Abstract
Background:
Continuous oxygen therapy is not recommended for emphysema patients who are not hypoxemic at rest, although it is often prescribed. Little is known regarding the clinical characteristics and survival of nonhypoxemic emphysema patients using continuous oxygen. Analysis of data from the National Emphysema Treatment Trial (NETT) offers insight into this population.
Methods:
We analyzed demographic and clinical characteristics of 1,215 participants of NETT, stratifying by resting Pao2 and reported oxygen use. Eight-year survival was evaluated in individuals randomized to medical therapy.
Results:
At enrollment, 33.8% (n = 260) of participants nonhypoxemic at rest reported continuous oxygen use. When compared to nonhypoxemic individuals not using oxygen (n = 226), those using continuous oxygen had worse dyspnea, lower quality of life, more frequent exercise desaturation, and higher case-fatality rate. After adjusting for age, body mass index, and FEV, percentage of predicted, the presence of exercise desaturation accounted for the differential mortality seen between these group.
Conclusions:
In the NETT, the use of continuous oxygen in resting nonhypoxemic emphysema patients was associated with worse disease severity and survival. The differential survival observed could nearly all be accounted for by the higher prevalence of exercise desaturation in those using continuous oxygen, suggesting that it is not a harmful effect of oxygen therapy contributing to mortality. It remains unclear whether continuous oxygen therapy improves survival in normoxic patients with exercise desaturation.
Trial registration:
Clinicaltrials.gov Identifier: NCT00000606.
Keywords: guideline adherence, pulmonary disease, chronic obstructive, supplemental oxygen
COPD remains a major public health issue, rdnking fourth in the United States as a cause of death, with a total estimated cost of $32.1 billion in 2002.1,2 The substantial impact of this disease on health-care cost and delivery within the United States and worldwide has stimulated development of international guidelines for the diagnosis and management of COPD.3–5 With the exception of smoking cessation, continuous oxygen therapy, and possibly some pharmacologic regimens, few interventions have been shown to improve mortality in patients with COPD.6–7 The Nocturnal Oxygen Therapy Trial (NOTT) and report of the Medical Research Council Working Party (MRC) evaluated long-term domiciliary oxygen therapy in patients with COPD and severe resting hypoxemia.8,9 These studies showed that continuous oxygen therapy increased survival and improved quality of life. Based primarily on the findings of the NOTT and MRC, current guidelines recommend oxygen therapy for some patients with COPD, although spec& recommendations vary among different organizations (Table 1).
Table 1–
Guidelines for Continuous Oxygen Therapy in COPD*
| Hypoxemia | ATS-ERS | GOLD | NCCCC-NICE |
|---|---|---|---|
| Severe | Pao2< 7.3 kPa (55 mm Hg) or SpO2≤ 88% | Pao2< 7.3 kPa (55 mm Hg) or Spo2 ≤ 88% | Pao2< 7.3 kPa |
| Moderate | Pao2of 7.3 to 8.0 kPa (55 to 59 mm Hg) or Spo2 of 89% and at least one of the following: cor pulmonale; peripheral edema; hematocrit > 55% | Pao2 of 7.3 to 8.0 kPa (55 to 59 mm Hg) or Spo2 of 88% and at least one of the following: pulmonary hypertension; peripheral edema; hematocrit > 55% | Pao2 of 7.3 to 8.0 kPa (55–59 mm Hg) and at least one of the following: pulmonary hypertension; peripheral edema; secondary polycythemia; nocturnal desaturation > 30% of sleep |
| None | Pao2≥ 8.0 kPa (60 mm Hg) or SpO2> 90% with severe nocturnal desaturation and lung-related dyspnea responsive to oxygen | No recommendation given | No recommendation given |
While the NOTT and MRC established the role of continuous oxygen therapy in patients with severe hypoxemia, few studies have evaluated continuous oxygen therapy in COPD patients with mild-to-moderate degrees of hypoxemia. Continuous oxygen therapy in this population has been shown to reduce the observed decline in exercise endurance but not impact survival.10–12 It remains unclear as to the potential benefit or harm of continuous oxygen use in nonhypoxemic emphysema patients. Most guidelines do not recommend continuous oxygen therapy for patients with resting and exertional Pao2 > 60 mm Hg (Table 1). Given the cost of therapy for COPD, it is important to understand the factors driving the use of continuous oxygen therapy in different populations of COPD patients and whether oxygen use affects survival. The National Emphysema Treatment Trial (NETT) provides an ideal data set to explore these issues. In this study, patients with severe emphysema were randomized to medical therapy or medical therapy plus lung volume reduction surgery.13 Extensive baseline demographic and clinical measurements were collected, including resting Pao2 and current oxygen use by self-report. Analysis of this study population provides insight into the characteristics of individuals with differing degrees of hypoxemia using continuous oxygen, and the potential effects of continuous oxygen in these individuals.
Our overall goal was to explore the relationship between mortality and use of oxygen in patients who did not meet conventional criteria. Therefore, in this study we address the following questions: Is there a survival difference in nonhypoxemic participants based on self-reported oxygen use pattern? Do clinical characteristics and survival differences exist based on self-reported oxygen use in participants exhibiting only exercise desaturation? How closely does self-reported oxygen use by NETT Participants follow current guidelines? How do the demographic and clinical characteristics of patients with resting Pao2 > 60 mm Hg prescribed continuous oxygen compare to those reporting no oxygen use?
Materials and Methods
Patient Selection
Data for this study were extracted from the initial and follow-up data of patients enrolled in the NETT. The design and methods of the NETT are published elsewhere.14 Briefly, former smokers with severe emphysema who were deemed to be candidates for lung volume reduction surgery were enrolled in 6 to 10 weeks of pulmonary rehabilitation. Oxygen therapy, when necessary, was prescribed by the rehabilitation center or primary care physician. After rehabilitation, the treatment plan including oxygen prescription was approved, by a NETT physician. Baseline measurements were obtained within 2 weeks of completing pulmonary rehabilitation but prior to randomization in the study. These measurements included resting room air arterial blood gas analysis and oxygen use by self-report. Three separate exercise tests were performed. A treadmill exercise test with pulse oximetry, performed by walking on a treadmill at one mile per hour for 3 min and then two to three miles per hour for 4 min, was used to test for exercise desaturation. Exercise desaturation was defined as oxygen saturation by pulse oximetry (Spo2) < 90% at any point during this test. A 6-inin walk test on supplemental oxygen (if needed based on treadmill walking) was conducted. Finally, a graded maximal cycle ergometry on 30% oxygen was performed to determine maximum exercise capacity. Short-acting bronchodilators were used at least 15 min and no more than 4 h before testing of oxygen desaturation. In order to assess severity and persistence of resting room air desaturation, two criteria were used. In the first S min of a resting period, desaturation was defined as presence of room air Spo2 ≤ 85% at any time. After 5 min of rest, desaturation was considered to be present if the Spo2 was < 90%. Self-administered questionnaires were used to assess disease-specific quality of life (St. George Respiratory Questionnaire [SGRQ]), general health-related quality of life (Quality of Well-Being Score [QWB]), and dyspnea (University of California San Diego Shortness of Breath Questionnaire [UCSD-SOBQ]). Eligible patients were then randomized to medical therapy or medical therapy plus lung volume reduction surgery. Each patient’s medical management was reviewed on an annual basis by one of the NETT pulmonologists and recommendations made to the treating physician based on American Thoracic Society guidelines. However, there was no requirement that changes he made consistent with these recommendations. The NETT study protocol was approved by local institutional review boards, and all patients provided informed consent.
