Abstract
Hashimoto thyroiditis (HT) is a pathology that often causes a gradual thyroid insufficiency in affected patients due to the autoimmune destruction of this gland. The cellular immune response mediated by T helper lymphocytes TH1 and TH17 can induce the HT disease. In this pathologic condition, there is an imbalance between the TH17 and Treg lymphocytes as well as a gut microbiota dysfunction. The objective of this work was to describe the interactions of the cell subpopulations that participate in HT. To achieve this goal, we generated a mathematical model that allowed the simulation of different scenarios for the dynamic interaction between thyroid cells, the immune system, and the gut microbiota. We used a hypothetical-deductive design of mathematical modeling based on a system of ordinary differential equations, where the state variables are the TH1, TH17, and Treg lymphocytes, the thyrocytes, and the bacteria from gut microbiota. This work generated a compartmental model of the cellular immune response occurring in the thyroid gland. It was observed that TH1 and TH17 lymphocytes could increase the immune cells’ activity, as well as activate effector cells directly and trigger the apoptosis and inflammation processes of healthy thyrocytes indirectly. Likewise, the model showed that a reduction in Treg lymphocytes could increase the activity of TH17 lymphocytes when an imbalance of the gut microbiota composition occurred. The numerical results highlight the TH1, TH17, and bacterial balance of the gut microbiota activities as important factors for the development of HT disease.
Keywords: autoimmune hypothyroidism, T helper cells, gut microbiota, thyrocytes, mathematical modeling, dynamic systems
The thyroid gland plays an important role in the metabolism, growth, and maintenance of human health (1, 2). Alterations in thyroid function cause important pathologies in humans (3). Hashimoto autoimmune thyroiditis (HT) disease is the most common cause of hypothyroidism in different regions of the world, mainly where the amount of iodine is sufficient. A higher incidence of HT is reported in women in contrast to men (3.5 per 1000 inhabitants/year and 0.8 per 1000 inhabitants/year, respectively) and its prevalence increases with age (4, 5). Gradual thyroid insufficiency characterizes HT clinically—even with or without goiter formation—due to autoimmune destruction of the gland that triggers apoptosis in thyroid epithelial cells (thyrocytes) (6, 7). Most patients with HT have elevated serum levels of antibodies against one or more thyroid antigens. In addition, they usually present with diffuse thyroid lymphocytic infiltration, mainly of thyroid-specific B and T lymphocytes, plasma cells, macrophages, and with follicular destruction, which all are characteristics of thyroiditis (4, 8). Currently HT is grouped with other autoimmune disorders, and the concept of autoimmune diathesis is widely accepted (9-12).
At the cellular level, HT has been characterized by an increase of TH1 lymphocytes activity that leads to cell-mediated immunity and thyrocyte death (13-15). However, a new subset of T helper (TH) lymphocytes called TH17 play an important role in thyroid autoimmunity, thus changing the traditional paradigm of the dichotomy between TH1 vs TH2 lymphocytes (16, 17). Evidence suggests that TH17 lymphocytes are responsible for the induction and development of chronic inflammatory responses in many autoimmune diseases, including HT (18-21). Additionally, patients with HT present with an imbalance between TH17 levels and regulatory T lymphocytes (Treg), as well as a dysfunction of Treg lymphocytes, as occurs in other autoimmune pathologies (22). Abnormal frequencies and functions of TH17 and Treg lymphocytes associated with thyroid autoimmunity underlie a complex genetic predisposition linked to factors including selenium deficiency, smoking, alcohol consumption, stressful events, infections, exposure to chemical compounds, and gut microbiota alterations (17, 23, 24).
The close relationship among the immune system, the gut microbiota, and the mechanisms for the generation of TH17 lymphocytes is well known. TH17 lymphocytes are cells responsible for promoting defense and facilitating the mutualism in the mucosa of the gut microbiota (25-27). The interactions between the host and the gut microbiota influence the host’s immunity and physiology, maintaining the homeostasis in that organ. Disruption in these interactions caused by a dysbiosis event can upset this balance and lead to a pathological state. The intestinal microbiota is essential for the differentiation and proliferation of TH17 lymphocytes in the normal lamina of the intestine (28, 29). Studies have highlighted the presence of Treg lymphocytes (30) associated with the intestinal microbiota (31). Furthermore, Treg lymphocytes maintain tolerance to self-antigens and prevent inflammatory responses that are induced by direct detection of microbial organisms and their metabolites (32).
In recent years, the knowledge generated about cellular mechanisms related to HT progression and the development of treatments with hormonal supplementation have provided a better understanding of HT (5). However, the mechanisms that drive the imbalance of immunity and facilitate the onset of inflammation, as well as cell apoptosis in the thyroid gland tissue, are still under study. Many therapeutic approaches in other autoimmune diseases aim to restore the balance of immunity (33, 34). Therefore, it is essential to improve the understanding of mechanisms involved in this immune imbalance for the development of new restorative treatments.
Mathematical modeling is a research tool considered as a complement to theory and experimentation in scientific research. In fact, it is an effective tool useful for the description and analysis of biological processes. Many studies have employed this tool to study behavior in—for example—heartbeat, viral infections, chronic disease treatment, cancer treatment and management, and recently, autoimmune diseases (35-40).
In this sense, this research developed and analyzed a mathematical model to study the balance of HT cellular immunity, changes in the autoimmunity induction process, and its relationship with alterations of the gut microbiota. This model used the Holling type II response function described by Iwami et al (41). This is the first report that attempts to predict part of the autoimmune process of HT using a mathematical model, which allows the simulation of different scenarios of the dynamic interactions among thyroid cells, the thyroid immune system, and the gut microbiota. This prediction will help in the understanding of HT pathogenesis. A hypothetical-deductive design of mathematical modeling was used based on a system of ordinary differential equations.
Materials and Methods
Mathematical Modeling
The mathematical model construction assumed a dynamic interaction between thyrocytes, the immune system, and the gut microbiota, which are represented in a system of 4 differential equations that describe the autoimmune dynamics of HT. This system is based on the hypothetical-deductive nature of mathematical modeling (42). The variables and parameters that compose this model describe the dynamics interactions of the cellular immune response and the development of this autoimmune disease. Likewise, the state variables used in this study are the thyrocytes (T), TH17 and TH1 lymphocytes, and typical bacteria of the intestinal microbiota (B). All these variables change over time (t), which is represented in months.
The model construction also considers that thyrocytes have an exponential growth rate in the absence of other variables. Furthermore, the growth of thyrocytes is affected by the intervention of the cellular immune response through the participation of the TH17 and TH1 lymphocytes. In this stage, TH17 lymphocytes stimulate the thyrocyte inflammation process at a rate of γ, while TH1 lymphocytes induce thyrocyte apoptosis at a rate of β.
Instead, the growth of TH17 and TH1 lymphocytes is represented by a functional response of the immune system (43), which in this model is assumed a Holling type II functional saturation response. This lymphocytic growth depends on the contribution of the differentiation process induced by the thyrocytes, with a maximum contribution of growth φ 1 and φ 2, respectively. Furthermore, the differentiation process of TH17 lymphocytes is strongly related to the existing balance between Treg lymphocytes and gut bacteria (28-31).
