Abstract
Cell-to-cell communication is a pivotal aspect of cancer biology. Recently, extracellular vesicles (EVs)have been shown to play essential roles in intercellular communications between cancer cells and the surrounding microenvironment owing to cancer development. EVs are small membrane-bound vesicles secreted by various cells containing proteins, lipids, mRNAs, and non-coding RNAs (microRNAs and long non-coding RNAs), which contribute to cancer cell development and progression. Here, we provide an overview of current research direction on EVs, especially biomolecules in EVs, and also point out the novel diagnostics, monitoring, predicting, and therapeutic aspects using EVs against cancer.
Key words: Extracellular vesicles, microRNAs, long non-coding RNAs, cancer diagnosis, cancer treatment, cancer monitoring
Introduction
Cell-to-cell communication is essential for normal cell development and cancer cell progression. The communication and exchange of cells were previously observed to occur through three known mechanisms as follows: i) via soluble mediators, such as growth factors, hormones, lipids, cytokines, and chemokines, which are secreted from cells and function in autocrine, paracrine, or endocrine signaling; ii) via cell to cell adhesion contacts between the donor and target cells; and iii) via the exchange of information through tunneling nanotubes.1,2 Evidence of the release and uptake of membrane-bound vesicles as the fourth cell communication mechanism has emerged.3 Extracellular vesicles (EVs) are small membrane-bound vesicles released from numerous cell types into the extracellular microenvironment; EVs are detectable in various body fluids, such as ascites, blood, saliva, urine, cerebrospinal fluid, and breast milk.4-7 EVs were initially observed as procoagulant platelet-derived particles in normal plasma; they were originally reported in 1946 by Chargaff and West8 and referred to as platelet dust by Wolf in 1967.9 In 1987, the term exosome was first used to describe small membrane vesicles formed by vesiculation of intracellular endosomes and released by exocytosis.10 However, the definition of extracellular vesicles was still indeterminate until 2018, during which the International Society has designated the term EV for extracellular vesicles as a generic term for all vesicles that are naturally released, are enclosed in a lipid bilayer membrane, and lack the ability to replicate. 11
EVs can be broadly organized into several categories regardless of the differences in size, density, subcellular origin, and component (Table 1) as follows: exosomes, microvesicles (MVs) or ectosome, and apoptotic bodies (ABs).6,7 Alternatively, EVs are classified according to their origins, as follows: oncosomes12 (Figure 1); telerosomes;13 melanosomes;14 and prostasomes.15 However, information on these EV types is rare.
Exosomes
Exosomes are membrane-enclosed vesicles with homogeneous sizes; their diameters range 30-150 nm. Exosomes are formed by the inward budding of the endosomal membrane system. 16 Exosome biogenesis includes four steps, namely, invagination, endocytosis, multivesicular bodies (MVBs), and exosome secretion.1,17 Early endosome formation starts with the invagination of the plasma membrane and fusing with endocytic vesicles.18 During early to late endosome maturation, intraluminal vesicles (ILVs) are formed by the inward budding of the endosomal membrane, and cytosolic materials are randomly incorporated. Late endosome-containing ILVs are referred to as MVBs, which either fuse with the plasma membrane and release their ILVs as exosomes into the extracellular space or fuse with the lysosome and are degraded by the contents of the lysosome.19-21 Moreover, the transformation process from the early to the late endosome stages can be observed by changing the shape and location inside the cell. The early endosome is tube-like and located in the outer portion of the cytoplasm. Meanwhile, the late endosome is spherical and found close to the nucleus.22,23 Cargo sorting into exosomes during ILVs formation is guided by either an endosomal sorting complex required for transport (ESCRT)-dependent pathway combined with other associated proteins, such as a programmed cell death 6 interacting protein (PDCD6IP) known as Alix and tumor susceptibility gene 101 protein (TSG101)24 or through an ESCRTindependent process, such as the ceramide/tetraspanin dependent pathway.25 Moreover, exosomes are secreted by docking and subsequent fusion of MVBs with the plasma membrane. Processes are regulated by the Rab family of small GTPase, SNAP, and SNARE proteins.26-29 For example, Rab3, Rab27, and Rab27b regulate the docking of the MVBs at the plasma membrane.27,29 Rab11, Rab31, R-SNARE protein YKT6, and V-SNARE protein VAMP7/TIVAMP are involved in fusing MVBs with the cell membrane. Interestingly, Rab27a was reported to play a role in docking and fusion.26,27 In addition to protein molecules, a high accumulation of intracellular Ca2+ and low pH in the microenvironment increases exosome secretion.30
Microvesicles
Compared with exosomes, MVs are more heterogeneous vesicles with diameters in the range of 100-1000 nm. MVs were initially identified as products of the activated blood platelets and erythrocytes involved in coagulation.31-33 The biogenesis of MVs is induced by the activation of the plasma membrane through intracellular Ca2+ influx. The alteration in asymmetric phospholipids distribution, such as repositioning phosphatidylserine (PS) on the outer side of the plasma membrane, appears to be one of the main features of MVs.34-36 Meanwhile, PS-negative MVs could also be produced by the membrane activation of non-Ca2+ agonists, such as collagen and adenosine diphosphate (ADP).37,38 This phenomenon was found in the state of chronic stimuli exposure or strictly in some cell types, such as endothelial cells.39,40 After that, MVs are released from cells by outward budding and fission of the plasma membrane, referred to as shedding.12 The process starts when outward buds form in specific sites of MV origin; then, vesicles undergo fission and are subsequent released to the extracellular space.1,41 ADP-ribosylation factor 6 (ARF6) has been reported as a key protein involved in MV formation and shedding.42 This protein triggers a signaling cascade that involves phospholipase D (PLD), the recruitment of extracellular signal-regulated kinase (ERK), the phosphorylation of myosin light-chain kinase (MLCK), and the activation of the myosin light chain.43,44 Moreover, the ARF6-regulated endosomal complex plays a vital role in the selective incorporation of molecular cargo into MVs.45
Apoptotic bodies
By contrast, ABs are distinct from exosomes and MVs. ABs are heterogeneous vesicles with diameter sizes in the range of 800-5000 nm. ABs contain cytosolic organelles and nuclear fragments as DNA and RNA. Formerly, ABs were considered simply as the cell remnant discarded from the apoptotic process.6,7,46 However, the investigation in ABs has become more apparent since the paradigm shift in apoptosis concepts as not merely the consequence of cell death but also methods of cells to reorchestrate the surrounding environment.47 The formation of ABs starts from cells that begin apoptosis, triggering by either intrinsic or extrinsic cascades.48 Then, the externalization of PS is induced by Ca2+ independent scramblases and activated by 3/7 cleaved caspase enzymes in the plasma membrane, which the production rate is regulated through different molecules, such as Rho-associated protein kinase (ROCK1) and myosin light chain kinase (MLCK).49,50 Considering ABs functions, as their name, ABs play a significant role in an apoptotic process. They contain several proteins, such as intercellular adhesion molecule 3 (ICAM-3), PS, and C-X-C motif chemokine ligand 3 (CXCL3), which can recruit phagocytes and activate their phagocytotic activity.51 In addition, ABs can also display antitumorigenic properties by manifesting as antigen-presenting cells to the dendritic cells, resulting in an immunogenic activation. 51 Finally, some studies found that ABs could promote tissue regeneration in cardiomyocytes, glomeruli, and bone tissue models through various mechanisms.50
Figure 1.
