Abstract
Introduction
Myofascial pain syndrome (MPS) is one of the most common disorders causing chronic muscle pain. Almost one-third of patients with musculoskeletal complaints meet the MPS criteria. The aim of this study is to evaluate the effectiveness of intramuscular electrical stimulation (IMES) in patients with MPS through a systematic review method.
Methods
PubMed, Scopus, Embase, ProQuest, PEDro, Web of Science, and CINAHL were systematically searched to find out the eligible articles without language limitations from 1990 to December 30, 2020. All relevant randomized controlled trials that compared the effectiveness of IMES with sham-IMES, dry needling, or exercise therapy in patients with MPS were included. Full texts of the selected studies were critically appraised using Revised Cochrane risk-of-bias tool for randomized trials (RoB2).
Results
Six studies (out of 397) had met our inclusion criteria (involving 158 patients) and were entered to the systematic review. Outcome measures examined in these studies included pain, range of motion, pressure pain threshold, biochemical factors, disability, and amount of analgesic use. In the most studies, it has been shown that IMES is more effective than the control group in improving some outcome measurements such as pain.
Conclusion
There is preliminary evidence from a few small trials suggesting the efficacy of IMES for the care of myofascial pain syndrome. The data support the conduct of larger trials investigating the efficacy of IMES.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12998-021-00396-z.
Keywords: Intramuscular electrical stimulation, Myofascial pain syndrome, Trigger point, Dry needling
Introduction
Myofascial pain syndrome (MPS) is one of the most frequent disorders causing chronic muscle pain that is usually overlooked [1]. Almost one-third of patients with musculoskeletal complaints meet the Simons and Travel MPS criteria [2]. Myofascial Pain Syndrome originates from a sensitive zone, referred to as a trigger point (TrP) [3, 4]. A trigger point is a painful point within a muscle contracture or taut band in the muscle belly, which is aggravated by a directly applied force, pressure, contraction, or stretching. A trigger point can cause referred pain to remote areas, limited range of motion (ROM), and reduced functional ability [2, 4–7].
Different physiotherapy interventions have been recommended to manage MPS, such as electrotherapy, manual therapy, exercises, and dry needling (DN) [8–12]. Current articles report evidence with different levels of effectiveness and long-term effects of physiotherapy interventions, including manual therapy, electrotherapy, and dry needling of TrPs. Therefore, it seems that further research is still needed to provide appropriate treatment for TrPs [8, 13]. Based on previous study results, DN positively affects the signs and symptoms of MPS [10]. There is also some evidence that electrical stimulation (ES) can increase blood flow to the muscle [14, 15]. Some researchers have combined DN with ES to achieve more effective treatment outcomes for blood flow, pain severity, and ROM, among others [16, 17].
There is some evidence of the effectiveness of intramuscular electrical stimulation (IMES) applied to various body regions in patients with MPS; however, these studies have much heterogeneity, making it difficult to draw definitive conclusions and apply the results in clinical practice. Therefore, we conducted a systematic review of randomized controlled trials (RCTs) to evaluate the effectiveness of IMES in the management of patients with MPS.
Methods
Inclusion/exclusion criteria
Type of studies
Any published RCTs reporting the effects of IMES on myofascial pain were included in this systematic review with no language restriction. Studies were considered eligible included patients with MPS based on Simons and Travel MPS criteria [2].
Also, only studies were included with patients with MPS.
Type of participants
Patients with MPS in any body region, sex, gender, and age were included.
Type of interventions
We include all RCTs applied IMES using DN with all types of wave properties. Anode or cathode use on TrP was not important for study including. All intervention types except IMES by DN, such as DN alone, ES insertion without DN use, or no intervention were considered proper for the control group.
Type of outcome measurements
Any quantitative outcome measurements like pain, ROM, functional disability score, etc., were accepted into the current study.
Search methods
Two researchers (MH & MJ) independently searched seven relevant databases to identify potentially relevant studies, including PubMed, Scopus, Embase, ProQuest, PEDro, Web of Science, and CINAHL from 1990 to December 2020. To identify keywords, the terms myofascial pain, trigger point, and intramuscular electrical stimulation were searched in medical subject heading (MeSH), and their synonyms were included in searching the databases. The searched keywords were ("Intramuscular electrical stimulation" OR "electrical intramuscular stimulation" OR "intramuscular stimulation" OR IMES OR EIMS OR "electrical twitch") AND ("trigger point" OR myofascial OR muscle OR muscular). The authors also searched the included articles' references and consulted the … University of Medical Sciences library to identify other relevant studies.
Study selection and data extraction
Two researchers (MH & MJ) independently screened the title and abstract of all identified articles. During the next stage, they reviewed the full texts of all potentially relevant studies. Researchers read the articles independently and extracted the data based on a pre-determined datasheet. The extracted data included study design, sample size, type of MPS disorder, age, interventions in experimental and control groups, number and frequency of treatment sessions, location of treatment, wave characteristics, needling method, outcome measures, and study results. We used Google Translate online software to extract data from non-English article [18, 19].
