Abstract
A multiplex PCR assay that detects the four commonest causes of viral meningitis and encephalitis in the United Kingdom (herpes simplex virus [HSV] type 1 [HSV-1], HSV type 2 [HSV-2], varicella-zoster virus [VZV], and enteroviruses) was developed, and its sensitivity was compared with those of similar assays described previously for this application. Compared to the previous assays, this single multiplex PCR assay had higher molecular sensitivities for the detection for each of the viruses and improved utility for routine use in a diagnostic laboratory. The assay was used to test a series of 1,683 consecutive cerebrospinal fluid (CSF) samples between June 1997 and March 1998 inclusively. Viral nucleic acid was detected in 138 (8.2%) of the CSF samples, including enteroviruses in 51 samples, HSV-2 in 33 samples, VZV in 28 samples, and HSV-1 in 25 samples. Compared to the accepted relative incidence of viral etiologies, aseptic meningitis due to HSV-2 infection was high, and in adult female patients with symptoms of aseptic meningitis, HSV-2 was the virus most commonly detected in the CSF.
Before the introduction of molecular techniques, laboratory diagnosis of viral infections of the central nervous system (CNS) relied on virus isolation in cell culture, detection of specific antibody production in cerebrospinal fluid (CSF), or, for encephalitis caused by herpes simplex virus (HSV), viral antigen detection in tissue from brain biopsy specimens. With the exception of the last procedure, which is highly invasive, the impact of laboratory diagnosis on acute patient management was relatively small because of the time taken for virus replication to produce a characteristic cytopathic effect in cell culture or for the development of a specific antibody response. Deficiencies in traditional laboratory techniques with regard to the diagnosis of viral CNS infection have meant that in many patients a clinical diagnosis of viral meningitis is made without supportive laboratory evidence of a viral etiology.
The use of PCR for the diagnosis of CNS disease has been well evaluated for HSV encephalitis (4, 6, 7, 9) and enterovirus meningitis (12, 15, 17, 19). The use of this highly sensitive technique has increased our understanding of the etiological role of viruses in CNS disease. For example, it has been demonstrated that varicella-zoster virus (VZV) and HSV type 2 (HSV-2) can cause meningitic symptoms without causing concurrent skin lesions (3, 13, 16).
A previous report (11) of a series of 2,233 consecutive CSF samples demonstrated the utility of using PCR as a first-line diagnostic test in a routine laboratory. It was shown that the use of three primer sets was sufficient for the PCR detection of the viruses responsible for 95% of the viral CNS disease in the United Kingdom. A subset of patients in that series was also used to identify the clinical and laboratory variables which independently predicted a positive PCR result (5). It wfas shown that a clinically diagnosed viral infection of the CNS was 88 times more likely to occur in a patient with a positive PCR result than in one with a negative PCR result. However, the possibility of a clinical diagnosis of viral CNS infection in a patient with a negative PCR result was determined to be moderate.
The aims of conducting the present study were twofold: to improve the utility of the PCR technique for routine use in a diagnostic laboratory by designing a single multiplex PCR assay capable of diagnosing nearly all cases of viral CNS disease occurring in the population in the United Kingdom and to increase the molecular sensitivities of the assays used, so raising confidence in a negative result.
MATERIALS AND METHODS
A PCR assay (screen 1) incorporating primers for the reverse transcription of enterovirus RNA and subsequent amplifications of HSV and VZV DNA and enterovirus cDNA was developed and evaluated for its sensitivity and specificity. Nested primer amplifications were used for each of the viruses. The HSV-specific primers were designed for this study, and the VZV-specific (18) and enterovirus-specific (14) primers have been described previously for different applications. Details of the primers used are given in Table 1.
TABLE 1.
