Abstract
RNAi is an effective tool for gene function analysis and a promising strategy to provide environmentally friendly control approaches for pathogens and pests. Recent studies support the utility of bacterium-mediated RNAi as a cost-effective method for gene function study and a suitable externally applied delivery mechanism for pest control. Here, we developed a bacterium-mediated RNAi system in Spodoptera frugiperda based on four target genes, specifically, Chitinase (Sf-CHI), Chitin synthase B (Sf-CHSB), Sugar transporter SWEET1 (Sf-ST), and Hemolin (Sf-HEM). RNAi conducted by feeding larvae with bacteria expressing dsRNAs of target genes or injecting pupae and adults with bacterially synthesized dsRNA induced silencing of target genes and resulted in significant negative effects on growth and survival of S. frugiperda. However, RNAi efficiency and effects were variable among different target genes and dsRNA delivery methods. Injection of pupae with dsCHI and dsCHSB induced a significant increase in wing malformation in adults, suggesting that precise regulation of chitin digestion and synthesis is crucial during wing formation. Injection of female moths with dsHEM resulted in lower mating, fecundity, and egg hatching, signifying a critical role of Sf-HEM in the process of egg production and/or embryo development. Our collective results demonstrate that bacterium-mediated RNAi presents an alternative technique for gene function study in S. frugiperda and a potentially effective strategy for control of this pest, and that Sf-CHI, Sf-CHSB, Sf-ST, and Sf-HEM encoding genes can be potent targets.
Keywords: Spodoptera frugiperda, bacterium-mediated RNAi, development, survival, reproductive success, egg hatching
The fall armyworm, Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae), is native to the tropical and subtropical regions of the western hemisphere from Argentina to USA (Johnson 1987, Kumar et al. 2009). This moth has been identified as a notorious agricultural pest due to its robust migration ability (Johnson 1987; Westbrook et al. 2016), strong pesticide resistance (Li et al. 2019, APRD 2021), polyphagous larval feeding habits (Capinera 2017, Montezano et al. 2018), high fecundity, and alternating generations (Montezano et al. 2018).
While S. frugiperda is a long-distance migratory pest, there have been no reports on its distribution outside the Americas before 2015. S. frugiperda was initially detected in Africa in 2016, with subsequent rapid expansion to almost the entire African continent (Abrahams et al. 2017, Feldmann et al. 2019). In Asia, S. frugiperda was first reported in India in May 2018 and identified in other countries shortly afterwards, including Myanmar, Thailand, Yemen and Sri Lanka (Kalleshwaraswamy et al. 2018, FAO 2019, CABI 2020a). S. frugiperda was subsequently detected in Yunnan Province in China at the end of 2018 (Guo et al. 2019a), which quickly spread to the north throughout the vast areas of China (Guo et al. 2019b, Xu et al. 2019, Zhang et al. 2019a). By the end of 2019, S. frugiperda had spread to 26 provinces, including major crop production areas along the Yangtze River Basin, Yellow River Basin, and Northeast China (Jiang et al. 2019, Yang et al. 2019b).
S. frugiperda has a wide range of host plants, involving 353 plant species from 76 families, primarily Poaceae (106) (Montezano et al. 2018). The most commonly damaged crops are corn, rice, sorghum and cotton (Capinera 2017, CABI 2020b). Other occasionally affected crops and plants are apple, grape, orange, papaya, peach, strawberry, hardy pecan, tea, eucalyptus, rubber, and pine (Casmuz et al. 2010, Capinera 2017, Montezano et al. 2018, CABI 2020b, Sun et al. 2020a). Two haplotypes of S. frugiperda have been identified to date: (1) the corn strain that feeds mainly on corn, cotton and sorghum and (2) the rice strain that principally consumes rice and grass weeds (Dumas et al. 2015). Substantial economic losses to corn production worldwide by S. frugiperda have been reported. In Brazil, the third largest global corn producer after United States and China, the cost of controlling S. frugiperda damage exceeds $600m annually (Shylesha et al. 2018). A report from the Department for International Development (Abrahams et al. 2017) showed that S. frugiperda from 12 countries in Africa has the potential to cause annual corn yield losses in the range of 21–53%, estimated between $2,481m and $6,187m. In China, evaluation of the potential economic effects of S. frugiperda suggests losses to the corn and wheat industries of $17,286m–$52,143m (Qin et al. 2020) and $15,571m–$90,143m (Xu et al. 2020), respectively.
At present, management of S. frugiperda primarily depends on broad-spectrum chemical insecticides, which are noxious to beneficial arthropods (Burtet et al. 2017, Li et al. 2019) and has led to the development of resistance of this insect to conventional insecticides and even Bacillus thuringiensis toxins (Burtet et al. 2017, Li et al. 2019, APRD 2021). Therefore, sustainable methods are urgently required for control of this highly invasive insect pest.
RNA interference (RNAi) is an effective tool for gene function determination, and also is an emerging technology to provide approaches for pest and disease control in agriculture and forestry (Xu et al. 2016, Goodfellow et al. 2019, Vogel et al. 2019). However, applications of RNAi in pest control are limited due to lack of stable delivery strategies (Goodfellow et al. 2019). Achievable systems for cost-effective production and external delivery of double-stranded RNA (dsRNA) to target invasive pests are crucial for effective deployment of RNAi in pest control (Zhang et al. 2019b). Generally, insect RNAi can be induced by injection or feeding of dsRNA (synthesized in vitro), feeding with bacteria expressing dsRNA in vivo (bacterium-mediated RNAi) or injection of extracted dsRNA produced by bacteria (Timmons and Fire 1998, Timmons et al. 2001). The use of bacterially expressed dsRNA is significantly more cost-effective than producing dsRNA in vitro with a kit, particularly for large-scale gene function analysis and pest control applications (Fraser et al. 2000, Solis et al. 2009, Xu et al. 2016, Zhang et al. 2019b). Previous studies on bacterium-mediated RNAi have validated its cost-effectiveness in gene function exploration and significant potential for agricultural applications (Solis et al. 2009, Xu et al. 2016, Goodfellow et al. 2019, Zhang et al. 2019b, Wang et al. 2021). To our knowledge, however, there is no report on the use of this technique in S. frugiperda so far.
In this study, we developed a bacterium-mediated RNAi system for S. frugiperda based on four target genes: Chitinase (Sf-CHI), Chitin synthase B (Sf-CHSB), Sugar transporter SWEET1 (Sf-ST) and Hemolin (Sf-HEM). Previous studies have shown that these genes play crucial roles in the growth, survival and reproduction of insects, representing as attractive targets for insecticides. Chitin degradation by chitinase is a key step in the insect life cycle that ensures periodic molting and plays essential roles in insect development (Pesch et al. 2016). Chitinase also function in digestion, defense, and immunity response (Zhao et al. 2018). Chitin synthase is an enzyme that transfers NDP-acetylglucosamine to polymerise chitin, catalyses the last step of chitin synthesis (Yang et al. 2019a). Insect Chitin synthases are divided into two different categories, CHSA and CHSB. CHSA is mainly located in the cuticle and trachea, while CHSB is mainly responsible for chitin synthesis in midgut and peritrophic membrane during the feeding stage (Zhang et al. 2012, Yang et al. 2019a). The sugar transporter SWEET1 (ST) functions in carbohydrate transport in a range of organisms from bacteria to mammals, and is responsible for movement of sugar into cells (Baldwin and Henderson 1989). Hemolin (HEM), an immunoglobulin-like peptide that plays a crucial role in surveillance for microbial pathogens and embryonic development, which has been identified only in lepidopteran insects (Su et al. 1998, Bettencourt et al. 2002, Jung et al. 2019). Owing to their key roles in insect development and reproduction, in the present study, these four genes were selected as targets for bacterium-mediated RNAi in S. frugiperda.
Here, RNAi was induced by feeding larvae with bacteria expressing dsRNA of target genes or injecting pupae and adults with bacterially produced dsRNA of target genes. RNAi efficiency and effects were examined via gene expression measurement and biological assays on development, survival, morphology and reproductive fitness of S. frugiperda. The functions of target genes and potential utility of bacterium-mediated RNAi in gene function testing and pest control were additionally explored.