Data Analysis
Of the 1,218 participants enrolled in the NETT, 3 patients were excluded from this data analysis because of missing baseline Pao2 measurements. For the purpose of this report, normoxia was defined as Pao2 > 60 mm Hg. For analysis, subjects were categorized based on postrehabilitation resting room air Pao2 and reported oxygen use at baseline postrehabilitation assessment. Because of the heterogeneous indications, usage patterns, and lack of detailed information about oxygen use duration in the participants (n = 283) using oxygen intermittently (rest, sleep, or exertion, but not all three), we evaluated their survival but otherwise did not include them in the analysis. Participants randomized to medical therapy were followed up for vital status until August 31, 2006, 8 years after trial initiation (n = 394). In order to avoid the confounding effect of lung volume reduction surgery, we did not include patients randomized to surgical therapy when evaluating survival. Clinical and demographic characteristics between groups were compared using t tests for continuous variables and Fisher exact tests for categorical variables. Kaplan-Meier curves were generated and compared for those individuals randomized to medical therapy, classified into two groups: resting Pao2 > 60 mm Hg using continuous oxygen and Pao2 > 60 mm Hg not using oxygen. A modified BODE (body mass index [BMI], obstruction, dyspnea; exercise capacity) [mBODE] score was calculated using the formula of Martinez et al.15 Because of multiple comparison, a p value of 0.01 was used to determine significance among groups treated with different oxygen therapies. Cox proportional hazard models were used to test for survival differences between groups, adjusting for known predictors of mortality (age, BMI, FEV1 percentage of predicted). Additional models included adjustment for exercise desaturation on 6-min walk test. All analyses were performed using statistical software (SAS version 9.1; SAS Institute; Cay, NC; and freeware R version 2.3.1; R Foundation for Statistical Computing; Vienna, Austria).
Results
Oxygen Use at Enrollment
At enrollment, 769 of the 1,215 participants had a resting baseline room air Pao2 > 60 mm Hg (Table 2). Of these, 33.8% reported continuous oxygen use, while 29.4% reported no oxygen use. The remaining 283 participants (36.8%) reported oxygen use for either rest, exercise, or sleep, but not all three (intermittent use). Resting baseline room Pao2 values (mean ± SD) for the three groups were 67.9 ± 6.5 mm Hg (continuous use), 70.3 ± 6.7 mm Hg (intermittent use), and 73.5 ± 7.8 mm Hg (no oxygen use); p < 0.0001 between groups (Kruskal Wallis test).
Table 2–
Oxygen Use at Enrollment (n = 1,215)*
| Baseline Pao2, mm Hg | |||
|---|---|---|---|
| Oxygen Use | ≤ 55 | 56 to 60 | >60 |
| None | 4 (1.5) | 7 (3.9) | 226 (29.4) |
| Intermittent | 22 (8.3) | 43 (23.6) | 283 (36.8) |
| Continuous | 238 (90.2) | 132 (72.5) | 260 (33.8) |
Data are presented as No. (% of patients in similar Pao2 group).
Baseline Demographics and Clinical Characteristics
We compared demographic and clinical characteristics of normoxic individuals using continuous oxygen to those not using oxygen (Table 3). Those using continuous oxygen had more advanced disease as evidenced by lower FEV1, FEV1 percentage of predicted, FVC, 6-min walk distance, and ergometry exercise. They also had worse dyspnea, lower quality of life, higher mBODE scores, and more frequent exercise desaturation. The continuous oxygen group was slightly younger with a higher BMI, prognostic variables generally considered favorable in COPD.
Table 3–
Demographic and Clinical Characteristics at Enrollment*
| Characteristics | Resting PaO2> 60 mm Hg and Continuous Oxygen (n = 260) | Resting Pao2> 60 mm Hg and No Oxygen (n = 226) | p Value |
|---|---|---|---|
| Age, yr | 65.5 ± 6.4 | 66.7 ± 6.5 | 0.04 |
| Race | |||
| White | 247 (95) | 214 (95) | > 0.99 |
| Nonwhite | 13 (5) | 12 (5) | |
| Gender | |||
| Female | 101 (39) | 72 (32) | 0.13 |
| Male | 159 (61) | 154 (68) | |
| Pack-yr† | 63.9 ± 30.1 | 63.8 ± 33.4 | 0.97 |
| Income, $ | |||
| < 15,000 | 54 (21) | 40 (18) | 0.37 |
| 15,000–29,999 | 90 (35) | 76 (35) | |
| 30,000–49,000 | 76 (29) | 65 (29) | |
| ≥ 50,000 | 34 (13) | 41 (18) | |
| Missing/no answer | 6 (2) | 1 (0.5) | |
| BMI, kg/m2 | 25,1 ± 3.8 | 24.1 ± 3.2 | 0.002 |
| FEV1, L | 0.75 ± 0.22 | 0.89 ± 0.26 | < 0.0001 |
| FEV1, % predicted‡ | 25.8 ± 6.8 | 29.8 ± 7.4 | < 0.0001 |
| FVC, L | 2.5 ± 0.8 | 2.8 ± 0.8 | 0.0002 |
| FVC, % predicted‡ | 65.7 ± 14.7 | 70.9 + 15.0 | 0.0002 |
| TLC, L | 7.7 ± 1.6 | 7.8 ± 1.5 | 0.17 |
| TLC, % predicted§ | 127.5 ± 15.0 | 127.1 ± 13.6 | 0.72 |
| RV, L | 5.0 ± 1.2 | 4.9 + 1.1 | 0.23 |
| RV, % predicted§ | 226.1 ± 48.8 | 214.8 ± 42.9 | 0.002 |
| IC, L | 1.7 ± 0.6 | 1.9 ± 0.6 | < 0.0001 |
| IC, % predicted | 60.1 ± 17.3 | 66.8 ± 17.3 | < 0.0001 |
| Dlco, mL/min/mm Hg | 7.7 ± 3.5 | 9.9 ± 3.4 | 0.25 |
| Dlco, % predicted‖ | 27.8 ± 11.6 | 30.4 ± 6.6 | 0.64 |
| 6-min walk distance, feet | 1143 ± 292 | 1363 ± 305 | < 0.0001 |
| Maximum exercise, W | 37.1 ± 21.1 | 47.3 ± 22.8 | < 0.0001 |
| Exercise desaturation | |||
| Present | 210 (81) | 79 (35) | < 0.0001 |
| Absent | 50 (19) | 147 (65) | |
| UCSD-SOBQ¶ | 68.2 ± 17.8 | 55.6 ± 19.6 | < 0.0001 |
| QWB# | 0.55 ± 0.13 | 0.58 ± 0.10 | 0.008 |
| SGRQ** | |||
| Symptoms | 58.8 ± 20.4 | 56.0 ± 20.0 | 0.14 |
| Activity | 81.7 ± 12.8 | 75.0 ± 15.0 | < 0.0001 |
| Impact | 40.2 ± 16.5 | 34.4 ± 14.5 | < 0.0001 |
| Total | 55.9 ± 13.3 | 50.4 ± 12.5 | < 0.0001 |
| mBODE score†† | |||
| 0–2 | 5 (1.9) | 18 (8.0) | < 0.0001 |
| 3–4 | 67 (25.8) | 115(51.1) | |
| 5–6 | 112 (43.1) | 71 (31.6) | |
| 7–10 | 76 (29.2) | 21 (9.3) |
Values are given as mean ± SD or No. (%). TLC = total lung capacity; RV = residual volume; IC = inspiratory capacity; Dlco = diffusing capacity of the lung for carbon monoxide.
No. of cigarettes smoked daily on average/20 times total years smoked.
