Abstract
A good point-of-care diagnostic test holds a promise to reduce inappropriate use of antibiotics by enabling early detection of the pathogen and facilitating rapid testing of antimicrobial susceptibility. India has taken many initiatives in the recent past to augment the development and deployment of diagnostics in Indian health care system. Funding opportunities to promote innovation in diagnostics development were started in early 2000s through various ministries and departments. India released National Essential Diagnostics List which enlists essential tests and there is now Free Diagnostics Service Initiative of Government of India under National Health Mission that mandates to provide all essential tests free of cost. We wanted to understand how these initiatives have impacted the diagnostics that could be of use in containment of antimicrobial resistance (AMR) and whether there is a smooth process for bringing indigenously developed products relevant to AMR into the healthcare system. We conducted a longitudinal survey (January 2019 and January 2021) to understand the availability of market ready indigenous rapid diagnostics for AMR in the country and their progress towards introduction in the private market or uptake in healthcare system. We found that many innovators and developers are working towards development of rapid tests that can be useful in the containment of AMR in India. While there are many promising diagnostics on the horizon, the pathway for uptake of indigenously developed diagnostics in healthcare system remains disjointed and needs to be harmonised for the investments made towards development to translate as tangible gains. Since most of these efforts are government funded, it is incumbent upon the government to also provide a seamless pathway to make these diagnostics available in health care system. In absence of this guidance, most of these diagnostics will sit with the innovators/developers and will never be used for the purpose they were intended to serve.
Keywords: public health, health systems, medical microbiology, diagnostics and tools
Summary box.
Rapid diagnostics for antimicrobial resistance (AMR) have enormous potential to support adoption of diagnostic stewardship in settings with restrained healthcare resources.
Huge efforts and investments have been made to develop rapid point-of-care diagnostics that can be effective in containment of AMR in India.
These efforts have not yet translated into ready to use AMR diagnostic products in the private market or government funded free diagnostics initiative.
We propose a three-step approach to expedite the availability of the AMR diagnostics in the healthcare system, (1) creating country-specific target product profiles for priority syndromes (2) creation of standard protocols for validation and systematic evaluation of tests and (3) a synchronised process to overcome the bottlenecks and facilitate expedited market introduction and clinical uptake.
Background: why are rapid diagnostic tests important for containment of antimicrobial resistance?
Antimicrobial resistance (AMR) has been recognised as a global public health emergency that is compromising the gains made towards control of infectious diseases.1–3 Rampant use and misuse of antimicrobials is one of the major drivers of AMR.4–6 Conventionally, diagnosis and susceptibility testing for bacterial pathogens depends on the culture, biochemical identification and diffusion or dilution methods of susceptibility testing, which is time consuming and leads to long turnaround time (TAT). The rapid point-of-care (POC) diagnostics for AMR have the potential to revolutionise the detection and treatment of bacterial infections1 7 8 and can be instrumental in preserving the efficacy of currently available antimicrobials by limiting unnecessary prescription and misuse of antimicrobials.7 9 The rapid diagnostics can be especially useful in secondary level hospitals in India, and below, as most of these hospitals do not have necessary infrastructure and human resources to support pathogen identification and antimicrobial susceptibility testing (AMST).10–12
Over the last decade, several tests using latest molecular techniques have been developed which identify the microorganism(s) and detect the presence or absence of genes(s) or gene mutations for resistance to antimicrobials.13 14 While they are helpful in expediting the diagnosis of infections, these tests are expensive and need specially trained staff to interpret the results, thus limiting their use only to the well-resourced hospitals. In low-income and middle-income countries (LMICs) like India, these imported tests even when available in private market are not used widely owing to their steep prices and stringent infrastructure and human resource requirements.12 15 Indigenously developed low cost diagnostics to contain AMR are, therefore, urgently needed to fill the diagnostic gap. Past decade saw several initiatives such as the Longitude Prize (UK), Horizon 2020 (European Commission) and AMR Diagnostic Challenge (USA) being launched globally, to stimulate the development of rapid tests for containment of AMR. In India too, government research funding bodies as well as many private enterprises funded development of diagnostics that can facilitate timely detection of infections relevant to AMR.
Typically, a diagnostic once developed undergoes systematic validation in laboratory for accuracy of analytical parameters (figure 1). If found satisfactory, it is approved by Indian regulator, that is, Drugs Controller General of India (DCGI), for market introduction. For diagnostics approved by the Indian regulator and evaluated through field demonstration studies for clinical usefulness, evidence of scalability and cost effectiveness, Government of India has created mechanisms like National Healthcare Innovations Portal (NHInP) (https://www.nhinp.org/index.php) and Health Technology Assessment in India (HTAIn) (https://htain.icmr.org.in/) to provide a framework for objective assessment and expedite their uptake in health system (figure 1). India also has a National Essential Diagnostics list (NEDL) and Free Diagnostics Service Initiative (FDI) to make diagnostics affordable and accessible to all populations and reduce out-of-pocket expenditure on diagnostics.16 17 The framework currently available in the country can augment deployment and uptake of AMR diagnostics tests by including them in NEDL and government-funded health programmes like FDI of National Health Mission.
