Abstract
Multiple myeloma (MM) is a common malignant tumor of plasma cells. Despite several treatment approaches in the past two decades, MM remains an aggressive and incurable disease in dire need of new treatment strategies. Approximately 70–80% of patients with MM have myeloma bone disease (MBD), often accompanied by pathological fractures and hypercalcemia, which seriously affect the prognosis of the patients. Calcium channels and transporters can mediate Ca2+ balance inside and outside of the membrane, indicating that they may be closely related to the prognosis of MM. Therefore, this review focuses on the roles of some critical calcium channels and transporters in MM prognosis, which located in the plasma membrane, endoplasmic reticulum and mitochondria. The goal of this review is to facilitate the identification of new targets for the treatment and prognosis of MM.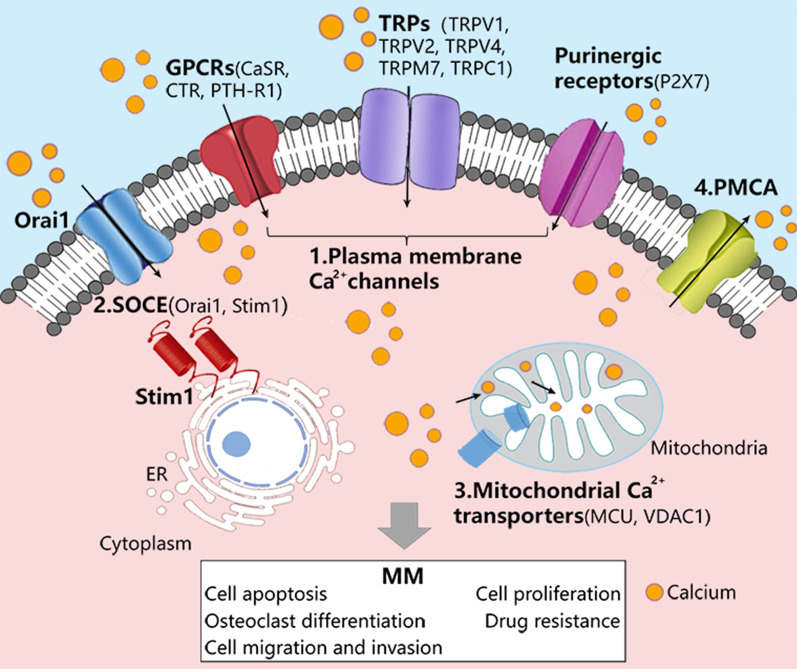
Video Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s12964-021-00781-4.
Keywords: Plasma membrane Ca2+ transporters, Store-operated Ca2+ entry, Mitochondrial Ca2+ transporters, Myeloma bone disease, Prognosis
Introduction
Multiple myeloma (MM), a malignant tumor with abnormal accumulation of terminally differentiated plasma cells, is the second most common hematologic cancer after lymphoma [1, 2]. Most patients with MM will have varying degrees of bone destruction [3], renal insufficiency and hypercalcemia [4], which is closely related to calcium regulation. Studies have confirmed that serum calcium level reflects the ability of bone resorption and bone formation [5]. Hypercalcemia is an adverse prognostic factor in MM [6], and it is most common in those myeloma patients who have the greatest tumor volume or patients with plasma cell leukemia (a late stage complication of myeloma) [7]. The reasons for this are still unclear, but they may be related to bone resorption activity produced by myeloma cells and glomerular filtration status. Because myeloma patients often have irreversible kidney damage and increased renal tubular calcium reabsorption, resulting in elevated serum calcium concentration and abnormal bone remodeling [7, 8]. In addition, previous studies have reported that the interaction between MM cells and osteoclasts accelerates bone destruction and bone remodeling in myeloma, which leads to an increase in calcium concentration in serum and bone marrow [7, 9]. Therefore, MM cells may be exposed to a high concentration of extracellular calcium in the bone marrow microenvironment, but so far, the relevant calcium transporters/channels and mechanisms of calcium regulation in MM are still unclear.
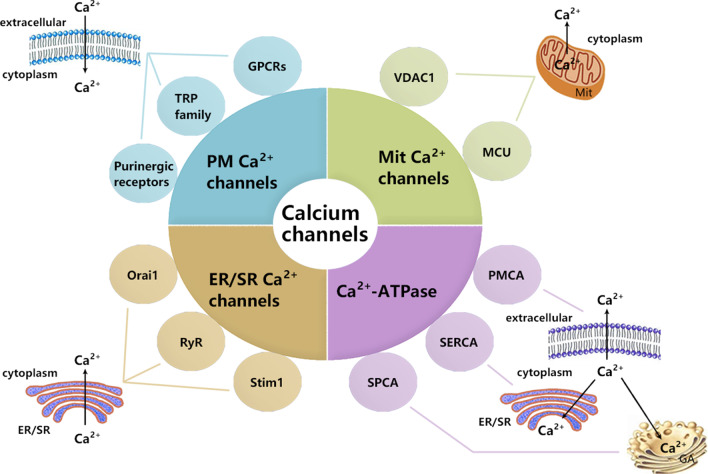
Calcium channels and transporters are protein structures that exist in cell membranes, endoplasmic reticulum membranes, and mitochondrial membranes. They can mediate Ca2+ balance inside and outside of the membrane, thus maintaining the physiological functions of the organism. The network control of calcium channels and transporters which regulate intracellular Ca2+ homeostasis includes: (1) calcium channels or transporters that allow Ca2+ influx from extracellular Ca2+ storage across the plasma membrane (PM), such as transient receptor potential channel (TRP), G Protein-Coupled Receptors (GPCRs) and purinergic receptor (ATP-gated cation channel P2X7 receptor); (2) Inositol 1,4,5-triphate receptor (IP3R) or ryanodine receptor (RyR) and stromal-interaction molecule1 (Stim1) combined with the plasma membrane calcium channel protein Orai1 (also known as CRACM1) to mediate Ca2+ release from the endoplasmic/sarcoplasmic reticulum (ER/SR); (3) Mitochondrial Voltage-Dependent Anion Channel 1 (VDAC1) and Ca2+ uniporter (MCU) regulate mitochondrial Ca2+ uptakes; (4) Ca2+-ATPase pumps Ca2+ from cytoplasm back to ER/SR or extracellular space [10, 11] (Fig. 1). Most features of cancer, if not all, involve calcium signaling to mediate critical cellular processes, including transcriptional regulation, which underlies the gene expression in a variety of pathways essential for tumorigenesis and metastasis [12]. So far, various studies have shown that changes in calcium channels and transporters are related to the proliferation, apoptosis, osteoclast differentiation and outcome of MM. For instance, the knockdown of the non-selective cation channel TRP proteins, known as a type of calcium channel on the cell membrane, was shown to result in the differentiation of osteoclast in MM [13, 14]. Purinergic receptor P2X7 activation induces cell death in human RPMI 8226 multiple myeloma cells [15]. Up-regulation of Stim1 or Orai1 [two critical regulators of Store-Operated Ca2+ Entry (SOCE)] was associated with the clinical outcome of MM, and Stim1 or Orai1 down-regulation reduce cell viability, cause cell apoptosis and cell cycle arrest in MM cell lines [16]. In human CD45+ U266 myeloma cells, VDAC1 might sensitize to many extracellular stimuli that trigger apoptosis via the mitochondrial pathway [17].
Fig. 1.
The composition of calcium channels and transporters. PM: plasmic membrane; Mit: mitochondrial; ER/SR: endoplasmic/sarcoplasmic reticulum; GA: Golgi apparatus; TRP: transient receptor potential channel; GPCRs: G protein-coupled receptors; Stim1: Stromal-interaction molecule1; RyR: ryanodine receptor; VDAC1: Voltage-Dependent Anion Channel 1; MCU: Mitochondrial Ca2+ uniporter; PMCA: Plasma membrane Ca2+-ATPase; SERCA: endoplasmic/sarcoplasmic reticulum Ca2+-ATPase; SPCA: secretory pathway Ca2+-ATPase
Therefore, in this review, we summarize the expression, localization and pathophysiological role of some essential calcium channels and transporters, including the transient receptor potential channel (TRP) family, GPCR family, Purinergic receptors, SOCE channels and Mitochondrial Ca2+ transporters, which have been reported to be altered in MM (Fig. 2, Table 1).
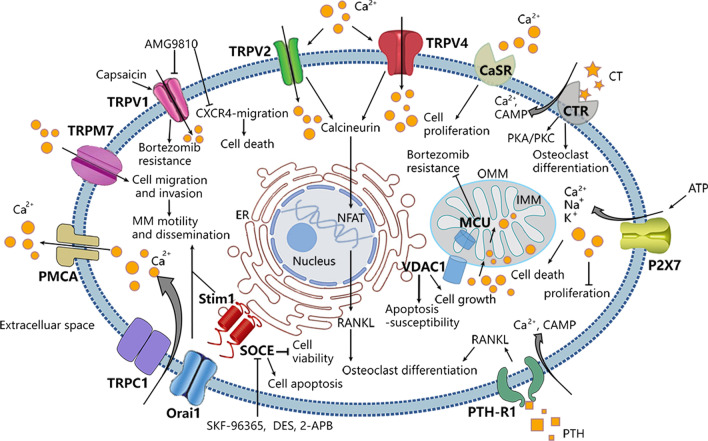
Fig. 2.
Important Ca2+ channels/transporters in multiple myeloma cells. ER: Endoplasmic reticulum; OMM: Outernal mitochondrial membranes; IMM: internal mitochondrial membranes. The intracellular Ca2+ is governed by a series of proteins: (1) plasma membrane Ca2+ channels or transporters, such as TRPs (TRPV1, TRPV2, TRPV4, TRPM7), G Protein-Coupled Receptors (CaSR, CTR, PTH-R1), Purinergic receptors (P2X7), which mediate Ca2+ influx into cells. (2) Store-Operated Ca2+ Entry, as one of the major pathways for Ca2+ influx across plasma membrane. (3) Mitochondrial Ca2+ transporters, including VDAC1 and MCU, mediate Ca2+ transport across internal and outernal mitochondrial membranes. (4) Ca2+-ATPases pumping Ca2+ from cytosol to extracellular space. Ca2+ can regulate various cellular events, including gene transcription, proliferation, migration and apoptosis. During development of multiple myeloma, the alteration of Ca2+ channels/transporters lead to changes in Ca2+ permeability and distribution inside and outside the cell membrane as well as activation of various signaling pathways, providing a suitable microenvironment for the growth of tumor cells. Targeting the dysregulated Ca2+ channels/transporters may improve the prognosis of patients with MM
Table 1.
