body fat distribution) across human populations (Lee et al., 2014; Luo et al., 2019; Sarink et al., 2021) due to genetic and environmental factors (Hur et al., 2008; Jelenkovic et al., 2016; Kang et al., 2010; Segal et al., 2009; Silventoinen et al., 2016). In particular, there is evidence that ancestry-specific genetic variation contributes partially to the predisposition to BMI and height, and other anthropometric traits (Guo et al., 2021). Although we show that genetic effects specific to African ancestry present associations linking anthropometric traits and PTSD concordant with those observed in individuals of African descent, these should be considered only as suggestive evidence. Indeed, the limited sample size available is likely to have strongly affected our findings. For example, UKB-AFR data included information regarding only 6570 participants, and this may have limited the possibility to identify putative causal effects of PTSD on the anthropometric traits tested. Additionally, the complex genetic background of recently admixed individuals such African-British participants from UKB and African-American individuals enrolled in the MVP and PGC studies cannot be fully modeled by the reference panels used for PRS and MR methods. With respect to both ancestry groups investigated, the high PTSD polygenicity likely affected the statistical power of our causal inference analysis due to the small average per-variant effect. Finally, although we used multiple causal inference methods to account for different pleiotropy scenarios, there may be still unaccounted biases that may affect our results.
In conclusion, our study represents the largest genetic investigation of the sex-specific relationships among posttraumatic stress disorder, traumatic experiences, and social support with respect to body size and composition. We observed consistent results between analyses conducted in different ancestry groups, although the limited sample size available did not permit us to explore sex differences in individuals of African descent. These findings suggest a complex network of associations where PTSD-related traits are affected or affect anthropometric traits due to their links with multiple biological pathways. These include both shared genetic mechanisms and putative causal effects that appear more pronounced in women than in men. This suggests that both biological mechanisms and pre-trauma risk factors contribute to the higher vulnerability to PTSD observed in women.
Author contributions
C.M.C., S.I.B. and R.P. conceived the ideas and designed the study. C.M.C and R.P. worked on analysis and interpretation of data for the study and wrote the original draft of manuscript. F.R.W. and G.A.P. contributed to the interpretation of data for the study and revised critically the manuscript. All authors provided important intellectual content critical review, helped in the interpretation of results, and approved the final manuscript.
Role of the funder/sponsor
The funders had no role in the designing, collecting, managing, analyzing, interpreting, writing, reviewing, or approving the materials presented in this study. Funders had no role in the decision to submit the manuscript for publication.
Data sharing
All data discussed in this study are provided in the article and in the Supplementary Material.
Declaration of competing interest
D.J.S. received personal fees from Lundbeck and Sun Pharmaceutical Industries. R.P. and J.G. are paid for their editorial work on the journal Complex Psychiatry. The other authors declare no competing interests.
Acknowledgements
This research was supported by the National Institutes of Health (R21 DC018098, R21 DA047527, and F32 MH122058). CMC and SIB were supported by Fundação de Amparo à Pesquisa do Estado de São Paulo international fellowship (FAPESP 2018/05995-4). Financial support for the PTSD PGC was provided by the Cohen Veterans Bioscience, Stanley Center for Psychiatric Research at the Broad Institute, One Mind, and the National Institute of Mental Health (NIMH; R01MH106595).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2021.100400.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
All data discussed in this study are provided in the article and in the Supplementary Material.
References
- Aliev G., Beeraka N.M., Nikolenko V.N., Svistunov A.A., Rozhnova T., Kostyuk S., Cherkesov I., Gavryushova L.V., Chekhonatsky A.A., Mikhaleva L.M., Somasundaram S.G., Avila-Rodriguez M.F., Kirkland C.E. Neurophysiology and psychopathology underlying PTSD and recent insights into the PTSD therapies—a comprehensive review. J. Clin. Med. 2020;9:2951. doi: 10.3390/jcm9092951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen N.E., Sudlow C., Peakman T., Collins R. UK biobank data: come and get it. Sci. Transl. Med. 2014 doi: 10.1126/scitranslmed.3008601. [DOI] [PubMed] [Google Scholar]
- Boscarino J.A. Posttraumatic stress disorder and physical illness: results from clinical and epidemiologic studies. Annals of the New York Academy of Sciences. 2004:141–153. doi: 10.1196/annals.1314.011. [DOI] [PubMed] [Google Scholar]
- Bowden J., Davey Smith G., Haycock P.C., Burgess S. Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet. Epidemiol. 2016;40:304–314. doi: 10.1002/gepi.21965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekman B.F.P., Olff M., Boer F. The genetic background to PTSD. Neurosci. Biobehav. Rev. 2007;31:348–362. doi: 10.1016/j.neubiorev.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Bulik-Sullivan B., Finucane H.K., Anttila V., Gusev A., Day F.R., Loh P.R., Duncan L., Perry J.R.B., Patterson N., Robinson E.B., Daly M.J., Price A.L., Neale B.M. An atlas of genetic correlations across human diseases and traits. Nat. Genet. 2015;47:1236–1241. doi: 10.1038/ng.3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulik-Sullivan B., Loh P.R., Finucane H.K., Ripke S., Yang J., Patterson N., Daly M.J., Price A.L., Neale B.M., Corvin A., Walters J.T.R., Farh K.H., Holmans P.A., Lee P., Collier D.A., Huang H., Pers T.H., Agartz I., Agerbo E., Albus M., Alexander M., Amin F., Bacanu S.A., Begemann M., Belliveau R.A., Bene J., Bergen S.E., Bevilacqua E., Bigdeli T.B., Black D.W., Bruggeman R., Buccola N.G., Buckner R.L., Byerley W., Cahn W., Cai G., Cairns M.J., Campion D., Cantor R.M., Carr V.J., Carrera N., Catts S.V., Chambert K.D., Chan R.C.K., Chen R.Y.L., Chen E.Y.H., Cheng W., Cheung E.F.C., Chong S.A., Cloninger C.R., Cohen D., Cohen N., Cormican P., Craddock N., Crespo-Facorro B., Crowley J.J., Curtis D., Davidson M., Davis K.L., Degenhardt F., Del Favero J., DeLisi L.E., Demontis D., Dikeos D., Dinan T., Djurovic S., Donohoe G., Drapeau E., Duan J., Dudbridge F., Durmishi N., Eichhammer P., Eriksson J., Escott-Price V., Essioux L., Fanous A.H., Farrell M.S., Frank J., Franke L., Freedman R., Freimer N.B., Friedl M., Friedman J.I., Fromer M., Genovese G., Georgieva L., Gershon E.S., Giegling I., Giusti-Rodríguez P., Godard S., Goldstein J.I., Golimbet V., Gopal S., Gratten J., De Haan L., Hammer C., Hamshere M.L., Hansen M., Hansen T., Haroutunian V., Hartmann A.M., Henskens F.A., Herms S., Hirschhorn J.N., Hoffmann P., Hofman A., Hollegaard M.V., Hougaard D.M., Ikeda M., Joa I., Juliá A., Kahn R.S., Kalaydjieva L., Karachanak-Yankova S., Karjalainen J., Kavanagh D., Keller M.C., Kelly B.J., Kennedy J.L., Khrunin A., Kim Y., Klovins J., Knowles J.A., Konte B., Kucinskas V., Kucinskiene Z.A., Kuzelova-Ptackova H., Kähler A.K., Laurent C., Keong J.L.C., Lee S.H., Legge S.E., Lerer B., Li M., Li T., Liang K.Y., Lieberman J., Limborska S., Loughland C.M., Lubinski J., Lönnqvist J., Macek M., Magnusson P.K.E., Maher B.S., Maier W., Mallet J., Marsal S., Mattheisen M., Mattingsdal M., McCarley R.W., McDonald C., McIntosh A.M., Meier S., Meijer C.J., Melegh B., Melle I., Mesholam-Gately R.I., Metspalu A., Michie P.T., Milani L., Milanova V., Mokrab Y., Morris D.W., Mors O., Murphy K.C., Murray R.M., Myin-Germeys I., Müller-Myhsok B., Nelis M., Nenadic I., Nertney D.A., Nestadt G., Nicodemus K.K., Nikitina-Zake L., Nisenbaum L., Nordin A., O'Callaghan E., O'Dushlaine C., O'Neill F.A., Oh S.Y., Olincy A., Olsen L., Van Os J., Pantelis C., Papadimitriou G.N., Papiol S., Parkhomenko E., Pato M.T., Paunio T., Pejovic-Milovancevic M., Perkins D.O., Pietiläinen O., Pimm J., Pocklington A.J., Powell J., Pulver A.E., Purcell S.M., Quested D., Rasmussen H.B., Reichenberg A., Reimers M.A., Richards A.L., Roffman J.L., Roussos P., Ruderfer D.M., Salomaa V., Sanders A.R., Schall U., Schubert C.R., Schulze T.G., Schwab S.G., Scolnick E.M., Scott R.J., Seidman L.J., Shi J., Sigurdsson E., Silagadze T., Silverman J.M., Sim K., Slominsky P., Smoller J.W., So H.C., Spencer C.C.A., Stahl E.A., Stefansson H., Steinberg S., Stogmann E., Straub R.E., Strengman E., Strohmaier J., Stroup T.S., Subramaniam M., Suvisaari J., Svrakic D.M., Szatkiewicz J.P., Söderman E., Thirumalai S., Toncheva D., Tooney P.A., Tosato S., Veijola J., Waddington J., Walsh D., Wang D., Wang Q., Webb B.T., Weiser M., Wildenauer D.B., Williams N.M., Williams S., Witt S.H., Wolen A.R., Wong E.H.M., Wormley B.K., Wu J.Q., Xi H.S., Zai C.C., Zheng X., Zimprich F., Wray N.R., Stefansson K., Visscher P.M., Adolfsson R., Andreassen O.A., Blackwood D.H.R., Bramon E., Buxbaum J.D., Børglum A.D., Cichon S., Darvasi A., Domenici E., Ehrenreich H., Esko T., Gejman P.V., Gill M., Gurling H., Hultman C.M., Iwata N., Jablensky A.V., Jönsson E.G., Kendler K.S., Kirov G., Knight J., Lencz T., Levinson D.F., Li Q.S., Liu J., Malhotra A.K., McCarroll S.A., McQuillin A., Moran J.L., Mortensen P.B., Mowry B.J., Nöthen M.M., Ophoff R.A., Owen M.J., Palotie A., Pato C.N., Petryshen T.L., Posthuma D., Rietschel M., Riley B.P., Rujescu D., Sham P.C., Sklar P., St Clair D., Weinberger D.R., Wendland J.R., Werge T., Sullivan P.F., O'Donovan M.C. LD score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat. Genet. 2015;47:291–295. doi: 10.1038/ng.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess S., Davies N.M., Thompson S.G. Bias due to participant overlap in two-sample Mendelian randomization. Genet. Epidemiol. 2016;40:597–608. doi: 10.1002/gepi.21998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buta E., Masheb R., Gueorguieva R., Bathulapalli H., Brandt C.A., Goulet J.L. Posttraumatic stress disorder diagnosis and gender are associated with accelerated weight gain trajectories in veterans during the post-deployment period. Eat. Behav. 2018;29:8–13. doi: 10.1016/j.eatbeh.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bycroft C., Freeman C., Petkova D., Band G., Elliott L.T., Sharp K., Motyer A., Vukcevic D., Delaneau O., O'Connell J., Cortes A., Welsh S., Young A., Effingham M., McVean G., Leslie S., Allen N., Donnelly P., Marchini J. The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018;562:203–209. doi: 10.1038/s41586-018-0579-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho C.M., Wendt F.R., Stein D.J., Stein M.B., Gelernter J., Belangero S.I., Polimanti R. Investigating causality between blood metabolites and emotional and behavioral responses to traumatic stress: a mendelian randomization study. Mol. Neurobiol. 2020;57:1542–1552. doi: 10.1007/s12035-019-01823-2. [DOI] [PubMed] [Google Scholar]