Figure 1.
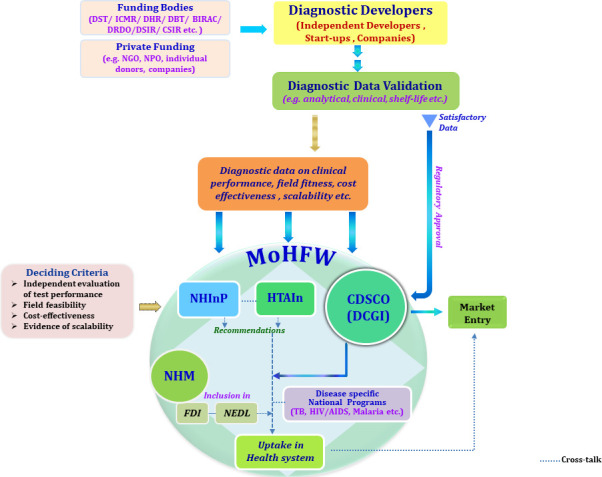
Framework and process flow illustrating the pathways of a new diagnostic development, its evaluation and uptake in health system of India. BIRAC, Biotechnology Industry Research Assistance Council; CDSCO, Central Drugs Standard Control Organisation; CSIR, Council of Scientific and Industrial Research; DBT, Department of Biotechnology; DCGI: Drugs Controller General of India; DHR, Department of Health and Research; DRDO, Defence Research and Development Organisation; DSIR, Department of Scientific and Industrial Research; DST, Department of Science and Technology; FD, Free Diagnostics Service Initiative; HTAIn, Health Technology Assessment in India; ICMR, Indian Council of Medical Research; MoHFW, Ministry of Health and Family Welfare; NEDL, National Essential Diagnostics list; NGO, non-government organisations; NHInP, National Health Innovation Portal; NHM, National Health Mission; NPO, non-profit organisations; TB, tuberculosis.
However, as of date both the NEDL and FDI recommend only culture and sensitivity for detection of bacterial infections at district hospital. The rapid diagnostics developed through any of the ongoing indigenous initiatives, for pathogen identification and AMST are yet to find their place in NEDL or FDI.16 17
This manuscript attempts to provide a landscape of Indian innovations in diagnostics that have potential to be useful for containment of AMR, the factors impeding their utilisation in healthcare system in the country and also suggests solutions to overcome these challenges.
Indian innovations in rapid diagnostics for AMR containment
We undertook web search using the search words ‘antimicrobial resistance, antibiotics rapid-test, indigenous, diagnostics, point-of-care test, device, instrument, kit, alternative, development, innovator, developer, India, pathogen identification and antimicrobial susceptibility’ to map the indigenously developed rapid AMR diagnostics in the country. We looked for Indian innovators/developers who have developed diagnostic test(s) that addresses pathogen identification and AMST. Diagnostics in early phases of development that is, at ideation stage, in research phase or at demonstration of proof of concept and were at Technology Readiness Level (TRL) 3 or below as per the Biotechnology Industry Research Assistance Council, TRL for medical devices including diagnostic devices18 were excluded from the analysis. Only the diagnostics which had crossed proof-of-concept stage and were available as market ready-products, that is, at TRL 4 or above were included in this survey for further analysis.
To understand the quality of diagnostics developed and assess their readiness to be available for actual use in healthcare system, we collected information through two longitudinal surveys undertaken in January 2019 (survey I) and January 2021 (survey II). Questionnaires designed to cover the process of diagnostic development and critical aspects of the test were shared with the developers. Survey I, (online supplemental file 1) collected information on characteristics of developed AMR diagnostic test, the type of technology used and novelty, stage of development, strength and weaknesses of the test and its intended use. Information was also sought on type of specimen used, pathogen(s) targeted, antibiotics panels used, hands-on-time per unit test, TAT, analytical parameters tested and stability of the test. To gauge the extent of analytical validation undertaken by the developer/innovator, information was sought on the number of samples tested, gold standard and quality controls used for evaluation of test. We also sought information on target product profile (TPP) followed, time and capital invested on diagnostic development to understand development process. Second survey (online supplemental file 2) sought information about any modification or improvement made in the diagnostic test since survey I, additional analytical or performance parameters analysed and whether any cost-effectiveness studies were undertaken. The innovators were given an option to withhold any confidential information.
bmjgh-2021-006628supp001.pdf (73.2KB, pdf)
A total of 16 indigenous diagnostics were identified through web search. Out of 16, 5 were under development at the time of survey (ie, at TRL 3 or below) and 8 diagnostics were fully developed (ie, at TRL 4 or above). For three rapid diagnostics, definitive information on the stage of development could not be ascertained based on information provided by the innovator hence they were dropped from the survey. Eight developers were contacted to share the information about their diagnostic but only seven developers (four public sector and three private sector) responded to our survey. One developer shared information on two different diagnostics. The characteristics, performance and analytical parameters of these eight indigenous diagnostics (Dx) are detailed in table 1.
