Abstract
Background
In view of the expected increase in expenditure on hip replacement treatment in Belgium, the complication rate and potential waste reduction, as estimated by the Organisation for Economic Cooperation and Development, we are not yet in a position to assess the efficiency of hip replacement treatment in Belgian hospitals. This objective study uses a cost–disability-adjusted life years (DALYs) ratio to propose a comparison of hip replacement surgery among 12 Belgian hospitals.
Methods
Our study seeks to innovate by proposing an interhospital comparison that simultaneously integrates the weighting of quality indicators and the costs of managing a patient. To this end, we associated a DALY impact with each patient safety indicator, readmission and mortality outcome. We then compared hospitals using both costs and DALYs adjusted to their case mix index. The adjusted values (costs and DALYs) were obtained by relating the observed value to the predicted value obtained from the linear regression model.
Results
We registered a total of 246.5 DALYs for the 12 hospital institutions, the average cost (SD) of a stay being €8013 (€4304). Our model allowed us to identify hospitals with observed values higher than those predicted. Out of the 12 hospitals evaluated, 4 need to reduce costs and DALYs impacts, 6 have to improve one of the two factors and 2 appear to have good results. The costs for the worst performing hospitals can rise to over €150 000.
Conclusion
Evaluating the rates of patient safety indicators, associated with cost, is a prerequisite for quality and cost improvement efforts on the part of managers and practitioners. However, it appears essential to evaluate the entire care chain using a comparable unit of measurement. The hospital’s case mix index must also be considered in benchmarking to avoid drawing the wrong conclusions. In addition, other indicators, such as the patient’s perception of the actual results, should be added to our study.
Keywords: efficiency, organisational, evaluation methodology, healthcare quality improvement, patient safety, pay for performance
Background
In 2015, Belgium registered more than 100 years of life lost (YLL) in good health (disability-adjusted life years: DALYs), including harm to patients, per 100 000 inhabitants. The Organisation for Economic Cooperation and Development (OECD) average is more than 70 DALYs/100 000 inhabitants.1 DALY is a factor that indicates the severity of the disease on a scale from 0 (perfect health) to 1 (equivalent to death). DALY is a unit of measurement commonly used in health economics studies to reflect the effectiveness of a treatment or intervention. A study by Hauck et al2 shows that, each year in England, approximately 36 000 DALYs (68 DALYs/100 000 inhabitants) are reported due to six types of adverse events: sepsis, pressure ulcer, hip fracture as a result of a hospitalised patient falling, deep vein thrombosis, central line infection and death in patients with a low probability of death. Faced with this situation and due to the limited availability of resources, the need to provide quality care combined with cost control is becoming increasingly prevalent in health policies. However, the causes of this harm are complex and difficult to identify in an institution such as a hospital.
In managing hip and knee surgery, Belgium saw 2.06% of such surgeries in 2013 result in venous thrombosis and 2.6% in pulmonary embolism, compared with the OECD average of 3.3% and 5%, respectively.1
In Belgium, with an average hospital cost of €9668 in 2016,3 and given the ageing population, hip replacements will generate an increase in financial expenditure borne by social security of more than €49 million (all other things being equal) by 2025.4 In addition, the severity of a complication is not currently expressed in benchmarking studies. However, certain complications have high financial and medical consequences. When we provide only cost data or complication data, providers resort to justifications such as, respectively, high-care quality or the severity of the case.
In view of the expected increase in expenditure on hip replacement treatment in Belgium,4 the complication rate1 and the potential waste reduction as estimated by the OECD,5 we are not yet in a position to assess the efficiency of hip replacement treatment in Belgian hospitals.
A simultaneous analysis of costs and outcomes appears therefore essential for reflecting areas of improvement in healthcare. In 2014, De Bethune et al6 compared Belgian interhospital practices and social security costs to cover these inpatients, and highlighted the need to work on guides to good practice combined with inter-hospital benchmarking.
Our study proposes an interhospital comparison that simultaneously integrates weighting complications (DALY) and hospital care costs adjusted to their case mix index.
Methods
Case selection
The study sample is based on data from 12 general hospitals in Belgium, including university hospitals from the ‘Associated Hospital Cost Analysis Project (PACHA)’.7 The hospitals involved have been anonymised. We focused our analyses on inpatients classified in the Diagnosis Related Group (DRG 301)—hip replacement (grouper 28)—and for whom we registered at least a one night stay in hospital in 2016. Within the DRG, we identified inpatients with the following admission diagnoses: trauma and chronic origin (osteoarthritis). Among these, we identified all patients who were readmitted to the same hospital within 30 days of discharge from the first inpatient stay. The inpatient stay in our study is therefore defined as the combination of the first inpatient stay and the readmission of the same patient to the same hospital. In the end, our total population is therefore estimated at 2411 inpatients.
Indicators of ‘patient safety’ and Charlson index
To develop the patient safety indicators, we used the construction methodology of the Agency for Healthcare Research and Quality (AHRQ), V.5.0.8 The AHRQ’s indicators are measures of healthcare quality, based on medico-administrative data available in hospital databases. Only the secondary diagnostic codes mentioned as ‘not present at admission’ were used to identify stay complications (box 1).
Box 1. List of patient safety indicators, AHRQ.
List of patient safety indicators (PSI) from AHRQ V.5.0 used in the study
PSI 03: pressure ulcer rate
PSI 06: iatrogenic pneumothorax rate
PSI 09: postoperative bleeding rate or hematoma rate
PSI 10: postoperative physiological and metabolic disorders rate
PSI 11: postoperative respiratory failure rate
PSI 12 :deep vein thrombosis rate or postoperative pulmonary embolism
PSI 13 :postoperative sepsis rate
PSI 16: number of transfusion reactions
The ‘infection’ indicator focused on identifying a list of codes from the ‘International Statistical Classification of Diseases and Related Health Problems’ (ICD-10). The Charlson index9 was applied to the entire population.
The calculation of DALYs
DALYs are calculated by adding the number of YLL due to premature death and the number of years of life lost due to disability (YLD)10 for each hospital stay.
Specifically, the number of YLD is calculated by multiplying incident cases by the duration and severity of the disability for a given disease. We used the disability weighting from the Institute for Health Metrics and Evaluation Reports from the Global Burden of Disease (2016)11 for decubitus ulcers (stage III and IV) and postoperative respiratory failure, while the disability weighting from the article by Jha et al12 was used for the remaining complications. When unable to find the DALY, we referred back to a pathology that was clinically similar to our complication (eg, respiratory failure or other severe cardiovascular diseases). The durations of short-term complications are derived from the literature review of the article by Jha et al. The calculation of the DALY was then applied to all stays of our population.