- Chen J.J., Roberson P.K., Schell M.J. The false discovery rate: a key concept in large-scale genetic studies. Cancer Control. 2010;17:58–62. doi: 10.1177/107327481001700108. [DOI] [PubMed] [Google Scholar]
- Choi S.W., Mak T.S.H., O'Reilly P.F. Tutorial: a guide to performing polygenic risk score analyses. Nat. Protoc. 2020 doi: 10.1038/s41596-020-0353-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelis M.C., Nugent N.R., Amstadter A.B., Koenen K.C. Genetics of post-traumatic stress disorder: review and recommendations for genome-wide association studies. Curr. Psychiatry Rep. 2010;12:313–326. doi: 10.1007/s11920-010-0126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallman M.F., Pecoraro N., Akana S.F., La Fleur S.E., Gomez F., Houshyar H., Bell M.E., Bhatnagar S., Laugero K.D., Manalo S. Chronic stress and obesity: a new view of “comfort food. Proc. Natl. Acad. Sci. U. S. A. 2003;100:11696–11701. doi: 10.1073/pnas.1934666100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey Smith G., Davies N., Dimou N., Egger M., Gallo V., Golub R., Higgins J.P., Langenberg C., Loder E., Richards J.B., Richmond R., Skrivankova V., Swanson S., Timpson N., Tybjaerg-Hansen A., VanderWeele T., Woolf B.A., Yarmolinsky J. STROBE-MR: guidelines for strengthening the reporting of Mendelian randomization studies. PeerJ Prepr. 2019;7 doi: 10.7287/peerj.preprints.27857. [DOI] [Google Scholar]
- Davies N.M., Holmes M.V., Davey Smith G. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ. 2018;362 doi: 10.1136/bmj.k601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis K.A.S., Coleman J.R.I., Adams M., Allen N., Breen G., Cullen B., Dickens C., Fox E., Graham N., Holliday J., Howard L.M., John A., Lee W., McCabe R., McIntosh A., Pearsall R., Smith D.J., Sudlow C., Ward J., Zammit S., Hotopf M. Mental Health in UK Biobank Revised – development, implementation and results from an online questionnaire completed by 157,366 participants. BJPsych Open. 2019;6:18. doi: 10.1101/19001214. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Dedert E.A., Becker M.E., Fuemmeler B.F., Braxton L.E., Calhoun P.S., Beckham J.C. Childhood traumatic stress and obesity in women: the intervening effects of PTSD and MDD. J. Trauma. Stress. 2010;23:785–793. doi: 10.1002/jts.20584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobie D.J., Kivlahan D.R., Maynard C., Bush K.R., Davis T.M., Bradley K.A. Posttraumatic stress disorder in female veterans: association with self-reported health problems and functional impairment. Arch. Intern. Med. 2004;164:394–400. doi: 10.1001/archinte.164.4.394. [DOI] [PubMed] [Google Scholar]
- Dong S.S., Zhang K., Guo Y., Ding J.M., Rong Y., Feng J.C., Yao S., Hao R.H., Jiang F., Chen J., Bin Wu, Chen X.F., Yang T.L. Phenome-wide investigation of the causal associations between childhood BMI and adult trait outcomes: a two-sample Mendelian randomization study. Genome Med. 2021;13 doi: 10.1186/s13073-021-00865-3. H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan A.E., Sartor C.E., Jonson-Reid M., Munn-Chernoff M.A., Eschenbacher M.A., Diemer E.W., Nelson E.C., Waldron M., Bucholz K.K., Madden P.A.F., Heath A.C. Associations between body mass index, post-traumatic stress disorder, and child maltreatment in young women. Child Abus. Negl. 2015;45:154–162. doi: 10.1016/j.chiabu.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y., Lin J., Su M., Zhang X., Fang D.Z. Interplays of estrogen receptor alpha gene rs2234693 with post-traumatic stress disorder influence serum glucose and lipids profiles in Chinese adolescents. J. Clin. Neurosci. 2019;61:36–43. doi: 10.1016/j.jocn.2018.11.021. [DOI] [PubMed] [Google Scholar]
- Gavranidou M., Rosner R. The weaker sex? Gender and post-traumatic stress disorder. Depress. Anxiety. 2003;17:130–139. doi: 10.1002/da.10103. [DOI] [PubMed] [Google Scholar]
- Gunstad J., Paul R.H., Spitznagel M.B., Cohen R.A., Williams L.M., Kohn M., Gordon E. Exposure to early life trauma is associated with adult obesity. Psychiatry Res. 2006;142:31–37. doi: 10.1016/j.psychres.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Guo J., Bakshi A., Wang Y., Jiang L., Yengo L., Goddard M.E., Visscher P.M., Yang J. Quantifying genetic heterogeneity between continental populations for human height and body mass index. Sci. Rep. 2021;11:5240. doi: 10.1038/s41598-021-84739-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper C. Diagnostic and statistical manual of mental disorders. A Companion to Criminal Justice, Mental Health & Risk. 2014 doi: 10.1097/00002093-198802020-00022. [DOI] [Google Scholar]
- Hartwig F.P., Smith G.D., Bowden J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int. J. Epidemiol. 2017;46:1985–1998. doi: 10.1093/ije/dyx102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemani G., Zheng J., Elsworth B., Wade K.H., Haberland V., Baird D., Laurin C., Burgess S., Bowden J., Langdon R., Tan V.Y., Yarmolinsky J., Shihab H.A., Timpson N.J., Evans D.M., Relton C., Martin R.M., Davey Smith G., Gaunt T.R., Haycock P.C. The MR-base platform supports systematic causal inference across the human phenome. Elife. 2018;7 doi: 10.7554/eLife.34408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hübel C., Gaspar H.A., Coleman J.R.I., Hanscombe K.B., Purves K., Prokopenko I., Graff M., Ngwa J.S., Workalemahu T., O'Reilly P.F., Bulik C.M., Breen G. Genetic correlations of psychiatric traits with body composition and glycemic traits are sex- and age-dependent. Nat. Commun. 2019;10:5765. doi: 10.1038/s41467-019-13544-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur Y.M., Kaprio J., Iacono W.G., Boomsma D.I., McGue M., Silventoinen K., Martin N.G., Luciano M., Visscher P.M., Rose R.J., He M., Ando J., Ooki S., Nonaka K., Lin C.C.H., Lajunen H.R., Cornes B.K., Bartels M., Van Beijsterveldt C.E.M., Cherny S.S., Mitchell K. Genetic influences on the difference in variability of height, weight and body mass index between Caucasian and East Asian adolescent twins. Int. J. Obes. 2008;32:1455–1467. doi: 10.1038/ijo.2008.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelenkovic A., Sund R., Hur Y.M., Yokoyama Y., Hjelmborg J.V.B., Möller S., Honda C., Magnusson P.K.E., Pedersen N.L., Ooki S., Aaltonen S., Stazi M.A., Fagnani C., D'Ippolito C., Freitas D.L., Maia J.A., Ji F., Ning F., Pang Z., Rebato E., Busjahn A., Kandler C., Saudino K.J., Jang K.L., Cozen W., Hwang A.E., Mack T.M., Gao W., Yu C., Li L., Corley R.P., Huibregtse B.M., Derom C.A., Vlietinck R.F., Loos R.J.F., Heikkilä K., Wardle J., Llewellyn C.H., Fisher A., McAdams T.A., Eley T.C., Gregory A.M., He M., Ding X., Bjerregaard-Andersen M., Beck-Nielsen H., Sodemann M., Tarnoki A.D., Tarnoki D.L., Knafo-Noam A., Mankuta D., Abramson L., Burt S.A., Klump K.L., Silberg J.L., Eaves L.J., Maes H.H., Krueger R.F., McGue M., Pahlen S., Gatz M., Butler D.A., Bartels M., Van Beijsterveldt T.C.E.M., Craig J.M., Saffery R., Dubois L., Boivin M., Brendgen M., Dionne G., Vitaro F., Martin N.G., Medland S.E., Montgomery G.W., Swan G.E., Krasnow R., Tynelius P., Lichtenstein P., Haworth C.M.A., Plomin R., Bayasgalan G., Narandalai D., Harden K.P., Tucker-Drob E.M., Spector T., Mangino M., Lachance G., Baker L.A., Tuvblad C., Duncan G.E., Buchwald D., Willemsen G., Skytthe A., Kyvik K.O., Christensen K., Öncel S.Y., Aliev F., Rasmussen F., Goldberg J.H., Sørensen T.I.A., Boomsma D.I., Kaprio J., Silventoinen K. Genetic and environmental influences on height from infancy to early adulthood: an individual-based pooled analysis of 45 twin cohorts. Sci. Rep. 2016;6 doi: 10.1038/srep28496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S.J., Chiang C.W.K., Palmer C.D., Tayo B.O., Lettre G., Butler J.L., Hackett R., Adeyemo A.A., Guiducci C., Berzins I., Nguyen T.T., Feng T., Luke A., Shriner D., Ardlie K., Rotimi C., Wilks R., Forrester T., McKenzie C.A., Lyon H.N., Cooper R.S., Zhu X., Hirschhorn J.N. Genome-wide association of anthropometric traits in African- and African-derived populations. Hum. Mol. Genet. 2010;19:2725–2738. doi: 10.1093/hmg/ddq154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick D.G., Resnick H.S., Milanak M.E., Miller M.W., Keyes K.M., Friedman M.J. National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-5 criteria. J. Trauma. Stress. 2013;26:537–547. doi: 10.1002/jts.21848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenen K.C., Fu Q.J., Ertel K., Lyons M.J., Eisen S.A., True W.R., Goldberg J., Tsuang M.T. Common genetic liability to major depression and posttraumatic stress disorder in men. J. Affect. Disord. 2008;105:109–115. doi: 10.1016/j.jad.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubzansky L.D., Bordelois P., Jun H.J., Roberts A.L., Cerda M., Bluestone N., Koenen K.C. The weight of traumatic stress: a prospective study of posttraumatic stress disorder symptoms and weight status in women. JAMA Psychiatry. 2014;71:44–51. doi: 10.1001/jamapsychiatry.2013.2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langdon R.J., Richmond R.C., Hemani G., Zheng J., Wade K.H., Carreras-Torres R., Johansson M., Brennan P., Wootton R.E., Munafo M.R., Smith G.D., Relton C.L., Vincent E.E., Martin R.M., Haycock P. A phenome-wide Mendelian randomization study of pancreatic cancer using summary genetic data. Cancer Epidemiol. Biomarkers Prev. 2019;28:2070–2078. doi: 10.1158/1055-9965.EPI-19-0036. [DOI] [PubMed] [Google Scholar]