Materials and Methods
Insects
Larvae of S. frugiperda were originally collected on corn from a field near Zhanyi town in Yunnan Province, China. The larvae were reared on an artificial diet (Li et al. 2006) under conditions of 28 ± 1°C, 60–80% relative humidity and a 14:10 h light:dark photoperiod. Insects were collected in July 2019 and six generations reared in the laboratory before this study, which was conducted in May 2020.
To ensure virginity and age, male and female pupae were sexed according to morphological characteristics (Dong et al. 2019) and caged separately until adult emergence. Adults were fed with a 10% honey solution.
Molecular Cloning and Characterization
Based on the previous full-length mRNA sequencing of S. frugiperda (Majorbio Biotechnology, Shanghai), we obtained mRNA (with complete cds) of the four target genes (Sf-CHI, Sf-CHSB, Sf-ST, and Sf-HEM). These mRNA sequences were further validated via PCR and sequencing. Sequence alignment and open reading frame (ORF) detection were performed with the aid of ClustalX 2.1, MEGA 4.0, and DNAMAN 7.0. Signal peptides were predicted using SignalP 5.0 (Petersen et al. 2011). The putative transmembrane (TM) domains (TM1–TM7) were predicted using TMHMM Server v.2.0. Conserved protein domains were predicted using Pfam (http://pfam.xfam.org) and the conserved domain database of NCBI (https://www.ncbi.nlm.nih.gov/cdd). Swiss model (https://swissmodel.expasy.org/interactive) and UCSF ChimeraX software version 1.13 (https://www.cgl.ucsf.edu/chimera/) were used for three-dimensional protein structure and superimposition analysis. Phylogenetic trees based on protein sequences were constructed using the neighbor-joining method with 1,000 bootstrap replicates using the MEGA 7.0 software program.
Vector Construction and dsRNA Preparation
RNAi target fragments were designed using siDirect version 2.0. A fragment of enhanced green fluorescence protein (EGFP) gene was used as the control. Gene fragments were amplified via PCR using gene-specific primers containing NotI and XhoI restriction sites (Table 1) under the following conditions: 30 cycles of 94°C for 30 s, 58°C for 30 s, and 72°C for 60 s, and a final extension step of 72°C for 10 min. Purified products were cloned into plasmid L4440 (Timmons and Fire 1998, Timmons et al. 2001) and the resulting recombinant vectors (L4440-Sf-CHI, L4440-Sf-CHSB, L4440-Sf-ST, and L4440-Sf-HEM) were introduced into competent HT115 (DE3) cells. Positive clones were selected for sequence verification and sequenced in both directions (Sangon Biotech, China).
Table 1.
Primers for RNAi and qPCR
| Primer names | Primer sequences (5′ to 3′)* | Usages |
|---|---|---|
| Sf-CHI-Qf | GCCGTTCGTTCACTCTGACA | qPCR |
| Sf-CHI-Qr | GTCCCACTTCTTAGTCCATCCT | qPCR |
| Sf-CHSB-Qf | GAATTTAGGAGCAGCGTGCG | qPCR |
| Sf-CHSB-Qr | GCAGCCAATGACCAATAGCG | qPCR |
| Sf-ST-Qf | ACTTGTTCGGTTTGGCCTTG | qPCR |
| Sf-ST-Qr | ATCTTCCATTTCGGCGTACG | qPCR |
| Sf-HEM-Qf | AATTTTGGCCGCGTGCATAG | qPCR |
| Sf-HEM-Qr | TTGTCGGCTTTGAACAGCAC | qPCR |
| Sf-Actin-Qf | AGCTTGTGTCATCGATGTCG | qPCR |
| Sf-Actin-Qr | AAACTGCGGTTCCTTGTTGC | qPCR |
| dsCHI-F | AAGGAAAAAAgcggccgcTCGCACAGAAACAAACGC | RNAi |
| dsCHI-R | CCGctcgagAAGGAACCACCACGGTCAGC | RNAi |
| dsCHSB-F | AAGGAAAAAAgcggccgcAATACGATCAAGGCGAGGAC | RNAi |
| dsCHSB-R | CCGctcgagTGAGGTTAGCGAGGAAGAG | RNAi |
| dsST-F | AAGGAAAAAAgcggccgcTTCCTTGGAGGTGTCGTTATG | RNAi |
| dsST-R | CCGctcgagCTCCGTGCTCTTGTTCTTGAT | RNAi |
| dsHEM-F | AAGGAAAAAAgcggccgcCCTGATGGCACGCTTTGGTT | RNAi |
| dsHEM-R | CCGctcgagTGCGGTTGTGGTCTGTTACTCTGT | RNAi |
| dsEGFP-F | AAGGAAAAAAgcggccgcCACCCTCGTGACCACCCTGAC | RNAi |
| dsEGFP-R | CCGctcgagACCTTGATGCCGTTCTTCTGC | RNAi |
*Underlined were protecting nucleotides and lower case letters were NotI or XhoI restriction sites.
To prepare dsRNA-expressing bacteria, single colonies of HT115 (DE3) containing recombinant vectors were grown for 14 h in liquid LB (containing 100 μg/mL ampicillin and 12.5 μg/mL tetracycline) at 37°C with shaking at 220 rpm. Synthesis of dsRNAs was induced by adding IPTG to a final concentration of 0.4 mM and bacteria were incubated for an additional 4 h under similar conditions. Expressed dsRNAs were extracted from bacteria using RNAiso Plus (TaKaRa, China) and the lengths of dsRNA confirmed via 1.5% agarose gel electrophoresis. The production level achieved using this bacterial incubation system was ~7–9 μg dsRNA/mL culture (Wang et al. 2021), as estimated by comparing the brightness of the dsRNA band and quantified RNA marker.
RNAi by Feeding Larvae With Bacteria Expressing the dsRNAs of Target Genes
Bacterial cultures of the four target genes and control EGFP were used for preparation of cells for feeding. In brief, 30 ml IPTG-induced bacterial culture was centrifuged for 3 min at 10,000 g and resuspended in 1 ml DEPC-water (Wang et al. 2021). Second-instar larvae (3-d-old) individually caged in plastic cells (4 × 4 × 4 cm) were fed with 20 µl of the prepared bacterial cell solution (dropped on food) once a day and kept for 8 d (Wang et al. 2021). After 8 d of feeding, the larvae are going to pupate soon. Larvae provided with 20 µl DEPC-water (dropped on food) once a day and kept for 8 d were used as the blank control. All larvae were collected for the RNAi efficiency assay. Three biological replicates were used for each treatment, with six larvae per replicate. Total RNA was extracted from larvae with TRIzol (Takara, China) according to the manufacturer’s protocol. The purity and concentration of RNA were determined using a spectrophotometer (NanoDrop 2000, USA). First-strand cDNA synthesis was performed using a PrimeScript RT reagent kit (Perfect Real Time; TaKaRa). Real-Time quantitative PCR (qPCR) was performed with gene-specific primers (Table 1) using SYBR Premix Ex Taq II (TaKaRa) in a total volume of 25 µl. Actin (NCBI ID: KT218672.1) was used as a reference gene. Reactions were run in triplicate on the QuantStudio 7 Flex system (Thermo Fisher Scientific, USA) using the following program: 95°C for 5 min, followed by 40 cycles of 95°C for 30 s, and 60°C for 34 s. Analysis of the dissociation curves for target and reference genes showed a single melt peak. The efficiencies of the target and reference genes were similar. The 2-ΔΔCT method (Livak and Schmittgen 2001) was applied to calculate the relative quantities of target genes.
To explore the effects of bacterial feeding-based RNAi targeting the four genes on the development and survival of S. frugiperda, the duration of larval and pupal stages, lifespan of adults, pupation rate (number of pupae/number of larvae%), eclosion rate (number of adults/number of pupae%), and survival rate from larvae to adults (number of adults/number of larvae%) were evaluated. Three replicates were used for each treatment, with 20 larvae per replicate.
RNAi by Injecting Pupae With Extracted dsRNAs of Target Genes
Expressed dsRNAs were extracted from bacteria as above, dissolved in DEPC-water and the concentration adjusted to 1 μg dsRNA/µl solution. One-day-old pupae were collected and injected with 5 µl dsRNA (1 μg/µl) of Sf-CHI or Sf-CHSB target genes. Pupae injected with 5 µl DEPC-water and 5 µl dsEGFP (1 μg/µl) were used as controls. The solution was injected into the abdominal cavity of pupae through the junction of the segment 5 and segment 6. Injected pupae were collected after 24 h for the RNAi efficiency test as described above. Three biological replicates were used for each treatment, with six pupae per replicate.
