Abstract
Background and Objectives
Peripheral injection of dexmedetomidine (DEX) has been widely used in regional anesthesia to prolong the duration of analgesia. However, the optimal perineural dose of DEX is still uncertain. It is important to elucidate this characteristic because DEX may cause dose-dependent complications. The aim of this meta-analysis was to determine the optimal dose of perineural DEX for prolonged analgesia after brachial plexus block (BPB) in adult patients undergoing upper limb surgery.
Method
A search strategy was created to identify suitable randomized clinical trials (RCTs) in Embase, PubMed and The Cochrane Library from inception date to Jan, 2021. All adult patients undergoing upper limb surgery under BPB were eligible. The RCTs comparing DEX as an adjuvant to local anesthetic (LA) with LA alone for BPB were included. The primary outcome was duration of analgesia for perineural DEX. Secondary outcomes included visual analog scale (VAS) in 12 and 24 h, consumption of analgesics in 24 h, and adverse events.
Results
Fifty-seven RCTs, including 3332 patients, were identified. The subgroup analyses and regression analyses revealed that perineural DEX dose of 30-50 μg is an appropriate dosage. With short−/intermediate-acting LAs, the mean difference (95% confidence interval [CI]) of analgesia duration with less than and more than 60 μg doses was 220.31 (153.13–287.48) minutes and 68.01 (36.37–99.66) minutes, respectively. With long-acting LAs, the mean differences (95% CI) with less than and more than 60 μg doses were 332.45 (288.43–376.48) minutes and 284.85 (220.31–349.39) minutes.
Conclusion
30-50 μg DEX as adjuvant can provides a longer analgesic time compared to LA alone and it did not increase the risk of bradycardia and hypotension.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12871-021-01452-0.
Keywords: Perineural dexmedetomidine, Adjuvant, Brachial plexus block, meta-analysis
Introduction
Upper limb surgery is often performed under brachial plexus block (BPB), which is a series of regional anesthesia techniques and also contributes to reliable postoperative analgesia [1]. Single block and continuous catheter-based block are two different anesthesia regimens. Compared with continuous catheter-based block, more and more anesthesiologists prefer single block, because the catheter placement requires additional time, cost, and increases the risk of infection and neurological complications [2]. In order to prolong the time of single nerve block analgesia, more and more anesthesiologists add adjuvants to local anesthetics (LAs) [3]. Over the past decade, adjuvants of local anesthetics such as opioids [4], epinephrine [5], clonidine [6], magnesium [7], midazolam [8], dexamethasone [9], buprenorphine [10] and dexmedetomidine (DEX) [11] have been proved to prolong the analgesic time of nerve block, and have achieved varying degrees of success. Among these different kinds of adjuvants, DEX is more widely used. However, these adjuvants have different defects, such as the need for special equipment and monitoring, or the risk of complications that may delay discharge or lead to readmission [12].
Several prior meta-analyses [13–18] draw a conclusion that DEX is an effective perineural adjunct to LAs for producing prolonged analgesia duration. However, the use of DEX is not risk-free and may lead to complications in a dose-dependent manner, including hypertension, hypotension, bradycardia, excessive sedation, sleepiness, etc. It is vital to evaluate the optimal dose of perineural DEX that maximizes the analgesic benefit while minimizing associated perioperative risk. Since the publication of the previous meta-analysis, a large number of papers have been published focusing on different doses of peripheral DEX for BPB. The objective of current systematic review and meta-analysis was therefore to define the optimal dose of perineural DEX that prolongs analgesia after BPB in adult patients undergoing upper limb surgery.
Materials and methods
This investigation followed the recommended process described in the “Preferred Reporting Items for Systematic Reviews and Meta-Analyses [19]” extension statement for reporting meta-analyses, and the protocol was registered on the International Platform of Registered Systematic Review and Meta-analysis Protocols (INPLASY; registration number: INPLASY202110066). A preliminary search suggested that vast majority of the published comparisons of interest have been conducted in the setting of BPB. Consequently, we decided to focus on the population of patients having upper limb surgery under BPB.
Search strategy
Two authors (H Cai and X Fan) independently searched the electronic database including Embase, PubMed, and Cochrane Library from inception date to Jan, 2021. The search was restricted to articles in the English language. The online literature was searched using the following combination of medical subject heading terms and entry terms: “Brachial Plexus Block” or “Block, Brachial Plexus” or “Blocks, Brachial Plexus” or “Brachial Plexus Blocks” or “Brachial Plexus Anesthesia” or “Anesthesia, Brachial Plexus” or “Brachial Plexus Blockade” or “Blockade, Brachial Plexus” or “Blockades, Brachial Plexus” or “Brachial Plexus Blockades” or “Plexus Blockade, Brachial” or “Plexus Blockades, Brachial”. These search results were combined with “Dexmedetomidine” or “Dexmedetomidine Hydrochloride” or “MPV-1440” or “MPV1440” or “Precedex” or “MPV 1440” or “Hydrochloride, Dexmedetomidine”. We limited our search to title and abstract. Furthermore, the two authors (H Cai and X Fan) looked through the references of the relative papers to find additional studies.
Including and excluding criteria
Studies were included if they met the following criteria: (1) only randomized clinical trials (RCTs); (2) comparison between perineural DEX with LA and only LA in single-injection BPB for upper limb surgery; (3) adult patients; and (4) in English.
Studies were excluded if they were (1) non-RCTs; (2) continuous or repeated nerve blocks; (3) DEX administered through non-perineural route or without LAs; (4) retracted articles; (5) Lack of relevant outcomes.
Four trials [20–23] investigated the effect of different dose of perineural DEX with LA by allocating patients into different separate groups were considered for the purpose of this meta-analysis. Trials [24–26] investigating the effect of perineural DEX with another perineural adjunct or without a placebo group, administering systemic DEX to all patients [27], or administering other α-2 agonist [28] than DEX were excluded.
Assessment of methodological quality
Two reviewers (H Cai and P Feng) independently applied inclusion criteria from a review of the titles, abstracts, and keywords. Inconsistencies were settled by discussion or through consultation with the supervisor (Y Xie) until a consensus was reached. References were then searched by hand by the reviewer (H Cai and P Feng).
The reviewers (H Cai and P Feng) independently evaluated the methodological quality of the included RCTs according to the Cochrane Collaboration’s Risk of Bias Tool [29]. Studies were assessed for random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessors, incomplete outcome data, selective reporting, and any other potential source of bias. The results of every trial were used following consensus between the 2 reviewers. Inconsistencies were settled by discussion or through consultation with the superior reviewer (Y Xie) until a consensus was reached.
Data extraction and outcome assessment
Two reviewers (H Cai and X Wang) independently extracted the data from articles including first author, publication year, sample size, nerve localization techniques, perineural DEX dosage or dosages per average body weight, LA concentration and volume, and types. If they disagreed with each other, disagreements were either discussed to reach a consensus between the 2 reviewers or decided by superior (Y Xie). The source study text and tables were used to extract means, standard deviations (SDs), number of events, and total number of participants. If the trials just provided graphs, we extract data using GetData Graph Digitizer software [30]. The median and interquartile range were used for mean and SD approximations as follows: the mean was estimated as equivalent to the median and the SD was approximated to be the interquartile range divided by 1.35 or the 95% CI range divided by 4 [31]. All opioids were converted into equianalgesic doses of intravenous (IV) morphine for analysis (IV morphine 10 mg = oral morphine 30 mg = IV hydromorphone 1.5 mg = oral hydromorphone 7.5 mg = IV pethidine 75 mg = oral oxycodone 20 mg = IV tramadol 100 mg = intramuscular diclofenac 100 mg) [32]. Pain scores reported as visual, verbal, or numeric rating scales were converted to a standardized 0–10 analog scale for quantitative evaluations.
The primary outcome was duration of analgesia, defined as the time interval between block performance or onset time of sensory blockade and the time of first analgesic request or initial pain report [33]. The secondary outcomes included VAS in 12 and 24 h postoperatively, cumulative IV morphine consumption at 24 h postoperatively, and adverse events such as bradycardia and hypotension.
Statistical analysis
One reviewer (H Cai) input the data and another (X Fan) checked its accuracy. Meta-analysis was implemented using Review Manager software (RevMan for Windows, version 5.4, Cochrane Collaboration, Oxford, UK). We estimated the mean differences for continuous data and risk difference for categorical data between groups, with an overall estimate of the pooled effect. The χ2 test was used for heterogeneity analysis, and heterogeneity was assessed by I2. If I2 < 50%, the fixed effects model was used; if I2 ≥ 50%, the random effects model was used and the heterogeneity was assessed [15]. Our primary outcome, duration of analgesia, was analyzed according to the dose of perineural DEX injected for each type of LA (short−/intermediate-acting LAs and long-acting LAs). We further undertook an exploratory analysis for each type of LAs in an attempt to account for heterogeneity and grouped trials by DEX dosage group (low doses: ≤ 60 μg; moderate doses: > 60 μg), by BPB localization (interscalene, supraclavicular, infraclavicular, axillary) and by regional anesthetic technique (anatomic landmarks, nerve stimulation, ultrasound). Finally, the relationship between dose of perineural DEX and mean increase in duration of analgesia was investigated for each type of local anesthetic with a regression analyses using the JMP 13 statistical package (SAS Institute, Cary, NC) [32]. The likelihood of publication bias was assessed by drawing a funnel plot of standard error of the mean difference (y-axis) as a function of the mean difference (x-axis) of our primary outcome [33]. This assessment was performed using STATA software (STATA for Windows, version 16.0, Stata Corp, Texas, USA). Results are presented as the mean difference or risk difference with 95% CI. A 2-sided P value < 0.05 was considered significant.
