Abstract
Background:
There is conflicting evidence about whether mortality after myocardial infarction (MI) is higher among women than among men. This study aimed to compare sex differences in post myocardial infarction mortality in the Veterans Affairs system, a setting where the predominant subjects are men.
Methods:
The Veterans Affairs Corporate Data Warehouse inpatient and laboratory chemistry databases were used to identify patients diagnosed with acute myocardial infarction from inpatient records from January 1st, 2005 to April 25th, 2015. Mortality data was obtained through the VA’s death registry.
Results:
A total of 130,241 patients were identified; 127,711 men (98%) and 2,530 women (2%). Men typically had more comorbidities including congestive heart failure (54% vs 46%, p value <0.001), diabetes mellitus (54% vs 48%, p value <0.001), and chronic kidney disease (39% vs 28%, p value <0.001). The peak troponin-I was significantly higher among men (16.0 vs 10.7 ng/mL, p value = 0.03). The mean follow-up time was 1490.67 ± 8 days. After adjusting for differences in demographics and comorbidities, women had a significantly lower risk of mortality (hazard ration [HR]: 0.747, p value <0.0001) as compared to men.
Conclusions:
In a health care system where the predominant subjects are men, women had better short- and long-term survival than men after an acute myocardial infarction. Further investigation is warranted to determine the reasons behind the improved outcomes in women post-MI in the veteran population.
Keywords: Cardiology, Myocardial Infarction, Mortality
INTRODUCTION
Despite advances in management of myocardial infarction (MI) with reduction in mortality, a review of the literature suggests that post MI mortality in the U.S. and abroad remains higher among women than men.1–4 A variety of reasons have been suggested to explain this including atypical presentation among women, delay in presentation, delay in recognition of MI, delay in treatment, and lower a procedure rate among women.1–4
The Veterans Affairs medical system serves a predominantly male patient population and provides equal access to care to all veterans. It is not clearly known whether a sex difference in post MI prognosis exists, or if it is even greater in the veteran population. Contrary to prior reports5, our prior attempt to address the question of differences of post-MI outcomes based on sex\ showed that despite a greater number of procedures performed on men, women fared better post MI in the veterans hospitals in long term follow up. Our initial study had several limitations, including lack of information on pharmacotherapy and size of infarct. Our new analysis utilizes one of the largest databases to address the sex difference in post MI prognosis among veterans. The updated database includes a greater number of patients, longer follow-up to 10 years, and information on drug use, procedures, and infarct size.
METHODS
Study Population
The Veterans Affairs (VA) administration provides care for 9 million veterans and their families in the United States. The study was approved by an institutional review committee. We queried the VA’s Corporate Data Warehouse (CDW) inpatient and laboratory chemistry databases. All hospital discharges of veterans with a primary ICD-9 code diagnosis of type 1 MI recorded, which have been shown previously to have excellent positive predictive value, were studied.6 Our sample contains patients who were discharged between January 1, 2005 and April 25, 2015 (Figure 1). For veterans with an eligible ICD-9 code during the study period who had multiple admissions, the first admission was considered to be the index admission. Patients without a VA measurement of troponin at any time were excluded (3%). Follow-up duration was defined as the time from the first inpatient diagnosis of myocardial infarction to death or to the end of the study.