The effects of RNAi-based injection on eclosion rate (number of adults/ number of pupae%) and adult morphology were further examined. Three replicates were used for each treatment, with 20 pupae per replicate.
RNAi by Injecting Adults With extracted dsRNAs of target genes
In this experiment, 1-d-old male or female adults were injected with 5 µl dsRNA (1 μg/µl) of the four target genes. Adults injected with 5 µl water and 5 µl dsEGFP (1 μg/µl) were used as controls. The solution was injected into adults through the junction of the segment 5 and segment 6 as above. To confirm silencing efficiency, injected adults were collected after 24 h and the transcription levels of target genes quantified via qPCR as described above. Three biological replicates were used for each treatment, with six adults per replicate.
Following the above injection treatments, the survival rates of adults within 3 d of injection were calculated. Three biological replicates were used for each treatment, with 20 adults per replicate.
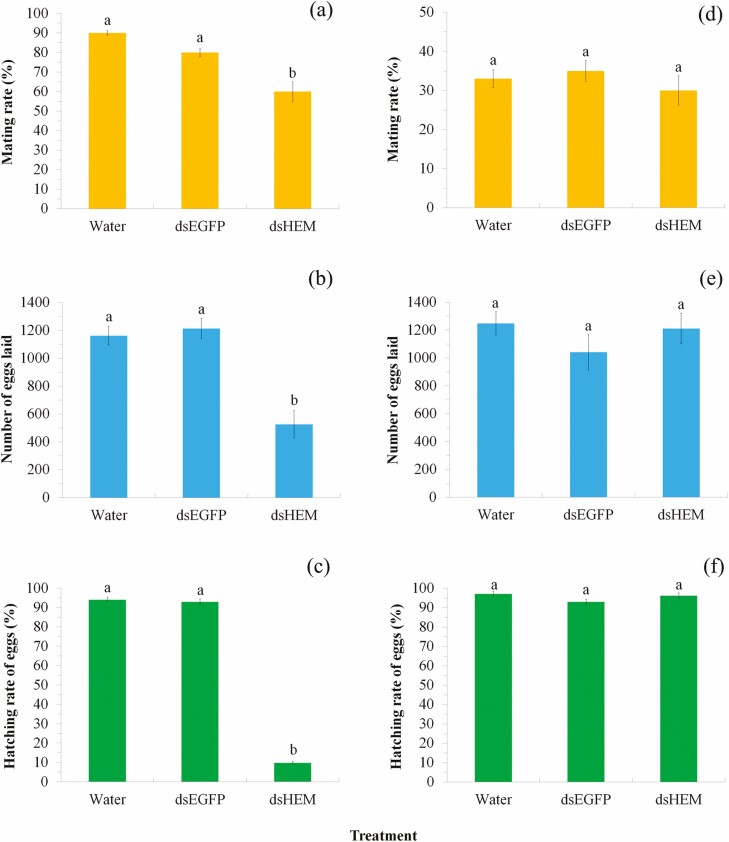
HEM plays an important role in the reproductive process in insects. Accordingly, we further examined whether RNAi of Sf-HEM affected male and female reproductive fitness and offspring viability and the underlying mechanisms. In the female RNAi trial, Sf-HEM dsRNA-injected females were paired with wild-type males the second night after injection in plastic boxes (25 cm long, 15 cm wide, and 8 cm high; one pair per box). Mating events within this night (10 h) of all females were recorded by rapid observation of treated insects every 30 min (mating duration was approximately 1 h) under 15 W red light illumination. Males were removed after mating. Each box was provided a paper strip (15 × 20 cm) folded in zigzag fashion as an oviposition substratum and 10% honey solution as food. Eggs were collected and incubated in petri dishes (8.5 × 1.5 cm) under the above conditions. The number of hatched eggs (larvae) was recorded 4 d after incubation. Mating was verified by counting the number of spermatophores in female bursa copulatrix after death under a dissecting microscope. Thirty females were used for the mating test. Up to 55% females did not mate during the test night (Fig. 6), and thus we only used 14 mated females from each treatment for reproductive output tests.
Fig. 6.
Effect of RNAi on reproductive success by injecting female adults (a–c) or male adults (d–f) with extracted dsRNAs targeting Sf-HEM. Mating rate (a), fecundity (b) and egg hatching rate (c) of dsHEM females mating with wild males; and mating rate (d), fecundity (e), and egg hatching rate (f) of wild females mating with dsHEM males. In each of the subgraphs, bars with different letters are significantly different (P < 0.05). Values were reported as mean ± SE.
In the male RNAi trial, Sf-HEM dsRNA-injected males were paired with wild-type females the second night after injection and their mating events and reproductive fitness were tested as above. Thirty injected males were used for the mating rate test. Only a few females mated with injected males during the test night (Fig. 6), which may be due to the side effects of male injection. We thus only used five mated females from each treatment for reproductive output tests.
Statistics
Data on the developmental duration of larvae and pupae and adult lifespans were not normally distributed even after transformation and thus were analyzed using the nonparametric Kruskal–Wallis test followed by Dunn’s procedure with Bonferroni correction for multiple comparisons (Zar 1999). Other data were analyzed using an ANOVA followed by Tukey’s studentized range (HSD) test for multiple comparisons. Percentage data were arcsin square root-transformed before ANOVA. All analyses were conducted using SPSS 25.0 software. The rejection level was set at α < 0.05. All values are expressed as mean ± SE.
Results
Molecular Cloning and Phylogenetic Analysis
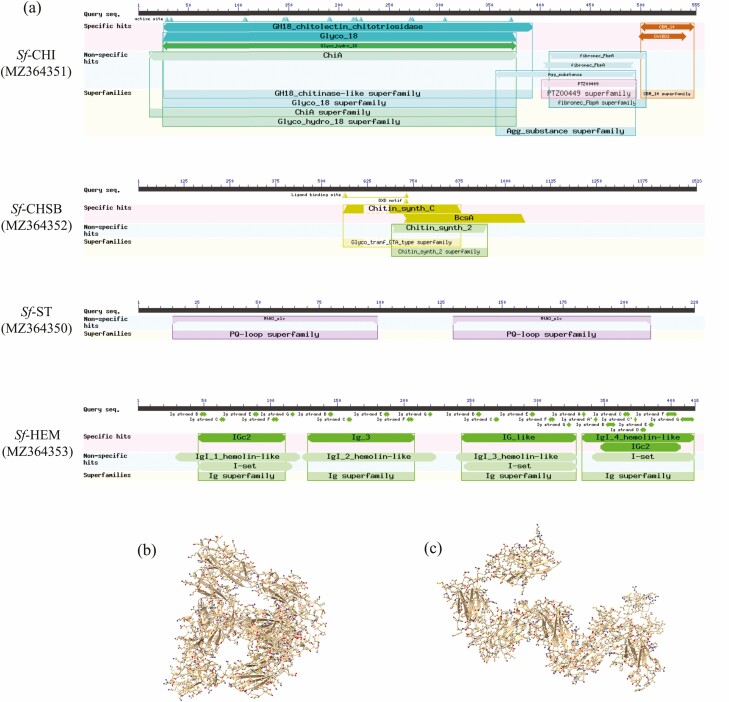
S. frugiperda CHI (Sf-CHI; GeneBank ID: MZ364351) contains an ORF of 1668 bp encoding a 555 residue protein, which has a glycosyl hydrolase family 18 domain (GH 18 domain: pfam00704) and a chitin-binding domain (CBD) (CBM-14: pfam01607) with an N-terminal signal peptide (Fig. 1a; Supp Fig. S1 [online only]). Sf-CHSB (MZ364352) contains an ORF of 4572 bp encoding a 1523 amino acid protein incorporating a central chitin synthase domain (pfam03142), N-terminal domain with seven TM helices and C-terminal domain with an additional seven TM helices (Fig. 1a; Supp Fig. S3 [online only]). Sf-ST (MZ364350) consists of a putative 690 bp ORF encoding a 229 residue protein with two copies of the sugar efflux transporter for intercellular exchange domain (pfam03083) and seven TM domains (Fig. 1a; Supp Fig. S5 [online only]). Sf-HEM (MZ364353) has a putative ORF of 1257 bp encoding a 418 amino acid protein with four immunoglobulin (Ig) domains and an 18 amino acid N-terminal signal peptide (Fig. 1a; Supp Fig. S7 [online only]). No TM domains were found in Sf-CHI and Sf-HEM and no signal peptides were found in Sf-ST and Sf-CHSB. Three-dimensional prediction of Sf-HEM protein showed that its four Ig domains form a characteristic horseshoe shape (Fig. 1b), distinct from the extended chain form of human Ig protein (Fig. 1c).