However, because Treg cells prevent the inflammatory response, they exhibit a suppressive effect on T lymphocytes (32). The model also assumes that thyrocytes, TH17 lymphocytes, and TH1 lymphocytes have mortality rates β 1, β 2, and β 3, respectively, resulting in their own half-lives in the body.
Additionally, the bacteria of the gut show logistic growth with a growth rate α 1 and a carrying capacity of the medium given by K, which is a dynamic characteristic of bacterial growth.
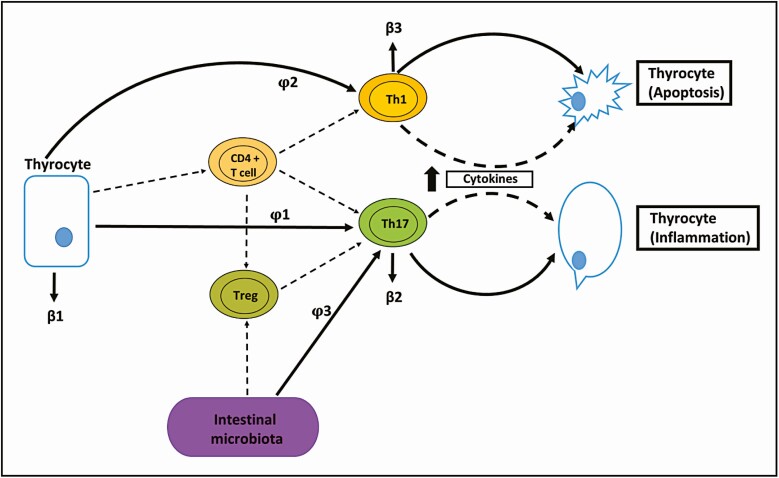
The conceptual diagram resulting from this cellular dynamic that describes the interaction between the variables and the model parameters is shown in Fig. 1. In this diagram, the dashed lines represent the cellular interaction and the solid lines represent the underlying dynamics in the mathematical model. The variables and parameters involved in the development of HT are described in Table 1.
Figure 1.
Schematic representation of simulation model for the Hashimoto autoimmune thyroiditis disease process. In the thyroid gland, thyrocytes mediate the differentiation of CD4+ T-helper (TH) cells into subpopulations of specific T cells (TH1, TH17, and Treg). The proliferation of TH1 and TH17 lymphocytes activate apoptosis and inflammation of the thyroid tissue, respectively. The gut microbiota regulates the relationship between the effector (TH17) and regulator (Treg) lymphocytes, leading to greater differentiation of pathogenic TH17 lymphocytes and subsequent inflammation. The model also assumes that thyrocytes, TH17 lymphocytes and TH1 lymphocytes have mortality rates β 1, β 2, and β 3. The lymphocytic differentiation process φ 1 and φ 2. The Treg and φ 3 parameters stimulate the differentiation of TH17 lymphocytes.
Table 1.
Variables and parameters for autoimmune Hashimoto thyroiditis disease
| Variable and parameter | Definition | Unit |
|---|---|---|
| T(t) | Thyrocytes concentration | cells/mL |
| TH17(t) | TH17 lymphocytes concentration | cells/mL |
| TH1(t) | TH1 lymphocytes concentration | cells/mL |
| B(t) | Bacteria from gut microbiota | cfu/mL |
| α | Thyrocytes growth rate | % |
| β | Predation rate of TH1 lymphocytes on thyrocytes | % |
| γ | Induction rate to the cellular apoptosis process | % |
| β 1 | Thyrocytes mortality rate | % |
| β 2 | TH17 mortality rate lymphocyte predation rate over thyrocytes | % |
| β 3 | TH1 mortality rate lymphocyte predation rate over thyrocytes | % |
| Treg | Treg lymphocyte concentration | cells/mL |
| φ 1 | TH17 lymphocyte differentiation rate | % |
| φ 2 | TH1 lymphocyte differentiation rate | % |
| φ 3 | Maximum contribution rate of bacteria to TH17 lymphocyte | % |
| α 1 | Growth rate of bacteria from gut microbiota | % |
| K | Bacterial carrying capacity in the gastrointestinal tract | bacteria/mL |
Abbreviations: TH, T helper cell; Treg, regulatory T cell.
Finally, the mathematical model proposed for HT disease, which has been built based on the cellular dynamics of the immune system, is represented by the following system of differential equations (equation 1):
Results
The mathematical model proposed in this work combines 3 dynamic and different patterns that are indicators of HT disease and represent 3 states: 1) the proliferation process of TH lymphocytes TH1 and TH17; 2) the alteration of the gut microbiota; and 3) apoptosis of thyroid epithelial cells (thyrocytes). The sensitivity of these parameters was analyzed. Additionally, the importance of their intercommunication in the involved processes was evaluated, showing different simulation scenarios. The sensitivity of the model evidenced the interrelation of the Treg and φ 3 parameters, which represents the balance between Treg lymphocytes and the gut bacteria that stimulate the differentiation process of TH17 lymphocytes (see Fig. 1).
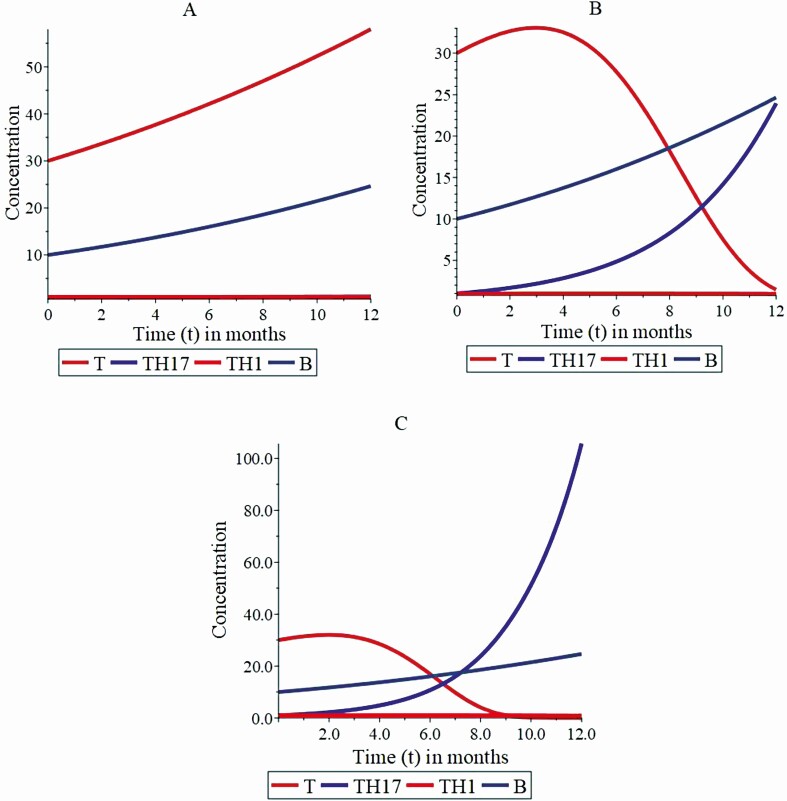
Fig. 2 shows the results of the simulations in different scenarios of the deterministic model, described by the equations system (equation 1). These simulations show the differentiation of thyrocytes over time (measured in months). This variable was analyzed over time during the development of the interaction dynamics between TH1, TH17 lymphocytes, and the gut microbiota. In addition, the dynamic is based on a growth rate of TH lymphocytes in the cellular immune response and on a gut microbiota that exhibits increasing behavior, tending to its carrying capacity K. For all situations, the initial conditions are identical. The initial condition for the concentration of bacteria in the gut microbiota is under the carrying capacity, with a positive growth rate.