Primer sequences used in screen 1 and in identification of HSV type
| Virus | Genome region amplified | Primer coordinates | Primer orientation | Primer sequence |
|---|---|---|---|---|
| HSV-1 and -2 | Glycoprotein D | 716–735 | Outer sense | ATCCGAACGCAGCCCCGCTG |
| 1131–1112 | Outer antisense | TCCGG(G/C)GGCAGCAGGGTGCT | ||
| 789–808 | Inner sense | GCGCCGTCAGCGAGGATAAC | ||
| 1070–1051 | Inner antisense | AGCTGTATA(G/C)GGCGACGGTG | ||
| VZV | Gene 29 | 51067–51087 | Outer sense | ACGGGTCTTGCCGGAGCTGGT |
| 51338–51315 | Outer antisense | AATGCCGTGACCACCAAGTATAAT | ||
| 51091–51111 | Inner sense | ACCTTAAAACTCACTACCAGT | ||
| 51298–51279 | Inner antisense | CTAATCCAAGGCGGGTGCAT | ||
| Enteroviruses | 5′ NTRa | 454–470 | Outer sense | CGGCCCCTGAATGCGGC |
| 647–630 | Outer antisense | CACCGGATGGCCAATCCA | ||
| 457–474 | Inner sense | CCCCTGAATGCGGCTAAT | ||
| 603–584 | Inner antisense | ATTGTCACCATAAGCAGCCA | ||
| HSV-1 | Glycoprotein D | 619–638 | Outer and inner sense | CGAAGACGTCCGGAAACAAC |
| 899–915 | Outer antisense | CGGTGCTCCAGGATAAA | ||
| Inner antisense | TCTCCGTCCAGTCGTTTATCTTC | |||
| HSV-2 | Glycoprotein D | 619–638 | Outer and inner sense | GGACGAGGCCCGAAAGCACA |
| 899–915 | Outer antisense | CGGTGCTCCAGGATAAA | ||
| Inner antisense | TCTCCGTCCAGTCGTTTATCTTC |
5′ NTR, 5′ nontranslated region.
Single procedures for the preparation of viral RNA and DNA for reverse transcription and/or PCR amplification were evaluated. The in-house protocol developed for the detection of RNA and described previously (1), with modifications (11), was compared to the commercially available QIAamp viral RNA kit (QIAGEN Ltd, Crawley, United Kingdom) for both DNA and RNA detection by PCR. The in-house protocol is based on guanidinium isothiocyanate lysis of virus particles and binding of nucleic acid to a slurry of silica particles. Extraction and purification of viral nucleic acid with the QIAamp extraction kit were performed according to the manufacturer’s instructions, but with the following modifications: 70 μl of CSF or the appropriate dilution of control virus-infected cell culture supernatant was processed, and the volumes of ethanol and the AVL kit buffer were adjusted proportionally. Nucleic acid was eluted with 70 μl of AE kit buffer. The sensitivity of screen 1 with the two nucleic acid extraction protocols was measured with cell culture-grown poliovirus type 2, HSV-1, and VZV by determining the highest dilutions of the viruses that gave a 50% detection rate by PCR (PCRD50). The infectivities (expressed as 50% tissue culture infective doses [TCID50s]) and the virus particle counts of these stocks as determined by electron microscopy had been investigated previously (11).
The specificities of the oligonucleotides designed for this study for the PCR amplification of HSV were evaluated by using supernatants of cell cultures infected with VZV, Epstein-Barr virus, cytomegalovirus, human herpesvirus 6, human herpesvirus 8, and poliovirus type 2. Control experiments with log10 dilution series of HSV-1, VZV, and poliovirus type 2 stocks were done to determine any difference in the sensitivity of detection between multiplex primer reactions and reactions with single primer pairs.
Screen 1 and HSV type-specific primary PCR amplifications were performed in a solution with a total volume of 50 μl containing 20 μl of extracted nucleic acid solution, 16 mM (NH4)2SO4, 67 mM Tris-HCl (pH 8.8 at 25°C), 0.01% (wt/vol) Tween 20 (ammonium sulfate buffer; Bioline Ltd., London, United Kingdom), 1.5 mM MgCl2, each deoxynucleotide triphosphate (Bioline Ltd.) at a concentration of 0.25 mM, 0.1 μM each oligonucleotide primer (PE Applied Biosystems, Warrington, United Kingdom), and 0.625 U of Taq polymerase (manufacturer’s units; Bioline Ltd.). In the screen 1 primary reaction, 0.1 U of Moloney murine leukemia virus reverse transcriptase (manufacturer’s units; Advanced Biotechnologies Ltd., Epsom, United Kingdom) was included for the specific antisense oligonucleotide primed reverse transcription of enterovirus RNA.