Fig. 1.
Domains of Sf-CHI, Sf-CHSB, Sf-ST, and Sf-HEM (a), and predicted three-dimensional structures of Sf-HEM (b) and immunoglobulin 1 (HsIg1) of Homo sapiens (GeneBank ID: CAA71535.1) (c).
Phylogenetic analysis revealed that Sf-CHI, Sf-CHSB, Sf-ST, and Sf-HEM cluster together with CHI, CHSB, ST, and HEM from other insect species, respectively (Supp Figs S2, S4, S6 and S8 [online only]), in which sequences from S. frugiperda show a shorter genetic distance to lepidopterans than insects from other orders, consistent with traditional taxonomy.
RNAi by Feeding Larvae With Bacteria Expressing the dsRNAs of Target Genes
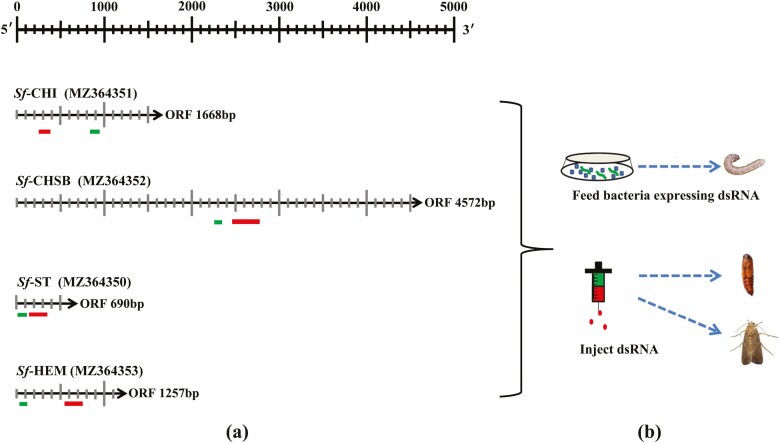
RNAi target fragments were designed using siDirect version 2.0 (Fig. 2a) and RNAi trials (Fig. 2b) conducted by feeding larvae with bacteria expressing the dsRNA of target genes or injecting pupae or adults with bacterially produced dsRNAs.
Fig. 2.
RNAi targets of Sf-CHI, Sf-CHSB, Sf-ST, and Sf-HEM (a) and dsRNA delivery methods (b). Red bands refer to RNAi targets and green bands refer to qPCR fragments.
In feeding trials, second-instar larvae were individually caged in plastic cells and fed with 20 µl prepared bacterial solution expressing dsRNA of Sf-CHI (dsCHI), Sf-CHSB (dsCHSB), Sf-ST (dsST), or Sf-HEM (dsHEM) each day for 8 d. Larvae administered water or bacteria expressing dsEGFP were used as controls. Notably, expression of target genes was significantly reduced in the treatment groups compared with controls (P < 0.05; Table 2). Sf-CHI showed the greatest reduction in expression (66.0–67.99%) followed by Sf-CHSB (35.00–36.89%), while Sf-HEM (14.00–16.50%) and Sf-ST (15.00–17.48) were only slightly reduced.
Table 2.
Relative expression levels of the target genes in RNAi treated larvae (Feeding), pupae (Injection), and adults (Injection)
| Treatments | Gene | Relative expression levels | |||||
|---|---|---|---|---|---|---|---|
| Water (Control)* | EGFP (Control) | Target gene | Reduction Percentages† | F-value (ANOVA) | P-value | ||
| Feed larvae | Sf-CHI | 1.00 ± 0.04a | 1.03 ± 0.04a | 0.34 ± 0.01b | 66.00; 66.99 | F2,6 = 94.30 | <0.001 |
| Feed larvae | Sf-CHSB | 1.00 ± 0.04a | 1.03 ± 0.04a | 0.65 ± 0.01b | 35.00; 36.89 | F2,6 = 318.30 | <0.001 |
| Feed larvae | Sf-ST | 1.00 ± 0.04a | 1.03 ± 0.04a | 0.85 ± 0.02b | 15.00; 17.48 | F2,6 = 15.08 | <0.01 |
| Feed larvae | Sf-HEM | 1.00 ± 0.04a | 1.03 ± 0.04a | 0.86 ± 0.01b | 14.00; 16.50 | F2,6 = 18.69 | <0.01 |
| Inject pupae | Sf-CHI | 1.00 ± 0.04a | 1.09 ± 0.05a | 0.29 ± 0.02b | 71.00; 73.39 | F2,6 = 208.09 | <0.001 |
| Inject pupae | Sf-CHSB | 1.00 ± 0.04a | 1.09 ± 0.05a | 0.45 ± 0.04b | 55.00; 58.72 | F2,6 = 81.98 | <0.001 |
| Inject adults | Sf-CHI | 1.00 ± 0.04a | 1.10 ± 0.07a | 0.60 ± 0.02b | 40.00; 45.45 | F2,6 = 37.95 | <0.001 |
| Inject adults | Sf-CHSB | 1.00 ± 0.04a | 1.10 ± 0.07a | 0.78 ± 0.01b | 22.00; 29.09 | F2,6 = 15.10 | <0.01 |
| Inject adults | Sf-ST | 1.00 ± 0.04a | 1.10 ± 0.07a | 0.64 ± 0.02b | 36.00; 41.81 | F2,6 = 32.12 | <0.01 |
| Inject adults | Sf-HEM | 1.00 ± 0.04a | 1.10 ± 0.07a | 0.26 ± 0.02b | 74.00; 76.36 | F2,6 = 111.46 | <0.0001 |
*Water-treated insects were used as calibrators. For each parameter (in each line), values with different letters are significantly different (P < 0.05).
†The first value is the Reduction Percentage relative to Water, and the second value is the Reduction Percentage relative to EGFP.
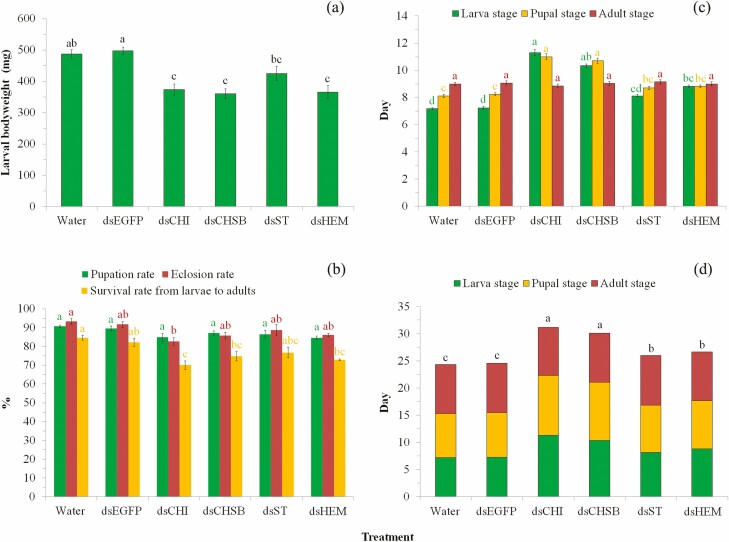
Knockdown of the four target genes by feeding larvae with bacteria expressing the respective dsRNAs led to a significant decrease in larval body weight (F5,85 = 13.05, P < 0.0001; Fig. 3a), with reduction rates of 12.96–28.10%. Post hoc multiple comparisons indicated that knockdown of Sf-CHI, Sf-CHSB, and Sf-HEM caused significantly lower larval body weight in comparison with controls (Water and dsEGFP) (P < 0.05), and knockdown of Sf-ST showed significant effects on larval body weight in comparison with the control of dsEGFP (P < 0.05) while did not show significant effects when compared with the control of Water (P > 0.05; Fig. 3a). Knockdown of the four target genes negatively affected the pupation rate of larvae, but not to a significant extent (F5,12 = 3.06, P = 0.052), while significantly reduced the eclosion rate of pupae (F5,12 = 4.60, P = 0.014) and survival rate from larvae to adults (F5,12 = 7.67, P = 0.002; Fig. 3b). Multiple comparisons indicated that knockdown of Sf-CHI resulted significantly negative effects on eclosion rate of pupae in comparison with the control of Water (P < 0.05) while did not show significant effects when compared with the control of dsEGFP (P > 0.05); knockdown of the other three genes did not show significant effects on eclosion rate of pupae in comparison with controls (P > 0.05; Fig. 3b). Knockdown of Sf-CHI showed significantly negative effects on survival rate from larvae to adults in comparison with controls (P < 0.05), while knockdown of the other three genes did not show significant effects when compared with controls (P > 0.05; Fig. 3b). RNAi additionally led to significant prolongation of the duration of larval (χ2 = 91.946, P < 0.0001; Fig. 3c) and pupal (χ2 = 73.194, P < 0.0001; Fig. 3c) stages and larva to adult (χ2 = 85.865, P < 0.0001; Fig. 3d) stages, but exerted no significant effect on longevity of adults (χ2 = 2.773, P = 0.735; Fig. 3c).