Figure 2.
The numerical simulation of the model parameters satisfies the condition of the disease state. The orange line represents the behavior of thyrocytes; the gray line shows the bacterial growth in the gastrointestinal tract; the blue and red lines represent the growth dynamics of TH17 and TH1 lymphocytes, respectively. Graphs A to C illustrate the interaction dynamics of the state variables, where the increase of the balance between Treg lymphocytes and the gut bacteria stimulates a higher differentiation of TH17 lymphocytes. In the different simulation scenarios shown in A to C, it is assumed that α = 0.25, β 1 = 0.05, β = 0.092, γ = 0.05, φ 1 = 0.05, Treg = 0.7, β 2 = 0.05, φ 2 = 0.05, β 3 = 0.05, α 1 = 0.09; and the value of φ 3 in the distinct scenarios A to C is φ 3 = 0.01, φ 3 = 0.2, and φ 3 = 0.3, respectively.
Regarding this result, (Fig. 2A) shows a rapid growth of thyrocytes in the initial 12 months due to an exponential growth for the concentration of thyrocytes in the diseased state. This finding can be explained once the concentration of bacteria slowly tends to stabilize at its carrying capacity. TH1 and TH17 lymphocytes multiply slowly and remain with this growth dynamic over time. For the concentration of bacteria in the gut microbiota, the dynamic assumes as an initial condition. The concentration of bacteria is lower than the carrying capacity. Therefore, as time passes, the concentration of bacteria tends to stabilize at its carrying capacity value. This behavior is observed in all the different simulation scenarios. However, in this situation, this process occurs slowly.
Fig. 2A shows a progressive increase in the bacterial concentration of the gut microbiota and its impact on the cells of the thyroid gland. Over time, this condition could progress to the state of autoimmunity and be associated with an increase in the inflammation rate of the thyrocytes. This rate is represented as γ in the equation 1, which is assumed to have an increase of 5%. Likewise, the relationship between Treg and gut bacteria contributes 1.4% to the differentiation process to TH17 lymphocytes. Considering that a normalized model is proposed, the relationship between Treg lymphocytes and bacteria of the intestinal microbiota is presented in the second term of the second equation, in this case, it is represented as the ratio between φ 3 (maximum contribution rate of bacteria to TH17) and Treg lymphocytes. Furthermore, the construction of the model assumes a constant value for Treg lymphocytes, which implies that the relationship between Treg lymphocytes and gut bacteria varies according to changes in the parameter of maximum contribution rate of bacteria to TH17, resulting in the different simulation scenarios.
Finally, (Fig. 2B and 2C) show that the concentration of TH17 lymphocytes is relatively high, which induces a process of apoptosis in thyrocytes. This finding implies a reduction in the concentration of thyrocytes that tends to reach zero, resulting in the disease state. The dynamics are observed when the relationship between Treg lymphocytes and the bacteria of the intestinal microbiota contributes to the differentiation process of TH17 lymphocytes, reaching values of 28.5% and 42.8%, respectively. These values result from the ratio between φ 3 and Treg lymphocytes, with contributions from the parameter of the maximum contribution rate of bacteria to TH17 lymphocyte reaching a percentage of 20% and 30%, respectively.
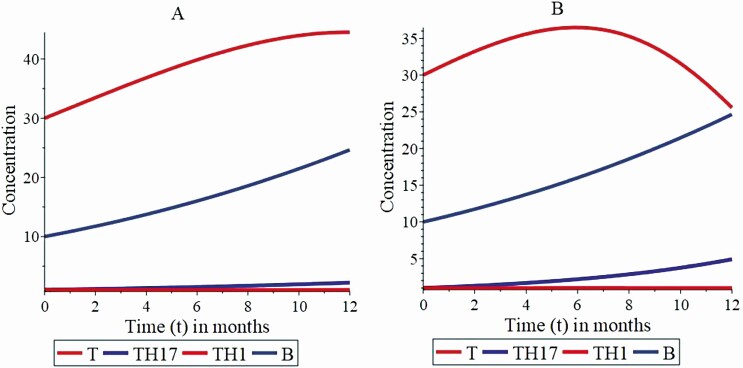
Fig. 3A shows a disease-free condition, where the thyrocytes concentration tends to stabilize after 12 months. The graph shows the dynamics of the model parameters, which satisfies the condition of disease-free stability. Fig. 3A shows the tolerance of the immune response, that is, the induction of immune cells cannot be activated, TH1 (t) and TH17 (t) disappear, and autoimmune disease does not develop. This result is the product of the relationship between Treg lymphocytes and bacteria of the intestinal microbiota that contributes with 7.1%. These values are obtained from the ratio between φ 3 and Treg lymphocytes, with a contribution of the parameter of the maximum contribution rate of bacteria to TH17 lymphocyte of 5%, which can influence the differentiation process to TH17 lymphocytes. Additionally, this result shows an intestinal microbiota in balance with the system.
Figure 3.
The numerical simulation of the model parameters satisfies stability condition of disease free. The orange line represents the behavior of thyrocytes; the gray line shows bacterial growth in the gastrointestinal tract; the blue and red lines show the growth dynamics of TH17 and TH1 lymphocytes, respectively. Graphs A and B illustrate the interaction dynamics of the state variables, where the increase in the balance between Treg lymphocytes and the bacteria of the intestinal microbiota stimulates a higher differentiation of TH17 lymphocytes. In the different simulation scenarios shown in A and B it is assumed that α = 0.25, β 1 = 0.05, β = 0.092, γ = 0.05, φ 1 = 0.05, Treg = 0.7, β 2 = 0.05, φ 2 = 0.05, β 3 = 0.05, α 1 = 0.09; and the value of φ 3 in the distinct scenarios A and B is φ 3 = 0.05 and φ 3 = 0.1, respectively.