Secondary amplifications with nested primers were performed with 2 μl of the primary reaction solution. Concentrations of reagents identical to those used in the primary PCR were used, but they were included in a total volume of 25 μl.
The PCR thermal cycling incubations used for screen 1 were as follows: reverse transcription and initial amplification were performed in a single reaction by incubation at 37°C for 15 min and 94°C for 40 s preceding 33 cycles of incubation at 94, 60, and 72°C for 20 s each; further amplification with the nested primers was by 33 cycles of incubation at 94, 55, and 72°C for 20 s each. HSV type-specific PCR was performed by incubation at 94°C for 40 s, followed by 33 cycles of incubation at 94, 50, and 72°C for 20 s each for initial amplification, followed by amplification with nested primers with 33 cycles of incubation at 94, 60, and 72°C for 20 s each. All thermal cycling was performed with PE Applied Biosystems 2400 machines. Amplification products were identified by their molecular weights following electrophoresis of 10 μl of the secondary reaction mixture through an ethidium bromide-stained 2% agarose gel and UV light transillumination.
Carryover contamination by the amplified products was avoided by strict physical separation of pre- and postamplification processes. This was sufficient precaution to avoid the problem of false-positive results which would have been detected with the use of multiple nucleic acid extraction and amplification controls incorporated into each assay batch. A positive control processed with each batch of samples consisted of a mixture of the virus stock supernatants equivalent to 10 PCRD50s of each nucleic acid (HSV-1, VZV, and poliovirus type 2).
Screen 1 was used as the first-line laboratory diagnostic test for CSF samples received between June 1997 and March 1998. Overall, 1,683 consecutive CSF samples were tested in this series, with 179 samples coming from Oxford city hospitals, 247 coming from hospitals in the Oxford region, and 1,257 (75%) referred from hospitals outside the Oxford region. Collection of clinical data on all the patients in this series would therefore have been difficult, but for the PCR-positive patients a brief description of signs and symptoms was sought. Overall, it was noted that 66 (4%) samples were from patients known to be infected with the human immunodeficiency virus. The authors applied no selection to the samples that were tested by the PCR assay; all samples received for a diagnosis of meningitis or encephalitis or with a request to test for either of the viruses were included in the study.
RESULTS
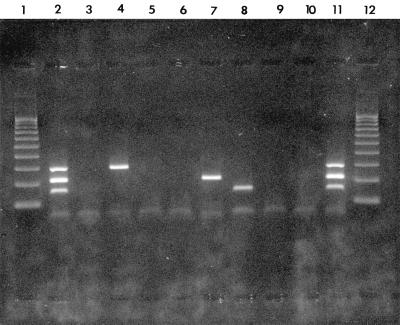
The molecular sensitivity of detection by screen 1 was estimated with reference to infectivity measurements and virus particle counts (Table 2). The number of intact virus particles, determined by electron microscopy, required for PCR detection of the nucleic acids after extraction with the QAIamp kit ranged from 2 to 16; this represents approximately a 10- to 100-fold greater sensitivity compared to those of the previous nucleic acid extraction and amplification protocols. The new PCR methods were 3- to 1,000-fold more sensitive than routine virus culture. In the control experiments, the oligonucleotides designed for the amplification of HSV were shown to be specific for HSV-1 and HSV-2 and did not amplify other related viruses (data not shown). The sensitivity of detection of each of the viral nucleic acids was equivalent by multiplex primer or single primer pair reactions (data not shown). An example of the viral nucleic acids amplified by screen 1 is shown in Fig. 1.