Fig. 3.
Bioassay after RNAi by feeding larvae with bacteria expressing the dsRNAs of target genes. (a) effect of RNAi on larval bodyweight; (b) effect of RNAi on pupation rate, eclosion rate, and survival rate from larvae to adults; (c) effect of RNAi on larval and pupal development durations, and adults longevity; and (d) effect of RNAi on percentile proportions of development stages. For each parameter in each of the subgraphs, bars with different letters are significantly different (P < 0.05); i.e., letters with the same color can be compared to derive the significance of difference between treatments in the same subgraph. Values were reported as mean ± SE.
RNAi by Injecting Pupae With Extracted dsRNAs of Target Genes
In this trial, 1-d-old pupae were injected with 5 μg of dsCHI or dsCHSB per pupa. Pupae injected with water or dsEGFP were used as the control. qPCR data disclosed that dsRNA injection led to significant silencing of the two target genes (P < 0.05; Table 2), with reduction rates of 71.00–73.39% for Sf-CHI and 55.00–58.72% for Sf-CHSB.
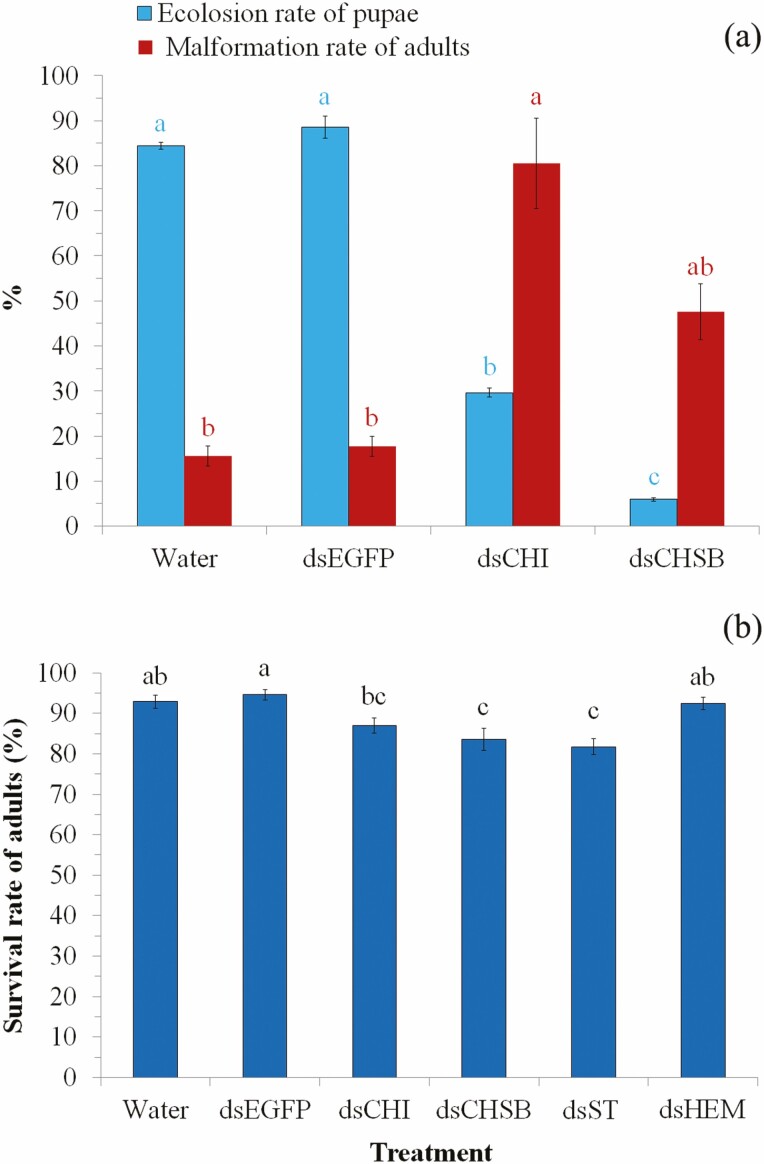
Data from the biological assays (Fig. 4a) showed that injection of both dsCHI and dsCHSB significantly reduced the eclosion rate of pupae (54.80–78.47%; F3,8 = 478.11, P < 0.0001) but increased the malformation rate of adults (32.07–65.03%; F3,8 = 12.68, P = 0.002). Adult malformation was mainly manifested in wing morphology (Fig. 5a–c), whereby wings of RNAi adults were bent, distorted and/or incomplete, either on one (Fig. 5b) or both sides (Fig. 5c).
Fig. 4.
Effect of RNAi on eclosion rate of pupae and malformation of adults by injecting pupae with extracted dsRNAs of target genes (a), and effect of RNAi on adult survival rate by injecting adults with extracted dsRNAs of target genes (b). For each parameter in each of the subgraphs, bars with different letters are significantly different (P < 0.05); i.e., letters with the same color can be compared to derive the significance of difference between treatments in the same subgraph. Values were reported as mean ± SE.
Fig. 5.
Effect of RNAi on adults morphology and embryo development. (a) normal adults from control; (b) and (c), adults with wing malformation from pupae injected with either dsCHI or dsCHSB; (d) newly laid eggs by control females; (e) 2-d incubated eggs of control females; (f) newly hatched larvae from 4 d incubated eggs of control females; (g) newly laid eggs by dsHEM females; (h) 2-d incubated eggs by dsHEM females; and (i) 4 d incubated eggs of dsHEM females. Bars = 1 mm.
RNAi by Injecting Adults With Extracted dsRNAs of Target Genes
In this trial, 1-d-old male or female adults were injected with 5 μg dsCHI, dsCHSB, dsHEM or dsST per adult. Adults injected with water or dsEGFP were used as control groups. Gene expression tests showed that dsRNA injection led to a significant reduction in target gene levels compared with controls (P < 0.05; Table 2). Sf-HEM was reduced by 74.00–76.36%, followed by Sf-CHI (40.00–45.45%), Sf-ST (36.00–41.81%), and Sf-CHSB (22.00–29.09%).
Examination of survival rates within 3 d after injection revealed that injection of dsRNAs significantly affected adult survival (F5,12 = 14.03, P < 0.0001; Fig. 4b). Post hoc tests showed that injection of dsCHSB and dsST led to a significant reduction (P < 0.05) in adult survival rate, but not dsCHI and dsHEM (P > 0.05).
In the reproductive fitness test, females injected with dsHEM had a significantly lower mating rate with wild-type males (F2,6 = 27.99, P = 0.001) and mated females laid significantly fewer eggs (F2,39 = 22.87, P < 0.0001) with a lower hatching rate (F2,39 = 624.84, P < 0.0001; Fig. 6a–c). However, males injected with dsHEM and paired with wild-type females displayed no significant differences in terms of mating rate (F2,6 = 0.73, P = 0.522), eggs laid by mates (F2,12 = 1.02, P = 0.391) and egg hatching rate (F2,12 = 2.52, P = 0.122; Fig. 6d–f).