Fig. 3B shows a slow increase in the concentration of TH1 lymphocytes. The TH17 lymphocytes show faster growth, an action that reflects the dynamics of TH17 cells. In particular, TH1 lymphocytes multiply slowly and remain in this tendency over time, while the concentration of TH17 lymphocytes increases and subsequently tends to stabilize, until reaching a stable state after 12 months. In this case, the initial condition for the concentration of bacteria in the intestinal microbiota is under the carrying capacity of the medium. Then, the bacterial growth begins to increase slowly at the rate α 1. Likewise, the concentration of bacteria in the intestinal microbiota increases progressively, whereas the concentration of thyrocytes decreases considerably, although the tendency to reach zero is not evident in the initial 12 months. In this simulation, a state of latent autoimmunity is generated, a condition defined by the relationship between Treg lymphocytes and bacteria of the intestinal microbiota, which contributes 14% to the differentiation process of TH17 lymphocytes. In this simulation scenario, the relationship between Treg lymphocytes and gut bacteria of the intestinal microbiota is obtained from the ratio between φ 3 and Treg lymphocytes, where the maximum contribution rate of bacteria to TH17 lymphocyte is around 10%.
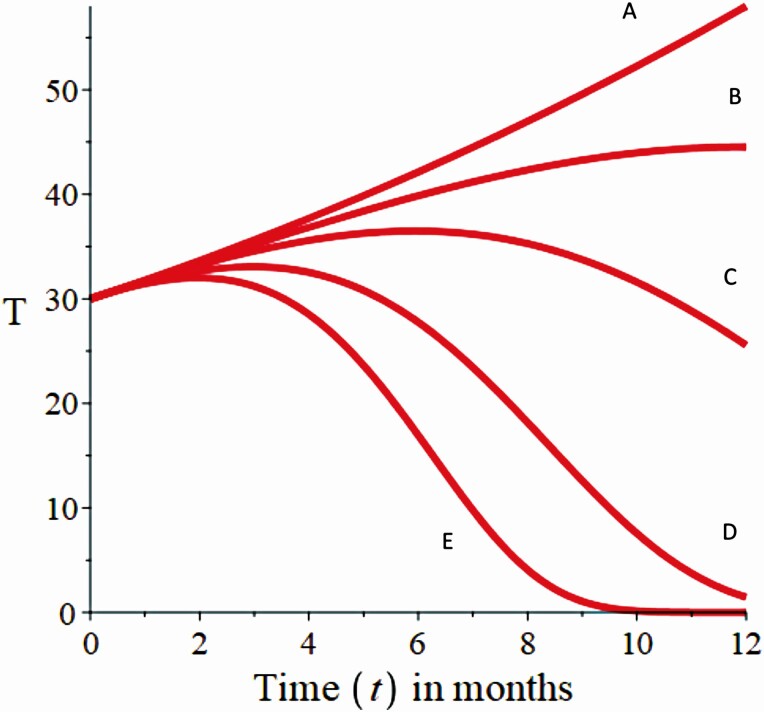
Regarding another aspect, the change in the growth dynamics of thyrocytes is represented in Fig. 4. This change reflects an increase in the balance between Treg lymphocytes and bacteria of the intestinal microbiota, which generates an increase in the contribution rates to the process of differentiation to TH17 lymphocytes. In Fig. 4A with a contribution rate of 1.4%, thyrocytes grow rapidly and uncontrollably, showing a pathological state. With a contribution rate of 7.1% Fig. 4B, the thyrocyte concentration stabilizes at 12 months, showing a state of equilibrium or homeostatic state of the system (healthy state). When a contribution rate of 14.2% (Fig. 4C) is observed, the homeostatic equilibrium begins to get lost, being a warning sign to adopt strategies to stabilize the equilibrium state. For this simulation result, the maximum contribution rate of bacteria to TH17 lymphocyte is represented as φ 3 in equation 1. The values for the Fig. 4A to 4E charts are 1%, 5%, 10%, 20%, and 30%, respectively. These results show a high sensitivity to the parameter φ 3.
Figure 4.
Numerical simulation for the state variable thyrocytes with the parameter values of Table 1, in identical initial condition. The orange line represents the behavior of the thyrocytes in different simulation scenarios. A to E illustrate the behavior of thyrocytes, as the balance between Treg lymphocytes and the bacteria of the intestinal microbiota, which increases their contribution percentage to TH17 lymphocyte differentiation. For all simulation scenarios, A-E assumes that α = 0.25, β 1 = 0.05, β = 0.092, γ = 0.05, φ 1 = 0.05, Treg = 0.7, β 2 = 0.05, φ 2 = 0.05, β 3 = 0.05, α 1 = 0.09; and the value of φ 3 in the different scenarios represented by A-E is φ 3 = 0.01, φ 3 = 0.05, φ 3 = 0.1, φ 3 = 0.2, and φ 3 = 0.3, respectively.
On the contrary, when the contribution rate is 28.5% (Fig. 4D) and 42.8% (Fig. 4E), the thyrocyte concentration rapidly tends to zero, which directly influences the appearance of the pathological state. Three conditions are presented for a disease state, either by increase (see Fig. 4A) or decrease (see Fig. 4D) and (see Fig. 4E) in the concentration of thyrocytes. These conditions show that the homeostatic balance between Tregs lymphocytes and gut bacteria has been lost.
Discussion
The established clinical course of HT can lead to thyrocytes apoptosis (autoimmune destruction of the thyroid gland) through a deterministic model based on an ordinary differential equations system. This model describes the specific dynamics that increase the cellular immune response after an alteration of the gut microbiota occurs. To build this model, clinical variables were employed, such as concentration of TH1, TH17, thyrocytes, and bacterial cells of the microbiota present in the gastrointestinal tract. In HT, the functional size of the thyroid gland was considered a hidden variable that could be obtained through the direct and indirect relationship of the 4 aforementioned variables. Different scenarios were presented for the HT process. First, thyrocyte growth T(t) was altered by the intervention of the cellular immune response, through the participation of TH lymphocytes TH1 and TH17. Both lymphocytes stimulated the process of cellular apoptosis and thyrocyte inflammation, at β and γ rates, respectively. Second, the differentiation and proliferation of TH17 could be influenced by its interaction with Treg lymphocytes and bacteria of the gut microbiota B(t). Furthermore, as a result of the thyrocytes differentiation at φ 1 and φ 2 growth rates, respectively, the model being developed predicted the key assumptions of the cellular immune response in HT condition, which was described by Iwami et al (41) as a classical Holling type II prey-predator functional response system. Finally, the differentiation process of TH17 lymphocytes was strongly related to the enrichment of the intestinal microbiota. Numerical dynamics describe how the balance alteration of the cellular immune system triggers severe lymphocyte proliferation leading to destruction of the thyroid follicles. As a result obtained from this system, it was predicted how the aforementioned processes lead to the development of HT (see equation 1, Fig. 1, and Table 1).
Braley-Mullen and Sharp (44) and Ben-Skowronek et al (45) highlight the importance and contribution of cytotoxic T lymphocytes and Tregs in the cellular immune process of HT. Treg lymphocyte dysfunction causes autoimmune reactions in the thyroid gland (46, 47). Specifically, a decrease in the number of Treg lymphocytes (especially CD25+) induces the development of lymphocytic infiltrations in the thyroid gland, which generates hypothyroidism (48). In patients with HT, numerous fibroblasts and collagen fibers can be present in thyroid tissue (45). This thyroid dysfunction is related to thyrocytes apoptosis that occurs near active TH1 and TH17 lymphocytes (49-51). The TH1/TH17 response type is dominant in patients with HT (52, 53). This dominance corresponds to a hyperfunction of these cells due the hypersecretion of certain proinflammatory cytokines, such as interleukin 17 (IL-17) and IL-17F (53, 54).