Results
Search results
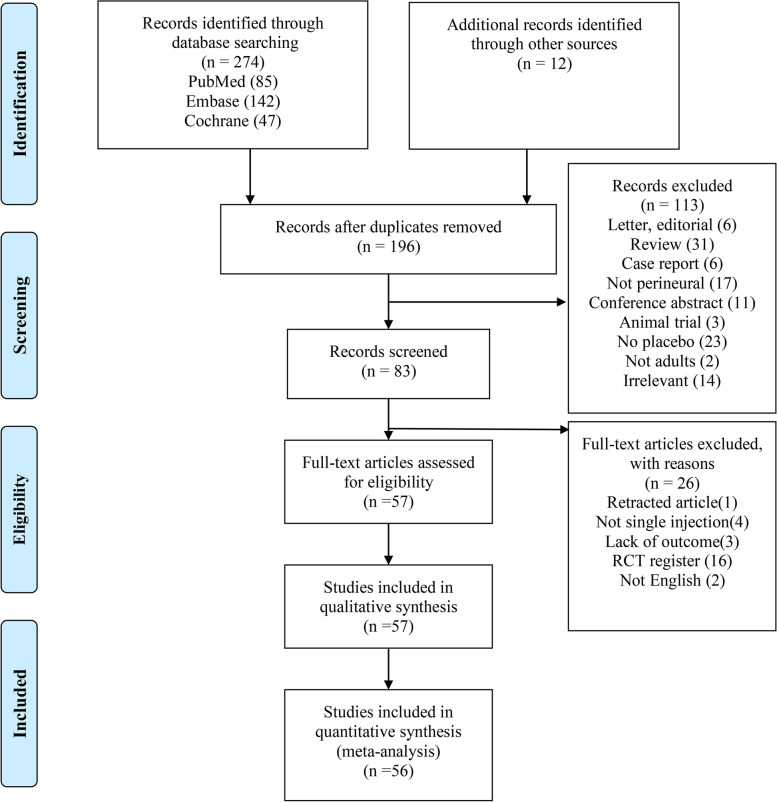
Of the 286 trials identified from the literature search strategy and other sources, 57 RCTs [20–23, 34–86] met the inclusion criteria, representing a total of 3332 patients. Among the 286 articles, 90 duplicate articles were excluded initially. Then, 113 articles were excluded after screened titles and abstracts. 26 articles were excluded after full-text reading for the following reasons: retracted article, not single injection, lack of required outcomes, RCT registration, not English. Finally, 57 RCTs remained eligible to meet the inclusion criteria for the current meta-analysis. And the flow diagram of study selection is shown in (Fig. 1).
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram summarizing included and excluded randomized controlled trials
Study characteristics
A detailed description of all the included studies is shown in (Table 1). All of the included studies were published between the years 2010 and 2020. The vast majority of the studies were conducted at international centers in Asia. Across all included studies, a total of 3332 patients were assessed. DEX was used as an adjuvant to several different local anesthetics, which included ropivacaine [23, 34, 39, 41, 42, 45–47, 49, 50, 52, 56, 57, 59, 63–66, 68, 72–74, 76, 78, 80, 81, 84, 85], bupivacaine [20, 35, 37, 38, 40, 51, 53–55, 67, 69, 71], levobupivacaine [21, 43, 44, 48, 60–62, 79, 83], and lidocaine [22, 36, 70, 77, 86]. Across the studies, the dose of DEX ranged from 0.5 μg/kg to a total of 150 μg. Local anesthetic dosages also varied across the studies.
Table 1.
Characteristics of including trials
| Author | Year | Country | Groups(n) | CON of LA-Total volume | DEX dose | Weight (kg) | Block/location | Technique | Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Hwang | 2020 | Korea | 1.Ropivacaine + DEX (25) 2.Ropivacaine + NS (25) | 0.75%-9 ml | 100 μg | 68.50 ± 13.20 | Interscalene | Ultrasound | DOA, VAS |
| Nicholas | 2020 | India | 1.Ropivacaine + DEX (27) 2.Ropivacaine + NS (27) | 0.5%-21 ml | 0.5 μg/kg | 68.89 | Axillary | Ultrasound | AE |
| Shahtaheri | 2020 | Iran | 1.Lidocaine + DEX (33) 2.Lidocaine + NS (33) | 1.5%-35 ml | 0.5 μg/kg | NR | Axillary | Nerve stimulator | OC, VAS |
| Singh | 2020 | India | 1.Ropivacaine + DEX (20) 2.Ropivacaine + NS (20) | 0.5%-30 ml | 1 μg/kg | 67.50 ± 9.30 | Supraclavicular | Ultrasound | DOA, OC, AE |
| Avula | 2019 | India | 1.Bupivacaine + DEX (30) 2.Bupivacaine + NS (30) | 0.5%-20.75 ml | 75 μg | 68.83 ± 5.38 | Supraclavicular | Ultrasound | DOA, AE |
| Hassan | 2019 | Egypt | 1.Bupivacaine + Lidocaine + DEX (15) 2.Bupivacaine + Lidocaine + NS (15) | 0.5–2%-25 ml | 100 μg | NR | Supraclavicular | Ultrasound | DOA |
| Nazir | 2019 | India | 1.Ropivacaine + DEX (30) 2.Ropivacaine + NS (30) | 0.5%-30 ml | 50 μg | 64.88 ± 6.70 | Supraclavicular | Nerve stimulator | DOA |
| Sharma | 2019 | Nepal | 1.Ropivacaine + DEX (30) 2.Ropivacaine + NS (30) | 0.5%-31 ml | 0.75 μg/kg | 64.30 ± 5.90 | Supraclavicular | Ultrasound | DOA, AE |
| Singh | 2019 | India | 1.Ropivacaine + DEX (30) 2.Ropivacaine + NS (30) | 0.5%-31 ml | 100 μg | 53.33 ± 8.21 | Supraclavicular | Nerve stimulator | DOA, AE |
| Yaghoobi | 2019 | Iran | 1.Lidocaine + DEX (25) 2.Lidocaine + NS (25) | 2%-30 ml | 1 μg/kg | NR | Infraclavicular | Ultrasound | DOA |
| Akhondzadeh | 2018 | Iran | 1.Lidocaine + DEX (36) 2.Lidocaine + NS (31) | 2%-30 ml | 1 μg/kg | 68.72 ± 7.87 | Supraclavicular | Ultrasound | DOA, VAS, OC |
| Elyazed | 2018 | Egypt | 1.Ropivacaine + DEX (35) 2.Ropivacaine + NS (35) | 0.5%-39 ml | 100 μg | 76.50 ± 8.90 | Infraclavicular | Ultrasound | DOA, VAS, OC, AE |
| Hamed | 2018 | Egypt | 1.Bupivacaine + DEX (20) 2.Bupivacaine + NS (20) | 1.5 mg/kg-40 ml | 1 μg/kg | 72.80 ± 7.30 | Supraclavicular | Ultrasound | DOA, AE |
| He | 2018 | China | 1.Ropivacaine + DEX (28) 2.Ropivacaine + NS (28) | 0.375%-40 ml | 1 μg/kg | 77.32 ± 14.18 | Coracoid approach | Nerve stimulator | DOA, VAS, OC |
| Jung | 2018 | Korea | 1.Levobupivacaine + DEX (25) 2.Levobupivacaine + DEX (25) 3.Levobupivacaine + DEX (24) 4.Levobupivacaine + NS (23) | 0.5%-22 ml | 1 μg/kg 1.5 μg/kg 2 μg/kg | 69.37 ± 11.33 63.81 ± 9.44 69.80 ± 16.85 | Interscalene | Ultrasound | DOA, VAS, OC |
| Kaur | 2018 | India | 1.Levobupivacaine + DEX (40) 2.Levobupivacaine + NS (40) | 0.5%-30 ml | 1 μg/kg | NR | Supraclavicular | Ultrasound | DOA, AE |
| Koraki | 2018 | Greece | 1.Ropivacaine + DEX (19) 2.Ropivacaine + NS (18) | 0.5%-16 ml | 100 μg | NR | Axillary | Ultrasound | DOA, AE |
| Liu | 2018 | China | 1.Ropivacaine + DEX (57) 2.Ropivacaine + NS (57) | 0.375%-20 ml | 100 μg | NR | NR | Nerve stimulator | DOA, VAS, AE |
| Mangal | 2018 | India | 1.Ropivacaine + DEX (44) 2.Ropivacaine + NS (43) | 0.75%-22 ml | 1 μg/kg | 60.36 ± 6.41 | Supraclavicular | Ultrasound | DOA, OC, AE |
| Mathew | 2018 | India | 1.Ropivacaine + DEX (20) 2.Ropivacaine + NS (20) | 0.5%-30 ml | 1 μg/kg | 68.35 ± 11.70 | Supraclavicular | Ultrasound | DOA |
| Pillai | 2018 | India | 1.Bupivacaine + DEX (33) 2.Bupivacaine + DEX (33) 3.Bupivacaine + NS (33) | 0.5%-27 ml | 20 μg 40 μg | 63.52 ± 14.14 66.64 ± 13.62 | Supraclavicular | Ultrasound | DOA |
| Aksu | 2017 | Turkey | 1.Bupivacaine + DEX (25) 2.Bupivacaine + NS (25) | 0.33%-15 ml | 1 μg/kg | 76.40 ± 10.80 | Supraclavicular | Ultrasound, Nerve stimulator | DOA, AE |
| Bisui | 2017 | India | 1.Levobupivacaine + DEX (34) 2.Levobupivacaine + NS (33) | 0.5%-30 ml | 0.75 μg/kg | 59.00 ± 7.64 | Supraclavicular | Nerve stimulator | DOA, VAS |
| Chinnappa | 2017 | UAE | 1.Ropivacaine + DEX (30) 2.Ropivacaine + NS (30) | 0.5%-31 ml | 1 μg/kg | 64.90 ± 11.30 | Supraclavicular | Nerve stimulator | DOA, AE |
| Farooq | 2017 | India | 1.Ropivacaine + DEX (35) 2.Ropivacaine + NS (35) | 0.75%-35 ml | 1 μg/kg | 50.90 ± 10.20 | Interscalene | Nerve stimulator | DOA |