- Lee S., Bountziouka V., Lum S., Stocks J., Bonner R., Naik M., Fothergill H., Well J.C.K. Ethnic variability in body size, proportions and composition in children aged 5 to 11 years: is ethnic-specific calibration of bioelectrical impedance required? PLoS One. 2014;9 doi: 10.1371/journal.pone.0113883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke A.E., Kahali B., Berndt S.I., Justice A.E., Pers T.H., Day F.R., Powell C., Vedantam S., Buchkovich M.L., Yang J., Croteau-Chonka D.C., Esko T., Fall T., Ferreira T., Gustafsson S., Kutalik Z., Luan J., Mägi R., Randall J.C., Winkler T.W., Wood A.R., Workalemahu T., Faul J.D., Smith J.A., Zhao J.H., Zhao W., Chen J., Fehrmann R., Hedman K., Karjalainen J., Schmidt E.M., Absher D., Amin N., Anderson D., Beekman M., Bolton J.L., Bragg-Gresham J.L., Buyske S., Demirkan A., Deng G., Ehret G., Feenstra B., Feitosa M., Fischer K., Goel A., Gong J., Jackson A.U., Kanoni S., Kleber M.E., Kristiansson K., Lim U., Lotay V., Mangino M., Leach I.M., Medina-Gomez C., Medland S.E., Nalls M.A., Palmer C.D., Pasko D., Pechlivanis S., Peters M.J., Prokopenko I., Shungin D., Stančáková A., Strawbridge R.J., Sung Y.J., Tanaka T., Teumer A., Trompet S., van der Laan S.W., van Setten J., Van Vliet-Ostaptchouk J.V., Wang Z., Yengo L., Zhang W., Isaacs A., Albrecht E., Ärnlöv J., Arscott G.M., Attwood A.P., Bandinelli S., Barrett A., Bas I.N., Bellis C., Bennett A.J., Berne C., Blagieva R., Blüher M., Böhringer S., Bonnycastle L.L., Böttcher Y., Boyd H.A., Bruinenberg M., Caspersen I.H., Chen Y.I., Clarke R., Daw E.W., de Craen A.J.M., Delgado G., Dimitriou M., Doney A.S.F., Eklund N., Estrada K., Eury E., Folkersen L., Fraser R.M., Garcia M., Geller F., Giedraitis V., Gigante B., Go A.S., Golay A., Goodall A., Gordon S.D., Gorski M., Grabe H.J., Grallert H., Grammer T.B., Gräßler J., Grönberg H., Groves C.J., Gusto G., Haessler J., Hall P., Haller T., Hallmans G., Hartman C.A., Hassinen M., Hayward C., Heard-Costa N.L., Helmer Q., Hengstenberg C., Holmen O., Hottenga J.J., James A.L., Jeff J., Johansson, Jolley J., Juliusdottir T., Kinnunen L., Koenig W., Koskenvuo M., Kratzer W., Laitinen J., Lamina C., Leander K., Lee N.R., Lichtner P., Lind L., Lindström J., Lo K.S., Lobbens S., Lorbeer R., Lu Y., Mach F., Magnusson P.K., Mahajan A., McArdle W.L., McLachlan S., Menni C., Merger S., Mihailov E., Milani L., Moayyeri A., Monda K.L., Morken M.A., Mulas A., Müller G., Müller-Nurasyid M., Musk A.W., Nagaraja R., Nöthen M.M., Nolte I.M., Pilz S., Rayner N.W., Renstrom F., Rettig R., Ried J.S., Ripke S., Robertson N., Rose L.M., Sanna S., Scharnagl H., Scholtens S., Schumacher F., Scott W.R., Seufferlein T., Shi J., Smith A.V., Smolonska J., Stanton A.V., Steinthorsdottir V., Stirrups K., Stringham H.M., Sundström J., Swertz M.A., Swift A.J., Syvänen A.C., Tan S.T., Tayo B., Thorand B., Thorleifsson G., Tyrer J., Uh H.W., Vandenput L., Verhulst F.C., Vermeulen S.H., Verweij N., Vonk J.M., Waite L.L., Warren H.R., Waterworth D.M., Weedon M.N., Wilkens L., Willenborg C., Wilsgaard T., Wojczynski M.K., Wong A., Wright A.F., Zhang Q., Brennan E.P., Choi M., Dastani Z., Drong A.W., Eriksson P., Franco-Cereceda A., Gådin J.R., Gharavi A.G., Goddard M.E., Handsaker R.E., Huang J., Karpe F., Kathiresan S., Keildson S., Kiryluk K., Kubo M., Lee J.Y., Liang L., Lifton R.P., Ma B., McCarroll S.A., McKnight A.J., Min J.L., Moffatt M.F., Montgomery G.W., Murabito J.M., Nicholson G., Nyholt D.R., Okada Y., Perry J.R., Dorajoo R., Reinmaa E., Salem R.M., Sandholm N., Scott R.A., Stolk L., Takahashi A., Van’t Hooft F.M., Vinkhuyzen A.A.E., Westra H.J., Zheng W., Zondervan K.T., Heath A.C., Arveiler D., Bakker S.J., Beilby J.P., Bergman R.N., Blangero J., Bovet P., Campbell H., Caulfield M., Cesana G., Chakravarti A., Chasman D., Chines P.S., Collins F.S., Crawford D., Cupples L., Cusi D., Danesh J., de Faire U., Den Ruijter H.M., Dominiczak A.F., Erbel R., Erdmann J., Eriksson J.G., Farrall M., Felix S.B., Ferrannini E., Ferrières J., Ford I., Forouhi N.G., Forrester T., Franco O.H., Gansevoort R.T., Gejman P.V., Gieger C., Gottesman O., Gudnason V., Gyllensten U.B., Hall A.S., Harris T.B., Hattersley A.T., Hicks A.A., Hindorff L., Hingorani A., Hofman A., Homuth G., Hovingh G., Humphries S.E., Hunt S.C., Hyppönen E., Illig T., Jacobs K.B., Jarvelin M.R., Jöckel K.H., Johansen B., Jousilahti P., Jukema J., Jula A., Kaprio J., Kastelein J.J., Keinanen-Kiukaanniemi S.M., Kiemeney L.A., Knekt P., Kooner J.S., Kooperberg C., Kovacs P., Kraja A.T., Kumari M., Kuusisto J., Lakka T., Langenberg C., Le Marchand L., Lehtimäki T., Lyssenko V., Männistö S., Marette A., Matise T., McKenzie C.A., McKnight B., Moll F.L., Morris A.D., Morris A.P., Murray J.C., Nelis M., Ohlsson C., Oldehinkel A.J., Ong K.K., Madden P.A.F., Pasterkamp G., Peden J.F., Peters A., Postma D.S., Pramstaller P.P., Price J.F., Qi L., Raitakari O., Rankinen T., Rao D.C., Rice T.K., Ridker P., Rioux J.D., Ritchie M., Rudan I., Salomaa V., Samani N., Saramies J., Sarzynski M.A., Schunkert H., Schwarz P.E., Sever P., Shuldiner A.R., Sinisalo J., Stolk R.P., Strauch K., Tönjes A., Trégouët D.A., Tremblay A., Tremoli E., Virtamo J., Vohl M.C., Völker U., Waeber G., Willemsen G., Witteman J.C., Zillikens M.C., Adair L.S., Amouyel P., Asselbergs F.W., Assimes T.L., Bochud M., Boehm B.O., Boerwinkle E., Bornstein S.R., Bottinger E.P., Bouchard C., Cauchi S., Chambers J.C., Chanock S.J., Cooper R.S., de Bakker P.I.W., Dedoussis G.V., Ferrucci L., Franks P.W., Froguel P., Groop L., Haiman C., Hamsten A., Hui J., Hunter D.J., Hveem K., Kaplan R.C., Kivimaki M., Kuh D., Laakso M., Liu Y., Martin N.G., März W., Melbye M., Metspalu A., Moebus S., Munroe P., Njølstad I., Oostra B.A., Palmer C.N., Pedersen N.L., Perola M., Pérusse L., Peters U., Power C., Quertermous T., Rauramaa R., Rivadeneira F., Saaristo T.E., Saleheen D., Sattar N., Schadt E., Schlessinger D., Slagboom P.E., Snieder H., Spector T.D., Thorsteinsdottir U., Stumvoll M., Tuomilehto J., Uitterlinden A.G., Uusitupa M., van der Harst P., Walker M.C., Wallaschofski H., Wareham N., Watkins H., Weir D.R., Wichmann H., Wilson J.F., Zanen P., Borecki I., Deloukas P., Fox C.S., Heid I.M., O’connell J.R., Strachan D.P., Stefansson K., Van Duijn C., Abecasis G., Franke L., Frayling T.M., McCarthy M.I., Visscher P.M., Scherag A., Willer C.J., Boehnke M., Mohlke K.L., Lindgren C.M., Beckmann J.S., Barroso I., North K.E., Ingelsson E., Hirschhorn J.N., Loos R.J., Speliotes E.K., Thompson J.R., Goldstein B.A., König I.R., Cazier J.B., Grundberg E., Havulinna A.S., Ho W.K., Hopewell J.C., Eriksson N., Lundmark P., Lyytikäinen L.P., Rafelt S., Tikkanen E., Van Zuydam N., Voight B.F., Ziegler A., Altshuler D., Balmforth A.J., Braund P.S., Burgdorf C., Claudi-Boehm S., Cox D., Do R., Doney A.S., El Mokhtari N., Fontanillas P., Hager J., Han B.G., Hunt S.E., Kang H.M., Kessler T., Knowles J.W., Kolovou G., Langford C., Lokki M.L., Lundmark A., Meisinger C., Melander O., Maouche S., Nikus K., Rasheed A., Rosinger S., Rubin D., Rumpf M.P., Schäfer A., Sivananthan M., Song C., Stewart A.F., Thorgeirsson G., van der Schoot C.E., Wagner P.J., Wells G.A., Wild P.S., Tsun-Po Y., Basart H.V., Brambilla P., Cambien F., Cupples A.L., Dehghan A., Diemert P., Epstein S.E., Evans A., Ferrario M., Gauguier D., Hazen S.L., Holm H., Iribarren C., Jang Y., Kähönen M., Kee F., Kim H.S., Klopp N., Kuulasmaa K., Laaksonen R., Ouwehand W., Parish S., Park J.E., Rader D.J., Shah S., Stark K., Wallentin L., Zimmermann M.E., Nieminen M.S., Sandhu M.S., Pastinen T., Zalloua P.A., Siegbahn A., Schreiber S., Ripatti S., Blankenberg S.S., O’donnell C., Reilly M., Collins R., Roberts R., Pattaro C., Köttgen A., Garnaas M., Böger C.A., Fuchsberger C., Olden M., Chen M.H., Tin A., Taliun D., Li M., Gao X., Yang Q., Hundertmark C., Foster M.C., O’seaghdha C.M., Glazer N., Liu C.T., Struchalin M., Li G., Johnson A.D., Gierman H.J., Hwang S.J., Atkinson E.J., Lohman K., Cornelis M.C., Chouraki V., Holliday E.G., Sorice R., Deshmukh H., Ulivi S., Chu A.Y., Murgia F., Imboden M., Kollerits B., Pistis G., Launer L., Aspelund T., Eiriksdottir G., Mitchell B.D., Schmidt H., Cavalieri M., Rao M., Hu F.B., de Andrade M., Turner S.T., Ding J., Andrews J.S., Freedman B.I., Döring A., Kolcic I., Zemunik T., Boban M., Minelli C., Wheeler H.E., Igl W., Zaboli G., Wild S.H., Ellinghaus D., Nöthlings U., Jacobs G., Biffar R., Endlich K., Ernst F., Kroemer H.K., Nauck M., Stracke S., Völzke H., Aulchenko Y.S., Polasek O., Hastie N., Vitart V., Helmer C., Wang J.J., Ruggiero D., Bergmann S., Viikari J., Nikopensius T., Province M., Ketkar S., Colhoun H.M., Doney A., Robino A., Giulianini F., Krämer B.K., Portas L., Buckley B.M., Adam M., Thun G.A., Paulweber B., Haun M., Sala C., Metzger M., Mitchell P., Ciullo M., Kim S.K., Vollenweider P., Palmer C., Gasparini P., Pirastu M., Probst-Hensch N.M., Kronenberg F., Toniolo D., Coresh J., Schmidt R., Siscovick D.S., Kardia S.L., Curhan G., Franke A., Parsa A., Goessling W., Kao W., de Boer I.H., Peralta C.A., Akylbekova E., Kramer H., Arking D.E., Franceschini N., Egan J., Hernandez D.G., Townsend R.R., Lumley T., Psaty B., Kestenbaum B., Haritunians T., Mooser V., Florez J.C., Meigs J.B., Lu X., Leak T.S., Aasarød K., Skorpen F., Baumert J., Devuyst O., Mychaleckyj J.C., Kedenko L., Coassin S., Hallan S., Navis G., Shlipak M.G., Bull S.B., Paterson A.D., Rotter J.I., Dreisbach A.W., Anderson C.A., Guo Q., Henders A., Lambert A., Lee S.H., Kraft P., Kennedy S.H., Macgregor S., Missmer S.A., Painter J.N., Roseman F., Treloar S.A., Wallace L., Forsblom C., Isakova T., McKay G.J., Williams W.W., Sadlier D.M., Mäkinen V.P., Swan E.J., Boright A.P., Ahlqvist E., Keller B.J., Huang H., Ahola A., Fagerholm E., Gordin D., Harjutsalo V., He B., Heikkilä O., Hietala K., Kytö J., Lahermo P., Lehto M., Österholm A.M., Parkkonen M., Pitkäniemi J., Rosengård-Bärlund M., Saraheimo M., Sarti C., Söderlund J., Soro-Paavonen A., Syreeni A., Thorn L.M., Tikkanen H., Tolonen N., Tryggvason K., Wadén J., Gill G.V., Prior S., Guiducci C., Mirel D., Taylor A., Hosseini M., Parving H.H., Rossing P., Tarnow L., Ladenvall C., Alhenc-Gelas F., Lefebvre P., Rigalleau V., Roussel R., Tregouet D.A., Maestroni A., Maestroni S., Falhammar H., Gu T., Möllsten A., Cimponeriu D., Mihai I., Mota M., Mota E., Serafinceanu C., Stavarachi M., Hanson R.L., Nelson R.G., Kretzler M., Panduru N.M., Gu H.F., Brismar K., Zerbini G., Hadjadj S., Marre M., Lajer M., Waggott D., Savage D.A., Bain S.C., Martin F., Godson C., Groop P.H., Maxwell A.P., Sengupta S., Peloso G.M., Ganna A., Mora S., Chang H.Y., Den Hertog H.M., Donnelly L.A., Freitag D.F., Gurdasani D., Heikkilä K., Johnson T., Kaakinen M., Kettunen J., Li X., Montasser M.E., Petersen A.K., Saxena R., Service S.K., Sidore C., Surakka I., Teslovich T.M., Van den Herik E.G., Volcik K.A., Wu Y., Asiki G., Been L.F., Burnett M.S., Doring A., Elliott P., Eyjolfsson G.I., Goodarzi M.O., Gravito