Figure 1:
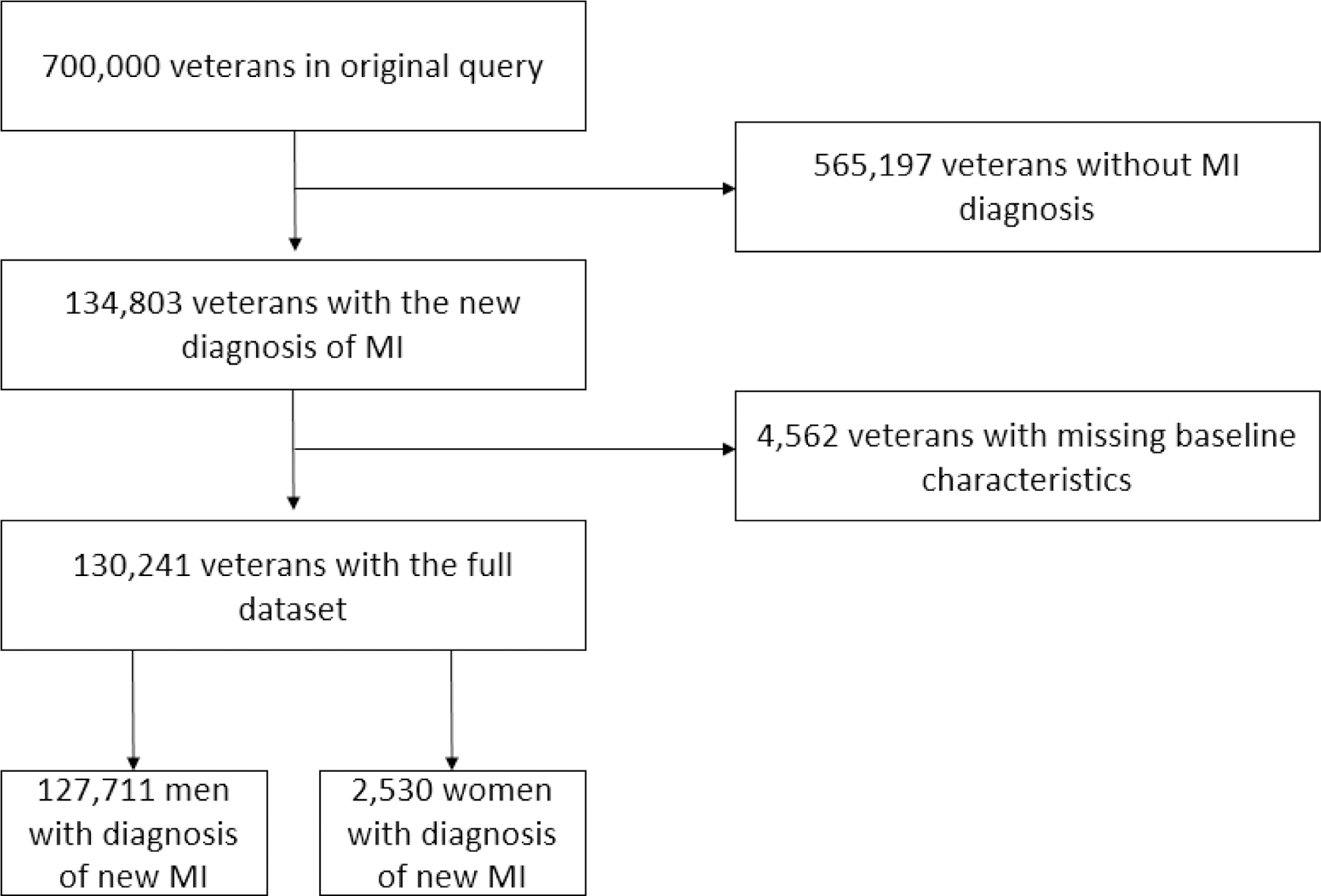
Consort diagram for retrospective data analysis. Abbreviation: MI, myocardial infarction.
Comorbidities were defined by inpatient or outpatient ICD-9 code diagnoses. All-cause mortality data was obtained through the VA’s death registry. Other patient characteristics considered as variables in our risk-adjustment models included age and sex. We chose not to include race in the models due to a large number of missing values.
