This randomized clinical trial measures whether methylphenidate compared with placebo decreases the severity of apathy in individuals with Alzheimer disease.
Key Points
Question
Does methylphenidate decrease apathy in individuals with Alzheimer disease?
Findings
In this randomized clinical trial of 200 participants, methylphenidate vs placebo was found to be safe and was associated with a decrease in apathy symptoms as measured by the Neuropsychiatric Inventory within 2 months that was sustained for 6 months.
Meaning
Methylphenidate may be useful for the treatment of apathy in individuals with Alzheimer disease, which can reduce symptoms and caregiver burden.
Abstract
Importance
Apathy, characterized by diminished will or initiative and one of the most prevalent neuropsychiatric symptoms in individuals with Alzheimer disease, is associated with significant caregiver burden, excess disability, increased medical costs, and mortality.
Objective
To measure whether methylphenidate compared with placebo decreases the severity of apathy in individuals with Alzheimer disease.
Design, Setting, and participants
This multicenter randomized placebo-controlled clinical trial was conducted from August 2016 to July 2020 in 9 US clinics and 1 Canadian clinic specializing in dementia care. A total of 307 potential participants were screened. Of those, 52 did not pass screening and 55 were not eligible. Participants with Alzheimer disease, mild to moderate cognitive impairment, and frequent and/or severe apathy as measured by the Neuropsychiatric Inventory (NPI) were included.
Interventions
Ten milligrams of methylphenidate, twice daily, vs matching placebo.
Main Outcomes and Measures
The coprimary outcomes included (1) change from baseline to 6 months in the NPI apathy subscale or (2) improved rating on the Alzheimer’s Disease Cooperative Study Clinical Global Impression of Change. Other outcomes include safety, change in cognition, and quality of life.
Results
Of 200 participants, 99 were assigned to methylphenidate and 101 to placebo. The median (interquartile range) age of study participants was 76 (71-81) years; 68 (34%) were female and 131 (66%) were male. A larger decrease was found from baseline to 6 months in the NPI apathy score in those receiving methylphenidate compared with placebo (mean difference, −1.25; 95% CI, −2.03 to −0.47; P = .002). The largest decrease in the NPI apathy score was observed in the first 100 days, with a significant hazard ratio for the proportion of participants with no apathy symptoms receiving methylphenidate compared with placebo (hazard ratio, 2.16; 95% CI, 1.19-3.91; P = .01). At 6 months, the odds ratio of having an improved rating on the Alzheimer’s Disease Cooperative Study Clinical Global Impression of Change for methylphenidate compared with placebo was 1.90 (95% CI, 0.95-3.84; P = .07). The difference in mean change from baseline to 6 months estimated using a longitudinal model was 1.43 (95% CI, 1.00-2.04; P = .048). Cognitive measures and quality of life were not significantly different between groups. Of the 17 serious adverse events that occurred during the study, none were related to the study drug. No significant differences in the safety profile were noted between treatment groups.
Conclusions and Relevance
This study found methylphenidate to be a safe and efficacious medication to use in the treatment of apathy in Alzheimer disease.
Trial Registration
ClinicalTrials.gov Identifier: NCT02346201
Introduction
Apathy is one of the most prevalent neuropsychiatric symptoms in individuals with Alzheimer disease (AD).1,2 Apathy is defined as diminished will and initiative, lack of interest in activities, and limited affective response to positive or negative events3 that is present for at least 4 weeks.4 Apathy affects up to 71% of people with dementia and results in excess disability.5
Apathy is associated with caregiver burden and distress,6 increased service utilization, accelerated institutionalization,7 increased mortality risk,8,9,10 and financial burden.8,9,10,11,12 No treatments are proven to treat apathy in AD, but catecholaminergic agents such as methylphenidate hold promise.13,14,15 Methylphenidate has been one of the most studied catecholaminergic compounds in older adults and has a good safety profile16 with 2 trials showing preliminary efficacy in apathy in AD. The Apathy in Dementia Methylphenidate Trial (ADMET) found that methylphenidate treatment of apathy in AD was associated with significant improvement in 2 of 3 efficacy outcomes, suggesting an improvement in global cognition and minimal adverse effects.17,18 The second study19 showed efficacy in all primary and secondary outcome measures as well as minimal adverse events. However, both trials were small and of short duration (6 and 12 weeks, respectively). To clarify the clinical efficacy of methylphenidate for apathy in AD more precisely, we conducted a larger, longer trial with more robust measures: the Apathy in Dementia Methylphenidate Trial 2 (ADMET 2), a phase 3, placebo-controlled, masked, 6-month, multicenter randomized clinical trial involving 200 participants with apathy and AD.
Methods
Participants
The design and rationale of ADMET 2 were recently published.20 The trial protocol is available in Supplement 1. Briefly, participants were recruited at 10 clinical centers (9 US clinics and 1 Canadian clinic) specializing in dementia care where they or their legally authorized representatives and the primary caregiver for the participant provided informed consent. The study adhered to the Declaration of Helsinki21 and was approved by the ethical review boards of each site. Participants were enrolled from May 2016 to December 2019 with the final visit in July 2020.
Participants included individuals of all race and ethnic groups as self-defined by the participant or their study partner. As required by National Institutes of Health policy, we conducted valid subgroup analyses of the primary and adverse event outcomes by sex and race and ethnicity to determine if there are possible differences (ie, interactions) in treatment effects.