| Rashmi | 2017 | India | 1.Ropivacaine + DEX (30) 2.Ropivacaine + NS (30) | 0.75%-30.5 ml | 50 μg | 61.00 ± 12.16 | Interscalene | Nerve stimulator | DOA |
| Thakur | 2017 | India | 1.Lidocaine + DEX (30) + adrenaline 2.Lidocaine + DEX (30) + adrenaline 3.Lidocaine + NS (30) + adrenaline | 2%-30 ml | 1 μg/kg 0.5 μg/kg | 50.77 ± 10.64 48.37 ± 10.48 | Axillary | Landmark | DOA |
| Wang | 2017 | China | 1.Ropivacaine + DEX (31) 2.Ropivacaine + NS (27) | 0.5%-25 ml | 0.75 μg/kg | 61.00 ± 5.00 | Interscalene | Nerve stimulator | AE |
| Abdallah | 2016 | Canada | 1.Ropivacaine + DEX (33) 2.Ropivacaine + NS (32) | 0.5%-16 ml | 0.5 μg/kg | 82.3 | Interscalene | Ultrasound | DOA, OC, AE, VAS |
| Arun | 2016 | India | 1.Ropivacaine + DEX (30) 2.Ropivacaine + NS (30) | 0.75%-26 ml | 50 μg | 68.57 ± 2.00 | Axillary | Nerve stimulator | DOA, AE |
| Bangera | 2016 | India | 1.Ropivacaine + DEX (40) 2.Ropivacaine + NS (40) | 0.375%-40 ml | 1 μg/kg | 60.33 ± 7.11 | Axillary | Nerve stimulator | DOA, AE |
| Lee | 2016 | Korea | 1.Ropivacaine + DEX (17) 2.Ropivacaine + NS (17) | 0.5%-22 ml | 100 μg | 65.6 ± 4.80 | Axillary | Ultrasound, Nerve stimulator | AE |
| Nazir | 2016 | India | 1.Bupivacaine + DEX (35) 2.Bupivacaine + NS (35) | 0.25%-40 ml | 1 μg/kg | 52.00 ± 8.70 | Supraclavicular | Ultrasound | DOA |
| Singh | 2016 | India | 1.Levobupivacaine + DEX (29) 2.Levobupivacaine + NS (28) | 0.5%-31 ml | 100 μg | NR | Supraclavicular | Nerve stimulator | DOA, AE |
| Tandon | 2016 | India | 1.Levobupivacaine + DEX (30) 2.Levobupivacaine + NS (30) | 0.5%-31 ml | 100 μg | 63.10 ± 4.28 | Supraclavicular | Nerve stimulator | DOA, AE |
| Bharti | 2015 | India | 1.Ropivacaine + lidocaine + DEX (27) + adrenaline 2.Ropivacaine + lidocaine + NS (27) + adrenaline | 0.75–2%-0.5 ml/kg | 1 μg/kg | 62.52 ± 9.22 | Supraclavicular | Ultrasound | DOA, AE |
| Gurajala | 2015 | India | 1.Ropivacaine + DEX (16) 2.Ropivacaine + NS (15) | 0.5%-35 ml | 50 μg | 62.13 ± 13.61 | Supraclavicular | Nerve stimulator | DOA, AE |
| Karthik | 2015 | India | 1.Levobupivacaine + DEX (50) 2.Levobupivacaine + NS (50) | 0.5%-40 ml | 1 μg/kg | 66.42 ± 5.34 | Axillary | Landmark | AE |
| Kathuria | 2015 | India | 1.Ropivacaine + DEX (20) 2.Ropivacaine + NS (20) | 0.5%-30 ml | 50 μg | 72.00 ± 11.67 | Supraclavicular | Ultrasound | DOA, OC |
| Kaur | 2015 | India | 1.Levobupivacaine+lidocaine+DEX (50) 2.Levobupivacaine+lidocaine+NS (50) | 0.25–1%-40 ml | 1 μg/kg | 66.18 ± 7.32 | Supraclavicular | Nerve stimulator | DOA |
| Manohar | 2015 | India | 1.Bupivacaine + DEX (30) 2.Bupivacaine + NS (30) | 0.5%-30 ml | 50 μg | 53.26 ± 10.49 | Supraclavicular | Nerve stimulator | DOA, AE |
| Tiwari | 2015 | India | 1.Ropivacaine + DEX (60) 2.Ropivacaine + NS (60) | 0.75%-20 ml | 50 μg | 60.27 ± 9.11 | Supraclavicular | Ultrasound | DOA, AE |
| Agarwal | 2014 | India | 1.Bupivacaine + DEX (25) 2.Bupivacaine + NS (25) | 0.325%-31 ml | 100 μg | 64.00 ± 9.40 | Supraclavicular | Nerve stimulator | DOA, AE |
| Biswas | 2014 | India | 1.Levobupivacaine + DEX (30) 2.Levobupivacaine + NS (30) | 0.5%-36 ml | 100 μg | 71.36 ± 9.38 | Supraclavicular | Nerve stimulator | DOA |
| Fritsch | 2014 | Austria | 1.Ropivacaine + DEX (16) 2.Ropivacaine + NS (15) | 0.5%-12 ml | 150 μg | NR | Interscalene | Ultrasound | VAS |
| Megha | 2014 | India | 1.Bupivacaine + lidocaine + DEX (20) 2.Bupivacaine + lidocaine + NS (20) | 0.5–2%-30 ml | 50 μg | NR | Supraclavicular | Nerve stimulator | DOA, AE |
| Mirkheshti | 2014 | Iran | 1.Lidocaine + DEX (34) 2.Lidocaine + NS (34) | 1.5%-30 ml | 100 μg | 72.00 ± 9.00 | Infraclavicular | Ultrasound | DOA |
| Nema | 2014 | India | 1.Ropivacaine + DEX (30) 2.Ropivacaine + NS (30) | 0.75%-30 ml | 50 μg | NR | Supraclavicular | Landmark | DOA |
| Song | 2014 | Korea | 1.Mepivacaine + DEX(10) 2.Mepivacaine + NS(10) | 1%-40 ml | 1 μg/kg | 64.80 ± 9.60 | Infraclavicular | Nerve stimulator | DOA |
| Zhang | 2014 | China | 1.Ropivacaine + DEX (15) 2.Ropivacaine + DEX (15) 3.Ropivacaine + NS (15) | 0.33%-41 ml | 100 μg 50 μg | 66.40 ± 7.85 65.47 ± 12.14 | Axillary | Nerve stimulator | AE |
| Dar | 2013 | India | 1.Ropivacaine + DEX (40) 2.Ropivacaine + NS (40) | 0.5%-41 ml | 50 μg | 72.20 ± 9.01 | Axillary | Nerve stimulator | DOA, AE |
| Patki | 2013 | India | 1.Ropivacaine + DEX (30) 2.Ropivacaine + NS (30) | 0.5%-30.5 ml | 50 μg | NR | Supraclavicular | Landmark | DOA |
| Ammar | 2012 | Egypt | 1.Bupivacaine + DEX (30) 2.Bupivacaine + NS (30) | 0.33%-30 ml | 0.75 μg/kg | 80.50 ± 9.50 | Infraclavicular | Ultrasound | DOA, OC |
| Gandhi | 2012 | India | 1.Bupivacaine + DEX (35) 2.Bupivacaine + NS (35) | 0.25%-40 ml | 30 μg | 51.40 ± 10.60 | Supraclavicular | Landmark | DOA, AE |
| Hanoura | 2012 | Egypt | 1.Bupivacaine + DEX (25) 2.Bupivacaine + NS (25) | 0.25%-41 ml | 100 μg | NR | Axillary | Nerve stimulator | DOA, VAS |
| Kaygusuz | 2012 | Turkey | 1.Levobupivacaine + DEX (30) 2.Levobupivacaine + NS (30) | 0.5%-40 ml | 1 μg/kg | 73.75 ± 8.11 | Axillary | Nerve stimulator | DOA |
| Esmaoglu | 2010 | Turkey | 1.Levobupivacaine + DEX (30) 2.Levobupivacaine + NS (30) | 0.5%-41 ml | 100 μg | 72.20 ± 9.01 | Axillary | Nerve stimulator | DOA, AE |
Abbreviations: CON, concentration; LA, local anesthetics; DEX, dexmedetomidine; kg, kilogram; NS, normal saline; ml, milliliter; μg, microgram; DOA, duration of analgesia; VAS, visual analogue scale; OC, Opioid consumption; AE, adverse event; NR, not reported
Risk-of-bias assessment of included studies
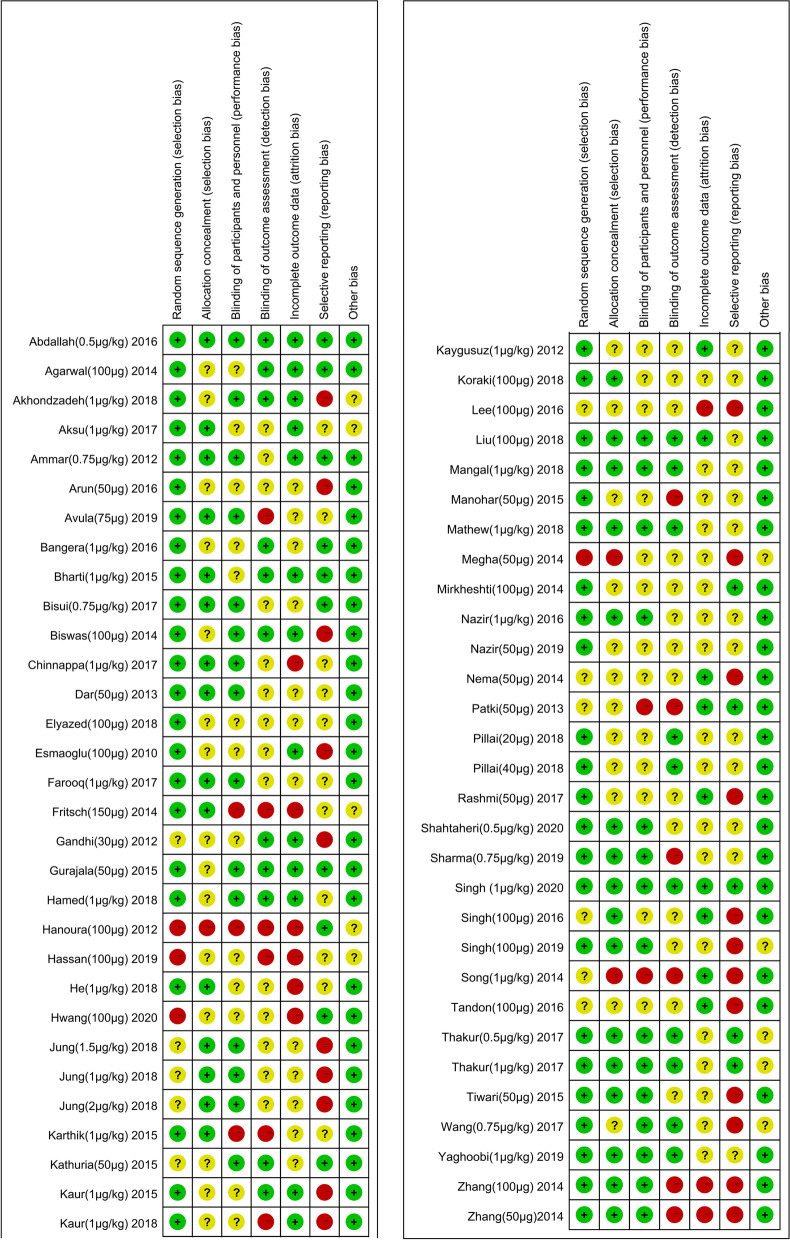
Two independent reviewers (H Cai and P Feng) assessed the risk-of-bias of all included studies. The vast majority of the studies had an unclear risk of bias due to the lack of sufficient methodological reporting. Several studies were classified as high risk of bias for allocation concealment due to the lack of clarity in methods used. A full risk-of-bias summary for all included studies is shown in (Fig. 2). Visual inspection of the funnel plot for primary outcomes suggests obviously publication bias.
Fig. 2.