Statistical Analysis
Using chi-square test and Student’s t-test, for categorical and continuous variables, respectively, men and women were compared in terms of demographics, medications, and comorbidities. The mortality rate difference by sex at 30 days, 60 days, and 1 year after admission was assessed using chi-square test, and the odds ratios (OR) were calculated. In order to consider the follow-up time and censored observations, Cox proportional hazards survival analysis was utilized to compare survival between men and women, and the hazard ratio (HR) was reported. The survival analysis was adjusted for the following variables: age, insurance status, peak serum troponin, ST elevation (STE) diagnosis, coronary artery bypass grafting (CABG), percutaneous coronary intervention (PCI), comorbidities previously mentioned, and medications that patient was discharged on. As a robustness test, we also performed propensity score matching of sex based on observed patient characteristics and comorbidities, and they were re-analyzed to test the consistency of results after matching.
All data analyses were performed using STATA 15.1. A p value < 0.05 was considered statistically significant.
RESULTS
A total of 130,241 patients were identified; 127,711 men (98%) and 2,530 women (2%). Table 1 summarizes the demographics and comorbidities of the two groups.
TABLE 1.
Summarizes the demographics and comorbidities of the two groups.
| Men | Women | P Value | |
|---|---|---|---|
| n = 127,711 | n = 2,530 | ||
| Age, years (mean) | 72.89 | 70.17 | <0.001 |
| Comorbidities | |||
| Smoking | 30.7% | 33.4% | 0.003 |
| Atrial fibrillation | 30.6% | 23.6% | <0.001 |
| Coronary artery disease | 88.8% | 80.6% | <0.001 |
| Congestive heart failure | 53.7% | 45.9% | <0.001 |
| Diabetes | 54.1% | 47.8% | <0.001 |
| Hypertension | 82.5% | 81.9% | 0.47 |
| Obstructive sleep apnea | 12% | 10.3% | 0.009 |
| Peripheral artery disease | 27.7% | 18.6% | <0.001 |
| Deep vein thrombosis | 6.2% | 5.9% | 0.56 |
| Pulmonary emboli | 3.4% | 3.8% | 0.22 |
| Cirrhosis | 2.6% | 1.9% | 0.03 |
| Chronic kidney disease | 39.4% | 28% | <0.001 |
| Chronic obstructive pulmonary disease | 38.1% | 32.3% | <0.001 |
| Cerebrovascular accident | 20.5% | 19.3% | 0.16 |
| Presentation | |||
| Peak troponin levels (normal < 0.05 ng/mL) | 16.00 | 10.70 | 0.03 |
| STE-MI | 24.2% | 24.3% | 0.91 |
| Medications a | |||
| Aspirin | 88.4% | 84% | <0.001 |
| Digoxin | 15.3% | 10.4% | <0.001 |
| Hydralazine | 1% | 1% | 0.85 |
| Beta blockers | 88.1% | 82.5% | <0.001 |
| P2Y 12 inhibitors | 67.2% | 59.4% | <0.001 |
| Loop diuretics | 59.3% | 54.2% | <0.001 |
| Non-dihydropyrdines Ca blockers | 18.3% | 19.8% | 0.05 |
| Dihydropyridine Ca Blockers | 38.9% | 41.4% | 0.01 |
| Statin | 91.7% | 87.3% | <0.001 |
| Nitrates | 42.6% | 36.8% | <0.001 |
| ACE inhibitor and ARB | 87.8% | 81.5% | <0.001 |
| Interventions | |||
| CABG | 4.30% | 2.1% | <0.0001 |
| PCI | 32.0% | 28.1% | <0.0001 |
Medications listed were prescribed on discharge.
Women were slightly younger (mean age 70 vs 73 years old). They were less likely to have significant comorbidities including atrial fibrillation, coronary artery disease history, congestive heart failure, diabetes mellitus, obstructive sleep apnea, peripheral artery disease, cirrhosis, chronic kidney disease, and chronic obstructive pulmonary disease. The peak troponin-I was significantly higher among men (16.0 vs 10.7 ng/mL). The rate of STEMI was comparable, but men were more likely to undergo CABG or PCI. Furthermore, men were prescribed more cardiac medications (Table 1).