Inclusion criteria included (1) diagnosis of possible or probable AD, using criteria from the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer Disease and Related Disorders Association22; (2) Mini-Mental State Examination23 scores between 10 and 28; (3) clinically significant apathy very frequently or frequently/often with a severity of moderate or marked for at least 4 weeks on the Neuropsychiatric Inventory (NPI)24; and (4) availability of a caregiver, who spent more than 10 hours a week with the potential participant. Exclusion criteria were (1) individuals experiencing a current or previously diagnosed major depressive episode by the Diagnostic and Statistical Manual of Mental Disorder; (2) clinically significant agitation/aggression, delusions, or hallucinations very frequently, frequently with severity of moderate, or marked on the NPI; (3) change in AD medication within the preceding 30 days; (4) change in use of antidepressants (except for trazodone for sleeping difficulties), lorazepam (except for sleeping difficulties), or benzodiazepines within preceding 30 days or within 5 half-lives of drug; (5) past failure of methylphenidate treatment; (6) current medication precluding safe use of methylphenidate; (7) current uncontrolled medical condition for which methylphenidate is contraindicated, including central nervous system abnormalities, hyperthyroidism, closed angle glaucoma, or cardiovascular or cerebrovascular abnormality; and (8) significant unintentional weight loss within previous 3 months.
Participants were randomized to methylphenidate or placebo in a 1:1 ratio using a randomization schedule stratified by clinical center with permuted length blocks and generated using a documented program in SAS statistical software version 9.2 (SAS Institute). Clinic staff obtained treatment assignments centrally using a web-based data system.
Intervention
Study drug was supplied as identical-appearing capsules containing either 5 mg of generic methylphenidate or placebo (cellulose) and dosed as 1 capsule twice a day (10 mg/d in the methylphenidate group) for 3 days, followed by 2 capsules twice a day (20 mg/d) for the remainder of the study. Study physicians could reduce the dose in the event of participant adverse events. Participants continued receiving all concomitant medications. All study partners and participants received a standardized psychosocial intervention, composed of a 20- to 30-minute counseling session at each visit, educational materials, and 24-hour availability of study staff for crisis management. In-person follow-up visits took place monthly for 6 months. Telephone contacts occurred at days 15, 45, and 75 after randomization. Virtual or telephone visits were allowed when COVID-19 precluded safe in-person visits.
Outcomes
Efficacy Outcomes
Two coprimary outcomes were prespecified: (1) mean change in the NPI apathy score from baseline to 6 months and (2) odds of improved rating on the Alzheimer’s Disease Cooperative Study Clinical Global Impression of Change (ADCS-CGIC)25 between baseline and 6 months, with attainment of significance for either outcome, signaling an efficacious treatment. A significant result for either primary outcome would indicate treatment efficacy. Secondary outcomes included change between baseline and 6 months in the Dementia Apathy Interview and Rating,26 a 16-item, informant rated scale that evaluates the change in motivation, engagement, and emotional response since disease onset. Higher scores indicate more apathy. Other outcomes included presence of adverse events and change in cognition and/or quality of life, described in detail below.
The NPI, administered as a structured interview with the caregiver, assessed the presence and severity of 12 neuropsychiatric symptoms, including apathy. Scores were calculated by multiplying frequency (range, 0-4) and severity (range, 0-3); higher scores indicate more frequent and/or severe symptoms. The ADCS-CGIC, administered by an independent clinician, was used to assess clinically meaningful change in apathy. Ratings were based on a 7-point Likert-type scale (ranging from very much worse to very much improved); a rating of 4 was equivalent to no change.
Cognitive and Other Outcomes
Cognitive tests were performed at baseline and at 2, 4, and 6 months and included the Mini-Mental State Examination,23 Hopkins Verbal Learning Test,27 Wechsler Adult Intelligence Scale—Revised Digit Span,28 Trail Making Test parts A and B,29 action verbal fluency test from the Parkinson’s Disease—Cognitive Rating Scale,30 Category Fluency Task—Animal Naming,31 and the Short Boston Naming32 as previously described.20 Other outcomes included the Alzheimer’s Disease Cooperative Study Activities of Daily Living Scale,33 Dependence Scale,34 EuroQol 5-dimension 5-level,35 and the Resource Utilization in Dementia—Lite.36
Adverse Events and Safety Monitoring
Adverse events data were collected by systematic, close-ended questions for known or expected adverse events of methylphenidate, open-ended questions for unexpected adverse events, and results of vital signs, electrolyte panels, and electrocardiogram results. Weight loss of 7% or more from baseline was considered an adverse event. Serious adverse events were defined as adverse events leading to hospitalization, emergency department visit, or death. A 3-person data safety and monitoring committee reviewed accumulating, unmasked data on the safety and efficacy of methylphenidate compared with placebo. An interim analysis was reviewed when half of the study participants had been enrolled. There were no formal stopping rules or reported P values for this analysis.
Statistical Methods
Based on the difference in NPI apathy change scores (1.8 points) and SD (3.2) previously observed,18 a sample size of 200 provided for greater than 90% power to detect a difference of 1.8 points in change on the NPI apathy subscale with 15% loss of participants. The power and sample size for the ADCS-CGIC outcome was determined using the method of Whitehead37 for proportional odds logistic regression and values from ADMET of −5 for moderate and −3 for minimal improvement. Assuming an odds ratio for better ratings in methylphenidate of 2.75 and 200 participants, the study would have greater than 85% power with 15% losses. We assumed a type I error rate of 0.025 to preserve an overall type I error rate of 0.05 over both primary comparisons.
We used means, SDs, medians, and proportions to describe baseline characteristics of the study participants following verification of symmetrical distribution of continuous variables. The assessment of efficacy was based on an intention-to-treat analysis of the 2 primary outcomes: mean change in the NPI score from baseline to 6 months and change in rating on the ADCS-CGIC at 6 months.
The primary comparison for the first coprimary outcome, difference in mean change from baseline to month 6, was evaluated longitudinally using a linear mixed-effects model with random intercept for each participant and adjusted for clinic, age, sex, and diabetes. The primary comparison for the second coprimary outcome was a proportional odds logistic regression implemented by the ‘popower’ in the Hmisc package in R comparing the ADCS-CGIC ratings of change at month 6 between treatment groups. We also used a generalized mixed model to evaluate the mean change in the ratings between the treatment groups from baseline to month 6. Prespecified subgroup analyses included comparison of clinics located in the United States vs Canada and valid analyses by sex and race and ethnicity as required by National Institutes of Health policy. We used Kaplan-Meier estimates to determine the timeline associated with the incident absence of any apathy symptoms as measured using the NPI. Statistical analyses were performed using SAS statistical software version 9.2 (SAS Institute) and R version 3.16.1 (R Foundation). All P values were 2-sided and P < .05 was used as the threshold for statistical significance.