Risk of bias summary. Review authors’ judgements about each risk of bias item for each included study. Green circle, low risk of bias; orange circle, high risk of bias; yellow circle, unclear risk of bias
Duration of analgesia
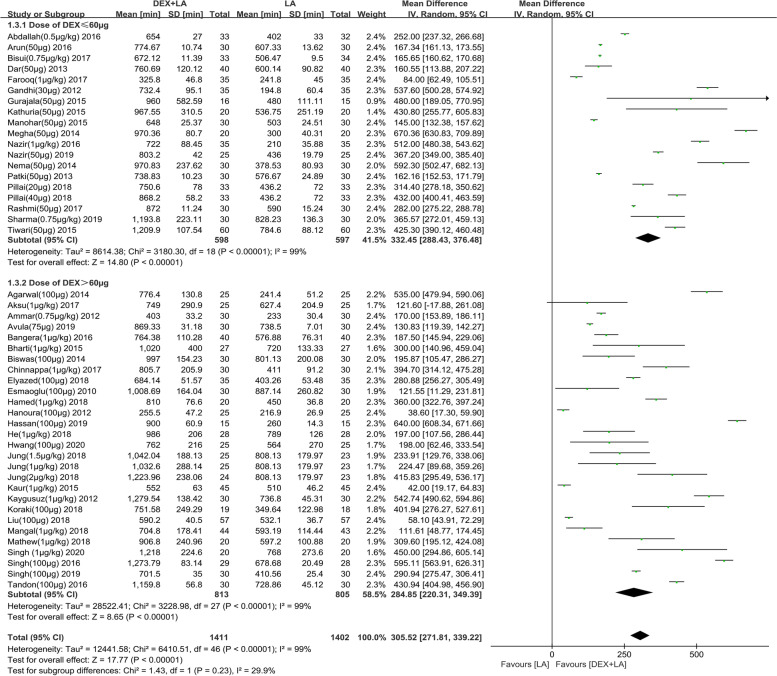
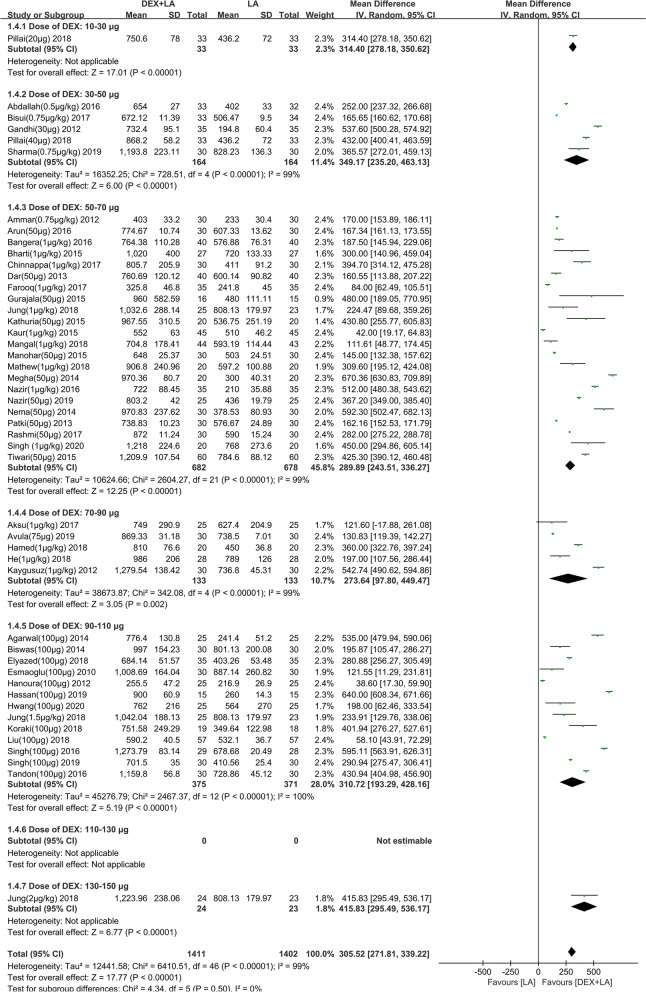
The duration of analgesia was assessed by 50 studies [20–22, 34–49, 51–57, 59–63, 65–72, 75, 76, 78–84, 86], all of them (n = 3218) had sufficient information to allow for pooling. With short−/intermediate-acting LAs, the mean difference (95% confidence interval [CI]) of duration of analgesia with ≤60 μg and >60 μg DEX were 220.31 (153.13 to 287.48) minutes and 68.01 (36.37 to 99.66) minutes, respectively (test for subgroup difference: P<0.0001) (Additional file 1). The forest plot for subgroup analysis of short−/intermediate-acting LAs by dose group was not available because of the lack of sufficient data. With long-acting LAs, the mean difference (95% CI) of duration of analgesia with ≤60 μg and>60 μg DEX were 332.45 (288.43 to 376.48) minutes and 284.85 (220.31 to 349.39) minutes, respectively (test for subgroup difference: P = 0.23) (Fig. 3). The forest plot for subgroup analysis of long-acting LAs by different dose group indicated that 30-50 μg DEX as adjuvant could prolong the duration of analgesia by 349.17 min compared with LA alone (95% CI: 235.20 to 463.13 min) (Fig. 4). With the obvious heterogeneity the subgroup analysis was conducted according to types of BPB approaches and location technology (Additional file 2). Unfortunately, we still did not find the source of heterogeneity. Regression analysis showed that the mean line and fitting line overlapped, and basically in the horizontal position when combined with long-acting LAs (R2 = 0.001408; P<0.0001) (Additional file 3). However, when combined with short−/intermediate-acting LAs, regression analysis showed that the angle between the mean line and the fitting line is large (R2 = 0.55371; P = 0.0465) (Additional file 4). The above indicated that DEX as LA adjuvants on BPB significantly prolonged the duration of analgesia. Subgroup analysis and regression analysis showed that 30-50 μg DEX could prolong the duration of analgesia up to about 5 h.
Fig. 3.
Effect of perineural DEX by dose administered (≤60 μg or>60 μg) on DOA when combined with long-acting LA. Abbreviations: DEX, dexmedetomidine; CI, confidence interval; DOA, duration of analgesia; LA, local anesthetic; IV, intravenous
Fig. 4.
Subgroup analysis by 20 μg increments of perineural DEX on DOA when combined with long-acting LA. Abbreviations: DEX, dexmedetomidine; CI, confidence interval; DOA, duration of analgesia; LA, local anesthetic; IV, intravenous
Pain-related outcome
Ten studies [21, 44, 47, 50, 54, 56, 57, 65, 77, 80] evaluated the pain score at 12 h postoperatively, and nine studies [21, 34, 47, 50, 56, 57, 65, 77, 80] for 24 h. It was found that the Pain score at rest at 12 h postoperatively was significantly reduced with perineural DEX. However, the pain score at rest at 24 h postoperatively was not statistically significant. Meanwhile, seven studies [21, 34, 36, 38, 47, 56, 59] accessed the anesthetic consumption in 24 h after surgery. It shows that DEX, no matter less than or more than 60 μg, can reduce the consumption of IV morphine in 24 h after operation. In conclusion, DEX as adjuvant can reduce postoperative pain score in 12 h and reduce the consumption of postoperative analgesics (Table 2).
Table 2.
Pain-related outcomes (Abbreviations: RCT, randomized Clinical Trial; DEX, dexmedetomidine; LA, local anesthetic; CI, confidence interval)
| Outcome | RCT | DEX + LA | LA | Mean Difference (95% CI) | I2(%) | P Value for Overall Effect | P Value for Subgroup Differences | Quality of Evidence (GRADE) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | N | Mean (SD) | N | |||||||
| Pain score at rest at 12 h postoperatively (analog scale, 0–10) | ||||||||||
| DEX ≤ 60 μg | Bisui (0.75 μg/kg) 2017 | 4.27(0.45) | 33 | 5.76(0.65) | 34 | −1.43(−1.88 to −0.99) | 8 | <0.00001 | ||
| Shahtaheri (0.5 μg/kg) 2020 | 0.50(4.76) | 33 | 1.02(2.33) | 33 | ||||||
| DEX>60 μg | Elyazed (100 μg) 2018 | 1.90(1.00) | 35 | 2.86(0.60) | 35 | − 1.63(−2.07 to − 1.20) | 76 | <0.00001 | ||
| Fritsch (150 μg) 2014 | 0.23(0.97) | 16 | 2.19(1.76) | 15 | ||||||
| Hanoura (100 μg) 2012 | 3.70(1.20) | 25 | 4.50(1.40) | 25 | ||||||
| He (1 μg/kg) 2018 | 0.93(0.98) | 28 | 1.93(1.15) | 28 | ||||||
| Hwang (100 μg) 2020 | 4.20(2.50) | 25 | 6.10(2.20) | 25 | ||||||
| Jung (1.5 μg/kg) 2018 | 0.61(0.99) | 25 | 2.80(1.40) | 23 | ||||||
| Jung (1 μg/kg) 2018 | 0.52(0.79) | 25 | 2.80(1.40) | 23 | ||||||
| Jung (2 μg/kg) 2018 | 0.00(0.00) | 24 | 2.80(1.40) | 23 | ||||||
| Liu (100 μg) 2018 | 2.20(0.90) | 57 | 4.20(1.10) | 57 | ||||||
| Singh (1 μg/kg) 2020 | 0.00(0.74) | 20 | 2.00(2.04) | 20 | ||||||
| total | −1.57(−1.91 to −1.23) | 71 | <0.00001 | 0.53 | Very low | |||||
| Pain score at rest at 24 h postoperatively (analog scale, 0–10) | ||||||||||
| DEX ≤ 60 μg | Abdallah (0.5 μg/kg) 2016 | 5.50(0.45) | 33 | 5.60(0.45) | 32 | −0.10(−0.32 to 0.12) | 0 | 0.38 | ||
| Shahtaheri (0.5 μg/kg) 2020 | 0.97(3.24) | 33 | 0.90(5.58) | 33 | ||||||
| DEX>60 μg | Elyazed (100 μg) 2018 | 1.60(0.50) | 35 | 1.80(0.60) | 35 | −0.71(−1.93 to 0.52) | 98 | 0.26 | ||
| Fritsch (150 μg) 2014 | 2.68(2.93) | 16 | 2.10(2.20) | 15 | ||||||
| He (1 μg/kg) 2018 | 1.54(0.84) | 28 | 4.36(1.31) | 28 | ||||||
| Hwang (100 μg) 2020 | 3.30(1.30) | 25 | 3.70(1.50) | 25 | ||||||
| Jung (1.5 μg/kg) 2018 | 5.13(1.15) | 25 | 4.35(1.01) | 23 | ||||||
| Jung (1 μg/kg) 2018 | 4.10(1.04) | 25 | 4.35(1.01) | 23 | ||||||
| Jung (2 μg/kg) 2018 | 4.90(1.20) | 24 | 4.35(1.01) | 23 | ||||||
| Liu (100 μg) 2018 | 2.10(0.40) | 57 | 5.40(0.80) | 57 | ||||||
| Singh (1 μg/kg) 2020 | 0.00(0.74) | 20 | 1.00(1.48) | 20 | ||||||
| total | −0.60(−1.61 to 0.42) | 98 | 0.25 | 0.34 | Very low | |||||
| Cumulative IV morphine consumption at 24 h postoperatively (mg) | ||||||||||
| DEX ≤ 60 μg | Abdallah (0.5 μg/kg) 2016 | 21.3(0.87) | 33 | 27.3(1.16) | 32 | −6.01(−6.50 to −5.52) | 0 | <0.00001 | ||
| Kathuria (50 μg) 2015 | 5.63(3.33) | 20 | 12.0(5.66) | 20 | ||||||
| DEX>60 μg | Akhondzadeh (1 μg/kg) 2018 | 12.04(6.74) | 36 | 26.62(7.58) | 36 | −5.03(−8.52 to −1.11) | 96 | 0.01 | ||
| Ammar (0.75 μg/kg) 2012 | 4.90(5.93) | 30 | 13.6(8.89) | 30 | ||||||
| Elyazed (100 μg) 2018 | 1.67(3.12) | 35 | 7.31(1.6) | 35 | ||||||
| He (1 μg/kg) 2018 | 7.30(4.40) | 28 | 15.9(7.60) | 28 | ||||||
| Jung (1.5 μg/kg) 2018 | 8.00(3.49) | 25 | 6.5(3.38) | 23 | ||||||
| Jung (1 μg/kg) 2018 | 7.00(3.16) | 25 | 6.5(3.38) | 23 | ||||||
| Jung (2 μg/kg) 2018 | 6.92(2.53) | 24 | 6.5(3.38) | 23 | ||||||