Women had lower unadjusted mortality at 30 days, 60 days, and 1 year from their initial MI (Table 3). The mortality rate was also lower among women over the entire follow-up period (1.5% vs 2.3%). In the 10-year follow-up period, women had higher survival rates compared to men (Figure 2). The mean follow-up time was 1490.67 ± 8 days, and there was no significant difference in follow-up time between men and women.
Figure 2:
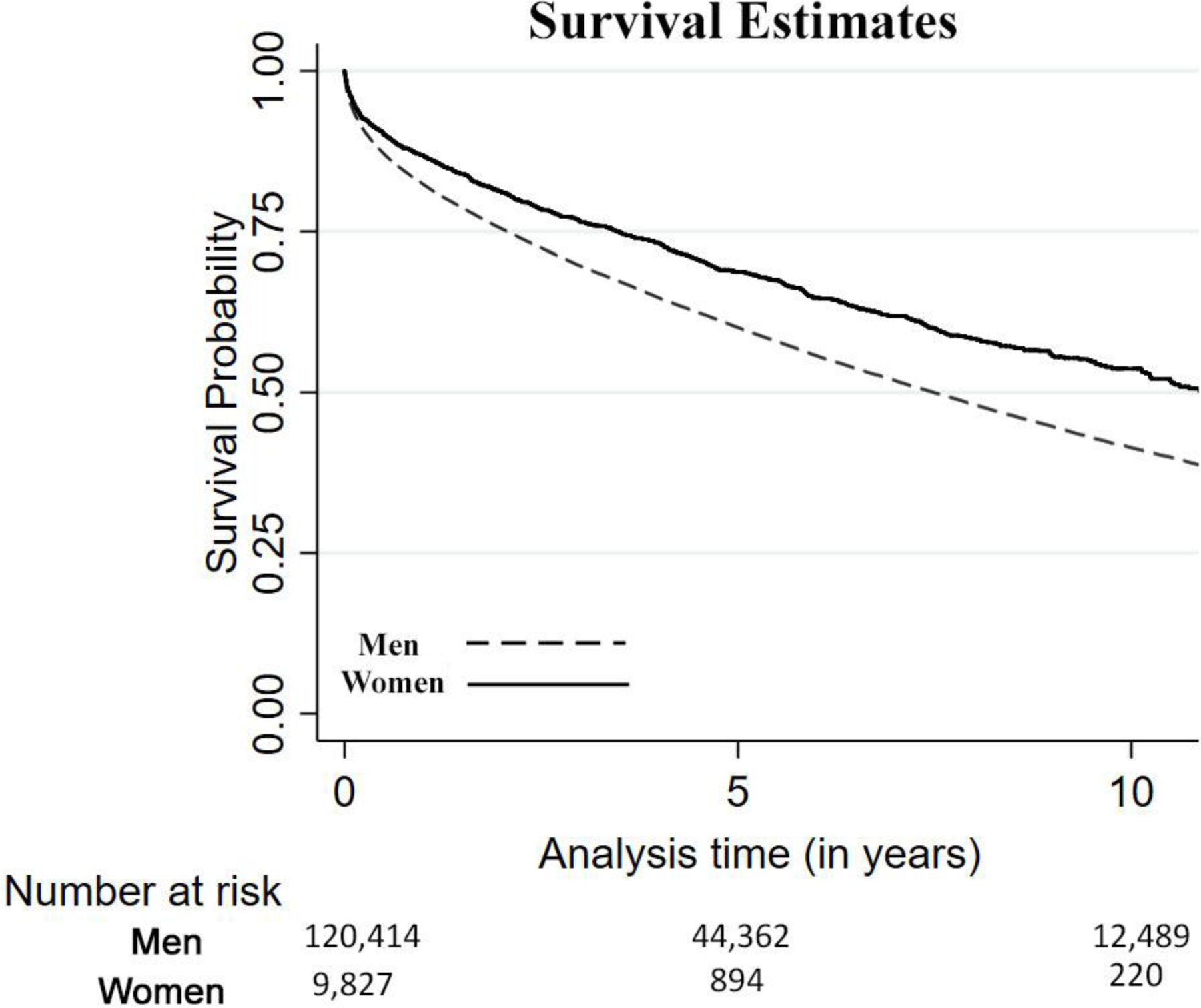
Kaplan-Meier survival estimates of men vs women.