TABLE 2.
Calculation of sensitivities of PCR or reverse transcription-PCR for detection of HSV-1, VZV, and poliovirus type 2 by screen 1
| Virus | Log infectivity (TCID50/ml)a | Log NVP/mlb | Log PCRD50/mlc | NVP/TCID50 | NVP/PCRD50 | TCID50/PCRD50 |
|---|---|---|---|---|---|---|
| HSV-1 | 6.5 | 8.2 | 7.0 | 50 | 16 (158)d | 0.3 (3) |
| Poliovirus type 2 | 7.0 | 8.74 | 8.0 | 55 | 5.5 (34) | 0.1 (3) |
| VZV | 5.0 | 8.3 | 8.0 | 1,995 | 2 (879) | 0.001 (0.1) |
TCID50, TCID at which 50% of inoculated monolayers become infected.
The number of virus particles (NVP) per milliliter was calculated from electron microscopic counts in parallel with counts of silicon beads by direct proportionality.
The reciprocal of the highest dilution positive for nucleic acid that was detectable by PCR adjusted to concentration per milliliter.
Data in parentheses refer to the same determinations obtained by multiplex methods previously described by Read et al. (11) and are included for comparison.
FIG. 1.
Screen 1-amplified viral nucleic acids from CSF samples and controls after agarose gel electrophoresis, ethidium bromide staining, and UV light transillumination. Lanes 1 and 12, 100- to 1,000-bp molecular ruler (Bio-Rad Laboratories Ltd., Hemel Hempstead, United Kingdom); lanes 2 and 11, control mixture containing HSV-1, VZV, and poliovirus type 2 nucleic acids at concentrations equivalent to those that give 10 PCRD50s (HSV, 280 bp; VZV, 200 bp; and enterovirus, 144 bp); lanes 3, 6, and 10, negative nucleic acid extraction and amplification controls; lanes 4, 5, and 7 to 9, CSF samples containing HSV DNA (lane 4), VZV DNA (lane 7), enterovirus RNA (lane 8), or no detectable specific nucleic acid (lanes 5 and 9).
Overall, 138 (8.2%) of the CSF samples tested positive in the multiplex reaction. Enterovirus RNA was detected in 51 patients (30 males). Seventeen of these were in babies aged less than 6 months, in whom the CNS infection was detected as part of a general infection screen; of the 34 older children and adults who were affected, 33 presented with meningitis and 1 had an encephalitic illness.
VZV DNA was detected in 28 people (16 males), all of whom were over the age of 6 months. When the information was available, it was noted that vesicular skin lesions accompanied the CNS manifestations in 14 patients but were absent from 11 patients. Among the patients in this group, 16 patients had meningitis and 10 had encephalitis. For two patients no information about their CNS illness or their signs or symptoms was available.
HSV DNA was detected in 59 patients. With HSV type-specific amplification, HSV-1 was detected in 25 of the patients, including 2 babies aged less than 6 months. Of the 23 older people affected (11 males), 22 had encephalitis and 1 had a benign lymphocytic meningitis. HSV-2 was detected in 33 patients, including 5 aged less than 6 months. It caused a meningoencephalitis in two adult patients, one of whom had a residual memory deficit. In the 26 other patients aged over 6 months, HSV-2 caused a benign lymphocytic meningitis. Seven of these patients gave a history of one to four previous episodes of aseptic meningitis. The sex ratio for the 28 adults with HSV-2 CNS infection was 6:1 (female to male). Five female patients were noted to have concurrent herpetic vesicular lesions (two with primary genital lesions, one with an oral lesion, one with an anal lesion, and one with whitlow). In addition, HSV was detected in one other baby aged less than 6 months, but there was insufficient CSF to enable the HSV type to be determined. The clinical presentations of the babies with a positive PCR result were nonspecific; in Table 3, therefore, clinical presentation is summarized for the patients who were older than 6 months and who had a positive PCR result.
TABLE 3.