Predicted values calculated from Crapo et al.16
Predicted values calculated from Crapo et al.17
Predicted values calculated from Crapo Morris.18
The UCSD-SOBQ is a 24-item questionnaire regarding dyspnea. Scores range from 0 to 120 with higher scores indicating more dyspnea.
The QWB scale is a 77-item questionnaire focusing on generic health-related quality of life. Scores range from 0 to 1, with higher scores indicate better quality of life.
The SGRQ is a 51-item questionnaire focusing on respiratory symptoms and quality of life. Scores range from 0 to 100, with higher scores indicating worse health-related quality of life.
The mBODE is a composite 11-point score.
Survival in Participants Enrolled in the Medical Therapy Arm
Limiting the analysis to participants randomized to medical therapy, we compared survival in normoxic participants using continuous oxygen normoxic participants not using oxygen (Fig 1). The case-fatality rate was substantially higher in normoxic individuals using continuous oxygen at the time of enrollment when compared to those reporting no oxygen use (61.4% vs 48.6%). The survival curve for normoxic patients using intermittent oxygen was intermediate between those using continuous oxygen and those using no supplemental oxygen (data not shown). The unadjusted hazard ratio for the groups using continuous oxygen and no oxygen was 1.63 (p = 0.005), with worse survival in those using continuous oxygen. In order to explore whether clinical prognostic indicators accounted for this difference in survival, we incorporated other variables into the proportional hazards model. After adjusting for BMI, age, arid FEV1 percentage of predicted, the difference between the two groups was no longer significant, with a hazard ratio of 1.38 (p = 0.078; Fig 1). The differential mortality was further attenuated by incorporating the presence of exercise desaturation into the model, with a resultant hazard ratio of 1.14 (p = 056).
Figure 1.
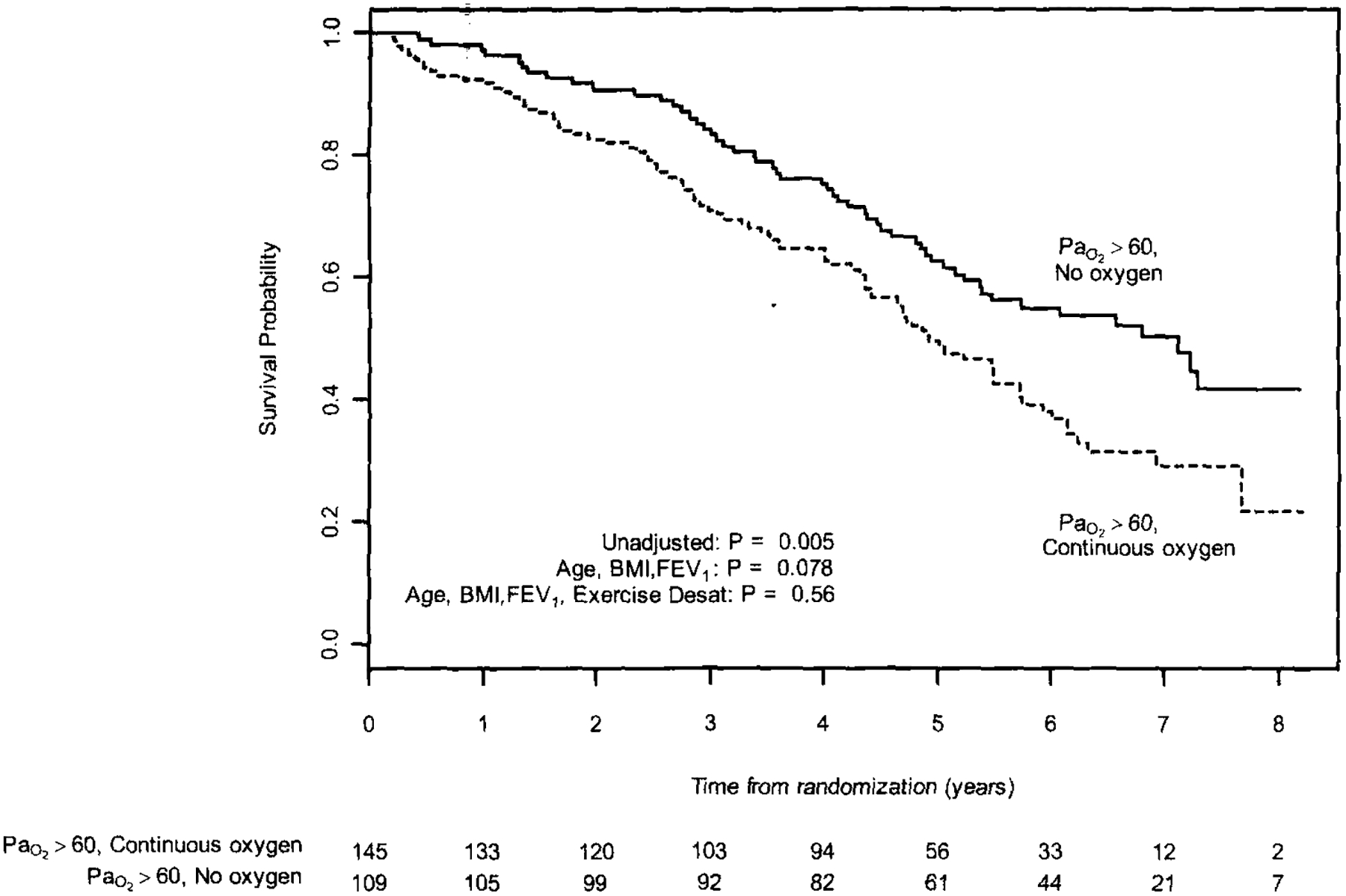
Multivariate 8-year survival analysis in normoxic participants randomized to medical therapy, startified bv oxygen use; p values are calculated from Cox models that adjust for BMI. age, FEV1 percentage of predicted, and exercise desaturation (Desat).
Normoxic Participants With Exercise Desaturation
Because exercise desaturation was highly correlated with mortality, we wanted to understand die characteristics and survival of those using continuous oxygen and demonstrating exercise desaturation (Table 4, Fig 2). Among the 471 participants with resting normoxia and exercise desaturation, 44.5% were using continuous oxygen, 38.6% were using oxygen intermittently, and 16.7% reported no oxygen use. More severe disease was seen in those using continuous oxygen, as demonstrated by lower FEV1, FEV1 percentage of predicted, FVC, FVC percentage of predicted, 6-min walk distance, ergometer exercise, and indexes of dyspnea and overall quality of life. Despite these differences, oxygen use was not associated with differences in survival in individuals randomized to medical therapy demonstrating resting normoxia and exercise desaturation (p = not significant for pair-wise comparisons by log-rank test) [Fig 2]. Thus, patients who had exercise desaturation had similar mortality regardless of whether they were using continuous, intermittent, or no oxygen.