| total | −5.03(−7.54 to −2.51) | 95 | <0.0001 | 0.53 | Very low | |||||
DEX-related adverse event
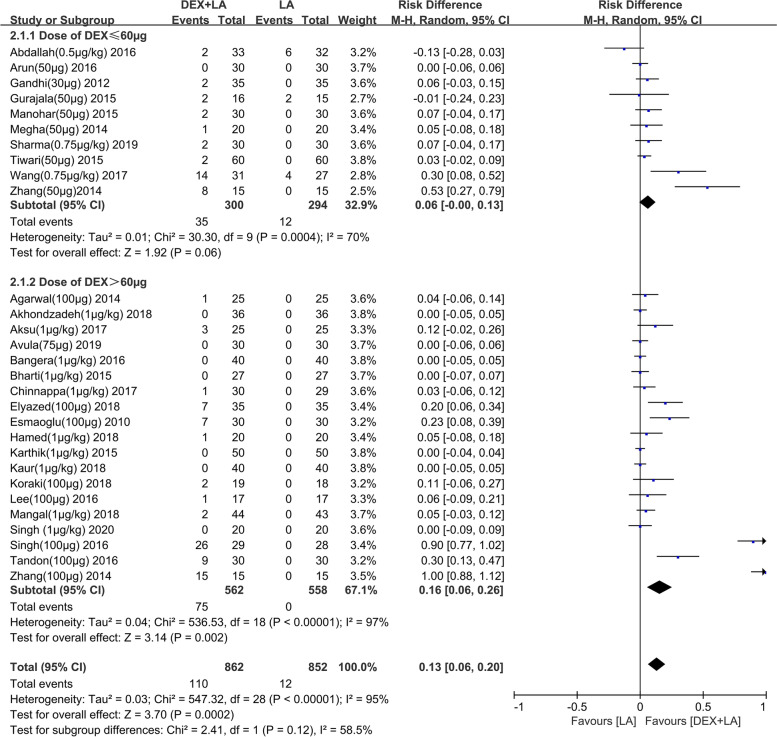
The incidence of bradycardia and hypotension was described in 28 studies [23, 34–37, 39–42, 45, 47, 48, 51–53, 58, 61, 63, 64, 66, 67, 69, 78–80, 83–85] and 26 studies [23, 34–36, 39–42, 45–48, 51–53, 58, 63, 64, 66, 67, 69, 78–80, 84, 85] respectively. Pooled analysis showed that perineural DEX>60 μg increased the risk of bradycardia (risk difference [RD]: 0.16, 95% CI: 0.06 to 0.26, I2 = 97%, P = 0.002) (Fig. 5) in comparison to control, and this result was robust to sensitivity analysis by eliminating two [23, 79] notable outliers (RD: 0.05, 95% CI: 0.01 to 0.05, I2 = 73%, P = 0.01) (Additional file 5). Nevertheless, perineural DEX ≤ 60 μg did not increase the risk of bradycardia (RD: 0.06, 95% CI: − 0.00 to 0.13, I2 = 70%, P = 0.06) (Fig. 5) when comparing to control, and this result was also robust to sensitivity analysis by eliminating two [23, 85] notable outliers (RD: 0.03, 95% CI: − 0.00 to 0.06, I2 = 0%, P = 0.09) (Additional file 5). With regard to hypotension, the meta-analysis concluded that DEX>60 μg as adjuvant obviously increased the risk of it (RD: 0.07, 95% CI: 0.01 to 0.13, I2 = 90%, P = 0. 02) (Additional file 6). However, perineural DEX ≤ 60 μg did not increased the risk of hypotension (RD: 0.01, 95% CI: − 0.01 to 0.04, I2 = 13%, P = 0.34) (Additional file 6). Overall, peripheral DEX>60 μg increases the risk of adverse events, such as bradycardia and hypotension.
Fig. 5.
Effect of perineural DEX by dose administered (≤60 μg or>60 μg) on bradycardia when combined with long-acting LA. Abbreviations: DEX, dexmedetomidine; CI, confidence interval; LA, local anesthetic; IV, intravenous
Publication bias
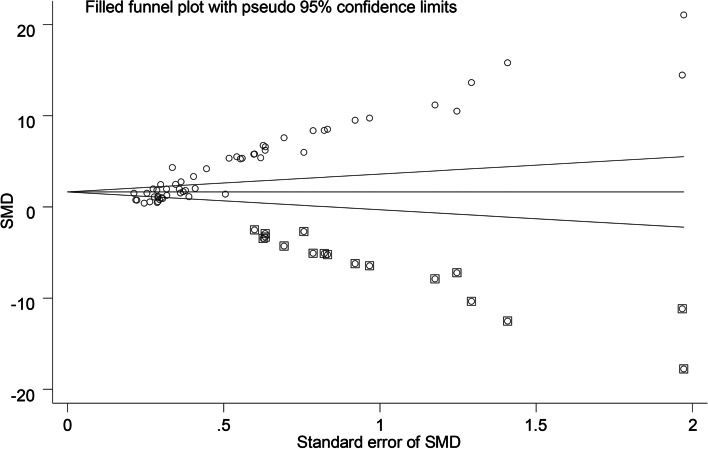
With regard to the funnel plot for our primary outcome, the Duval and Tweedie’s trim and fill test showed the standardized mean difference for the combined studies to be 4.20 (95% CI: 3.63 to 4.78), suggesting that 17 studies are missing (Fig. 6). We rated the quality of evidence for each outcome following the Grades of Recommendation, Assessment, Development, and Evaluation (GRADE) Working Group system [87] (Table 3).
Fig. 6.
Trim and fill test. It showed significant publication bias in the primary outcome (duration of analgesia) (P = 0.00). Abbreviations: SMD, standardized mean difference
Table 3.
Summary of findings
| Quality assessment | Summary of findings | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Outcome | Limitations | Inconsistency | Indirectness | Imprecision | Publication Bias | Number of Patients in DEX Group |
Number of Patients in Control Group | Mean Difference or Relative Risk (95% CI) |
P Value for Overall Effect | Quality of Evidence (GRADE) |
| DOA when combined with short−/intermediate-acting LA (min) | Concealment not clear in most studies | Serious inconsistency | Moderate indirectness | No serious imprecision | 7 studies missing for our primary outcome | 140 | 135 | 126.01(44.22 to 207.81) | 0.003 | Very low quality |
| DOA when combined with long-acting LA (min) | Concealment not clear in most studies | Serious inconsistency | Moderate indirectness | No serious imprecision | 7 studies missing for our primary outcome | 1411 | 1402 | 305.52(271.81 to 339.22) | <0.00001 | Very low quality |
| Pain score at rest at 12 h postoperatively (analog scale, 0–10) | Concealment not clear in most studies | Serious inconsistency | No serious indirectness | Moderate imprecision | 7 studies missing for our primary outcome | 346 | 341 | −1.57(−1.91 to −1.23) | <0.00001 | Very low quality |
| Pain score at rest at 24 h postoperatively (analog scale, 0–10) | Concealment not clear in most studies | Serious inconsistency | No serious indirectness | Moderate imprecision | 7 studies missing for our primary outcome | 321 | 314 | −0.60(−1.61 to 0.42) | 0.25 | Very low quality |
| Cumulative IV morphine consumption at 24 h postoperatively (mg) | Concealment not clear in most studies | Serious inconsistency | No serious indirectness | Moderate imprecision | 7 studies missing for our primary outcome | 256 | 250 | −5.03(−7.54 to −2.51) | <0.0001 | Very low quality |
| Rate of bradycardia | 28 of 57 trials reported that outcome | Serious inconsistency | No serious indirectness | Serious imprecision | 7 studies missing for our primary outcome | 862 | 852 | 0.13(0.06 to 0.20) | 0.0002 | Very low quality |
| Rate of hypotension | 26 of 57 trials reported that outcome | Serious inconsistency | No serious indirectness | Serious imprecision | 7 studies missing for our primary outcome | 802 | 789 | 0.05(0.01 to 0.09) | 0.007 | Very low quality |
Abbreviations: DEX, dexmedetomidine; CI, confidence interval; GRADE, Grades of Recommendation, Assessment, Development, and Evaluation; DOA, duration of analgesia; LA, local anesthetic; IV, intravenous
Discussion
This systematic review and meta-analysis explored the optimal dose of DEX as an adjuvant to prolong the duration of analgesia after BPB in adult patients undergoing upper limb surgery. Based on 58 RCTs, including a total of 3332 patients, our subgroup analysis and regression analysis suggest that 30-50 μg of DEX as an adjuvant represents an optimal dose and prolongs analgesia by 5 h, when combined with long-acting local anesthetics; higher doses may lead to DEX-related adverse events such as bradycardia and hypotension.
The first meta-analysis focused on DEX as an adjuvant, published in 2013 [13], indicated that there are presently insufficient safety data to support the use of perineural DEX in the clinical setting. Four years later, in 2017, the same team in an updated meta-analysis [16] confirmed that using perineural DEX improves BPB onset, quality, and analgesia. After that, four other meta-analysis [14, 15, 17, 18] further confirmed the efficacy of DEX as adjuvant. One of the them found that DEX, particularly at doses greater than 50 μg, holds a great potential for clinicians wishing to quicken the onset and prolong the duration of anesthesia [14]. In our meta-analysis, the interaction between dose of perineural DEX and mean increase in duration of analgesia was explored by grouping every 20 micrograms of DEX. Regression analysis was used to predict the relationship between them. Finally, we come to our conclusion.
The quality of evidence for our primary outcome was rated as very low due to the lack of clear allocation concealment, high coefficient of heterogeneity, absence of consistent definition of the primary outcome and significant publication bias. This means that we have little confidence in the effect estimation, and the real effect is likely to be very different from the effect estimation.
