Key Points
Question
What individual- and community-level factors are associated with detectable blood lead levels (BLLs) in children?
Findings
This cross-sectional study linking Quest Diagnostics childhood lead testing and US Census data captured individual- and community-level disparities in lead exposure from October 2018 through February 2020. In adjusted models, the proportion of children with detectable (≥1.0 μg/dL) and elevated (≥5.0 μg/dL) BLLs increased significantly among those with public insurance and for progressive quintiles of community pre-1950s housing and poverty.
Meaning
Similar individual- and community-level disparities were seen among children with detectable BLLs and those with elevated BLLs.
Abstract
Importance
No safe level of exposure to lead has been identified.
Objective
To evaluate individual- and community-level factors associated with detectable and elevated blood lead levels (BLLs) in children.
Design, Setting, and Participants
This cross-sectional, retrospective study analyzed deidentified results from blood lead tests performed at a large clinical laboratory from October 1, 2018, to February 29, 2020. Participants were 1 141 441 children younger than 6 years living in all 50 US states and the District of Columbia who underwent blood lead testing during the study period. Children who underwent lead testing of unknown source and those with elevated BLLs who received capillary blood lead testing without confirmatory venous testing were excluded.
Exposures
Individual demographic categories included sex, age, and insurance type; community-level demographic categories included pre-1950s housing, poverty, predominant race and ethnicity, and geographical regions.
Main Outcomes and Measures
Proportions of children with detectable (≥1.0 μg/dL) and elevated (≥5.0 μg/dL) BLLs, by exposure category.
Results
Of the 1 141 441 children (586 703 boys [51.4%]; mean [SD] age, 2.3 [1.4] years) in the study, more than half of the children tested (576 092 [50.5%; 95% CI, 50.4%-50.6%]) had detectable BLLs, and 21 172 children (1.9% [95% CI, 1.8%-1.9%]) had BLLs of 5.0 μg/dL or more. In multivariable analyses, children with public insurance had greater odds of having detectable BLLs (adjusted odds ratio [AOR], 2.01 [95% CI, 1.99-2.04]) and elevated BLLs (AOR, 1.08 [95% CI, 1.04-1.12]). The proportion of children with detectable and elevated BLLs increased significantly for progressive pre-1950s housing and poverty quintiles (P < .001). The odds of detectable BLLs were significantly higher among children in the highest vs lowest quintile of pre-1950s housing (AOR, 1.65 [95% CI, 1.62-1.68]) and of poverty (AOR, 1.89 [95% CI, 1.86-1.93]). A similar association was found for those with elevated BLLs, with an AOR of 3.06 (95% CI, 2.86-3.27) for the highest vs lowest quintile of pre-1950 housing and 1.99 (95% CI, 1.88-2.11) for the highest quintile of poverty. Children residing in zip codes with predominantly Black non-Hispanic and non-Latinx populations had higher odds of detectable BLLs (AOR, 1.13 [95% CI, 1.11-1.15]) but lower odds for elevated BLL (AOR, 0.83 [95% CI, 0.80-0.88]).
Conclusions and Relevance
This study suggests that, despite progress in reducing pediatric lead exposure, substantial individual- and community-level disparities persist.
This cross-sectional study evaluates individual- and community-level factors associated with detectable and elevated blood lead levels in children.
Introduction
Lead has no biological role in the body. Any detectable lead level is abnormal and potentially harmful, particularly in young children; no safe level of exposure to lead for children has been identified.1,2,3,4 The existing literature on risk factors for lead exposure in children is extensive but focuses largely on elevated blood lead levels (BLLs), defined as 5.0 μg/dL or more (to convert to micromoles per liter, multiply by 0.0483). Less evaluation is available on lead exposure at levels that are lower but still detectable (≥1.0 μg/dL), even though BLLs in this range are associated with adverse effects in infants and children.5,6 Understanding individual- and neighborhood-level risk factors that are associated with any exposure to lead in children may be important in targeting efforts to mitigate adverse effects.
During the past nearly 40 years, BLLs have decreased because of successful policies aimed at controlling lead in the environment and by eliminating it from gasoline, paint, plumbing fixtures, and consumer products.7,8 As a result of these policies, millions of children have been spared from the very high BLLs once ubiquitous in the United States.9 Nevertheless, numerous environmental sources of legacy lead still exist. Because of their developmentally appropriate hand-to-mouth exploratory behaviors, children are at particular risk in environments contaminated with lead.10
Disparities persist, meaning that lead exposure is disproportionately associated with adverse effects in vulnerable groups including immigrant children, low-income families, and young children of ethnic and racial minorities,11 based on age, socioeconomic, occupational, developmental, and cultural risk factors.12 Children living at or below the poverty line in older housing or in communities with high concentrations of poverty are at the greatest risk of the toxic effects from lead.12,13,14 In addition, low socioeconomic status places children at increased risk of nutritional problems. For example, iron deficiency has been associated with a 4- to 5-fold increase in baseline risk of harm from lead because of increased absorption of lead by the divalent metal transporter in the gastrointestinal tract.15 Less is known about whether these disparities and other factors apply to BLLs that are detectable at lower levels or whether particular community-level factors can be identified to refine strategies for the primary prevention of exposure to the toxic effects of lead.