Age ranges and medians, sex ratio, and clinical manifestations of viral infection for PCR-positive patients ages >6 months
| Characteristic | HSV-1 | HSV-2 | VZV | Enteroviruses |
|---|---|---|---|---|
| No. of patients positive | 23 | 28 | 28 | 34 |
| No. of patients with the following: | ||||
| Meningitis | 1 | 26 | 16 | 33 |
| Meningoencephalitis | 0 | 2 | 0 | 0 |
| Encephalitis | 22 | 0 | 10 | 1 |
| Not described | 0 | 0 | 2 | 0 |
| Age range (median age [yr]) of patients | 4–80 (46) | 7–50 (29) | 3–79 (35) | 1–74 (25) |
| Sex ratio (male:female) | 1:1.1 | 1:6.0 | 1:0.8 | 1:1.6 |
DISCUSSION
For this series of investigations of CSF, a multiplex PCR assay was developed. The assay had a higher molecular sensitivity for the viruses that were amplified than the sensitivities of the duplex assays for HSV and VZV and for enteroviruses and echovirus type 22 previously described for this application. In a comparison of the two protocols, this assay was between approximately 10- and 100-fold more sensitive for the detection of identical isolates of HSV-1, VZV, and poliovirus type 2. The result was that in this study, detection by screen 1 was more sensitive than testing for viral infectivity. In addition, because the detection of these viruses is now performed with a single nucleic acid extraction and amplification screen, it is convenient and cost-effective for use on a routine basis. The assay takes approximately 4 h to complete.
By screen 1, the relative rate of detection of HSV-2 showed a marked increase in comparison to the previous series (2 versus 0.3%). The rate of detection of HSV-1 and VZV also increased slightly (1.5 versus 0.9% and 1.7 versus 0.7%, respectively), but the rate of detection of enteroviruses decreased slightly. These differences may reflect differences in the population groups of these two series, and it is noteworthy that for enteroviruses, which cause seasonal infections in temperate climates, the relative detection rate may have been affected by the shorter time span of the latter series. The detection rate of 3% for enteroviruses is comparable to the 4.1% found by viral culture of CSF from patients with suspected cases of viral meningitis in the 1995 to 1997 United Kingdom Public Health Laboratory Service survey for the eradication of polio (10a).
The data from this series may be compared to those of Meyer et al. (8), who reported rates of 1.8% for HSV encephalitis and 0.84% for HSV meningitis in a series of 713 patients with acute CNS infection of presumed viral etiology (8). With the HSV typing data derived from this series, we suggest that it is reasonable to assume that in the earlier series the encephalitis was due mainly to HSV-1 and the meningitis was due mainly to HSV-2.
Of particular interest in this series is the prevalence of CNS disease caused by HSV-2 infection. The seroprevalence of HSV-2 antibody in the population of the United Kingdom indicates that 5% of adult women are infected (1a); however, the results for the present series of patients suggest that this virus is the commonest cause of benign lymphocytic meningitis in young adult women. Twenty-two of the 26 patients (85%) who were aged greater than 6 months and who had this diagnosis were female, and 7 (27%) gave a history of one to four similar previous episodes of aseptic meningitis; while it is not possible to exclude other causes for those earlier illnesses, in none of the patients was any virus identified by conventional laboratory tests. It is likely that those seven patients had recurrent benign lymphocytic meningitis, as described by Mollaret (10). The absence of herpetic skin lesions could not be used to exclude a diagnosis because only five patients had noticeable lesions. In only 1 of the 28 patients aged greater than 6 months was HSV-2 isolated from the CSF by cell culture. This was from a patient with a primary genital infection. In all cases of HSV-2 meningitis the patients made a complete recovery within 3 to 4 days without any specific antiviral treatment.
Among the HSV-2 PCR-positive patients aged older than 6 months, there were no cases of pure encephalitis, but two patients developed meningoencephalitis. One patient made a complete recovery following a primary infection, while the other patient was left with a mild residual memory loss following reactivation of infection. It is therefore essential to give antiviral therapy if there is an encephalitic component to the illness. In contrast to HSV-2, 22 of 23 patients in whom HSV-1 DNA was detected in their CSF had a severe encephalitic illness, with one patient described as having meningitis.