Table 4–
Characteristics of Individuals With Resting Normoxia and Exercise Desaturation*
| p Value | ||||||
|---|---|---|---|---|---|---|
| Characteristics | Continuous Oxygen (n = 210) | Intermittent Oxygen (n = 182) | No Oxygen (n = 79) | Continuous vs Intermittent | Continuous vs None | Intermittent vs None |
| Age, yr | 65.5 ± 6.2 | 66.9 ± 5.9 | 66.2 ± 5.9 | 0.02 | 0.40 | 0.33 |
| Race | ||||||
| White | 202 (96.2) | 170 (61.5) | 75 (94.9) | 0.25 | 0.74 | 0.78 |
| Nonwhite | 8 (3.8) | 12 (6.6) | 4(5.1) | |||
| Gender | ||||||
| Female | 84 (40.0) | 70 (38.5) | 25 (31.7) | 0.84 | 0.22 | 0.33 |
| Male | 126 (60.0) | 112 (61.5) | 54 (68.4) | |||
| Pack-yr | 64.0 ± 27.1 | 62.0 ± 29.3 | 67.4 ± 36.2 | 0.49 | 0.39 | 0.21 |
| Income, $ | ||||||
| < 15,000 | 41 (19.5) | 33 (18.1) | 17 (21.5) | 0.12 | 0.93 | 0.71 |
| 15,000–29,999 | 74 (35.2) | 54 (29.7) | 24 (30.4) | |||
| 30,000–49,000 | 62 (29.5) | 54 (29.7) | 25 (31.7) | |||
| ≥ 50,000 | 29 (13.8) | 41 (22.5) | 13 (16.5) | |||
| Missing/no answer | 4 (1.9) | 0 (0.0) | 0 (0.0) | |||
| BM1, kg/m2 | 25.3 ± 3.8 | 24.5 ± 3.4 | 24.3 ± 3.1 | 0.03 | 0.05 | 0.77 |
| FEV1, L | 0.73 ± 0.22 | 0.77 ± 0.21 | 0.86 ± 0.28 | 0.03 | < 0.0001 | 0.004 |
| FEV1, % predicted | 25.1 ± 6.6 | 27.7 ± 6.8 | 28.3 ± 8.3 | 0.002 | 0.001 | 0.28 |
| FVC, L | 2.4 ± 0.78 | 2.5 ± 0.78 | 2.7 ± 0.84 | 0.17 | 0.005 | 0.08 |
| FVC, % predicted | 64.1 ± 14.3 | 68.0 ± 15.4 | 68.1 + 15.7 | 0.01 | 0.04 | 0.94 |
| TLC, L | 7.7 ± 1.6 | 7.7 ± 1.5 | 8.1 ± 1.6 | 0.68 | 0.06 | 0.10 |
| TLC, % predicted | 127.6 ± 15.5 | 129.1 ± 13.0 | 127.8 ± 12.9 | 0.31 | 0.90 | 0.48 |
| RV, L | 5.1 ± 1.2 | 5.0 ± 1.1 | 5.1 ± 1.2 | 0.40 | 0.95 | 0.47 |
| RV, % predicted | 228.2 ± 49.6 | 221.4 ± 43.7 | 221.7 ± 49.7 | 0.15 | 0.32 | 0.96 |
| IC, L | 1.6 ± 0.60 | 1.8 ± 0.56 | 1.9 ± 0.63 | 0.05 | 0.0002 | 0.02 |
| IC, % predicted | 58.8 ± 17.3 | 63.4 ± 16.4 | 66.1 ± 16.7 | 0.008 | 0.001 | 0.22 |
| Dlco, mL/min/mm Hg | 7.2 ± 3.4 | 8.3 ± 2.5 | 9.4 ± 3.7 | 0.49 | 0.32 | 0.58 |
| Dlco, % predicted | 25.4 ± 10.9 | 29.7 ± 7.9 | 28.8 ± 6.3 | 0.39 | 0.58 | 0.84 |
| 6-min walk distance, feet | 347.9 ± 87.8 | 395.3 ± 95.2 | 400.8 ± 100 | < 0.0001 | < 0.0001 | 0.67 |
| Maximum exercise, W | 36.7 ± 21.0 | 42.0 ± 19.8 | 45.1 ± 23.7 | 0.01 | 0.004 | 0.27 |
| UCSD-SOBQ | 68.2 ± 16.9 | 60.6 ± 16.8 | 57.7 ± 19.4 | < 0.0001 | < 0.0001 | 0.23 |
| QWB | 0.55 ± 0.12 | 0.59 ± 0.11 | 0.57 ±0.10 | 0.002 | 0.28 | 0.16 |
| SGRQ | ||||||
| Symptoms | 58.5 ± 20.0 | 53.5 ± 18.7 | 54.7 ± 18.8 | 0.01 | 0.15 | 0.64 |
| Activity | 81.9 ± 12.3 | 79.8 + 13.1 | 78.1 ± 13.7 | 0.10 | 0.02 | 0.35 |
| Impact | 39.6 ± 15.9 | 35.4 ± 15.2 | 35.9 ± 14.4 | 0.01 | 0.08 | 0.80 |
| Total | 55.6 ± 12.7 | 51.9 ± 12.0 | 51.9 ± 12.3 | 0.004 | 0.03 | 0.98 |
| mBODE score | ||||||
| 0–2 | 2 (1.0) | 6 (3.3) | 7 (8.9) | < 0.0001 | < 0.0001 | 0.30 |
| 3–4 | 55 (26.2) | 84 (46.2) | 33 (41.8) | |||
| 5–6 | 92 (43.8) | 66 (36.3) | 27 (34.2) | |||
| 7–10 | 61 (29.1) | 26 (14.3) | 12 (15.2) | |||
Date we presented as mean ± SD or No. See Table 3 for expansion of abbreviations and definitions.
Figure 2.
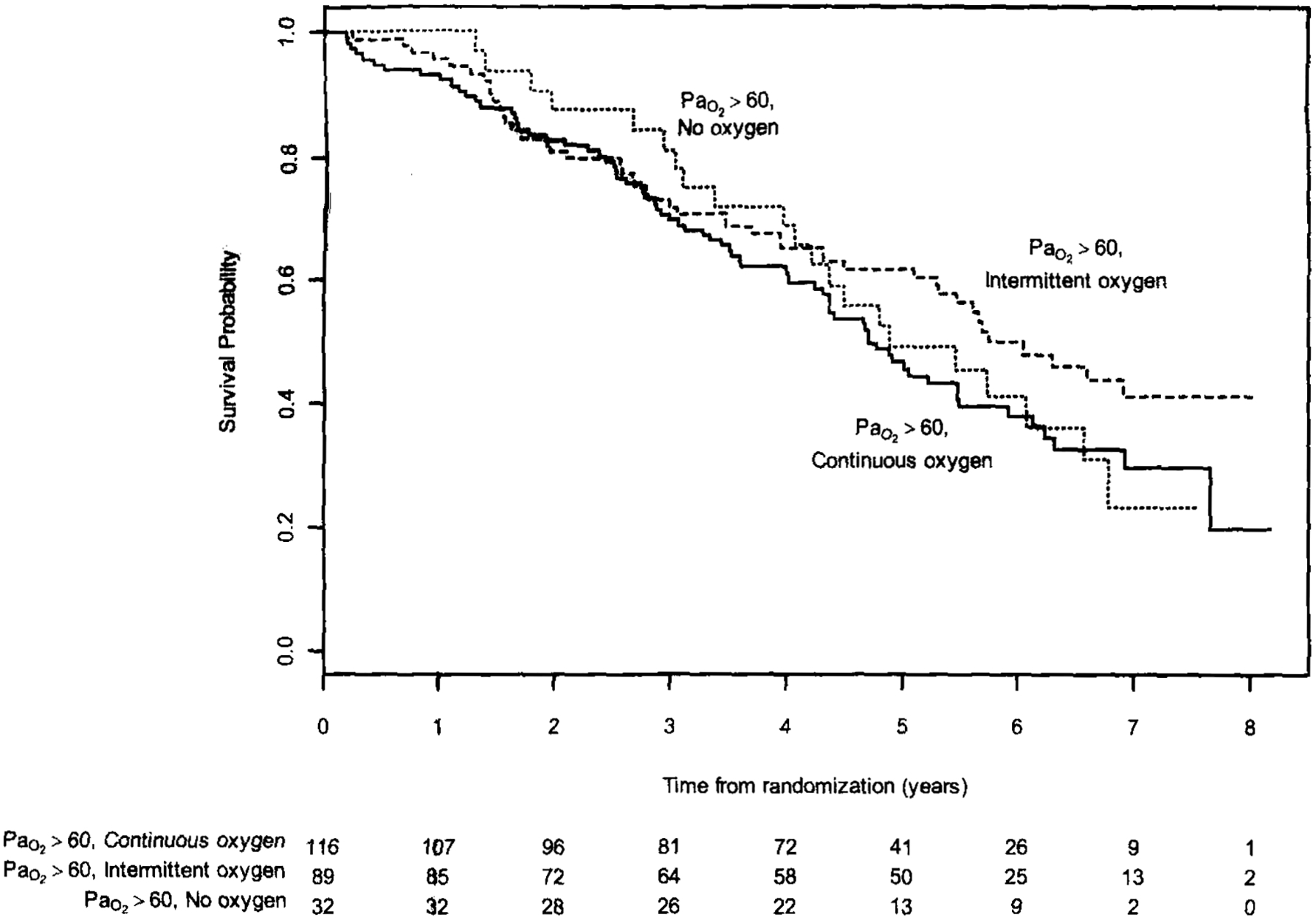
Multivariate 8-year survival analysis for participants with resting normoxia and exercise desaturation, randomized to medical therapy. Groups are stratified by oxygen use.