Two models were included for a patient’s risk of mortality due to MI using Cox proportional hazard survival analysis (Table 2). Model 1 controls for patient characteristics, presentation, interventions, and comorbidities of patients. Even after adjusting for differences in demographics and comorbidities, women had a significantly lower risk of death (HR: 0.747, p value <0.0001). In addition, to account for the differences in mortality due to specific medications, in Model 2 we controlled for medications prescribed and found that the results of sex remain robust to their inclusion in the model.
Table 2.
Two models were included for a patient’s risk of mortality due to MI using Cox proportional hazard survival analysis.
| Parameter | Model 1: baseline model | Model 2: with medications | ||||
|---|---|---|---|---|---|---|
| Hazard ratio | P Value | CI | Hazard Ratio | P Value | CI | |
| Patient Characteristics | ||||||
| Women | 0.747 | <0.0001 | 0.696–0.801 | 0.682 | <0.0001 | 0.636–0.732 |
| Age | 1.011 | <0.0001 | 1.01–1.012 | 1.008 | <0.0001 | 1.007–1.009 |
| Presentation | ||||||
| Log-peak troponin | 1 | <0.0001 | 0.999–1 | 1 | <0.0001 | 0.999–1 |
| STE+MI | 0.693 | <0.0001 | 0.679–0.708 | 0.686 | <0.0001 | 0.672–0.7 |
| Interventions | ||||||
| CABG | 0.462 | <0.0001 | 0.432–0.494 | 0.481 | <0.0001 | 0.449–0.514 |
| PCI | 0.613 | <0.0001 | 0.601–0.626 | 0.712 | <0.0001 | 0.696–0.728 |
| Comorbidities | ||||||
| Smoking | 0.814 | <0.0001 | 0.797–0.831 | 0.826 | <0.0001 | 0.809–0.844 |
| Atrial fibrillation | 1.123 | <0.0001 | 1.103–1.144 | 1.074 | <0.0001 | 1.053–1.095 |
| Chronic artery disease | 0.466 | <0.0001 | 0.454–0.48 | 0.637 | <0.0001 | 0.618–0.657 |
| Congestive heart failure | 1.684 | <0.0001 | 1.65–1.718 | 1.643 | <0.0001 | 1.607–1.679 |
| Deep vein thrombosis | 1.103 | <0.0001 | 1.067–1.139 | 1.079 | <0.0001 | 1.045–1.115 |
| Diabetes mellitus | 1.021 | 0.025 | 1.003–1.039 | 1.075 | <0.0001 | 1.055–1.095 |
| Hypertension | 0.804 | <0.0001 | 0.785–0.823 | 0.892 | <0.0001 | 0.871–0.914 |
| Obstructive sleep apnea | 0.699 | <0.0001 | 0.679–0.72 | 0.722 | <0.0001 | 0.701–0.743 |
| Peripheral artery disease | 1.182 | <0.0001 | 1.16–1.205 | 1.234 | <0.0001 | 1.21–1.257 |
| Pulmonary emboli | 1.094 | <0.0001 | 1.047–1.143 | 1.059 | 0.01 | 1.014–1.107 |
| Cirrhosis | 1.316 | <0.0001 | 1.255–1.379 | 1.179 | <0.0001 | 1.124–1.236 |
| Chronic kidney disease | 1.134 | <0.0001 | 1.111–1.157 | 1.217 | <0.0001 | 1.192–1.242 |
| Chronic obstructive pulmonary disease | 1.274 | <0.0001 | 1.252–1.297 | 1.237 | <0.0001 | 1.215–1.2 |
| Cerebrovascular accident | 1.109 | <0.0001 | 1.087–1.131 | 1.166 | <0.0001 | 1.143–1.19 |
| Medications | ||||||
| Aspirin | – | – | 0.823 | <0.0001 | 0.799–0.848 | |
| Digoxin | – | – | 1.077 | <0.0001 | 1.053–1.101 | |
| Hydralazine | – | – | 0.99 | 0.771 | 0.922–1.062 | |
| Beta blocker | – | – | 0.677 | <0.0001 | 0.658–0.698 | |
| P2y12 inhibitor | – | – | 0.8 | <0.0001 | 0.784–0.817 | |
| Loop diuretics | 1.191 | <0.0001 | 1.164–1.218 | |||
| Non-dihydropyrdines Ca blockers | – | – | 1.023 | 0.031 | 1.002–1.045 | |
| Dihydropyridine Ca blockers | – | – | 0.738 | <0.0001 | 0.724–0.752 | |
| Statin | – | – | 0.609 | <0.0001 | 0.59–0.629 | |
| Nitrates | – | – | 0.961 | <0.0001 | 0.943–0.979 | |
| ACE inhibitor/ARB | – | – | 0.795 | <0.0001 | 0.772–0.819 | |
Further, robustness tests with propensity score matched sample also yielded consistent results using Cox proportional hazard survival analysis (HR: 0.782, p value <0.0001). Table 4 compares the hazard ratio of men and women across various parameters of the Cox proportional survival model. All variables were included as independent predictors of mortality for a time period of 10-year post-MI follow up.