Results
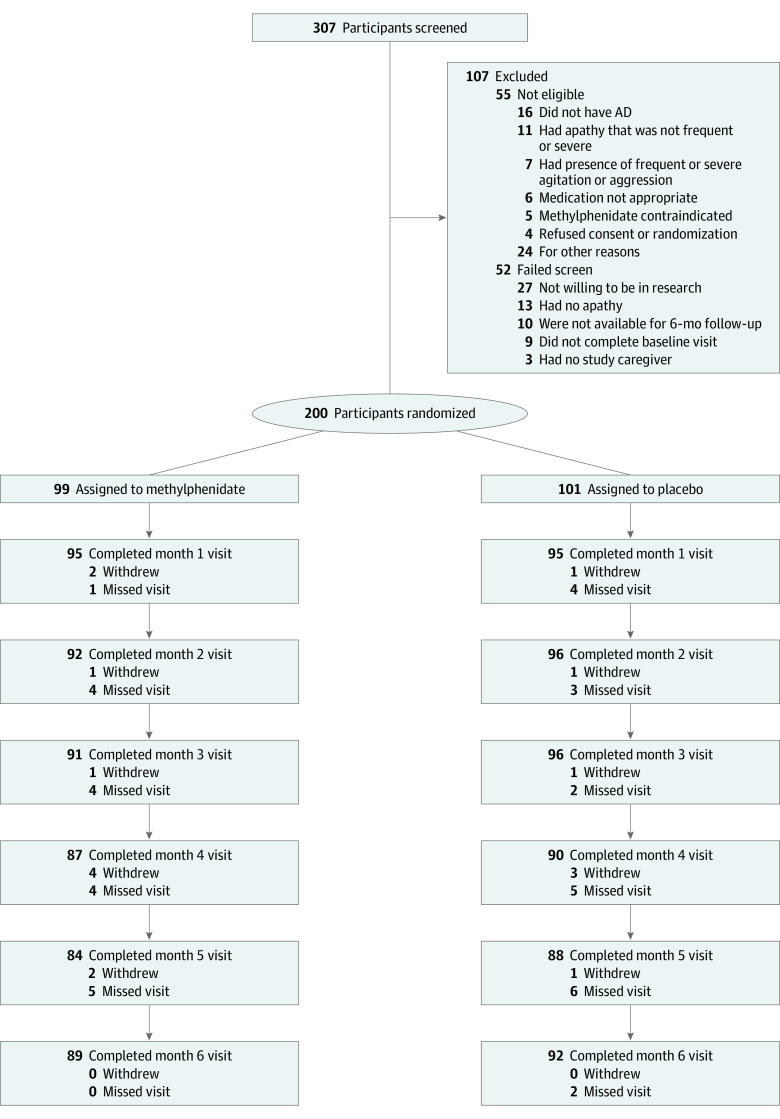
Of 307 persons screened, 52 did not pass the screen and 55 were not eligible following the baseline eligibility visit. Of the 200 individuals randomized, 99 were assigned to methylphenidate and 101 to placebo (Figure 1). Ten study participants in the methylphenidate group and 7 in the placebo group withdrew from the study; missed visits occurred throughout the study, with some delayed or missed owing to COVID-19. The intention-to-treat analysis at month 6 included 89 participants in the methylphenidate and 92 in the placebo group. Baseline data were missing for 1 participant.
Figure 1. CONSORT Flowchart.
AD indicates Alzheimer disease.
By participant/caregiver self-report, 69 participants (78%) in the methylphenidate group and 81 (88%) in the placebo group reported using drugs most of the time or always at all visits. Of those who received treatment, 72 (85.8%) in the methylphenidate group and 80 (90.7%) in the placebo group reported that they received the full 20-mg dosage across all visits. Nine study participants (5 in the methylphenidate and 4 in the placebo group) opted to discontinue medication at an intervening study visit but completed follow-up visits.
Baseline Characteristics
Participant demographics and baseline characteristics were similar across treatment groups (Table 1). The median (interquartile range) age of study participants was 76 (71-81) years. Most participants were male (131 [66%]), non-Hispanic (197 [99%]), White (181 [90%]), and had been diagnosed with dementia for about 3 years. Dementia was moderately severe with mean (SD) Mini-Mental State Examination scores of 19.2 (5) in the methylphenidate group and 18.5 (5) in the placebo group. The majority (157 [79%]) of study participants were being treated with dementia medications, including cholinesterase inhibitors (145 [73%]) and/or memantine (76 [38%]). Mean (SD) scores for the apathy subscale of the NPI were 8.0 (2.5) and 7.6 (2.3) in the methylphenidate and placebo groups, respectively, and 1.9 (0.5) on the Dementia Apathy Interview and Rating scale for both groups. A total of 71 participants (36%) were taking selective serotonin reuptake inhibitors at baseline.