Discussion
The main finding in this exploratory study is that use of oxygen in normoxic patients identifies a high-risk group of emphysema patients. Norimoxic participants using continuous oxygen demonstrated more frequent exercise desaturation, lower spirometric values, poorer exercise performance, more dyspnea, and worse survival. These observations indicate that normoxic individuals using continuous oxygen were a population with more severe disease. While the increased mortality in those using oxygen could be caused directly by the use of oxygen, the observed difference in mortality could be partially accounted for by adjusting for FEV1, age, and BMI. This suggests that patient characteristics rather than oxygen use contributed to the observed increase in mortality. Therefore, oxygen use appears to be a surrogate marker for other risk factors for mortality.
A significant proportion of the risk of death could be accounted for in the hazard model by the presence of exercise desaturation. Because the presence of exercise desaturation correlated strongly with continuous oxygen use in normoxic individuals, the ability to confidently infer die impact of exercise desaturation on survival is limited. There are several potential explanations for why exercise desaturation may be a strong predictor of mortality in COPD patients with resting normoxia. Given the frequent presence of comorbidities in individuals with COPD,7 acute desaturation with exercise may pose imminent threat by increasing the risk of cardiac dysrhythmias and ischemia. Other insults such as pneumonia or acute exacerbations of COPD may induce more frequent or more severe hypoxemia. Exercise desaturation may be a marker of more extensive, undiagnosed pulmonary hypertension, which is associated with shorter survival in COPD patients.19 Exercise desaturation has been shown previously to correlate with severity of pulmonary vascular disease in COPD patients with no or mild resting hypoxemia.20 Although it is unclear which of these mechanisms is playing a role in the poorer survival observed in those with exercise desaturation, our analysis suggests that exercise desaturation is a predictor of mortality in patients with severe emphysema and resting normoxia. This conclusion supports the findings of Takigawa and colleagues,21 who reported on the ability of oxygen desaturation during 6-min walk test to predict mortality in a group of 144 COPD patients. Previous analysis of the NETT cohort demonstrated that increased mortality was independently associated with use of oxygen supplementation.15 The present study extends the analysis of Martinez et al15 by analyzing only the subgroup that was normoxic at rest. Our findings add exercise desaturation to the list of factors impacting mortality in this population. Although oxygen supplementation may be helpful in patients with exercise desaturation, we did not see a significant difference in survival based on oxygen use. Because the retrospective nature of our analysis limits full evaluation of causal mechanisms, the potential benefit of continuous oxygen therapy in emphysema patients with resting normoxia and exercise desaturation needs further evaluation in a prospective randomized manner.
A second finding of this study is that 21.4% of the 1,215 NETT participants reported oxygen use outside of current guidelines despite having been recently enrolled in supervised pulmonary rehabilitation. This observation highlights the challenge of monitoring oxygen prescription and use. While one could speculate that the patient’s clinical status changed from the time of physician assessment at rehabilitation discharge to the baseline Pao2 measurement, this is unlikely given that the Pao2 assessment occurred within 2 weeks of discharge from rehabilitation. Higher altitudes could have impacted on the appropriateness of oxygen use, but of the 49 participants enrolled at a high altitude site (Denver, CO), only 1 of the 4 patients with a baseline Pao2 > 60 mm Hg reported continuous oxygen use. Several centers enrolled patients from large recruitment areas with wide variations in altitude, which may have led to differing degrees of hypoxia at home compared to the screening site, resulting in oxygen prescriptions that were appropriate at home, but not when visiting the study center. Using participant home zip codes and reference altitudes for those zip codes, we found no trend in guideline adherence associated with altitude in a center with a large recruitment area of various topography (Seattle, WA).
What could explain the use of continuous oxygen in normoxic participants? Physician may be inclined to treat a patient with worsening functional status and quality of life more aggressively. Physicians may attempt to minimize symptoms of dyspnea and exercise intolerance by prescribing continuous oxygen therapy for this subset of patients despite lack of indication by Pao2 measurements. There is support for this approach because oxygen therapy can yield higher training intensity and improve exercise tolerance in nonhypoxic patients with COPD.22 Alternatively, physicians may have responded to requests for oxygen from patients with worsening functional status and quality of life. We do not have data regarding the specific rationale and indication for individual oxygen prescription in NETT participants. Moreover, it seems likely that oxygen prescriptions changed over time, and this may have influenced survival, either positively or negatively. Another limitation of this study is that we relied on patient self-report of oxygen use, which may have underestimated actual oxygen use. This has been well documented for continuous oxygen use, and may also apply to patients who report intermittent oxygen use.23 Despite this, this study observes that within a group of normoxic emphysema patients, physicians recognized a sicker subset of individuals and prescribed them continuous oxygen therapy.
In conclusion, this study shows that continuous oxygen use in a population of patients with severe emphysema and resting normoxia is common. The use of continuous oxygen identifies a high-risk subset of emphysema patients. Exercise desaturation is a substantial contributor to mortality in this population. The findings of this study highlight the ongoing need for prospective trials focusing on the use of oxygen therapy in emphysema patients not meeting conventional criteria for continuous oxygen use.
Abbreviations:
- RMI
body mass index
- mBODE
modified body mass index, obstruction, dyspnea; exercise capacity
- MRC
Medical Research Council Working Party
- NETT
National Emphysema Treatment Trial
- NOTT
Nocturnal Oxygen Treatment Trial
- QWB
quality of well-being score
- SGRQ
St. George Respiratory Questionnaire
- Spo2
oxygen saturation by pulse oximetry
- UCSD-SOBQ
University of California Sari Diego Shortness of Breath Questionnaire
Appendix: Members of the NETT Research Group
Office of the Chair of the Steering Committee, University of Pennsylvania, Philadelphia, PA: Alfred P. Fishman, MD (Chair), Betsy Ann Bozzarello, and Ameena Al-Amin.