Expression, localization and phthophysiological function of calcium channels and transporters in MM
| Name | Related channels/transporters | Main localization | Compound | Mechanism | Pathophysiological role in MM | References |
|---|---|---|---|---|---|---|
| TRPVs | TRPV1 | Plasma membrane | Capsaicin | Activator | TRPV1 inhibitor has synergistic anti-MM activity with bortezomib | [47] |
| TRPV2 | Plasma membrane | SKF96365 | Inhibitor | TRPV2 promotes osteoclast differentiation | [13] | |
| TRPV4 | Plasma membrane | – | – | TRPV4 activation promotes osteoclast differentiation and bone resorption | [60] | |
| TRPMs | TRPM7 | Plasma membrane | – | – | TRPM7 regulates MM cell motility and dissemination | [68] |
| GPCRs | CaSR | Plasma membrane |
CaCl2 Gadolinium Neomycin |
Activator Activator Activator |
CaSR promotes the mitosis of MM cells | [57] |
| CTR | Plasma membrane | Calcitonin | Activator | CTR inhibits bone resorption by neutralizing OC migration and shape retraction, and may participate in the osteoclast differentiation of MM |
[85] [89] |
|
| PTH-R1 | Plasma membrane | PTHrP | Activator | PTHrP stimulates the secretion of PTH-R1, promotes proliferation of MM cells and the production of osteoclastogenesis factors |
[90] [92] |
|
| Purinergic receptors | P2X7 | Plasma membrane | ATP | Activator | Activation of P2X7 may induce the apoptosis and prevent the proliferation of MM cells | [15] |
| SOCE | Stim1 | Plasma membrane, |
SKF-96365 DES 2-APB |
Inhibitor Inhibitor Inhibitor |
Silencing Stim1 reduces cell viability, leading to apoptosis and cell cycle arrest of MM cells, and the high expression of Stim1 affects the clinical outcome of MM. In addition, Stim1 could regulate the motility and dissemination of diffuse large B-cell lymphoma (DLBCL) cells and MM cells |
[16] [68] [126] |
| Endoplasmic reticulum | ||||||
| Orai1 | Plasma membrane, |
SKF-96365 DES 2-APB |
Inhibitor Inhibitor Inhibitor |
Silencing Orai1 reduces cell viability, leading to apoptosis and cell cycle arrest of MM cells. And Orai1 regulates the motility and dissemination of diffuse large B-cell lymphoma (DLBCL) cells and MM cells |
[16] [68] [111] [126] |
|
| Cytosol | AnCoA4 | |||||
| TRPC1 | Plasma membrane | – | – | Knockout of TRPC1 inhibits the death of MM cells | [132] | |
| Mitochondrial Ca2+ transporters | VDAC1 |
Mitochondrion, Nucleus |
– | – | VDAC1 promotes the growth of MM cells, accelerates the development of MM, and affects the prognosis of patients |
[17] [144] |
| MCU | Mitochondrion | Ruthenium red | Inhibitor | MCU can reduce MM bortezomib resistance and promote MM cell apoptosis |
[151] [152] |
Plasma membrane Ca2+ channels
TRP channels
The TRP superfamily channels consist of many non-selective cation channels, including more than 30 members and can be further divided into seven subgroups, i.e. TRPV (vanilloid), TRPC (canonical), TRPM (melastatin), TRPML (mucolipin), TRPP (polycystic), TRPN (no mechanoreceptor potential C), and TRPA (ankyrin) [18]. Each subgroup contains several channel subtypes, which have different Ca2+ selectivity, activation mechanisms and interacting proteins [19]. These channels consist of six transmembrane domain segments (S1–S6) and intracellular carboxy (C-) and amino (N-) termini in the pore region between S5 and S6 [20]. TRP family is one of the leading calcium channels [21], which regulates intracellular Ca2+ concentration and plays a vital role in various physiological functions, including mediating pain transmission, bone metabolism, and tumor occurrence and metastasis [22, 23]. For example, the TRPV family is related to the onset and progression of MM and chronic myeloid leukemia [24]. The TRPM family, especially TRPM7, has been shown to regulate B cell development and antigen recognition [25]. The TRPC family is involved in the occurrence and development of acute T-cell leukemia tumors [26]. Next, we aim to outline the role of TRPV and TRPM ion channels in MM, and the role of TRPC in MM will be introduced in the part of endoplasmic reticulum SOCE.
TRPV channel structures have six highly conserved transmembrane domain architecture. The functional TRPV channels are tetrameric assemblies that surround a central permeation pathway [27]. The six members of TRPV family can be further divided into three distinct groups based on functional characteristics: fairly non-selective cation channels TRPV1-3 [28], cation-selective efflux channel TRPV4 [29], and epithelial channels TRPV5 and TRPV6 [30, 31]. In TRPV family, studies have reported the importance of TRPV1, TRPV2, and TRPV4 in MM.
TRPM family is the largest and most diverse subfamily in the TRP superfamily [32], composed of eight members, TRPM1 to TRPM8. The TRPM channels have a large cytosolic domain with 732–1611 amino acids for per subunit [33]. Most of the TRPM channels are non-selective Ca2+ permeable cation channels; only TRPM4 and TRPM5 are impermeable to Ca2+[34, 35]. In this part, we mainly discuss the role of TRPM7 in MM tumorigenesis.
TRPV1 channel
TRPV1 is the first member of the TRPV family, which respond to low pH value (< 5), capsaicin noxious heat (> 43 °C) and so on [36, 37]. TRPV1 was initially discovered in rat dorsal root ganglion [38], and involved in the regulation of calcium signaling, inflammation and metabolism, and was closely related to cancer [39, 40]. The functional expression of TRPV1 has been confirmed in several human malignancies including breast, prostate, urothelial cancer and glioma [41–44], yet remained largely unknown in hematologic malignancies especially in MM. At present, drug resistance remains a major challenge for MM cure and calcium signaling was proposed to play a role in drug resistance of cancer cells [45]. TRPV1 has been recognized as an important regulator of intracellular calcium levels, and it was found to be highly expressed in MM cell lines (RPMI8226, INA6 and MM.1S) in a chip analysis of GSE5900 and GSE2658. Recently, a study proposed the role of TRPV1 in MM tumor progression and bortezomib resistance. It is known that induction of endoplasmic reticulum (ER) stress is one of the main mechanisms of bortezomib-mediated cell death. In response to ER stress conditions, the unfolded protein response (UPR) signaling cascade is activated to counteract the occurring damage [46]. Beider et al. found that TRPV1 inhibition (using a pharmacological inhibitor AMG9810) resulted in calcium-dependent accumulation of mitochondrial reactive oxygen species (ROS), followed by mitochondrial instability and MM cell death [47]. These results are consistent with previous findings, indicating that calcium is a key regulator of mitochondrial function, and that calcium overload can impair electron transport leading to ROS generation [48]. In addition, TRPV1 inhibitor (AMG9810) interferes with calcium signaling and suppresses chemokine receptor CXCR4 (CXC-Motif Receptor 4)-mediated migration and stromal protection. It acts synergistically with bortezomib to target ubiquitin pathway and cytoprotective mitochondrial UPR, impairs mitochondria, destabilizes lysosome and promotes MM cell death. On the contrary, TRPV1 agonist (capsaicin) promotes calcium influx, resulting in transient increase in cytosolic calcium levels, thus supporting CXCR4-mediated activity [47]. Importantly, the combination of TRPV1 inhibitor (AMG9810) and bortezomib showed superior anti-MM activity in vivo model of CXCR4-driven human MM engrafting in murine bone marrow [47]. Altogether, these results reveal the mechanism mediating the synergistic anti-MM activity of bortezomib in combination with TRPV1 inhibition which may be translated into clinical practice.
TRPV2 channel
TRPV2 is widely expressed in different cells and tissues [49], such as B lymphocyte [50], CD34+ hematopoietic stem cells [51], and is a Ca2+ permeable channel that contributes to calcium homeostasis [28]. TRPV2 expression in some tumor cells is significantly higher than that in normal cells [52–54], and according to the Cancer Cell Line Encyclopedia (CCLE) database, the expression of TRPV2 is higher in MM cell lines compared to other tumor cell lines. In the study of TRPV2 expression, the protein was detected to be highly expressed in MM patient and MM cell lines (ARP-1, LP-1) by Immunohistochemistry and Western-blot analysis technology, indicating that TRPV2 played a role in MM development [13]. Numerous studies have shown that TRPV2 expression is related to tumor prognosis. In bladder cancer, TRPV2 promotes tumor cell migration and invasion through metalloproteinase 2 (MMP2) [55]; in oesophagal squamous cell carcinoma, high expression of TRPV2 has confirmed to be related to the patient’s disease stage and overall survival [56]. Bai et al. analyzed public gene expression data of bone marrow plasma cells from GSE24080. They found that MM patients with asymptomatic survival and overall survival less than 24 months, the transcriptional level of TRPV2 was significantly higher than that in patients with approximately 24 months [13]. In addition, TRPV2 is related to the occurrence of bone lesions in MM. Myeloma cells often expose to high levels of extracellular calcium concentrations (Cao2+) in the microenvironment surrounding destructive bone lesion [57]. Laboratory studies have shown that a high concentration of Cao2+ could increase the expression of TRPV2 in ARP-1 and LP-1 cell lines. TRPV2 up-regulates intracellular calcineurin protein, promoting the dephosphorylation of NFATc3 and accelerating NFATc3 to enter the nucleus, thereby enhancing the synthesis and secretion of osteoclast-related factors (such as Receptor Activator of Nuclear Factor-κ B Ligand (RANKL)) in MM cells, ultimately leading to MBD [13]. SKF96365, an inhibitor of TRPV2, proved to be able to inhibit RANKL-mediated osteoclast differentiation, suggesting that SKF96365 may be used as a new type of treatment for MBD [13].
TRPV4 channel
TRPV4, a Ca2+‐permeable channel of the TRP family, regulates the homeostasis of intracellular calcium concentrations (Cai2+) [58]. It mediates Ca2+ influx in the late stage of osteoclast differentiation, thus regulating Ca2+ signal, which is crucial for cellular events during osteoclast differentiation [59]. MM patients often accompany by abnormal bone metabolism, and the occurrence of MBD is related to the differentiation of osteoclasts [13]. Studies have shown that TRPV4 gene knocked out mice increase bone mass by impairing bone resorption, and TRPV4 activation promotes osteoclast differentiation and bone resorption [60]. In recent years, autophagy has been reported not only as an essential mechanism for maintaining cell homeostasis but also play a vital role in the regulation of osteoclastogenesis [61]. Cao and his colleagues found that TRPV4 up-regulated autophagy-related proteins and Ca2+-calcineurin-NFATc1 signaling, thereby promoting the secretion of osteoclast-related factors and modulating osteoporosis [14]. In summary, TRPV4 can be used as a potential therapeutic target for MBD and other bone resorption diseases.
TRPM7 channel
TRPM7 is an ion channel that regulates cellular magnesium and calcium homeostasis [62], though it specifically promotes Ca2+ influx in various cancer cells [63, 64]. TRPM7 is essential for embryonic development, and deletion of TRPM7 in the chicken B cell line DT40 results in growth arrest and cell death [65]. Earlier, Krishnamoorthy et al. demonstrated for the first time that TRPM7 can regulate B cell development and antigen recognition [25], suggesting its role in MM tumorigenesis.
Recently, growing evidence has demonstrated that MM originates from BM and disseminates throughout the body (also called extramedullary MM), which are closely correlated with poor prognosis with an overall survival period of less than 6 months [66, 67]. It is generally believed that the BM microenvironment provides support for MM cell growth and survival and for the acquisition of aggressive phenotypes. High Ca2+ and altered Ca2+ signals in the BM microenvironment may be a key contributing factor to the pathological process [68]. Therefore, Ca2+ channels and transporters, which are molecular participants of Ca2+ homeostasis, could be significantly involve with MM progression in term of MM cell motility and dissemination. Samart et al. observed that the expression of Ca2+ influx channels (such as TRPM7) in patient-derived MM cells are upregulated compared with normal plasma cells in a bioinformatics database. They used the CRISPR/Cas9 system to repress TRPM7 gene expression in MM cells, which can significantly inhibit the migration and invasion of MM cells [68]. Such result can reduce the intravasation of MM cells from the BM into nearby blood vessels and for their subsequent extravasation into distant tissues [69, 70]. In addition, vitro and vivo experiments show that TRPM7, Orai1 and Stim1 (Orai1 and Stim1 will be described in detail in SOCE) mediated Ca2+ influx regulate MM cell motility and dissemination by orchestrating O-GlcNAylation homeostasis that targets integrin α4 and integrin β7 [68]. In summary, TRPM7 is a key regulator of MM cell motility and dissemination, and may be a potential predictive biomarker and therapeutic targets for advanced MM. Further studies on the role of novel TRPM7 small molecule inhibitors in disseminated MM xenograft models in vivo may be beneficial to future MM treatments to achieve long-term disease control.