We considered readmission as a source of pain/discomfort for the patient, which explains why we also allocated a DALY for stays for which readmission occurred within 30 days and was related to the initial reason for hospitalisation. The duration of invalidity for readmissions corresponds to the sum of the duration of the first stay and the period before the beginning of the second admission. Mortality was calculated on the basis of Belgian mortality and life expectancy tables.13 The disability weighting of death corresponding to 1 in our study was multiplied by life expectancy according to the individual’s age.
If a patient experiences a complication followed by death during their stay, we only count this as a death.
Hospital cost data
The costs in this study refer to expenses for the acute management of hospital stays from the hospital perspective, not social security. The cost from the hospital perspective is calculated using a cost accounting analytical methodology in full costing.7 As not all hospitals have a revalidation service, and in order to compare them objectively, we did not consider cost data related to activities that occurred in the revalidation department. The isolated costs of revalidation have been subtracted from the total cost of the stay.
Statistical analyses
The statistical analyses were conducted using SPSS software, V.25. We used the descriptive ‘mean/SD’ statistics to provide a univariate description of all the variables in our study. Despite the asymmetry of the quantitative variables, we did not use ‘medians—confidence intervals’ due to the possible lack of interpretation of small values such as DALYs. The Kruskal-Wallis and Mann-Whitney tests were used to verify significant differences in dependent variables (cost–DALY) in relation to ordinal and dichotomous independent variables.
The indicators structured according to the Donabedian model in this table (table 1) were constructed by first using the literature review and then the availability of data in our database.14 15
Table 1.
Comparison of Donabedian indicators for the management of hip replacement surgery among the 12 Belgian hospitals in the study
| Hospitals | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
| Structure | Number of stays* | 109 | 92 | 47 | 108 | 376 | 113 | 639 | 183 | 251 | 179 | 184 | 130 |
| Gender (%) | |||||||||||||
| Female | 59.63 | 58.7 | 57.4 | 61.1 | 61.4 | 55.8 | 56.5 | 58.5 | 59.0 | 55.9 | 63.0 | 74.6 | |
| Male | 40.4 | 41.3 | 42.3 | 38.9 | 38.6 | 44.2 | 43.5 | 41.5 | 41.0 | 44.1 | 37.0 | 25.4 | |
| Diagnostic type (%) | |||||||||||||
| Chronic | 63.3 | 67.4 | 72.3 | 66.7 | 71.0 | 78.8 | 87.2 | 81.4 | 80.1 | 81.6 | 61.4 | 61.5 | |
| Traumatology | 36.7 | 32.6 | 27.7 | 33.3 | 29.0 | 21.2 | 12.8 | 18.6 | 19.9 | 18.4 | 38.6 | 38.5 | |
| Age* average (SD) | 69.9 (14) |
71.9 (14.6) |
63.9 (15.4) |
70.6 (11.9) |
68.7 (16.1) |
68.3 (14) |
69.7 (11.5) |
67.8 (12.7) |
69.1 (13.1) |
69.8 (12.8) |
73.5 (13.2) |
73.7 (13.2) |
|
| Charlson average* (SD) | 1.02 (1.48) |
1.02 (1.42) |
0.85 (2.32) |
0.93 (1.62) |
0.67 (1.24) |
0.64 (1.02) |
0.56 (1.03) |
0.84 (1.41) |
0.58 (0.99) |
0.62 (1.03) |
0.61 (1.19) |
0.75 (1.32) |
|
| Process | Number of days between admission day and operating day (day)* average (SD) | 1.6 (1.9) |
1.7 (3.3) |
1.4 (2.6) |
2.2 (4.5) |
1.1 (1.2) |
1.2 (1) |
1.1 (0.9) |
1 (2) |
0.5 (1.12) |
0.3 (0.9) |
0.8 (1.5) |
1.5 (1.6) |
| Chronic* average (SD) | 1.1 (0.34) |
1.1 (0.65) |
0.7 (2.4) |
0.5 (0.6) |
1 (0.9) |
1 (0) |
0.9 (0.6) |
0.5 (0.5) |
0.2 (0.6) |
0.2 (0.7) |
0.3 (1) |
1 (0.7) |
|
| Trauma* average (SD) | 2.6 (2.8) |
2.9 (5.6) |
3 (2.4) |
5.4 (6.6) |
1.2 (2.3) |
1.8 (1.9) |
1.9 (1.7) |
3.1 (4) |
1.9 (1.5) |
1 (1.5) |
1.7 (1.8) |
2.3 (2.1) |
|
| Length of stay (without revalidation) average (SD) | 11.6 (8.8) |
8.3 (5.1) |
9.5 (6.9) |
9.8 (8.5) |
6.5 (4.3) |
7.6 (5.4) |
5.1 (3.7) |
6.7 (5.43) |
6.9 (5.9) |
6.2 (4.2) |
8.9 (5.8) |
9 (4.6) |
|
| Length of stay in surgical care unit* average (SD) | 11.3 (8) |
7.8 (4.9) |
7.7 (5.2) |
6.4 (6.9) |
6.3 (3.1) |
6.7 (3) |
4.8 (3.3) |
6.5 (4.8) |
6 (3) |
6 (4) |
7.5 (4.6) |
8.8 (4.5) |
|
| Geriatric | |||||||||||||
| Inpatient geriatric liaison (%) | 13.76 | 25.00 | 17.02 | 27.78 | 10.37 | 17.7 | 0.47 | 6.56 | 2.79 | 0.00 | 24.46 | 0.00 | |
| Geriatric care unit (%) | 1.83 | 4.35 | 10.64 | 17.59 | 0.8 | 4.42 | 2.19 | 0.55 | 5.18 | 1.68 | 8.7 | 2.31 | |
| Length of stay in geriatric care unit* average (SD) | 21 (28.3) |
11 (7.4) |
17.6 (7.2) |
19.3 (8) |
26.3 (30.3) |
20.4 (7.8) |
13.9 (7.9) |
29 (-) |
19.5 (11) |
12.3 (6) |
16.1 (7.1) |
6.7 (6) |
|
| Intensive care unit (%) | 4.58% | 14.13 | 4.26 | 1.85 | 1.33 | 1.76 | 1.41 | 0.55 | 1.99 | 2.23 | 3.26 | 9.23 | |
| Physiotherapy session | |||||||||||||
| After the operation (%) | 88.99 | 96.74 | 100.00 | 65.74 | 9.31 | 98.23 | 68.08 | 99.45 | 99.6 | 96.09 | 96.74 | 15.38 | |