Our review comes with several strengths and potential limitations. Firstly, ours is the first review to pool a large number of RCTs on this topic and provide greater insights into the optimal dose of DEX. While the prior review [18] in 2018 just included 12 RCTs, we were able to include an additional 45. Secondly, there was a high consistency in the evaluation of each parameter in this meta-analysis. Finally, we successfully analyzed the influencing factors of DEX on duration of analgesia, including different doses, BPB approaches and positioning techniques; however, since these factors were not randomized in the included studies, there was an inherent risk of bias in this analysis.
It is worth noting that one of the limitations of our review is the high heterogeneity of primary outcome. Furthermore, even subgroup analysis could not successfully solve the problem of heterogeneity attributed to the smaller sample sizes of individual studies, the potential variation in the study populations, and the different methods that could have been used to measure the outcomes in question. Secondly, most of included trials were performed in developing countries and published in non-anesthesia journals. This may also be the reason for the high heterogeneity.
Conclusion
In conclusion, there is very low quality evidence that 30-50 μg of perineural DEX represents an appropriate dosage, which prolongs analgesia duration by a mean period of 5 h when combined with long-acting LAs. Perineural DEX above 60 μg can significantly increase the incidence of adverse events such as bradycardia or hypotension. More high-quality methodological and strictly defined RCTs are urgently needed to further evaluate the advantages and disadvantages of DEX as an adjuvant.
Supplementary Information
Additional file 1. Effect of perineural DEX by dose administered (≤60 μg or>60 μg) on DOA when combined with short−/intermediate-acting. Abbreviations: DEX, dexmedetomidine; CI, confidence interval; DOA, duration of analgesia; LA, local anesthetic; IV, intravenous.
Additional file 2. Subgroup analyses of DEX on DOA by BPB approaches and localization techniques. Abbreviations: DEX, dexmedetomidine; DOA, duration of analgesia; BPB, brachial plexus block; MD, mean difference; CI, confidence interval; LA, local anesthetic.
Additional file 3. Regression analysis of perineural DEX dose and mean increase in DOA when combined with long-acting LAs (pink line: mean line; green line: fitting line). Abbreviations: DEX, dexmedetomidine; DOA, duration of analgesia; LA, local anesthetic.
Additional file 4. Regression analysis of perineural DEX dose and mean increase in DOA when combined with short−/intermediate-acting LAs (pink line: mean line; green line: fitting line). Abbreviations: DEX, dexmedetomidine; DOA, duration of analgesia; LA, local anesthetic.
Additional file 5. Sensitivity analysis by eliminating two notable outliers respectively. Abbreviations: DEX, dexmedetomidine; LA, local anesthetic; CI, confidence interval.
Additional file 6. Effect of perineural DEX by dose administered (≤60 μg or>60 μg) on hypotension. Abbreviations: DEX, dexmedetomidine; LA, local anesthetic; CI, confidence interval.
Abbreviations
- LA
local anesthetic
- BPB
brachial plexus block
- RCTs
randomized clinical trials
- CI
confidence interval
- DEX
dexmedetomidine
- RD
risk difference
- MD
mean difference
Authors’ contributions
(I) Conception and design: Y Xie; (II) Administrative support: Y Xie; (III) Provision of study materials or patients: H Cai, X Fan; (IV) Collection and assessment of data: H Cai, P Feng; (V) Data analysis and interpretation: H Cai, X Wang; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors.
Funding
This work was supported by the Program of Guangxi Key Research and Development (No. AB18221031), Key Project of Guangxi Natural Science Foundation (No. 2020GXNSFDA238025), the National Natural Science Foundation of China (No. 81373498) and Planning Subject of Health Committee of Guangxi Zhuang Autonomous Region (No. Z20190146).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Samar P, Dhawale TA, Pandya S. Comparative study of intravenous Dexmedetomidine sedation with Perineural Dexmedetomidine on supraclavicular approach brachial plexus block in upper limb Orthopaedic surgery. Cureus. 2020;12(10):e10768. doi: 10.7759/cureus.10768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stephan SR, Garlich JM, Debbi EM, Johnson CR, Polakof LS, Noorzad AS, Moak ZB, Yalamanchili DR, Stephenson SK, Anand KK, et al. A comparison in outcomes of preoperative single-shot versus continuous catheter fascia Iliaca regional anesthesia in geriatric hip fracture patients. Injury. 2020;51(6):1337–1342. doi: 10.1016/j.injury.2020.04.024. [DOI] [PubMed] [Google Scholar]
- 3.Krishna Prasad GV, Khanna S, Jaishree SV. Review of adjuvants to local anesthetics in peripheral nerve blocks: current and future trends. Saudi J Anaesth. 2020;14(1):77–84. doi: 10.4103/sja.SJA_423_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Majedi MA, Kavehmajd S, Yousefi F, Ahsan B. Comparision between the effects of dexmedtomedian and fentanyl as adjuvants to lidocain on axillary plexus block. Sci J Kurdistan Univ Med Sci. 2019;24(1):1–10. [Google Scholar]
- 5.Kim D, Jeong JS, Park MJ, Ko JS. The effect of epinephrine on the perfusion index during ultrasound-guided supraclavicular brachial plexus block: a randomized controlled trial. Sci Rep. 2020;10(1):11585. doi: 10.1038/s41598-020-68475-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Das A, Dutta S, Chattopadhyay S, Chhaule S, Mitra T, Banu R, Mandal P, Chandra M. Pain relief after ambulatory hand surgery: a comparison between dexmedetomidine and clonidine as adjuvant in axillary brachial plexus block: a prospective, double-blinded, randomized controlled study. Saudi J Anaesth. 2016;10(1):6–12. doi: 10.4103/1658-354X.169443. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Sun J, Feng X, Zhu Q, Lin W, Guo H, Ansong E, Liu L. Analgesic effect of perineural magnesium sulphate for sciatic nerve block for diabetic toe amputation: a randomized trial. PLoS One. 2017;12(5):e0176589. doi: 10.1371/journal.pone.0176589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knight JB, Schott NJ, Kentor ML, Williams BA. Neurotoxicity of common peripheral nerve block adjuvants. Curr Opin Anaesthesiol. 2015;28(5):598–604. doi: 10.1097/ACO.0000000000000222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shekar M, Kanakalakshmi ST, Mathew S, Muhamed S, Duggappa AKH, Herekar B. Comparison of ultrasound guided interscalene brachial plexus block using 0.2% ropivacaine with dexmedetomidine and 0.2% ropivacaine with dexamethasone-a prospective observational study. Sri Lankan J Anaesthesiol. 2020;28(2):114–118. doi: 10.4038/slja.v28i2.8574. [DOI] [Google Scholar]
- 10.Lomate PA, Mane MV. Efficacy of multimodal analgesia with perineural buprenorphine or dexmedetomidine for surgeries performed under ultrasound-guided infraclavicular brachial plexus block. J Anaesthesiol Clin Pharmacol. 2020;36(1):66–71. doi: 10.4103/joacp.JOACP_30_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hong B, Kim B, Yun S, Youngkwon K. Prolonged analgesic duration of brachial plexus block by addition of dexamethasone to dexmedetomidine for sedation. Reg Anesth Pain Med. 2019;44(10):A271–A273. [Google Scholar]
- 12.Prabhakar A, Lambert T, Kaye RJ, Gaignard SM, Ragusa J, Wheat S, Moll V, Cornett EM, Urman RD, Kaye AD. Adjuvants in clinical regional anesthesia practice: a comprehensive review. Best Pract Res Clin Anaesthesiol. 2019;33(4):415–423. doi: 10.1016/j.bpa.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Abdallah FW, Brull R. Facilitatory effects of perineural dexmedetomidine on neuraxial and peripheral nerve block: a systematic review and meta-analysis. Br J Anaesth. 2013;110(6):915–925. doi: 10.1093/bja/aet066. [DOI] [PubMed] [Google Scholar]
- 14.Hussain N, Grzywacz VP, Ferreri CA, Atrey A, Banfield L, Shaparin N, Vydyanathan A. Investigating the efficacy of dexmedetomidine as an adjuvant to local anesthesia in brachial plexus block a systematic review and meta-analysis of 18 randomized controlled trials. Reg Anesth Pain Med. 2017;42(2):184–196. doi: 10.1097/AAP.0000000000000564. [DOI] [PubMed] [Google Scholar]
- 15.Ping Y, Ye Q, Wang W, Ye P, You Z. Dexmedetomidine as an adjuvant to local anesthetics in brachial plexus blocks a meta-analysis of randomized controlled trials. Medicine (United States). 2017;96(4):e5846. [DOI] [PMC free article] [PubMed]
- 16.Vorobeichik L, Brull R, Abdallah FW. Evidence basis for using perineural dexmedetomidine to enhance the quality of brachial plexus nerve blocks: a systematic review and meta-analysis of randomized controlled trials. Br J Anaesth. 2017;118(2):167–181. doi: 10.1093/bja/aew411. [DOI] [PubMed] [Google Scholar]
- 17.Xiu H, Zhang Y, Shan Z, Wen J, Xu K, Zhang Y. Effects of dexmedetomidine as a local anesthetic adjuvant for brachial plexus block: a systematic review and meta-analysis. Int J Clin Exp Med. 2017;10(1):357–366. [Google Scholar]
- 18.Dai W, Tang M, He K. The effect and safety of dexmedetomidine added to ropivacaine in brachial plexus block: A meta-analysis of randomized controlled trials. Medicine (United States). 2018;97(41):e12573. [DOI] [PMC free article] [PubMed]
- 19.Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA: Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ (Clinical research ed). 2015;350:g7647. [DOI] [PubMed]
- 20.Pillai AR, Palanisamy N, Ranjan RV, George SK, Ramachandran TR, Oommen TG, Chinthavalli S, Valasareddy SK. Supraclavicular brachial plexus block: Comparision of two different doses of dexmeditomidine as an adjunct to bupivacaine: a study. J Clin Diagn Res. 2018;12(10):UC01–UC04. [Google Scholar]
- 21.Jung HS, Seo KH, Kang JH, Jeong JY, Kim YS, Han NR. Optimal dose of perineural dexmedetomidine for interscalene brachial plexus block to control postoperative pain in patients undergoing arthroscopic shoulder surgery: A prospective, double-blind, randomized controlled study. Medicine (United States). 2018;97(16):e0440. [DOI] [PMC free article] [PubMed]
- 22.Thakur A, Singh J, Kumar S, Rana S, Sood P, Verma V. Efficacy of dexmedetomidine in two different doses as an adjuvant to lignocaine in patients scheduled for surgeries under axillary block. J Clin Diagn Res. 2017;11(4):UC16–UC21. doi: 10.7860/JCDR/2017/23540.9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y, Wang CS, Shi JH, Sun B, Liu SJ, Li P, Li EY. Perineural administration of dexmedetomidine in combination with ropivacaine prolongs axillary brachial plexus block. Int J Clin Exp Med. 2014;7(3):680–685. [PMC free article] [PubMed] [Google Scholar]
- 24.Sombabu K, Ruth MS, Mohanarangan T, Kulasekar A. Comparison of two different doses of dexmedetomidine combined with 0.5% levobupivacine for post operative analgesia in ultrasound guided supraclavicular brachial plexus block - A double blinded randomized controlled study. Annals Trop Med Public Health. 2020;23(15):SP231516.