The hyperfunction of TH1 and TH17 is observed when both numerical simulations (see Fig. 2) are analyzed. The increase in the proliferation rate of TH1 and TH17 lymphocytes can indirectly alter the apoptosis rate of thyrocytes (see Fig. 2). In fact, the quantitative results indicate those conditions that present a high proliferation rate for TH lymphocytes could contribute to the development of the apoptotic process (see Fig. 2B and 2C) and inflammatory effects (see Fig. 2A) in thyrocytes. Patients with HT show hypothyroidism that is associated with apoptosis of alveolar epithelial cells induced by the activity of some cytokines and the indiscriminate proliferation of TH1 and TH17 lymphocytes (55, 56). Blood cell analyses of HT patients indicate an elevated presence of interferon γ (a type of TH1 cytokine) and IL-17A (a type of TH17 cytokine) (57-59). Various studies show how adhesive molecules, such as IL-8, IP-10/CXCL1, MIG/CXCL9, and MCP-1, that transfer the activation and proliferation signals of the thyrocytes, are part of this migration process of self-reactive lymphocytes toward the thyroid gland (60, 61).
The autoimmune response in the HT cellular microenvironment is a complex and dynamic process that, depending on the context, could present with transient or constant behavior. Recent research highlights the relationship between autoimmune disease onset and gastrointestinal microbiota disorders (62). The gut microbiota is acquired after birth; it is essential for human homeostasis and remains relatively stable during life. The ability of the gut microbiota to modulate the immune system has been widely studied, as has the interaction between bacteria and the gastrointestinal epithelium. This interaction could generate some effects at the cellular level, having a systemic impact on many metabolic processes (63, 64). In this context, the quantitative disorder of the gut microbiota, the appearance of apoptosis, and inflammation in the thyroid gland were simulated and analyzed. In patients with HT, the intestinal microbiota has a special significance due to the metabolic activity of microorganisms (65, 66). Metabolic functions include food processing, digestion and synthetization of vitamin B12 and short-chain fatty acids (SCFAs) (67). SCFAs are a source of energy for epithelial cells (68), which can accelerate colonic transit 2 by stimulating intestinal motility through serotonin (69). In addition, SCFAs can regulate the activity of the sympathetic nervous system at the level of the sympathetic ganglion (70). Among SCFAs, butyrate in particular modulates immunity and exerts an anti-inflammatory effect (71). The dysfunction of the bacterial microbiota was considered as an imbalance of microorganisms’ charge (see Figs. 2 and 3B). In both cases, an altered concentration of the typical bacteria from the intestinal microbiota was observed, as well as a reduction in the growth rate of thyrocyte concentration and an increase in their inflammation rate (see Figs. 2A-2C). The participation of the intestinal microbiota in autoimmune thyroiditis has been determined since 1988 (72). Some studies highlight the presence of an alteration in the intestinal microbiota in HT patients regardless of thyroid functional status (73, 74). In addition, some authors point out that the charge alterations of specific microorganisms decrease the anti-inflammatory activities by reducing the polarization of TH17 lymphocytes and promoting the differentiation of anti-inflammatory Treg lymphocytes at the intestinal level (74, 75). Furthermore, the simulation presented in Fig. 3 demonstrates a successful compensation of resistance to autoimmunity and the failure to trigger HT disease, which shows the conditions for interventions that reverse the established HT. These characteristics are the result of normal and positive feedback (failure) for large alterations (ie, the balance between Treg lymphocytes and gut bacteria). Such characteristics are ubiquitous in dynamic systems and can naturally lead to a threshold that separates 2 coexisting stationary states (bistability): health or disease free (see Fig. 3) and disease. This finding is consistent with published empirical work (4, 17). This autoimmune process would be affected by an increase in the TH17/Treg lymphocyte ratio (76). Both lymphocytic cells perform opposite functions in the progression of autoimmune and inflammatory diseases. Whereas TH17 lymphocytes promote autoimmunity, Treg lymphocytes participate in its control (77). Therefore, this balance plays an important role in the pathogenesis of HT by maintaining self-tolerance and controlling the expansion and activation of a T lymphocytes type: CD4 + effector autoreactive.
The intestinal microbiota has several effects on the gut environment that could influence it, among them, distant organs and metabolism, as well as lead to pathological processes such as various autoimmune diseases (26, 27, 64). Here, we assess the balance between the gut microbiota and Treg lymphocytes in HT, and we observed an HT-associated gastrointestinal microbiota imbalance (Fig. 4). Fig. 4 shows the imbalance between anti-inflammatory and proinflammatory effector cells, in favor of the latter cells, resulting in the breakdown of self-tolerance and the direct attack of T lymphocytes on thyrocytes. In this case, inflammation of the gland can occur, with exposure to antigens, which are normally hidden, leading to the production of specific autoantibodies. The proinflammatory change in the intestinal mucosa called intestinal dysbiosis could lead to an overgrowth of TH17 lymphocytes and thus contribute to autoimmunity and the development of HT (Fig. 4 A). Our simulation found similar results reported by Ishaq et al (73) and Zhao et al (74). In these studies, HT patients were shown to have an altered gut microbiota profile compared to the healthy population (normal plasma levels of free 3,5,3′-triiodothyronine [T3], free thyroxine [T4], and thyrotropin [TSH] without hormonal therapy). Both studies were conducted to establish a relationship between the thyroid autoimmunity of the study groups and their gut microbiota. The patients studied presented ultrastructural morphological changes of the enterocytes of the distal duodenum, a variation in the thickness of the microvilli, a leaky gut condition, and changes in the levels of TSH and T3. The T3 hormone is considered one of the most important regulators of the development and differentiation of the epithelial cells of the intestinal mucosa (78). Clinically, the variation in the blood concentration of thyroid hormones is responsible for gastrointestinal symptoms, and this finding is evidenced by the gastrointestinal alterations frequently documented, both in hypofunction and in hyperfunction of the gland (79, 80). Another factor influencing the microbiota is its effect on neurotransmitters such as dopamine, which can inhibit TSH, indicating that the gut microbiota is closely related to HT (81). When the data show a balance between the concentration of bacteria and Treg lymphocytes, the concentration of thyrocytes stabilizes (see Fig. 3B), which corresponds to a healthy state. The gut microbiota regulates homeostasis and the development of immune cells. In addition, it is capable of enhancing anti-inflammatory activities by reducing the polarization of TH17 and promoting the differentiation of anti-inflammatory Treg lymphocytes at the gut level. Furthermore, it modulates both the innate and adaptive immune systems and plays an important role in the development of tolerance to autoantigens (82). In (see Fig. 2C), the homeostatic equilibrium decreases and, in the presence of rates of contributions of 28.5% (D) and 42.8% (E), the thyrocyte concentration tends toward zero rapidly. This fact is in accordance with the presence of thyrocyte apoptosis (6, 7). A damaged microbiota can negatively influence the immune system and inflammatory regulation–promoting apoptosis of thyrocytes and therefore lead to the development of HT. This is the most common thyroid disorder and is characterized by chronic inflammation, with production of autoantibodies against thyroid peroxidase and thyroglobulin, leading to hypothyroidism and often destruction of the thyroid gland (7, 60). Therefore, there are 3 conditions for a disease state, either by increase (A) or decrease (D) and (E) in the concentration of thyrocytes.