G protein-coupled receptors (GPCRs)
GPCRs are the largest family of membrane signaling proteins in the human body. The core structure of all GPCRs are very similar: 7 transmembrane helix domains, extracellular N-terminus, and intracellular C-terminus [71]. There are two extra-membrane loops (Loop) and three intra-membrane loops. The C-terminus and the intracellular loop connecting the fifth and sixth transmembrane helices have binding sites for protein G (guanylate binding protein). Most GPCRs belong to class A [71], which are rhodopsin-like receptors. Class B GPCRs can interact with peptides. Class C GPCRs are dimers composed of two 7 TM units, which are homologous to bacterial proteins involved in the transport of amino acids and ions [72]. And there are no receptors belong to class D, E, F or O in the human genome [71]. GPCRs usually localize on the plasma membrane, which mediates the response of cells to various stimuli, ultimately cause cellular responses [73]. Here, we mainly explain the role of GPCRs in the Ca2+ transport process.
Calcium sensitive receptor (CaSR)
Calcium-Sensing Receptor (CaSR) is a class C GPCRs discovered in 1993 by Brown and coworkers [74]. It is widely distributed in the human body and participates in numerous physiological and pathological processes, monitoring extracellular Ca2+ concentration and responding to various signals stimulate, and then regulating Ca2+ homeostasis [74]. CaSR is a homodimer, and each monomer composes an N-terminal extracellular domain (ECD), a seven-transmembrane domain (TTM) and an intracellular C-tail domain (C-tail) [75]. Ca2+ is the primary agonist of CaSR and can bind to the ECD region [76], and is thought to be involved in regulating mitotic transitions [77]. Yamaguchi et al. found that CaSR can promote the mitogenic response of mouse osteoblastic MC3T3-E1 cell [78]. CaSR agonists (such as Ca2+, gadolinium (Gd3+), and neomycin) increased levels of inositol triphosphate (IP3), cytosolic calcium concentration and DNA synthesis through a mechanism coupled to the activation of G protein and PKC [74, 79]. Besides, the mitogen-activated protein kinase (MAPK) family members, p42/44 and p38 MAPK, have been confirmed to be involved in CaSR-stimulated mitogenic response in mouse osteoblastic MC3T3-E1 cell. These responses may serve to ensure the availability of adequate numbers of osteoblastic cells at sites of recent bone resorption [80]. MM often accompanies by an increase in Cao2+, which leads to changes of CaSR expression in hematopoietic precursor cells, and ultimately red blood cell precursors, megakaryocytes (the precursors of blood platelets), monocytes, and macrophages, all of which express higher levels of the CaSR than white blood cells precursors [81]. In human myeloma cells, such as U266, IM-9 and RPMI8226 cells, exposure to high Ca2+ concentration augmented cell proliferation through CaSR on their surfaces, and further participate in a vicious cycle by expanding myeloma cell mass in destructive bone lesions [57]. Studies have reported that some basal or constitutive activity of MAPK, Interleukin-6 (IL-6), insulin-like growth factor-1 (IGF-1) or other signaling pathways have been found in these myeloma cells, which can promot cell proliferation [82, 83]. This is how myeloma cells survive in bone marrow microenvironment, indicating that CaSR, at least in part, might mediate these survival signals to regulate the mitosis of myeloma cells. However, CaSR activators (such as Ca2+, NPS, R467) did not enhance IL-6 expression in these cells, indicating that CaSR agonists exerting a mitogenic effect on myeloma cells seemed to be independent of IL-6 actions [57]. In addition, CaSR agonists have shown to stimulate proliferation of osteoblasts, monocyte-macrophages, bone marrow stromal cells and fibroblasts [57], which further supports the view that CaSR activation can promote MM mitosis.
Calcitonin receptor (CTR)
Calcitonin receptor (CTR) belonged to class B GPCR and identified originally in porcine kidney epithelial cell line [84]. CTR primarily expressed in osteoclasts (OCs), K562 myeloid leukemia cells, human breast cancer, kidney, and mouse and human lymphocytes, with a down-regulating effect on its function in response to hypercalcemia [85]. CTR contains seven transmembrane regions, four extracellular loops (ECL1-4) and four intracellular loops. It has proved that the ECL2 and ECL3 region are the binding sites of calcitonin (CT) [86]. Six structural variants have described, i.e. CTR-1 to -6 isoforms with most unknown functions. CTR structural isoforms can activate several heterotrimeric G proteins in response to CT stimulation, trigger various signal transduction mechanisms, that lead to intracellular Ca2+ isoform or adenylate cyclase accumulation throughout PKC or PKA pathway, respectively [87]. The CTR-2 isoform was detected on Ocs, with 16 amino acid deletions in the first intracellular loop [88]. Its main functions include the inhibition of bone resorption by neutralizing OCs migration and a shape retraction [89]. Silvestris et al. have shown that myeloma cell lines (U-266, MCC-2 and RPMI 8226 cells) express CTR-2 isoform and activate both cAMP and Ca2+ flow, suggesting the activation of PKA and PKC pathways. In addition, in myeloma cells, CT effectively inhibits bone resorption in vitro, supports the OC-like behavior of myeloma cells, indicating that CT or CTR may participate in the osteoclast differentiation of MM [85].
Parathyroid hormone-related protein Receptor (PTH-R1)
Parathyroid hormone-related protein (PTHrP) is functionally similar to parathyroid hormone (PTH), which is the main regulator of Ca2+ homeostasis and an OC activator. The NH2 terminal fragment of PTHrP can bind to PTH-R1, resulting in a significant increase in intracellular Ca2+ influx [90]. PTH-R1 is mainly distributed in bones and kidneys, and belongs to class B GPCRs. The receptor has seven transmembrane regions, longer extracellular amino acids, and contains more than three pairs of disulfide bonds folded into a network. The extracellular region cooperates with the helical structure of the transmembrane area and participates in the binding of ligands, and PTHrP is the only known PTH-R1 endogenous ligand [91]. There is little information about the role of PTH-R1 in MM. Previous studies reported that PTH-R1 highly expressed in bone marrow plasma cells of MM patients and MM cell lines, and PTHrP stimulated PTH-R1 in MM cells, promoting the proliferation of MM cells, and eventually the increase of Ca2+ influx, CAMP content [92]. Cafforio used ELISA and flow cytometry to demonstrated that in U266, MUS and RPMI 8226 cells, PTH-R1 directly participated in reinforcing both RANKL and monocyte chemoattractant protein 1 (MCP-1) production, while the opposite effect could be observed after PTH-R1 silenced [92]. The above results have clarified that PTHrP promotes MM tumor biological behavior and development through autocrine or paracrine stimulation of PTH-R1, thereby reinforcing the production of osteoclastogenic factors. In addition, they further verified that the PTHrP NH2 fragment (aa 1–40) reacted with PTH-R1 in RPMI 8226 cells, but not the NLS-middle region (aa 67–86), NLS middle region (aa 38–94) and COOH end (aa 107–139) fragments. PTH-R1 systematic analysis is still needed in MM patients to determine whether the receptor is a new marker of disease progression.
Purinergic receptors
Purinergic receptors, namely P1 and P2 receptors, are widely expressed in mammalian cells and are involved in several physiological functions like nociception, platelet aggregation, inflammatory reaction etc. [93]. The P2 receptors are divided into two groups: P2X and P2Y, each containing some members with different ion selectivity and regulatory properties. The P2X receptors have a trimeric topology with two transmembrane domains. The P2X7 receptor is a member of P2X, mainly expressed in bones, epithelial cells and normal B lymphocytes [94]. ATP can activate P2X7, leading to the absorption of cations such as Ca2+, Na+ and K+[95]. Low activation of P2X7 receptor promotes cell division, while an acute stimulation by massive ATP causes cell death [96]. For instance, activation of P2X7 in chronic lymphocytic leukaemia (CLL) results in cell death [97, 98] and induces the rapid release of cell-surface CD23 from CLL B lymphocytes [99]. CD23 is a ‘low affinity’, transmembrane receptor for IgE that can be shed from the cell surface to form soluble CD23 (sCD23), which also binds IgE and exerts cytokine-like activities on B cells and other leukocytes [100]. sCD23 maintains B-cell precursors growth, promotes B and T cell differentiation, and drives monocytes to release cytokines [101]. Compared with the plasma sCD23 levels of normal individuals, the sCD23 concentration of CLL patients may be 5 to 300-flod higher [102]. In addition, individuals with low plasma sCD23 concentrations have a more positive prognosis than those with high sCD23 concentrations [103].
Although P2X7 receptor is playing a role in neoplasia, long-term lack of malignant cell lines expressing this receptor, resulting in progress limited of P2X7 receptor in tumorigenesis. In recent years, Farrell et al. found functional P2X7 on the RPMI 8226 MM cell line. In this studies, P2X7 activation-induced death of RPMI 8226 cells, prevented the proliferation of RPMI 8226 cells and also generated CD23 shedding from myeloma cells [15]. As a useful marker in either diagnosis or prognosis of disease [104–106], sCD23 can provide useful value for further study of the mechanism of P2X7 in myeloma. In addition to P2X7, RT-PCR also showed that P2X4 and P2X5 are high expressions and P2X1 is low expression in RPMI 8226 cells, but it is still unknown whether these mRNAs are translated to proteins or lead to functional P2X channels [15]. At present, the physiological and pathophysiological effects of P2X in myeloma remain unclear and requires further research to elucidate.
Store-operated Ca2+ entry (SOCE)
Store-Operated Ca2+ Entry (SOCE), as a calcium ion channel, has been widely recognized as one of the major pathways for Ca2+ influx across PM of cells [107, 108]. The main components of SOCE are Stim proteins and Orai proteins (also known as CRAC). Stim proteins, located in the ER, are single-pass transmembrane proteins. Their EF-hand motif of N-terminal domain serves as a Ca2+ sensor within the ER [109]. In response to ER Ca2+ depletion, Stim proteins undergo a conformational change, forming self-aggregation and CRAC channels at the ER-PM junction through a diffusion trap mechanism, which promotes Stim proteins binding and activating hexamers of the Orai pore-forming proteins to trigger Ca2+ entry [110]. Stim proteins have two subtypes, Stim1 and Stim2, and there are three subtypes of Orai proteins in mammals, namely Orai1, Orai2 and Orai3 [110]. Orai proteins are four-time transmembrane membrane proteins, and their N-terminus and C-terminus are both in the cytoplasm, and TM1 forms the pore of the ion channel. Stim and Orai proteins widely expressed in various organs and tissues in the human body. Their deficiency or malfunction can lead to alterations in Ca2+ handling and ultimately cause oncogenic transformation [111]. In addition, the TRPC channel has suggested to be a component of SOCE channels [112]. It has reported that Orai1 is indispensable to TRPC1 function through the dynamic signal complex composed of Stim1- Orai1- TRPC1 [113, 114]. At rest, TRPC1 docked in the trafficking vesicle of the PM. After the storage is depleted, TRPC1 inserts into the PM, which is then gated and activated by Stim1, allowing TRPC1 mediated Ca2+ influx. TRPC1 plays a vital role in the overall function of the SOCE pathway [111]. Altered SOCE activity or the remodelling of SOCE-expression profile have reported in numerous cancers, such as colorectal cancers [115], hepatocellular carcinoma [116], melanoma [117], breast cancer [118], glioblastoma [119] and clear cell renal carcinoma [120]. Until now, tumor cell migration is considered as a Ca2+ dependent process, and calcium channels are major regulators of this process [121]. In non-excitable cells, calcium entry is mainly due to SOCE. Orai1 and Stim1 are the main calcium channels responsible for Ca2+ entry in lymphocytes [122, 123], and both have been described to be involved in the mediation of actomyosin assembly and the focal adhesions of cancer cell migration [124, 125]. Latour el al have demonstrated that Orai1 and Stim1 could regulate diffuse large B cell lymphoma (DLBCL) cell motility and dissemination, promoting homing of tumor B cells to extra-nodal sites [126]. Although in-depth researches have conducted, little is known about the role of SOCE in MM. Stim1 and Orai1 are the critical components of CRAC channels that highly expressed in MM bone marrow tissues. And compared with healthy controls, the mRNA levels and protein level of Stim1 and Orai1 were higher in primary MM cells, KM3 and U266 cells [16]. Moreover, U937 and RIMP 8226 MM cell lines also express higher levels of Orai3 [127]. Wang et al. demonstrated that SOCE inhibitors (SKF-96365, DES and 2-APB) affect the biological functions of human MM cell lines, not only inhibited the viability of MM cell lines, caused MM cell cycle arrest, but also induced MM cell apoptosis. Consistently, pretreatment of KM3 and U266 cells with the siRNAs of Stim1 or Orai1 for 48 h also reduced cell viability, leading to apoptosis and cell cycle arrest in MM cell lines, indicating that SOCE plays a role in MM development [16]. Moreover, clinical data found that Stim1/Orai1 highly expressed in the stage III group than stage II and I group in MM. Progression-free survival (PFS) (15.33 months) of patients in low Stim1 expression was greater than that of PFS (13.34 months) high Stim1 expression group [16]. The above results indicate that Stim1/Orai1 participates in the pathogenesis of MM, but the mechanism by which it works requires further research.