- 25.Nallam SR, Chiruvella S, Karanam S. Supraclavicular brachial plexus block: comparison of varying doses of dexmedetomidine combined with levobupivacaine: a double-blind randomised trial. Indian J Anaesthesia. 2017;61(3):256–261. doi: 10.4103/ija.IJA_700_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumari I, Gehlot RK, Verma R, Narang A, Verma V, Suwalka P. Comparison of two different doses of dexmedetomidine added to ropivacaine in patients posted for upper limb surgery under supraclavicular brachial plexus block. Anaesthesia Pain Intensive Care. 2017;21(2):141–146. [Google Scholar]
- 27.Kang RA, Jeong JS, Yoo JC, Lee JH, Choi SJ, Gwak MS, Hahm TS, Huh J, Ko JS. Effective dose of intravenous Dexmedetomidine to prolong the analgesic duration of Interscalene brachial plexus block: a single-center, prospective, double-blind, randomized controlled trial. Reg Anesth Pain Med. 2018;43(5):488–495. doi: 10.1097/AAP.0000000000000773. [DOI] [PubMed] [Google Scholar]
- 28.Tripathi A, Sharma K, Somvanshi M, Samal RL. A comparative study of clonidine and dexmedetomidine as an adjunct to bupivacaine in supraclavicular brachial plexus block. J Anaesthesiol Clin Pharmacol. 2016;32(3):344–348. doi: 10.4103/0970-9185.188819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA: The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ (Clinical research ed) 2011, 343:d5928. [DOI] [PMC free article] [PubMed]
- 30.Sun T, Wang Y, Hui D, Jing X, Feng W. Vertical distributions of soil microbial biomass carbon: a global dataset. Data in brief. 2020;32:106147. doi: 10.1016/j.dib.2020.106147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, Thomas J: Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Systematic Rev 2019, 10:Ed000142. [DOI] [PMC free article] [PubMed]
- 32.Kirkham KR, Jacot-Guillarmod A, Albrecht E. Optimal dose of Perineural dexamethasone to prolong analgesia after brachial plexus blockade: a systematic review and Meta-analysis. Anesth Analg. 2018;126(1):270–279. doi: 10.1213/ANE.0000000000002488. [DOI] [PubMed] [Google Scholar]
- 33.Albrecht E, Vorobeichik L, Jacot-Guillarmod A, Fournier N, Abdallah FW. Dexamethasone is superior to dexmedetomidine as a perineural adjunct for supraclavicular brachial plexus block: systematic review and indirect meta-analysis. Anesth Analg. 2019;128(3):543–554. doi: 10.1213/ANE.0000000000003860. [DOI] [PubMed] [Google Scholar]
- 34.Abdallah FW, Dwyer T, Chan VWS, Niazi AU, Ogilvie-Harris DJ, Oldfield S, Patel R, Oh J, Brull R. IV and perineural dexmedetomidine similarly prolong the duration of analgesia after interscalene brachial plexus block: a randomized, three-arm, triple-masked, placebo-controlled trial. Anesthesiology. 2016;124(3):683–695. doi: 10.1097/ALN.0000000000000983. [DOI] [PubMed] [Google Scholar]
- 35.Agarwal S, Aggarwal R, Gupta P. Dexmedetomidine prolongs the effect of bupivacaine in supraclavicular brachial plexus block. J Anaesthesiol Clin Pharmacol. 2014;30(1):36–40. doi: 10.4103/0970-9185.125701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Akhondzadeh R, Rashidi M, Gousheh M, Olapour A, Baniahmad A. The effect of adding dexmedetomidine as an adjuvant to lidocaine in forearm fracture surgeries by supraclavicular block procedure under ultrasound-guided. Anesthesiol Pain Med. 2018;8(4):e74355. [DOI] [PMC free article] [PubMed]
- 37.Aksu R, Bicer C. Addition of dexmedetomidine to bupivacaine in supraclavicular brachial plexus block. Clin Invest Med. 2017;40(3):E111–E116. doi: 10.25011/cim.v40i3.28390. [DOI] [PubMed] [Google Scholar]
- 38.Ammar AS, Mahmoud KM. Ultrasound-guided single injection infraclavicular brachial plexus block using bupivacaine alone or combined with dexmedetomidine for pain control in upper limb surgery: a prospective randomized controlled trial. Saudi J Anaesth. 2012;6(2):109–114. doi: 10.4103/1658-354X.97021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arun S: Effect of dexmedetomidine as an adjuvant to 0.75% ropivacaine in axillary brachial plexus block for forearm and hand surgeries. Int J Biomed Res 2016, 7(4):187–192
- 40.Avula RR, Vemuri NN, Puthi S. Ultrasound-guided Subclavian perivascular brachial plexus block using 0.5% bupivacaine with Dexmedetomidine as an adjuvant: a prospective randomized controlled trial. Anesth Essays Res. 2019;13(4):615–619. doi: 10.4103/aer.AER_122_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bangera A, Manasa M, Krishna P. Comparison of effects of ropivacaine with and without dexmedetomidine in axillary brachial plexus block: a prospective randomized double-blinded clinical trial. Saudi J Anaesth. 2016;10(1):38–44. doi: 10.4103/1658-354X.169473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bharti N, Sardana DK, Bala I. The analgesic efficacy of dexmedetomidine as an adjunct to local anesthetics in supraclavicular brachial plexus block: a randomized controlled trial. Anesth Analg. 2015;121(6):1655–1660. doi: 10.1213/ANE.0000000000001006. [DOI] [PubMed] [Google Scholar]
- 43.Bisui B, Samanta S, Ghoshmaulik S, Banerjee A, Ghosh TR, Sarkar S. Effect of locally administered Dexmedetomidine as adjuvant to Levobupivacaine in supraclavicular brachial plexus block: double-blind controlled study. Anesth Essays Res. 2017;11(4):981–986. doi: 10.4103/aer.AER_55_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Biswas S, Das RK, Mukherjee G, Ghose T. Dexmedetomidine an adjuvant to levobupivacaine in supraclavicular brachial plexus block: a randomized double blind prospective study. Ethiop J Health Sci. 2014;24(3):203–208. doi: 10.4314/ejhs.v24i3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chinnappa J, Shivanna S, Pujari VS, Anandaswamy TC. Efficacy of dexmedetomidine with ropivacaine in supraclavicular brachial plexus block for upper limb surgeries. J Anaesthesiol Clin Pharmacol. 2017;33(1):81–85. doi: 10.4103/0970-9185.202196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dar FA, Najar MR, Jan N. Dexmedetomidine added to Ropivacaine prolongs axillary brachial plexus block. Int J Biomed Adv Res. 2013;4(10):719–722. doi: 10.7439/ijbar.v4i10.501. [DOI] [Google Scholar]
- 47.Elyazed MMA, Mogahed MM. Comparison of magnesium sulfate and Dexmedetomidine as an adjuvant to 0.5% Ropivacaine in Infraclavicular brachial plexus block. Anesth Essays Res. 2018;12(1):109–115. doi: 10.4103/aer.AER_70_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Esmaoglu A, Yegenoglu F, Akin A, Turk CY. Dexmedetomidine added to levobupivacaine prolongs axillary brachial plexus block. Anesth Analg. 2010;111(6):1548–1551. doi: 10.1213/ANE.0b013e3181fa3095. [DOI] [PubMed] [Google Scholar]
- 49.Farooq N, Singh RB, Sarkar A, Rasheed MA, Choubey S. To evaluate the efficacy of fentanyl and Dexmedetomidine as adjuvant to Ropivacaine in brachial plexus block: a double-blind, prospective, randomized study. Anesth Essays Res. 2017;11(3):730–739. doi: 10.4103/aer.AER_30_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fritsch G, Danninger T, Allerberger K, Tsodikov A, Felder TK, Kapeller M, Gerner P, Brummett CM. Dexmedetomidine added to ropivacaine extends the duration of interscalene brachial plexus blocks for elective shoulder surgery when compared with ropivacaine alone: a single-center, prospective, triple-blind, randomized controlled trial. Reg Anesth Pain Med. 2014;39(1):37–47. doi: 10.1097/AAP.0000000000000033. [DOI] [PubMed] [Google Scholar]
- 51.Gandhi R, Shah A, Patel I. Use of dexmedetomidine along with bupivacaine for brachial plexus block. National J Med Res. 2012;2(1):67–69. [Google Scholar]
- 52.Gurajala I, Thipparampall AK, Durga P, Gopinath R. Effect of perineural dexmedetomidine on the quality of supraclavicular brachial plexus block with 0.5% ropivacaine and its interaction with general anaesthesia. Indian J Anaesthesia. 2015;59(2):89–95. doi: 10.4103/0019-5049.151369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hamed MA, Ghaber S, Reda A. Dexmedetomidine and fentanyl as an adjunct to bupivacaine 0.5% in supraclavicular nerve block: a randomized controlled study. Anesth Essays Res. 2018;12(2):475–479. doi: 10.4103/aer.AER_50_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hanoura SE, Elsayed MM, Abdullah AA, Elsayed HO, Eldeen TMN. Dexmedetomidine improves the outcome of a bupivacaine brachial plexus axillary block: a prospective comparative study. Ain-Shams J Anesthesiol. 2012;6:58–62. [Google Scholar]
- 55.Hassan M, Abaza KA, Sayouh EF, Kamel ASA. The effect of various additives to local anesthetics on the duration of analgesia of supraclavicular brachial plexus block. J Anest Inten Care Med. 2019;9(2):24–28. [Google Scholar]
- 56.He WS, Liu Z, Wu ZY, Sun HJ, Yang XC, Wang XL. The effect of dexmedetomidine in coracoid approach brachial plexus block under dual stimulation. Med (United States). 2018;97(39):e12240. [DOI] [PMC free article] [PubMed]
- 57.Hwang JT, Jang JS, Lee JJ, Song DK, Lee HN, Kim DY, Lee SS, Hwang SM, Kim YB, Lee S. Dexmedetomidine combined with interscalene brachial plexus block has a synergistic effect on relieving postoperative pain after arthroscopic rotator cuff repair. Knee Surg Sports Traumatol Arthroscopy. 2020;28(7):2343–2353. doi: 10.1007/s00167-019-05799-3. [DOI] [PubMed] [Google Scholar]
- 58.Karthik AV, Bhupal JPS, Grewal TK, Bindra TK, Verma R. DEXMEDETOMIDINE prolongs the effect of 0.5% isobaric LEVOBUPIVACAINE in axillary brachial plexus block. J Evol Med Dent Sci. 2015;4(6):1015–1022. doi: 10.14260/jemds/2015/142. [DOI] [Google Scholar]