Together, the mathematical model and the simulations developed here simulate how the imbalance between anti-inflammatory and proinflammatory effector cells results in the self-tolerance breakdown and the direct attack of T lymphocytes on thyrocyte cells. Consequently, this alteration can produce inflammation of the glands or tissue atrophy, scenarios that have been simulated in this research. Furthermore, the homeostasis of the proinflammatory TH17 and Treg lymphocytes is closely related to the bacterial balance of the gut microbiota. An imbalance among these 3 factors could trigger inflammation and thyroid tissue damage.
Conclusion
This study predicted the process of the cellular immune response for HT through an ordinary system of differential equations. Different simulation scenarios were generated under an alteration behavior of the bacterial logistic, with a carrying capacity of the medium K and an α 1 growth rate. This work presents a dynamic simulation that described the interactions between various cell populations involved in HT disease and a compartmental model of the cellular immune response within the thyroid gland. Numerical simulations illustrate several typical courses of interactions when the immune system malfunctions and destroys healthy cells, such as thyrocytes. In these proposed scenarios, it was observed that TH1 and TH17 lymphocytes could increase the activity of immune cells, activate effector cells directly, and trigger apoptosis of healthy thyrocytes indirectly. Likewise, the model shows the increase in the activity of TH17 lymphocytes when there is an imbalance of the intestinal microbiota. Finally, the numerical results highlight the activity of TH1, TH17, and the bacterial balance of the gut microbiota as important factors for the development of HT disease (see Fig. 4). Mathematical models are useful for multiple purposes: to screen individuals at high risk for autoimmune diseases, to predict future disease events, and to aid in medical decisions.
Acknowledgments
J.G.V.S. thanks the “UCM Doctoral Scholarship,” awarded in 2018 by the Vice-Rectory for Research and Postgraduate of the Universidad Católica Del Maule, Talca, Chile.
Financial Support: The authors received no financial support for this research.
Glossary
Abbreviations
- HT
Hashimoto autoimmune thyroiditis
- IL
interleukin
- SCFA
short-chain fatty acid
- T3
3,5,3′-triiodothyronine
- T4
thyroxine
- TH
T helper cell
- Treg
regulatory T cell
- TSH
thyrotropin.
Additional Information
Disclosures : The authors have nothing to disclose.
Data Availability
Data sharing is not applicable to this article because no data sets were generated or analyzed during the present study.
References
- 1. Arthur JR, Beckett GJ. Thyroid function. Br Med Bull. 1999;55(3):658-668. [DOI] [PubMed] [Google Scholar]
- 2. Angell TE, Huang SA, Alexander EK. The thyroid. In: Belfiore A., LeRoith D, eds. Principles of Endocrinology and Hormone Action. Endocrinology. Springer; 2018:353-366. 10.1007/978-3-319-44675-2_14 [DOI] [Google Scholar]
- 3. Gaitan E, Nelson NC, Poole GV. Endemic goiter and endemic thyroid disorders. World J Surg. 1991;15(2):205-215. [DOI] [PubMed] [Google Scholar]
- 4. Wiersinga WM. Hashimoto’s thyroiditis. In: Vitti P, Hegedüs L, eds. Thyroid Diseases. Endocrinology. Springer; 205-247. ISBN 978-3-319-45013-1. 10.1007/978-3-319-45013-1_7 [DOI] [Google Scholar]
- 5. Mincer DL, Jialal I. Hashimoto thyroiditis. In: StatPearls. StatPearls Publishing; 2020. [PubMed] [Google Scholar]
- 6. Lumachi F, Basso S. Apoptosis: life through planned cellular death regulating mechanisms, control systems, and relations with thyroid diseases. Thyroid. 2002;12(1):27-34. [DOI] [PubMed] [Google Scholar]
- 7. Yu X, Li L, Li Q, Zang X, Liu Z. TRAIL and DR5 promote thyroid follicular cell apoptosis in iodine excess-induced experimental autoimmune thyroiditis in NOD mice. Biol Trace Elem Res. 2011;143(2):1064-1076. [DOI] [PubMed] [Google Scholar]
- 8. Weetman A. Autoimmune thyroid disease. Endocrine. 2020;68(2):258-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Weetman AP. Non-thyroid autoantibodies in autoimmune thyroid disease. Best Pract Res Clin Endocrinol Metab. 2005;19(1):17-32. [DOI] [PubMed] [Google Scholar]
- 10. Weetman AP. The genetics of autoimmune thyroid disease. Horm Metab Res. 2009;41(6):421-425. [DOI] [PubMed] [Google Scholar]
- 11. Weetman AP. Thyroid disease in pregnancy in 2011: thyroid function—effects on mother and baby unraveled. Nat Rev Endocrinol. 2011;8(2):69-70. [DOI] [PubMed] [Google Scholar]
- 12. Effraimidis G, Wiersinga WM. Mechanisms in endocrinology: autoimmune thyroid disease: old and new players. Eur J Endocrinol. 2014;170(6):R241-R252. [DOI] [PubMed] [Google Scholar]
- 13. Giordano C, Stassi G, De Maria R, et al. Potential involvement of Fas and its ligand in the pathogenesis of Hashimoto’s thyroiditis. Science. 1997;275(5302):960-963. [DOI] [PubMed] [Google Scholar]
- 14. Weetman AP. Cellular immune responses in autoimmune thyroid disease. Clin Endocrinol. 2004;61(4):405-413. [DOI] [PubMed] [Google Scholar]
- 15. Ramos-Leví AM, Marazuela M. Pathogenesis of thyroid autoimmune disease: the role of cellular mechanisms. Endocrinol Nutr. 2016;63(8):421-429. [DOI] [PubMed] [Google Scholar]
- 16. Li Q, Wang B, Mu K, Zhang JA. The pathogenesis of thyroid autoimmune diseases: new T lymphocytes—cytokines circuits beyond the Th1-Th2 paradigm. J Cell Physiol. 2019;234(3):2204-2216. [DOI] [PubMed] [Google Scholar]
- 17. Zaķe T, Skuja S, Lejnieks A, Groma V, Konrāde I. Immunological mechanisms of autoimmune thyroid diseases: a shift in the traditional TH1/TH2 paradigm. Proc Latv Acad Sci B Nat Exact Appl Sci. 2019;73(2):67-77. [Google Scholar]
- 18. Ghoreschi K, Laurence A, Yang XP, et al. Generation of pathogenic T(H)17 cells in the absence of TGF-β signalling. Nature. 2010;467(7318):967-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shi Y, Wang H, Su Z, et al. Differentiation imbalance of Th1/Th17 in peripheral blood mononuclear cells might contribute to pathogenesis of Hashimoto’s thyroiditis. Scand J Immunol. 2010;72(3):250-255. [DOI] [PubMed] [Google Scholar]
- 20. Li D, Cai W, Gu R, et al. Th17 cell plays a role in the pathogenesis of Hashimoto’s thyroiditis in patients. Clin Immunol. 2013;149(3):411-420. [DOI] [PubMed] [Google Scholar]
- 21. Yasuda K, Takeuchi Y, Hirota K. The pathogenicity of Th17 cells in autoimmune diseases. Semin Immunopathol. 2019;41(3):283-297. [DOI] [PubMed] [Google Scholar]
- 22. Papp G, Boros P, Nakken B, Szodoray P, Zeher M. Regulatory immune cells and functions in autoimmunity and transplantation immunology. Autoimmun Rev. 2017;16(5):435-444. [DOI] [PubMed] [Google Scholar]