| Number of days between first intervention and first physiotherapy session* average (SD) | 2.2 (1.6) |
1 (0.6) |
1.1 (0.4) |
1.5 (4.2) |
2.1 (1.6) |
1.3 (1.3) |
1.5 (1.4) |
1 (0.4) |
1.1 (0.4) |
1.3 (1.1) |
0.9 (1.1) |
4.4 (3.4) |
|
| Outcomes 1 | Complication (%) | 11.93 | 14.13 | 4.26 | 7.41 | 2.93 | 10.62 | 2.66 | 14.21 | 3.98 | 8.38 | 14.67 | 10.00 |
| Postoperative respiratory failure (%) | 0.92 | 1.09 | 0.00 | 0.00 | 0.53 | 1.77 | 0.16 | 0.00 | 0.00 | 0.56 | 0.00 | 0.77 | |
| Perioperative haemorrhage or haematoma (%) | 8.26 | 8.7 | 2.13 | 2.78 | 1.06 | 6.19 | 0.78 | 12.02 | 1.99 | 4.47 | 5.43 | 3.85 | |
| Infection (%) | 1.83 | 5.43 | 2.13 | 2.78 | 0.53 | 1.77 | 1.25 | 1.64 | 1.99 | 3.91 | 7.61 | 3.08 | |
| Pressure ulcer (III or IV) (%) | 0.92 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.63 | 0.55 | 0.4 | 0.00 | 3.26 | 0.00 | |
| Perioperative pulmonary embolism or deep venous thrombosis (%) | 0.92 | 0.00 | 0.00 | 0.93 | 0.00 | 0.00 | 0.31 | 0.00 | 0.00 | 0.00 | 0.54 | 0.77 | |
| Sepsis (%) | 1.83 | 0.00 | 0.00 | 0.00 | 0.00 | 0.88 | 0.00 | 0.00 | 0.00 | 0.56 | 0.54 | 0.00 | |
| Postoperative physiologic and metabolic derangement (%) | 2.75 | 2.17 | 2.13 | 0.93 | 0.53 | 3.54 | 0.31 | 0.55 | 0.4 | 0.56 | 0.00 | 2.31 | |
| Transfusion reaction (%) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.4 | 0.00 | 0.00 | 0.00 | |
| Iatrogenic pneumothorax (%) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| Outcomes 2 | Case mix index average* | 1.69 (0.29) |
1.72 (0.43) |
1.66 (0.22) |
1.78 (0.31) |
1.71 (0.36) |
1.71 (0.40) |
1.67 (0.22) |
1.65 (0.18) |
1.64 (0.26) |
1.68 (0.28) |
1.77 (0.43) |
1.7 (0.45) |
| Mortality* (%) | 1.83 | 4.35 | 0.00 | 1.85 | 1.06 | 2.65 | 0.31 | 0.00 | 1.2 | 1.12 | 1.63 | 3.08 | |
| Readmission <30 days (11 months) | |||||||||||||
| Total readmissions (%) | 6.42 | 11.96 | 2.13 | 12.04 | 5.85 | 3.54 | 3.29 | 8.2 | 4.78 | 5.59 | 7.61 | 3.85 | |
| Readmissions related to arthroplasty (%) | 2.75 | 8.7 | 0.00 | 8.33 | 3.99 | 3.54 | 2.19 | 6.01 | 3.19 | 4.47 | 2.72 | 0.77 | |
| Mortality following readmission* (%) | 14.29 | 18.18 | 0.00 | 4.76 | 0.00 | 0.00 | 0.00 | 8.33 | 10.00 | 7.14 | 0.00 | 0.00 | |
| DALY-cost | DALY (YLL + YLD) total | 17.64 | 40.06 | 0.03 | 14.00 | 27.52 | 30.8 | 14.3 | 0.73 | 25.37 | 38.6 | 18.36 | 19.11 |
| DALY (YLL + YLD)* average (SD) | 0.162 | 0.435 | 0.001 | 0.13 | 0.073 | 0.273 | 0.022 | 0.004 | 0.101 | 0.216 | 0.1 | 0.147 | |
| 0.941 | 1.855 | 0.003 | 0.933 | 0.643 | 1.766 | 0.373 | 0.022 | 0.785 | 2.192 | 0.625 | 0.842 | ||
| Total average cost excluding revalidation* (SD) | €11 137 (€7778) |
€10 246 (€4003) |
€7828 (€3538) |
€9099 (€4465) |
€8322 (€3492) |
€9027 (€4357) |
€7078 (€1795) |
€8343 (€2389) |
€6413 (€3540) |
€7634 (€8379) |
€7943 (€3260) |
€9052 (€5126) |
|
| Total average cost including cost of readmission (excluding revalidation)* (SD) | €11 506 (€7937) |
€12 241 (€9557) |
€7935 (€3790) |
€9982 (€5990) |
€8799 (€4337) |
€9276 (€4603) |
€7210 (€2086) |
€8898 (€3416) |
€6693 (€4209) |
€8049 (€8760) |
€8391 (€4130) |
€9326 (€5323) |
|
*Kruskal-Wallis = p>0.05.
DALYs, disability-adjusted life years; YLD, years lost due to disability; YLL, years of life lost.
To correct the distribution of our dependent variables such as DALY and cost, we conducted a logarithmic transformation. We then recoded our independent variables into dummy variables. Stepwise linear regression was then carried out on these new dependent variables to identify the predicted hospital values. We chose this statistical model to adjust the data according to the hospital’s case mix. The predictors used for the model are the Charlson index, age, admission diagnosis, gender, type of admission, type of discharge destination, move to intensive care unit, move to geriatric unit, geriatric assessment during hospitalisation, readmission within 30 days of the end of the first stay, time between date of admission and date of operation, time between date of operation and first physiotherapy session and patient safety indicators during the inpatient stay. We selected these independent variables based on indicators from the literature14 15 and on the significance of the data from the univariate analysis. Homoscedasticity was controlled using a graph. Preference was given to using the Charlson index in the regression, rather than the relative weight (case mix index), since the Charlson index includes comorbidities present at admission and not complications encountered during the hospital stay. Finally, ratios between the observed value and predicted value of the inpatient stay were calculated.
Results
Table 1 describes the main results from the univariate analysis and hospital comparison. Tables 2 and 3 summarise the main regression results.
Table 2.