As a potential regulator of SOCE, TRPC1 widely expressed in most tissues, and its dysregulated activity may be a hallmark of many types of cancers, including glioblastoma multiforme [128], breast cancer [129], colon cancer [130], multiple myeloma [131]. Some studies on vitro mechanisms of TRPC1 inducing an increase in intracellular Ca2+ in the MM cell lines, indicating that TRPC1 is related to the development of MM. Knockout of TRPC1 expression showed a reduction in MTI-101(an frist-in-class peptidomimetic that triggers cell death in MM)-induced cell death in U266 and MM.1S MM cell lines [132]. This study suggests that TRPC1 plays an important role in treating MM. However, there is still needed to develop more specific drugs to inhibit or activate TRPC1 function, so that the efficacy of TRPC1 as a potential target for cancer treatment can be verified.
Mitochondrial Ca2+ transporters
Mitochondria have shown to have a significant contribution to the activity of SOCE, and the ability to chelate Ca2+ locally at the sites of Ca2+ release and entry was supposed to rule SOCE by promoting Ca2+ depletion of the ER and removal from Ca2+ inhibitory channels, respectively [133]. The uptake of mitochondrial Ca2+ must be precisely regulated, because excessive mitochondrial Ca2+ load can cause cell death [134]. Several proteins mediate Ca2+ transport across internal and outernal mitochondrial membranes (IMM, OMM, respectively), including VDAC1 on OMM, and MCU and Na+ dependent mitochondrial Ca2+ efflux transporter (NCLX) on IMM. Ca2+ is transferred to the IMM through OMM. VDAC1 allows Ca2+ to enter MCU, thus promoting Ca2+ transport to the matrix and also from IMM to cytoplasm [11]. This section focuses on VDAC1 and MCU function in the uptake and release of mitochondrial Ca2+ and their effects on MM.
Voltage-dependent anion channel 1 (VDAC1)
VDAC1 is located on the outer membrane of mitochondria in all eukaryotes and is a pore type protein. VDAC1 is structurally organized into a transmembrane β-bucket with an N-terminal domain as an α helix [135]. It acts as a voltage-gated channel to control the exchange of small ions and metabolites on the OMM, maintaining many organelle functions [136, 137]. VDAC1 is the only known Ca2+ transporter on OMM [11, 138], which leading to mitochondrial Ca2+ overload, is a marker of cell apoptosis [139]. Recent studies have shown that apoptosis signal up-regulates VDAC1 in a Ca2+ dependent manner, leading to cell death [140, 141]. It is now clear that VDAC1 is the main target against cancer. Compared with normal cell lines, the expression of VDAC1 increases in much human cancer cell lines [142]. However, research on the effect of VDAC1 on MM cell apoptosis is still relatively rare. Previous studies have indicated that the expression of CD45 in human myeloma cells is necessary for IL-6-induced proliferation [143]. Liu et al. found that susceptibility to apoptosis by apoptotic stimulis (such as oxidative stress and ER stress) are increased in CD45+ myeloma cell line U266, which highly expressed VDAC1 [17]. Together with the information that expression of VDAC1 in patients with stage III was significantly higher than that in patients with stage I and stage II [144], one could imply that VDAC1 seems to accelerate MM tumor growth, while increase susceptibility to apoptosis, making it a good target candidate for MM therapy.
Ca2+uniporter (MCU)
In IMM, the rapid absorption of Ca2+ is mediated by the MCU. MCU can actively shape Ca2+ signaling throughout the cell [145, 146]. MCU is composed of two transmembrane and N-terminal domains and forms a complex in the IMM with multiple protein regulators, which have an impact on gating [147–149]. It is known that the disregulation of intracellular Ca2+ is among the first hallmarks of apoptosis [150], and some reports indicate that MCU plays an important role in bortezomib-induced apoptosis [151, 152]. Bortezomib is a first-in-class selective PI (proteasome inhibitor), which has been proven to be effective in the treatment of MM. However, most patients eventually develop disease recurrence, and the bortezomib resistance becomes the primary cause of recurrence and incurability of myeloma [153]. Therefore, there is an urgent need to identify the potential mechanism of bortezomib resistance, and find some novel therapeutic targets that can reduce bortezomib resistance in MM. Song et al. found that mitochondrial activity is a determining factor in regulating apoptosis resistance in response to bortezomib, and compared with bortezomib-resistant KMS20 cells, the expression of MCU in bortezomib-sensitive KMS28BM cells were significantly higher under bortezomib stimulation [152]. Furthermore, the cytotoxicity of bortezomib against myeloma cells H929, U266 and MM.1S can be inhibited by mitochondrial Ca2+ uptake inhibitor ruthenium red (a cationic dye that blocks MCU) [151], indicating that changes in MCU were related with the resistance of bortezomib. Landowski's team proposed a model in which bortezomib evokes an instantaneous release of Ca2+ stores, leading mitochondrial Ca2+ influx. Mitochondrial Ca2+ sensors associated with uniporters initiate capacitative Ca2+ influx, also called CCI, a phenomenon that IP3R-activated depletion of ER stores is also involved in the regulation of Ca2+ influx from the extracellular environment [154], which is enhanced in bortezomib-treated cells, leading to apoptotic pathways activation [151]. Then, MUC blocker would be protective by blocking mitochondrial loading and CCI activation and the Ca2+-dependent signal transduction pathways that initiate cell death [155]. Together, MCU is expected to become a new therapeutic target to reduce MM bortezomib resistance. However, the specific mechanism of MUC participation in resistance still needs intense investigation.
Ca2+-ATPases
Ca2+-ATPases, which belong to the P-type pump superfamily, are involved in maintaining low cytoplasmic Ca2+ levels at rest and initiating organelle stores [156]. They quickly pump cytosolic Ca2+ ions back to intracellular organelles, such as ER, or to squeeze Ca2+ ions into extracellular space [10]. According to their subcellular location, Ca2+-ATPases can be divided into three subtypes: plasma membrane (plasma membrane Ca2+ -ATPase or PMCA), endoplasmic reticulum (Sarco/endoplasmic reticulum Ca2+-ATPase or SERCA) and Golgi apparatus / Golgi-derived vesicles (secretory pathway Ca2+-ATPase or SPCA) [157] (Fig. 1). Among them, SERCA is the most distinctive one, which is responsible for replenishing ER Ca2+ storage and maintaining correct folding of proteins. SERCA imbalance leads to decreased or overloaded Ca2+ storage in the ER lumen, increased ER stress, chaperones protein imbalance and lipid synthesis [10, 158]. Their expression levels and tissue distribution in the body are different [159]. Mutations and changes in SERCA subtypes expression levels have confirmed in a variety of cancers, such as colon cancer, gastric cancer, lung cancer, myeloid leukemia, and choroid plexus tumors [160]. Roti and his colleagues showed that SERCA inhibition preferentially damages the maturation of leukemia-related mutant NOTCH receptors, leading to G0/G1 arrest [161]. PMCA is a calcium pump that relies on ATP to drive Ca2+ out of the cell. When PMCA expression is abnormal, it will cause the disorder of Ca2+ balance in the cell. Studies have found that plasma membrane PMCA is abnormally expressed in various malignant tumors, including breast cancer [162], colon cancer [163], pancreatic cancer [164], and so on. PMCA proved to be a part of the Ca2+ exclusion system, but the role of each subtype of PMCA has not studied extensively. The main reason is the lack of specific inhibitors of each subtype of PMCA. The third newly discovered subtype is SPCA, a freshly discovered subtype, include calcium pumps located in the Golgi compartments and post-Golgi vesicles [156]. Changed expression of SPCA subtypes occurs in various types of cancer including breast, prostate and colon cancer [165]. The results of the above studies have shown that Ca2+ -ATPase is used to promote the escape of specific cancer cells from normal cellular control and accelerate tumorigenesis. Although clear evidence linking Ca2+-ATPases to several malignancies, rare reports about the study of Ca2+-ATPase in MM. Further study the role of Ca2+-ATPase in MM will contribute to understanding the complex intracellular Ca2+ signaling network in MM.
Conclusion
Calcium channels and transporters play a vital role in the regulation of Ca2+ transport and participate in multiple physiological and pathological processes, including the progression of cancer. Although various studies have reported the role of ion transporters in MM, the mechanism by which Ca2+ imbalance caused by defective ion transporter function leads to the occurrence of MM remains unclear. Therefore, it is particularly significant to elucidate the aetiology and pathogenesis of MM and explore new tumor markers for early diagnosis and treatment. In this review, we concerned about the effects of various calcium channels and transporters abnormalities on the development of MM, including many plasma membrane Ca2+ channels, SOCE, Mitochondrial Ca2+ transporters and Ca2+-ATPases. This review aims to stimulate people's interest in and to provide a basic, systematic overview of the research in this field, thereby providing new directions for the prognosis and treatment of MM by targeting Ca2+ channels or transporters.
Acknowledgements
Not applicable.
Abbreviations
- Cao2+
Extracellular calcium concentrations
- Cai2+
Intracellular calcium concentrations
- CaSR
Calcium sensitive receptor
- CCLE
Cancer cell line encyclopedia
- CCI
Capacitative Ca2+ influx
- CLL
Chronic lymphocytic leukaemia
- CT
Calcitonin
- CTR
Calcitonin receptor
- DLBCL
Diffuse large B cell lymphoma
- ECD
Extracellular domain
- ER/SR
Endoplasmic/sarcoplasmic reticulum
- GA
Golgi apparatus
- GPCRs
G protein-coupled receptors
- IMM
Internal mitochondrial membranes
- IP3R
Inositol 1,4,5-triphate receptor
- IGF-1
Interleukin-6 (IL-6), insulin-like growth factor-1
- MAPK
Mitogen-activated protein kinase
- MBD
Myeloma bone disease
- MCP-1
Monocyte chemoattractant protein 1
- MCU
Mitochondrial Ca2+ uniporter
- Mit
Mitochondrial
- MM
Multiple myeloma
- MMP2
Metalloproteinase 2
- NCLX
Na+ dependent mitochondrial Ca2+ efflux transporter
- OCs
Osteoclasts
- OMM
Outernal mitochondrial membranes
- IMM
Internal mitochondrial membranes
- PFS
Progression-free survival
- PM
Plasma membrane
- PMCA
Plasma membrane Ca2+ -ATPase
- PTH
Parathyroid hormone
- PTH-R1
Parathyroid hormone-related protein Receptor
- PTHrP
Parathyroid hormone-related protein
- RA NKL
Nuclear factor-κ B ligand
- RyR
Ryanodine receptor
- ROS
Reactive oxygen species
- SOCE
Store-operated Ca2+ entry
- SERCA
Sarco/endoplasmic reticulum Ca2+-ATPase
- STAT3
Activator of transcription 3
- Stim1
Stromal-interaction molecule1
- SPCA
Secretory pathway Ca2+-ATPase
- TTM
Transmembrane domain
- TRP
Transient receptor potential channel
- TRPV
Transient receptor potential channel subfamily V
- UPR
Unfolded protein response
- VDAC1
Voltage-dependent anion channel 1
Authors' contributions
T.T.L conceived and wrote the manuscript. J.M.C and Z.Y.Z revised the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by grants from the National Natural Science Foundation of China (Nos. 81400160 and 82070218), the Natural Science Foundation of Fujian Province (No. 2017J01185), and Clinical research project of Wu Jieping Medical Foundation (Nos. 320.6750.19094-41 and 320.6750.19094-58).