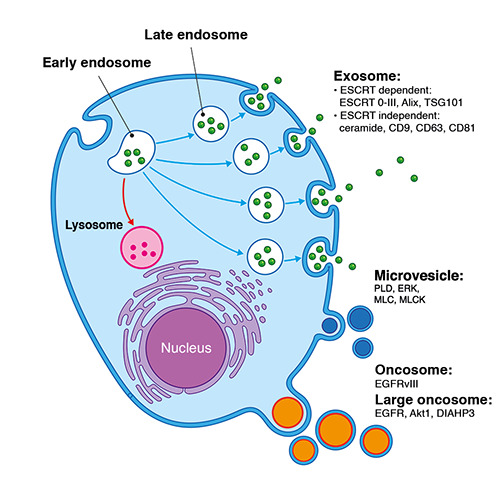
Biogenesis of EV and their main components. Exosomes (50-150 nm) primarily originated from the endosomal membrane system. Invagination of the plasma membrane led to the formation of early endosomes. Then, endosomes are either destined to degrade in lysosomes or to mature into late endosomes and subsequently released into the circulation. Microvesicles (100-1000 nm) are formed by outward cell membrane budding, which is triggered by various cell states. Some cargos could be recruited into either exosomes or microvesicles during the processes. Oncosomes (100-400 nm) and large oncosomes (1-10 μm) are triggered by specific mechanisms only in cancer cells. ESCRT, endosomal sorting complex required for transport; TSG101, tumor susceptibility gene 101; CD, cluster of differentiate; PLD, phospholipase D; ERK, extracellular signalregulated kinases; MLC, myosin light chain; MLCK, myosin light chain kinases; EGFR, epidermal growth factor receptor; Akt, protein kinase B; DIAHP3, diaphanous related formin 3.
Table 1.
The key features of the main extracellular vesicle populations.
| Feature | Exosome | Microvesicle | Apoptotic body |
|---|---|---|---|
| Size (nm) | 30-100 | 100-1000 | 800-5000 |
| Density in sucrose (g/mL) | 1.13-1.22 | ND | 1.16-1.28 |
| Sedimentation (×g) | 100,000 | 10,000 | 1200; 10,000; or 100,000 |
| Appearance by Electron microscopy | Cup shape | Irregular shape and electron-dense | Heterogenous |
| Lipid composition | Enriched in cholesterol, sphingomyelin, and ceramide; contain lipid raft; expose phosphatidylserine | Expose phosphatidylserine | ND |
| Main protein markers | Tetraspanin (CD81, CD9, CD63), Alix and TSG101 | Integrins, selectins, and CD40 ligand | Histones |
| Intracellular origin | Internal compartment (endosome) | Plasma membrane | Programmed cell death |
ND, no data; CD, cluster of differentiate; TSG, tumor susceptibility gene. Modified from Mincheva-Nilsson and Baranov3 and Mathivanan et al.4
Oncosomes and large oncosomes
Apart from the classification mentioned above, EV subtypes are classified according to their origins.15 Specifically for cancer, oncosomes were named in 2008 following the discovery of Epidermal Growth Factor Receptor (EGFR) vIII containing vesicles derived from EGFRvIII expressed glioma cell lines. EGFRvIII is an oncogenic form of EGFR. These oncosomes can make inert glioma cells engage in an oncogenic activity.12 Fundamentally, the term oncosomes indicates vesicles of 100-400 nm sizes that are released from cancer cells via an unknown underlying mechanism.52 Meanwhile, the large oncosome (LO) subpopulation was initially discovered in 2009 by Vizio et al. The prostate cancer cell lines at amoeboid state could release extraordinarily large EVs (1-10 μm) in response to specific EGFR and Akt1 induction.53 The biogenesis of LO resembles MVs in terms of budding membrane origination.54 However, induction signaling occurred specifically via oncogenic signaling pathways, such as EGFR, Akt induction, or diaphanous-related formin-3 gene (DIAPH3) silencing.55 In addition, LO contains oncogenic molecular cargos, such as caveolin- 1, αV-integrin, and miR-1227 in prostate cancer, which are distinct from typical cell vesicles56-58 V-ATPase V1G1, and homeobox genes in glioblastoma.59 The LO release amounts are related to cancer cells’ size and aggressiveness.53,56 Nevertheless, the utility of LO is still limited in cell line studies. In clinical samples, the tumor cell population is heterogeneous; they do not only stay in the amoeboid state. Therefore, they can release both LO and non-LO large EVs (including MVs and ABs). Distinguishing these two subgroups is difficult because of the lack of specific LO markers and isolation methods. Thus, many publications use the term large EVs, which does not accurately reflect the LO sub-population.60,61
Extracellular vesicles components in cancer development
Intercellular communication between EVs and the target cells can occur by direct interaction via EV surface proteins with the receptors on the recipient cells. Such interaction leads to the activation of intracellular signaling pathways. Moreover, EVs can be pocketed through membrane fusion by the target cells (endocytosis or phagocytosis), and the bioactive molecules can be transferred.24 The specific contents, such as membrane receptors, ligands, proteins, lipids, and nucleic acids (DNA, RNA, and microRNA), can be accommodated into EVs depending on their parental cells.25,62 Although common biomarkers of EVs have been established, such as endosome-associated proteins (SNAREs, ALIX, and TSG101) and tetraspanin proteins (CD9, CD63, and CD81), the specific content of different EV subtypes and the manner of sorting molecules into EVs still need to be explored.11
Most cancer cells release various EV types that differ from healthy cells in terms of content and quantity. Cancer-derived EVs affect located cells and other distal cells, resulting in the transfer of metastatic capability. EVs from noncancerous neighboring epithelial cells can suppress cancer cell progression.22 EVs from malignant cells, primarily exosomes, can induce normal cell transformation into malignant phenotypes (Figure 2). For example, exosomes derived from breast cancer cells and sera of patients could induce normal epithelial cells to form cancer in a Dicer-dependent state.63 Prostate cancer cell-derived exosomes contain various oncogenic factors, such as H-ras and K-ras mRNA and oncogenic microRNAs (miRNAs; miR-125b, miR-130b, and miR-155) that cause neoplastic reprogramming of adipose-derived stem cells.64