Table 1.
Characteristics of indigenously developed rapid diagnostics (Dx) for pathogen identification and antimicrobial susceptibility
| Characteristics | Diagnostic (Dx) | |||||||
| Dx 1 | Dx 2 | Dx 3 | Dx 4 | Dx 5 | Dx 6 | Dx 7 | Dx 8 | |
| Syndrome targeted | Sepsis (bacteraemia detection) |
Sepsis | Bacteraemia | Urinary tract infection (UTI) | Identification of Pathogen and resistance markers | Urinary Tract Infection | Pulmonary and pleural tuberculosis (TB), TB meningitis | TB |
| Level of healthcare (HC) targeted | Primary HC, general practitioner, hospitals, | Primary HC, general practitioner, | Primary HC, general practitioner, | Primary HC, general practitioner, Hospitals |
Primary HC, general practitioner, | Primary HC, general practitioner, | Primary HC, general practitioner, | Microscopy technicians in the DOTS centre |
| Sample type used | Plasma, serum | Saliva | Blood | Urine | Blood, urine | Urine | Cerebrospinal fluid, pleural fluid and sputum | Sputum |
| Pathogen targeted | Gram-positive and gram-negative bacteria | NA (detect biomarkers) | Salmonella Typhi | Escherichia coli, Klebsiella, Enterococcus, Staphylococcus, Pseudomonas, Proteus | Gram negative: Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Acinetobacter baumannii Gram positive: Enterococcus spp, Staphylococcus aureus |
Escherichia coli, Klebsiella, Pseudomonas aeruginosa, Enterococci |
NA (detect antigens) | Mycobacterium tuberculosis (MTB) |
| Antibiotics or genes tested | Nil | NA | Cephalosporin Piperacillin Imipenem Cefotaxime Ceftriaxone |
A customisable and prefunctionalised panel of 42 antibiotics; | A customisable panel of resistance markers: NDM, KPC, VIM, IMP, OXA-48 family, OXA-58, OXA-23; | Nil | Nil | Nil |
| Output type | Colorimetric; semiquantitative | Quantitative | Qualitative and quantitative | Colorimetric and nephlometric | Qualitative | Qualitative | Qualitative and quantitative | Qualitative |
| Hands-on-time | 10–15 min | 3 min | 5 min | 20 min | ~10 min | 2–3 min | No information shared by developer | 15 min |
| Turnaround time (TAT) | 5 min | 2 min | 5 hours for bacterial identification and 2 hours for AST | 4 hours for105 bacterial per mL | 2–3 hours | 20 min | 5 hours | 1 hour |
| Control/ reference method used | White Blood Cells count; procalcitonin, culture, ELISA | Quantitative blood CRP | Blood culture and VITEK | Kirby Bauer VITEK |
Culture method | Culture method; Urine dipsticks |
No information shared by developer | GeneXpert, MGIT liquid culture; |
| Clinical validation of test* | Yes, 150 clinical samples |
Yes, 100 samples |
No | Yes, 2324 samples |
Yes, 300 patients |
Yes, 500 patients |
No | Yes, tested at a DOTS centre but details of sample size not provided |
| Strength | Rapid detection of gram positive and gram negatives in sepsis | Potential of good clinical utility for rapid diagnosis of neonatal sepsis, and use of low sample volume (~40 µL) | Potential of good clinical utility for rapid diagnosis of bacteraemia (eg, Salmonella) | Rapid quantitative detection of UTI pathogens, ready-to-use antibiogram, No need for accessory equipment |
Clinically promising for rapid detection of bacteraemia, UTI and sepsis, and culture free identification of common bacterial pathogens |
Rapid and point of care test to detect four common uropathogens in a single test | Rapid qualitative and quantitative detection of TB antigen, pulmonary and pleural TB and TB meningitis | Useful in converting a bright field microscope into a fluorescence microscope which can improve the outcome of smear-based microscopic analysis, and promising for resource limited settings |
| Weakness† | Does not test antibacterial sensitivity or detect resistant genes, Does not detect fungal pathogens causing sepsis like Candida |
Few aspects such as effect of milk contamination, dehydration, etc need to be addressed | Does not detect resistant genes, Targets only bacteria, Field feasibility in hospital wards or non-laboratory locations need to be ascertained |
Does not detect priority pathogens like Candida, Enterobacter, Acinetobacter, Low probability of not detecting pathogen if bacterial load is less than 102 cells/mL |
Does not detect Candida, Streptococcus pneumoniae | Does not test antibacterial sensitivity or detect resistant genes, Does not detect Candida |
Does not detect AST, Not suitable for the detection of TB from tissue samples, May not detect infections of Mycobacterium other than TB |
High TAT, Does not differentiate non-TB mycobacteria from MTB |
*For understanding the extent of validation of diagnostic undertaken by developer, details were sought on samples tested and population, control/reference method used and analytical parameters that had been evaluated. Details of analytical parameters that have been evaluated was sought qualitatively as ‘yes’ or ‘no’ to ensure the data confidentiality.