Declarations
Competing interests
The authors have declared that no competing interest exists.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Junmin Chen, Email: drjunminchen@hotmail.com.
Zhiyong Zeng, Email: zengzhiyong049@163.com.
References
- 1.Mimura N, Hideshima T, Anderson KC. Novel therapeutic strategies for multiple myeloma. Exp Hematol. 2015;43:732–741. doi: 10.1016/j.exphem.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar SK, Rajkumar V, Kyle RA, van Duin M, Sonneveld P, Mateos MV, et al. Multiple myeloma. Nat Rev Disease Primers. 2017;3:17046. doi: 10.1038/nrdp.2017.46. [DOI] [PubMed] [Google Scholar]
- 3.Rasch S, Lund T, Asmussen JT, Lerberg Nielsen A, Faebo Larsen R, Østerheden Andersen M, et al. Multiple myeloma associated bone disease. Cancers. 2020;12:2113. doi: 10.3390/cancers12082113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364:1046–1060. doi: 10.1056/NEJMra1011442. [DOI] [PubMed] [Google Scholar]
- 5.Stewart AF. Clinical practice. Hypercalcemia associated with cancer. N Engl J Med. 2005;352:373–379. doi: 10.1056/NEJMcp042806. [DOI] [PubMed] [Google Scholar]
- 6.Zagouri F, Kastritis E, Zomas A, Terpos E, Katodritou E, Symeonidis A, et al. Hypercalcemia remains an adverse prognostic factor for newly diagnosed multiple myeloma patients in the era of novel antimyeloma therapies. Eur J Haematol. 2017;99:409–414. doi: 10.1111/ejh.12923. [DOI] [PubMed] [Google Scholar]
- 7.Oyajobi BO. Multiple myeloma/hypercalcemia. Arthritis Res Therapy. 2007;9(Suppl 1):S4. doi: 10.1186/ar2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tuttle KR, Kunau RT, Loveridge N, Mundy GR. Altered renal calcium handling in hypercalcemia of malignancy. J Am Soc Nephrol. 1991;2:191–199. doi: 10.1681/ASN.V22191. [DOI] [PubMed] [Google Scholar]
- 9.Silver IA, Murrills RJ, Etherington DJ. Microelectrode studies on the acid microenvironment beneath adherent macrophages and osteoclasts. Exp Cell Res. 1988;175:266–276. doi: 10.1016/0014-4827(88)90191-7. [DOI] [PubMed] [Google Scholar]
- 10.Cui C, Merritt R, Fu L, Pan Z. Targeting calcium signaling in cancer therapy. Acta pharmaceutica Sinica B. 2017;7:3–17. doi: 10.1016/j.apsb.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shoshan-Barmatz V, De S. Mitochondrial VDAC, the Na(+)/Ca(2+) exchanger, and the Ca(2+) uniporter in Ca(2+) dynamics and signaling. Adv Exp Med Biol. 2017;981:323–347. doi: 10.1007/978-3-319-55858-5_13. [DOI] [PubMed] [Google Scholar]
- 12.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/S0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 13.Bai H, Zhu H, Yan Q, Shen X, Lu X, Wang J, et al. TRPV2-induced Ca(2+)-calcineurin-NFAT signaling regulates differentiation of osteoclast in multiple myeloma. Cell Commun Signal. 2018;16:68. doi: 10.1186/s12964-018-0280-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao B, Dai X, Wang W. Knockdown of TRPV4 suppresses osteoclast differentiation and osteoporosis by inhibiting autophagy through Ca(2+) -calcineurin-NFATc1 pathway. J Cell Physiol. 2019;234:6831–6841. doi: 10.1002/jcp.27432. [DOI] [PubMed] [Google Scholar]
- 15.Farrell AW, Gadeock S, Pupovac A, Wang B, Jalilian I, Ranson M, et al. P2X7 receptor activation induces cell death and CD23 shedding in human RPMI 8226 multiple myeloma cells. Biochem Biophys Acta. 2010;1800:1173–1182. doi: 10.1016/j.bbagen.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 16.Wang W, Ren Y, Wang L, Zhao W, Dong X, Pan J, et al. Orai1 and stim1 mediate the majority of store-operated calcium entry in multiple myeloma and have strong implications for adverse prognosis. Cell Physiol Biochem: Int J Exp Cell Physiol Biochem Pharmacol. 2018;48:2273–2285. doi: 10.1159/000492645. [DOI] [PubMed] [Google Scholar]
- 17.Liu S, Ishikawa H, Tsuyama N, Li FJ, Abroun S, Otsuyama KI, et al. Increased susceptibility to apoptosis in CD45(+) myeloma cells accompanied by the increased expression of VDAC1. Oncogene. 2006;25:419–429. doi: 10.1038/sj.onc.1208982. [DOI] [PubMed] [Google Scholar]
- 18.Wu LJ, Sweet TB, Clapham DE. International union of basic and clinical pharmacology. LXXVI. Current progress in the mammalian TRP ion channel family. Pharmacol Rev. 2010;62:381–404. doi: 10.1124/pr.110.002725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clapham DE. SnapShot: mammalian TRP channels. Cell. 2007;129:220. doi: 10.1016/j.cell.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 20.Minke B. TRP channels and Ca2+ signaling. Cell Calcium. 2006;40:261–275. doi: 10.1016/j.ceca.2006.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taberner FJ, Devesa I, Ferrer-Montiel A. Calcium entry through thermosensory channels. Adv Exp Med Biol. 2016;898:265–304. doi: 10.1007/978-3-319-26974-0_12. [DOI] [PubMed] [Google Scholar]
- 22.Eda H, Santo L, David Roodman G, Raje N. Bone disease in multiple myeloma. Cancer Treat Res. 2016;169:251–270. doi: 10.1007/978-3-319-40320-5_14. [DOI] [PubMed] [Google Scholar]
- 23.Roodman GD. Targeting the bone microenvironment in multiple myeloma. J Bone Miner Metab. 2010;28:244–250. doi: 10.1007/s00774-009-0154-7. [DOI] [PubMed] [Google Scholar]
- 24.Fabris S, Todoerti K, Mosca L, Agnelli L, Intini D, Lionetti M, et al. Molecular and transcriptional characterization of the novel 17p11.2-p12 amplicon in multiple myeloma. Genes Chromosomes Cancer. 2007;46:1109–1118. doi: 10.1002/gcc.20494. [DOI] [PubMed] [Google Scholar]
- 25.Krishnamoorthy M, Wasim L, Buhari FHM, Zhao T, Mahtani T, Ho J, et al. The channel-kinase TRPM7 regulates antigen gathering and internalization in B cells. Science signaling. 2018;11:eaah6692. doi: 10.1126/scisignal.aah6692. [DOI] [PubMed] [Google Scholar]
- 26.Launay P, Cheng H, Srivatsan S, Penner R, Fleig A, Kinet JP. TRPM4 regulates calcium oscillations after T cell activation. Science (New York, NY) 2004;306:1374–1377. doi: 10.1126/science.1098845. [DOI] [PubMed] [Google Scholar]
- 27.van Goor MK, de Jager L, Cheng Y, van der Wijst J. High-resolution structures of transient receptor potential vanilloid channels: Unveiling a functionally diverse group of ion channels. Protein Sci: Publ Protein Soc. 2020;29:1569–1580. doi: 10.1002/pro.3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- 29.Guarino BD, Paruchuri S, Thodeti CK. The role of TRPV4 channels in ocular function and pathologies. Exp Eye Res. 2020;201:108257. doi: 10.1016/j.exer.2020.108257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Goor MKC, Hoenderop JGJ, van der Wijst J. TRP channels in calcium homeostasis: from hormonal control to structure-function relationship of TRPV5 and TRPV6. Biochim Biophys Acta. 2017;1864:883–893. doi: 10.1016/j.bbamcr.2016.11.027. [DOI] [PubMed] [Google Scholar]
- 32.Fleig A, Penner R. The TRPM ion channel subfamily: molecular, biophysical and functional features. Trends Pharmacol Sci. 2004;25:633–639. doi: 10.1016/j.tips.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 33.Huang Y, Fliegert R, Guse AH, Lü W, Du J. A structural overview of the ion channels of the TRPM family. Cell Calcium. 2020;85:102111. doi: 10.1016/j.ceca.2019.102111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Launay P, Fleig A, Perraud AL, Scharenberg AM, Penner R, Kinet JP. TRPM4 is a Ca2+-activated nonselective cation channel mediating cell membrane depolarization. Cell. 2002;109:397–407. doi: 10.1016/S0092-8674(02)00719-5. [DOI] [PubMed] [Google Scholar]
- 35.Hofmann T, Chubanov V, Gudermann T, Montell C. TRPM5 is a voltage-modulated and Ca(2+)-activated monovalent selective cation channel. Current Biol: CB. 2003;13:1153–1158. doi: 10.1016/S0960-9822(03)00431-7. [DOI] [PubMed] [Google Scholar]
- 36.Winter Z, Buhala A, Ötvös F, Jósvay K, Vizler C, Dombi G, et al. Functionally important amino acid residues in the transient receptor potential vanilloid 1 (TRPV1) ion channel–an overview of the current mutational data. Mol Pain. 2013;9:30. doi: 10.1186/1744-8069-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang F, Zheng J. Understand spiciness: mechanism of TRPV1 channel activation by capsaicin. Protein Cell. 2017;8:169–177. doi: 10.1007/s13238-016-0353-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 39.Marrone MC, Morabito A, Giustizieri M, Chiurchiù V, Leuti A, Mattioli M, et al. TRPV1 channels are critical brain inflammation detectors and neuropathic pain biomarkers in mice. Nat Commun. 2017;8:15292. doi: 10.1038/ncomms15292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Riera CE, Huising MO, Follett P, Leblanc M, Halloran J, Van Andel R, et al. TRPV1 pain receptors regulate longevity and metabolism by neuropeptide signaling. Cell. 2014;157:1023–1036. doi: 10.1016/j.cell.2014.03.051. [DOI] [PubMed] [Google Scholar]
- 41.Lozano C, Córdova C, Marchant I, Zúñiga R, Ochova P, Ramírez-Barrantes R, et al. Intracellular aggregated TRPV1 is associated with lower survival in breast cancer patients. Breast Cancer (Dove Medical Press) 2018;10:161–168. doi: 10.2147/BCTT.S170208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sanchez MG, Sanchez AM, Collado B, Malagarie-Cazenave S, Olea N, Carmena MJ, et al. Expression of the transient receptor potential vanilloid 1 (TRPV1) in LNCaP and PC-3 prostate cancer cells and in human prostate tissue. Eur J Pharmacol. 2005;515:20–27. doi: 10.1016/j.ejphar.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 43.Amantini C, Ballarini P, Caprodossi S, Nabissi M, Morelli MB, Lucciarini R, et al. Triggering of transient receptor potential vanilloid type 1 (TRPV1) by capsaicin induces Fas/CD95-mediated apoptosis of urothelial cancer cells in an ATM-dependent manner. Carcinogenesis. 2009;30:1320–1329. doi: 10.1093/carcin/bgp138. [DOI] [PubMed] [Google Scholar]
- 44.Amantini C, Mosca M, Nabissi M, Lucciarini R, Caprodossi S, Arcella A, et al. Capsaicin-induced apoptosis of glioma cells is mediated by TRPV1 vanilloid receptor and requires p38 MAPK activation. J Neurochem. 2007;102:977–990. doi: 10.1111/j.1471-4159.2007.04582.x. [DOI] [PubMed] [Google Scholar]