Previous studies have investigated several proteins in EVs that are derived from cancer cells. For example, amphiregulin identified from breast and colorectal cancer EVs play a role as invasive factors;65 EGFR identified in brain and pancreatic cancer EVs is involved in cell proliferation.12,66,67 EV Hsp90 derived from cancer cells is responsible for their motility and invasiveness.68 In addition to EV proteins, EV miRNAs can promote cancer cell metastasis through angiogenesis.69 Moreover, growth-inhibitory miRNAs released from normal cells are hypothesized to kill transformed cells during cancer initiation, thereby reviving the tissue to a healthy state. For example, EV miRNA-143 isolated from normal prostate cells could inhibit cancer cell growth in vitro and in vivo.70 Tumor-suppressive miRNAs are commonly down-regulated in cancer cells. Thus, continuous secretion of tumor-suppressive miRNAs via EVs derived from normal cells helps the homeostatic mechanism. Other types of non-coding RNA have potential as biomarkers. Long non-coding RNAs (lncRNAs) have attracted considerable attention, especially in EVs. Chen et al. reported that several kinds of the antisense lncRNAs, such as RP5-940J5.9, RP11-290D2.6, 5 C17orf76AS1, and ZFAS1, are enriched in EVs derived from colorectal cancer cell lines.71 Meanwhile, ZFAS1 has been reported as an oncogene by destabilization of p53, leading to cell cycle progression and inhibition of apoptosis in breast, liver, and colorectal cancers.72-74 Moreover, lncRNA-TUC339 found in EVs released from hepatocellular cancer is functionally implicated in modulating tumor cell growth, adhesion, and cell cycle progression. 75 lncRNA-MALAT1 enriched in EVs derived from HeLa cervical and MCF-7 breast cancer cells is related to tumor metastasis and invasion.76,77 In addition to abilities of cancer EVs in transforming normal cells into malignant forms, cancer EVs can also reorganize the surrounding tumor microenvironment (TME) to facilitate tumor cells’ survival, growth, and metastatic capabilities (Figure 3). Particularly, EVs from cancer cells can indoctrinate nearby fibroblasts to execute the glycolysis process for them via the reverse Warburg effect,78,79 with a similar process occurring in adipocytes through enhancing the lipolysis activity80 - the manipulation results in transforming fibroblasts and adipocytes into major ATP powerhouses for cancer cells. Moreover, cancer EVs facilitate a metastatic environment by promoting angiogenesis and inducing vascular leakiness,81 as demonstrated in EV-associated miRNA-9, which can reduce the suppressor of cytokine signaling and activating the JAK/STAT pathway,19 and EV miRNA-210, which regulates neutral sphingomyelinase 2 (nSMase2)69,82,83 and inhibit angiogenic inhibitor ephrin-A3 in endothelial cells, resulting in angiogenesis promotion.84 Finally, tumor EVs carry the ability to evade the immune response system by inducing immune cell apoptosis via death ligands, such as TNF-α and Fas ligand,78 or directly evade the immune checkpoint by expressing protein deathligand 1 (PD-L1), rendering cytotoxic T-cells to ignore the tumor cells completely.85
Figure 2.
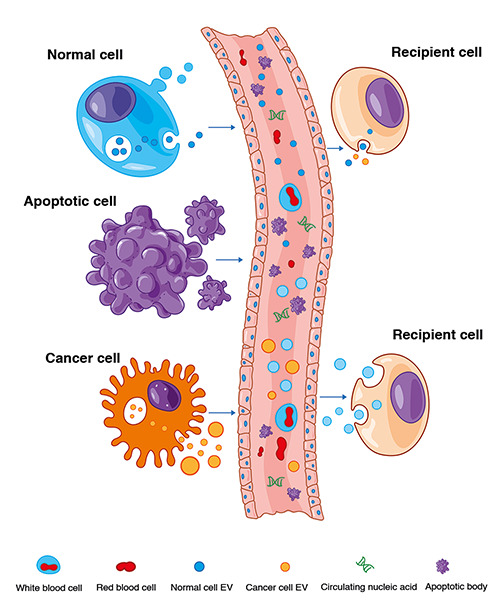
EV components in cancer development. Both normal and cancer cells can release EVs to the target recipient cells. Cancer cell EVs can convert recipient cells into malignant cells. Conversely, normal cells can suppress the transforming cells and convert them back into the normal state. Apoptotic bodies are released from apoptotic cells and have less significant functions.
Extracellular vesicles utilization
Since cancer-derived EVs contain a cancer-specific molecule signature, they are dramatically gaining interest as potential tools for aiding cancer treatment in various aspects, mainly for diagnostic, treatment monitoring, and drug delivery (Figure 4).
Extracellular vesicles as cancer diagnostic tools
Given that pain from a traditional cancer diagnosis is caused by needle or tissue biopsy, blood-based minimally invasive and non-invasive diagnoses using either urine or saliva samples are attractive alternatives. Traditional biomarkers, such as prostatespecific antigen (PSA) has low specificity for early detection, and their use does not absolutely distinguish cancer patients from normal persons.86 Thus, novel biomarkers with high specificity are currently required to corroborate the early cancer diagnosis. Incidentally, as EVs are present in various body fluids, they can act as non-invasive biomarkers that reflect the physiological and pathological status of the cells they originated from by delivering bioactive molecules into neighboring or distant cells.87 Circulating EVs from blood were the most investigated as the potential cancer diagnostic biomarker. For instance, the level of plasma EV survivin, an inhibitor of apoptosis protein, is significantly increased in prostate cancer patients compared with patients with benign prostate hyperplasia and normal controls.88 Serum EVs Glypican- 1 (GPC-1) from pancreatic ductal carcinoma (PDAC) patients can distinguish cancers from benign tumors and controls with 100% sensitivity and specificity.89 Moreover, several kinds of EV miRNAs and lncRNAs have been proposed as cancer diagnostic and prognostic indicators for lung cancers. Examples are as follows: miR-17-3p, miR-21, miR-106a, and lncRNA-MALAT1.90,91 In addition, urine EVs have been extensively studied in urological cancers, such as EVd-catenin, caveolin-1, and CD59 in prostate cancer92 and miR-720, miR-205, mi-2003p, and miR-29b-3p in bladder cancer.93 Other biofluids study mostly focused on recruitment from local tissue. For example, EVs lncRNA-AA174084 from gastric lavage in gastric cancers94 and EVs miR-21, miR-146, and lncRNA MALAT1, HOTAIR, and MEG3 in cervicovaginal lavage of cervical cancer, were found to be significantly elevated compared with their benign counterparts.95,96
Figure 3.
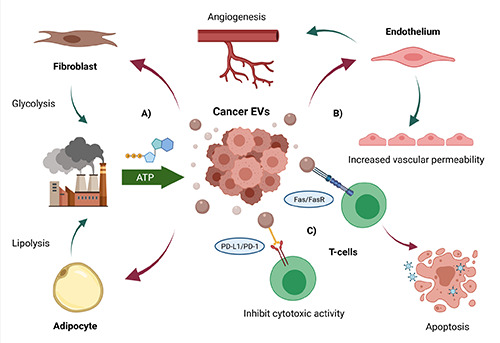
Roles of EVs in tumor microenvironment. A) Cancer EVs can manipulate fibroblast and adipocyte to serve as the ATP powerhouse for the tumor via glycolysis and lipolysis. B) Cancer EVs induce the endothelium to increase permeability state and promote angiogenesis. C) Some cancer EVs express the death ligands Fas, which induce T-cells apoptosis when pair with T-cell Fas receptor (FasR). Meanwhile, some EVs present protein death ligand 1 (PD-L1) which can pair with protein death 1 (PD-1) on T-cells, rendering them to ignore tumor in the cytotoxic process. Created with BioRender.com
Figure 4.