In 2012, the Advisory Committee on Childhood Lead Poisoning Prevention of the Centers for Disease Control and Prevention (CDC) cited new scientific data indicating that there is no known lower threshold for lead-based health effects.16 In response, the CDC lowered the BLL reference value to 5.0 μg/dL (from 10 μg/dL) to trigger public health action and, at the same time, emphasized the importance of primary prevention efforts given the lack of an identified threshold for the deleterious neurodevelopmental effects of lead.7,17,18 The reference level of 5.0 μg/dL is based on the 97.5th percentile of the BLL distribution in children reported by the National Health and Nutrition Examination Survey (NHANES), with a recommendation to reevaluate this reference level every 5 years.7 More recently, the CDC subcommittee on lead poisoning prevention recommended further lowering the reference value for BLL in children to 3.5 μg/dL, based on the 2011-2014 NHANES report.19
Honoring the spirit of the Advisory Committee on Childhood Lead Poisoning Prevention recommendation, this study follows up on an earlier report of national trends in pediatric lead exposure,13 using a lower BLL threshold to analyze data from one of the largest national reference clinical laboratories in the United States. Like the earlier study on which it builds, the present study examines factors that may be associated with patterns of BLLs, including insurance type, pre-1950 housing, poverty, latitude, age, sex, and geography (US states).
Methods
Study Population
In this retrospective, cross-sectional, observational analysis of deidentified results from a clinical laboratory, blood lead testing results from children younger than 6 years, obtained from October 1, 2018, through February 29, 2020, were selected for potential inclusion. October 2018 was selected as the first full month after Quest Diagnostics began reporting blood lead testing results with a detection threshold of 1.0 μg/dL. February 2020 was chosen to eliminate the potential effects associated with the COVID-19 pandemic. A Quest Diagnostics unique patient identifier was required to assess the number of test results per patient. If a participant had 2 or more tests within the data set, only the first result within the data set was included. However, if the first specimen was from capillary blood and the result was 5.0 μg/dL or more, a confirmatory specimen was required within 90 days; in this case, only the results from the confirmatory specimens were considered in this analysis, consistent with the CDC definition of “confirmed elevated BLL.”20 Capillary blood test results of 5.0 μg/dL or more without confirmation were excluded from analysis because of the possibility of contamination.21 Specimens from an unknown source (capillary or venous) were also excluded. This Quest Diagnostics Health Trends study was deemed exempt by the Western Institutional Review Board and parental consent was not required, as the study is of aggregated deidentified data.
Laboratory Methods
Venous blood specimens were collected into evacuated tubes certified for lead testing, such as tan-top and royal blue–top tubes containing the anticoagulant EDTA. Capillary blood specimens were collected in lavender-top capillary tubes. Specimens were analyzed using either inductively coupled plasma mass spectrometry or Zeeman graphite furnace atomic absorption spectroscopy. Instrument calibrations were performed using National Institute of Standards and Technology traceable Standard Reference Material 3128.22 The coefficient of variation of the lead measurement of quality control material of approximately 3.0 μg/dL was consistently less than 10%.
Estimates by Zip Code
To analyze demographic factors that were not available for individual children, estimates from the US Census were obtained for each zip code and linked to patient residential zip code. For race and ethnicity,23 poverty,24 and pre-1950s housing,25 estimates were obtained from the 2018 5-year American Community Survey. Zip codes with estimated proportions of Black non-Hispanic and non-Latinx patients of more than 50% were classified as predominately Black non-Hispanic and non-Latinx (hereafter referred to as Black). The same pattern was followed for zip codes with predominantly Hispanic and Latinx and predominantly White non-Hispanic and non-Latinx populations (hereafter referred to as White) in a manner consistent with previous literature.26 Pre-1950s housing was analyzed because, although the manufacture of lead-based paint was banned nationally in 1978, some cities had enacted legislation on lead-based paint as early as the 1950s, and some manufacturers had initiated voluntary reduction of lead concentrations in paints.27 As a result, paint produced after the 1950s tended to have lower concentrations of lead than that produced earlier.28 Poverty is defined as the proportion of the community population below the poverty threshold, defined by the poverty to income ratio of less than 1.0. A latitude for each zip code was assigned using SAS Institute reference data, and the results were stratified into 3 groups: above 40 degrees (northern), 32 to 40 degrees (central), and below 32 degrees (southern).
Reporting Thresholds and BLL Groups
Any value at or above the reporting threshold of 1.0 μg/dL was considered a detectable BLL. Any value of 5.0 μg/dL or more was considered an elevated BLL. Also, the proportion was analyzed among children with BLL values of 1.0 to 1.9, 2.0 to 2.9, 3.0 to 3.9, 4.0 to 4.9, 5.0 to 9.9 μg/dL (between the 2012 CDC reference level and the previous 1991 CDC level of concern), and 10 μg/dL or more.
Geographical Categories
This study includes children from all 50 US states and the District of Columbia. The proportion of children with detectable BLLs of 1.0 μg/dL or more and BLLs of 5.0 μg/dL or more (elevated BLLs) are reported for US states with results from at least 500 children.
Statistical Analysis
The Cochran-Armitage test was used to analyze trends in proportions of children with different BLLs for quintile groups. Testing for the statistical significance of differences between 2 groups was conducted using the χ2 test. Multivariable logistic regression models to evaluate characteristics associated with detectable BLLs and elevated BLLs are reported. Variables in both models were chosen based on plausibility and/or statistical significance in previous studies13,14 and remained in the final model using a stepwise entry criterion of P < .01. Model performance was assessed using the area under the receiver operating curve. Analyses were performed using SAS Studio 3.6 on SAS, version 9.4 (SAS Institute Inc).