- 45.Büsselberg D, Florea AM. Targeting intracellular calcium signaling ([Ca(2+)](i)) to overcome acquired multidrug resistance of cancer cells: a mini-overview. Cancers. 2017;9:48. doi: 10.3390/cancers9050048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Obeng EA, Carlson LM, Gutman DM, Harrington WJ, Jr, Lee KP, Boise LH. Proteasome inhibitors induce a terminal unfolded protein response in multiple myeloma cells. Blood. 2006;107:4907–4916. doi: 10.1182/blood-2005-08-3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beider K, Rosenberg E, Dimenshtein-Voevoda V, Sirovsky Y, Vladimirsky J, Magen H, et al. Blocking of Transient Receptor Potential Vanilloid 1 (TRPV1) promotes terminal mitophagy in multiple myeloma, disturbing calcium homeostasis and targeting ubiquitin pathway and bortezomib-induced unfolded protein response. J Hematol Oncol. 2020;13:158. doi: 10.1186/s13045-020-00993-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol. 2004;287:C817–C833. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- 49.Everaerts W, Gevaert T, Nilius B, De Ridder D. On the origin of bladder sensing: Tr(i)ps in urology. Neurourol Urodyn. 2008;27:264–273. doi: 10.1002/nau.20511. [DOI] [PubMed] [Google Scholar]
- 50.Santoni G, Farfariello V, Liberati S, Morelli MB, Nabissi M, Santoni M, et al. The role of transient receptor potential vanilloid type-2 ion channels in innate and adaptive immune responses. Front Immunol. 2013;4:34. doi: 10.3389/fimmu.2013.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park KS, Pang B, Park SJ, Lee YG, Bae JY, Park S, et al. Identification and functional characterization of ion channels in CD34(+) hematopoietic stem cells from human peripheral blood. Mol Cells. 2011;32:181–188. doi: 10.1007/s10059-011-0068-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu G, Xie C, Sun F, Xu X, Yang Y, Zhang T, et al. Clinical significance of transient receptor potential vanilloid 2 expression in human hepatocellular carcinoma. Cancer Genet Cytogenet. 2010;197:54–59. doi: 10.1016/j.cancergencyto.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 53.Zhou K, Zhang SS, Yan Y, Zhao S. Overexpression of transient receptor potential vanilloid 2 is associated with poor prognosis in patients with esophageal squamous cell carcinoma. Med Oncol (Northwood, London, England) 2014;31:17. doi: 10.1007/s12032-014-0017-5. [DOI] [PubMed] [Google Scholar]
- 54.Monet M, Lehen'kyi V, Gackiere F, Firlej V, Vandenberghe M, Roudbaraki M, et al. Role of cationic channel TRPV2 in promoting prostate cancer migration and progression to androgen resistance. Can Res. 2010;70:1225–1235. doi: 10.1158/0008-5472.CAN-09-2205. [DOI] [PubMed] [Google Scholar]
- 55.Zhang W, Chu X, Tong Q, Cheung JY, Conrad K, Masker K, et al. A novel TRPM2 isoform inhibits calcium influx and susceptibility to cell death. J Biol Chem. 2003;278:16222–16229. doi: 10.1074/jbc.M300298200. [DOI] [PubMed] [Google Scholar]
- 56.Narayan G, Bourdon V, Chaganti S, Arias-Pulido H, Nandula SV, Rao PH, et al. Gene dosage alterations revealed by cDNA microarray analysis in cervical cancer: identification of candidate amplified and overexpressed genes. Genes Chromosom Cancer. 2007;46:373–384. doi: 10.1002/gcc.20418. [DOI] [PubMed] [Google Scholar]
- 57.Yamaguchi T, Yamauchi M, Sugimoto T, Chauhan D, Anderson KC, Brown EM, et al. The extracellular calcium Ca2+o-sensing receptor is expressed in myeloma cells and modulates cell proliferation. Biochem Biophys Res Commun. 2002;299:532–538. doi: 10.1016/S0006-291X(02)02690-6. [DOI] [PubMed] [Google Scholar]
- 58.White JP, Cibelli M, Urban L, Nilius B, McGeown JG, Nagy I. TRPV4: molecular conductor of a diverse orchestra. Physiol Rev. 2016;96:911–973. doi: 10.1152/physrev.00016.2015. [DOI] [PubMed] [Google Scholar]
- 59.Li P, Bian X, Liu C, Wang S, Guo M, Tao Y, et al. STIM1 and TRPV4 regulate fluid flow-induced calcium oscillation at early and late stages of osteoclast differentiation. Cell Calcium. 2018;71:45–52. doi: 10.1016/j.ceca.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 60.Masuyama R, Mizuno A, Komori H, Kajiya H, Uekawa A, Kitaura H, et al. Calcium/calmodulin-signaling supports TRPV4 activation in osteoclasts and regulates bone mass. J Bone Miner Res: Off J Am Soc Bone Miner Res. 2012;27:1708–1721. doi: 10.1002/jbmr.1629. [DOI] [PubMed] [Google Scholar]
- 61.Shapiro IM, Layfield R, Lotz M, Settembre C, Whitehouse C. Boning up on autophagy: the role of autophagy in skeletal biology. Autophagy. 2014;10:7–19. doi: 10.4161/auto.26679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Duan J, Li Z, Li J, Hulse RE, Santa-Cruz A, Valinsky WC, et al. Structure of the mammalian TRPM7, a magnesium channel required during embryonic development. Proc Natl Acad Sci USA. 2018;115:E8201–E8210. doi: 10.1073/pnas.1810719115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yee NS. Role of TRPM7 in cancer: potential as molecular biomarker and therapeutic target. Pharmaceuticals (Basel, Switzerland). 2017;10:39. doi: 10.3390/ph10020039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu L, Wu N, Wang Y, Zhang X, Xia B, Tang J, et al. TRPM7 promotes the epithelial-mesenchymal transition in ovarian cancer through the calcium-related PI3K / AKT oncogenic signaling. J Exp Clin Cancer Re: CR. 2019;38:106. doi: 10.1186/s13046-019-1061-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nadler MJ, Hermosura MC, Inabe K, Perraud AL, Zhu Q, Stokes AJ, et al. LTRPC7 is a Mg.ATP-regulated divalent cation channel required for cell viability. Nature. 2001;411:590–595. doi: 10.1038/35079092. [DOI] [PubMed] [Google Scholar]
- 66.Weinstock M, Ghobrial IM. Extramedullary multiple myeloma. Leukemia Lymphoma. 2013;54:1135–1141. doi: 10.3109/10428194.2012.740562. [DOI] [PubMed] [Google Scholar]
- 67.de Haart SJ, Willems SM, Mutis T, Koudijs MJ, van Blokland MT, Lokhorst HM, et al. Comparison of intramedullary myeloma and corresponding extramedullary soft tissue plasmacytomas using genetic mutational panel analyses. Blood Cancer J. 2016;6:e426. doi: 10.1038/bcj.2016.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Samart P, Luanpitpong S, Rojanasakul Y, Issaragrisil S. O-GlcNAcylation homeostasis controlled by calcium influx channels regulates multiple myeloma dissemination. J Exp Clin Cancer Res: CR. 2021;40:100. doi: 10.1186/s13046-021-01876-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ghobrial IM. Myeloma as a model for the process of metastasis: implications for therapy. Blood. 2012;120:20–30. doi: 10.1182/blood-2012-01-379024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bhutani M, Foureau DM, Atrash S, Voorhees PM, Usmani SZ. Extramedullary multiple myeloma. Leukemia. 2020;34:1–20. doi: 10.1038/s41375-019-0660-0. [DOI] [PubMed] [Google Scholar]
- 71.Fredriksson R, Lagerström MC, Lundin LG, Schiöth HB. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol Pharmacol. 2003;63:1256–1272. doi: 10.1124/mol.63.6.1256. [DOI] [PubMed] [Google Scholar]
- 72.Pin JP, Bettler B. Organization and functions of mGlu and GABA(B) receptor complexes. Nature. 2016;540:60–68. doi: 10.1038/nature20566. [DOI] [PubMed] [Google Scholar]
- 73.Gurevich VV, Gurevich EV. GPCR signaling regulation: the role of GRKs and arrestins. Front Pharmacol. 2019;10:125. doi: 10.3389/fphar.2019.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brown EM, Gamba G, Riccardi D, Lombardi M, Butters R, Kifor O, et al. Cloning and characterization of an extracellular Ca(2+)-sensing receptor from bovine parathyroid. Nature. 1993;366:575–580. doi: 10.1038/366575a0. [DOI] [PubMed] [Google Scholar]
- 75.Zhang C, Zhang T, Zou J, Miller CL, Gorkhali R, Yang JY, et al. Structural basis for regulation of human calcium-sensing receptor by magnesium ions and an unexpected tryptophan derivative co-agonist. Sci Adv. 2016;2:e1600241. doi: 10.1126/sciadv.1600241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ranieri M. Renal Ca(2+) and water handling in response to calcium sensing receptor signaling: physiopathological aspects and role of CaSR-regulated microRNAs. Int J Mol Sci. 2019;20:5341. doi: 10.3390/ijms20215341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Whitaker M, Larman MG. Calcium and mitosis. Semin Cell Dev Biol. 2001;12:53–58. doi: 10.1006/scdb.2000.0217. [DOI] [PubMed] [Google Scholar]
- 78.Yamaguchi T, Chattopadhyay N, Kifor O, Butters RR, Jr, Sugimoto T, Brown EM. Mouse osteoblastic cell line (MC3T3-E1) expresses extracellular calcium (Ca2+o)-sensing receptor and its agonists stimulate chemotaxis and proliferation of MC3T3-E1 cells. J Bone Miner Res: Off J Am Soc Bone Miner Res. 1998;13:1530–1538. doi: 10.1359/jbmr.1998.13.10.1530. [DOI] [PubMed] [Google Scholar]
- 79.Quarles LD, Hartle JE, 2nd, Middleton JP, Zhang J, Arthur JM, Raymond JR. Aluminum-induced DNA synthesis in osteoblasts: mediation by a G-protein coupled cation sensing mechanism. J Cell Biochem. 1994;56:106–117. doi: 10.1002/jcb.240560115. [DOI] [PubMed] [Google Scholar]
- 80.Yamaguchi T, Chattopadhyay N, Kifor O, Sanders JL, Brown EM. Activation of p42/44 and p38 mitogen-activated protein kinases by extracellular calcium-sensing receptor agonists induces mitogenic responses in the mouse osteoblastic MC3T3-E1 cell line. Biochem Biophys Res Commun. 2000;279:363–368. doi: 10.1006/bbrc.2000.3955. [DOI] [PubMed] [Google Scholar]
- 81.House MG, Kohlmeier L, Chattopadhyay N, Kifor O, Yamaguchi T, Leboff MS, et al. Expression of an extracellular calcium-sensing receptor in human and mouse bone marrow cells. J Bone Miner Res: Off J Am Soc Bone Miner Res. 1997;12:1959–1970. doi: 10.1359/jbmr.1997.12.12.1959. [DOI] [PubMed] [Google Scholar]
- 82.Mitsiades CS, Mitsiades N, Poulaki V, Schlossman R, Akiyama M, Chauhan D, et al. Activation of NF-kappaB and upregulation of intracellular anti-apoptotic proteins via the IGF-1/Akt signaling in human multiple myeloma cells: therapeutic implications. Oncogene. 2002;21:5673–5683. doi: 10.1038/sj.onc.1205664. [DOI] [PubMed] [Google Scholar]
- 83.Podar K, Tai YT, Davies FE, Lentzsch S, Sattler M, Hideshima T, et al. Vascular endothelial growth factor triggers signaling cascades mediating multiple myeloma cell growth and migration. Blood. 2001;98:428–435. doi: 10.1182/blood.V98.2.428. [DOI] [PubMed] [Google Scholar]