Table 1. Baseline Characteristics of Participants.
| Characteristic | No./total No. (%) | ||
|---|---|---|---|
| Total (N = 200)a | Methylphenidate group (n = 99) | Placebo group (n = 101) | |
| Age, median (IQR), y | 76 (71-81) | 77 (71-81) | 76 (70-81) |
| Women | 68/199 (34) | 33/98 (34) | 35/101 (35) |
| Men | 131/199 (66) | 65/98 (66) | 66/101 (65) |
| Race and ethnicity | |||
| Black/African American | 12/199 (6) | 6/98 (6) | 6/101 (6) |
| Hispanic/Latino | 1/199 (0.50) | 0/98 (0) | 1/101 (1) |
| White | 181/199 (90) | 90/98 (92) | 91/101 (90) |
| Otherb | 5/199 (2.5) | 1/98 (1) | 4/101 (4) |
| Unknown or not reported | 1/199 (0.5) | 1/98 (1) | 0 |
| Marital status | |||
| Married | 171/199 (86) | 83/98 (85) | 88/101 (87) |
| Single | 28/199 (14) | 15/98 (15) | 13/101 (13) |
| Education | |||
| <High school | 10/199 (5) | 4/98 (4) | 6/101 (6) |
| High school diploma or GED | 39/199 (20) | 20/98 (20) | 19/101 (19) |
| Some college or associate degree | 40/199 (20) | 17/98 (17) | 23/101 (23) |
| College degree | 58/199 (29) | 30/98 (31) | 28/101 (28) |
| Postgraduate degree | 52/199 (26) | 27/98 (28) | 25/101 (25) |
| BMI, mean (SD) | |||
| Women | 25.9 (4.5) | 25.5 (5.1) | 26.3 (3.9) |
| Men | 27.6 (4.3) | 27.0 (4.2) | 28.2 (4.4) |
| Age at onset of AD, median (IQR), y | 73 (67-78) | 73 (67-77) | 72 (67-79) |
| Missing | 18/199 (9.0) | 13/98 (13.3) | 5/101 (5) |
| Personal psychiatric history before onset of AD | |||
| Other dementia | 8/199 (4) | 4/98 (4) | 4/101 (4) |
| Other mood disorder | 6/199 (3) | 2/98 (2) | 4/101 (4) |
| No psychiatric disorder reported | 143/199 (72) | 71/98 (72) | 72/101 (71) |
| Hypertension | 117/199 (59) | 55/98 (56) | 62/101 (61) |
| Diabetes | 31/199 (16) | 10/98 (10) | 21/101 (21) |
| Blood pressure level, median (IQR), mm Hg | |||
| Systolic | 134 (125-149) | 132 (124-148) | 136.0 (126-150) |
| Diastolic | 77 (70-82) | 76 (70-82) | 77 (70-82) |
| Abnormal ECG results | |||
| Not clinically significant | 118/199 (65) | 61/98 (67) | 57/101 (63) |
| Clinically significant | 2/199 (1) | 1/98 (1) | 1/101 (1) |
| Cigarette smoking behavior | |||
| Never | 111/199(56) | 57/98 (58) | 54/101 (54) |
| Ever | 82/199 (41) | 38/98 (39) | 44/101 (44) |
| Current | 5/199 (3) | 2/98 (2) | 3/101 (3) |
| Current medications | |||
| Memantine | 75/199 (38) | 38/98 (39) | 37/101 (37) |
| Cholinesterase inhibitor | 145/199 (73) | 75/98 (76) | 70/101 (69) |
| Other AD medication | 3/199 (1.5) | 1/98 (1) | 2/101 (2) |
| Not using any AD medication | 42/199 (21) | 19/98 (19) | 23/101 (23) |
| Selective serotonin reuptake inhibitor | 71/199 (36) | 32/98 (32) | 39/101 (39) |
| Serotonin-norepinephrine reuptake inhibitor | 13/199 (7) | 7/98 (7) | 6/101 (6) |
| Neuropsychiatric Inventory score, mean (SD) | |||
| Total | 16.4 (9.8) | 15.2 (7.5) | 17.6 (11.5) |
| Apathy subscale | 7.8 (2.4) | 8.0 (2.5) | 7.6 (2.3) |
| DAIR score, mean (SD) | 1.9 (0.5) | 1.9 (0.5) | 1.9 (0.5) |
| MMSE score, mean (SD) | 18.8 (5) | 19.2 (5) | 18.5 (5) |
Abbreviations: AD, Alzheimer disease; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); DAIR, Dementia Apathy Interview and Rating; ECG, electrocardiogram; IQR, interquartile range; MMSE, Mini-Mental State Examination.
Baseline data are missing for 1 participant.
Other included American Indian, Asian, Black, Native Hawaiian, White, and other race.
Hypertension was prevalent across both groups at 56% (55 of 98) and 61% (62 of 101) in the methylphenidate and placebo groups, respectively. The proportion of participants reporting a history of diabetes was lower in the methylphenidate compared with the placebo group (10 of 98 [10%] vs 21 of 101 [21%]).
Apathy Outcomes
At 6 months, using an adjusted longitudinal model, we observed a significant difference between treatment groups in change in the NPI apathy score from baseline to 6 months with a larger difference in the methylphenidate compared with the placebo group (mean difference = −1.25; 95% CI, −2.03 to −0.47; P = .002), equivalent to Cohen d of 0.365 (Table 2; eTable 1 in Supplement 2).
Table 2. Measures of Apathy at 6 Months in Persons With Alzheimer Disease Participating in the ADMET 2 Trial.
| Characteristic | No. (%) | |
|---|---|---|
| Methylphenidate group | Placebo group | |
| No. of participants with data at 6 mo | 89 | 91 |
| NPI apathy subscale | ||
| Score at 6 mo, mean (SD) | 3.5 (3.4) | 4.6 (3.3) |
| Change from baseline to 6 mo, mean (SD) | −4.5 (4.1) | −3.1 (3.6) |
| Estimated treatment effect (methylphenidate – placebo), mean (95% CI)a,b | −1.25 (−2.03 to −0.47) | |
| P value | .002 | |
| Cohen d | 0.365 | |
| ADCS-CGIC change in apathy at 6 mo | ||
| Improvement | ||
| Marked | 2 (2.2) | 2 (2.2) |
| Moderate | 14 (15.7) | 10 (11.0) |
| Minimal | 23 (25.8) | 20 (22.0) |
| No change | 42 (47.2) | 40 (44.0) |
| Worsening | ||
| Minimal | 7 (7.9) | 16 (17.6) |
| Moderate | 1 (1.1) | 3 (3.3.) |
| Marked | 0 (.00) | 0 (0.0) |
| Estimated treatment effect (methylphenidate vs placebo), OR (95% CI)b | 1.90 (0.95-3.84) | |
| P value | .07 | |
| Estimated difference in mean change from baseline to 6 mo (methylphenidate vs placebo), OR (95% CI)c | 1.43 (1.00-2.04) | |
| P value | .048 | |
Abbreviations: ADCS-CGIC, Alzheimer’s Disease Cooperative Study Clinical Global Impression of Change; ADMET, Apathy in Dementia Methylphenidate Trial; NPI, Neuropsychiatric Inventory; OR, odds ratio.