DISCUSSION
This study analyzed data from a national database in the setting in which predominantly male patients are treated and found that women had a better survival compared to men after acute myocardial infarction despite a greater number of cardiac procedures performed among men. Our findings provide unexpected results in disagreement with prior studies,1–4 but are similar to our previous investigation and in a larger and more recent data set with more complete information including therapy and size of myocardial infarction.5
Better survival in women may be associated with a variety of reasons. First, women seem to have smaller sized infarcts as determined by peak troponins. This could translate to better survival. Second, men were more aggressively treated both pharmacologically and with PCI and CABG procedures. Although this may suggest better management for men, it may also represent more comorbidities not accounted for in our analysis that could result in a worse prognosis.
The literature on sex differences in post MI survival has conflicting information. A number of older studies have reported lower post MI survival in women.1–3, 7–11 Recently in comparable sized studies, Swedish and British reports also suggested this trend on long term follow up with another report from the United States showing that risk of readmission after MI is higher for women than men.12, 13,14 In contrast, Nauta and colleagues reported that adjusted mortality rates for men and women were similar in the intensive care unit in the Netherlands.15
In general, men have more risk factors for coronary disease making them higher risk for poor outcomes. The large US study by Dreyer and colleagues confirmed our findings that invasive procedures were higher among men.14 Many reasons have been put forward for worse survival among women post MI including differences in presentation with more atypical presentation in women, delay in diagnosis, and less use of invasive procedures among women.16 Irrespective of the cause of the MI, there is disparity in diagnostic evaluation between the sexes, and our study confirms fewer invasive procedures were performed on women. However, despite this, survival was better among women.
Men also have higher coronary atherosclerotic burden than women in the setting of acute coronary syndrome when referred for percutaneous coronary intervention.7, 17–19 It has been hypothesized that endothelial and microvascular dysfunction is one potential mechanism to explain why women frequently have non-obstructive coronary artery disease. Female cardiomyocytes has been demonstrated to be more protected against apoptosis and cell death as compared to male cardiomyocytes,20 even in mice models which demonstrated a delay in myocardial healing and higher infarct re-expansion and increased risk of cardiac rupture.20, 21,22 Decreased apoptosis were linked to 17-B-estradiol which is significantly higher in women. 23, 24
Better outcomes in women do not appear to be related as a result of varying access to care, given uniform access to the VA health system among veterans. Consistent with previous observations and reports,1–4, 25–28 women underwent fewer invasive cardiac procedures in our study without an effect on outcomes. The female population is significantly smaller compared to men that are treated in the veteran’s system, and it may translate to increase focus and improved overall quality of care.