Clinical Centers
Baylor College of Medicine, Houston, TX:
Marcia Katz, MD (Principal Investigator); Carolyn Wheeler, RN, BSN (Principal Clinic Coordinator); Elaine Baker, RRT, RPFT; Peter Barnard, PhD, RPFT; Phil Cagle, MD; James Carter, MD; Sophia Chatziioannou, MD; Karla Conejo-Gonzales; Kimberly Dubose, RRT; John Haddad, MD; David Hicks, RRT, RPFT; Neal Kleiman, MD; Mary Milburn-Barnes, CRTT; Chinh Nguyen, RPFT; Michael Reardon, MD; Joseph Reeves-Viets, MD; Steven Sax, MD; Amir Sharafkhaneh, MD; Owen Wilson, PhD; Christine Young PT; Rafael Espada, MD (Principal Investigator from 1996 to 2002); Rose Butanda (from 1999 to 2001); Minnie Ellisor (2002); Pamela Fox, MD (from 1999 to 2001); Katherine Hale, MD (from 1998 to 2000); Everett Hood, RPFT (from 1998 to 2000); Amy Jahn (from 1998 to 2000); Satish Jhingran, MD (from 1998 to 2001); Karen King, RPF’T (from 1998 to 1999); Charles Miller III, PhD (from 1996 to 1999); Imran Nizami, MD (Co-Principal Investigator, from 2000 to 2001); Todd Officer (from 1998 to 2000); Jeannie Ricketts (from 1998 to 2000); Joe Rodarte, MD (Co-Principal Investigator from 1996 to 2000); Robert Teague, MD (Co-Principal Investigator from 1999 to 2000); and Kedren Williams (from 1998 to 1999).
Brigham and Women’s Hospital, Boston, MA:
John Reilly, MD (Principal Investigator); David Sugarbaker, MD (Co-Principal Investigator); Carol Fanning, RRT (Principal Clinic Coordinator); Simon Body, MD; Sabine Duffy, MD; Vladmir Formanek, MD; Anne Fuhlbrigge, MD; Philip Hartigan, MD; Sarah Hooper, EP; Andetta Hunsaker, MD; Francine Jacobson, MD; Marilyn Moy, MD; Susan Peterson, RRT; Roger Russell, MD; Diane Saunders; and Scott Swanson, MD (Co-Principal Investigator, from 1996 to 2001).
Cedars-Sinai Medical Center, Los Angeles, CA:
Rob McKenna, MD (Principal Investigator); Zab Mohsenifar, MD (Co-Principal Investigator); Carol Geaga, RN (Principal Clinic Coordinator); Manmohan Biring, MD; Susan Clark, RN, MN; Jennifer Cutler, MD; Robert Frantz, MD; Peter Julien, MD; Michael Lewis, MD; Jennifer Minkoff-Rau, MSW; Valentina Yegyan, BS, CPFT; arid Milton Joyner, BA (from 1996 to 2002).
Cleveland Clinic Foundation, Cleveland OH:
Malcolm De-Camp, MD (Principal Investigator); James Stoller, MD (Co-Principal Investigator); Yvonne Meli, RN, C (Principal Clinic Coordinator); John Apostolakis, MD; Darryl Atwell, MU: Jeffrey Chapman, MD; Pierre DeVilliers, MD; Raed Dweik, MD: Erik Kraenzler, MD; Rosemary Lann, LISW; Nancy Kurokawa, RRT, CPFT; Scott Marlow, RRT; Kevin McCarthy, RCPT; Pricilla McCreight, RRT, CPFT; Atul Mehta, MD; Moulay Meziane, MD; Omar Minai, MD; Mindi Steiger, RRT; Kenneth White, RPFT; Janet Maurer, MD (Principal Investigator, from 1996 to 2001); Terri Durr, RN (from 2000 to 2001); Charles Hearn. DO (from 1998 to 2001); Susan Lubell, PA-C (froin 1999 to 2000); Peter O’Donovan, MD (from 1998 to 2003); and Robert Schilz, DO (from 1998 to 2002).
Columbia University, New York, NY, in consortium with Long Island Jewish Medical Center, New Hyde Park, NY:
Mark Ginsburg, MD (Principal Investigator); Byron Thomashow, MD (Co-Principal Investigator); Patricia Jellen, MSN, RN (Principal Clinic Coordinator); John Austin, MD; Matthew Bartels, MD: Yahya Berkmen, MD; Patricia Berkoski, MS, RRT (Site coordinator, LIJ); Frances Brogan, MSN, RN; Amy Chong, BS, CRT; Glenda DeMercado, BSN; Angela DiMango, MD; Sandy Do. MS, PT; Bessie Kachulis, MD; Arfa Khan, MD); Berend Mets, MD; Mitchell O’ Shea, BS, RT, CPFT: Gregory Pearson, MD; Leonard Rossoff, MD; Steven Scharf, MD, PhD (Co-Principal Investigator, from 1998 to 2002); Maria Shiau, MD; Paul Simonelli, MD; Kim Stavrolakes, MS, PT; Donna Tsang, BS; Denise Vilotijevic, MS, PT; Chun Yip, MD; Mike Mantinaos, MD (from 1998 to 2001); Kerri McKeon, BS, RRT, RN (from 1998 to 1999); and Jacqueline Pfeffer, MPH, PT (from 1997 to 2002).
Duke University Medical Center, Durha, NC:
Neil Mac-Intyre, MD (Principal Investigator); R. Duane Davis, MD (Co-Principal Investigator); John Howe, RN (Principal Clinic Coordinator); R. Edward Coleman, MD; Rebecca Crouch, RPT: Dora Greene; Katherine Grichnik, MD; David Harpole, Jr., MD; Abby Krichman, RRT; Brian Lawlor, RRT; Holman McAdams, MD; John Plankeel, MD; Susan Rinaldo-Gallo, MED; Sheila Shearer, RRT; Jeanne Smith, ACSW; Mark Stafford-Smith, MD; Victor Tapson, MD; Mark Steele, MD (from 1998 to 1999); and Jennifer Norten, MD (from 1998 to 1999).
Mayo Foundation, Rochester, MN:
James Utz, MD (Principal Investigator); Claude Deschamps, MD (Co-Principal Investigator); Kathy Mieras, CCRP (Principal Clinic Coordinator); Martin Abel, MD; Mark Allen, MD; Deb Andrist, RN; Gregory Augh-enbaugh, MD; Sharon Bendel, RN; Eric Edell, MD; Marlene Edgar; Bonnie Edwards; Beth Elliot, MD; James Garrett, RRT; Delmar Gillespie, MD; Judd Gurney, MD; Boleyn Hammel; Karen Hanson, RRT; Lori Hanson, RRT; Gordon Harms, MD; June Hart; Thomas Hartman, MD; Robert Hyatt. MD; Eric Jensen, MD: Nicole Jenson, RRT; Sanjay Kalra, MD; Phlip Karsell, MD; Jennifer Lamb; David Midthun, MD; Carl Mottram, RRT; Stephen Swensen, MD: Anne-Marie Sykes, MD: Karen Taylor; Norman Torres, MD; Rolf Hubmayr, MD (from 1998 to 2000); Daniel Miller, MD (from 1999 to 2002); Sara Bartling, RN (from 1998 to 2000); and Kris Bradt (from 1998 to 2002).
National Jewish Medical and Research Center, Denver, CO:
Barry Make, MD (Principal Investigator): Marvin Pomerantz, MD (Co-Principal Investigator); Mary Gilmartin, RN, RRT (Principal Clinic Coordinator); Joyce Canterbury; Martin Carlos; Phyllis Dibbern, PT; Enrique Fernandez, MD; Lisa Geyman, MSPT; Connie Hudson; David Lynch, MD; John Newell, MD; Robert Quaife, MD; Jennifer Propst, RN; Cynthia Raymond, MS; Jane Whalen-Price, PT; Kathy Winner, OTR; Martin Zamora, MD; and Reuben Cherniack, MD (Principal Investigator, from 1997 to 2000).