Estimated in a longitudinal model for mean difference between arms in change from baseline, using a linear mixed model with random intercept for each participant, adjusting for clinic, age, sex, and diabetes condition.
Estimated difference in proportional odds of improvement at 6 months.
Estimated in a longitudinal model for mean difference between arms in change from baseline, for all follow-up visits, adjusted for clinic, participant sex, and diabetes condition.
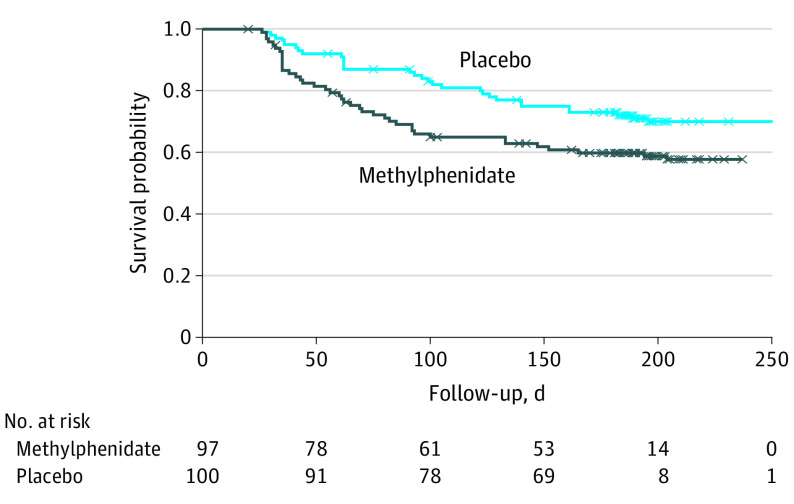
The largest change in NPI apathy occurred during the first 2 months of treatment (Figure 2). At month 6, 27% (24 of 89) of participants in the methylphenidate group had an NPI apathy score of 0 compared with 14% (13 of 90) in the placebo group. We estimated the rate at which participants achieved an NPI apathy score of 0, using Kaplan-Meier curves and observed a hazard ratio of 1.57 (95% CI, 0.97-2.53; P = .07) over the entire follow-up period (Figure 3). We observed a significant difference in the hazard ratio change (2.16; 95% CI, 1.19-3.91; P = .01) for the first 100 days, suggesting that the methylphenidate group demonstrated a decrease in the NPI apathy subscale sooner than the placebo group during this initial time period.
Figure 2. Mean (SE) Change in Neuropsychiatric Inventory (NPI) Apathy Subscale Score by Visit.
Figure 3. Kaplan-Meier Estimates of Proportion of Participants Achieving a Neuropsychiatric Inventory Apathy Score of 0.
Censored events are noted by X’s. Over the complete follow-up period of 6 months, the methylphenidate group had a 57% increase in the hazard ratio compared with the placebo group (hazard ratio, 1.57; 95% CI, 0.97-2.53; P = .07). For the first 100 days of follow-up, the methylphenidate group had more than twice the increase in the hazard ratio compared with the placebo group (hazard ratio, 2.16; 95% CI, 1.19-3.91; P = .01). The model was adjusted for age, sex, and presence of diabetes.
At 6 months, 43.8% (39 of 89) of participants in the methylphenidate group showed improvement compared with 35.2% (32 of 91) of study participants in the placebo group in the ADCS-CGIC (Table 2). The odds ratio of having an improved rating on the ADCS-CGIC for methylphenidate compared with placebo was 1.90 (95% CI, 0.95-3.84; P = .07), favoring methylphenidate over placebo. Using an adjusted longitudinal model, the odds ratio for difference in mean change from baseline to 6 months between treatment groups was 1.43 (95% CI, 1.00-2.04; P = .048). It should be noted that there was a strong association between improvement in NPI apathy and ADCS-CGIC apathy subscales (odds ratio, 2.95; 95% CI, 1.48-5.97; P = .002) and between improvement in NPI apathy subscale and overall ADCS-CGIC (odds ratio, 6.10; 95% CI, 1.35-56.67; P = .009) (eTables 2 and 3 in Supplement 2). Both groups showed a decrease in apathy from baseline to 6 months on the Dementia Apathy Interview and Rating (mean [SD]; −0.5 [0.8] in the methylphenidate and −0.4 [0.7] in the placebo group).
There were no significant treatment interactions by sex or race other than White. The results were similar for all other sensitivity analyses (data not shown).
Other Neuropsychiatric Symptoms
We observed no change in other neuropsychiatric symptoms, except for increased NPI aberrant motor behavior in the methylphenidate compared with the placebo group (mean difference 0.69; 95% CI, 0.09-1.25; P = .03) (eTable 4 and eFigures 1 and 2 in Supplement 2).
Adverse Events
There were 17 serious adverse events in the methylphenidate group and 10 in the placebo group (eTable 5 in Supplement 2), all hospitalizations for nonrelated events. More participants in the methylphenidate group lost more than 7% of their baseline body weight at follow-up (10 vs 6, respectively). Of these, 4 in each group had a body mass index (calculated as weight in kilograms divided by height in meters squared) greater than 25 at baseline. Other adverse events showed no important differences (eTable 6 in Supplement 2).
Cognitive and Other Measures
We found no treatment difference in cognitive measures (eTable 7 in Supplement 2), Alzheimer’s Disease Cooperative Study Activities of Daily Living Scale, Dependence Scale, or EuroQol (eTable 8 in Supplement 2). Resource utilization data will be presented in a subsequent article.