- 84.Lin HY, Harris TL, Flannery MS, Aruffo A, Kaji EH, Gorn A, et al. Expression cloning of an adenylate cyclase-coupled calcitonin receptor. Science (New York, NY) 1991;254:1022–1024. doi: 10.1126/science.1658940. [DOI] [PubMed] [Google Scholar]
- 85.Silvestris F, Cafforio P, De Matteo M, Quatraro C, Dammacco F. Expression and function of the calcitonin receptor by myeloma cells in their osteoclast-like activity in vitro. Leuk Res. 2008;32:611–623. doi: 10.1016/j.leukres.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 86.Dal Maso E, Glukhova A, Zhu Y, Garcia-Nafria J, Tate CG, Atanasio S, et al. The molecular control of calcitonin receptor signaling. ACS Pharmacol Transl Sci. 2019;2:31–51. doi: 10.1021/acsptsci.8b00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shyu JF, Zhang Z, Hernandez-Lagunas L, Camerino C, Chen Y, Inoue D, et al. Protein kinase C antagonizes pertussis-toxin-sensitive coupling of the calcitonin receptor to adenylyl cyclase. Eur J Biochem. 1999;262:95–101. doi: 10.1046/j.1432-1327.1999.00346.x. [DOI] [PubMed] [Google Scholar]
- 88.Ikegame M, Rakopoulos M, Zhou H, Houssami S, Martin TJ, Moseley JM, et al. Calcitonin receptor isoforms in mouse and rat osteoclasts. J Bone Miner Res: Off J Am Soc Bone Miner Res. 1995;10:59–65. doi: 10.1002/jbmr.5650100110. [DOI] [PubMed] [Google Scholar]
- 89.Bowler WB, Gallagher JA, Bilbe G. G-protein coupled receptors in bone. Front Biosci. 1998;3:d769–d780. doi: 10.2741/A320. [DOI] [PubMed] [Google Scholar]
- 90.Kremer R, Li J, Camirand A, Karaplis AC. Parathyroid hormone related protein (PTHrP) in tumor progression. Adv Exp Med Biol. 2011;720:145–160. doi: 10.1007/978-1-4614-0254-1_12. [DOI] [PubMed] [Google Scholar]
- 91.Fujita T, Meguro T, Fukuyama R, Nakamuta H, Koida M. New signaling pathway for parathyroid hormone and cyclic AMP action on extracellular-regulated kinase and cell proliferation in bone cells. Checkpoint of modulation by cyclic AMP. J Biol Chem. 2002;277:22191–22200. doi: 10.1074/jbc.M110364200. [DOI] [PubMed] [Google Scholar]
- 92.Cafforio P, Savonarola A, Stucci S, De Matteo M, Tucci M, Brunetti AE, et al. PTHrP produced by myeloma plasma cells regulates their survival and pro-osteoclast activity for bone disease progression. J Bone Miner Res: Off J Am Soc Bone Miner Res. 2014;29:55–66. doi: 10.1002/jbmr.2022. [DOI] [PubMed] [Google Scholar]
- 93.Pacheco PAF, Dantas LP, Ferreira LGB, Faria RX. Purinergic receptors and neglected tropical diseases: why ignore purinergic signaling in the search for new molecular targets? J Bioenerg Biomembr. 2018;50:307–313. doi: 10.1007/s10863-018-9761-0. [DOI] [PubMed] [Google Scholar]
- 94.Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledgebase of drug targets and their ligands. Nucleic Acids Res. 2014;42:D1098–D1106. doi: 10.1093/nar/gkt1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Qu Y, Dubyak GR. P2X7 receptors regulate multiple types of membrane trafficking responses and non-classical secretion pathways. Purinergic Signal. 2009;5:163–173. doi: 10.1007/s11302-009-9132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Adinolfi E, Pizzirani C, Idzko M, Panther E, Norgauer J, Di Virgilio F, et al. P2X(7) receptor: death or life? Purinergic Signal. 2005;1:219–227. doi: 10.1007/s11302-005-6322-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Adinolfi E, Melchiorri L, Falzoni S, Chiozzi P, Morelli A, Tieghi A, et al. P2X7 receptor expression in evolutive and indolent forms of chronic B lymphocytic leukemia. Blood. 2002;99:706–708. doi: 10.1182/blood.V99.2.706. [DOI] [PubMed] [Google Scholar]
- 98.Wiley JS, Dao-Ung LP, Gu BJ, Sluyter R, Shemon AN, Li C, et al. A loss-of-function polymorphic mutation in the cytolytic P2X7 receptor gene and chronic lymphocytic leukaemia: a molecular study. Lancet (London, England) 2002;359:1114–1119. doi: 10.1016/S0140-6736(02)08156-4. [DOI] [PubMed] [Google Scholar]
- 99.Gu B, Bendall LJ, Wiley JS. Adenosine triphosphate-induced shedding of CD23 and L-selectin (CD62L) from lymphocytes is mediated by the same receptor but different metalloproteases. Blood. 1998;92:946–951. doi: 10.1182/blood.V92.3.946. [DOI] [PubMed] [Google Scholar]
- 100.Acharya M, Borland G, Edkins AL, Maclellan LM, Matheson J, Ozanne BW, et al. CD23/FcεRII: molecular multi-tasking. Clin Exp Immunol. 2010;162:12–23. doi: 10.1111/j.1365-2249.2010.04210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pupovac A, Geraghty NJ, Watson D, Sluyter R. Activation of the P2X7 receptor induces the rapid shedding of CD23 from human and murine B cells. Immunol Cell Biol. 2015;93:77–85. doi: 10.1038/icb.2014.69. [DOI] [PubMed] [Google Scholar]
- 102.Sarfati M, Bron D, Lagneaux L, Fonteyn C, Frost H, Delespesse G. Elevation of IgE-binding factors in serum of patients with B cell-derived chronic lymphocytic leukemia. Blood. 1988;71:94–98. doi: 10.1182/blood.V71.1.94.94. [DOI] [PubMed] [Google Scholar]
- 103.Sarfati M, Chevret S, Chastang C, Biron G, Stryckmans P, Delespesse G, et al. Prognostic importance of serum soluble CD23 level in chronic lymphocytic leukemia. Blood. 1996;88:4259–4264. doi: 10.1182/blood.V88.11.4259.4259. [DOI] [PubMed] [Google Scholar]
- 104.Barna G, Reiniger L, Tátrai P, Kopper L, Matolcsy A. The cut-off levels of CD23 expression in the differential diagnosis of MCL and CLL. Hematol Oncol. 2008;26:167–170. doi: 10.1002/hon.855. [DOI] [PubMed] [Google Scholar]
- 105.Schlette E, Fu K, Medeiros LJ. CD23 expression in mantle cell lymphoma: clinicopathologic features of 18 cases. Am J Clin Pathol. 2003;120:760–766. doi: 10.1309/XV4AG7EMWQU7ER67. [DOI] [PubMed] [Google Scholar]
- 106.Walters M, Olteanu H, Van Tuinen P, Kroft SH. CD23 expression in plasma cell myeloma is specific for abnormalities of chromosome 11, and is associated with primary plasma cell leukaemia in this cytogenetic sub-group. Br J Haematol. 2010;149:292–293. doi: 10.1111/j.1365-2141.2009.08042.x. [DOI] [PubMed] [Google Scholar]
- 107.Parekh AB, Putney JW., Jr Store-operated calcium channels. Physiol Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- 108.Pan Z, Brotto M, Ma J. Store-operated Ca2+ entry in muscle physiology and diseases. BMB Rep. 2014;47:69–79. doi: 10.5483/BMBRep.2014.47.2.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhou Y, Cai X, Nwokonko RM, Loktionova NA, Wang Y, Gill DL. The STIM-Orai coupling interface and gating of the Orai1 channel. Cell Calcium. 2017;63:8–13. doi: 10.1016/j.ceca.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lewis RS. Store-operated calcium channels: from function to structure and back again. Cold Spring Harbor Perspect Biol. 2020;12:a035055. doi: 10.1101/cshperspect.a035055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pierro C, Sneyers F, Bultynck G, Roderick HL. ER Ca(2+) release and store-operated Ca(2+) entry - partners in crime or independent actors in oncogenic transformation? Cell Calcium. 2019;82:102061. doi: 10.1016/j.ceca.2019.102061. [DOI] [PubMed] [Google Scholar]
- 112.Lopez JJ, Jardin I, Sanchez-Collado J, Salido GM, Smani T, Rosado JA. TRPC channels in the SOCE scenario. Cells. 2020;9:126. doi: 10.3390/cells9010126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ambudkar IS, de Souza LB, Ong HL. TRPC1, Orai1, and STIM1 in SOCE: friends in tight spaces. Cell Calcium. 2017;63:33–39. doi: 10.1016/j.ceca.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cheng KT, Ong HL, Liu X, Ambudkar IS. Contribution of TRPC1 and Orai1 to Ca(2+) entry activated by store depletion. Adv Exp Med Biol. 2011;704:435–449. doi: 10.1007/978-94-007-0265-3_24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zuccolo E, Laforenza U, Ferulli F, Pellavio G, Scarpellino G, Tanzi M, et al. Stim and Orai mediate constitutive Ca(2+) entry and control endoplasmic reticulum Ca(2+) refilling in primary cultures of colorectal carcinoma cells. Oncotarget. 2018;9:31098–31119. doi: 10.18632/oncotarget.25785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yang N, Tang Y, Wang F, Zhang H, Xu D, Shen Y, et al. Blockade of store-operated Ca(2+) entry inhibits hepatocarcinoma cell migration and invasion by regulating focal adhesion turnover. Cancer Lett. 2013;330:163–169. doi: 10.1016/j.canlet.2012.11.040. [DOI] [PubMed] [Google Scholar]
- 117.Stanisz H, Saul S, Müller CS, Kappl R, Niemeyer BA, Vogt T, et al. Inverse regulation of melanoma growth and migration by Orai1/STIM2-dependent calcium entry. Pigment Cell Melanoma Res. 2014;27:442–453. doi: 10.1111/pcmr.12222. [DOI] [PubMed] [Google Scholar]
- 118.McAndrew D, Grice DM, Peters AA, Davis FM, Stewart T, Rice M, et al. ORAI1-mediated calcium influx in lactation and in breast cancer. Mol Cancer Ther. 2011;10:448–460. doi: 10.1158/1535-7163.MCT-10-0923. [DOI] [PubMed] [Google Scholar]
- 119.Motiani RK, Hyzinski-García MC, Zhang X, Henkel MM, Abdullaev IF, Kuo YH, et al. STIM1 and Orai1 mediate CRAC channel activity and are essential for human glioblastoma invasion. Pflugers Arch: Eur J Physiol. 2013;465:1249–1260. doi: 10.1007/s00424-013-1254-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kim JH, Lkhagvadorj S, Lee MR, Hwang KH, Chung HC, Jung JH, et al. Orai1 and STIM1 are critical for cell migration and proliferation of clear cell renal cell carcinoma. Biochem Biophys Res Commun. 2014;448:76–82. doi: 10.1016/j.bbrc.2014.04.064. [DOI] [PubMed] [Google Scholar]
- 121.Iamshanova O, Fiorio Pla A, Prevarskaya N. Molecular mechanisms of tumour invasion: regulation by calcium signals. J Physiol. 2017;595:3063–3075. doi: 10.1113/JP272844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Feske S, Wulff H, Skolnik EY. Ion channels in innate and adaptive immunity. Annu Rev Immunol. 2015;33:291–353. doi: 10.1146/annurev-immunol-032414-112212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yeromin AV, Zhang SL, Jiang W, Yu Y, Safrina O, Cahalan MD. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature. 2006;443:226–229. doi: 10.1038/nature05108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chen YF, Chiu WT, Chen YT, Lin PY, Huang HJ, Chou CY, et al. Calcium store sensor stromal-interaction molecule 1-dependent signaling plays an important role in cervical cancer growth, migration, and angiogenesis. Proc Natl Acad Sci USA. 2011;108:15225–15230. doi: 10.1073/pnas.1103315108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chen YT, Chen YF, Chiu WT, Wang YK, Chang HC, Shen MR. The ER Ca2+ sensor STIM1 regulates actomyosin contractility of migratory cells. J Cell Sci. 2013;126:1260–1267. doi: 10.1242/jcs.121129. [DOI] [PubMed] [Google Scholar]