M.L., Hartikainen A.L., Hung Y.J., Jones M.R., Kaleebu P., Khaw K.T., Kim E., Komulainen P., Lehtimaki T., Lin S.Y., Lindstrom J., Muller G., Narisu N., Nieminen T.V., Nsubuga R.N., Olafsson I., Palotie A., Papamarkou T., Pomilla C., Pouta A., Ruokonen A., Seeley J., Silander K., Tiret L., van Pelt L., Wainwright N., Wijmenga C., Young E.H., Bennett F., Boomsma D.I., Burnier M., Chen Y.D., Feranil A.B., Ferrieres J., Freimer N.B., Hsiung C.A., Kesäniemi A., Koudstaal P.J., Krauss R.M., Kyvik K.O., Meneton P., Moilanen L., Sanghera D.K., Sheu W.H., Whitfield J.B., Wolffenbuttel B.H., Ordovas J.M., Rich S.S., Johnson A., Johnson L., Larson M.G., Levy D., Newton-Cheh C., O’reilly P.F., Palmas W., Rice K., Smith A., Snider H., Tobin M., Verwoert G., Rice K.M., Verwoert G.C., Pihur V., Heath S., Sõber S., Arora P., Zhang F., Lucas G., Milaneschi Y., Parker A.N., Fava C., Fox E.R., Go M.J., Sjögren M., Vinay D., Alexander M., Tabara Y., Shaw-Hawkins S., Whincup P.H., Shi G., Seielstad M., Sim X., Nguyen K.D., Matullo G., Gaunt T.R., Onland-Moret N.C., Cooper M.N., Platou C.G., Org E., Hardy R., Dahgam S., Palmen J., Kuznetsova T., Uiterwaal C.S., Adeyemo A., Ludwig B., Tomaszewski M., Tzoulaki I., Palmer N.D., Chang Y.P., Steinle N.I., Grobbee D.E., Morrison A.C., Najjar S., Hadley D., Brown M.J., Connell J.M., Day I.N., Lawlor D.A., Lawrence R.W., Ongen H., Li Y., Young J.H., Bis J.C., Bolton J.A., Chaturvedi N., Islam M., Jafar T.H., Kulkarni S.R., Howard P., Guarrera S., Ricceri F., Emilsson V., Plump A., Weder A.B., Sun Y.V., Scott L.J., Peltonen L., Vartiainen E., Brand S.M., Staessen J.A., Wang T.J., Burton P.R., Artigas M.S., Dong Y., Wang X., Zhu H., Rudock M.E., Heckbert S.R., Smith N.L., Wiggins K.L., Doumatey A., Shriner D., Veldre G., Viigimaa M., Kinra S., Prabhakaran D., Tripathy V., Langefeld C.D., Rosengren A., Thelle D.S., Corsi A.M., Singleton A., Hilton G., Salako T., Iwai N., Kita Y., Ogihara T., Ohkubo T., Okamura T., Ueshima H., Umemura S., Eyheramendy S., Meitinger T., Cho Y.S., Kim H.L., Scott J., Sehmi J.S., Hedblad B., Nilsson P., Smith G.D., Raffel L.J., Yao J., Schwartz S.M., Ikram M., W L., Mosley T.H., Seshadri S., Shrine N.R., Wain L.V., Zitting P., Cooper J.A., van Gilst W.H., Janipalli C.S., Mani K., Yajnik C.S., Mattace-Raso F.U., Lakatta E.G., Orru M., Scuteri A., Ala-Korpela M., Kangas A.J., Soininen P., Tukiainen T., Würtz P., Ong R.T., Dörr M., Galan P., Hercberg S., Lathrop M., Zelenika D., Zhai G., Meschia J.F., Sharma P., Terzic J., Kumar M., Denniff M., Zukowska-Szczechowska E., Wagenknecht L.E., Fowkes F., Charchar F.J., Guo X., Rotimi C., Bots M.L., Brand E., Talmud P.J., Nyberg F., Laan M., Palmer L.J., van der Schouw Y.T., Casas J.P., Vineis P., Ganesh S.K., Wong T.Y., Tai E.S., Morris R.W., Marmot M.G., Miki T., Chandak G.R., Zhu X., Elosua R., Soranzo N., Sijbrands E.J., Uda M., Vasan R.S., Alizadeh B.Z., de Boer R.A., Boezen H.M., Hillege H.L., van der Klauw M.M., Ormel J., Rosmalen J.G., Slaets J.P., Lagou V., Welch R.P., Wheeler E., Rehnberg E., Rasmussen-Torvik L.J., Lecoeur C., Johnson P.C., Sennblad B., Salo P., Timpson N.J., Evans D.M., St Pourcain B., Bielak L.F., Horikoshi M., Navarro P., Raychaudhuri S., Chen H., Rybin D., Willems S.M., Song K., An P., Marullo L., Jansen H., Pankow J.S., Edkins S., Varga T.V., Oksa H., Antonella M., Kong A., Herder C., Antti J., Small K., Miljkovic I., Atalay M., Kiess W., Smit J.H., Campbell S., Fowkes G.R., Rathmann W., Maerz W., Watanabe R.M., de Geus E.J., Penninx B.W., Toenjes A., Peyser P.A., Körner A., Dupuis J., Cucca F., Balkau B., Bouatia-Naji N., Purcell S., Musunuru K., Ardissino D., Mannucci P.M., Anand S., Engert J.C., Morgan T., Spertus J.A., Stoll M., Girelli D., McKeown P.P., Patterson C.C., Merlini P.A., Berzuini C., Bernardinelli L., Peyvandi F., Tubaro M., Celli P., Fetiveau R., Marziliano N., Casari G., Galli M., Ribichini F., Rossi M., Bernardi F., Zonzin P., Piazza A., Yee J., Friedlander Y., Marrugat J., Subirana I., Sala J., Ramos R., Williams G., Nathan D.M., Macrae C.A., Berglund G., Asselta R., Duga S., Spreafico M., Daly M.J., Nemesh J., Korn J.M., Surti A., Gianniny L., Parkin M., Burtt N., Gabriel S.B., Wright B.J., Ball S.G., Schunkert I., Linsel-Nitschke P., Lieb W., Fischer M., Grosshennig A., Preuss M., Scholz M., Chen Z., Wilensky R., Matthai W., Qasim A., Hakonarson H.H., Devaney J., Pichard A.D., Kent K.M., Satler L., Lindsay J.M., Waksman R., Knouff C.W., Scheffold T., Berger K., Huge A., Martinelli N., Olivieri O., Corrocher R., Hólm H., Xie C., Ahmadi K.R., Ainali C., Bataille V., Bell J.T., Buil A., Dermitzakis E.T., Dimas A.S., Durbin R., Glass D., Hassanali N., Ingle C., Knowles D., Krestyaninova M., Lowe C.E., Meduri E., Di Meglio P., Montgomery S.B., Nestle F.O., Nica A.C., Nisbet J., O’rahilly S., Parts L., Potter S., Sekowska M., Shin S.Y., Surdulescu G., Travers M.E., Tsaprouni L., Tsoka S., Wilk A., Yang T.P., Higashio J., Williams R., Nato A., Ambite J.L., Deelman E., Manolio T., Heiss G., Taylor K., Avery C., Graff M., Lin D., Quibrera M., Cochran B., Kao L., Umans J., Cole S., Maccluer J., Person S., Gross M., Fornage M., Durda P., Jenny N., Patsy B., Arnold A.M., Buzkova P., Haines J., Murdock D., Glenn K., Brown-Gentry K., Thornton-Wells T., Dumitrescu L., Bush W.S., Mitchell S.L., Goodloe R., Wilson S., Boston J., Malinowski J., Restrepo N., Oetjens M., Fowke J., Spencer K., Pendergrass S., Park L., Tiirikainen M., Kolonel L., Cheng I., Wang H., Shohet R., Stram D., Henderson B., Monroe K., Anderson G., Carlson C., Prentice R., Lacroix A., Wu C., Carty C., Rosse S., Young A., Kocarnik J., Lin Y., Jackson R., Duggan D., Kuller L., He C., Sulem P., Barbalic M., Broer L., Byrne E.M., Gudbjartsson D.F., McArdle P.F., Porcu E., van Wingerden S.W., Zhuang W.V., Lauc L.B., Broekmans F.J., Burri A., Chen C., Corre T., Coviello A.D., D’adamo P., Davies G., Deary I.J., Ebrahim S., Fauser B.C., Ferreli L., Folsom A.R., Hankinson S.E., Hass M., Janssens A.C., Karasik D., Keyzer J., Kiel D.P., Lahti J., Lai S., Laisk T., Laven J.S., Liu J., Lopez L.M., Louwers Y.V., Marongiu M., Klaric I.M., Masciullo C., Melzer D., Newman A.B., Paré G., Peeters P.H., Pop V.J., Räikkönen K., Salumets A., Stacey S.N., Starr J.M., Stathopoulou M.G., Styrkarsdottir U., Tenesa A., Tryggvadottir L., Tsui K., van Dam R.M., van Gils C.H., van Nierop P., Vink J.M., Voorhuis M., Widen E., Wijnands-Van Gent C.J., Yerges-Armstrong L.M., Zgaga L., Zygmunt M., Buring J.E., Crisponi L., Demerath E.W., Streeten E.A., Murray A., Visser J.A., Lunetta K.L., Elks C.E., Cousminer D.L., Koller D.L., Lin P., Smith E.N., Warrington N.M., Alavere H., Berenson G.S., Blackburn H., Busonero F., Chen W., Couper D., Easton D.F., Eriksson J., Foroud T., Kilpeläinen T.O., Li S., Murray S.S., Ness A.R., Northstone K., Peacock M., Pennell C.E., Pharoah P., Rafnar T., Rice J.P., Ring S.M., Schork N.J., Segrè A.V., Sovio U., Srinivasan S.R., Tammesoo M.L., van Meurs J.B., Young L., Bierut L.J., Econs M.J. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518:197–206. doi: 10.1038/nature14177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J., Hendryx M., Laddu D., Phillips L.S., Chlebowski R., LeBlanc E.S., Allison D.B., Nelson D.A., Li Y., Rosal M.C., Stefanick M.L., Manson J.A.E. Racial and ethnic differences in anthropometric measures as risk factors for diabetes. Diabetes Care. 2019;42:126–133. doi: 10.2337/dc18-1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luppino F.S., De Wit L.M., Bouvy P.F., Stijnen T., Cuijpers P., Penninx B.W.J.H., Zitman F.G. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch. Gen. Psychiatry. 2010 doi: 10.1001/archgenpsychiatry.2010.2. [DOI] [PubMed] [Google Scholar]
- Mamun A.A., Lawlor D.A., O'Callaghan M.J., Bor W., Williams G.M., Najman J.M. Does childhood sexual abuse predict young adult's BMI? A birth cohort study. Obesity. 2007;15:2103–2110. doi: 10.1038/oby.2007.250. [DOI] [PubMed] [Google Scholar]
- Masodkar K., Johnson J., Peterson M.J. A review of posttraumatic stress disorder and obesity: exploring the link. Prim. Care Companion J. Clin. Psychiatry. 2016;18 doi: 10.4088/PCC.15r01848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B.S. Protective and damaging effects of stress mediators. N. Engl. J. Med. 1998;338:171–179. doi: 10.1056/nejm199801153380307. [DOI] [PubMed] [Google Scholar]
- Midei A.J., Matthews K.A. Interpersonal violence in childhood as a risk factor for obesity: a systematic review of the literature and proposed pathways. Obes. Rev. 2011;12 doi: 10.1111/j.1467-789X.2010.00823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell K.S., Porter B., Boyko E.J., Field A.E. Longitudinal associations among posttraumatic stress disorder, disordered eating, and weight gain in military men and women. Am. J. Epidemiol. 2016;184:33–47. doi: 10.1093/aje/kwv291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muniz Carvalho C., Wendt F.R., Maihofer A.X., Stein D.J., Stein M.B., Sumner J.A., Hemmings S.M.J., Nievergelt C.M., Koenen K.C., Gelernter J., Belangero S.I., Polimanti R. Dissecting the genetic association of C-reactive protein with PTSD, traumatic events, and social support. Neuropsychopharmacology. 2021;46:1071–1077. doi: 10.1038/s41386-020-0655-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nievergelt C.M., Maihofer A.X., Klengel T., Atkinson E.G., Chen C.Y., Choi K.W., Coleman J.R.I., Dalvie S., Duncan L.E., Gelernter J., Levey D.F., Logue M.W., Polimanti R., Provost A.C., Ratanatharathorn A., Stein M.B., Torres K., Aiello A.E., Almli L.M., Amstadter A.B., Andersen S.B., Andreassen O.A., Arbisi P.A., Ashley-Koch A.E., Austin S.B., Avdibegovic E., Babić D., Bækvad-Hansen M., Baker D.G., Beckham J.C., Bierut L.J., Bisson J.I., Boks M.P., Bolger E.A., Børglum A.D., Bradley B., Brashear M., Breen G., Bryant R.A., Bustamante A.C., Bybjerg-Grauholm J., Calabrese J.R., Caldas- de- Almeida J.M., Dale A.M., Daly M.J., Daskalakis N.P., Deckert J., Delahanty D.L., Dennis M.F., Disner S.G., Domschke K., Dzubur-Kulenovic A., Erbes C.R., Evans A., Farrer L.A., Feeny N.C., Flory J.D., Forbes D., Franz C.E., Galea S., Garrett M.E., Gelaye B., Geuze E., Gillespie C., Uka A.G., Gordon S.D., Guffanti G., Hammamieh R., Harnal S., Hauser M.A., Heath A.C., Hemmings S.M.J., Hougaard D.M., Jakovljevic M., Jett M., Johnson E.O., Jones I., Jovanovic T., Qin X.J., Junglen A.G., Karstoft K.I., Kaufman M.L., Kessler R.C., Khan A., Kimbrel N.A., King A.P., Koen N., Kranzler H.R., Kremen W.S., Lawford B.R., Lebois L.A.M., Lewis C.E., Linnstaedt S.D., Lori A., Lugonja B., Luykx J.J., Lyons M.J., Maples-Keller J., Marmar C., Martin A.R., Martin N.G., Maurer D., Mavissakalian M.R., McFarlane A., McGlinchey R.E., McLaughlin K.A., McLean S.A., McLeay S., Mehta D., Milberg W.P., Miller M.W., Morey R.A., Morris C.P., Mors O., Mortensen P.B., Neale B.M., Nelson E.C., Nordentoft M., Norman S.B., O'Donnell M., Orcutt H.K., Panizzon M.S., Peters E.S., Peterson A.L., Peverill M., Pietrzak R.H., Polusny M.A., Rice J.P., Ripke S., Risbrough V.B., Roberts A.L., Rothbaum A.O., Rothbaum B.O., Roy-Byrne P., Ruggiero K., Rung A., Rutten B.P.F., Saccone N.L., Sanchez S.E., Schijven D., Seedat S., Seligowski A.V., Seng J.S., Sheerin C.M., Silove D., Smith A.K., Smoller J.W., Sponheim S.R., Stein D.J., Stevens J.S., Sumner J.A., Teicher M.H., Thompson W.K., Trapido E., Uddin M., Ursano R.J., van den Heuvel L.L., Van Hooff M., Vermetten E., Vinkers C.H., Voisey J., Wang Y., Wang Z., Werge T., Williams M.A., Williamson D.E., Winternitz S., Wolf C., Wolf E.J., Wolff J.D., Yehuda R., Young R.M.D., Young K.A., Zhao H., Zoellner L.A., Liberzon I., Ressler K.J., Haas M., Koenen K.C. International meta-analysis of PTSD genome-wide association studies identifies sex- and ancestry-specific genetic risk loci. Nat. Commun. 