Result of cost stepwise linear regression
| Model summary | ||||||||
| Model | R | R2 | Adjusted R2 | SE of the estimate | ||||
| 21 | 0.716 | 0.513 | 0.509 | 0.24262 | ||||
| Coefficients* | ||||||||
| Model | Unstandardised coefficients | Standardised coefficients | t | Significance | 95 CI for B | |||
| B | SE | Beta | Lower bound | Upper bound | ||||
| 21 | (Constant) | 8.929 | 0.048 | 184.830 | 0.000 | 8.834 | 9.024 | |
| Readmission | 0.568 | 0.022 | 0.377 | 26.024 | 0.000 | 0.525 | 0.611 | |
| Complication | 0.273 | 0.022 | 0.200 | 12.325 | 0.000 | 0.230 | 0.317 | |
| Unit care | Intensive | 0.432 | 0.033 | 0.204 | 13.066 | 0.000 | 0.367 | 0.497 |
| Geriatric | 0.283 | 0.029 | 0.153 | 9.582 | 0.000 | 0.225 | 0.340 | |
| Inpatient geriatric liaison | 0.106 | 0.020 | 0.085 | 5.370 | 0.000 | 0.067 | 0.145 | |
| Charlson index | 1 | 0.034 | 0.012 | 0.040 | 2.714 | 0.007 | 0.009 | 0.058 |
| 2 | 0.047 | 0.018 | 0.038 | 2.566 | 0.010 | 0.011 | 0.083 | |
| 3 | 0.116 | 0.029 | 0.059 | 4.005 | 0.000 | 0.059 | 0.173 | |
| 4 | 0.137 | 0.040 | 0.052 | 3.409 | 0.001 | 0.058 | 0.216 | |
| 5 | 0.144 | 0.039 | 0.055 | 3.643 | 0.000 | 0.066 | 0.221 | |
| Days between admission day and operating day | 1 | 0.129 | 0.011 | 0.185 | 11.555 | 0.000 | 0.107 | 0.151 |
| 2 | 0.140 | 0.028 | 0.083 | 5.019 | 0.000 | 0.086 | 0.195 | |
| 3 | 0.188 | 0.034 | 0.089 | 5.584 | 0.000 | 0.122 | 0.254 | |
| 4 | 0.338 | 0.028 | 0.206 | 12.238 | 0.000 | 0.284 | 0.393 | |
| Diagnosis | Osteoarthritis | −0.092 | 0.024 | −0.112 | −3.794 | 0.000 | −0.139 | −0.044 |
| Physiotherapy session | 4 | 0.145 | 0.024 | 0.088 | 6.091 | 0.000 | 0.099 | 0.192 |
| Emergency | Without ambulance | −0.072 | 0.025 | −0.082 | −2.912 | 0.004 | −0.120 | −0.024 |
| Destination | Deceased | −0.181 | 0.052 | −0.057 | −3.470 | 0.001 | −0.283 | −0.079 |
| Transfer to care home | −0.062 | 0.020 | −0.051 | −3.087 | 0.002 | −0.101 | −0.023 | |
| Transfer to hospital | 0.140 | 0.020 | 0.105 | 6.967 | 0.000 | 0.100 | 0.179 | |
| Age | 51–60 years old | −0.044 | 0.014 | −0.045 | −3.115 | 0.002 | −0.072 | −0.016 |
*Dependent variable: LN_cost.
Table 3.
Result of DALY stepwise linear regression
| Model summary | ||||||||
| Model | R | R2square | Adjusted R2square | SE of the estimate | ||||
| 6 | 0.945f | 0.893 | 0.893 | 0.63105 | ||||
| Coefficients* | ||||||||
| Model | Unstandardised coefficients | Standardised coefficients | t | Significance | 95% CI for B | |||
| B | SE | Beta | Lower bound | Upper bound | ||||
| 6 | (Constant) | −9.204 | 0.014 | −658.221 | 0.000 | −9.232 | −9.177 | |
| Complication | 4.436 | 0.056 | 0.584 | 79.075 | 0.000 | 4.326 | 4.546 | |
| Readmission | 3.837 | 0.056 | 0.457 | 68.301 | 0.000 | 3.727 | 3.948 | |
| Destination | Deceased | 6.342 | 0.132 | 0.358 | 48.092 | 0.000 | 6.083 | 6.601 |
| Unit care | Intensive | 0.337 | 0.082 | 0.029 | 4.091 | 0.000 | 0.176 | 0.499 |
| Addressed by | General practitioner | 0.164 | 0.072 | 0.015 | 2.282 | 0.023 | 0.023 | 0.305 |
| Emergency room | Without ambulance | 0.170 | 0.070 | 0.016 | 2.413 | 0.016 | 0.032 | 0.308 |
*Dependent Variable: LN_DALY.
Description of the inpatients
We have 2411 inpatients with an average age (SD) of 69 years (13.5 years) (table 1). More than 59% of the population is female (table 1). The complication rate during hospital stays is estimated at 6.93%, while the mortality rate is 1.20% (table 1). Over 76% of inpatients are admitted for chronic conditions (osteoarthritis and so on) (table 1). Eighty-nine per cent of deceased patients were admitted for trauma. The mortality rate for inpatients with a ‘Patient Safety’ complication is 20%. Patients who died in hospital have a mean Charlson index (SD) of 2.19 (1.68), compared with 0.63 (1.047) for patients who did not die (p<0.001). The average rate of haemorrhage haematomas is 3.61%, infection 2.32%, physiological complications 0.87%, pressure ulcer 0.54%, respiratory arrest 0.37%, deep vein thrombosis 0.25% and sepsis 0.21% (table 1). The group admitted for trauma, which represents just under 24% of inpatients, accounts for more than half of the complications (54%); 2.74% of inpatients go through an intensive care unit; 3.65% of the population goes to a geriatric unit; 62% of inpatients have no comorbidity according to the Charlson index; 5.60% of inpatients are readmitted to the same institution within 30 days (table 1); and 67% of these inpatients return for a reason related to their previous hospitalisations. Seven patients died following these readmissions.
Evaluation of duration and cost of stay
The average cost (SD) of an inpatient stay is €8013 (€4304) (table 1). It also seems to increase with age (p<0.001) from €7446 (€2453) in the 18–50 age category to €9148 (€5039) in the 81–102 age category. Inpatients admitted for a ‘chronic’ condition, such as osteoarthritis, have an average total cost (SD) of €7414 (€3087), while inpatients admitted in the ‘trauma’ group, for example due to a femur fracture, represent an average cost (SD) of €9939 (€6529) (p<0.001). The average cost (SD) of readmission is estimated at €6953 (€7873).
The average cost (SD) of an inpatient stay that does not go through the intensive care unit is €7725 (€2627), while the average cost (SD) of an inpatient stay that does go through the intensive care unit is €18 250 (€18 124). The average cost (SD) of an inpatient stay that does not go through a geriatric unit is €7743, while the average cost (SD) of an inpatient stay that does go through a geriatric unit is €15 141 (€10 725).