Discussion
In this study, methylphenidate treatment was associated with a small to medium reduction in apathy in patients with AD as shown by the NPI apathy subscale. These findings were first observed 2 months after treatment initiation and sustained over 6 months. The ADCS-CGIC did not show a statistically significant difference, but there was a trend favoring methylphenidate (eTable 9 in Supplement 2). Measures of caregiver distress did not show a statistically significant difference. However, both improvement in ADCS-CGIC and in measures of caregiver distress (eFigure 3 in Supplement 2) were associated with improvement in apathy, suggesting the clinical relevance of improvement in apathy as shown in this study. Adverse events were generally modest and consistent with those expected with methylphenidate.
The development of this study was based on data suggesting that the use of catecholamine/dopamine-enhancing agents is a promising and feasible approach to treat apathy in AD. This approach is based on the understanding that motivated behavior relies on subregions of the prefrontal cortex (dorsolateral, orbital-ventromedial, dorsomedial), which degenerate in AD resulting in apathetic behaviors. Methylphenidate treatment may ameliorate those symptoms by boosting norepinephrine and dopamine actions in prefrontal-striatal-thalamocortical circuits.15 Studies evaluating potential treatments for apathy in AD are limited and the results of some of them have been disappointing.38,39,40 However, 2 small trials evaluating the treatment of apathy in AD with methylphenidate have shown evidence of safety and efficacy in treating apathy.17,18,19 ADMET 2 was designed to examine the efficacy and safety of methylphenidate as treatment for clinically significant apathy in participants with AD, where efficacy was measured by looking at changes in apathy and cognition. ADMET 2 results are similar to prior studies showing a small to medium efficacy of methylphenidate as soon as 2 months after initiating treatment. Additionally, it is important to note that there were no group differences in any of the cognitive measures, suggesting that the effect of the treatment is specific to the treatment of apathy and not a secondary effect of improvement in cognition.
Patients experiencing major depressive episode as diagnosed by the Diagnostic and Statistical Manual of Mental Disorders may experience some of the same symptoms as those experiencing apathy, those are 2 very distinct clinical entities. Apathy is characterized by a lack of affect, while depression is characterized by an overwhelming presence of a negative affect and mood. Furthermore, literature has shown the lack of response of symptoms of apathy to selective serotonin reuptake inhibitors, supporting the importance of the distinction and providing evidence of 2 very different biological pathways.41,42 Most individuals in the study were also receiving currently approved AD treatments. The effect of concomitant medication, including cholinesterase inhibitors, memantine, and/or antidepressants, was examined, and we did not observe any effect in study results. Furthermore, doing so reflects clinical practice, where apathy would remain after stabilization on cognitive enhancers.
Limitations
Limitations of the study include the following: (1) participants composed a convenience sample of mainly White individuals in US and Canadian academic medical centers that may not generalize to other settings or to non-AD forms of dementia; (2) we did not obtain biomarker confirmation for the diagnosis of AD; (3) treatment was limited to 6 months; and (4) lack of data on potential participants who declined to participate or failed screening.
Conclusions
Apathy is a prevalent and clinically significant neuropsychiatric symptom in AD. Methylphenidate offers a treatment approach providing a modest but potentially clinically significant benefit for patients and caregivers. The efficacy has a relatively early onset and its effect is sustainable for at least 6 months. Clinicians should be aware of the small to medium treatment effects sizes and the lack of effect on activities of daily living.
Trial Protocol
eTable 1. Apathy/indifference from the Neuropsychiatric Inventory (NPI) by visit and treatment assignment
eTable 2. CGIC apathy (any vs no improvement) by change in NPI apathy score from baseline, categorized at median change at F6
eTable 3. Overall CGIC (any vs no improvement) by change in NPI apathy score from baseline, categorized at median change at F6
eTable 4. Average change in NPI neuropsychiatric symptom by treatment assignment and difference in change between treatment assignment at F6, observed and modelled
eTable 5. Participants reporting serious adverse events during follow-up by treatment assignment
eTable 6. Participants reporting non-serious adverse events during follow-up by treatment assignment
eTable 7. Change in cognitive score at 6 months by treatment group
eTable 8. EuroQol scores at baseline and 6 months follow-up by treatment group
eTable 9. Average change in apathy based on Alzheimer’s Disease Cooperative Study (ADCS) - Clinical Global Impression of Change (CGIC) classification by treatment assignment and difference in change between treatment assignment at F6, observed and modelled, and by visit
eFigure 1. Proportion of participants with neuropsychiatric symptoms other than apathy from the Neuropsychiatric Inventory (NPI) by visit and treatment assignment
eFigure 2. Severity of neuropsychiatric symptoms other than apathy from the Neuropsychiatric Inventory (NPI) by visit and treatment assignment