An improved understanding of the etiology of viral CNS infection with the use of molecular assays has enabled the design of a multiplex PCR assay that detects the four commonest viral causes of CNS disease in the United Kingdom, making it appropriate for use for any patient with a suspected CNS infection. The multiplex PCR has many advantages over other types of laboratory tests including single target PCR assays. It allows for economic screening to detect a number of viruses in patients with suspected CNS infections, and consequently, our knowledge of the etiologies and spectra of CNS disease is being broadened. The data from this series suggest that the incidence of meningitis caused by HSV-2 infection is being underestimated. Unlike the detection of HSV-1 DNA in patients with encephalitis, for which PCR has been demonstrated to be a highly sensitive technique in many studies, it is probable that the concentration of HSV-2 DNA is lower in patients with meningitis and, consequently, that assays of very high sensitivity are required for this diagnosis. The data presented here suggest that it is not sufficient for the virology laboratory to investigate meningitis by cell culture isolation from CSF alone, on the basis of the assumption that enteroviruses are the only significant cause of viral meningitis.
ACKNOWLEDGMENTS
We thank Ulrich Desselberger for valued support and advice; Mike Alder, Lee Fulton, Terry Lee, and Sue Wareing for expert technical support; and Charles Bangham and Katie Jeffery for the collaboration that led to this work.
Support for this work was obtained from the Public Health Laboratory Service, London, United Kingdom.
REFERENCES
- 1.Boom R, Sol C J A, Salimans M M M, Jansen C L, Wertheim-van Dillen P M E, van de Noorda J. Rapid and simple method for purification of nucleic acids. J Clin Microbiol. 1990;28:495–503. doi: 10.1128/jcm.28.3.495-503.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1a.Brown, D. Personal communication.
- 2.Burke D G, Kalayjian R C, Vann V R, Madreperla S A, Shick H E, Leonard D G. Polymerase chain reaction detection and clinical significance of varicella-zoster virus in cerebrospinal fluid from human immunodeficiency virus-infected patients. J Infect Dis. 1997;176:1080–1084. doi: 10.1086/516516. [DOI] [PubMed] [Google Scholar]
- 3.Echeverria J M, Casas I, Tenorio T, de Ory F, Martinez-Martin P. Detection of varicella-zoster virus-specific DNA sequences in cerebrospinal fluid from patients with acute aseptic meningitis and no cutaneous lesions. J Med Microbiol. 1994;43:331–335. doi: 10.1002/jmv.1890430403. [DOI] [PubMed] [Google Scholar]
- 4.Guffond T, Dewilde A, Lobert P-E, Caparros-Lefebvre D, Hober D, Wattre P. Significance and clinical relevance of the detection of herpes simplex virus DNA by the polymerase chain reaction in cerebrospinal fluid from patients with presumed encephalitis. Clin Infect Dis. 1994;18:744–749. doi: 10.1093/clinids/18.5.744. [DOI] [PubMed] [Google Scholar]
- 5.Jeffery K J M, Read S J, Peto T E A, Mayon-White R T, Bangham C R M. Diagnosis of viral infections of the central nervous system: clinical interpretation of PCR results. Lancet. 1997;349:313–317. doi: 10.1016/S0140-6736(96)08107-X. [DOI] [PubMed] [Google Scholar]
- 6.Koskiniemi M, Piiparinen H, Mannonen L, Rantalaiho T, Vaheri A. Herpes encephalitis is a disease of middle aged and elderly people: polymerase chain reaction for detection of herpes simplex virus in the CSF of 516 patients with encephalitis. J Neurol Neurosurg Psychiatry. 1996;60:174–178. doi: 10.1136/jnnp.60.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lakeman F D, Whitley R J the National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. Diagnosis of herpes simplex encephalitis: application of polymerase chain reaction to cerebrospinal fluid from brain-biopsied patients and correlation with disease. J Infect Dis. 1995;171:857–863. doi: 10.1093/infdis/171.4.857. [DOI] [PubMed] [Google Scholar]
- 8.Meyer H M, Johnson R T, Crawford I P, Dascomb H E, Rodgers N G. Central nervous system syndromes of ‘viral’ aetiology. Am J Med. 1960;29:334–347. doi: 10.1016/0002-9343(60)90029-2. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell P S, Espy M J, Smith T F, Toal D R, Rys P N, Berbari E F, Osmon D R, Persing D H. Laboratory diagnosis of central nervous system infections with herpes simplex virus by PCR performed with cerebrospinal fluid specimens. J Clin Microbiol. 1997;35:2873–2877. doi: 10.1128/jcm.35.11.2873-2877.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mollaret P. La méningite endothélio-leucocytaire multirécurrente benigne: syndrome nouveau ou maladie nouvelle? Rev Neurol (Paris) 1944;76:57–76. [Google Scholar]
- 10a.Ramsay, M. Personal communication.