The average length of stay (LOS) (SD) is 7.1 days (5.5 days) (table 1). The LOS (SD) is 5.67 days (3.78 days) if the admission diagnosis is chronic and 11.48 days (7.53 days) if the admission diagnosis is trauma based (p<0.001). In the case of a patient who dies while in hospital (p<0.001), the LOS (SD) is 12.41 days (9.58 days).
About 11.92 days is the LOS (SD) that elapses between the date of discharge of the first inpatient stay and the date of admission for the second inpatient stay (readmission) (8.89 days).
Evaluation of LOS and cost associated with complications
About 54% of inpatients admitted through emergency services have a complication. Among the 62% of inpatients who have a Charlson comorbidity index of 0, 3.78% suffer at least one complication during their stay (p<0.001). Among the 5.4% of stays with a Charlson index greater than 2, 36% suffer at least one complication (p<0.001). The average duration (SD) increases from 6.53 days (4.43 days), if the inpatient does not encounter a complication, to 14 days (11.14 days), if the inpatient experiences at least one complication during hospitalisation (p<0.001). The average cost (SD) increases from €7611 (€2566), if the inpatient does not experience at least one complication, to €13 419 (€12 181), if the inpatient experiences at least one complication during hospitalisation (p<0.001).
Impact of DALYs
We registered a total of 246.5 DALYs for these inpatients in the 12 hospitals in our study (table 1). Deaths alone represent more than 240 DALYs (YLL). Complications and readmissions represent 6.5 DALYs for the entire group (YLD) (figure 1). The average number (SD) of DALYs per inpatient is estimated at more than 0.102 (0.97). The average number (SD) of DALYs increases from 0.03 (0.51 DALYs) with a Charlson index of 0–1.43 (4.91 DALYs) with a Charlson index of 5 (p<0.001).
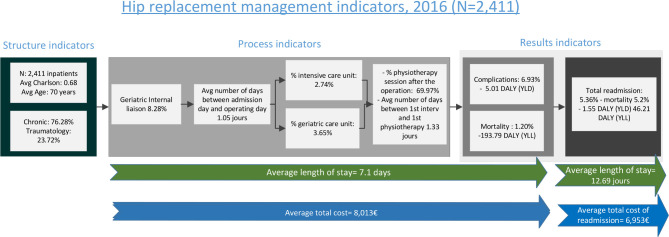
Figure 1.
Process overview—indicators and data for hip replacement management, for the 12 Belgian hospitals in the study, N=2411. DALYs, disability-adjusted life years; YLD, years lost due to disability; YLL, years of life lost.
Benchmarking
Process and results indicators
Hospitals 1, 11 and 12 have the highest number of inpatients admitted for trauma treatment (between 36.70% and 38.59%) (table 1). The average LOS (SD) varies from 5.1 days (3.73 days) for hospital 7 to 11.6 days (8.78 days) for hospital 1 (table 1). In particular, geriatric care and intensive care vary from one hospital to another, with hospital 4 seeing more than 17% of stays in geriatric care, compared with hospital 2, which sees more than 14% of its inpatients in an intensive care unit. Our hospital comparison also shows variability in the use of inpatient geriatric liaison services, from 0% in hospitals 10 and 12% to 25% in hospital 2 (table 1). The average intervention time in all hospitals is less than 48 hours for admissions related to chronic conditions. When the reason for admission is trauma, seven hospitals report an average duration of more than 2 days before the operation. The inpatients admitted for a trauma diagnosis experience the longest intervention time (SD), particularly hospital 4 with 5.4 days (6.6 days). Only 9% of hospital 5 inpatients receive physiotherapy sessions, while hospital 3 provides 100% of its patients with physiotherapy (table 1). The complication rate, all complications considered, varies between 2.6% (hospital 7) and more than 14% (hospitals 2, 8, 11) (table 1). Finally, readmission rates can be high in some hospitals, with hospitals 2 and 4, respectively, reporting more than 11% and 18%. The mortality rate also varies by hospital, from 0% for hospitals 3 and 8% to 4.5% for hospital 2 (table 1).
Table 1 below shows the DALYs and hospital costs reported by the 12 hospitals in managing hip replacements in 2016. The average DALY (SD) varies by hospital, with hospital 3 at 0.001 (0.003) and hospital 2 at 0.435 (1.855) (table 1). Hospitals 2, 10, 6, 5 and 9 have the highest number of DALYs. These five hospitals report over 136 DALYs covering 41% of inpatients. Hospitals 2, 6 and 10 have the highest average number of DALYs (YLD + YLL) per inpatient (table 1).
Hospital 2, which has an important number of DALYs, also has high total costs. Hospitals with the lowest average cost do not systematically have lower DALYs as shown by hospitals 6 and 11 (table 1). However, when we analyse our data, we see that the average cost of benefits varies between 58% for hospitals 1, 2, 4 and 12 and more than 73% for hospitals 7 and 8.
Data adjustment according to hospital profile
Stepwise linear regression was carried out to determine the impact of our predictors (see statistical analyses) on the hospital cost of hip replacements. In our model, a significant positive link was found: R² is calculated at 0.509 (table 2).
In our model, the independent variables that influence the logarithm of cost (table 2) are readmission within 30 days, complications, move to intensive care, inpatient geriatric liaison, transfer to another hospital, Charlson index, number of days between the date of admission and the surgical intervention of more than 1 day, intervention by a physiotherapist after 4 days and transfer to hospital. However, admission in emergency department, admission diagnosis, patient death, transfer to a rest or care home and age category between 51 and 60 years show a significant but negative relationship with the total cost (table 2).
When DALY is our dependent variable, our linear regression has an R² of 0.893 (table 3). For DALYs, significant variables are complications, readmission, death, move to intensive care, patient referral by a general practitioner and admission through the emergency department without an ambulance (table 3).
Table 4 shows the hospital ratios obtained from our regression models on cost and DALYs. The ratios per hospital are calculated by dividing the observed mean value by the predicted mean value from the regression model. When the ratio is above 1, this means that the observed value is higher than the value predicted by our model.
Table 4.
Results of observed cost/predicted cost and observed DALY/predicted DALY from linear regression for 12 hospitals
| Hospitals | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
| Observed/predicted cost | 1.015 | 1.017 | 0.997 | 1.001 | 1.005 | 1.009 | 0.992 | 1.010 | 0.985 | 1.003 | 0.998 | 1.006 |
| Observed/predicted DALY | 0.989 | 0.995 | 1.011 | 1.003 | 1.003 | 0.998 | 1.003 | 1.001 | 0.997 | 0.099 | 0.980 | 1.008 |
DALY, disability-adjusted life years.