†All diagnostic tests need to undergo field feasibility and cost-effectiveness studies.
AST, antibiotic sensitivity testing; CRP, C reactive protein; DOTS, directly observed therapy, short course; IMP, imipenemase; KPC, Klebsiella pneumoniae carbapenemase; LoD, limit of detection; MGIT, mycobacterial growth indicator tube; NA, not applicable; NDM, New Delhi metallo-β-lactamase; NPV, negative predictive value; OXA, oxacillinase; PPV, positive predictive value; VIM, Verona- intergon-encoded metallo-β-lactamase.
Test characteristics and evaluation
Eight diagnostics chosen for analysis dealt with the rapid identification of bacteria for conditions such as bacteraemia (n=1), sepsis (n=2), tuberculosis (n=2) and urinary tract infection (UTI) (n=3) (table 1). Six out of eight diagnostics were developed with the financial support from the government organisations and three of these also had other sponsors. All diagnostics were instrument-based tests except one (Dx 1) which was an instrument-free disposable kit. The instrument-based diagnostics did not have any auxiliary need of equipment such as air conditioner, centrifuge, incubator, laminar-flow, etc or a laboratory. Five rapid diagnostics used non-invasive samples (saliva, sputum and urine) for testing of targeted pathogen or biomarker of interest. Only three diagnostics offered rapid testing of antibiotic susceptibility or detection of resistance markers directly from the samples. The hands-on time per unit test ranged from 2 min to 15 min. Three of the rapid diagnostic tests could process more than 100 samples in a single batch. The TAT for these rapid indigenous diagnostics varied from 2 min to 5 hours for bacterial identification and 2–7 hours for AMST. Four out of eight tests also provided quantitative assessment. Different developers had used different gold standards for evaluating analytical parameters of diagnostics. Blood culture, Kirby Bauer, GeneXpert, VITEK, etc were used as gold standards. Three developers had obtained accreditation of their tests, either in the form of TM (Trademark) (Dx1 and Dx 6) or CE (European conformity) approval (Dx 4) but none had Indian regulator’s (DCGI) approval.
During survey I, it was observed that half of the diagnostics were not evaluated for the stability or shelf life and none of the diagnostics had undergone independent/third party evaluation. One innovator (Dx2) cited lack of facilities/labs to undertake third party evaluation for saliva based test in India. All the diagnostics lacked evidence of scalability and none had undergone cost-effectiveness studies (table 2). Most of these diagnostics were developed with feedback from microbiologists and clinicians. At the time of survey II, two diagnostics (Dx 2 and Dx 5) had undergone modification to further improve the test performance and, two diagnostics (Dx 6 and Dx 8) had undertaken the stability studies. Four diagnostics had undertaken evaluation for the scalability and two developers had developed the price per device calculation for diagnostic based on the cost of raw materials used in test development (table 2). However, a cost-effectiveness analysis had not been performed for any of the diagnostic. It is important to mention that none of the diagnostic had reached the market or had been taken up by healthcare system within this time span of 2 years.
Table 2.
Validation of indigenously developed diagnostics
| Dx | Syndrome targeted | Survey* | Analytical/performance parameter tested | Stability of product tested | Evidence on scalability | Evaluation of cost effectiveness | Technology readiness level (TRL)† |
| 1 | Sepsis | I | Sensitivity, LoD, LoQ, PPV, specificity, NPV, accuracy | No | No | No | TRL 4 |
| II | No additional parameter tested | No | No | No | TRL 4 | ||
| 2 | Sepsis | I | Sensitivity, LoQ, specificity, NPV, PPV, accuracy, reproducibility | Yes, 6 months at room temperature | No | No | TRL 5 |
| II |
|
Same as in survey I | Yes | No | TRL 7 | ||
| 3 | Bacteraemia | I | Sensitivity, LoD | No | No | No | TRL 4 |
| II | No additional parameter tested | No | No | No‡ | TRL 4 | ||
| 4 | Urinary tract infection | I | Sensitivity, LoD, LoQ, PPV, specificity, NPV, accuracy, linearity, reproducibility | Yes, 1 year at 4°C | No | No | TRL 7 |
| II | No additional parameter tested | Same as in survey I | Yes | No‡ | TRL 8 | ||
| 5 | Identification of Pathogen and resistance markers | I | No information provided | Yes, 1 month | No | No | TRL 4 |
| II | Calorimetric format developed | Same as in survey I | No | No‡ | TRL 6 | ||
| 6 | Urinary tract infection | I | Sensitivity, LoD, reproducibility | No | No | No | TRL 5 |
| II | No additional parameter tested | Yes, details not provided | No | No | TRL 6 | ||
| 7 | Pulmonary, pleural tuberculosis (TB), TB meningitis | I | Sensitivity, LoD, PPV, NPV, specificity, accuracy, linearity, reproducibility | Yes, 6 months | No | No | TRL 5 |
| II | Details not provided | Same as in survey I | Yes | No | TRL 6 | ||
| 8 | TB | I | Sensitivity, LoD, NPV, specificity, reproducibility | Yes, | No | No | TRL 6 |
| II |
|
Same as in survey I | Yes | No‡ | TRL 7 |
Green colour box indicates the progress made in particular characteristic in survey II.