2019;10 doi: 10.1038/s41467-019-12576-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg J.T., Johnstone J.M., Musser E.D., Long H.G., Willoughby M., Shannon J. Attention-deficit/hyperactivity disorder (ADHD) and being overweight/obesity: new data and meta-analysis. Clin. Psychol. Rev. 2016 doi: 10.1016/j.cpr.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor L.J., Price A.L. Distinguishing genetic correlation from causation across 52 diseases and complex traits. Nat. Genet. 2018;50:1728–1734. doi: 10.1038/s41588-018-0255-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olff M. Sex and gender differences in post-traumatic stress disorder: an update. Eur. J. Psychotraumatol. 2017;8:1351204. doi: 10.1080/20008198.2017.1351204. [DOI] [Google Scholar]
- Olff M., Langeland W., Draijer N., Gersons B.P.R. Gender differences in posttraumatic stress disorder. Psychol. Bull. 2007;133:183–204. doi: 10.1037/0033-2909.133.2.183. [DOI] [PubMed] [Google Scholar]
- Perkonigg A., Owashi T., Stein M.B., Kirschbaum C., Wittchen H.U. Posttraumatic stress disorder and obesity. Evidence for a risk association. Am. J. Prev. Med. 2009;36:1–8. doi: 10.1016/j.amepre.2008.09.026. [DOI] [PubMed] [Google Scholar]
- Peters T., Nüllig L., Antel J., Naaresh R., Laabs B.H., Tegeler L., Amhaouach C., Libuda L., Hinney A., Hebebrand J. The role of genetic variation of BMI, body composition, and fat distribution for mental traits and disorders: a look-up and mendelian randomization study. Front. Genet. 2020;11 doi: 10.3389/fgene.2020.00373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polimanti R., Amstadter A.B., Stein M.B., Almli L.M., Baker D.G., Bierut L.J., Bradley B., Farrer L.A., Johnson E.O., King A., Kranzler H.R., Maihofer A.X., Rice J.P., Roberts A.L., Saccone N.L., Zhao H., Liberzon I., Nievergelt C.M., Koenen K.C., Gelernter J., Duncan L.E., Chen C.Y., Ratanatharathorn A., Aiello A.E., Ashley-Koch A.E., Garrett M.E., Hauser M.A., Beckham J.C., Kimbrel N.A., Bisson J., Dalvie S., Galea S., Gelernter J.E., Guffanti G., Ressler K.J., Kessler R.C., Koen N., Stein D.J., Logue M.W., Miller M.W., Martin A.R., Morey R.A., Nugent N.R., Ripke S., Smoller J.W., Sumner J.A., Uddin M., Ursano R.J., Wildman D.E., Yehuda R., Daly M.J. A putative causal relationship between genetically determined female body shape and posttraumatic stress disorder. Genome Med. 2017;9:99. doi: 10.1186/s13073-017-0491-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polimanti R., Peterson R.E., Ong J.S., MacGregor S., Edwards A.C., Clarke T.K., Frank J., Gerring Z., Gillespie N.A., Lind P.A., Maes H.H., Martin N.G., Mbarek H., Medland S.E., Streit F., Agrawal A., Edenberg H.J., Kendler K.S., Lewis C.M., Sullivan P.F., Wray N.R., Gelernter J., Derks E.M. Evidence of causal effect of major depression on alcohol dependence: findings from the psychiatric genomics consortium. Psychol. Med. 2019;49:1218–1226. doi: 10.1017/S0033291719000667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polimanti R., Ratanatharathorn A., Maihofer A.X., Choi K.W., Stein M.B., Morey R.A., Logue M.W., Nievergelt C.M., Stein D.J., Koenen K.C., Gelernter J. Association of economic status and educational attainment with posttraumatic stress disorder a mendelian randomization study. JAMA Netw. Open. 2019;2 doi: 10.1001/jamanetworkopen.2019.3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polimanti R., Ratanatharathorn A., Maihofer A.X., Choi K.W., Stein M.B., Morey R.A., Logue M.W., Nievergelt C.M., Stein D.J., Koenen K.C., Gelernter J. Association of economic status and educational attainment with posttraumatic stress disorder a mendelian randomization study. JAMA Netw. Open. 2019;2 doi: 10.1001/jamanetworkopen.2019.3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polimanti R., Yang B.Z., Zhao H., Gelernter J. Evidence of polygenic adaptation in the systems genetics of anthropometric traits. PLoS One. 2016;11 doi: 10.1371/journal.pone.0160654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooley A.E., Benjamin R.C., Sreedhar S., Eagle A.L., Robison A.J., Mazei-Robison M.S., Breedlove S.M., Jordan C.L. Sex differences in the traumatic stress response: the role of adult gonadal hormones. Biol. Sex Differ. 2018;9 doi: 10.1186/s13293-018-0192-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramikie T.S., Ressler K.J. Stress-related disorders, pituitary adenylate cyclase-activating peptide (PACAP)ergic system, and sex differences. Dialogues Clin. Neurosci. 2016;18:403–413. doi: 10.31887/dcns.2016.18.4/kressler. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall J.C., Winkler T.W., Kutalik Z., Berndt S.I., Jackson A.U., Monda K.L., Kilpeläinen T.O., Esko T., Mägi R., Li S., Workalemahu T., Feitosa M.F., Croteau-Chonka D.C., Day F.R., Fall T., Ferreira T., Gustafsson S., Locke A.E., Mathieson I., Scherag A., Vedantam S., Wood A.R., Liang L., Steinthorsdottir V., Thorleifsson G., Dermitzakis E.T., Dimas A.S., Karpe F., Min J.L., Nicholson G., Clegg D.J., Person T., Krohn J.P., Bauer S., Buechler C., Eisinger K., Bonnefond A., Froguel P., Hottenga J.J., Prokopenko I., Waite L.L., Harris T.B., Smith A.V., Shuldiner A.R., McArdle W.L., Caulfield M.J., Munroe P.B., Grönberg H., Chen Y.D.I., Li G., Beckmann J.S., Johnson T., Thorsteinsdottir U., Teder-Laving M., Khaw K.T., Wareham N.J., Zhao J.H., Amin N., Oostra B.A., Kraja A.T., Province M.A., Cupples L.A., Heard-Costa N.L., Kaprio J., Ripatti S., Surakka I., Collins F.S., Saramies J., Tuomilehto J., Jula A., Salomaa V., Erdmann J., Hengstenberg C., Loley C., Schunkert H., Lamina C., Wichmann H.E., Albrecht E., Gieger C., Hicks A.A., Johansson Å., Pramstaller P.P., Kathiresan S., Speliotes E.K., Penninx B., Hartikainen A.L., Jarvelin M.R., Gyllensten U., Boomsma D.I., Campbell H., Wilson J.F., Chanock S.J., Farrall M., Goel A., Medina-Gomez C., Rivadeneira F., Estrada K., Uitterlinden A.G., Hofman A., Zillikens M.C., den Heijer M., Kiemeney L.A., Maschio A., Hall P., Tyrer J., Teumer A., Völzke H., Kovacs P., Tönjes A., Mangino M., Spector T.D., Hayward C., Rudan I., Hall A.S., Samani N.J., Attwood A.P., Sambrook J.G., Hung J., Palmer L.J., Lokki M.L., Sinisalo J., Boucher G., Huikuri H., Lorentzon M., Ohlsson C., Eklund N., Eriksson J.G., Barlassina C., Rivolta C., Nolte I.M., Snieder H., van der Klauw M.M., van Vliet-Ostaptchouk J.V., Gejman P.V., Shi J., Jacobs K.B., Wang Z., Bakker S.J.L., Mateo Leach I., Navis G., van der Harst P., Martin N.G., Medland S.E., Montgomery G.W., Yang J., Chasman D.I., Ridker P.M., Rose L.M., Lehtimäki T., Raitakari O., Absher D., Iribarren C., Basart H., Hovingh K.G., Hyppönen E., Power C., Anderson D., Beilby J.P., Hui J., Jolley J., Sager H., Bornstein S.R., Schwarz P.E.H., Kristiansson K., Perola M., Lindström J., Swift A.J., Uusitupa M., Atalay M., Lakka T.A., Rauramaa R., Bolton J.L., Fowkes G., Fraser R.M., Price J.F., Fischer K., KrjutÅkov K., Metspalu A., Mihailov E., Langenberg C., Luan J., Ong K.K., Chines P.S., Keinanen-Kiukaanniemi S.M., Saaristo T.E., Edkins S., Franks P.W., Hallmans G., Shungin D., da Morris A., Palmer C.N.A., Erbel R., Moebus S., Nöthen M.M., Pechlivanis S., Hveem K., Narisu N., Hamsten A., Humphries S.E., Strawbridge R.J., Tremoli E., Grallert H., Thorand B., Illig T., Koenig W., Müller-Nurasyid M., Peters A., Boehm B.O., Kleber M.E., März W., Winkelmann B.R., Kuusisto J., Laakso M., Arveiler D., Cesana G., Kuulasmaa K., Virtamo J., Yarnell J.W.G., Kuh D., Wong A., Lind L., de Faire U., Gigante B., Magnusson P.K.E., Pedersen N.L., Dedoussis G., Dimitriou M., Kolovou G., Kanoni S., Stirrups K., Bonnycastle L.L., Njølstad I., Wilsgaard T., Ganna A., Rehnberg E., Hingorani A., Kivimaki M., Kumari M., Assimes T.L., Barroso I., Boehnke M., Borecki I.B., Deloukas P., Fox C.S., Frayling T., Groop L.C., Haritunians T., Hunter D., Ingelsson E., Kaplan R., Mohlke K.L., O'Connell J.R., Schlessinger D., Strachan D.P., Stefansson K., van Duijn C.M., Abecasis G.R., McCarthy M.I., Hirschhorn J.N., Qi L., Loos R.J.F., Lindgren C.M., North K.E., Heid I.M. Sex-stratified genome-wide association studies including 270,000 individuals show sexual dimorphism in genetic loci for anthropometric traits. PLoS Genet. 2013;9 doi: 10.1371/journal.pgen.1003500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravera S., Carrasco N., Gelernter J., Polimanti R. Phenomic impact of genetically-determined euthyroid function and molecular differences between thyroid disorders. J. Clin. Med. 2018;7:296. doi: 10.3390/jcm7100296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roenholt S., Beck N.N., Karsberg S.H., Elklit A. Post-traumatic stress symptoms and childhood abuse categories in a national representative sample for a specific age group: associations to body mass index. Eur. J. Psychotraumatol. 2012;3 doi: 10.3402/ejpt.v3i0.17188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarink D., Wilkens L.R., White K.K., Le Marchand L., Wu A.H., Setiawan V.W., Park S.L., Park S.Y., Killeen J.L., Merritt M.A. Racial/ethnic differences in anthropometric and hormone-related factors and endometrial cancer risk: the Multiethnic Cohort Study. Br. J. Cancer. 2021;124:1724–1733. doi: 10.1038/s41416-021-01292-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor C.E., McCutcheon V.V., Pommer N.E., Nelson E.C., Grant J.D., Duncan A.E., Waldron M., Bucholz K.K., Madden P.A.F., Heath A.C. Common genetic and environmental contributions to post-traumatic stress disorder and alcohol dependence in young women. Psychol. Med. 2011;41:1497–1505. doi: 10.1017/S0033291710002072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal N.L., Feng R., McGuire S.A., Allison D.B., Miller S. Genetic and environmental contributions to body mass index: comparative analysis of monozygotic twins, dizygotic twins and same-age unrelated siblings. Int. J. Obes. 2009;33:37–41. doi: 10.1038/ijo.2008.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segman R.H., Shalev A.Y. Genetics of posttraumatic stress disorder. CNS Spectr. 2003;8:693–698. doi: 10.1017/S1092852900008889. [DOI] [PubMed] [Google Scholar]