The strengths of our study include having a nationwide analysis, large number of patients, uniform access of care among veterans, long follow up, and involvement of all patients who receive their care through the VA. This study has several limitations that affect its generalizability. Although we adjusted for baseline characteristics, not all confounders can be adjusted in a retrospective study and is therefore subject to residual confounding. In addition, patients were identified by ICD codes along with troponins, however electrocardiographic and cardiac catheterization information were not provided. We were unable to classify the cause of MI and to assess cardiovascular mortality. Being a VA study, women were underrepresented.
CONCLUSIONS
Our study showed that in the predominantly male veteran population, post-MI mortality is significantly lower in women even after adjusting for comorbidities. Further investigation is warranted to determine the reasons behind the improved survival in women post-MI in the veteran population.
Supplementary Material
Acknowledgments:
This material is the result of work supported with resources and the use of facilities at the Richard L. Roudebush VA Medical Center.
M.F. is supported by an American Heart Association Grant, 17MCPRP33460225 and NIH T32 grant 5T32HL007101. M.K. has received research support from Amgen.
Footnotes
The contents do not represent the views of the U.S. Department of Veterans Affairs or Unites States Government.
REFERENCES
- 1.Jousilahti P, Vartiainen E, Tuomilehto J, et al. Sex, age, cardiovascular risk factors, and coronary heart disease: a prospective follow-up study of 14 786 middle-aged men and women in Finland. Circulation. 1999;99:1165–72. [DOI] [PubMed] [Google Scholar]
- 2.Albert CM, McGovern BA, Newell JB, et al. Sex differences in cardiac arrest survivors. Circulation. 1996;93:1170–6. [DOI] [PubMed] [Google Scholar]
- 3.Wenger NK. You’ve come a long way, baby: cardiovascular health and disease in women: problems and prospects. Circulation. 2004;109:558–60. [DOI] [PubMed] [Google Scholar]
- 4.Goldberg RJ, Larson M, Levy D. Factors associated with survival to 75 years of age in middle-aged men and women. The Framingham Study. Arch Intern Med. 1996;156:505–9. [PubMed] [Google Scholar]
- 5.Kamalesh M, Subramanian U, Ariana A, et al. Paradoxical lower postmyocardial infarction mortality among veteran women--does a sex bias exist in the Veterans Affairs medical system? Can J Cardiol. 2008;24:691–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petersen LA, Wright S, Normand SL, et al. Positive predictive value of the diagnosis of acute myocardial infarction in an administrative database. J Gen Intern Med. 1999;14:555–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berger JS, Elliott L, Gallup D, et al. Sex differences in mortality following acute coronary syndromes. JAMA. 2009;302:874–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simon T, Mary-Krause M, Cambou JP, et al. Impact of age and gender on in-hospital and late mortality after acute myocardial infarction: increased early risk in younger women: results from the French nation-wide USIC registries. Eur Heart J. 2006;27:1282–8. [DOI] [PubMed] [Google Scholar]
- 9.Vaccarino V, Parsons L, Every NR, et al. Sex-based differences in early mortality after myocardial infarction. National Registry of Myocardial Infarction 2 Participants. N Engl J Med. 1999;341:217–25. [DOI] [PubMed] [Google Scholar]
- 10.Stock EO, Redberg R. Cardiovascular disease in women. Curr Probl Cardiol. 2012;37:450–526. [DOI] [PubMed] [Google Scholar]