Newly laid eggs by dsHEM females (Fig. 5g) were similar to those of controls (Fig. 5d), which were round and full, grey or light green, and nontransparent. After 2 d of incubation, eggs laid by control females retained their round and full shape and become darker, indicative of normal embryonic development (Fig. 5e). In contrast, eggs laid by dsHEM females became deflated and transparent (Fig. 5h). After 4 d of incubation, eggs laid by control females hatched normally (Fig. 5f) while those laid by dsHEM females become even more deflated and ultimately died (Fig. 5i).
Discussion
In the present study, we cloned the Sf-CHI, Sf-CHSB, Sf-ST, and Sf-HEM encoding genes in S. frugiperda and then verified the sequences and predicted their possible functions through sequence BLAST, phylogeny, and protein structure analysis. The four sequences from S. frugiperda show a shorter genetic distance to lepidopterans than insects from other orders, which consistent with traditional taxonomy. Sf-HEM shares homology with other immunoglobulin (Ig)-like genes of invertebrates and mammals, which are classified into different families (Jung et al. 2019). The four Ig domains of Sf-HEM show a horseshoe shape, which is similar to the result found in S. exigua (Jung et al. 2019). The horseshoe shape of HEM from lepidopteran is different to the extended chain form of Ig molecule from human and other species other than lepidopteran (Jung et al. 2019). However, whether this difference is related to their functional differences in different taxa remains to be clarified.
Accumulating studies have shown that bacterium-mediated RNAi achieves effective silencing of target genes in different insect species, resulting in substantial effects on larval development and survival and/or adult reproduction (reviewed in Xu et al. 2016, Goodfellow et al. 2019). For example, a recent study in Plagiodera versicolora, a key pest of Salicaceae plants, showed that feeding bacteria-expressed dsRNA successfully triggered the silencing of the five target genes (Actin, Signal recognition particle protein 54k, Heat shock protein 70, Cactus and N-ethylmaleimide-sensitive fusion attachment protein) and the suppression of Actin and Signal recognition particle protein 54k genes caused significant mortality (Zhang et al. 2019b).
In the present study, we further developed a bacterium-mediated RNAi system in S. frugiperda using the four genes as targets. In the first round of trials, second-instar larvae were fed with bacteria expressing dsRNA of the four target genes and successfully induced gene silencing (Table 2). The growth and development of insects is strictly dependent on the precise regulation of chitin synthesis and digestion (Pesch et al. 2016, Zhao et al. 2018). Consistently, the following bioassay test showed that knockdown of Sf-CHI and Sf-CHSB by feeding larvae with bacteria expressing dsRNA resulted in lower larval body weight and lower survival rate, as well as longer developmental duration in S. frugiperda (Fig. 3). ST plays a crucial role in the cell-to-cell transport of sugars in insects (Baldwin and Henderson 1989) and HEM plays roles in immunity and reproduction in lepidopteran insects (Su et al. 1998). In this study, although the reduction of gene expression due to RNAi is slight, knockdown of Sf-ST and Sf-HEM by feeding larvae with bacteria expressing dsRNAs also showed significantly negative effect on larval growth and body weight in S. frugiperda (Fig. 3). These results demonstrate that feeding larvae with bacteria expressing dsRNAs effectively achieved RNAi of target genes and caused significantly negative effects on larval development and survival in S. frugiperda, although the efficacy and effects of RNAi differed between genes.
Moreover, bacterium-mediated RNAi also has been suggested as a cost-effective method for gene function analysis (Xiang et al. 2009, Zhang et al. 2012, Xu et al. 2016, Wang et al. 2021). Therefore, we further injected pupae and adults with bacterially expressed dsRNAs of the four target genes, which induced faster (tested 24 h after injection) and greater reduction (up to 76.36%) in gene expression (Table 2). Interestingly, injection of pupae with dsCHI significantly reduced eclosion rate of pupae but increased wing malformation in adults (P < 0.05; Figs 4a and 5a–c). Chitin is the main substance of insect wings, and the precise regulation of chitin synthesis and digestion may be crucial for the development of wings (Zhang et al. 2012, Pesch et al. 2016, Zhao et al. 2018), which may explain the result of adult wing malformation after the knockdown of Sf-CHI. Previous studies have indicated that CHSB is mainly responsible for chitin synthesis in midgut and peritrophic membrane of larvae (Zhang et al. 2012, Yang et al. 2019a). In the present study, injection of pupae with dsCHSB resulted in significant lower eclosion rate of pupae (P < 0.05; Fig. 4a). Although not statistically significant (P > 0.05), knockdown of Sf-CHSB also clearly increased wing malformation rate in adults (Figs 4a and 5a–c). These results suggest that Sf-CHSB also is crucial for the survival of pupae and may play a function in wing formation in S. frugiperda. Moreover, injection of female adults with dsHEM caused lower mating rate and lower egg hatching rate in S. frugiperda (Fig. 6a–c), which is consistence to the result found in the giant silkmoth, Hyalophora cecropia, by injection of pupae with HEM dsRNA (Bettencourt et al. 2002). These results suggest that Sf-HEM is likely to play vital function in the process of egg production (which may thus affect mating behavior as egg production positively correlated to female receptivity [Ringo 1996, Wedell 2005]) and/or embryo development. However, the mechanism of Sf-HEM in reproduction is still unclear and thus warrants further study. These evidences suggest that bacterium-mediated RNAi can also be a reliable technique for gene function analysis in S. frugiperda.
Above results demonstrated that both feeding bacteria expressing dsRNAs or injecting extracted dsRNAs produced by bacteria can induce RNAi of target genes in S. frugiperda while the efficacy of RNAi can be various between genes and between dsRNA delivery ways (Table 2). The efficacy of dsRNA-based RNAi can be affected by a number of factors, including the target gene itself and where it is expressed, the length and amount of dsRNA, the insect species and the life stage of the insect, and the technique for delivery of exogenous dsRNA into organisms (Xu et al. 2016, Goodfellow et al. 2019). In Tribolium castaneum, RNAi efficacy is relative to the length of dsRNA and the amount of dsRNA used (Tomoyasu and Denell 2004), while in Drosophila, sometimes a lower dsRNA dose may be more effective than a higher one in gene silencing (Yang et al. 2000). Therefore, by optimizing these influencing factors, a better RNAi effect of target genes can be obtained. Bacterium-mediated RNAi through insects feeding with bacteria expression dsRNA is a cost-effective and natural way to introduce dsRNA-based insecticides into pest insects. However, bacterial survival and dsRNA degradation in insect gut may have significant effects on the efficacy of RNAi (Huvenne and Smagghe 2010, Burand and Hunter 2013, Xu et al. 2016, Goodfellow et al. 2019). Therefore, re-application by application more than once or continuously may achieve better silencing of target genes (Goodfellow et al. 2019). For example, in S. litura, one-time feeding of bacteria expressing dsRNA led to lower silencing efficiency (<30%) while continuous feeding (from the third instar to mature larvae) resulted in higher efficacy (up to 83%). Moreover, application of bacteria expressing dsRNAs targeting multiple genes is likely to archive higher interference efficiency and greater control efficacy (Huvenne and Smagghe 2010, Xu et al. 2016, Goodfellow et al. 2019). However, increased dose of bacteria and dsRNA may induce high immune responses in insect (Eigenbrod and Dalpke 2015), which may bring side effects on gene function study using RNAi. Therefore, future studies should improve our understanding on bacterial RNA secretion and delivery and insect–microbial interactions, as well as target gene screen and bacterium selection, which will facilitate optimization of bacterium-mediated RNAi technology for pest control applications (Goodfellow et al. 2019).
Further, studies also suggested that extracts of bacterially expressed dsRNA can be applied in pest control, particularly when the use of live bacteria is limited or the RNAi efficiency of use of bacteria expressing dsRNA is dissatisfied (Leelesh and Rieske 2020). And recent studies also showed that nanomaterial-wrapped dsRNA has higher stability and better cellular uptake, providing a potential RNAi-based strategy for pest control (Sun et al. 2020b). Our injection trials indicated that the extracted bacterially expressed dsRNAs have high RNAi efficacies, signifying the potential use as dsRNA-based biopesticides in S. frugiperda.