- Shen X., Howard D.M., Adams M.J., Hill W.D., Clarke T.K., McIntosh A.M., Deary I.J., Wray N.R., Ripke S., Mattheisen M., Trzaskowski M., Byrne E.M., Abdellaoui A., Agerbo E., Air T.M., Andlauer T.F.M., Bacanu S.A., Bækvad-Hansen M., Beekman A.T.F., Bigdeli T.B., Binder E.B., Bryois J., Buttenschøn H.N., Bybjerg-Grauholm J., Cai N., Castelao E., Christensen J.H., Coleman J.R.I., Colodro-Conde L., Couvy-Duchesne B., Craddock N., Crawford G.E., Davies G., Degenhardt F., Derks E.M., Direk N., Dolan C.V., Dunn E.C., Eley T.C., Escott-Price V., Kiadeh F.F.H., Finucane H.K., Foo J.C., Forstner A.J., Frank J., Gaspar H.A., Gill M., Goes F.S., Gordon S.D., Grove J., Hall L.S., Hansen C.S., Hansen T.F., Herms S., Hickie I.B., Hoffmann P., Homuth G., Horn C., Hottenga J.J., Hougaard D.M., Ising M., Jansen R., Jones I., Jones L.A., Jorgenson E., Knowles J.A., Kohane I.S., Kraft J., Kretzschmar W.W., Kutalik Z., Li Y., Lind P.A., MacIntyre D.J., MacKinnon D.F., Maier R.M., Maier W., Marchini J., Mbarek H., McGrath P., McGuffin P., Medland S.E., Mehta D., Middeldorp C.M., Mihailov E., Milaneschi Y., Milani L., Mondimore F.M., Montgomery G.W., Mostafavi S., Mullins N., Nauck M., Ng B., Nivard M.G., Nyholt D.R., O'Reilly P.F., Oskarsson H., Owen M.J., Painter J.N., Pedersen C.B., Pedersen M.G., Peterson R.E., Pettersson E., Peyrot W.J., Pistis G., Posthuma D., Quiroz J.A., Qvist P., Rice J.P., Riley B.P., Rivera M., Mirza S.S., Schoevers R., Schulte E.C., Shen L., Shi J., Shyn S.I., Sigurdsson E., Sinnamon G.C.B., Smit J.H., Smith D.J., Stefansson H., Steinberg S., Streit F., Strohmaier J., Tansey K.E., Teismann H., Teumer A., Thompson W., Thomson P.A., Thorgeirsson T.E., Traylor M., Treutlein J., Trubetskoy V., Uitterlinden A.G., Umbricht D., Auwera S. Van der, van Hemert A.M., Viktorin A., Visscher P.M., Wang Y., Webb B.T., Weinsheimer S.M., Wellmann J., Willemsen G., Witt S.H., Wu Y., Xi H.S., Yang J., Zhang F., Arolt V., Baune B.T., Berger K., Boomsma D.I., Cichon S., Dannlowski U., de Geus E.J.C., DePaulo J.R., Domenici E., Domschke K., Esko T., Grabe H.J., Hamilton S.P., Hayward C., Heath A.C., Kendler K.S., Kloiber S., Lewis G., Li Q.S., Lucae S., Madden P.A.F., Magnusson P.K., Martin N.G., Metspalu A., Mors O., Mortensen P.B., Müller-Myhsok B., Nordentoft M., Nöthen M.M., O'Donovan M.C., Paciga S.A., Pedersen N.L., Penninx B.W.J.H., Perlis R.H., Porteous D.J., Potash J.B., Preisig M., Rietschel M., Schaefer C., Schulze T.G., Smoller J.W., Stefansson K., Tiemeier H., Uher R., Völzke H., Weissman M.M., Werge T., Lewis C.M., Levinson D.F., Breen G., Børglum A.D., Sullivan P.F., Whalley H.C. A phenome-wide association and Mendelian Randomisation study of polygenic risk for depression in UK Biobank. Nat. Commun. 2020;11 doi: 10.1038/s41467-020-16022-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shungin D., Winkler T., Croteau-Chonka D.C., Ferreira T., Locke A.E., Mägi R., Strawbridge R.J., Pers T.H., Fischer K., Justice A.E., Workalemahu T., Wu J.M.W., Buchkovich M.L., Heard-Costa N.L., Roman T.S., Drong A.W., Song C., Gustafsson S., Day F.R., Esko T., Fall T., Kutalik Z., Luan J., Randall J.C., Scherag A., Vedantam S., Wood A., Chen J., Fehrmann R., Karjalainen J., Kahali B., Liu C.T., Schmidt E.M., Absher D., Amin N., Anderson D., Beekman M., Bragg-Gresham J.L., Buyske S., Demirkan A., Ehret G., Feitosa M., Goel A., Jackson A.U., Johnson T., Kleber M.E., Kristiansson K., Mangino M., Leach I.M., Medina-Gomez C., Palmer C.D., Pasko D., Pechlivanis S., Peters M.J., Prokopenko I., Stančáková A., Sung Y.J., Tanaka T., Teumer A., Van Vliet-Ostaptchouk J.V., Yengo L., Zhang W., Albrecht E., Ärnlöv J., Arscott G.M., Bandinelli S., Barrett A., Bellis C., Bennett A.J., Berne C., Blüher M., Böhringer S., Bonnet F., Böttcher Y., Bruinenberg M., Carba D.B., Caspersen I.H., Clarke R., Daw E.W., Deelen J., Deelman E., Delgado G., Doney A., Eklund N., Erdos M.R., Estrada K., Eury E., Friedrich N., Garcia M., Giedraitis V., Gigante B., Go A.S., Golay A., Grallert H., Grammer T.B., Gräßler J., Grewal J., Groves C.J., Haller T., Hallmans G., Hartman C.A., Hassinen M., Hayward C., Heikkilä K., Herzig K.H., Helmer Q., Hillege H.L., Holmen O., Hunt S.C., Isaacs A., Ittermann T., James A.L., Johansson I., Juliusdottir T., Kalafati I.P., Kinnunen L., Koenig W., Kooner I.K., Kratzer W., Lamina C., Leander K., Lee N.R., Lichtner P., Lind L., Lindström J., Lobbens S., Lorentzon M., Mach F., Magnusson P.K., Mahajan A., McArdle W.L., Menni C., Merger S., Mihailov E., Milani L., Mills R., Moayyeri A., Monda K.L., Mooijaart S.P., Mühleisen T.W., Mulas A., Müller G., Müller-Nurasyid M., Nagaraja R., Nalls M.A., Narisu N., Glorioso N., Nolte I.M., Olden M., Rayner N.W., Renstrom F., Ried J.S., Robertson N., Rose L.M., Sanna S., Scharnagl H., Scholtens S., Sennblad B., Seufferlein T., Sitlani C.M., Smith A.V., Stirrups K., Stringham H.M., Sundström J., Swertz M.A., Swift A.J., Syvänen A.C., Tayo B., Thorand B., Thorleifsson G., Tomaschitz A., Troffa C., van Oort F.V., Verweij N., Vonk J.M., Waite L.L., Wennauer R., Wilsgaard T., Wojczynski M.K., Wong A., Zhang Q., Zhao J.H., Brennan E.P., Choi M., Eriksson P., Folkersen L., Franco-Cereceda A., Gharavi A.G., Hedman K., Hivert M.F., Huang J., Kanoni S., Karpe F., Keildson S., Kiryluk K., Liang L., Lifton R.P., Ma B., McKnight A.J., McPherson R., Metspalu A., Min J.L., Moffatt M.F., Montgomery G.W., Murabito J.M., Nicholson G.C., Nyholt D.R., Olsson C., Perry J.R., Reinmaa E., Salem R.M., Sandholm N., Schadt E., Scott R.A., Stolk L., Vallejo E.E., Westra H.J., Zondervan K.T., Amouyel P., Arveiler D., Bakker S.J., Beilby J.P., Bergman R.N., Blangero J., Brown M.J., Burnier M., Campbell H., Chakravarti A., Chines P.S., Claudi-Boehm S., Collins F.S., Crawford D., Danesh J., de Faire U., de Geus E.J., Dörr M., Erbel R., Eriksson J.G., Farrall M., Ferrannini E., Ferrières J., Forouhi N.G., Forrester T., Franco O.H., Gansevoort R.T., Gieger C., Gudnason V., Haiman C., Harris T., Hattersley A.T., Heliövaara M., Hicks A.A., Hingorani A., Hoffmann W., Hofman A., Homuth G., Humphries S.E., Hyppönen E., Illig T., Jarvelin M.R., Johansen B., Jousilahti P., Jula A., Kaprio J., Kee F., Keinanen-Kiukaanniemi S.M., Kooner J., Kooperberg C., Kovacs P., Kraja A.T., Kumari M., Kuulasmaa K., Kuusisto J., Lakka T.A., Langenberg C., Le, Marchand L., Lehtimäki T., Lyssenko V., Männistö S., Marette A., Matise T., McKenzie C.A., McKnight B., Musk A.W., Möhlenkamp S., Morris A.D., Nelis M., Ohlsson C., Oldehinkel A.J., Ong K.K., Palmer L.J., Penninx B.W., Peters A., Pramstaller P.P., Raitakari O., Rankinen T., Rao D.C., Rice T.K., Ridker P., Ritchie M., Rudan I., Salomaa V., Samani N., Saramies J., Sarzynski M.A., Schwarz P.E., Shuldiner A.R., Staessen J.A., Steinthorsdottir V., Stolk R.P., Strauch K., Tönjes A., Tremblay A., Tremoli E., Vohl M.C., Völker U., Vollenweider P., Wilson J.F., Witteman J.C., Adair L.S., Bochud M., Boehm B.O., Bornstein S.R., Bouchard C., Cauchi S., Caulfield M., Chambers J., Chasman D., Cooper R.S., Dedoussis G.V., Ferrucci L., Froguel P., Grabe H.J., Hamsten A., Hui J., Hveem K., Jöckel K.H., Kivimaki M., Kuh D., Laakso M., Liu Y., März W., Munroe P., Njølstad I., Oostra B., Palmer C.N., Pedersen N.L., Perola M., Pérusse L., Peters U., Power C., Quertermous T., Rauramaa R., Rivadeneira F., Saaristo T.E., Saleheen D., Sinisalo J., Slagboom P., Snieder H., Spector T.D., Stefansson K., Stumvoll M., Tuomilehto J., Uitterlinden A.G., Uusitupa M., van der Harst P., Veronesi G., Walker M., Wareham N., Watkins H., Wichmann H.E., Abecasis G., Assimes T.L., Berndt S.I., Boehnke M., Borecki I., Deloukas P., Franke L., Frayling T.M., Groop L.C., Hunter D.J., Kaplan R.C., O’connell J.R., Qi L., Schlessinger D., Strachan D.P., Thorsteinsdottir U., van Duijn C., Willer C.J., Visscher P.M., Yang J., Hirschhorn J.N., Zillikens M.C., McCarthy M.I., Speliotes E.K., North K.E., Fox C.S., Barroso I., Franks P.W., Ingelsson E., Heid I.M., Loos R.J., Cupples L., Morris A.P., Lindgren C.M., Mohlke K.L., Dastani Z., Timpson N., Yuan X., Henneman P., Kizer J.R., Lyytikainen L.P., Fuchsberger C., Small K.S., Coassin S., Lohman K., Pankow J.S., Uh H.W., Wu Y., Bidulescu A., Rasmussen-Torvik L.J., Greenwood C.M., Ladouceur M., Grimsby J., Manning A.K., Mooser V.E., Kapur K.A., Frants R., Willemsvan-Vandijk K., Willems S.M., Psaty B., Tracy R.P., Brody J., Chen I., Viikari J., Kähönen M., Evans D.M., St Pourcain B., Sattar N., Carlson O.D., Egan J., van Heemst D., Kedenko L., Nuotio M.L., Loo B.M., Kanaya A., Haun M., Klopp N., Katsareli E., Couper D.J., Duncan B.B., Kloppenburg M., Borja J.B., Wilson J.G., Musani S., Guo X., Semple R., Teslovich T.M., Allison M.A., Redline S., Buxbaum S.G., Meulenbelt I., Ballantyne C.M., Hu F.B., Paulweber B., Florez J.C., Smith G.D., Siscovick D.S., Kronenberg F., Waterworth D., Meigs J.B., Dupuis J., Richards J.B., Willenborg C., Thompson J.R., Erdmann J., Goldstein B.A., König I.R., Cazier J.B., Johansson, Hall A.S., Lee J.Y., Grundberg E., Havulinna A.S., Ho W.K., Hopewell J.C., Eriksson N., Lundmark P., Lyytikäinen L.P., Rafelt S., Tikkanen E., Van Zuydam N., Voight B.F., Ziegler A., Altshuler D., Balmforth A.J., Braund P.S., Burgdorf C., Cox D., Dimitriou M., Do R., El Mokhtari N., Fontanillas P., Groop L., Hager J., Han B.G., Hunt S.E., Kang H.M., Kessler T., Knowles J.W., Kolovou G., Langford C., Lokki M.L., Lundmark A., Meisinger C., Melander O., Maouche S., Nikus K., Peden J.F., Rasheed A., Rosinger S., Rubin D., Rumpf M.P., Schäfer A., Sivananthan M., Stewart A.F., Tan S.T., Thorgeirsson G., van der Schoot C.E., Wagner P.J., Wells G.A., Wild P.S., Yang T.P., Basart H.V., Boerwinkle E., Brambilla P., Cambien F., Cupples A.L., Dehghan A., Diemert P., Epstein S.E., Evans A., Ferrario