- 59.Kathuria S, Gupta S, Dhawan I. Dexmedetomidine as an adjuvant to ropivacaine in supraclavicular brachial plexus block. Saudi J Anaesth. 2015;9(2):148–154. doi: 10.4103/1658-354X.152841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kaur H, Singh G, Rani S, Gupta KK, Kumar M, Rajpal AS, Aggarwal S. Effect of dexmedetomidine as an adjuvant to levobupivacaine in supraclavicular brachial plexus block: a randomized double-blind prospective study. J Anaesthesiol Clin Pharmacol. 2015;31(3):333–338. doi: 10.4103/0970-9185.161668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kaur M, Kaur R, Kaur S, Baghla N, Bansal A, Kalia A, Kumar S, Lall A. A study to compare the analgesic efficacy of Dexmedetomidine and fentanyl as adjuvants to Levobupivacaine in ultrasound-guided supraclavicular brachial plexus block. Anesth Essays Res. 2018;12(3):669–673. doi: 10.4103/aer.AER_64_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kaygusuz K, Kol IO, Duger C, Gursoy S, Ozturk H, Kayacan U, Aydin R, Mimaroglu C. Effects of adding Dexmedetomidine to Levobupivacaine in axillary brachial plexus block. Curr Therapeutic Res Clin Experimental. 2012;73(3):103–111. doi: 10.1016/j.curtheres.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Koraki E, Stachtari C, Kapsokalyvas I, Stergiouda Z, Katsanevaki A, Trikoupi A. Dexmedetomidine as an adjuvant to 0.5% ropivacaine in ultrasound-guided axillary brachial plexus block. J Clin Pharm Ther. 2018;43(3):348–352. doi: 10.1111/jcpt.12657. [DOI] [PubMed] [Google Scholar]
- 64.Lee MJ, Koo DJ, Choi YS, Lee KC, Kim HY. Dexamethasone or dexmedetomidine as local anesthetic adjuvants for ultrasound-guided axillary brachial plexus blocks with nerve stimulation. Korean J Pain. 2016;29(1):29–33. doi: 10.3344/kjp.2016.29.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu Z, Jiang M, Xu T, Hua H. Analgesic effect of Ropivacaine combined with Dexmedetomidine on brachial plexus block. BMC Anesthesiol. 2018;18(1):107. [DOI] [PMC free article] [PubMed]
- 66.Mangal V, Mistry T, Sharma G, Kazim M, Ahuja N, Kulshrestha A. Effects of dexmedetomidine as an adjuvant to ropivacaine in ultrasound-guided supraclavicular brachial plexus block: a prospective, randomized, double-blind study. J Anaesthesiol Clin Pharmacol. 2018;34(3):357–361. doi: 10.4103/joacp.JOACP_182_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Manohar P, Prakash M. Comparison of the effects of fentanyl and Dexmedetomidine in supraclavicular brachial plexus block achieved with 0.5% bupivacaine in Karpaga Vinayaga medical college and hospital, Maduranthagam. JMSCR. 2015;3(8):7131–7138. [Google Scholar]
- 68.Mathew S, Prasad S, Krishna R, Kumar A, Shiyad M. Ultrasound guided supraclavicular brachial plexus block using plain ropivacaine and ropivacaine with additives. Sri Lankan J Anaesthesiol. 2018;26(1):15–21. doi: 10.4038/slja.v26i1.8261. [DOI] [Google Scholar]
- 69.Megha GH, Sanjeev GM. Dexmeditomidine as an adjuvant to bupivacaine in supraclavicular brachial plexus block. IJSR. 2014;3(7):378–381. doi: 10.15373/22778179/July2014/116. [DOI] [Google Scholar]
- 70.Mirkheshti A, Saadatniaki A, Salimi A, Manafi Rasi A, Memary E, Yahyaei H. Effects of dexmedetomidine versus ketorolac as local anesthetic adjuvants on the onset and duration of infraclavicular brachial plexus block. Anesth Pain Med. 2014;4(3):e17620. doi: 10.5812/aapm.17620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nazir N, Jain S. A randomized controlled trial study on the effect of adding Dexmedetomidine to bupivacaine in supraclavicular block using ultrasound guidance. Ethiop J Health Sci. 2016;26(6):561–566. doi: 10.4314/ejhs.v26i6.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nazir O, Bhat AH, Sharma T, Khatuja A, Misra R. Comparison of clonidine and dexmedetomidine as adjuvants for ropivacaine in supraclavicular brachial plexus block. Sri Lankan J Anaesthesiol. 2019;27(1):53–58. doi: 10.4038/slja.v27i1.8393. [DOI] [Google Scholar]
- 73.Nema N, Badgaiyan H, Raskaran S, Kujur S, Vaskle P, Mujalde M, Verma J, Berde R. Effect of addition of dexmedetomidine to ropivacaine hydrochloride (0.75%) in brachial plexus block through supraclavicular route in upper limb surgeries: a clinical comparative study. J Evolution Med Dent Sci. 2014;3(55):12612–12621. doi: 10.14260/jemds/2014/3670. [DOI] [Google Scholar]
- 74.Nicholas SRK, D’souza PH. Dexmedetomidine as an adjuvant agent to 0.5% ropivacaine in ultra sound guided axillary brachial plexus block in orthopaedic surgeries. Indian J Public Health Res Development. 2020;11(5):406–412. [Google Scholar]
- 75.Patki YS, Bengali R, Patil T. Efficacy of Dexmedetomidine as an adjuvant to 0.5% Ropivacaine in supraclavicular brachial plexus block for postoperative analgesia. IJSR. 2013;4(1):2345–2351. [Google Scholar]
- 76.Rashmi HD, Komala HK. Effect of Dexmedetomidine as an adjuvant to 0.75% Ropivacaine in Interscalene brachial plexus block using nerve stimulator: a prospective, randomized double-blind study. Anesth Essays Res. 2017;11(1):134–139. doi: 10.4103/0259-1162.181431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shahtaheri SY, Rad MT, Yazdi B, Azami M, Kamali A. Clinical comparison of adding sulfate magnesium and Dexmedetomidine in axillary plexus block for prolonging the duration of sensory and motor block: study protocol for a double-blind randomized clinical trial. Folia Med. 2020;62(1):124–132. doi: 10.3897/folmed.62.e49805. [DOI] [PubMed] [Google Scholar]
- 78.Sharma S, Shrestha A, Koirala M. Effect of dexmedetomidine with ropivacaine in supraclavicular brachial plexus block. Kathmandu Univ Med J. 2019;17(67):173–183. [PubMed] [Google Scholar]
- 79.Singh AP, Mahindra M, Gupta R, Bajwa SJ. Dexmedetomidine as an adjuvant to levobupivacaine in supraclavicular brachial plexus block: a novel anesthetic approach. Anesth Essays Res. 2016;10(3):414–419. doi: 10.4103/0259-1162.176404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Singh N, Gupta S, Kathuria S. Dexmedetomidine vs dexamethasone as an adjuvant to 0.5% ropivacaine in ultrasound-guided supraclavicular brachial plexus block. J Anaesthesiol Clin Pharmacol. 2020;36(2):238–243. doi: 10.4103/joacp.JOACP_130_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Singh R, Singam A. Comparative evaluation of dexmedetomedine versus clonidine as an adjuvant in supraclavicular brachial plexus block. J Krishna Institute Med Sci Univ. 2019;8(3):53–65. [Google Scholar]
- 82.Song JH, Shim HY, Lee TJ, Jung JK, Cha YD, Lee DI, Kim GW, Han JU. Comparison of dexmedetomidine and epinephrine as an adjuvant to 1% mepivacaine in brachial plexus block. Korean J Anesthesiol. 2014;66(4):283–289. doi: 10.4097/kjae.2014.66.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tandon N, Gupta M, Agrawal J, Mathur A, Gupta S, Yadav S, Goyal P, Choudhary B. A comparative clinical study to evaluate the efficacy of levobupivacaine with clonidine and levobupivacaine with dexmedetomidine in supraclavicular brachial plexus block. J Evolution Med Dent Sci. 2016;5(19):925–929. doi: 10.14260/jemds/2016/215. [DOI] [Google Scholar]
- 84.Tiwari P, Jain M, Rastogi B, Aggarwal S, Gupta K, Singh V. A comparative clinical study of perineural administration of 0.75% ropivacaine with and without dexmedetomidinein upper limb surgery by ultrasound guided single injection supraclavicular brachial plexus block. Glob Anesth Perioper Med. 2015;1(5):131–133. doi: 10.15761/GAPM.1000133. [DOI] [Google Scholar]
- 85.Wang X, Yu F, Li K. Which is the better adjuvant to ropivacaine in brachial plexus block: Dexmedetomidine or morphine? A prospective, randomized, double-blinded, comparative study. Int J Clin Exp Med. 2017;10(7):10813–10819. [Google Scholar]
- 86.Yaghoobi S, Shahamat H, Alizadeh A, Khezri MB. Comparing postoperative analgesic effect of Dexmedetomidine or dexamethasone added to Lidocaine through Infraclavicular block in forearm surgery. Clin J Pain. 2019;35(9):766–771. doi: 10.1097/AJP.0000000000000736. [DOI] [PubMed] [Google Scholar]
- 87.Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, Vist GE, Falck-Ytter Y, Meerpohl J, Norris S, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Effect of perineural DEX by dose administered (≤60 μg or>60 μg) on DOA when combined with short−/intermediate-acting. Abbreviations: DEX, dexmedetomidine; CI, confidence interval; DOA, duration of analgesia; LA, local anesthetic; IV, intravenous.
Additional file 2. Subgroup analyses of DEX on DOA by BPB approaches and localization techniques. Abbreviations: DEX, dexmedetomidine; DOA, duration of analgesia; BPB, brachial plexus block; MD, mean difference; CI, confidence interval; LA, local anesthetic.
Additional file 3. Regression analysis of perineural DEX dose and mean increase in DOA when combined with long-acting LAs (pink line: mean line; green line: fitting line). Abbreviations: DEX, dexmedetomidine; DOA, duration of analgesia; LA, local anesthetic.
Additional file 4. Regression analysis of perineural DEX dose and mean increase in DOA when combined with short−/intermediate-acting LAs (pink line: mean line; green line: fitting line). Abbreviations: DEX, dexmedetomidine; DOA, duration of analgesia; LA, local anesthetic.
Additional file 5. Sensitivity analysis by eliminating two notable outliers respectively. Abbreviations: DEX, dexmedetomidine; LA, local anesthetic; CI, confidence interval.
Additional file 6. Effect of perineural DEX by dose administered (≤60 μg or>60 μg) on hypotension. Abbreviations: DEX, dexmedetomidine; LA, local anesthetic; CI, confidence interval.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.