*Two surveys were conducted in January 2019 (survey I) and in January 2021 (survey II) to collect the data.
†TRL has been refereed as per the criteria mentioned by Biotechnology Industry Research Assistance Council, Department of Biotechnology, Government of India for medical devices including diagnostic devices and for in vitro diagnostic kits and reagents.18 Available from https://www.birac.nic.in/webcontent/birac_trl_doc5_medical_devices_and_diagnosis_12_09_2018.pdf.
‡The cost per test has been estimated for the diagnostic based on the cost of raw materials but cost-effectiveness study was not performed.
LoD, limit of detection; LoQ, limit of quantitation; NPV, negative predictive value; PPV, positive predictive value.
Understanding challenges in the development and validation process
We followed up with the developers for steps and processes followed towards the development of each diagnostic, which have been summarised in table 3. Three developers consulted available TPPs from the Foundation for Innovative New Diagnostics (FIND) and four referred to the TPPs of WHO. Although developers mentioned the use of TPPs, but TPPs outlining the requirements specifically for rapid diagnostics for conditions such as sepsis, neonatal sepsis and UTI, etc do not exist with the sources (https://www.who.int/research-observatory/analyses/tpp/en/, https://www.finddx.org/tpps/)19 mentioned by developers. Four developers mentioned using as Clinical and Laboratory Standards Institute and the International Organization for Standardization standards to evaluate performance of analytical parameters (table 3). Absence of gold standards for baseline comparisons was identified as one of the major hurdles for evalution of the biomarker-based diagnostics. Using imperfect standards for comparison has implications on the reported analytical parameters underscoring the importance of field validation studies to demonstrate their clinical usefulness.
Table 3.
Overview of the steps followed by developers for diagnostic development
| S no. | Characteristics | Diagnostic (Dx) | |||||||
| Dx 1 | Dx 2 | Dx 3 | Dx 4 | Dx 5 | Dx 6 | Dx 7 | Dx 8 | ||
| Targeted syndrome | Sepsis | Sepsis | Bacteraemia | Urinary tract infection | Identification of Pathogen and resistance markers | Urinary tract infection | Pulmonary and pleural tuberculosis (TB), TB meningitis | TB | |
| i | Target product profiles (TPP) consulted for Dx development | No information shared by developer | Yes, FIND* |
Yes, WHO* |
Yes, WHO* |
Yes, FIND |
Yes, FIND* |
Yes, WHO |
Yes, WHO |
| ii | Standard or guidelines consulted to evaluate performance parameters | Yes, CLSI, ISO |
Yes, CLSI |
Yes, CLSI |
No | No | Yes, | No | |
| iii | Time taken for diagnostic development (in years) | 3.5 | 4 | 4 | 3 | 3 | 8 | 3 | |
| iv | Approximate grant for diagnostic development (in USD)† | Approx 100–150 k | 100–150 k | 221 197 200–250 k |
350–400 k | 150–200 k | 200–250 k | 200–250 k | |
| v | Source of funding | Government, industry and non-profit organisation |
Government and multiple collaborators | Government and industry |
Government | Government | Government | Industry | Industry |
| vi | Whether Dx submitted in National Healthcare Innovations Portal (NHInP)‡ | No | Yes | No | No | No | No | No | No |
| vii | Diagnostic development team | Biochemist, biochemical engineer | Biophysicist, biochemist |
Biophysicists, molecular biologist | Medical biochemist molecular biologists, engineers | Biotechnologist, molecular biologist, engineers, | Biotechnologist, biochemist, molecular biologist, microbiologist, critical care clinician, engineers | Biotechnologist, molecular biologist, protein biochemist, virologist | Biophysicist, biochemist |
| viii | Key challenges in progress |
|
|
Limited opportunity for engagement with regulators | Multisite validation |
|
Multisite field validation | Multisite field validation | Completing regulatory approvals |
* A TPP on ‘Simplified Blood Culture’ is available from FIND but no separate TPPs are available for sepsis or neonatal sepsis either from FIND (https://www.finddx.org/tpps/) or WHO. TPPs on guidance on therapy for different syndromes/conditions are available from WHO (https://apps.who.int/iris/discover?query=Target+product+profile). TPP for antibacterial resistance diagnostics and uncomplicated enteric fever is published in 2020 by WHO.19
†Conversion rate US$1=72.34 as on 24 February 2021; the cost of diagnostic development is self-reported by the innovator/developer.
‡(NHInP aims to provide innovative programmes designs, practices, technology solutions and products across public and private healthcare sector of India. National Health Systems Resource Centre under the National Health Mission of Government of India serves as its technical secretariat. Link to NHInP https://www.nhinp.org/index.php.