M.M., Gauguier D., Goodall A.H., Hazen S., Holm H., Iribarren C., Jang Y., Kim H.S., Laaksonen R., Ouwehand W.H., Parish S., Park J.E., Rader D.J., Shah S., Stark K., Trégouët D.A., Virtamo J., Wallentin L., Zimmermann M.E., Nieminen M.S., Hengstenberg C., Sandhu M.S., Pastinen T., Hovingh G.K., Zalloua P.A., Siegbahn A., Schreiber S., Ripatti S., Blankenberg S.S., O’donnell C., Reilly M., Collins R., Kathiresan S., Roberts R., Schunkert H., Pattaro C., Köttgen A., Garnaas M., Böger C.A., Chen M.H., Tin A., Taliun D., Li M., Gao X., Gorski M., Yang Q., Hundertmark C., Foster M.C., O’seaghdha C.M., Glazer N., Struchalin M., Li G., Johnson A.D., Gierman H.J., Hwang S.J., Atkinson E.J., Cornelis M.C., Chouraki V., Holliday E.G., Sorice R., Deshmukh H., Ulivi S., Chu A.Y., Murgia F., Trompet S., Imboden M., Kollerits B., Pistis G., Launer L.J., Aspelund T., Eiriksdottir G., Mitchell B.D., Schmidt H., Cavalieri M., Rao M., de Andrade M., Turner S.T., Ding J., Andrews J.S., Freedman B.I., Döring A., Kolcic I., Zemunik T., Boban M., Minelli C., Wheeler H.E., Igl W., Zaboli G., Wild S.H., Wright A.F., Ellinghaus D., Nöthlings U., Jacobs G., Biffar R., Endlich K., Ernst F., Kroemer H.K., Nauck M., Stracke S., Völzke H., Aulchenko Y.S., Polasek O., Hastie N., Vitart V., Helmer C., Wang J.J., Ruggiero D., Bergmann S., Nikopensius T., Province M.A., Ketkar S., Colhoun H., Robino A., Giulianini F., Krämer B.K., Portas L., Ford I., Buckley B.M., Adam M., Thun G.A., Sala C., Metzger M., Mitchell P., Ciullo M., Kim S.K., Palmer C., Gasparini P., Pirastu M., Jukema J.W., Probst-Hensch N.M., Toniolo D., Coresh J., Schmidt R., Kardia S.L., Curhan G.C., Gyllensten U.B., Franke A., Rettig R., Parsa A., Goessling W., Kao W., de Boer I.H., Peralta C.A., Akylbekova E., Kramer H., Arking D.E., Franceschini N., Hernandez D., Townsend R.R., Lumley T., Kestenbaum B., Haritunians T., Waeber G., Lu X., Leak T.S., Aasarød K., Skorpen F., Baumert J., Devuyst O., Mychaleckyj J.C., Hallan S., Navis G., Shlipak M.G., Bull S.B., Paterson A.D., Rotter J.I., Beckmann J.S., Dreisbach A.W., Styrkarsdottir U., Evangelou E., Hsu Y.H., Duncan E.L., Ntzani E.E., Oei L., Albagha O.M., Kemp J.P., Koller D.L., Minster R.L., Vandenput L., Willner D., Xiao S.M., Yerges-Armstrong L.M., Zheng H.F., Alonso N., Eriksson J., Kammerer C.M., Kaptoge S.K., Leo P.J., Wilson S.G., Aalto V., Alen M., Aragaki A.K., Center J.R., Dailiana Z., Duggan D.J., Garcia-Giralt N., Giroux S., Hocking L.J., Husted L.B., Jameson K.A., Khusainova R., Kim G.S., Koromila T., Kruk M., Laaksonen M., Lacroix A.Z., Lee S.H., Leung P.C., Lewis J.R., Masi L., Mencej-Bedrac S., Nguyen T.V., Nogues X., Patel M.S., Prezelj J., Scollen S., Siggeirsdottir K., Svensson O., Trummer O., van Schoor N.M., Woo J., Zhu K., Balcells S., Brandi M.L., Cheng S., Christiansen C., Cooper C., Frost M., Goltzman D., González-Macías J., Karlsson M., Khusnutdinova E., Koh J.M., Kollia P., Langdahl B.L., Leslie W.D., Lips P., Ljunggren, Lorenc R.S., Marc J., Mellström D., Obermayer-Pietsch B., Olmos J.M., Pettersson-Kymmer U., Reid D.M., Riancho J.A., Rousseau F., Tang N.L., Urreizti R., Van Hul W., Zarrabeitia M.T., Castano-Betancourt M., Herrera L., Ingvarsson T., Johannsdottir H., Kwan T., Li R., Luben R., Medina-Gómez C., Palsson S.T., Reppe S., Sigurdsson G., van Meurs J.B., Verlaan D., Williams F.M., Zhou Y., Gautvik K.M., Raychaudhuri S., Cauley J.A., Clark G.R., Cummings S.R., Danoy P., Dennison E.M., Eastell R., Eisman J.A., Jackson R., Jones G., Khaw K.T., McCloskey E., Nandakumar K., Peacock M., Pols H., Prince R.L., Reid I.R., Robbins J., Sambrook P.N., Sham P.C., Tylavsky F.A., Econs M.J., Kung A.W., Reeve J., Streeten E.A., Karasik D., Brown M.A., Ralston S.H., Ioannidis J.P., Kiel D.P., Forsblom C., Isakova T., McKay G.J., Williams W.W., Sadlier D.M., Mäkinen V.P., Swan E.J., Boright A.P., Ahlqvist E., Keller B.J., Huang H., Ahola A., Fagerholm E., Gordin D., Harjutsalo V., He B., Heikkilä O., Hietala K., Kytö J., Lahermo P., Lehto M., Österholm A.M., Parkkonen M., Pitkäniemi J., Rosengård-Bärlund M., Saraheimo M., Sarti C., Söderlund J., Soro-Paavonen A., Syreeni A., Thorn L.M., Tikkanen H., Tolonen N., Tryggvason K., Wadén J., Gill G.V., Prior S., Guiducci C., Mirel D.B., Taylor A., Hosseini M., Parving H.H., Rossing P., Tarnow L., Ladenvall C., Alhenc-Gelas F., Lefebvre P., Rigalleau V., Roussel R., Tregouet D.A., Maestroni A., Maestroni S., Falhammar H., Gu T., Möllsten A., Cimponeriu D., Mihai I., Mota M., Mota E., Serafinceanu C., Stavarachi M., Hanson R.L., Nelson R.G., Kretzler M., Panduru N.M., Gu H., Brismar K., Zerbini G., Hadjadj S., Marre M., Lajer M., Waggott D., Savage D.A., Bain S.C., Martin F., Godson C., Groop P.H., Maxwell A.P., Sengupta S., Peloso G.M., Ganna A., Mora S., Chang H.Y., Den Hertog H.M., Donnelly L.A., Fraser R.M., Freitag D.F., Gurdasani D., Kaakinen M., Kettunen J., Li X., Montasser M.E., Petersen A.K., Saxena R., Service S.K., Sidore C., Surakka I., Van den Herik E.G., Volcik K.A., Asiki G., Been L.F., Bolton J.L., Bonnycastle L.L., Burnett M.S., Cesana G., Elliott P., Eyjolfsson G.I., Goodarzi M.O., Gravito M.L., Hartikainen A.L., Hung Y.J., Jones M.R., Kaleebu P., Kastelein J.J., Kim E., Komulainen P., Lin S.Y., Nieminen T.V., Nsubuga R.N., Olafsson I., Palotie A., Papamarkou T., Pomilla C., Pouta A., Ruokonen A., Seeley J., Silander K., Tiret L., van Pelt L., Wainwright N., Wijmenga C., Willemsen G., Young E.H., Bennett F., Boomsma D.I., Bovet P., Chen Y.D., Feranil A.B., Freimer N.B., Hsiung C.A., Kesäniemi A., Koudstaal P.J., Krauss R.M., Kyvik K.O., Martin N.G., Meneton P., Moilanen L., Price J.F., Sanghera D.K., Sheu W.H., Whitfield J.B., Wolffenbuttel B.H., Ordovas J.M., Rich S.S., Johnson A., Johnson L., Larson M., Levy D., Newton-Cheh C., O’reilly P., Palmas W., Rice K., Smith A., Snider H., Tobin M., Verwoert G., Rice K.M., Verwoert G.C., Pihur V., Heath S., Sõber S., Arora P., Zhang F., Lucas G., Milaneschi Y., Parker A.N., Fava C., Fox E.R., Go M.J., Sjögren M., Vinay D., Alexander M., Tabara Y., Shaw-Hawkins S., Whincup P.H., Shi G., Seielstad M., Sim X., Nguyen K.D., Matullo G., Gaunt T.R., Onland-Moret N.C., Cooper M.N., Platou C.G., Org E., Hardy R., Dahgam S., Palmen J., Kuznetsova T., Uiterwaal C.S., Adeyemo A., Ludwig B., Tomaszewski M., Tzoulaki I., Palmer N.D., Chang Y.P., Steinle N.I., Grobbee D.E., Morrison A.C., Najjar S., Hadley D., Connell J.M., Day I.N., Lawlor D.A., Lawrence R.W., Ongen H., Li Y., Young J.H., Bis J.C., Bolton J.A., Chaturvedi N., Islam M., Jafar T.H., Kulkarni S.R., Howard P., Guarrera S., Ricceri F., Emilsson V., Plump A., Weder A.B., Sun Y.V., Scott L.J., Peltonen L., Vartiainen E., Brand S.M., Wang T.J., Burton P.R., Artigas M.S., Dong Y., Wang X., Zhu H., Rudock M.E., Heckbert S.R., Smith N.L., Wiggins K.L., Doumatey A., Shriner D., Veldre G., Viigimaa M., Kinra S., Prabhakaran D., Tripathy V., Langefeld C.D., Rosengren A., Thelle D.S., Corsi A.M., Singleton A., Hilton G., Salako T., Iwai N., Kita Y., Ogihara T., Ohkubo T., Okamura T., Ueshima H., Umemura S., Eyheramendy S., Meitinger T., Cho Y.S., Kim H.L., Scott J., Sehmi J.S., Hedblad B., Nilsson P., Stanèáková A., Raffel L.J., Yao J., Schwartz S.M., Ikram M., Longstreth W., Mosley T.H., Seshadri S., Shrine N.R., Wain L.V., Morken M.A., Laitinen J., Zitting P., Cooper J.A., van Gilst W.H., Janipalli C.S., Mani K., Yajnik C.S., Mattace-Raso F.U., Lakatta E.G., Orru M., Scuteri A., Ala-Korpela M., Kangas A.J., Soininen P., Tukiainen T., Würtz P., Ong R.T., Galan P., Hercberg S., Lathrop M., Zelenika D., Zhai G., Meschia J.F., Sharma P., Terzic J., Kumar M., Denniff M., Zukowska-Szczechowska E., Wagenknecht L.E., Fowkes F., Charchar F.J., Rotimi C., Bots M.L., Brand E., Talmud P.J., Nyberg F., Laan M., van der Schouw Y.T., Casas J.P., Vineis P., Ganesh S.K., Wong T.Y., Tai E.S., Morris R.W., Dominiczak A.F., Marmot M.G., Miki T., Chandak G.R., Zhu X., Elosua R., Soranzo N., Sijbrands E.J., Uda M., Vasan R.S., Anderson C.A., Gordon S., Guo Q., Henders A., Lambert A., Kraft P., Kennedy S.H., Macgregor S., Missmer S.A., Painter J.N., Roseman F., Treloar S.A., Wallace L., Alizadeh B., de Boer R.A., Boezen H.M., van der Klauw M.M., Ormel J., Postma D.S., Rosmalen J.G., Slaets J.P., Lagou V., Welch R.P., Wheeler E., Rehnberg E., Lecoeur C., Johnson P.C., Hottenga J.J., Salo P., Bielak L.F., Zhao W., Horikoshi M., Navarro P., Chen H., Rybin D., Song K., An P., Marullo L., Jansen H., Edkins S., Varga T.V., Oksa H., Antonella M., Kong A., Herder C., Antti J., Miljkovic I., Atalay M., Kiess W., Smit J.H., Campbell S., Fowkes G.R., Rathmann W., Maerz W., Watanabe R.M., Toenjes A., Peyser P.A., Körner A., Cucca F., Balkau B., Bouatia-Naji N., Ahmadi K.R., Ainali C., Bataille V., Bell J.T., Buil A., Dermitzakis E.T., Dimas A.S., Durbin R., Glass D., Hassanali N., Ingle C., Knowles D., Krestyaninova M., Lowe C.E., Meduri E., Di Meglio P., Montgomery S.B., Nestle F.O., Nica A.C., Nisbet J., O’rahilly S., Parts L., Potter S., Sekowska M., Shin S.Y., Surdulescu G., Travers M.E., Tsaprouni L., Tsoka S., Wilk A., Higashio J., Williams R., Nato A., Ambite J.L., Manolio T., Hindorff L., Heiss G., Taylor K., Avery C., Graff M., Lin D., Quibrera M., Cochran B., Kao L., Umans J., Cole S., Maccluer J., Person S., Gross M., Fornage M., Durda P., Jenny N., Patsy B., Arnold A.M., Buzkova P., Haines J., Murdock D., Glenn K., Brown-Gentry K., Thornton-Wells T., Dumitrescu L., Jeff J., Bush W.S., Mitchell S.L., Goodloe R., Boston J., Malinowski J., Restrepo N., Oetjens M., Fowke J., Zheng W., Spencer K., Pendergrass S., Wilkens L., Park L., Tiirikainen M., Kolonel L., Lim U., Cheng I., Wang H., Shohet R., Stram D., Henderson B., Monroe K., Schumacher F., Anderson G., Carlson C., Prentice R., Wu C., Carty C., Gong J., Rosse S., Young A., Haessler J., Kocarnik J., Lin Y., Duggan D., Kuller L., He C., Sulem P., Barbalic M., Broer L., Byrne E.M., Gudbjartsson D.F., McArdle P.F., Porcu E., van Wingerden S., Zhuang W., Lauc L.B., Broekmans F.J., Burri A., Chanock S.J., Chen C., Corre T., Coviello A.D., D’adamo P., Davies G., Deary I.J., Ebrahim S., Fauser B.C., Ferreli L., Folsom A.R., Hall P., Hankinson S.E., Hass M., Heath A.C., Janssens A.C., Keyzer J., Lahti J., Lai S., Laisk T., Laven J.S., Liu J., Lopez L.M., Louwers Y.V., Marongiu M., Klaric I.M., Masciullo C., Medland S.E., Melzer D., Newman A.B., Paré G., Peeters P.H., Pop V.J., Räikkönen K., Salumets A., Smith J.A., Stacey S.N., Starr J.M., Stathopoulou M.G., Tenesa A., Tryggvadottir L., Tsui K., van Dam R.M., van Gils C.H., van Nierop P., Vink J.M., Voorhuis M., Wallaschofski H., Widen E., Wijnands-Van Gent C.J., Zgaga L., Zygmunt M., Buring J.E., Crisponi L., Demerath E.W., Murray A., Visser J.A., Lunetta K.L., Elks C.E., Cousminer D.L., Feenstra B., Lin P., Smith E.N., Warrington N.M., Alavere H., Berenson G.S., Blackburn H., Busonero F., Chen W., Easton D.F., Foroud T., Geller F., Kilpeläinen T.O., Li S., Melbye M., Murray J.C., Murray S.S., Ness A.R., Northstone K., Pennell C.E., Pharoah P., Rafnar T., Rice J.P., Ring S.M., Schork N.J., Segrè A.V., Sovio U., Srinivasan S.R., Tammesoo M.L., Tyrer J., Weedon M.N., Young L., Bierut L.J., Boyd H.A. New genetic loci link adipose and insulin biology to body fat distribution. Nature. 