In an attempt to more easily identify hospitals with higher than expected ratios, we translate this data into a fourzone graph. The upper right area shows hospitals with a higher cost and DALY than predicted. The lower left area shows hospitals with a lower cost and DALY than their predicted case mix (figure 2).
Figure 2.
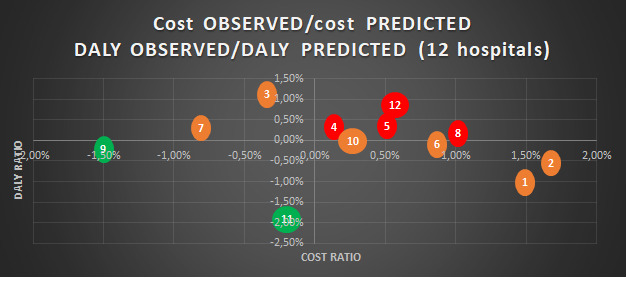
Graphic of observed cost/predicted cost and observed DALY/predicted DALY from linear regression among 12 Belgian hospitals. This graph identifies the position of hospitals regarding their performance in terms of costs and DALYs. A bubble represents a hospital and is based on the data from table 4. Hospitals with a red colour suggest that both variables are unfavourable, hospitals with a green colour suggest that both variables are favourable and hospitals with an orange colour suggest that one of the two variables of the hospital is favourable. DALY, disability-adjusted life years.
Hospitals 4, 5, 8 and 12 appear to have higher costs and DALYs than the predicted values in our model (table 4). The difference between the observed cost and the predicted cost for hospital 1 is €1626. With 109 stays, the excessive hospital cost therefore amounts to more than €170 000. Only hospitals 9 and 11 have observed values lower than our model for both costs and DALYs. The observed costs for hospitals 3 and 7 are lower than our model. Hospitals 1, 2, 6, 9, 10 and 11 have observed DALYs lower than our model.
Discussion
The objective of our study was to conduct a cost–DALY comparison between 12 Belgian general hospitals with respect to the management of hip replacement surgery. In order to position the hospitals, we identified the hospital costs of acute inpatient stays as well as the DALY impact of their management by the hospital.
In order to identify the indicators in our article, and despite the absence of a guide to good practice in Belgium,6 we drew on the literature and, in particular, references already available in many countries: England, the USA, Scotland and countries.16 17 The review of good practice guidelines points to the need for ortho-geriatric management, an intervention time of less than 48 hours, and the rapid mobilisation of patients.16–20 The simultaneous management of orthopaedics and geriatrics is highlighted as reducing the cost of stay.21 22 Only the geriatric aspect could be explored in our study. As such, our study shows divergent practices regarding the care provided to the elderly population. This variability is partly explained by the funding of stays in geriatric care in Belgium.23
The average length of our inpatient stays (7.1 days) is lower than in the Belgian technical report3 due to the selection of our population and the exclusion of data related to rehabilitation care. This exclusion was carried out in order to make a more reliable comparison between hospitals, since not all of them have a rehabilitation service. Our average overall LOS is within the ranges of the literature, which reports that variability can be encountered depending on the different postoperative procedures or the lack of social support.24 25 This organisation of care is reflected in our study by the number of returns home and the number of transfers from stays to rest homes, which can vary from 60% (hospital 1) to 89% (hospital 10) and from 3.54% (hospital 6) to 20.77% (hospital 11), respectively. Nevertheless, our results seem in line with Maeda et al26 who obtain high costs for stays on average longer and with a high complication rate.
In identifying process indicators, we did not have the opportunity, due to the lack of data, to analyse pain management, the impact of access routes and surgical techniques, or post-revision, as considered in other studies.6
In terms of result indicators, the haemorrhage/haematoma indicator has a high frequency in our hospitals, potentially explained by the systematic presence of postoperative haematoma in the surgeon’s operating protocol. Belgian funding rules may indeed encourage certain hospitals to overcode or undercode medical information in order to optimise this funding.23 Using DALYs to weight this complication moderates the magnitude of the frequency. As a previous study has shown, the weighting of complications and the adjustment of the result to the case mix are essential27 in positioning hospitals. The DALYs have therefore allowed us to weight the complications encountered during stays, with it being understood that they do not all have the same impact on the patient. The use of DALYs in favour of quality-adjusted life years is justified because of the unfavourable impact of medical complications. For this reason, hospital mortality as a function of age is the most important indicator, particularly due to the early loss of a patient.
Furthermore, this cost–DALY impact of readmissions is not negligible for some hospitals and must be considered when assessing hospital performance. Our average readmission rate of 5.6% is comparable to Dundon et al,28 which is 5%. The results of the study by Cary et al29 are similar to our results with respect to reason for readmission: periprosthetic fractures, dislocations, infections, and so on.
However, eliminating all complications does not appear to reduce the observed cost variations.26 According to the OECD report, there is wide variability in the care provided to correct these errors, resulting in a high consumption of care that is sometimes considered unnecessary or even harmful to the patient.5 The hospital costs in our study are quite close to the literature reviewed,30 31 with an average cost ranging from €7816 in Austria to €8805 in Sweden. The differences can be explained partly by the studies’ methodology and partly by the care organisation models in the different countries.24 25 Nevertheless, our study shows that some hospitals have a cost of up to 23% higher than expected to cover their inpatients. Given the diversity of medical practices,32 our article highlights the value of combining both costs and quality indicators in one single weighted measure to objectively assess the performance of hospitals according to their case mix. Nonetheless, it is necessary to identify criteria for quality assessment and risk adjustment methods.33 Decision-makers should reward the relevance of care rather than the quantity of treatment provided.1
The literature reports a significant improvement in outcomes as a result of the introduction of, and compliance with, evidence-based medicine,20–34 specifically when the comparison of structural, process and outcome indicators identified by providers is used to improve hospital and patient performance.14–35 However, this performance also requires the patient’s involvement through the evaluation of their satisfaction. Gjertsen et al36 suggest that a high proportion of patients report postoperative problems that are not mentioned in the preoperative phase: pain, walking problems.
In the absence of benchmarks and communication,37 Belgian hospital managers and healthcare providers are therefore not in a position to assess the quality, the achievement of an outcome or a performance in the overall management of a pathology in their institution. For this reason, our study proposes a benchmarking process that reflects the organisation of hospitals outside good practice guidelines and before weighting the results of the care provided.38 39 Our study reveals that, in 2025, complications in prosthetic treatment in Belgian hospitals (all other things being equal) could result in just over 3270 YLL.4 We believe that resorting to DALYs is a good approach for assessing health outcomes in hospitals. Furthermore, translating these adverse events offers us a common unit of comparison in the field of quality management.