- 11.Shaw LJ, Bugiardini R, Merz CN. Women and ischemic heart disease: evolving knowledge. J Am Coll Cardiol. 2009;54:1561–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nielsen S, Bjorck L, Berg J, et al. Sex-specific trends in 4-year survival in 37 276 men and women with acute myocardial infarction before the age of 55 years in Sweden, 1987–2006: a register-based cohort study. BMJ Open. 2014;4:e004598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smolina K, Wright FL, Rayner M, et al. Long-term survival and recurrence after acute myocardial infarction in England, 2004 to 2010. Circ Cardiovasc Qual Outcomes. 2012;5:532–40. [DOI] [PubMed] [Google Scholar]
- 14.Dreyer RP, Ranasinghe I, Wang Y, et al. Sex Differences in the Rate, Timing, and Principal Diagnoses of 30-Day Readmissions in Younger Patients with Acute Myocardial Infarction. Circulation. 2015;132:158–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nauta ST, Deckers JW, van Domburg RT, et al. Sex-related trends in mortality in hospitalized men and women after myocardial infarction between 1985 and 2008: equal benefit for women and men. Circulation. 2012;126:2184–9. [DOI] [PubMed] [Google Scholar]
- 16.Gabizon I, Lonn E. Young Women With Acute Myocardial Infarction and the Posthospital Syndrome. Circulation. 2015;132:149–51. [DOI] [PubMed] [Google Scholar]
- 17.Argulian E, Patel AD, Abramson JL, et al. Gender differences in short-term cardiovascular outcomes after percutaneous coronary interventions. Am J Cardiol. 2006;98:48–53. [DOI] [PubMed] [Google Scholar]
- 18.Rosengren A, Wallentin L, A KG, et al. Sex, age, and clinical presentation of acute coronary syndromes. Eur Heart J. 2004;25:663–70. [DOI] [PubMed] [Google Scholar]
- 19.Lansky AJ, Ng VG, Maehara A, et al. Gender and the extent of coronary atherosclerosis, plaque composition, and clinical outcomes in acute coronary syndromes. JACC Cardiovasc Imaging. 2012;5:S62–72. [DOI] [PubMed] [Google Scholar]
- 20.Guerra S, Leri A, Wang X, et al. Myocyte death in the failing human heart is gender dependent. Circ Res. 1999;85:856–66. [DOI] [PubMed] [Google Scholar]
- 21.Wang F, He Q, Sun Y, et al. Female adult mouse cardiomyocytes are protected against oxidative stress. Hypertension. 2010;55:1172–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cavasin MA, Tao Z, Menon S, et al. Gender differences in cardiac function during early remodeling after acute myocardial infarction in mice. Life Sci. 2004;75:2181–92. [DOI] [PubMed] [Google Scholar]
- 23.Patten RD, Pourati I, Aronovitz MJ, et al. 17beta-estradiol reduces cardiomyocyte apoptosis in vivo and in vitro via activation of phospho-inositide-3 kinase/Akt signaling. Circ Res. 2004;95:692–9. [DOI] [PubMed] [Google Scholar]
- 24.Bouma W, Noma M, Kanemoto S, et al. Sex-related resistance to myocardial ischemia-reperfusion injury is associated with high constitutive ARC expression. Am J Physiol Heart Circ Physiol. 2010;298:H1510–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gu K, Cowie CC, Harris MI. Diabetes and decline in heart disease mortality in US adults. JAMA. 1999;281:1291–7. [DOI] [PubMed] [Google Scholar]
- 26.Zuanetti G, Latini R, Maggioni AP, Set al. Influence of diabetes on mortality in acute myocardial infarction: data from the GISSI-2 study. J Am Coll Cardiol. 1993;22:1788–94. [DOI] [PubMed] [Google Scholar]
- 27.Hanes DS, Weir MR, Sowers JR. Gender considerations in hypertension pathophysiology and treatment. Am J Med. 1996;101:10S–21S. [DOI] [PubMed] [Google Scholar]
- 28.Barrett-Connor EL, Cohn BA, Wingard DL, et al. Why is diabetes mellitus a stronger risk factor for fatal ischemic heart disease in women than in men? The Rancho Bernardo Study. JAMA. 1991;265:627–31. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