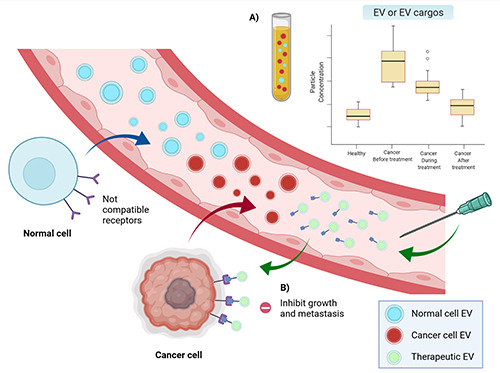
Utilization of EVs in different aspects. A) Difference in EVs or EV cargo concentrations can distinguish healthy patients from patients with cancer. Moreover, the level of EVs in cancer patients can be monitored for disease status after treatment. B) Biosynthetic EVs can be used to deliver the specific substance to target cancer. Created with BioRender.com
Intriguingly, several EV-based diagnostic tools are currently being used. Initiated by Caris Life Sciences, Carisome®Prostate cMV 1.0 was launched in late 2010. The test uses a suitable method to capture and analyze blood-based circulating microvesicles (cMV) by detecting specific protein markers on the cMV of prostate cancer. Additionally, ExoDxTM IntelliScore, launched by Exomed, analyzes the EV expression levels of mRNA PCA3, ERG, and SPDEF to calculate the risk of high-grade prostate cancer. 97 The diagnostic kit is more accurate than a standard method in virgin patients or patients with a prior negative biopsy with PSA 2-10 ng/mL.97,98 Consequently, ExoDxTM lung (EGFR T790M) launch plan was released in 2016 from the same company. When combined with cell-free DNA, the kit can detect EGFR mutation in non-small cell lung cancer (NSCLC) patients with 92% sensitivity and 89% specificity.99
EVs as a marker that predicts and monitors treatments
EVs’ characteristic mirrors the current status of cancer cells. Thus, its specificity leads to the potential use of EVs as novel monitoring biomarkers before, during, and after cancer treatment, especially in cancers that currently have limited biomarkers. This concept was demonstrated in various cancer treatment modalities as the EVs amounts in post-treatment patients correlate with diminished tumor volume. For surgery, The GPC-1 exosome concentration in patients with PDAC was significantly reduced from preresection to the seventh day post-resection.89 The exosome levels in patients with glioblastoma significantly decreased after the resection compared with pre-operation levels and were elevated back during the recurrence phase.100 For radiation and chemotherapy treatment modalities, EV levels correspond with the tumor volume according to imaging or post-surgery pathology findings. Therefore, EVs could be used to assess patients’ response status without invasive procedures or exposure to hazardous radiologic contrasts. For instance, in an analysis of serum EVs from breast cancer patients who received neoadjuvant chemotherapy (NAC) followed by surgery, patients who responded to NAC exhibited significantly lower EV concentration in the post-treatment period. However, the EV level in non-responders remained constant.101 Breast cancer resistance protein (BCRP), a multi-chemotherapy resistance protein that functions by expelling xenobiotics out of cells,102 was more highly expressed in tissue and serum EVs retrieved from chemo-resist patients compared with those from patients who did not require NAC.103 Moreover, EV concentration can also be used in conjunction with other markers to improve treatment monitoring accuracy. In a study conducted by Kassam et al., serum circulating tumor cells (CTC) and EV concentration, together with computed tomography-perfusion, were used to accurately assess NAC response status before resection when compared with Ryan’s histopathological criteria evaluated after resection.104
EV cargos, such as RNA and protein, are also promising targets for monitoring cancer therapy. For instance, the different expressions of EV miRNA hsa-let-7a-5p, hsa-miR-21-5p, miR- 493-5p, miR-323a-3p, and miR-411-5p can be used as predictors of radiation response in patients with prostate cancer, or miR-423-3p can be used as a predictor of platinum sensitivity in NSCLC.105,106 Similarly, lncRNA-GC1, a known marker of gastric cancer progression, was significantly reduced after surgical resection. 107 Furthermore, EVs PD-L1, an immune checkpoint receptor inhibitor stated in the earlier section, is receiving substantial attention at the moment as a promising tool to monitor cancer progression. In functional studies, EV PD-L1 manifested the same immunosuppressive property with tissue PD-L1 by inhibiting Tcell activation, but it had a better detection rate than tissue immunochemistry.108,109 Consequently, many clinical studies have found that the decreased/increased expression of EV PD-L1 correlated with patients’ disease status in melanoma, head and neck cancer, and NSCLC.109-111
Interestingly, EV panel analysis, also known as EV phenotype analysis, is currently being developed. Phenotype analysis allows the detection of multiple EV markers by embedding multiple antibody markers in a single chip, thereby enhancing the speed and specificity of EV characterization compared with conventional methods, such as Western blot and ELISA. Wang et al. developed an automatic EV phenotype analyzer chip consisting of the EV markers that commonly increased during the melanoma treatment course, such as MCSP, MCAM, ErbB3, and LNGFR. The panel chip successfully analyzed the increased expression of all markers during the targeted therapy period and decreased expression in resistant patients with melanoma.112 Jin et al. utilized an aptamerbased exosome-activated DNA molecular machine (ExoADM), which allows the simultaneous dual detection of CD63 and PD-L1 with an accuracy comparable with ELISA profiling.113 However, validation needs to be performed in a large sample size in future research. If the verification is successful, these biosensor chips can lead to a point-of-care paradigm in cancer treatment monitoring.