Results
Test results from 1 150 948 children were selected for potential inclusion. Of these results, 9507 were excluded because the first specimen was from an unknown blood source (n = 7508) or because a child with an initial capillary blood test result of 5.0 μg/dL or more did not undergo confirmation testing within 90 days (n = 1999). The final analytic cohort thus comprised 1 141 441 children (99.2% of the children considered; 586 703 boys [51.4%]; mean [SD] age, 2.3 [1.4] years). Most children in this study had BLL test results obtained from venous blood specimens (844 961 [74.0%]) and were between 1.0 and 2.9 years of age (699 359 [61.3%]). The demographic characteristics of children included in the study and the distribution of BLL results are shown in the Table.
Table. Demographic Characteristics of Children by Blood Lead Level.
| Characteristic | Total No. | Blood lead level, μg/dL, No. (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| <1.0 | 1.0-1.9 | 2.0-2.9 | 3.0-3.9 | 4.0-4.9 | 5.0-9.9 | ≥10.0 | ||
| All children | 1 141 441 | 565 349 (49.5) | 408 689 (35.8) | 99 885 (8.8) | 30 839 (2.7) | 15 507 (1.4) | 15 963 (1.4) | 5209 (0.5) |
| Blood source | ||||||||
| Venous | 844 961 | 441 738 (52.3) | 279 960 (33.1) | 68 616 (8.1) | 22 016 (2.6) | 11 622 (1.4) | 15 843 (1.9) | 5166 (0.6) |
| Capillary | 296 480 | 123 611 (41.7) | 128 729 (43.4) | 31 269 (10.6) | 8823 (3.0) | 3885 (1.3) | 120 (0.04) | 43 (0.01) |
| Sexa | ||||||||
| Female | 554 172 | 278 213 (50.2) | 197 849 (35.7) | 47 024 (8.5) | 14 279 (2.6) | 7121 (1.3) | 7313 (1.3) | 2373 (0.4) |
| Male | 586 703 | 286 770 (48.9) | 210 721 (35.9) | 52 816 (9.0) | 16 548 (2.8) | 8373 (1.4) | 8646 (1.5) | 2829 (0.5) |
| Age group, y | ||||||||
| <1.0 | 114 685 | 65 888 (57.5) | 34 953 (30.5) | 8528 (7.4) | 2517 (2.2) | 1220 (1.1) | 1220 (1.1) | 359 (0.3) |
| 1.0-1.9 | 411 833 | 201 190 (48.9) | 151 288 (36.7) | 35 931 (8.7) | 10 907 (2.7) | 5348 (1.3) | 5407 (1.3) | 1762 (0.4) |
| 2.0-2.9 | 287 526 | 131 750 (45.8) | 109 203 (38.0) | 27 920 (9.7) | 8469 (3.0) | 4301 (1.5) | 4402 (1.5) | 1481 (0.5) |
| 3.0-3.9 | 129 162 | 61 161 (47.4) | 47 268 (36.6) | 11 840 (9.2) | 3947 (3.1) | 1966 (1.5) | 2214 (1.7) | 766 (0.6) |
| 4.0-4.9 | 120 411 | 61 951 (51.5) | 41 900 (34.8) | 9732 (8.1) | 3110 (2.6) | 1621 (1.4) | 1599 (1.3) | 498 (0.4) |
| 5.0-5.9 | 77 824 | 43 409 (55.8) | 24 077 (30.9) | 5934 (7.6) | 1889 (2.4) | 1051 (1.4) | 1121 (1.4) | 343 (0.4) |
| Latitude (degrees)b | ||||||||
| Northern (>40) | 505 841 | 262 765 (52.0) | 156 846 (31.0) | 50 106 (9.9) | 15 898 (3.1) | 8048 (1.6) | 9058 (1.8) | 3120 (0.6) |
| Central (32-40) | 482 470 | 207 780 (43.1) | 207 133 (42.9) | 41 138 (8.5) | 12 523 (2.6) | 6276 (1.3) | 5881 (1.2) | 1739 (0.4) |
| Southern (<32) | 151 206 | 94 172 (62.3) | 43 822 (29.0) | 8426 (5.6) | 2351 (1.6) | 1143 (0.8) | 954 (0.6) | 338 (0.2) |
SI conversion factor: To convert blood lead level to micromoles per liter, multiply by 0.0483.
Missing data for 566 patients.
Missing data for 1924 patients.