- 23. Maddur MS, Miossec P, Kaveri SV, Bayry J. Th17 cells: biology, pathogenesis of autoimmune and inflammatory diseases, and therapeutic strategies. Am J Pathol. 2012;181(1):8-18. [DOI] [PubMed] [Google Scholar]
- 24. Shao S, Yu X, Shen L. Autoimmune thyroid diseases and Th17/Treg lymphocytes. Life Sci. 2018;192:160-165. [DOI] [PubMed] [Google Scholar]
- 25. Ivanov II, de Llanos Frutos R, Manel N, et al. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe. 2008;4(4):337-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Maynard CL, Elson CO, Hatton RD, Weaver CT. Reciprocal interactions of the intestinal microbiota and immune system. Nature. 2012;489(7415):231-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Honda K, Littman DR. The microbiota in adaptive immune homeostasis and disease. Nature. 2016;535(7610):75-84. [DOI] [PubMed] [Google Scholar]
- 28. Shaw MH, Kamada N, Kim YG, Núñez G. Microbiota-induced IL-1β, but not IL-6, is critical for the development of steady-state TH17 cells in the intestine. J Exp Med. 2012;209(2):251-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sano T, Huang W, Hall JA, et al. An IL-23R/IL-22 circuit regulates epithelial serum amyloid A to promote local effector Th17 responses [published correction appears in Cell. 2016;164(1-2):324. dosage error in article text]. Cell. 2015;163(2):381-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Omenetti S, Pizarro TT. The Treg/Th17 Axis: a dynamic balance regulated by the gut microbiome. Front Immunol. 2015;6:639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sano T, Kageyama T, Fang V, et al. Redundant cytokine requirement for intestinal microbiota-induced Th17 cell differentiation in draining lymph nodes. Cell Rep. 2021;36(8):109608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Buc M. Role of regulatory T cells in pathogenesis and biological therapy of multiple sclerosis. Mediators Inflamm. 2013;2013:963748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Flores-Borja F, Mauri C, Ehrenstein MR. Restoring the balance: harnessing regulatory T cells for therapy in rheumatoid arthritis. Eur J Immunol. 2008;38(4):934-937. [DOI] [PubMed] [Google Scholar]
- 34. Smilek DE, Ehlers MR, Nepom GT. Restoring the balance: immunotherapeutic combinations for autoimmune disease. Dis Model Mech. 2014;7(5):503-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jones DS, Plank M, Sleeman BD.. Differential Equations and Mathematical Biology. 2nd ed. Chapman and Hall/CRC; 2009. [Google Scholar]
- 36. Ogunlaran OM, Oukouomi Noutchie SC. Mathematical model for an effective management of HIV infection. Biomed Res Int. 2016;2016:4217548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Elettreby MF, Ahmed E, Safan M. A simple mathematical model for Guillain-Barré syndrome. Adv Differ Equ. 2019;208(2019). doi:10.1186/s13662-019-2146-9 [Google Scholar]
- 38. Shtylla B, Gee M, Do A, Shabahang S, Eldevik L, de Pillis L. A mathematical model for DC vaccine treatment of type I diabetes. Front Physiol. 2019;10:1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hara A, Iwasa Y. Autoimmune diseases initiated by pathogen infection: mathematical modeling. J Theor Biol. 2020;498:110296. [DOI] [PubMed] [Google Scholar]
- 40. Makhlouf AM, El-Shennawy L, Elkaranshawy HA. Mathematical modelling for the role of CD4+T cells in tumor-immune interactions. Comput Math Methods Med. 2020;2020:7187602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Iwami S, Takeuchi Y, Iwamoto K, Naruo Y, Yasukawa M. A mathematical design of vector vaccine against autoimmune disease. J Theor Biol. 2009;256(3):382-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vergaño-Salazar JG, Pastenes L, Córdova-Lepe F, Mardones-Precth P. Impulsive simulation model for the analysis of allergy dynamics. J Phys Conf Ser. 2020;1702(1):012009. [Google Scholar]
- 43. Macfarlane FR, Chaplain MAJ, Eftimie R. Quantitative predictive modelling approaches to understanding rheumatoid arthritis: a brief review. Cells. 2019;9(1):74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Braley-Mullen H, Sharp GC. Adoptive transfer murine model of granulomatous experimental autoimmune thyroiditis. Int Rev Immunol. 2000;19(6):535-555. [DOI] [PubMed] [Google Scholar]
- 45. Ben-Skowronek I, Szewczyk L, Ciechanek R, Korobowicz E. Interactions of lymphocytes, thyrocytes and fibroblasts in Hashimoto’s thyroiditis: an immunohistochemical and ultrastructural study. Horm Res Paediatr. 2011;76(5):335-342. [DOI] [PubMed] [Google Scholar]
- 46. Itoh M, Takahashi T, Sakaguchi N, et al. Thymus and autoimmunity: production of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J Immunol. 1999;162(9):5317-5326. [PubMed] [Google Scholar]
- 47. Kristensen B, Hegedüs L, Lundy SK, Brimnes MK, Smith TJ, Nielsen CH. Characterization of regulatory B cells in Graves’ disease and Hashimoto’s thyroiditis. PLoS One. 2015;10(5):e0127949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. McLachlan SM, Nagayama Y, Pichurin PN, et al. The link between Graves’ disease and Hashimoto’s thyroiditis: a role for regulatory T cells. Endocrinology. 2007;148(12):5724-5733. [DOI] [PubMed] [Google Scholar]
- 49. Kotani T, Aratake Y, Hirai K, Fukazawa Y, Sato H, Ohtaki S. Apoptosis in thyroid tissue from patients with Hashimoto’s thyroiditis. Autoimmunity. 1995;20(4):231-236. [DOI] [PubMed] [Google Scholar]
- 50. Stassi G, De Maria R. Autoimmune thyroid disease: new models of cell death in autoimmunity. Nat Rev Immunol. 2002;2(3):195-204. [DOI] [PubMed] [Google Scholar]
- 51. Guo Y, Zynat J, Xing S, et al. Immunological changes of T helper cells in flow cytometer-sorted CD4+ T cells from patients with Hashimoto’s thyroiditis. Exp Ther Med. 2018;15(4):3596-3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mazziotti G, Sorvillo F, Naclerio C, et al. Type-1 response in peripheral CD4+ and CD8+ T cells from patients with Hashimoto’s thyroiditis. Eur J Endocrinol. 2003;148(4):383-388. [DOI] [PubMed] [Google Scholar]
- 53. Figueroa-Vega N, Alfonso-Pérez M, Benedicto I, Sánchez-Madrid F, González-Amaro R, Marazuela M. Increased circulating pro-inflammatory cytokines and Th17 lymphocytes in Hashimoto’s thyroiditis. J Clin Endocrinol Metab. 2010;95(2):953-962. [DOI] [PubMed] [Google Scholar]
- 54. Martinez GJ, Nurieva RI, Yang XO, Dong C. Regulation and function of proinflammatory TH17 cells. Ann N Y Acad Sci. 2008;1143:188-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Akamizu T, Amino N, De Groot LJ. Thyroid manager, chapter 8: Hashimoto’s Thyroiditis. Updated August 2008. www.Thyroidmanager.org/Chapter8/chapter8.pdf. Accessed February 09, 2021.