CLSI, Clinical and Laboratory Standards Institute; FIND, Foundation for Innovative New Diagnostics; ISO, International Organization for Standardization; NA, not applicable.;
The average time invested in the development of a rapid test ranged from 3 to 4 years for most diagnostics with only one diagnostic (Dx 7) reporting 8 years for its development and evaluation (table 3). Six out of eight diagnostic developers invested more than US$200 000 towards the diagnostic development (table 3). Only one developer had submitted diagnostic to the NHInP of Government of India, specifically dedicated for the assessment of new health innovations, and was awaiting response.
Discussion
Rapid diagnostics have a huge potential to influence prevention and treatment of a disease and this has been previously very well documented for diseases like malaria and diabetes.20 21 For containment of AMR, rapid diagnostics can strengthen diagnostic stewardship and substantially reduce indiscriminate use of antimicrobials. As highlighted by the findings of the survey, it is promising to note that the innovators in our country do understand the challenges of delivering test at a resource-constrained setting and this is reflected in specifications of diagnostics being developed. The tests developed are able to function without requirement of auxiliary instrument using non-invasive samples (table 1) which can work well even at a primary healthcare centre. This could be postive outcome of clinician engagement for development and feedback. Tests are user friendly, require short hands-on-time (<30 min) and also promise fast TAT, both critical for field use of any diagnostic in India. In the strength-weakness assessment (table 1), the strengths outweigh the weaknesses. This needs to be ascertained through the field feasibility studies which most of the diagnostics included in the survey had missed, as the sensitivity and specificity derived from a controlled laboratory environment may not be replicated in field settings. Unfortunately, despite the availability of many indigenously developed rapid tests for pathogen identification or AMST in the country, none were ready to be included in the Indian NEDL and FDI list.22
Developers have done well at focusing on UTI and sepsis as both the syndromes warrant excessive antimicrobial use, in community and in hospitals/ICUs, respectively. Although the diagnostics developed addressed the important syndromes and pathogens relevant to India,23 24 they also missed important country specific requirements. For example, viral respiratory infections are recognised as the most common cause for unnecessary prescription of antibiotics, in community practice or urgent care settings.25 However, no diagnostic focusing on respiratory bacterial pathogens had been developed or was under development in our survey. We, also, did not come across any test that can facilitate the rapid detection of fungal pathogens like Candida, and other endemic and re-emerging infections prevalent in India such as scrub typhus, murine typhus and leptospirosis, etc. One diagnostic (Dx 3) was developed for the detection and susceptibility testing of Salmonella Typhi, causative agent of typhoid fever, which remains a high priority public health concern in India.26
Most diagnostics in this survey had been evaluated for sensitivity, specificity, negative predictive value, positive predictive value, limit of detection and other analytical parameters. None had undergone third party validation, field feasibility and the cost-effectiveness studies which are absolutely essential to convince policy-makers on the potential advantage of using these diagnostics in healthcare (box 1). The absence of guidance on gold standards for baseline comparisons, access to clinical specimens, undertaking multisite field evaluations and lack of knowledge on steps to regulatory approval were other challenges cited by Indian innovators interviewed in this survey. Previous studies and reviews have identified absence of adequate funding, access to specimens and reagents, weak political commitment and regulatory harmonisation as the key challenges to diagnostic development.27–29 A push from Government of India to fund development of new diagnostics through diverse funding mechanisms seems to have helped innovators who were part of this survey. Six out of eight developers acknowledged having received government funding and none of the developers cited lack of funding as major challenge. It is important that the information on initiatives like NHInP and HTAIn, which have been specially created to expedite the uptake of useful rapid diagnostics in our country, is widely disseminated among the developers. In current survey, only one developer had submitted the diagnostic in NHInP for further assessment in 2019, and was awaiting response at the time of survey. None of the developers had approached HTAIn for any evaluation. Once there is a clear guidance/Standard Operating Procedures (SOP) on the next steps to guide regulatory approvals and market entry, these innovators may need additional funding to support field feasibility, cost effectiveness studies, etc.
Box 1. Key challenges impacting the completion of validation of rapid diagnostic tests for antimicrobial resistance containment.
Absence of target product profiles (TPP) to guide the development process.
Absence of gold standards for comparison.
Long duration of development process.
Absence of clearly defined process for validation of indigenously developed diagnostic.
Lack of guidance to undertake field feasibility or validation studies.
Funding to support all the steps of validation.
Though this survey has been helpful in identifying key challenges blocking the use of indigenously developed diagnostics which can be useful in containment of AMR, this survey had many limitations. This is not an exhaustive survey of all available diagnostics under development relevant to AMR in India and we may have missed out the other fully developed diagnostics which are not yet in public domain in any form, for example, research or news publications, etc. While we cite and bring to light the challenges faced by developers at advanced stage of development, we may be missing the early-stage diagnostic development challenges leaving open the possibility of introducing self-bias to the assessment. Although we did try to design the questionnaires for objective assessment of all the critical aspects of the diagnostics and their development process. The survey could also have benefited from participation of other stakeholder groups who are part of supply chain or associated with test service and delivery, both in public and private sector, and who may become relevant as these innovations develop further and get closer to deployment. We could not include their opinions in this survey.