Combined with the patient-reported outcome measures, the automatic availability of our methodology in the daily life of stakeholders seems to be a concrete approach to translate the value brought to patients and society. However, this method is intended to be progressive, since the analysis allows data to move from the inter-hospital to the patient level.
Our multidimensional reporting (regression, Donabedian model) first identifies the best performing hospitals in terms of cost and patient safety. It then gives stakeholders the opportunity to pinpoint the elements of the management process that have the greatest impact, in comparison to other institutions, for the same pathology: the transition to intensive care, readmissions, number of days between admission and operation, patient safety indicators and so on.
After identifying and prioritising the indicators that justify the hospital’s position, the managers of the facilities can mobilise all the stakeholders (manager, doctor, nurse, pharmacist, financial analyst, etc.) to analyse and understand these indicators, more specifically using a less aggregated set of data. In view of the results of the benchmarking, setting common objectives between the stakeholders becomes easier.
In the long term, regular and proactive consultations of these results extended to other pathologies, in a computerised file or during budgetary meetings, would make it possible to open up the debate between players in order to activate the levers of change in hospitals.
Limitations
The DALY impact is probably greater than we estimated in our study. The scope of the study was essentially hospital-based, which is why the calculation of the DALY was limited to complications during hospital stays. In addition, it appears essential to refine these weighting keys to improve the quality of the comparisons. Since we only had data from 2016 from the hospitals taking part in the PACHA benchmarking, we were unable to identify readmissions:
within 30 days for stays where the person was taken to hospital after 2 December 2016.
of inpatients who could have taken a place in a facility different from the first.
Due to a lack information, we did not identify any ‘patient safety’ medical complications that might have occurred during readmissions or at home. Furthermore, in the absence of disability weights for some complications, we summarised pathologies that were clinically similar to our medical complications.
Unfortunately, neither the ICD-10-PCS coding system nor the Belgian nomenclature allowed us to distinguish between the surgical approaches used in hospitals.
Conclusion
Evaluating the rates of patient safety indicators associated with costs is a prerequisite for quality and cost improvement efforts on the part of managers and practitioners. However, the availability of benchmarking to assess hospital care costs must be refined and incorporated into improving the quality of care provided by the hospitals. It appears essential to evaluate the entire care chain using a comparable unit of measurement. The hospital’s case mix index must also be considered in the benchmarking process at the risk of drawing the wrong conclusions. In addition, other indicators should be added to our study, including the patient’s perception of the actual results they experienced. In view of the increasing demand from the field for medicoeconomic tools, we believe that our approach is an opportunity to open a new door.
Footnotes
Contributors: Different sections of the article were contributed by for following authors. Background: FD. Methods: FD, MG, BD, BB, PVW, BM, PL and MP. Data: PL and MP. Interpretation of results: FD, MG, BD, BB and MP. Revised discussion: FD, MG, PL and MP. Revised conclusion: FD, MG and MP.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data may be obtained from a third party and are not publicly available. The data sets generated and/or analysed during the current study are not publicly available, as this study is partly based on cost data from a hospital’s benchmarking of cost by pathology. That being said, they are available from the relevant author upon reasonable request and after being rendered anonymous.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Slawomirski L, Auraaen A, Klazinga N. The Economics of Patient Safety. Strengthening a value-based approach to reducing patient harm at national level’, OECD Health Working Paper. 96, 2017: 20. http://www.oecd.org/health/patient-safety.htm [Google Scholar]
- 2.Hauck KD, Wang S, Vincent C, et al. Healthy life-years lost and excess Bed-Days due to 6 patient safety incidents: empirical evidence from English hospitals. Med Care 2017;55:125–30. 10.1097/MLR.0000000000000631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Service public fédéral Santé Publique, . Feedback financier par pathologie, 2016. Available: https://tct.fgov.be/webetct/etct-web/anonymous?lang=fr
- 4.Van de Voorde C, Van Den Heede K, Beguin C. Centre Fédéral d’expertise des soins de santé, ‘Capacité hospitalière nécessaire en 2025 et critères de la maîtrise de l’offre pour la chirurgie oncologique complexe, la radiothérapie et la maternité’, KCE REPORT 289Bs 2017.
- 5.The Organisation for Economic Co-operation and Development (OECD), ‘Tacking Wasteful Spending on Health’, OECD Publishing 2017.
- 6.De Bethune X, Ackaert K, Gillet P, et al. Total hip arthroplasty in Belgium: the contribution of a social health insurer to the debate. Acta Orthop Belg 2014;80:348–56. [PubMed] [Google Scholar]
- 7.Pirson M, Leclercq P. Un projet pilote d’évaluation des coûts par pathologie, le projet PACHA. HealthCare Executive 2014;78:12–14. [Google Scholar]
- 8.Agency for Healthcare Research and Quality . Patient safety indicators technical specifications, 2018. Available: https://www.qualityindicators.ahrq.gov/modules/psi_resources.aspx
- 9.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005;43:1130–9. 10.1097/01.mlr.0000182534.19832.83 [DOI] [PubMed] [Google Scholar]
- 10.Health statistics and information systems, metrics: Disability-Adjusted life year (DALY), 2018. Available: https://www.who.int/healthinfo/global_burden_disease/metrics_daly/en/
- 11.Institute for Health Metrics and Evaluation, . Global burden of disease study 2016, data resources, 2018. Available: http://ghdx.healthdata.org/gbd-2016
- 12.Jha AK, Larizgoitia I, Audera-Lopez C, et al. The global burden of unsafe medical care: Analytic modelling of observational studies. BMJ Qual Saf 2013;22:809–15. 10.1136/bmjqs-2012-001748 [DOI] [PubMed] [Google Scholar]