Supplementary Data
Supplementary data are available at Journal of Insect Science online.
Fig. S1 Prediction of the signal peptide of Sf-CHI.
Fig. S2 Phylogenetic analysis of Sf-CHI. The phylogenetic tree was constructed using the neighbor joining method based on amino acid sequences with 1000 bootstrap replicates. The percentage bootstrap support was presented by the number above the branches.
Fig. S3 Prediction of transmenbrane dormains of Sf-CHSB.
Fig. S4 Phylogenetic analysis of Sf-CHSB. The phylogenetic tree was constructed using the neighbor joining method based on amino acid sequences with 1000 bootstrap replicates. The percentage bootstrap support was presented by the number above the branches.
Fig. S5 Prediction of transmenbrane dormains of Sf-ST.
Fig. S6 Phylogenetic analysis of Sf-ST. The phylogenetic tree was constructed using the neighbor joining method based on amino acid sequences with 1000 bootstrap replicates. The percentage bootstrap support was presented by the number above the branches.
Fig. S7 Prediction of the signal peptide of Sf-HEM.
Fig. S8 Phylogenetic analysis of Sf-HEM. The phylogenetic tree was constructed using the neighbor joining method based on amino acid sequences with 1000 bootstrap replicates. The percentage bootstrap support was presented by the number above the branches.
Acknowledgments
We acknowledge Science and Technology Planning Project in Key Areas of Yunnan Province (202001BB050002), Joint Special Project of Yunnan Province for Agricultural Basic Research (2018FG001-002) and National Natural Science Foundation Program of P.R. China (31860140; 31760635; 31560606) for funding this research at Southwest Forestry University, China.
Author Contributions
Conceptualization, J.X., J.H.L., X.S.W., and M.R.S.; methodology, J.X., M.R.S, X.S.W., and H.Y.; investigation, X.S.W., M.R.S, J.H.L., and J.X.; data analysis, X.S.W., M.R.S, J.X., and H.Y.; writing, X.S.W., M.R.S, J.X., and J.H.L. All authors have read and agreed to the published version of the manuscript. X.-S.W. and M.-R.S. contributed equally to this work.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
References Cited
- Abrahams, P., Bateman M., Beale T., Clottey V., Cock M., Colmenarez Y., Corniani N., Day R., Early R., Godwin J., . et al. 2017. Fall armyworm: impacts and implications for Africa. Outlooks Pest Manag. 28: 196–201. [Google Scholar]
- Aprd. 2021. Arthropod pesticide resistance database.Accessed 18 May 2021. http://www.pesticide-resistance.org/
- Baldwin, S. A., and Henderson P. J.. . 1989. Homologies between sugar transporters from eukaryotes and prokaryotes. Annu. Rev. Physiol. 51: 459–471. [DOI] [PubMed] [Google Scholar]
- Bettencourt, R., Terenius O., and Faye I.. . 2002. Hemolin gene silencing by ds-RNA injected into Cecropia pupae is lethal to next generation embryos. Insect Mol. Biol. 11: 267–271. [DOI] [PubMed] [Google Scholar]
- Burand, J. P., and Hunter W. B.. . 2013. RNAi: future in insect management. J. Invertebr. Pathol. 112(Suppl): S68–S74. [DOI] [PubMed] [Google Scholar]
- Burtet, L. M., Bernardi O., Melo A. A., Pes M. P., Strahl T. T., and Guedes J. V.. . 2017. Managing fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae), with Bt maize and insecticides in southern Brazil. Pest Manag. Sci. 73: 2569–2577. [DOI] [PubMed] [Google Scholar]
- Cabi. 2020a. Invasive species compendium: fall armyworm portal.Accessed 26 Nov. 2020. https://www.cabi.org/isc/fallarmyworm
- Cabi. 2020b. Invasive species compendium: Spodoptera frugiperda (fall armyworm) Datasheet.Accessed 26 Nov. 2020. https://www.cabi.org/isc/datasheet/29810
- Capinera, J. L. 2017. Fall Armyworm, Spodoptera frugiperda (J.E. Smith) (Insecta: Lepidoptera: Noctuidae).Accessed 26, Nov. 2020. http://entnemdept.ufl.edu/creatures/field/fall_armyworm.htm
- Casmuz, A., Laura Juarez M., Guillermina Socias M., Gabriela Murua M., Prieto S., Medina S., Willink E. and Gastaminza G.. . 2010. Review of the host plants of fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae). Revis. Socied. Entom. Argentina 69: 209–231. [Google Scholar]
- Dong, Q.-J., Zhou J.-C., Zhu K.-H., Z-T Z. and Dong H.. . 2019. A simple method for identifiying sexuality of Spodoptera frugiperd (J. E. Smith) pupae and adults. Plant Prot. (China) 45: 96–98. [Google Scholar]
- Dumas, P., Legeai F., Lemaitre C., Scaon E., Orsucci M., Labadie K., Gimenez S., Clamens A. L., Henri H., Vavre F., . et al. 2015. Spodoptera frugiperda (Lepidoptera: Noctuidae) host-plant variants: two host strains or two distinct species? Genetica. 143: 305–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eigenbrod, T., and Dalpke A. H.. . 2015. Bacterial RNA: an underestimated stimulus for innate immune responses. J. Immunol. 195: 411–418. [DOI] [PubMed] [Google Scholar]
- Fao. 2019. FAO statement on fall armyworm in Sri Lanka.Accessed 26 Nov. 2020. http://www.fao.org/srilanka/news/detail-events/en/c/1177796/
- Feldmann, F., Rieckmann U. and Winter S.. . 2019. The spread of the fall armyworm Spodoptera frugiperda in Africa: what should be done next? J. Plant Diseas. Prot. 126: 97–101. [Google Scholar]
- Fraser, A. G., Kamath R. S., Zipperlen P., Martinez-Campos M., Sohrmann M., and Ahringer J.. . 2000. Functional genomic analysis of C. elegans chromosome I by systematic RNA interference. Nature. 408: 325–330. [DOI] [PubMed] [Google Scholar]
- Goodfellow, S., Zhang D., Wang M.-B. and Zhang R.. . 2019. Bacterium-mediated RNA interference: potential application in plant protection. Plants-Basel 8: 572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, J. F., He K. L. and Wang Z. Y.. . 2019a. Biological characteristics, trend of fall armyworm Spodoptera frugiperda, and the strategy for management of the pest. J. Appl. Entom. (China) 56: 361–369. [Google Scholar]
- Guo, Z. B., Jiang R. X., Tang Y. L., Gu R. C., Li Q. Y., Xing T., Xiang L., Wu Y. Y., Hu Y., Liu X., . et al. 2019b. Identification of new islolates of gut bacteria of Spodoptera frugiperda feeding on sorghum in Chongqing area. J. Southwest Univ. 41: 9–16. [Google Scholar]
- Huvenne, H., and Smagghe G.. . 2010. Mechanisms of dsRNA uptake in insects and potential of RNAi for pest control: a review. J. Insect Physiol. 56: 227–235. [DOI] [PubMed] [Google Scholar]
- Jiang, Y. Y., Liu J., Xie M., Li Y. H., Yang J. J., Zhang M. L. and Qiu K.. . 2019. Observation on law of diusion damage of Spodoptera frugiperdain in China in 2019. Plant Prot. (China) 45: 10–1l9. [Google Scholar]
- Johnson, S. J. 1987. Migration and the life history strategy of the fall armyworm, Spodoptera frugiperda in the western hemisphere. Int. J. Trop. Insect. Sci. 8: 543–549. [Google Scholar]
- Jung, J., Sajjadian S. M. and Kim Y.. . 2019. Hemolin, an immunoglobulin-like peptide, opsonizes nonself targets for phagocytosis and encapsulation in Spodoptera exigua, a lepidopteran insect. J. Asia-Pacific Entomol. 22: 947–956. [Google Scholar]
- Kalleshwaraswamy, C. M., Asokan R., Swamy H. M., Maruthi M. S., Pavithra H. B., Hegde K., Navi S., Prabhu S. T. and Goergen G.. . 2018. First report of the fall armyworm, Spodoptera frugiperda (J E Smith) (Lepidoptera: Noctuidae), an alien invasive pest on maize in India. Pest Manag. Horticul. Ecosyst. 24: 23–29. [Google Scholar]