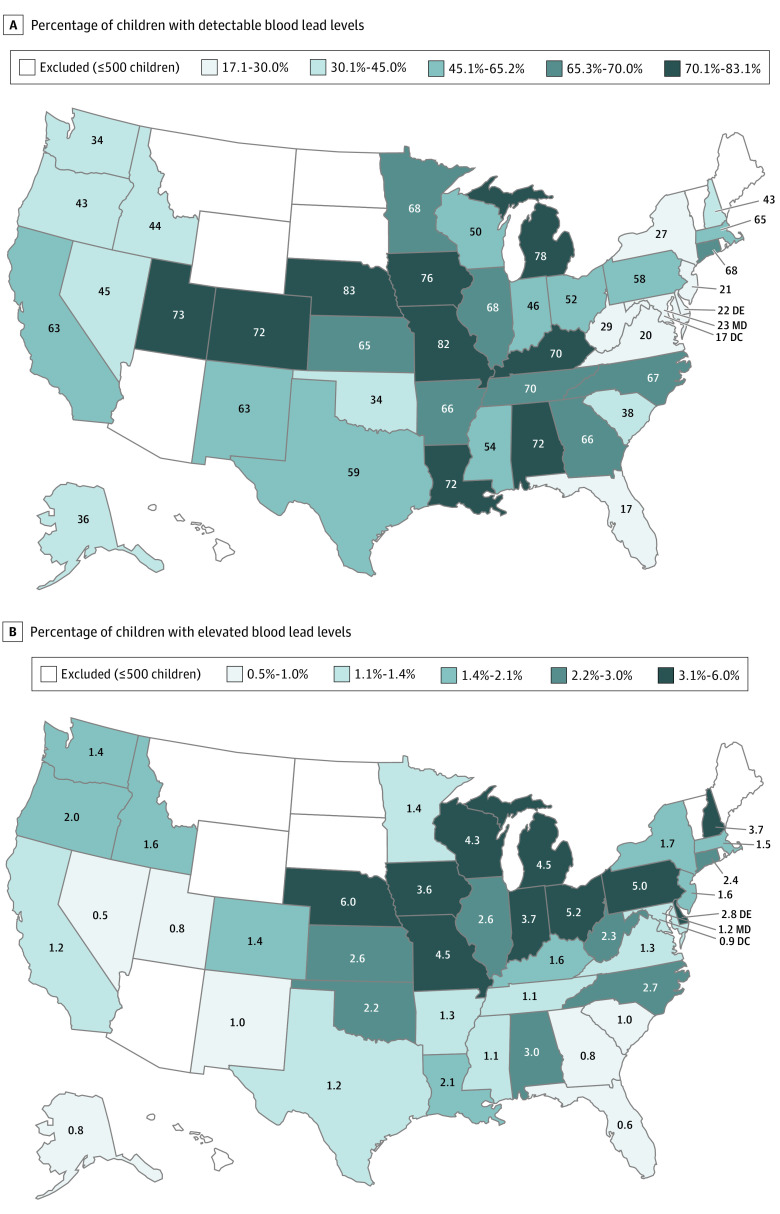
More than half of the children (576 092 [50.5%; 95% CI, 50.4%-50.6%]) had detectable BLLs, including most children from 24 different states (Figure 1), and 21 172 children (1.9% [95% CI, 1.8%-1.9%]) had BLLs of 5.0 μg/dL or more. The highest proportions of children with detectable BLLs were found in Nebraska (83% [95% CI, 81%-85%]), Missouri (82% [95% CI, 82%-83%]), Michigan (78% [95% CI, 78%-79%]), Iowa (76% [95% CI, 74%-77%]), and Utah (73% [95% CI, 70%-76%]). Six states had proportions of elevated BLLs more than double the national rate of 1.9% (95% CI, 1.8%-1.9%): Nebraska (6.0% [95% CI, 4.8%-7.2%]), Ohio (5.2% [95% CI, 4.7%-5.6%]), Pennsylvania (5.0% [95% CI, 4.9%-5.2%]), Missouri (4.5% [95% CI, 4.2%-4.8%]), Michigan (4.5% [95% CI, 4.1%-4.9%]), and Wisconsin (4.3% [95% CI, 3.8%-4.8%]). Results for detectable BLLs and BLLs of 5.0 μg/dL or more from additional states are displayed in Figure 1. These differences persisted in multivariate regression models, adjusting for individual- and community-level factors.
Figure 1. Children With Detectable or Elevated Blood Lead Levels by State.
A, Percentage of children with detectable blood lead levels. B, Percentage of children with elevated blood lead levels. Analysis limited to states and the Discrict of Columbia with results for more than 500 children. Detectable blood lead level, 1.0 μg/dL or more; elevated blood lead level, 5.0 μg/dL or more (to convert to micromoles per liter, multiply by 0.0483).
The proportion of children with detectable BLLs increased significantly with progressive quintiles of pre-1950s housing (from 42.6% [95% CI, 42.4%-42.8%] to 57.2% [95% CI, 57.0%-57.4%]; P < .001) (Figure 2A) and of poverty (from 38.8% [95% CI, 38.6%-39.0%] to 60.2% [95% CI, 60.0%-60.4%]; P < .001) (Figure 2B). In addition, the proportion of children with BLLs of 5.0 μg/dL or more increased significantly with progressive quintiles of pre-1950s housing (from 0.9% [95% CI, 0.8%-0.9%] to 3.5% [95% CI, 3.4%-3.6%]; P < .001) and of poverty (from 1.2% [95% CI, 1.1%-1.2%] to 2.9% [95% CI, 2.8%-2.9%]; P < .001). Zip codes with predominately Black populations had significantly higher proportions of children with detectable BLLs and BLLs of 5.0 μg/dL or more than did zip codes with predominately White populations (detectable BLLs, 57.6% [95% CI, 57.3%-57.9%] vs 48.7% [95% CI, 48.6%-48.9%]; P < .001; BLLs ≥5.0 μg/dL, 2.5% [95% CI, 2.4%-2.6%] vs 2.0% [95% CI, 2.0%-2.1%]; P < .001) (Figure 2C). Zip codes with predominately Hispanic and Latinx populations had a significantly higher proportion of children with detectable BLLs than did zip codes with predominately White populations (56.0% [95% CI, 55.8%-56.2%] vs 48.7% [95% CI, 48.6%-48.9%]; P < .001), but they had a significantly lower proportion of children with BLLs of 5.0 μg/dL or more (1.1% [95% CI, 1.1%-1.2%] vs 2.0% [95% CI, 2.0%-2.1%]; P < .001).
Figure 2. Distribution of Blood Lead Levels Based on Risk Factor Quintiles Assessed by Zip Code.