Extracellular vesicles as a new therapeutic target in cancer
In addition to standard cancer treatments, including surgery, radiation, chemotherapy, immune checkpoint blockades, and molecularly targeted drugs, targeting EVs has currently become a promising therapeutic approach for personalized medicine. Considering that the delivery of selected molecules from cancer cells to target cells by EVs leads to cancer progression, EVs have alternative therapeutic potential. Hence, EV research for cancer therapy focuses on using native EVs and artificially engineered vesicles as new tools for drug delivery.19,69 For example, the suppression of targeting molecules related to EV production and secretion from tumor cells, such as nSMase2, RAB27A, RAB27B, and RAB22A, leads to cancer development inhibition.19,27,69,114-116 However, the impairment of EV secretion may affect cancer and normal cells by affecting the homeostatic function of EVs. Thus, understanding the contribution of these molecules in the biogenesis and secretion of EVs from both normal and cancer cells is essential. Dendritic cell-derived EVs (dexosomes) have been proposed as immunotherapeutic anticancer agents used in clinical trials on several tumors, such as colorectal cancer, NSCLC, and metastatic melanoma.116,117 Moreover, the elimination of circulating EVs from advanced cancer patients could potentially prevent cancer metastasis. The HER2osome medical device strategy was launched by Aethlon Medical. This device can reduce the circulatory presence of cancer-secreted HER2-positive exosomes by immobilizing a HER2 antibody and an exosome-targeted affinity agent in the outer-capillary space of plasma filtration integrated within dialysis machines. This approach resulted in the inhibition of HER2-positive breast cancer progression.118
Using EVs as a delivery vesicle for therapeutic agents, such as nucleic acid or drug molecules that inhibit the functions of targeted cancer cells, has attracted considerable attention because of EV’s excellent biodistribution, bioavailability, and biocompatibility.119 The expression of EGFR was highly elevated in various human cancers originating from epithelial cells, thereby suggesting that the EGFR ligand, which includes epidermal growth factor and EGFR-specific peptide (GE11), was considered to be a cancer drug target.120 Ohno et al. conducted a study in which EVs targeted EGFR-expressing breast cancer cells. In their study, GE11 was incorporated onto the surface of EVs carrying lethal 7a (let-7a miRNA), and these incorporated molecules were subsequently injected intravenously into tumor-bearing mice. The targeting of EGFR-expressing breast cancer cells by GE11-EVs could significantly reduce tumor formation in mice because the delivery of let- 7a miRNA inhibited the expression of high-mobility group AThook protein (HMGA2) in cancer cells.121 Besides transporting endogenous miRNA, EVs can efficiently deliver artificial short interfering RNA (siRNA) to the target cells and induce cell death through post-transcriptional target gene regulation. For example, siRNA against RAD51, i.e., the eukaryote gene that assists in the repair of DNA double-strand breaks in abnormally proliferating cells, was incorporated into EVs by electroporation, and a significant reduction of the RAD51 transcript led to the inhibition of tumor growth after the delivery of EV carrying siRNA against RAD51 to HEK293 and HCT116 colon cancer cell.122 In addition to nucleic acid, EVs have become an effective vehicle for drug reagent delivery. Curcumin was previously used as an anti-inflammatory, anti-neoplastic, and antioxidant agent with chemopreventive properties.123-125 However, curcumin is significantly limited by its low solubility due to its hydrophobic nature and preferential interaction with lipid membranes.126 Interestingly, curcumin showed increasing solubility, stability, and bioavailability when incorporated into EVs.127 Moreover, the fusion of integrin αv-specific peptide onto the surface membrane of EV-containing doxorubicin led to the highly efficient targeting of chemotherapeutics to integrin αv-positive breast cancer cells and to the inhibition of tumor growth in vivo without overt toxicity.128
Conclusions
EV is gradually attracting attention as a novel class of intercellular signal mediators that deliver bioactive molecules of parental cells into adjacent or distant cells and influence target cells’ physiological and pathological state. In tumor progression, circulating EVs are increased in cancer patients, and this phenomenon is correlated with the tumor stage. EVs have potential as biomarkers for diagnosis, monitoring, prediction, and targeting therapeutic targets. Nonetheless, a validation method of EV cargos as a biomarker in cancer patient specimens is required. In addition, the bioactive molecule sorted in EVs is investigated in various cancers. Loading of EVs with small molecules or drugs has also been utilized for cancer therapy. A large number of EVs from proper donor cells with efficiently introduced therapeutic agents demonstrated the use of EVs as a natural carrier in cancer therapy.
Funding Statement
Funding: this work was financially supported by the Research fund from Faculty of Medicine, Prince of Songkla University (REC-63-465-4-2) and Thailand research fund (MRG6180125).
References
- 1.Ratajczak J, Wysoczynski M, Hayek F, et al. Membranederived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia 2006;20:1487-95. [DOI] [PubMed] [Google Scholar]
- 2.Rechavi O, Goldstein I, Kloog Y.Intercellular exchange of proteins: The immune cell habit of sharing. FEBS Lett 2009;583:1792-9. [DOI] [PubMed] [Google Scholar]
- 3.Mincheva-Nilsson L, Baranov V.The role of placental exosomes in reproduction. Am J Reprod Immunol 2010;63:520-33. [DOI] [PubMed] [Google Scholar]
- 4.Cocucci E, Racchetti G, Meldolesi J.Shedding microvesicles: artefacts no more. Trends Cell Biol 2009;19:43-51. [DOI] [PubMed] [Google Scholar]
- 5.Vlassov AV, Magdaleno S, Setterquist R, Conrad R.Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta BBA - Gen Subj 2012;1820:940-8. [DOI] [PubMed] [Google Scholar]
- 6.Théry C, Boussac M, Véron P, et al. Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J Immunol. 2001;166:7309-18. [DOI] [PubMed] [Google Scholar]
- 7.Mathivanan S, Simpson RJ. ExoCarta: A compendium of exosomal proteins and RNA. Proteomics 2009;9:4997-5000. [DOI] [PubMed] [Google Scholar]
- 8.Chargaff E, West R.The biological significance of the thromboplastic protein of blood. J Biol Chem 1946;166:189-97. [PubMed] [Google Scholar]
- 9.Wolf P. The nature and significance of platelet products in human plasma. Br J Haematol 1967;13:269-88. [DOI] [PubMed] [Google Scholar]
- 10.Johnstone RM, Adam M, Hammond JR, et al. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem 1987;262:9412-20. [PubMed] [Google Scholar]
- 11.Théry C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 2018;7:1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Nedawi K, Meehan B, Micallef J, et al. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol 2008;10:619-24. [DOI] [PubMed] [Google Scholar]
- 13.Östman S, Taube M, Telemo E.Tolerosome-induced oral tolerance is MHC dependent. Immunology 2005;116:464-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wasmeier C, Hume AN, Bolasco G, Seabra MC. Melanosomes at a glance. J Cell Sci 2008;121:3995-9. [DOI] [PubMed] [Google Scholar]
- 15.Stegmayr B, Ronquist G.Promotive effect on human sperm progressive motility by prostasomes. Urol Res 1982;10:253-7. [DOI] [PubMed] [Google Scholar]