eFigure 3. Proportion of caregiver distress by visit and treatment assignment
Nonauthor Collaborators. The ADMET 2 Research Group
Data Sharing Statement
References
- 1.Brodaty H, Altendorf A, Withall A, Sachdev P. Do people become more apathetic as they grow older? a longitudinal study in healthy individuals. Int Psychogeriatr. 2010;22(3):426-436. doi: 10.1017/S1041610209991335 [DOI] [PubMed] [Google Scholar]
- 2.Benoit M, Berrut G, Doussaint J, et al. Apathy and depression in mild Alzheimer’s disease: a cross-sectional study using diagnostic criteria. J Alzheimers Dis. 2012;31(2):325-334. doi: 10.3233/JAD-2012-112003 [DOI] [PubMed] [Google Scholar]
- 3.Benoit M, Robert P. [Depression and apathy in Alzheimer's disease]. Presse Med. 2003;32(24 suppl):S14-S18. [PubMed] [Google Scholar]
- 4.Miller DS, Robert P, Ereshefsky L, et al. Diagnostic criteria for apathy in neurocognitive disorders. Alzheimers Dement. Published online May 5, 2021. doi: 10.1002/alz.12358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steinberg M, Shao H, Zandi P, et al. ; Cache County Investigators . Point and 5-year period prevalence of neuropsychiatric symptoms in dementia: the Cache County Study. Int J Geriatr Psychiatry. 2008;23(2):170-177. doi: 10.1002/gps.1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dauphinot V, Delphin-Combe F, Mouchoux C, et al. Risk factors of caregiver burden among patients with Alzheimer’s disease or related disorders: a cross-sectional study. J Alzheimers Dis. 2015;44(3):907-916. doi: 10.3233/JAD-142337 [DOI] [PubMed] [Google Scholar]
- 7.Smyth K, Neundorfer M, Stuckey J. Progression of Alzheimer's disese, caregiver quality of life, and resource use. Paper presented at: Eighth Congress of the International Psychogeriatric Association; August 17-22, 1997; Jerusalem, Israel. [Google Scholar]
- 8.Nijsten JMH, Leontjevas R, Pat-El R, Smalbrugge M, Koopmans RTCM, Gerritsen DL. Apathy: risk factor for mortality in nursing home patients. J Am Geriatr Soc. 2017;65(10):2182-2189. doi: 10.1111/jgs.15007 [DOI] [PubMed] [Google Scholar]
- 9.Vilalta-Franch J, Calvó-Perxas L, Garre-Olmo J, Turró-Garriga O, López-Pousa S. Apathy syndrome in Alzheimer’s disease epidemiology: prevalence, incidence, persistence, and risk and mortality factors. J Alzheimers Dis. 2013;33(2):535-543. doi: 10.3233/JAD-2012-120913 [DOI] [PubMed] [Google Scholar]
- 10.Spalletta G, Long JD, Robinson RG, et al. Longitudinal neuropsychiatric predictors of death in Alzheimer’s disease. J Alzheimers Dis. 2015;48(3):627-636. doi: 10.3233/JAD-150391 [DOI] [PubMed] [Google Scholar]
- 11.Herrmann N, Rothenburg LS, Black SE, et al. Methylphenidate for the treatment of apathy in Alzheimer disease: prediction of response using dextroamphetamine challenge. J Clin Psychopharmacol. 2008;28(3):296-301. doi: 10.1097/JCP.0b013e318172b479 [DOI] [PubMed] [Google Scholar]
- 12.Storga D, Vrecko K, Birkmayer JG, Reibnegger G. Monoaminergic neurotransmitters, their precursors and metabolites in brains of Alzheimer patients. Neurosci Lett. 1996;203(1):29-32. doi: 10.1016/0304-3940(95)12256-7 [DOI] [PubMed] [Google Scholar]
- 13.Salamone JD, Correa M. The mysterious motivational functions of mesolimbic dopamine. Neuron. 2012;76(3):470-485. doi: 10.1016/j.neuron.2012.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruthirakuhan MT, Herrmann N, Abraham EH, Chan S, Lanctôt KL. Pharmacological interventions for apathy in Alzheimer’s disease. Cochrane Database Syst Rev. 2018;5(5):CD012197. doi: 10.1002/14651858.CD012197.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Dyck CH, Arnsten AFT, Padala PR, et al. Neurobiologic rationale for treatment of apathy in Alzheimer's disease with methylphenidate. Am J Geriatr Psychiatry. 2021;29(1):51-62. doi: 10.1016/j.jagp.2020.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Epstein T, Patsopoulos NA, Weiser M. Immediate-release methylphenidate for attention deficit hyperactivity disorder (ADHD) in adults. Cochrane Database Syst Rev. 2014;(9):CD005041. doi: 10.1002/14651858.CD005041.pub2 [DOI] [PubMed] [Google Scholar]
- 17.Drye LT, Scherer RW, Lanctot KL, et al. Designing a trial to evaluate potential treatments for apathy in dementia: the apathy in dementia methylphenidate trial (ADMET). Am J Geriatr Psychiatry. 2013;21(6):549-559. doi: 10.1016/j.jagp.2012.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenberg PB, Lanctôt KL, Drye LT, et al. ; ADMET Investigators . Safety and efficacy of methylphenidate for apathy in Alzheimer’s disease: a randomized, placebo-controlled trial. J Clin Psychiatry. 2013;74(8):810-816. doi: 10.4088/JCP.12m08099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Padala PR, Padala KP, Lensing SY, et al. Methylphenidate for apathy in community-dwelling older veterans with mild Alzheimer’s disease: a double-blind, randomized, placebo-controlled trial. Am J Psychiatry. 2018;175(2):159-168. doi: 10.1176/appi.ajp.2017.17030316 [DOI] [PubMed] [Google Scholar]
- 20.Scherer RW, Drye L, Mintzer J, et al. ; ADMET 2 Research Group . The Apathy in Dementia Methylphenidate Trial 2 (ADMET 2): study protocol for a randomized controlled trial. Trials. 2018;19(1):46. doi: 10.1186/s13063-017-2406-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 22.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939-944. doi: 10.1212/WNL.34.7.939 [DOI] [PubMed] [Google Scholar]
- 23.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198. doi: 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 24.Cummings JL. The Neuropsychiatric Inventory: assessing psychopathology in dementia patients. Neurology. 1997;48(5)(suppl 6):S10-S16. doi: 10.1212/WNL.48.5_Suppl_6.10S [DOI] [PubMed] [Google Scholar]