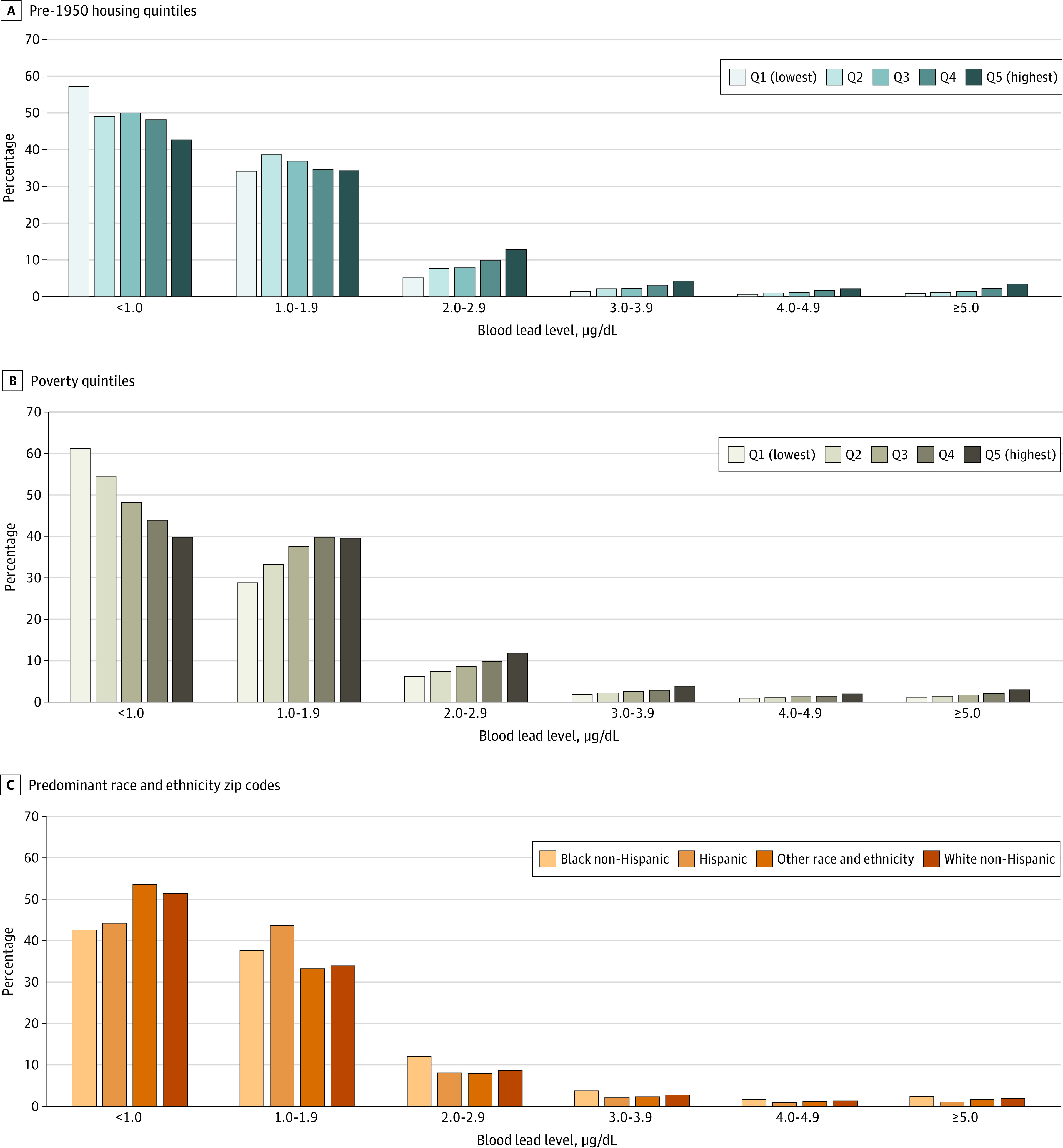
A, Pre-1950s housing quintile; 6778 missing estimates. B, Poverty quintile; 6901 missing estimates. C, Predominant race and ethnicity. “Predominant” refers to zip codes with estimated proportions of the given race and ethnicity over 50%. To convert blood lead levels to micromoles per liter, multiply by 0.0483. Cochran-Armitage test for trend, P < .001 for progressive quintiles of pre-1950s housing construction (A) and poverty (B) within each blood lead measurement level.