- 16.Xu R, Rai A, Chen M, et al. Extracellular vesicles in cancer - implications for future improvements in cancer care. Nat Rev Clin Oncol 2018;15:617-38. [DOI] [PubMed] [Google Scholar]
- 17.Zhang X, Yuan X, Shi H, et al. Exosomes in cancer: small particle, big player. J Hematol OncolJ Hematol Oncol 2015;8:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grant BD, Donaldson JG. Pathways and mechanisms of endocytic recycling. Nat Rev Mol Cell Biol 2009;10:597-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhuang G, Wu X, Jiang Z, et al. Tumour-secreted miR-9 promotes endothelial cell migration and angiogenesis by activating the JAK-STAT pathway. EMBO J 2012;31:3513-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abels ER, Breakefield XO. Introduction to extracellular vesicles: biogenesis, RNA cargo selection, content, release, and uptake. Cell Mol Neurobiol 2016;36:301-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buschow SI, Hoen ENMN-‘t, Niel GV, et al. MHC II in dendritic cells is targeted to lysosomes or t cell-induced exosomes via distinct multivesicular body pathways. Traffic 2009;10:1528-42. [DOI] [PubMed] [Google Scholar]
- 22.Wolfers J, Lozier A, Raposo G, et al. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med 2001;7:297-303. [DOI] [PubMed] [Google Scholar]
- 23.Beach A, Zhang H-G, Ratajczak MZ, Kakar SS. Exosomes: an overview of biogenesis, composition and role in ovarian cancer. J Ovarian Res 2014;7:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vader P, Breakefield XO, Wood MJA. Extracellular vesicles: emerging targets for cancer therapy. Trends Mol Med 2014;20:385-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. J Proteomics. 2010;73:1907-20. [DOI] [PubMed] [Google Scholar]
- 26.Bobrie A, Colombo M, Raposo G, Théry C.Exosome secretion: molecular mechanisms and roles in immune responses. Traffic 2011;12:1659-68. [DOI] [PubMed] [Google Scholar]
- 27.Ostrowski M, Carmo NB, Krumeich S, et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol 2010;12:19-30. [DOI] [PubMed] [Google Scholar]
- 28.Baietti MF, Zhang Z, Mortier E, et al. Syndecan-syntenin- ALIX regulates the biogenesis of exosomes. Nat Cell Biol 2012;14:677-85. [DOI] [PubMed] [Google Scholar]
- 29.Hsu C, Morohashi Y, Yoshimura S, et al. Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C. J Cell Biol 2010;189:223-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parolini I, Federici C, Raggi C, et al. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J Biol Chem 2009;284:34211-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aatonen MT, Öhman T, Nyman TA, et al. Isolation and characterization of platelet-derived extracellular vesicles. J Extracell Vesicles 2014;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fourcade O, Simon M-F, Viodé C, et al. Secretory phospholipase A2 generates the novel lipid mediator lysophosphatidic acid in membrane microvesicles shed from activated cells. Cell 1995;80:919-27. [DOI] [PubMed] [Google Scholar]
- 33.Müller I, Klocke A, Alex M, et al. Intravascular tissue factor initiates coagulation via circulating microvesicles and platelets. FASEB J 2003;17:1-20. [DOI] [PubMed] [Google Scholar]
- 34.Al-Nedawi K, Meehan B, Rak J.Microvesicles: messengers and mediators of tumor progression. Cell Cycle. 2009;8:2014-8. [DOI] [PubMed] [Google Scholar]
- 35.Lima LG, Chammas R, Monteiro RQ, et al. Tumor-derived microvesicles modulate the establishment of metastatic melanoma in a phosphatidylserine-dependent manner. Cancer Lett 2009;283:168-75. [DOI] [PubMed] [Google Scholar]
- 36.Larson MC, Karafin MS, Hillery CA, Hogg N.Phosphatidylethanolamine is progressively exposed on RBCs during storage. Transfus Med Oxf Engl 2017;27:136-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barteneva NS, Fasler-Kan E, Bernimoulin M, et al. Circulating microparticles: square the circle. BMC Cell Biol 2013;14:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Connor DE, Exner T, Ma DDF, Joseph JE. The majority of circulating platelet-derived microparticles fail to bind annexin V, lack phospholipid-dependent procoagulant activity and demonstrate greater expression of glycoprotein Ib. Thromb Haemost 2010;103:1044-52. [DOI] [PubMed] [Google Scholar]
- 39.Horstman LL, Jy W, Jimenez JJ, et al. New horizons in the analysis of circulating cell-derived microparticles. Keio J Med 2004;53:210-30. [DOI] [PubMed] [Google Scholar]
- 40.Al-Massarani G, Najjar F, Aljapawe A, Ikhtiar A.Evaluation of circulating microparticles in healthy medical workers occupationally exposed to ionizing radiation: A preliminary study. Int J Occup Med Environ Health 2018;31:783-93. [DOI] [PubMed] [Google Scholar]
- 41.Muralidharan-Chari V, Clancy JW, Sedgwick A, D’Souza-Schorey C. Microvesicles: mediators of extracellular communication during cancer progression. J Cell Sci 2010;123:1603-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muralidharan-Chari V, Clancy J, Plou C, et al. ARF6-regulated shedding of tumor cell-derived plasma membrane microvesicles. Curr Biol 2009;19:1875-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Akers JC, Gonda D, Kim R, et al. Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J Neurooncol 2013;113:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rashmi R, Shikha J, Kirti C, et al. Role of extracellular vesicles in glioma progression: deciphering cellular biological processes to clinical applications. Curr Top Med Chem 2021;21:696-704. [DOI] [PubMed] [Google Scholar]
- 45.D’Souza-Schorey C, Chavrier P.ARF proteins: roles in membrane traffic and beyond. Nat Rev Mol Cell Biol 2006;7:347-58. [DOI] [PubMed] [Google Scholar]
- 46.Kerr JFR, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wideranging implications in tissue kinetics. Br J Cancer 1972;26:239-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kakarla R, Hur J, Kim YJ, et al. Apoptotic cell-derived exosomes: messages from dying cells. Exp Mol Med 2020;52:1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol 2007;35:495-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mariño G, Kroemer G.Mechanisms of apoptotic phosphatidylserine exposure. Cell Res 2013;23:1247-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li M, Liao L, Tian W.Extracellular vesicles derived from apoptotic cells: an essential link between death and regeneration. Front Cell Dev Biol 2020;8:1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Caruso S, Poon IKH. Apoptotic cell-derived extracellular vesicles: more than just debris. Front Immunol 2018;9:1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meehan B, Rak J, Di Vizio D.Oncosomes - large and small: what are they, where they came from? J Extracell Vesicles 2016;5:33109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vizio DD, Kim J, Hager MH, et al. Oncosome formation in prostate cancer: association with a region of frequent chromosomal deletion in metastatic disease. Cancer Res 2009;69:5601-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Minciacchi VR, Freeman MR, Di Vizio D.Extracellular vesicles in cancer: exosomes, microvesicles and the emerging role of large oncosomes. Semin Cell Dev Biol 2015;40:41-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jaiswal R, Sedger LM. Intercellular vesicular transfer by exosomes, microparticles and oncosomes - implications for cancer biology and treatments. Front Oncol 2019;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Di