- Kumar, M., Gupta G. P., and Rajam M. V.. . 2009. Silencing of acetylcholinesterase gene of Helicoverpa armigera by siRNA affects larval growth and its life cycle. J. Insect Physiol. 55: 273–278. [DOI] [PubMed] [Google Scholar]
- Leelesh, R. S. and Rieske L. K.. . 2020. Oral ingestion of bacterially expressed dsRNA can silence genes and cause mortality in a highly invasive, tree-killing pest, the emerald ash borer. Insects 11: 440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, W., Zou W. J. and Wang L. H.. . 2006. The bionomics and control of Prodenia litura in Kunming. Southwest China J. Agric. Sci. 19: 85–89. [Google Scholar]
- Li, Y., Zhang S., Wang X., Xie X., Liang P., Zhang L., Gu S. and Gao X.. . 2019. Current status of insecticide resistance in Spodoptera frugiperda and strategies for its chemical control. Plant Prot. (China) 45: 14–19. [Google Scholar]
- Livak, K. J., and Schmittgen T. D.. . 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 25: 402–408. [DOI] [PubMed] [Google Scholar]
- Montezano, D. G., Specht A., Sosa-Gomez D. R., Roque-Specht V. F., Sousa-Silva J. C., Paula-Moraes S. V., Peterson J. A. and Hunt T. E.. . 2018. Host plants of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas. Afr. Entomol. 26: 286–300. [Google Scholar]
- Pesch, Y. Y., Riedel D., Patil K. R., Loch G., and Behr M.. . 2016. Chitinases and Imaginal disc growth factors organize the extracellular matrix formation at barrier tissues in insects. Sci. Rep. 6: 18340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen, T. N., Brunak S., von Heijne G., and Nielsen H.. . 2011. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat. Methods. 8: 785–786. [DOI] [PubMed] [Google Scholar]
- Qin, Y., Yang D., Kang D., Zhao Z., Zhao Z., Yang P. and Li Z.. . 2020. Potential economic loss assessment of maize industry caused by fall armyword (Spodoptera frugiperda) in china. Plant Prot. (China) 46: 69–73. [Google Scholar]
- Ringo, J. 1996. Sexual receptivity in insects. Annu. Rev. Entomol. 41: 473–494. [DOI] [PubMed] [Google Scholar]
- Shylesha, A. N., Jalali S. K., Gupta A., Varshney R., Venkatesan T., Shetty P., Ojha R., Ganiger P. C., Navik O., Subaharan K., . et al. 2018. Studies on new invasive pest Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae) and its natural enemies. J. Biol. Control 32: 145–151. [Google Scholar]
- Solis, C. F., Santi-Rocca J., Perdomo D., Weber C., and Guillén N.. . 2009. Use of bacterially expressed dsRNA to downregulate Entamoeba histolytica gene expression. PLoS One. 4: e8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su, X. D., Gastinel L. N., Vaughn D. E., Faye I., Poon P., and Bjorkman P. J.. . 1998. Crystal structure of hemolin: a horseshoe shape with implications for homophilic adhesion. Science. 281: 991–995. [DOI] [PubMed] [Google Scholar]
- Sun, X.-L., Chen C.-C., Li N., Liu F.-J., Dong Y.-N., Qian X.-N., Xing Y.-X., Liu M.-M. and Li X.-W.. . 2020a. The fall armyworm Spodoptera frugiperda may transfer to damage tea plant (Camellia sinesis). J. Tea Sci. (China) 40: 105–112. [Google Scholar]
- Sun, Y., Wang P., Abouzaid M., Zhou H., Liu H., Yang P., Lin Y., Hull J. J., and Ma W.. . 2020b. Nanomaterial-wrapped dsCYP15C1, a potential RNAi-based strategy for pest control against Chilo suppressalis. Pest Manag. Sci. 76: 2483–2489. [DOI] [PubMed] [Google Scholar]
- Timmons, L., and Fire A.. . 1998. Specific interference by ingested dsRNA. Nature. 395: 854. [DOI] [PubMed] [Google Scholar]
- Timmons, L., Court D. L., and Fire A.. . 2001. Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene. 263: 103–112. [DOI] [PubMed] [Google Scholar]
- Tomoyasu, Y., and Denell R. E.. . 2004. Larval RNAi in Tribolium (Coleoptera) for analyzing adult development. Dev. Genes Evol. 214: 575–578. [DOI] [PubMed] [Google Scholar]
- Vogel, E., Santos D., Mingels L., Verdonckt T.-W. and Vanden Broeck J.. . 2019. RNA interference in insects: protecting beneficials and controlling pests. Front. Physiol. 9: 1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X., Chen Z., Wang X., Xu J., Chen P. and Ye H.. . 2021. Bacterialmediated RNAi and functional analysis of Natalisin in a moth. Sci. Rep. 11: 4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedell, N. 2005. Female receptivity in butterflies and moths. J. Exp. Biol. 208: 3433–3440. [DOI] [PubMed] [Google Scholar]
- Westbrook, J. K., Nagoshi R. N., Meagher R. L., Fleischer S. J., and Jairam S.. . 2016. Modeling seasonal migration of fall armyworm moths. Int. J. Biometeorol. 60: 255–267. [DOI] [PubMed] [Google Scholar]
- Xiang, S., Keates A. C., Fruehauf J., Yang Y., Guo H., Nguyen T., and Li C. J.. . 2009. In vitro and in vivo gene silencing by TransKingdom RNAi (tkRNAi). Methods Mol. Biol. 487: 147–160. [DOI] [PubMed] [Google Scholar]
- Xu, J., Wang X. F., Chen P., Liu F. T., Zheng S. C., Ye H. and Mo M. H.. . 2016. RNA interference in moths: mechanisms, applications, and progress. Genes 7: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, L. N., Hu B. J., Su X. Y., Qi R. D., Su W. H., Qiu K., Zhou Z. Y., Zheng Z. Y., Zhang Q. Y., Hu F., . et al. 2019. Genetic analysis of the fall armyworm Spodoptera frugiperda invaded in Anhui province. Plant Prot. (China) 45: 47–53. [Google Scholar]
- Xu, Y., Li Z., Chen J., Li Z. and Qin Y.. . 2020. Assessment of potential economic loss of wheat industry caused by the fall armyworm Spodoptera frugiperda in China. J. Plant Prot. 47: 740–746. [Google Scholar]
- Yang, D., Lu H., and Erickson J. W.. . 2000. Evidence that processed small dsRNAs may mediate sequence-specific mRNA degradation during RNAi in Drosophila embryos. Curr. Biol. 10: 1191–1200. [DOI] [PubMed] [Google Scholar]
- Yang, X., Yin Q., Xu Y., Li X., Sun Y., Ma L., Zhou D., and Shen B.. . 2019a. Molecular and physiological characterization of the chitin synthase B gene isolated from Culex pipiens pallens (Diptera: Culicidae). Parasit. Vectors. 12: 614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, X. L., Liu Y. C., Luo M. Z., Li Y., Wang W. H., Wan F. and Jiang H.. . 2019b. Spodoptera frugiperda moved into southwestern China for the first time in Jiangcheng County, Yunnan Province. Yunnan Agricul. 1: 72. [Google Scholar]
- Zar, J. H. 1999. Biostatistical analysis. Prentice Hall, Upper Saddle River, New Jersey. [Google Scholar]
- Zhang, X., Zhang J., Park Y., and Zhu K. Y.. . 2012. Identification and characterization of two chitin synthase genes in African malaria mosquito, Anopheles gambiae. Insect Biochem. Mol. Biol. 42: 674–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, L., Liu B., Jiang Y. Y., Liu J., Wu K. M. and Xiao Y. T.. . 2019a. Molecular characterization analysis of fall armyworm populations in China. Plant Prot. (China) 45: 20–27. [Google Scholar]
- Zhang, Y., Xu L., Li S. and Zhang J.. . 2019b. Bacteria-mediated RNA interference for management of Plagiodera versicolora (Coleoptera: Chrysomelidae). Insects 10: 415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, X., Situ G., He K., Xiao H., Su C., and Li F.. . 2018. Functional analysis of eight chitinase genes in rice stem borer and their potential application in pest control. Insect Mol. Biol. 27: 835–846. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.