- 13.Statbel . Tables de mortalité et espérance de vie 2016, 2019. Available: https://data.gov.be/fr/dataset/72c1db031defb669a78ea81ddba786bc3238a78a
- 14.Moore L, Lavoie A. Donabedian’s structure-process-outcome quality of care model: Validation in an integrated trauma system. J Trauma Acute Care Surg 2005;78:1168–75. [DOI] [PubMed] [Google Scholar]
- 15.Petrosyan Y, Sahakyan Y, Barnsley JM, et al. Quality indicators for care of osteoarthritis in primary care settings: a systematic literature review. Fam Pract 2018;35:151–9. 10.1093/fampra/cmx090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Institute for Health and Care Excellence . Nice Pathways - Hip fracture overview, 2018. Available: https://pathways.nice.org.uk/pathways/hip-fracture
- 17.Roberts KC, Brox WT, Jevsevar DS, et al. Management of hip fractures in the elderly. J Am Acad Orthop Surg 2015;23:131–7. 10.5435/JAAOS-D-14-00432 [DOI] [PubMed] [Google Scholar]
- 18.Voeten SC, Krijnen P, Voeten DM, et al. Quality indicators for hip fracture care, a systematic review. Osteoporos Int 2018;29:1963–85. 10.1007/s00198-018-4558-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scottish Intercollegiate Guidelines Network, . Prevention and management of hip fracture in older people: a national clinical guideline (2009), 2018. Available: https://www.sign.ac.uk/sign-111-management-of-hip-fracture-in-older-people.html
- 20.Farrow L, Hall A, Wood AD, et al. Quality of care in hip fracture patients: the relationship between adherence to national standards and improved outcomes. J Bone Joint Surg Am 2018;100:751–7. 10.2106/JBJS.17.00884 [DOI] [PubMed] [Google Scholar]
- 21.Hughson J, Newman J, Pendleton RC. Hip fracture management for the hospital-based clinician: a review of the evidence and best practices. Hosp Pract 2011;39:52–61. 10.3810/hp.2011.02.374 [DOI] [PubMed] [Google Scholar]
- 22.Coventry LL, Pickles S, Sin M, et al. Impact of the orthopaedic nurse practitioner role on acute hospital length of stay and cost-savings for patients with hip fracture: a retrospective cohort study. J Adv Nurs 2017;73:2652–63. 10.1111/jan.13330 [DOI] [PubMed] [Google Scholar]
- 23.Service public fédéral, Santé Publique, sécurité de la chaine alimentaire et environnement, Financement des hôpitaux - Réforme du paysage hospitalier et du financement des hôpitaux, 2018. Available: https://www.health.belgium.be/fr/sante/organisation-des-soins-de-sante/hopitaux/financement-des-hopitaux/reforme-du-paysage
- 24.Geissler A, Scheller-Kreinsen D, Quentin W, et al. Do diagnosis-related groups appropriately explain variations in costs and length of stay of hip replacement? A comparative assessment of DRG systems across 10 European countries. Health Econ 2012;21(Suppl 2):103–15. 10.1002/hec.2848 [DOI] [PubMed] [Google Scholar]
- 25.Murphy Benjamin P d'S, Dowsey MM, Choong PFM. The impact of advanced age on the outcomes of primary total hip and knee arthroplasty for osteoarthritis: a systematic review. JBJS Rev 2018;6:e6. 10.2106/JBJS.RVW.17.00077 [DOI] [PubMed] [Google Scholar]
- 26.Maeda JLK, Mosher Henke R, Marder WD, et al. Variation in hospital inpatient prices across small geographic areas. Am J Manag Care 2014;20:907–16. [PubMed] [Google Scholar]
- 27.Dehanne F, Van Wilder P, Leclercq P, et al. Benchmarking de la prothèse hanche dans 7 hôpitaux. Journal de gestion et d'économie de la santé 2019;5:410–30. [Google Scholar]
- 28.Dundon JM, Bosco J, Slover J, et al. Improvement in total joint replacement quality metrics: year one versus year three of the bundled payments for care improvement initiative. J Bone Joint Surg Am 2016;98:1949–53. 10.2106/JBJS.16.00523 [DOI] [PubMed] [Google Scholar]
- 29.Cary MP, Goode V, Crego N, et al. Hospital readmission in total hip replacement patients in 2009 and 2014. Arch Phys Med Rehabil 2018;99:1213–6. 10.1016/j.apmr.2017.12.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koeck CM, Schwappach DL, Niemann FM, et al. Incidence and costs of osteoporosis-associated hip fractures in Austria. Wien Klin Wochenschr 2001;113:371–7. [PubMed] [Google Scholar]
- 31.Bouee S, Lafuma A, Fagnani F, et al. Estimation of direct unit costs associated with non-vertebral osteoporotic fractures in five European countries. Rheumatol Int 2006;26:1063–72. 10.1007/s00296-006-0180-x [DOI] [PubMed] [Google Scholar]
- 32.Carlisle DM, Valdez RB, Shapiro MF, et al. Geographic variation in rates of selected surgical procedures within Los Angeles County. Health Serv Res 1995;30:27–42. [PMC free article] [PubMed] [Google Scholar]
- 33.Antonova E, Boye ME, Sen N, et al. Can bundled payment improve quality and efficiency of care for patients with hip fractures? J Aging Soc Policy 2015;27:1–20. 10.1080/08959420.2015.970844 [DOI] [PubMed] [Google Scholar]
- 34.Dellinger EP, Hausmann SM, Bratzler DW, et al. Hospitals collaborate to decrease surgical site infections. Am J Surg 2005;190:9–15. 10.1016/j.amjsurg.2004.12.001 [DOI] [PubMed] [Google Scholar]
- 35.Merle V, Moret L, Pidhorz L, et al. Does comparison of performance lead to better care? A pilot observational study in patients admitted for hip fracture in three French public hospitals. Int J Qual Health Care 2009;21:321–9. 10.1093/intqhc/mzp029 [DOI] [PubMed] [Google Scholar]
- 36.Gjertsen J-E, Baste V, Fevang JM, et al. Quality of life following hip fractures: results from the Norwegian hip fracture register. BMC Musculoskelet Disord 2016;17:265. 10.1186/s12891-016-1111-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thakker A, Briggs N, Maeda A, et al. Reducing the rate of post-surgical urinary tract infections in orthopedic patients. BMJ Open Qual 2018;7:e000177. 10.1136/bmjoq-2017-000177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Service public fédéral, Santé Publique, sécurité de la chaine alimentaire et environnement . Programme Pay for Performance 2018 pour les hôpitaux généraux, note d’accompagnement, 2019. Available: https://www.health.belgium.be/sites/default/files/uploads/fields/fpshealth_theme_file/note_daccompagnement_p4p_24_avril_2018_1.pdf
- 39.Annemans L, Boeckxstaens P, Borgermans L. ‘Avantages, désavantages et faisabilité de l’introduction de programmes “P4Q” en Belgique’, Centre Fédéral d’expertise des soins de santé, reports 118B (2009).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data may be obtained from a third party and are not publicly available. The data sets generated and/or analysed during the current study are not publicly available, as this study is partly based on cost data from a hospital’s benchmarking of cost by pathology. That being said, they are available from the relevant author upon reasonable request and after being rendered anonymous.