Vizio D, Morello M, Dudley AC, et al. Large oncosomes in human prostate cancer tissues and in the circulation of mice with metastatic disease. Am J Pathol 2012;181:1573-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ciardiello C, Leone A, Lanuti P, et al. Large oncosomes overexpressing integrin alpha-V promote prostate cancer adhesion and invasion via AKT activation. J Exp Clin Cancer Res 2019;38:317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Morello M, Minciacchi V, de Candia P, et al. Large oncosomes mediate intercellular transfer of functional microRNA. Cell Cycle 2013;12:3526-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bertolini I, Terrasi A, Martelli C, et al. A GBM-like VATPase signature directs cell-cell tumor signaling and reprogramming via large oncosomes. EBio Med 2019;41:225-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Słomka A, Urban SK, Lukacs-Kornek V, et al. Large extracellular vesicles: have we found the holy grail of inflammation? Front Immunol 2018;9:2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pezzicoli G, Tucci M, Lovero D, et al. Large extracellular vesicles - a new frontier of liquid biopsy in oncology. Int J Mol Sci 2020;21:6543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ogorevc E, Kralj-Iglic V, Veranic P.The role of extracellular vesicles in phenotypic cancer transformation. Radiol Oncol 2013;47:197-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Melo SA, Sugimoto H, O’Connell JT, et al. Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis. Cancer Cell 2014;26:707-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Elmageed ZYA, Yang Y, Thomas R, et al. Neoplastic reprogramming of patient-derived adipose stem cells by prostate cancer cell-associated exosomes. Stem Cells 2014;32:983-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Higginbotham JN, Demory Beckler M, Gephart JD, et al. Amphiregulin exosomes increase cancer cell invasion. Curr Biol 2011;21:779-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Graner MW, Alzate O, Dechkovskaia AM, et al. Proteomic and immunologic analyses of brain tumor exosomes. FASEB J 2009;23:1541-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Adamczyk KA, Klein-Scory S, Tehrani MM, et al. Characterization of soluble and exosomal forms of the EGFR released from pancreatic cancer cells. Life Sci 2011;89:304-12. [DOI] [PubMed] [Google Scholar]
- 68.McCready J, Sims JD, Chan D, Jay DG. Secretion of extracellular hsp90α via exosomes increases cancer cell motility: a role for plasminogen activation. BMC Cancer 2010;10:294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kosaka N, Iguchi H, Hagiwara K, et al. Neutral sphingomyelinase 2 (nSMase2)-dependent exosomal transfer of angiogenic microRNAs regulate cancer cell metastasis. J Biol Chem 2013;288:10849-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Clapé C, Fritz V, Henriquet C, et al. Mir-143 interferes with ERK5 signaling, and abrogates prostate cancer progression in mice. PLoS One 2009;4:e7542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen M, Xu R, Ji H, et al. Transcriptome and long noncoding RNA sequencing of three extracellular vesicle subtypes released from the human colon cancer LIM1863 cell line. Sci Rep 2016;6:38397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gerbasi FR, Bottoms S, Farag A, Mammen EF. Changes in hemostasis activity during delivery and the immediate postpartum period. Am J Obstet Gynecol 1990;162:1158-63. [DOI] [PubMed] [Google Scholar]
- 73.Li T, Xie J, Shen C, et al. Amplification of long noncoding RNA ZFAS1 promotes metastasis in hepatocellular carcinoma. Cancer Res 2015;75:3181-91. [DOI] [PubMed] [Google Scholar]
- 74.Thorenoor N, Faltejskova-Vychytilova P, Hombach S, et al. Long non-coding RNA ZFAS1 interacts with CDK1 and is involved in p53-dependent cell cycle control and apoptosis in colorectal cancer. Oncotarget 2015;7:622-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kogure T, Yan IK, Lin W-L, Patel T.Extracellular vesiclemediated transfer of a novel long noncoding RNA TUC339: a mechanism of intercellular signaling in human hepatocellular cancer. Genes Cancer 2013;4:261-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gezer U, Özgür E, Cetinkaya M, et al. Long non-coding RNAs with low expression levels in cells are enriched in secreted exosomes. Cell Biol Int 2014;38:1076-9. [DOI] [PubMed] [Google Scholar]
- 77.Sun R, Qin C, Jiang B, et al. Down-regulation of MALAT1 inhibits cervical cancer cell invasion and metastasis by inhibition of epithelial-mesenchymal transition. Mol Biosyst 2016;12:952-62. [DOI] [PubMed] [Google Scholar]
- 78.Lucchetti D, Ricciardi Tenore C, Colella F, Sgambato A.Extracellular vesicles and cancer: a focus on metabolism, cytokines, and immunity. Cancers 2020;12:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kobayashi Y, Banno K, Kunitomi H, et al. Warburg effect in gynecologic cancers. J Obstet Gynaecol Res 2019;45:542-8. [DOI] [PubMed] [Google Scholar]
- 80.Robado de Lope L, Alcíbar OL, Amor López A, et al. Tumour-adipose tissue crosstalk: fuelling tumour metastasis by extracellular vesicles. Philos Trans R Soc B Biol Sci 2018;373:20160485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim J, Afshari A, Sengupta R, et al. Replication study: Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. eLife 2018;7:e39944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kosaka N, Iguchi H, Yoshioka Y, et al. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem 2010;285:17442-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Trajkovic K, Hsu C, Chiantia S, et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 2008;319:1244-7. [DOI] [PubMed] [Google Scholar]
- 84.Fasanaro P, D’Alessandra Y, Di Stefano V, et al. MicroRNA- 210 modulates endothelial cell response to hypoxia and inhibits the receptor tyrosine kinase ligand Ephrin-A3. J Biol Chem 2008;283:15878-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kim JW, Wieckowski E, Taylor DD, et al. Fas ligand-positive membranous vesicles isolated from sera of patients with oral cancer induce apoptosis of activated T lymphocytes. Clin Cancer Res Off J Am Assoc Cancer Res 2005;11:1010-20. [PubMed] [Google Scholar]
- 86.Katsuda T, Kosaka N, Ochiya T.The roles of extracellular vesicles in cancer biology: Toward the development of novel cancer biomarkers. Proteomics 2014;14:412-25. [DOI] [PubMed] [Google Scholar]
- 87.Raposo G, Stoorvogel W.Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol 2013;200:373-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Khan S, Jutzy JMS, Valenzuela MMA, et al. Plasma-derived exosomal survivin, a plausible biomarker for early detection of prostate cancer. PLoS One 2012;7:e46737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Melo SA, Luecke LB, Kahlert C, et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature 2015;523:177-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rabinowits G, Gerçel-Taylor C, Day JM, et al. Exosomal microRNA: a diagnostic marker for lung cancer. Clin Lung Cancer 2009;10:42-6. [DOI] [PubMed] [Google Scholar]