2015;518:187–196. doi: 10.1038/nature14132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silventoinen K., Jelenkovic A., Sund R., Hur Y.M., Yokoyama Y., Honda C., Hjelmborg J.B., Moller S., Ooki S., Aaltonen S., Ji F., Ning F., Pang Z., Rebato E., Busjahn A., Kandler C., Saudino K.J., Jang K.L., Cozen W., Hwang A.E., MacK T.M., Gao W., Yu C., Li L., Corley R.P., Huibregtse B.M., Christensen K., Skytthe A., Kyvik K.O., Derom C.A., Vlietinck R.F., Loos R.J., Heikkila K., Wardle J., Llewellyn C.H., Fisher A., McAdams T.A., Eley T.C., Gregory A.M., He M., Ding X., Bjerregaard-Andersen M., Beck-Nielsen H., Sodemann M., Tarnoki A.D., Tarnoki D.L., Stazi M.A., Fagnani C., D'Ippolito C., Knafo-Noam A., Mankuta D., Abramson L., Burt S.A., Klump K.L., Silberg J.L., Eaves L.J., Maes H.H., Krueger R.F., McGue M., Pahlen S., Gatz M., Butler D.A., Bartels M., Van Beijsterveldt T.C.E.M., Craig J.M., Saffery R., Freitas D.L., Maia J.A., Dubois L., Boivin M., Brendgen M., Dionne G., Vitaro F., Martin N.G., Medland S.E., Montgomery G.W., Chong Y., Swan G.E., Krasnow R., Magnusson P.K.E., Pedersen N.L., Tynelius P., Lichtenstein P., Haworth C.M.A., Plomin R., Bayasgalan G., Narandalai D., Harden K.P., Tucker-Drob E.M., Oncel S.Y., Aliev F., Spector T., Mangino M., Lachance G., Baker L.A., Tuvblad C., Duncan G.E., Buchwald D., Willemsen G., Rasmussen F., Jack H.G., Sørensen T.I., Boomsma D.I., Kaprio J. Genetic and environmental effects on body mass index from infancy to the onset of adulthood: an individual-based pooled analysis of 45 twin cohorts participating in the COllaborative project of Development of Anthropometrical measures in Twins (CODATwins) Am. J. Clin. Nutr. 2016;104:371–379. doi: 10.3945/ajcn.116.130252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon Z. PTSD and social functioning - a three year prospective study. Soc. Psychiatry Psychiatr. Epidemiol. 1989;24:127–133. doi: 10.1007/BF01788021. [DOI] [PubMed] [Google Scholar]
- Solomon Z., Tsur N., Levin Y., Uziel O., Lahav M., Ohry A. The implications of war captivity and long-term psychopathology trajectories for telomere length. Psychoneuroendocrinology. 2017;81:122–128. doi: 10.1016/j.psyneuen.2017.04.004. [DOI] [PubMed] [Google Scholar]
- Speed M.S., Jefsen O.H., Børglum A.D., Speed D., Østergaard S.D. Investigating the association between body fat and depression via Mendelian randomization. Transl. Psychiatry. 2019;9 doi: 10.1038/s41398-019-0516-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein M.B., Jang K.L., Taylor S., Vernon P.A., Livesley W.J. Genetic and environmental influences on trauma exposure and posttraumatic stress disorder symptoms: a twin study. Am. J. Psychiatry. 2002;159:1675–1681. doi: 10.1176/appi.ajp.159.10.1675. [DOI] [PubMed] [Google Scholar]
- Stein M.B., Levey D.F., Cheng Z., Wendt F.R., Harrington K., Pathak G.A., Cho K., Quaden R., Radhakrishnan K., Girgenti M.J., Ho Y.L.A., Posner D., Aslan M., Duman R.S., Zhao H., Stein M.B., Levey D.F., Cheng Z., Wendt F.R., Pathak G.A., Radhakrishnan K., Aslan M., Zhao H., Polimanti R., Concato J., Gelernter J., Stein M.B., Levey D.F., Cheng Z., Wendt F.R., Harrington K., Pathak G.A., Cho K., Quaden R., Ho Y.L.A., Posner D., Polimanti R., Concato J., Gelernter J., Polimanti R., Concato J., Gelernter J. Genome-wide association analyses of post-traumatic stress disorder and its symptom subdomains in the Million Veteran Program. Nat. Genet. 2021;53:174–184. doi: 10.1038/s41588-020-00767-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suliman S., Anthonissen L., Carr J., du Plessis S., Emsley R., Hemmings S.M.J., Lochner C., McGregor N., van den Heuvel L., Seedat S. Posttraumatic stress disorder, overweight, and obesity: a systematic review and meta-analysis. Harv. Rev. Psychiatry. 2016 doi: 10.1097/HRP.0000000000000106. [DOI] [PubMed] [Google Scholar]
- Tambs K., Czajkowsky N., Røysamb E., Neale M.C., Reichbom-Kjennerud T., Aggen S.H., Harris J.R., Ørstavik R.E., Kendler K.S. Structure of genetic and environmental risk factors for dimensional representations of DSM-IV anxiety disorders. Br. J. Psychiatry. 2009;195:301–307. doi: 10.1192/bjp.bp.108.059485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamman A.J.F., Wendt F.R., Pathak G.A., Krystal J.H., Montalvo-Ortiz J.L., Southwick S.M., Sippel L.M., Gelernter J., Polimanti R., Pietrzak R.H. Attachment style moderates polygenic risk for posttraumatic stress in United States military veterans: results from the national health and resilience in veterans study. Biol. Psychiatry. 2021;89:878–887. doi: 10.1016/j.biopsych.2020.09.018. [DOI] [PubMed] [Google Scholar]
- Taylor S.E. Mechanisms linking early life stress to adult health outcomes. Proc. Natl. Acad. Sci. U. S. A. 2010 doi: 10.1073/pnas.1003890107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolin D.F., Foa E.B. Sex differences in trauma and posttraumatic stress disorder: a quantitative review of 25 years of research. Psychol. Bull. 2006;132:959–992. doi: 10.1037/0033-2909.132.6.959. [DOI] [PubMed] [Google Scholar]
- True W.R., Rice J., Eisen S.A., Heath A.C., Goldberg J., Lyons M.J., Nowak J. A twin study of genetic and environmental contributions to liability for posttraumatic stress symptoms. Arch. Gen. Psychiatry. 1993;50:257–264. doi: 10.1001/archpsyc.1993.01820160019002. [DOI] [PubMed] [Google Scholar]
- Turner H.A., Finkelhor D., Ormrod R., Hamby S., Leeb R.T., Mercy J.A., Holt M. Family context, victimization, and child trauma symptoms: variations in safe, stable, and nurturing relationships during early and middle childhood. Am. J. Orthopsychiatry. 2012;82:209–219. doi: 10.1111/j.1939-0025.2012.01147.x. [DOI] [PubMed] [Google Scholar]
- Tyrrell J., Jones S.E., Beaumont R., Astley C.M., Lovell R., Yaghootkar H., Tuke M., Ruth K.S., Freathy R.M., Hirschhorn J.N., Wood A.R., Murray A., Weedon M.N., Frayling T.M. Height, body mass index, and socioeconomic status: mendelian randomisation study in UK Biobank. BMJ. 2016;352 doi: 10.1136/bmj.i582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rheenen W., Peyrot W.J., Schork A.J., Lee S.H., Wray N.R. Genetic correlations of polygenic disease traits: from theory to practice. Nat. Rev. Genet. 2019 doi: 10.1038/s41576-019-0137-z. [DOI] [PubMed] [Google Scholar]
- Verbanck M., Chen C.Y., Neale B., Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 2018;50:693–698. doi: 10.1038/s41588-018-0099-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieweg W.V.R., Julius D.A., Fernandez A., Tassone D.M., Narla S.N., Pandurangi A.K. Posttraumatic stress disorder in male military veterans with comorbid overweight and obesity: psychotropic, antihypertensive, and metabolic medications. Prim. Care Companion J. Clin. Psychiatry. 2006 doi: 10.4088/pcc.v08n0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendt Carvalho, Pathak, Gelernter, Polimanti Deciphering the biological mechanisms underlying the genome-wide associations between computerized device use and psychiatric disorders. J. Clin. Med. 2019;8:2040. doi: 10.3390/jcm8122040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendt F.R., Pathak G.A., Lencz T., Krystal J.H., Gelernter J., Polimanti R. Dissecting the genetic overlap of education, socioeconomic status, and mental health. medRxiv. 2020 doi: 10.1101/2020.01.09.20017079. 2020.01.09.20017079. [DOI] [Google Scholar]
- Wichers M., Schrijvers D., Geschwind N., Jacobs N., Myin-Germeys I., Thiery E., Derom C., Sabbe B., Peeters F., Delespaul P., Van Os J. Mechanisms of gene-environment interactions in depression: evidence that genes potentiate multiple sources of adversity. Psychol. Med. 2009;39:1077–1086. doi: 10.1017/S0033291708004388. [DOI] [PubMed] [Google Scholar]
- Wolf E.J., Miller D.R., Logue M.W., Sumner J., Stoop T.B., Leritz E.C., Hayes J.P., Stone A., Schichman S.A., McGlinchey R.E., Milberg W.P., Miller M.W. Contributions of polygenic risk for obesity to PTSD-related metabolic syndrome and cortical thickness. Brain. Behav. Immun. 2017;65:328–336. doi: 10.1016/j.bbi.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xian H., Chantarujikapong S.I., Scherrer J.F., Eisen S.A., Lyons M.J., Goldberg J., Tsuang M., True W.R. Genetic and environmental influences on posttraumatic stress disorder, alcohol and drug dependence in twin pairs. Drug Alcohol Depend. 2000;61:95–102. doi: 10.1016/S0376-8716(00)00127-7. [DOI] [PubMed] [Google Scholar]
- Yang J., Zeng J., Goddard M.E., Wray N.R., Visscher P.M. Concepts, estimation and interpretation of SNP-based heritability. Nat. Genet. 2017;49:1304–1310. doi: 10.1038/ng.3941. [DOI] [PubMed] [Google Scholar]
- Zhao Q., Wang J., Hemani G., Bowden J., Small D.S. Statistical inference in two-sample summary-data Mendelian randomization using robust adjusted profile score. Ann. Stat. 2020;48:1742–1769. doi: 10.1214/19-AOS1866. [DOI] [Google Scholar]
- Zheng J., Baird D., Borges M.-C., Bowden J., Hemani G., Haycock P., Evans D.M., Smith G.D. Recent developments in mendelian randomization studies. Curr. Epidemiol. Reports. 2017;4:330–345. doi: 10.1007/s40471-017-0128-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J., Haberland V., Baird D., Walker V., Haycock P.C., Hurle M.R., Gutteridge A., Erola P., Liu Y., Luo S., Robinson J., Richardson T.G., Staley J.R., Elsworth B., Burgess S., Sun B.B., Danesh J., Runz H., Maranville J.C., Martin H.M., Yarmolinsky J., Laurin C., Holmes M.V., Liu J.Z., Estrada K., Santos R., McCarthy L., Waterworth D., Nelson M.R., Smith G.D., Butterworth A.S., Hemani G., Scott R.A., Gaunt T.R. Phenome-wide Mendelian randomization mapping the influence of the plasma proteome on complex diseases. Nat. Genet. 2020;52:1122–1131. doi: 10.1038/s41588-020-0682-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data discussed in this study are provided in the article and in the Supplementary Material.