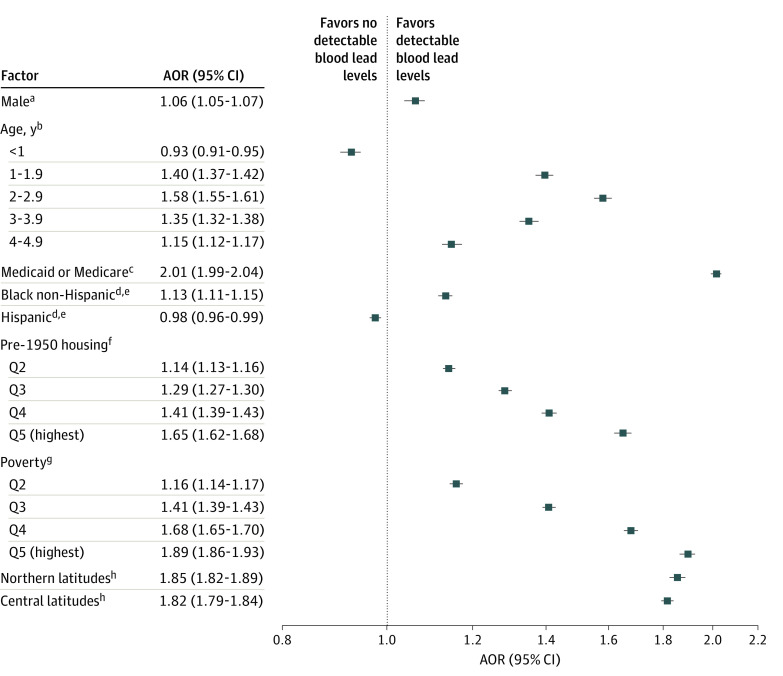
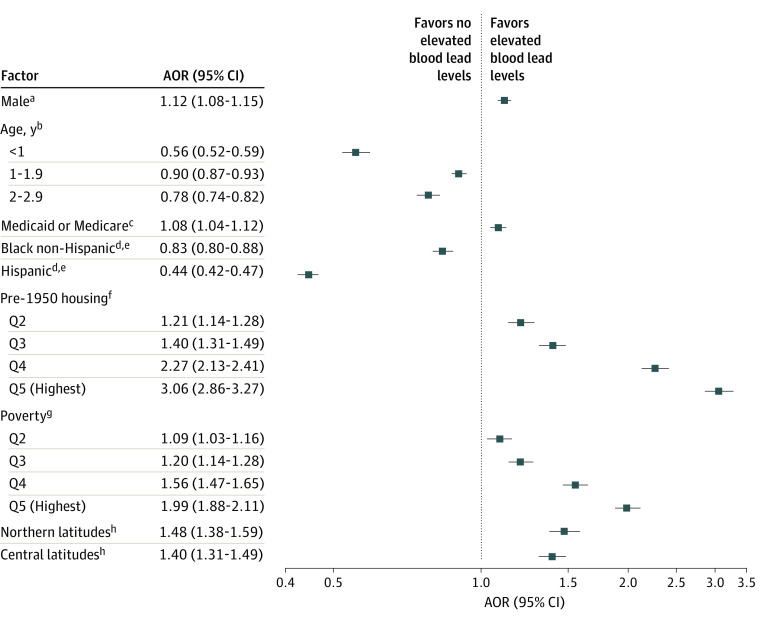
To assess the association of these factors when considered together, multivariable models were created with detectable BLLs (Figure 3) and elevated BLLs (Figure 4) as the dependent variables. In general, the associations with detectable BLLs for most factors found by looking at simple proportions were confirmed in adjusted analyses. The adjusted odds ratio (AOR) of having a detectable BLL was 1.65 (95% CI, 1.62-1.68) for the highest quintile of pre-1950 housing compared with the lowest quintile and was 1.89 (95% CI, 1.86-1.93) for the highest quintile of poverty compared with the lowest quintile. Children with public insurance had an AOR of 2.01 (95% CI, 1.99-2.04) for a detectable BLL compared with children with private insurance. An exception was children residing in zip codes with predominately Hispanic and Latinx populatons, with an AOR of 0.98 (95% CI, 0.96-0.99) for detectable BLLs, compared with children residing in zip codes with predominantly White populations. In adjusted models, children residing in zip codes with predominantly Hispanic and Latinx populations had an AOR of 0.44 (95% CI, 0.42-0.47) for elevated BLLs compared with children residing in zip codes with predominantly White populations. In adjusted models, children residing in zip codes with predominantly Black populations had an AOR of 1.13 (95% CI, 1.11-1.15) for detectable BLLs, but this finding did not hold for elevated BLLs (AOR, 0.83 [95% CI, 0.80-0.88]) compared with children living in predominantly White populations. In the model showing adjusted associations with BLLs of 5.0 μg/dL or more, children with public insurance had an AOR of 1.08 (95% CI, 1.04-1.12) for elevated BLLs compared with children with private insurance. The AOR for BLLs of 5.0 μg/dL or more was 3.06 (95% CI, 2.86-3.27) for children residing in the highest quintile of pre-1950 housing compared with those in the lowest quintile and 1.99 (95% CI, 1.88-2.11) for those in the highest quintile of poverty compared with those in the lowest quintile.
Figure 3. Factors Associated With Detectable Blood Lead Levels in Children Younger Than 6 Years: Multivariable Model.
Included in this model were 942 428 children, with no values missing for any variable (83% of those included). Area under receiver operator curve = 0.66. AOR indicates adjusted odds ratio.
aReference, female.
bReference, 5 to 5.9 years.
cReference, private payers.
dPredominantly White non-Hispanic and non-Latinx populations.
eReference, all other zip codes.
fReference, pre-1950 housing quintile (Q) 1.
gReference, poverty Q1.
hReference, southern latitudes.
Figure 4. Factors Associated With Elevated Blood Lead Levels in Children Younger Than 6 Years: Multivariable Model.
Included in this model were 942 428 children, with no values missing for any variable (83% of those included). Area under receiver operator curve = 0.68. AOR indicates adjusted odds ratio.
aReference, female.
bReference, 3 to 5.9 years.
cReference, private payers.
dPredominantly White non-Hispanic and non-Latinx populations.
eReference, all other zip codes.
fReference, pre-1950 housing quintile (Q) 1.
gReference, poverty Q1.
hReference, southern latitudes.