- 126.Latour S, Mahouche I, Cherrier F, Azzi-Martin L, Velasco V, Soubeyran P, et al. Calcium independent effect of orai1 and STIM1 in non-Hodgkin B cell lymphoma dissemination. Cancers. 2018;10:402. doi: 10.3390/cancers10110402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yanamandra N, Buzzeo RW, Gabriel M, Hazlehurst LA, Mari Y, Beaupre DM, et al. Tipifarnib-induced apoptosis in acute myeloid leukemia and multiple myeloma cells depends on Ca2+ influx through plasma membrane Ca2+ channels. J Pharmacol Exp Ther. 2011;337:636–643. doi: 10.1124/jpet.110.172809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lepannetier S, Zanou N, Yerna X, Emeriau N, Dufour I, Masquelier J, et al. Sphingosine-1-phosphate-activated TRPC1 channel controls chemotaxis of glioblastoma cells. Cell Calcium. 2016;60:373–383. doi: 10.1016/j.ceca.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 129.Faouzi M, Hague F, Geerts D, Ay AS, Potier-Cartereau M, Ahidouch A, et al. Functional cooperation between KCa3.1 and TRPC1 channels in human breast cancer: role in cell proliferation and patient prognosis. Oncotarget. 2016;7:36419–36435. doi: 10.18632/oncotarget.9261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Guéguinou M, Harnois T, Crottes D, Uguen A, Deliot N, Gambade A, et al. SK3/TRPC1/Orai1 complex regulates SOCE-dependent colon cancer cell migration: a novel opportunity to modulate anti-EGFR mAb action by the alkyl-lipid Ohmline. Oncotarget. 2016;7:36168–36184. doi: 10.18632/oncotarget.8786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Elzamzamy OM, Penner R, Hazlehurst LA. The role of TRPC1 in modulating cancer progression. Cells. 2020;9:338. doi: 10.3390/cells9020388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Emmons MF, Anreddy N, Cuevas J, Steinberger K, Yang S, McLaughlin M, et al. MTI-101 treatment inducing activation of Stim1 and TRPC1 expression is a determinant of response in multiple myeloma. Sci Rep. 2017;7:2685. doi: 10.1038/s41598-017-02713-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Demaurex N, Poburko D, Frieden M. Regulation of plasma membrane calcium fluxes by mitochondria. Biochem Biophys Acta. 2009;1787:1383–1394. doi: 10.1016/j.bbabio.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 134.Duchen MR, Verkhratsky A, Muallem S. Mitochondria and calcium in health and disease. Cell Calcium. 2008;44:1–5. doi: 10.1016/j.ceca.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 135.Colombini M. VDAC structure, selectivity, and dynamics. Biochem Biophys Acta. 2012;1818:1457–1465. doi: 10.1016/j.bbamem.2011.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Magrì A, Reina S, De Pinto V. VDAC1 as Pharmacological target in cancer and neurodegeneration: focus on its role in apoptosis. Front Chem. 2018;6:108. doi: 10.3389/fchem.2018.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Seo JH, Agarwal E, Chae YC, Lee YG, Garlick DS, Storaci AM, et al. Mitochondrial fission factor is a novel Myc-dependent regulator of mitochondrial permeability in cancer. EBioMedicine. 2019;48:353–363. doi: 10.1016/j.ebiom.2019.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tan W, Colombini M. VDAC closure increases calcium ion flux. Biochem Biophys Acta. 2007;1768:2510–2515. doi: 10.1016/j.bbamem.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mazure NM. VDAC in cancer. Biochim Biophys Acta. 2017;1858:665–673. doi: 10.1016/j.bbabio.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 140.Keinan N, Pahima H, Ben-Hail D, Shoshan-Barmatz V. The role of calcium in VDAC1 oligomerization and mitochondria-mediated apoptosis. Biochem Biophys Acta. 2013;1833:1745–1754. doi: 10.1016/j.bbamcr.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 141.Weisthal S, Keinan N, Ben-Hail D, Arif T, Shoshan-Barmatz V. Ca(2+)-mediated regulation of VDAC1 expression levels is associated with cell death induction. Biochem Biophys Acta. 2014;1843:2270–2281. doi: 10.1016/j.bbamcr.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 142.Shoshan-Barmatz V, Ben-Hail D. VDAC, a multi-functional mitochondrial protein as a pharmacological target. Mitochondrion. 2012;12:24–34. doi: 10.1016/j.mito.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 143.Mahmoud MS, Ishikawa H, Fujii R, Kawano MM. Induction of CD45 expression and proliferation in U-266 myeloma cell line by interleukin-6. Blood. 1998;92:3887–3897. doi: 10.1182/blood.V92.10.3887. [DOI] [PubMed] [Google Scholar]
- 144.Sharaf el dein O, Gallerne C, Brenner C, Lemaire C. Increased expression of VDAC1 sensitizes carcinoma cells to apoptosis induced by DNA cross-linking agents. Biochem Pharmacol. 2012;83:1172–1182. doi: 10.1016/j.bcp.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 145.Baughman JM, Perocchi F, Girgis HS, Plovanich M, Belcher-Timme CA, Sancak Y, et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011;476:341–345. doi: 10.1038/nature10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.De Stefani D, Raffaello A, Teardo E, Szabò I, Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2011;476:336–340. doi: 10.1038/nature10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Marchi S, Pinton P. The mitochondrial calcium uniporter complex: molecular components, structure and physiopathological implications. J Physiol. 2014;592:829–839. doi: 10.1113/jphysiol.2013.268235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kamer KJ, Sancak Y, Mootha VK. The uniporter: from newly identified parts to function. Biochem Biophys Res Commun. 2014;449:370–372. doi: 10.1016/j.bbrc.2014.04.143. [DOI] [PubMed] [Google Scholar]
- 149.Lee Y, Min CK, Kim TG, Song HK, Lim Y, Kim D, et al. Structure and function of the N-terminal domain of the human mitochondrial calcium uniporter. EMBO Rep. 2015;16:1318–1333. doi: 10.15252/embr.201540436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol. 2003;4:552–565. doi: 10.1038/nrm1150. [DOI] [PubMed] [Google Scholar]
- 151.Landowski TH, Megli CJ, Nullmeyer KD, Lynch RM, Dorr RT. Mitochondrial-mediated disregulation of Ca2+ is a critical determinant of Velcade (PS-341/bortezomib) cytotoxicity in myeloma cell lines. Can Res. 2005;65:3828–3836. doi: 10.1158/0008-5472.CAN-04-3684. [DOI] [PubMed] [Google Scholar]
- 152.Song IS, Kim HK, Lee SR, Jeong SH, Kim N, Ko KS, et al. Mitochondrial modulation decreases the bortezomib-resistance in multiple myeloma cells. Int J Cancer. 2013;133:1357–1367. doi: 10.1002/ijc.28149. [DOI] [PubMed] [Google Scholar]
- 153.Ria R, Vacca A. Bone marrow stromal cells-induced drug resistance in multiple myeloma. Int J Mol Sci. 2020;21:613. doi: 10.3390/ijms21020613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Vazquez G, Wedel BJ, Bird GS, Joseph SK, Putney JW. An inositol 1,4,5-trisphosphate receptor-dependent cation entry pathway in DT40 B lymphocytes. EMBO J. 2002;21:4531–4538. doi: 10.1093/emboj/cdf467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Glitsch MD, Bakowski D, Parekh AB. Store-operated Ca2+ entry depends on mitochondrial Ca2+ uptake. EMBO J. 2002;21:6744–6754. doi: 10.1093/emboj/cdf675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Dang D, Rao R. Calcium-ATPases: Gene disorders and dysregulation in cancer. Biochem Biophys Acta. 2016;1863:1344–1350. doi: 10.1016/j.bbamcr.2015.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Vangheluwe P, Sepúlveda MR, Missiaen L, Raeymaekers L, Wuytack F, Vanoevelen J. Intracellular Ca2+- and Mn2+-transport ATPases. Chem Rev. 2009;109:4733–4759. doi: 10.1021/cr900013m. [DOI] [PubMed] [Google Scholar]
- 158.Verkhratsky A. Physiology and pathophysiology of the calcium store in the endoplasmic reticulum of neurons. Physiol Rev. 2005;85:201–279. doi: 10.1152/physrev.00004.2004. [DOI] [PubMed] [Google Scholar]
- 159.Brini M, Calì T, Ottolini D, Carafoli E. Calcium pumps: why so many? Compr Physiol. 2012;2:1045–1060. doi: 10.1002/cphy.c110034. [DOI] [PubMed] [Google Scholar]
- 160.Yadav J, Muend S, Zhang Y, Rao R. A phenomics approach in yeast links proton and calcium pump function in the Golgi. Mol Biol Cell. 2007;18:1480–1489. doi: 10.1091/mbc.e06-11-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Roti G, Carlton A, Ross KN, Markstein M, Pajcini K, Su AH, et al. Complementary genomic screens identify SERCA as a therapeutic target in NOTCH1 mutated cancer. Cancer Cell. 2013;23:390–405. doi: 10.1016/j.ccr.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Varga K, Hollósi A, Pászty K, Hegedűs L, Szakács G, Tímár J, et al. Expression of calcium pumps is differentially regulated by histone deacetylase inhibitors and estrogen receptor alpha in breast cancer cells. BMC Cancer. 2018;18:1029. doi: 10.1186/s12885-018-4945-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ribiczey P, Papp B, Homolya L, Enyedi Á, Kovács T. Selective upregulation of the expression of plasma membrane calcium ATPase isoforms upon differentiation and 1,25(OH)2D3-vitamin treatment of colon cancer cells. Biochem Biophys Res Commun. 2015;464:189–194. doi: 10.1016/j.bbrc.2015.06.113. [DOI] [PubMed] [Google Scholar]
- 164.Richardson DA, Sritangos P, James AD, Sultan A, Bruce JIE. Metabolic regulation of calcium pumps in pancreatic cancer: role of phosphofructokinase-fructose-bisphosphatase-3 (PFKFB3) Cancer Metab. 2020;8:2. doi: 10.1186/s40170-020-0210-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Monteith GR, Davis FM, Roberts-Thomson SJ. Calcium channels and pumps in cancer: changes and consequences. J Biol Chem. 2012;287:31666–31673. doi: 10.1074/jbc.R112.343061. [DOI] [PMC free article] [PubMed] [Google Scholar]