From our study findings, we are hopeful that diagnostics developed by Indian innovators have the potential to be helpful in containment of AMR, not only in India but also in other LMICs. Government can take series of initiatives that can fill the prevailing gaps and expedite the market entry of indigenously developed quality AMR diagnostics in country. First, TPPs on country-specific priority conditions such as typhoid, sepsis, neonatal sepsis and fever need to be developed urgently by engaging clinicians, microbiologists, epidemiologists, etc to address the unmet clinical needs and ensure the development of ‘fit-for-purpose’ translatable products. TPPs for rapid detection of many serious conditions like sepsis, neonatal sepsis, acute febrile illness, differentiating viral and bacterial infections etc. are non-existent and the ones developed by WHO (https://apps.who.int/iris/discover?query=Target+product+profile) and FIND (https://www.finddx.org/tpps/) do not adequately address the healthcare needs in Indian healthcare system.
Second, there is a need to create guidelines explaining the process and framework of validation of AMR diagnostics with inputs from relevant stakeholders. The innovators in the country have made phenomenal progress towards creating desired diagnostics, however, they need to be supported with funding and guidance to achieve the final validation for regulatory approvals so that the investments made so far can be harnessed for the clinical advantage. An expert group comprising of technical experts, regulators and policy makers should be brought together to address these bottlenecks and create a pathway for effective translation and utilisation of diagnostics under development or the ones already developed.
Third, a systematic evaluation of the cost effectiveness and potential clinical utility of the indigenously developed diagnostics needs to be undertaken and documented through the HTAIn or NHInP. This evaluation can be useful in highlighting the value of using rapid diagnostics in Indian settings and for creating opportunities for enhanced funding and investment in AMR diagnostics.
In conclusion, the availability of many developed or under-development rapid POC diagnostics, which can be helpful in containment of AMR, instils confidence. How we take advantage of this opportunity to strengthen diagnostic stewardship will depend on the efficiency with which all the links in the pathway for uptake of indigenously developed diagnostics in healthcare system function. Unless we enable this, it will be a lost opportunity not only for our country but also for the other LMICs who could have benefited from affordable good quality rapid diagnostics for containment of AMR.
Conclusions
Many countries, including India, are supporting efforts towards development of rapid POC diagnostics, which can be helpful in containment of AMR. Despite tremendous progress in this area, no diagnostic has yet been put to clinical use in the country. Development of rapid tests for AMR containment is time, capital and resource-intensive endeavour. In order for these efforts to make a clinical difference, there is an urgent need to develop the country-specific TPPs and, a well-defined pathway for validation of these diagnostics which will ascertain their actual utility in the field and facilitate regulatory approvals. All efforts should be made to address these bottlenecks to ensure that the investment towards new product development is well used and the diagnostics being developed are made available for use at the earliest.
bmjgh-2021-006628supp002.pdf (225.5KB, pdf)
Acknowledgments
We would like to thank all the developers for their participation in the survey.
Footnotes
Handling editor: Seye Abimbola
Contributors: KW conceived the idea and supervised the study. MS carried out study and analysed the data. RRG and SB provided the clinical and microbiological inputs, respectively, in the data analysis.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not required.
References
- 1.O'Neil J. Tackling drug-resistant infections globally: final report and recommendations. The review on antimicrobial resistance chaired by Jim O’Neill. 1. London, UK: Wellcome Trust, 2016: 84. [Google Scholar]
- 2.Khan MS, Rothman-Ostrow P, Spencer J, et al. The growth and strategic functioning of one health networks: a systematic analysis. Lancet Planet Health 2018;2:e264–73. 10.1016/S2542-5196(18)30084-6 [DOI] [PubMed] [Google Scholar]
- 3.Pokharel S, Raut S, Adhikari B. Tackling antimicrobial resistance in low-income and middle-income countries. BMJ Glob Health 2019;4:e002104. 10.1136/bmjgh-2019-002104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holmes AH, Moore LSP, Sundsfjord A, et al. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet 2016;387:176–87. 10.1016/S0140-6736(15)00473-0 [DOI] [PubMed] [Google Scholar]
- 5.Laxminarayan R, Chaudhury RR. Antibiotic resistance in India: drivers and opportunities for action. PLoS Med 2016;13:e1001974. 10.1371/journal.pmed.1001974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhatia R, Walia K. Combating antimicrobial resistance in India: Technical challenges & opportunities. Indian J Med Res 2017;146:683–7. 10.4103/ijmr.IJMR_19_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reali S, Najib EY, Treuerné Balázs KE, et al. Novel diagnostics for point-of-care bacterial detection and identification. RSC Adv 2019;9:21486–97. 10.1039/C9RA03118A [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang C, Liu M, Wang Z, et al. Point-Of-Care diagnostics for infectious diseases: from methods to devices. Nano Today 2021;37:101092. 10.1016/j.nantod.2021.101092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Belkum A, Bachmann TT, Lüdke G, et al. Developmental roadmap for antimicrobial susceptibility testing systems. Nat Rev Microbiol 2019;17:51–62. 10.1038/s41579-018-0098-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chokshi M, Patil B, Khanna R, et al. Health systems in India. J Perinatol 2016;36:S9–12. 10.1038/jp.2016.184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National health policy, 2017 MoFHW. Available: https://www.nhp.gov.in/nhpfiles/national_health_policy_2017.pdf [Accessed 30 Jul 2021].