Discussion
This large, cross-sectional, retrospective national study, using both mapping techniques and multivariate analyses, demonstrates that there is an association between where a child lives and the risk of any lead exposure, an important health issue with long-term outcomes at a population level. Most children evaluated had detectable BLLs. Similar associations were seen with individual- and community-level factors and associations with both detectable and elevated BLLs. This result is important because prior studies have demonstrated that, if the US achieved BLLs of zero in US children born in 2018, it would result in an overall benefit of approximately $84 billion during the lifetimes of these children.29 These projected savings are primarily the result of the expected increases in productivity among children who will be able to achieve their full potential ($77.2 billion). The associations between lead exposure and elevated BLLs in children residing in zip codes with predominantly Black or Hispanic and Latinx populations were not consistent. Further studies are needed to characterize these associations.
Limitations
This study has limitations, especially with regard to conclusions about the prevalence of lead exposure, owing to potential selection bias. These data should only be interpreted as a large sample of national data because Quest Diagnostics does not perform all lead testing in the US. Specimen type could create a selection bias because some clinical practices rely on venous blood testing as a confirmatory test after a capillary blood point-of-care test is conducted in the office. Unfortunately, we cannot determine which venous blood specimens were confirmatory as the result of testing that was conducted outside of Quest Diagnostics. The rates reported in the results represent the data of clinical facilities that send laboratory testing to this reference laboratory. The proportion of overall lead testing represented by these facilities varies by state owing to multiple factors, including differing levels of Quest Diagnostics market share. There is significant state-level variability in how health care professionals determine who to test for lead exposure.30 For example, 10 US states and the District of Columbia require universal testing, generally for all children at the ages 1 and 2 years; 8 states require targeted testing (eg, based on receipt of public insurance, or living in a community with high proportions of older housing or a high proportion of elevated BLLs in children); 27 states provide only recommendations; and 5 states have no requirements or recommendations listed on their websites. These differences were likely associated with significant variability in the rate of children represented in this study population. Sectors of the population determined to be at higher risk of lead exposure may be tested more frequently; clinical judgement, variability in state and regional guidance, and socioenvironmental factors likely factor in to determining who is tested for lead. Another study limitation was the use of zip code–level data instead of individual-level patient data or a smaller geographically aggregated area for race and ethnicity estimates, pre-1950s housing, and poverty levels. The precision of the laboratory is critical in identifying any detectable lead exposure; our method reports a 1.0-μg/dL limit of detection. Last, the multivariable models displayed poor overall fit statistics. The intent of the model was to evaluate the relative importance of each of the factors assessed in this study after adjustment for all other factors.
Conclusions
To eliminate the effect of lead exposure on all children’s health, the US must focus efforts to prevent children from being exposed to lead, beginning in areas where risk is highest. Children, families, and society achieve the most benefit from interventions that ensure that the US mitigates lead exposures in homes and other settings before a child is ever exposed. To our knowledge, this is one of the first comprehensive national analyses investigating the association of lead exposure with individual- and community-level factors. In adjusted models, the proportions of children with detectable and elevated BLLs increased significantly among children with public insurance and for progressive quintiles of communities with pre-1950s housing and poverty rates. We did not see consistent associations between lead exposure and elevated BLLs in children residing in zip codes with predominantly Black or Hispanic and Latinx populations. There has been significant progress in reducing lead exposure throughout the country; this study demonstrates, however, that there are still substantial individual- and community-level disparities that have important implications for addressing childhood lead exposure.
References
- 1.Lanphear BP, Hornung R, Khoury J, et al. Low-level environmental lead exposure and children’s intellectual function: an international pooled analysis. Environ Health Perspect. 2005;113(7):894-899. doi: 10.1289/ehp.7688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Canfield RL, Henderson CRJ Jr, Cory-Slechta DA, Cox C, Jusko TA, Lanphear BP. Intellectual impairment in children with blood lead concentrations below 10 microg per deciliter. N Engl J Med. 2003;348(16):1517-1526. doi: 10.1056/NEJMoa022848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nigg JT, Nikolas M, Mark Knottnerus G, Cavanagh K, Friderici K. Confirmation and extension of association of blood lead with attention-deficit/hyperactivity disorder (ADHD) and ADHD symptom domains at population-typical exposure levels. J Child Psychol Psychiatry. 2010;51(1):58-65. doi: 10.1111/j.1469-7610.2009.02135.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Toxicology Program, US Department of Health and Human Services . NTP monograph: health effects of low-level lead. Accessed February 25, 2021. https://ntp.niehs.nih.gov/ntp/ohat/lead/final/monographhealtheffectslowlevellead_newissn_508.pdf