Ohio State University, Columbus, OH:
Philip Diaz, MD (Principal Investigator); Patrick Ross, MD (Co-Principal Investigator): Tina Bees (Principal Clinic Coordinator); Jan Drake; Charles Emery, PhD; Mark Gerhardt, MD, PhD; Mark King, MD; David Rittinger; and Mahasti Rittinger.
Saint Louis University, Saint Louis, MO:
Keith Naunheim, MD (Principal Investigator): Robert Gerber, MD (Co-Principal Investigator); Joan Osterloh, RN, MSN (Principal Clinic Coordinator); Susan Borosh; Willard Chamberlain, DO; Sally Frese; Alan Hibbit; Mary Ellen Kleinhenz, MD: Gregg Ruppel; Cary Stolar, MD; Janice Willey: Francisco Alvarez, MD (Co-Principal Investigator. from 1999 to 2002); and Cesar Keller, MD (Co-Principal Investigator, from 1996 to 2000).
Temple University, Philadelphia, PA:
Gerard Criner, MD (Principal Investigator); Satoshi Furukawa, MD (Co-Principal Investigator); Anne Marie Kuzma, RN, MSN (Principal Clinic Coordinator); Roger Barnette, MD; Neil Brister, MD: Kevin Carney, RN, CCTC; Wissain Chatila, MD; Francis Cordova, MD: Gilbert D’Alonzo, DO: Michael Keresztury, MD; Karen Kirsch; Chul Kwak, MD; Kathy Lautensack, RN, BSN; Madelina Lorenzon, CPFT; Ubaldo Martin. MD: Peter Rising, MS; Scott Schartel, MD; John Travaline, MD; Gwendolyn Vance. RN, CCTC: Phillip Boiselle, MD (from 1997 to 2000); and Gerald O’ Brien, MD (from 1997 to 2000).
University of California, San Diego, San Diego, CA:
Andrew Ries, MD, MPH (Principal Investigator); Robert Kaplan, PhD (Co-Principal Investigator); Catherine Ramirez, BS, RCP (Principal Clinic Coordinator); David Frankville, MD; Paul Friedinan, MD; James Harrell, MD; Jeffery Johnson; David Kapelanski, MD; David Kupferberg, MD, MPH; Catherine Larsen, MPH; Trina Limberg, RRT; Michael Magliocca, RN, CNP; Frank J. Papatheofanis, MD, PhD; Dawn Sassi-Dambron, RN; and Melissa Weeks.
University of Maryland at Baltimore, Baltimore, MD, in consortium with Johns Hopkins Hospital, Baltimore, MD:
Mark Krasna, MD (Principal Investigator); Henry Fessler, MD (Co-Principal Investigator); Iris Moskowitz (Principal Clinic Coordinator); Timothy Gilbert, MD; Jonathan Orens, MD; Steven Scharf, MD, PhD; David Shade; Stanley Siegelman, MI); Kenneth Silver, MD; Clarence Weir: and Charles White, MD.
University of Michigan, Ann Arbor, MI:
Fernando Martinez, MD (Principal Investigator): Mark Iannettoni, MD (Co-Principal Investigator); Catherine Meldruin, BSN, RN, CCRN (Principal Clinic Coordinator); William Bria, MD; Kelly Campbell; Paul Christensen, MD; Kevin Flaherty, MD; Steven Gay, MD; Paramjit Gill, RN; Paul Kazanjian, MD; Ella Kazerooni, MD; Vivian Knieper; Tammy Ojo. MD; Lewis Poole; Leslie Quint, MD; Paul Rysso: Thomas Sisson, MD; Mercedes True; Brian Woodcock, MD: and Lori Zaremba, RN.
University of Pennsylvania, Philadelphia, PA:
Larry Kaiser, MD (Principal Investigator); John Hansen-Flaschen, MD (Co-Principal Investigator); Mary Louise Dempsey, BSN. RN (Principal Clinic Coordinator); Abass Alavi, MD; Theresa Alcorn, Selim Arcasoy, MD; Judith Aronchick, MD; Stanley Aukberg, MD; Bryan Benedict, RRT; Susan Craemer, BS, RRT, CPFT; Ron Daniele, MD; Jeffrey Edelman, MD; Warren Gefter, MD; Laura Kotler-Klein, MSS; Robert Kotloff, MD; David Lipson, MD; Wallace Miller, Jr., MD; Richard O’ Connell, RPFT; Staci Opelman, MSW; Harold Palevsky, MD; William Russell. RPFT; Heather Sheaffer, MSW: Rodney Simcox, BSRT, RRT; Susanne Snedeker, RRT, CPFT; Jennifer Stone-Wynne, MSW; Gregory Tino, MD; Peter Wahl; James Walter, RPFT; Patricia Ward; David Zisman, MD; James Mendez, MSN, CRNP (from 1997 to 2001); and Angela Wurster, MSN, CRNP (from 1997 to 1999).
University of Pittsburgh, Pittsburgh, PA:
Frank Sciurba, MD (Principal Investigator); James Luketich, MI) (Co-Principal Investigator); Colleen Witt, MS (Principal Clinic Coordinator); Gerald Ayes; Michael Donahoe, MD; Carl Fuhrman, MD; Robert Hoffman, MD; Joan Lacomis, MD; Joan Sexton; William Slivka; Diane Strollo, MD; Erin Sullivan, MD; Tomeka Simon; Catherine Wrona, RN, BSN; Gerene Bauldoff, RN, MSN (from 1997 to 2000); Manuel Brown, MD (from 1997 to 2002); Elisabeth George, RN, MSN (Principal Clinic Coordinator from 1997 to 2001); Robert Keenan, MD (Co-Principal Investigator from 1997 to 2000); Theodore Kopp, MS (from 1997 to 1999); and Laurie Silfies (from 1997 to 2001).
University of Washington, Seattle, WA:
Joshua Benditt, MD (Principal Investigator), Douglas Wood, MD (Co-Principal Investigator); Margaret Snyder, MN (Principal Clinic Coordinator); Kymberley Anable; Nancy Battaglia; Louie Boitano; Andrew Bowdle, MD; Leighton Chan, MD; Cindy Chwalik; Bruce Culver, MD; Thurman Gillespy, MD; David Godwin, MD; Jeanne Hoffman; Andra Ibrahim, MD; Diane Lockhart; Stephen Marglin, MD; Kenneth Martay, MD; Patricia McDowell; Donald Oxorn, MD; Liz Roessler; Michelle Toshim; and Susan Golden (from 1998 to 2000).
Other Participants
Agency for Healthcare Research and Quality, Rockville, MD:
Lynn Bosco, MD, MPH; Yen-Pin Chiang, PhD; Carolyn Clancy, MD; and Harry Handelsman, DO.
Centers for Medicare and Medicaid Services, Baltimore, MD:
Steven M Berkowitz, PhD; Tanisha Carino, PhD; Joe Chin, MD; JoAnna Baldwin; Karen McVearry; Anthony Norris; Sarah Shirey; Claudette Sikora; and Steven Sheingold, PhD (from 1997 to 2004).
Coordinating Center, The John Hopkins University, Baltimore, MD:
Steven Piantadosi, MD, PhD (Principal Investigator); James Tonascia, PhD (Co-Principal Investigator); Patricia Belt; Amanda Blackford, ScM; Karen Collins; Betty Collison; Ryan Colvin, MPH; John Dodge; Michele Donithan, MHS; Vera Edmonds; Gregory L. Foster, MA; Julie Fuller; Judith Harle; Rosetta Jackson; Shing Lee. ScM; Charlene Levine; Hope Livingston; Jill Meinert; Jennifer Meyers; Deborah Nowakowski; Kapreena Owens: Shangqian Qi, MD; Michael Smith; Brett Simon, MD; Paul Smith: Alice Sternberg, ScM; Mark Van Natta, MHS; Laura Wilson, ScM: and Robert Wise, MD.