- 25.Schneider LS, Olin JT, Doody RS, et al. Validity and reliability of the Alzheimer’s Disease Cooperative Study-Clinical Global Impression of Change: the Alzheimer’s Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;11(suppl 2):S22-S32. doi: 10.1097/00002093-199700112-00004 [DOI] [PubMed] [Google Scholar]
- 26.Strauss ME, Sperry SD. An informant-based assessment of apathy in Alzheimer disease. Neuropsychiatry Neuropsychol Behav Neurol. 2002;15(3):176-183. [PubMed] [Google Scholar]
- 27.Brandt J, Benedict RHB. Hopkins Verbal Learning Test-Revised Professional Manual. Psychological Assessment Resources; 2001. [Google Scholar]
- 28.Wechsler D. Wechsler Adult Intelligence Scale: Revised Manual. The Psychological Corporation; 1981. [Google Scholar]
- 29.Stuss DT, Bisschop SM, Alexander MP, Levine B, Katz D, Izukawa D. The Trail Making Test: a study in focal lesion patients. Psychol Assess. 2001;13(2):230-239. doi: 10.1037/1040-3590.13.2.230 [DOI] [PubMed] [Google Scholar]
- 30.Piatt AL, Fields JA, Paolo AM, Tröster AI. Action (verb naming) fluency as an executive function measure: convergent and divergent evidence of validity. Neuropsychologia. 1999;37(13):1499-1503. doi: 10.1016/S0028-3932(99)00066-4 [DOI] [PubMed] [Google Scholar]
- 31.Isaacs B, Akhtar AJ. The set test: a rapid test of mental function in old people. Age Ageing. 1972;1(4):222-226. doi: 10.1093/ageing/1.4.222 [DOI] [PubMed] [Google Scholar]
- 32.Franzen MD, Haut MW, Rankin E, Keefover R. Empirical comparison of alternate forms of the Boston Naming Test. Clinical Neuropsychol. 1995;9(3):225-229. doi: 10.1080/13854049508400484 [DOI] [Google Scholar]
- 33.Galasko D, Bennett D, Sano M, et al. An inventory to assess activities of daily living for clinical trials in Alzheimer’s disease: the Alzheimer’s Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;11(suppl 2):S33-S39. doi: 10.1097/00002093-199700112-00005 [DOI] [PubMed] [Google Scholar]
- 34.Stern Y, Albert SM, Sano M, et al. Assessing patient dependence in Alzheimer’s disease. J Gerontol. 1994;49(5):M216-M222. doi: 10.1093/geronj/49.5.M216 [DOI] [PubMed] [Google Scholar]
- 35.Johnson JA, Coons SJ, Ergo A, Szava-Kovats G. Valuation of EuroQOL (EQ-5D) health states in an adult US sample. Pharmacoeconomics. 1998;13(4):421-433. doi: 10.2165/00019053-199813040-00005 [DOI] [PubMed] [Google Scholar]
- 36.Wimo A, Gustavsson A, Jönsson L, Winblad B, Hsu MA, Gannon B. Application of Resource Utilization in Dementia (RUD) instrument in a global setting. Alzheimers Dement. 2013;9(4):429-435.e17. doi: 10.1016/j.jalz.2012.06.008 [DOI] [PubMed] [Google Scholar]
- 37.Whitehead J. Sample size calculations for ordered categorical data. Stat Med. 1993;12(24):2257-2271. doi: 10.1002/sim.4780122404 [DOI] [PubMed] [Google Scholar]
- 38.Seltzer B, Zolnouni P, Nunez M, et al. ; Donepezil “402” Study Group . Efficacy of donepezil in early-stage Alzheimer disease: a randomized placebo-controlled trial. Arch Neurol. 2004;61(12):1852-1856. doi: 10.1001/archneur.61.12.1852 [DOI] [PubMed] [Google Scholar]
- 39.Rea R, Carotenuto A, Fasanaro AM, Traini E, Amenta F. Apathy in Alzheimer’s disease: any effective treatment? ScientificWorldJournal. 2014;2014:421385. doi: 10.1155/2014/421385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frakey LL, Salloway S, Buelow M, Malloy P. A randomized, double-blind, placebo-controlled trial of modafinil for the treatment of apathy in individuals with mild-to-moderate Alzheimer’s disease. J Clin Psychiatry. 2012;73(6):796-801. doi: 10.4088/JCP.10m06708 [DOI] [PubMed] [Google Scholar]
- 41.Barnhart WJ, Makela EH, Latocha MJ. SSRI-induced apathy syndrome: a clinical review. J Psychiatr Pract. 2004;10(3):196-199. doi: 10.1097/00131746-200405000-00010 [DOI] [PubMed] [Google Scholar]
- 42.Padala PR, Padala KP, Majagi AS, Garner KK, Dennis RA, Sullivan DH. Selective serotonin reuptake inhibitors-associated apathy syndrome: a cross sectional study. Medicine (Baltimore). 2020;99(33):e21497. doi: 10.1097/MD.0000000000021497 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Apathy/indifference from the Neuropsychiatric Inventory (NPI) by visit and treatment assignment
eTable 2. CGIC apathy (any vs no improvement) by change in NPI apathy score from baseline, categorized at median change at F6
eTable 3. Overall CGIC (any vs no improvement) by change in NPI apathy score from baseline, categorized at median change at F6
eTable 4. Average change in NPI neuropsychiatric symptom by treatment assignment and difference in change between treatment assignment at F6, observed and modelled
eTable 5. Participants reporting serious adverse events during follow-up by treatment assignment
eTable 6. Participants reporting non-serious adverse events during follow-up by treatment assignment
eTable 7. Change in cognitive score at 6 months by treatment group
eTable 8. EuroQol scores at baseline and 6 months follow-up by treatment group
eTable 9. Average change in apathy based on Alzheimer’s Disease Cooperative Study (ADCS) - Clinical Global Impression of Change (CGIC) classification by treatment assignment and difference in change between treatment assignment at F6, observed and modelled, and by visit
eFigure 1. Proportion of participants with neuropsychiatric symptoms other than apathy from the Neuropsychiatric Inventory (NPI) by visit and treatment assignment
eFigure 2. Severity of neuropsychiatric symptoms other than apathy from the Neuropsychiatric Inventory (NPI) by visit and treatment assignment
eFigure 3. Proportion of caregiver distress by visit and treatment assignment
Nonauthor Collaborators. The ADMET 2 Research Group
Data Sharing Statement