- 12.Ombelet S, Ronat J-B, Walsh T, et al. Clinical bacteriology in low-resource settings: today's solutions. Lancet Infect Dis 2018;18:e248–58. 10.1016/S1473-3099(18)30093-8 [DOI] [PubMed] [Google Scholar]
- 13.Fluit AC, Visser MR, Schmitz F-J. Molecular detection of antimicrobial resistance. Clin Microbiol Rev 2001;14:836–71. 10.1128/CMR.14.4.836-871.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vasala A, Hytönen VP, Laitinen OH. Modern tools for rapid diagnostics of antimicrobial resistance. Front Cell Infect Microbiol 2020;10:308. 10.3389/fcimb.2020.00308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okeke IN, Feasey N, Parkhill J, et al. Leapfrogging laboratories: the promise and pitfalls of high-tech solutions for antimicrobial resistance surveillance in low-income settings. BMJ Glob Health 2020;5:e003622. 10.1136/bmjgh-2020-003622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Essential Diagnostics List . New Delhi: Indian Council of medical research, 2019. Available: https://www.nhp.gov.in/NHPfiles/NEDL_2019_Final_V2.pdf [Accessed 30 Jul 2021].
- 17.Free drugs & diagnostics service initiative . New Delhi: National health mission (NHM), Ministry of health and family welfare, 2018. Available: https://nhm.gov.in/New_Updates_2018/NHM_Components/Health_System_Stregthening/Comprehensive_primary_health_care/letter/Guidance_document_for_Free_Laboratory_Services.pdf [Accessed 30 Jul 2021].
- 18.BIRAC-TRLS: technology readiness levels by BIRAC across areas under biotechnology. technology readiness levels (TRLs) details: medical devices and diagnosis. Available: https://www.birac.nic.in/desc_new.php?id=443 [Accessed 15 Jun 2021].
- 19.World Health Organization . Target product profiles for antibacterial resistance diagnostics, 2020. Available: https://apps.who.int/iris/handle/10665/331054
- 20.Mukkala AN, Kwan J, Lau R, et al. An update on malaria rapid diagnostic tests. Curr Infect Dis Rep 2018;20:49. 10.1007/s11908-018-0655-4 [DOI] [PubMed] [Google Scholar]
- 21.Schnell O, Crocker JB, Weng J. Impact of HbA1c testing at point of care on diabetes management. J Diabetes Sci Technol 2017;11:611–7. 10.1177/1932296816678263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vijay S, Gangakhedkar RR, Shekhar C, et al. Introducing a national essential diagnostics list in India. Bull World Health Organ 2021;99:236–8. 10.2471/BLT.20.268037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.India State-Level Disease Burden Initiative Collaborators . Nations within a nation: variations in epidemiological transition across the states of India, 1990-2016 in the global burden of disease study. Lancet 2017;390:2437–60. 10.1016/S0140-6736(17)32804-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gandra S, Tseng KK, Arora A, et al. The mortality burden of multidrug-resistant pathogens in India: a retrospective, observational study. Clin Infect Dis 2019;69:563–70. 10.1093/cid/ciy955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Escadafal C, Geis S, Siqueira AM, et al. Bacterial versus non-bacterial infections: a methodology to support use-case-driven product development of diagnostics. BMJ Glob Health 2020;5:e003141. 10.1136/bmjgh-2020-003141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mukhopadhyay B, Sur D, Gupta SS, et al. Typhoid fever: Control & challenges in India. Indian J Med Res 2019;150:437–47. 10.4103/ijmr.IJMR_411_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Engel N, Wachter K, Pai M, et al. Addressing the challenges of diagnostics demand and supply: insights from an online global health discussion platform. BMJ Glob Health 2016;1:e000132. 10.1136/bmjgh-2016-000132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ditiu & Boehme . Crossing the Valleys of death in TB: from development to roll-out, 2017. Available: http://gbchealth.org/crossing-the-valleys-of-death-in-tb-from-development-to-roll-out/#_ftnref2 [Accessed 30 Jul 2021].
- 29.Engel N, Wolffs PFG. Aligning diagnostics to the point-of-care: lessons for innovators, evaluators and decision-makers from tuberculosis and HIV. BMJ Glob Health 2020;5:e003457. 10.1136/bmjgh-2020-003457 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgh-2021-006628supp001.pdf (73.2KB, pdf)
bmjgh-2021-006628supp002.pdf (225.5KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.