- 91.Weber DG, Johnen G, Casjens S, et al. Evaluation of long noncoding RNA MALAT1 as a candidate blood-based biomarker for the diagnosis of non-small cell lung cancer. BMC Res Notes 2013;6:518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lu Q, Zhang J, Allison R, et al. Identification of extracellular δ-catenin accumulation for prostate cancer detection. Prostate 2009;69:411-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Armstrong DA, Green BB, Seigne JD, et al. MicroRNA molecular profiling from matched tumor and bio-fluids in bladder cancer. Mol Cancer 2015;14:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shao Y, Ye M, Jiang X, et al. Gastric juice long noncoding RNA used as a tumor marker for screening gastric cancer. Cancer 2014;120:3320-8. [DOI] [PubMed] [Google Scholar]
- 95.Eichelser C, Stückrath I, Müller V, et al. Increased serum levels of circulating exosomal microRNA-373 in receptor-negative breast cancer patients. Oncotarget 2014;5:9650-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang J, Liu S-C, Luo X-H, et al. Exosomal long noncoding RNAs are differentially expressed in the cervicovaginal lavage samples of cervical cancer patients. J Clin Lab Anal 2016;30:1116-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.McKiernan J, Noerholm M, Tadigotla V, et al. A urine-based Exosomal gene expression test stratifies risk of high-grade prostate Cancer in men with prior negative prostate biopsy undergoing repeat biopsy. BMC Urol 2020;20:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.McKiernan J, Donovan MJ, Margolis E, et al. A prospective adaptive utility trial to validate performance of a novel urine exosome gene expression assay to predict high-grade prostate cancer in patients with prostate-specific antigen 2-10 ng/mL at initial biopsy. Eur Urol 2018;74:731-8. [DOI] [PubMed] [Google Scholar]
- 99.Castellanos-Rizaldos E, Grimm DG, Tadigotla V, et al. Exosome-based detection of EGFR T790M in plasma from non-small cell lung cancer patients. Clin Cancer Res 2018;24:2944-50. [DOI] [PubMed] [Google Scholar]
- 100.Osti D, Bene MD, Rappa G, et al. Clinical significance of extracellular vesicles in plasma from glioblastoma patients. Clin Cancer Res 2019;25:266-76. [DOI] [PubMed] [Google Scholar]
- 101.König L, Kasimir-Bauer S, Bittner A-K, et al. Elevated levels of extracellular vesicles are associated with therapy failure and disease progression in breast cancer patients undergoing neoadjuvant chemotherapy. OncoImmunology 2018;7:e1376153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nakanishi T, Ross DD. Breast cancer resistance protein (BCRP/ABCG2): its role in multidrug resistance and regulation of its gene expression. Chin J Cancer 2012;31:73-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen Y, Wang L, Zhu Y, et al. Breast cancer resistance protein (BCRP)-containing circulating microvesicles contribute to chemoresistance in breast cancer. Oncol Lett 2015;10:3742-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kassam Z, Burgers K, Walsh JC, et al. A prospective feasibility study evaluating the role of multimodality imaging and liquid biopsy for response assessment in locally advanced rectal carcinoma. Abdom Radiol 2019;44:3641-51. [DOI] [PubMed] [Google Scholar]
- 105.Malla B, Aebersold DM, Dal Pra A. Protocol for serum exosomal miRNAs analysis in prostate cancer patients treated with radiotherapy. J Transl Med 2018;16:223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yu Q, Li P, Weng M, et al. Nano-vesicles are a potential tool to monitor therapeutic efficacy of carbon ion radiotherapy in prostate cancer. J Biomed Nanotechnol 2018;14:168-78. [DOI] [PubMed] [Google Scholar]
- 107.Guo X, Lv X, Ru Y, et al. Circulating exosomal gastric cancer- associated long noncoding rna1 as a biomarker for early detection and monitoring progression of gastric cancer: a multiphase study. JAMA Surg 2020;155:572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kim DH, Kim H, Choi YJ, et al. Exosomal PD-L1 promotes tumor growth through immune escape in non-small cell lung cancer. Exp Mol Med 2019;51:1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cordonnier M, Nardin C, Chanteloup G, et al. Tracking the evolution of circulating exosomal-PD-L1 to monitor melanoma patients. J Extracell Vesicles 2020;9:1710899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Theodoraki M-N, Yerneni S, Gooding WE, et al. Circulating exosomes measure responses to therapy in head and neck cancer patients treated with cetuximab, ipilimumab, and IMRT. Oncoimmunology 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Perez D de M, Russo A, Gunasekaran M, et al. 31 Dynamic change of PD-L1 expression on extracellular vesicles predicts response to immune-checkpoint inhibitors in non-small cell lung cancer patients. J Immunother Cancer 2020;8:A30. [Google Scholar]
- 112.Wang J, Wuethrich A, Sina AAI, et al. Tracking extracellular vesicle phenotypic changes enables treatment monitoring in melanoma. Sci Adv 2020;6:eaax3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jin D, Peng X-X, Qin Y, et al. Multivalence-actuated DNA nanomachines enable bicolor exosomal phenotyping and PDL1- guided therapy monitoring. Anal Chem 2020;92:9877-86. [DOI] [PubMed] [Google Scholar]
- 114.Ostenfeld MS, Jeppesen DK, Laurberg JR, et al. Cellular disposal of miR23b by RAB27-dependent exosome release is linked to acquisition of metastatic properties. Cancer Res 2014;74:5758-71. [DOI] [PubMed] [Google Scholar]
- 115.Wang T, Gilkes DM, Takano N, et al. Hypoxia-inducible factors and RAB22A mediate formation of microvesicles that stimulate breast cancer invasion and metastasis. Proc Natl Acad Sci USA 2014;111:E3234-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pitt JM, Charrier M, Viaud S, et al. Dendritic cell-derived exosomes as immunotherapies in the fight against cancer. J Immunol 2014;193:1006-11. [DOI] [PubMed] [Google Scholar]
- 117.Tan A, Peña HDL, Seifalian AM. The application of exosomes as a nanoscale cancer vaccine. Int J Nanomedicine 2010;5:889-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Alvarez-Erviti L, Seow Y, Yin H, et al. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol 2011;29:341-5. [DOI] [PubMed] [Google Scholar]
- 119.van den Boorn JG, Daßler J, Coch C, et al. Exosomes as nucleic acid nanocarriers. Adv Drug Deliv Rev 2013;65:331-5. [DOI] [PubMed] [Google Scholar]
- 120.Jr W. The epidermal growth factor receptor and its inhibition in cancer therapy. Pharmacol Ther 1999;82:241-50. [DOI] [PubMed] [Google Scholar]
- 121.Ohno S, Takanashi M, Sudo K, et al. Systemically injected exosomes targeted to EGFR deliver antitumor microrna to breast cancer cells. Mol Ther 2013;21:185-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shtam TA, Kovalev RA, Varfolomeeva EY, et al. Exosomes are natural carriers of exogenous siRNA to human cells in vitro. Cell Commun Signal 2013;11:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ravindran J, Prasad S, Aggarwal BB. Curcumin and cancer cells: how many ways can curry kill tumor cells selectively? AAPS J 2009;11:495-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Aggarwal BB, Harikumar KB. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int J Biochem Cell Biol 2009;41:40-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Anand P, Sundaram C, Jhurani S, et al. Curcumin and cancer: An ‘‘old-age” disease with an ‘‘age-old” solution. Cancer Lett 2008;32. [DOI] [PubMed] [Google Scholar]
- 126.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm 2007;4:807-18. [DOI] [PubMed] [Google Scholar]
- 127.Sun D, Zhuang X, Xiang X, et al. A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol Ther 2010;18:1606-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tian Y, Li S, Song J, et al. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials 2014;35:2383-90. [DOI] [PubMed] [Google Scholar]