- 5.Trasande L, Liu Y. Reducing the staggering costs of environmental disease in children, estimated at $76.6 billion in 2008. Health Aff (Millwood). 2011;30(5):863-870. doi: 10.1377/hlthaff.2010.1239 [DOI] [PubMed] [Google Scholar]
- 6.Council on Environmental Health . Prevention of childhood lead toxicity. Pediatrics. 2016;138(1):e20161493. doi: 10.1542/peds.2016-1493 [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention . CDC response to Advisory Committee on Childhood Lead Poisoning Prevention recommendations in “Low Level Lead Exposure Harms Children: A Renewed Call of Primary Prevention.” Accessed February 18, 2021. https://www.cdc.gov/nceh/lead/acclpp/cdc_response_lead_exposure_recs.pdf
- 8.Centers for Disease Control and Prevention . Blood lead levels (μg/dL) among U.S. children <72 months of age, by state, year, and blood lead level (BLL) group. Accessed February 18, 2021. https://www.cdc.gov/nceh/lead/data/CBLS-National-Table-508.pdf
- 9.Mahaffey KR, Annest JL, Roberts J, Murphy RS. National estimates of blood lead levels: United States, 1976-1980: association with selected demographic and socioeconomic factors. N Engl J Med. 1982;307(10):573-579. doi: 10.1056/NEJM198209023071001 [DOI] [PubMed] [Google Scholar]
- 10.Hauptman M, Bruccoleri R, Woolf AD. An update on childhood lead poisoning. Clin Pediatr Emerg Med. 2017;18(3):181-192. doi: 10.1016/j.cpem.2017.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teye SO, Yanosky JD, Cuffee Y, et al. Exploring persistent racial/ethnic disparities in lead exposure among American children aged 1-5 years: results from NHANES 1999-2016. Int Arch Occup Environ Health. 2021;94(4):723-730. doi: 10.1007/s00420-020-01616-4 [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention . Populations at higher risk. Accessed February 18, 2021. https://www.cdc.gov/nceh/lead/prevention/populations.htm
- 13.McClure LF, Niles JK, Kaufman HW. Blood lead levels in young children: US, 2009-2015. J Pediatr. 2016;175:173-181. doi: 10.1016/j.jpeds.2016.05.005 [DOI] [PubMed] [Google Scholar]
- 14.Vivier PM, Hauptman M, Weitzen SH, Bell S, Quilliam DN, Logan JR. The important health impact of where a child lives: neighborhood characteristics and the burden of lead poisoning. Matern Child Health J. 2011;15(8):1195-1202. doi. doi: 10.1007/s10995-010-0692-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wright RO, Tsaih S-W, Schwartz J, Wright RJ, Hu H. Association between iron deficiency and blood lead level in a longitudinal analysis of children followed in an urban primary care clinic. J Pediatr. 2003;142(1):9-14. doi: 10.1067/mpd.2003.mpd0344 [DOI] [PubMed] [Google Scholar]
- 16.Advisory Committee on Childhood Lead Poisoning Prevention, Centers for Disease Control and Prevention . Low level lead exposure harms children: a renewed call for primary prevention. Accessed February 19, 2021. https://www.cdc.gov/nceh/lead/acclpp/final_document_030712.pdf
- 17.Jacobs DE, Clickner RP, Zhou JY, et al. The prevalence of lead-based paint hazards in U.S. housing. Environ Health Perspect. 2002;110(10):A599-A606. doi: 10.1289/ehp.021100599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lanphear BP, Matte TD, Rogers J, et al. The contribution of lead-contaminated house dust and residential soil to children’s blood lead levels: a pooled analysis of 12 epidemiologic studies. Environ Res. 1998;79(1):51-68. doi: 10.1006/enrs.1998.3859 [DOI] [PubMed] [Google Scholar]
- 19.Work Group on Revision of the Blood Lead Reference Value, BSC Lead Poisoning Prevention Subcommittee . Consensus recommendations on revision of the blood lead reference value. Accessed February 25, 2021. https://www.atsdr.cdc.gov/science/lpp/docs/Consensus-Report-LPP-RV-work-group-report-01-13-2017.pdf
- 20.Centers for Disease Control and Prevention . Standard surveillance definitions and classifications. Accessed December 12, 2015. https://www.cdc.gov/nceh/lead/data/definitions.htm
- 21.Clinical and Laboratory Standards Institute . Measurement Procedures for the Determination of Lead Concentrations in Blood and Urine; Approved Guideline—Second Edition. Clinical and Laboratory Standards Institute; 2013. [Google Scholar]
- 22.National Institute of Standards & Technology. Certificate of analysis: standard reference material 3128: lead (Pb) standard solution. Accessed August 23, 2021. https://www-s.nist.gov/srmors/certificates/3128.pdf
- 23.US Census Bureau . Table B03002: American Community Survey, 5-year estimates. Accessed August 23, 2021. https://data.census.gov/cedsci/table?q=Table B03002&tid=ACSDT1Y2019.B03002
- 24.US Census Bureau . Table C17002: American Community Survey, 5-year estimates. Accessed August 23, 2021. https://data.census.gov/cedsci/table?q=Table C17002&tid=ACSDT1Y2019.C17002
- 25.US Census Bureau . Table DP04: American Community Survey, 5-year estimates. Accessed August 23, 2021. https://data.census.gov/cedsci/table?q=Table DP04&tid=ACSDP1Y2019.DP04
- 26.Kaufman HW, Niles JK, Kroll MH, Bi C, Holick MF. SARS-CoV-2 positivity rates associated with circulating 25-hydroxyvitamin D levels. PLoS One. 2020;15(9):e0239252. doi: 10.1371/journal.pone.0239252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dignam T, Kaufmann RB, LeStourgeon L, Brown MJ. Control of lead sources in the United States, 1970-2017: public health progress and current challenges to eliminating lead exposure. J Public Health Manag Pract. 2019;25(suppl 1, Lead Poisoning Prevention):S13-S22. doi: 10.1097/PHH.0000000000000889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention . Preventing lead poisoning in young children. Accessed August 23, 2021. https://www.cdc.gov/mmwr/preview/mmwrhtml/00000659.htm
- 29.Morley R, Lenhart A, Illa G, Brown MJ. 10 Policies to prevent and respond to childhood lead exposure. Accessed February 19, 2021. https://www.pewtrusts.org/en/research-and-analysis/reports/2017/08/10-policies-to-prevent-and-respond-to-childhood-lead-exposure
- 30.Dickman J. Children at risk: gaps in state lead screening policies. Accessed February 19, 2021. https://saferchemicals.org/wp-content/uploads/2017/01/saferchemicals.org_children-at-risk-report.pdf