Cost-Effectiveness Subcommittee:
Robert M, Kaplan, PhD (Chair): J. Sanford Schwartz, MD (Co-Chair); Yen-Pin Chiang, PhD; Marianne C. Fahs, PhD; A. Mark Fendrick, MD; Alan J. Moskowitz, MD; Dev Pathak, PhD; Scott Ramsey, MD, PhD; Steven Sheingold, PhD; A. Laurie Shroyer, PhD; Judith Wagner, PhD; and Roger Yusen, MD.
Cost-Effectiveness Data Center, Fred Hutchinson Cancer Research Center, Seattle, WA:
Scott Ramsey, MD, PhD (Principal Investigator); Ruth Etzioni, PhD; Sean Sullivan, PhD; Douglas Wood, MD; Thomas Schroeder, MA; Karma Kreizenbeck; Kristin Berry, MS; and Nadia Howlader, MS.
CT Scan Image Storage and Analysis Center, University of Iowa, Iowa City, IA:
Eric Hoffman, PhD (Principal Investigator): Janice Cook-Granroth, BS; Angela Delsing, RT; Junfeng Guo, PhD; Geoffrey McLennan, MD; Brian Mullan, MD; Chris Piker, BS; Joseph Reinhardt, PhD; Blake Robinswood; Jered Sieren, RTR; and William Stanford, MD.
Data and Safety Monitoring Board:
John A. Waldhausen, MD (Chair); Gordon Bernard, MD; David DeMets, PhD; Mark Ferguson, MD: Eddie Hoover, MD; Robert Levine, MD; Donald Mahler, MD; A. John McSweeny, PhD; Jeanine Wiener-Kronish, MD; O. Dale Williams, PhD; and Magdy Younes, MD.
Marketing Center, Temple University, Philadelphia, PA:
Gerard Criner, MD (Principal Investigator); and Charles Soltoff, MBA.
Project office, National Heart, Lung, and Blood Institute, Bethesda, MD:
Gail Weinmann, MD (Project Officer); Joanne Deshler (Contracting Officer); Dean Follmann, PhD; James Kiley, PhD; and Margaret Wu, PhD (from 1996 to 2001).
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Hoyert DL, Heron MP, Murphy SL, et al. Deaths: final data for 2003. Natl Vital Stat Rep 2006: 54:1–120 [PubMed] [Google Scholar]
- 2.National Heart, Lung, and Blood Institute. Chronic Obstructive Lung Disease Fact Sheet. Bethesda, MD: US Government Printing Office, 2003; NIH publication No. 03–5229 [Google Scholar]
- 3.Celli BR, MacNee W. Standards for the diagnosis and treatment of patients COPD: a summary of the ATS/ERS position paper. Eur Respir J 2004; 23:932–946 [DOI] [PubMed] [Google Scholar]
- 4.Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management and prevention of COPD, 2006 update. Am J Respir Crit Care Med 2007; 176:532–555 [DOI] [PubMed] [Google Scholar]
- 5.National Collaborating Centre for Chronic Conditions. Chronic obstructive pulmonary disease: national clinical guidelines on management of chronic obstructive pulmonary disease in adults in primary and secondary care. Thorax 2004; 59:1–232 [PMC free article] [PubMed] [Google Scholar]
- 6.Wedzicha JA, Calverley PM, Seemungal TA, et al. The prevention of chronic obstructive pulmonary disease exacerbations by salmeterol/fluticasone propionate or tiotropium bromide, Am J Respir Crit Care Med 2008; 177:19–26 [DOI] [PubMed] [Google Scholar]
- 7.Anthonisen NR, Skeans MA, Wise RA, et al. Lung Health Study Research Group: the effects of a smoking cessation intervention on 14.5-year mortality; a randomized clinical trial. Ann Intern Med 2005; 142:233–239 [DOI] [PubMed] [Google Scholar]
- 8.Nocturnal Oxygen Therapy Trial Group. Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a clinical trial. Ann Intern Med 1980; 93:391–398 [DOI] [PubMed] [Google Scholar]
- 9.Report of the Medical Research Council Working Party. Long-term damiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Lancet 1981; 1:681–686 [PubMed] [Google Scholar]
- 10.Gorecka D, Gorzelak K, Sliwinski P, et al. Effect of long-term oxygen therapy on survival in patients with chronic obstructive pulmonary disease with moderate hypoxemia. Thorax 1997: 52:574–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haidl P, Clement C, Wiese C, et al. Long-term oxygen therapy stops the natural decline of endurance in COPD patients with reversible hypercapnia. Respiration 2004; 71: 342–347 [DOI] [PubMed] [Google Scholar]
- 12.Cranston JM, Crockett AJ, Moss JR, et al. Domiciliary oxygen for chronic obstructive pulmonary disease. Coclirane Database of Systematic Reviews 2005; 4:CD001744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Emphysema Treatment Trial Research Group. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med 2003; 348:2059–2073 [DOI] [PubMed] [Google Scholar]
- 14.National Emphysema Treatment Trial Research Group. Rationale and design of the National Emphysema Treatment Trial: a prospective randomized trial of lung volume reduction surgery. Chest 1999; 116:1750–1761 [DOI] [PubMed] [Google Scholar]
- 15.Martinez FJ, Foster G, Curtis JL, et al. Predictors of mortality in patients with emphysema and severe airflow obstruction. Am J Respir Crit Care Med 2006; 173:1326–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crapo RO, Morris AH, Gardner RM. Reference spirometric values using techniques and equipment that meet ATS recommendations. Am Rev Respir Dis 1981; 123:659–664 [DOI] [PubMed] [Google Scholar]
- 17.Crapo RO, Morris AH, Clayton PD, et al. Lung volumes in healthy nonsmoking adults. Bull Eur Physiopathol Respir 1982; 18:419–425 [PubMed] [Google Scholar]
- 18.Crapo RO, Morris AH. Standardized single breath normal values for carbon monoxide diffusing capacity. Am Rev Respir Dis 1981; 132:185–189 [DOI] [PubMed] [Google Scholar]
- 19.Weitzenblum E, Hirth C, Ducolone A, et al. Prognostic due of pulmonary artery pressure in chronic obstructive pulmonary disease. Thorax 1981; 36:752–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Christensen CC, Ryg MS, Edvardsen A, et al. Relationship between exercise desaturation and pulmonary haemodynamics in COPD patients. Eur Respir J 2004; 24:580–586 [DOI] [PubMed] [Google Scholar]
- 21.Takigawa N, Tada A, Soda R, et al. Distance and oxygen desaturation in 6-min walk test predict prognosis in COPD patients. Respir Med 2007; 101:561–567 [DOI] [PubMed] [Google Scholar]
- 22.Emtner M, Porszasz J, Burns M, et al. Benefits of supplemental oxygen in exercise training in nonhypoxemic chronic obstructive pulmonary disease patients. Am J Respir Crit Care Med 2003; 168:1034–1042 [DOI] [PubMed] [Google Scholar]
- 23.Pepin JL, Bajhoux CE, Deschaux C, et al. Long-term oxygen therapy at home: compliance with medical prescription and effective use of therapy: ANTADIR Working Group on Oxygen Therapy; Association Nationale de Traitement a Domicile des Insuffisants Respiratories. Chest 1996; 109: 1144–1150 [DOI] [PubMed] [Google Scholar]