- 11.Read S J, Jeffery K J M, Bangham C R M. Aseptic meningitis and encephalitis: the role of PCR in the diagnostic laboratory. J Clin Microbiol. 1997;35:691–696. doi: 10.1128/jcm.35.3.691-696.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sawyer M H, Holland D, Aintablian N, Connor J D, Keyser E F, Waeker N J. Diagnosis of enteroviral central nervous system infection by polymerase chain reaction during a large community outbreak. Paediatr Infect Dis J. 1996;13:177–182. doi: 10.1097/00006454-199403000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Schlesinger Y, Tebas P, Gaudreault-Keener M, Buller R S, Storch G A. Herpes simplex virus type 2 meningitis in the absence of genital lesions: improved recognition with use of the polymerase chain reaction. Clin Infect Dis. 1995;20:842–848. doi: 10.1093/clinids/20.4.842. [DOI] [PubMed] [Google Scholar]
- 14.Shen S, Desselberger U, McKee T A. The development of an antigen capture polymerase chain reaction assay to detect and type human enteroviruses. J Virol Methods. 1997;65:139–144. doi: 10.1016/s0166-0934(97)02181-2. [DOI] [PubMed] [Google Scholar]
- 15.Tanel R E, Kao S Y, Niemiec T M, Loeffelholz M J, Holland D T, Shoaf L A, Sturky E R, Burns J C. Prospective comparison of culture vs genome detection for diagnosis of enteroviral meningitis in childhood. Arch Pediatr Adolesc Med. 1996;150:919–924. doi: 10.1001/archpedi.1996.02170340033006. [DOI] [PubMed] [Google Scholar]
- 16.Tedder D G, Ashley R, Tyler K L, Levin M J. Herpes simplex virus infection as a cause of benign recurrent lymphocytic meningitis. Ann Intern Med. 1994;121:334–338. doi: 10.7326/0003-4819-121-5-199409010-00004. [DOI] [PubMed] [Google Scholar]
- 17.Thoren A, Widell A. PCR for the diagnosis of enteroviral meningitis. Scand J Infect Dis. 1994;26:249–254. doi: 10.3109/00365549409011792. [DOI] [PubMed] [Google Scholar]
- 18.Wakefield A J J, Fox D, Sawyer A M, Taylor J E, Sweenie C H, Smith M, Emery V C. Detection of herpesvirus DNA in the large intestine of patients with ulcerative colitis and Crohn’s disease using the nested polymerase chain reaction. J Med Virol. 1992;38:183–190. doi: 10.1002/jmv.1890380306. [DOI] [PubMed] [Google Scholar]
- 19.Yerly S, Gervaix A, Simonet V, Catflisch M, Perrin L, Wunderli W. Rapid and sensitive detection of enteroviruses in specimens from patients with aseptic meningitis. J Clin Microbiol. 1996;34:199–201. doi: 10.1128/jcm.34.1.199-201.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]