- 56. Qin Q, Liu P, Liu L, et al. The increased but non-predominant expression of Th17- and Th1-specific cytokines in Hashimoto’s thyroiditis but not in Graves’ disease. Braz J Med Biol Res. 2012;45(12):1202-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Weetman AP, Bright-Thomas R, Freeman M. Regulation of interleukin-6 release by human thyrocytes. J Endocrinol. 1990;127(2):357-361. [DOI] [PubMed] [Google Scholar]
- 58. Ajjan RA, Watson PF, Weetman AP. Cytokines and thyroid function. Adv Neuroimmunol. 1996;6(4):359-386. [DOI] [PubMed] [Google Scholar]
- 59. Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75(2):163-189. [DOI] [PubMed] [Google Scholar]
- 60. Gianoukakis AG, Cao HJ, Jennings TA, Smith TJ. Prostaglandin endoperoxide H synthase expression in human thyroid epithelial cells. Am J Physiol Cell Physiol. 2001;280(3):C701-C708. [DOI] [PubMed] [Google Scholar]
- 61. Gianoukakis AG, Martino LJ, Horst N, Cruikshank WW, Smith TJ. Cytokine-induced lymphocyte chemoattraction from cultured human thyrocytes: evidence for interleukin-16 and regulated upon activation, normal T cell expressed, and secreted expression. Endocrinology. 2003;144(7):2856-2864. [DOI] [PubMed] [Google Scholar]
- 62. Severance EG, Yolken RH, Eaton WW. Autoimmune diseases, gastrointestinal disorders and the microbiome in schizophrenia: more than a gut feeling. Schizophr Res. 2016;176(1):23-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wu HJ, Wu E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes. 2012;3(1):4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sprouse ML, Bates NA, Felix KM, Wu HJ. Impact of gut microbiota on gut-distal autoimmunity: a focus on T cells. Immunology. 2019;156(4):305-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Köhling HL, Plummer SF, Marchesi JR, Davidge KS, Ludgate M. The microbiota and autoimmunity: Their role in thyroid autoimmune diseases. Clin Immunol. 2017;183:63-74. [DOI] [PubMed] [Google Scholar]
- 66. Virili C, Fallahi P, Antonelli A, Benvenga S, Centanni M. Gut microbiota and Hashimoto’s thyroiditis. Rev Endocr Metab Disord. 2018;19(4):293-300. [DOI] [PubMed] [Google Scholar]
- 67. Tremaroli V, Bäckhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489(7415):242-249. [DOI] [PubMed] [Google Scholar]
- 68. Topping DL, Clifton PM. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol Rev. 2001;81(3):1031-1064. [DOI] [PubMed] [Google Scholar]
- 69. Grider JR, Piland BE. The peristaltic reflex induced by short-chain fatty acids is mediated by sequential release of 5-HT and neuronal CGRP but not BDNF. Am J Physiol Gastrointest Liver Physiol. 2007;292(1):G429-G437. [DOI] [PubMed] [Google Scholar]
- 70. Kimura I, Inoue D, Maeda T, et al. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc Natl Acad Sci U S A. 2011;108(19):8030-8035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Davie JR. Inhibition of histone deacetylase activity by butyrate. J Nutr. 2003;133(7 Suppl):2485S-2493S. [DOI] [PubMed] [Google Scholar]
- 72. Penhale WJ, Young PR. The influence of the normal microbial flora on the susceptibility of rats to experimental autoimmune thyroiditis. Clin Exp Immunol. 1988;72(2):288-292. [PMC free article] [PubMed] [Google Scholar]
- 73. Ishaq HM, Mohammad IS, Guo H, et al. Molecular estimation of alteration in intestinal microbial composition in Hashimoto’s thyroiditis patients. Biomed Pharmacother. 2017;95:865-874. [DOI] [PubMed] [Google Scholar]
- 74. Zhao F, Feng J, Li J, et al. Alterations of the gut microbiota in Hashimoto’s Thyroiditis patients. Thyroid. 2018;28(2):175-186. [DOI] [PubMed] [Google Scholar]
- 75. Cosorich I, Dalla-Costa G, Sorini C, et al. High frequency of intestinal TH17 cells correlates with microbiota alterations and disease activity in multiple sclerosis. Sci Adv. 2017;3(7):e1700492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Li C, Yuan J, Zhu YF, et al. Imbalance of Th17/Treg in different subtypes of autoimmune thyroid diseases. Cell Physiol Biochem. 2016;40(1-2):245-252. [DOI] [PubMed] [Google Scholar]
- 77. Noack M, Miossec P. Th17 and regulatory T cell balance in autoimmune and inflammatory diseases. Autoimmun Rev. 2014;13(6):668-677. [DOI] [PubMed] [Google Scholar]
- 78. Meng S, Badrinarain J, Sibley E, Fang R, Hodin R. Thyroid hormone and the D-type cyclins interact in regulating enterocyte gene transcription. J Gastrointest Surg. 2001;5(1):49-55. [DOI] [PubMed] [Google Scholar]
- 79. Wegener M, Wedmann B, Langhoff T, Schaffstein J, Adamek R. Effect of hyperthyroidism on the transit of a caloric solid-liquid meal through the stomach, the small intestine, and the colon in man. J Clin Endocrinol Metab. 1992;75(3):745-749. [DOI] [PubMed] [Google Scholar]
- 80. Devdhar M, Ousman YH, Burman KD. Hypothyroidism. Endocrinol Metab Clin North Am. 2007;36(3):595-615, v. [DOI] [PubMed] [Google Scholar]
- 81. Sasso FC, Carbonara O, Torella R, et al. Ultrastructural changes in enterocytes in subjects with Hashimoto’s thyroiditis. Gut. 2004;53(12):1878-1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. de Oliveira GLV, Leite AZ, Higuchi BS, Gonzaga MI, Mariano VS. Intestinal dysbiosis and probiotic applications in autoimmune diseases. Immunology. 2017;152(1):1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article because no data sets were generated or analyzed during the present study.