Risk of bias assessment
We used Revised Cochrane risk-of-bias tool for randomized trials (RoB2) to evaluate the quality of included studies. This tool has five parts that include the Randomization process, Deviations from the intended interventions, Missing outcome data, Measurement of the outcome and Selection of the reported result. The overall bias for each study is based on the bias level obtained in each of these sections [20]. Any disagreements between the two researchers regarding the inclusion and quality assessment processes were resolved by an expert researcher (AR).
Statistical analysis
In this study, descriptive statistics are presented, including the means and SDs and statistical significance for between-groups comparisons for each outcome at each follow-up time point (“Appendix 1: Table 3”). Because of the small number of included studies and clinical heterogeneity discussed under the limitations section, data could not be pooled and meta-analysis on the results.
Table 3.
Details of included studies outcome measurements in assessment times (means with standard deviations)
| First author (year) | Outcome measurements | Scale ranges | Treatment groups | Before (Mean ± SD) |
Three days (Mean ± SD) |
One week (Mean ± SD) |
Three weeks (Mean ± SD) |
After (Mean ± SD) |
Fallow up | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| One week (Mean ± SD) |
Four weeks (Mean ± SD) |
Twelve weeks (Mean ± SD) |
||||||||||
| Byeon (2003) | VAS | 0–10 | Exp | 6.2 ± 1.4 | 5.1 ± 0.9 | 3.9 ± 1.9 | 3.1 ± 1.5(2w) | |||||
| Cont | 6.2 ± 1.1 | 5.7 ± 1.5 | 5.6 ± 1.5 | 4.5 ± 1.4 (2w) | ||||||||
| MPQ | 0–78 | Exp | 28.1 ± 15.9 | 27.3 ± 15.1 | 24.19 ± 15 | 22.9 ± 15.5 (2w) | ||||||
| Cont | 27.6 ± 8 | 25.3 ± 9 | 24.6 ± 9.7 | 20.8 ± 7.3 (2w) | ||||||||
| ROM | NA | Exp | 42.8 ± 3.9 | 43.6 ± 2.7 | 44.2 ± 3.3 | 44.3 ± 2.9 (2w) | ||||||
| Cont | 42.9 ± 2.6 | 43.4 ± 2.8 | 43.9 ± 3.2 | 43.5 ± 3.1 (2w) | ||||||||
| Sumen (2015) | VAS | 0–10 | Exp | 6.80 ± 1.69 | 3.40 ± 1.50(2w) * | 2.33 ± 1.63* | ||||||
| Cont | 6.80 ± 1.32 | 5.00 ± 1.77(2w) | 5.20 ± 1.14 | |||||||||
| PPT | NA | Exp | 21.80 ± 7.25 | 29.73 ± 8.85(2w) * | 35.93 ± 13.68* | |||||||
| Cont | 25.30 ± 8.38 | 26.66 ± 8.37(2w) | 25.26 ± 6.54 | |||||||||
| ROM | NA | Exp | 32.46 ± 3.71 | 39.60 ± 5.12(2w) | 40.20 ± 4.73 | |||||||
| Cont | 37.00 ± 6.78 | 41.13 ± 5.12(2w) | 40.00 ± 5.42 | |||||||||
| NDI | 0–100 | Exp | 39.33 ± 12.86 | 26.86 ± 13.33(2w) | 21.33 ± 13.65 | |||||||
| Cont | 35.46 ± 9.24 | 28.40 ± 11.01(2w) | 27.73 ± 10.59 | |||||||||
| Medeiros (2016) | VAS | 0–10 | Exp | 6.21 ± 2.87 | 1.95 ± 1.85(10d) * | |||||||
| Cont | 5.05 ± 2.36 | 2.38 ± 1.69(10d) | ||||||||||
| PB | BDNF | NA | Exp | 19.75 ± 14.06 | 21.98 ± 11.88(10d) | |||||||
| Cont | 35.05 ± 42.01 | 27.78 ± 29.36(10d) | ||||||||||
| S100β | NA | Exp | 11.28 ± 4.55 | 11.87 ± 5.87(10d) | ||||||||
| Cont | 16.68 ± 10.31 | 18.25 ± 13.72(10d) | ||||||||||
| TNF-α | NA | Exp | 28.18 ± 3.34 | 27.50 ± 16.32(10d) | ||||||||
| Cont | 28.28 ± 7.69 | 28.72 ± 5.89(10d) | ||||||||||
| IL10 | NA | Exp | 0.34 ± 1.27 | 1.17 ± 1.78(10d) | ||||||||
| Cont | 1.11 ± 2.63 | 1.38 ± 2.47(10d) | ||||||||||
| IL6 | NA | Exp | 0.48 ± 0.58 | 0.41 ± 0.44(10d) | ||||||||
| Cont | 0.50 ± 0.60 | 0.55 ± 0.59(10d) | ||||||||||
| LDH | NA | Exp | 89.46 ± 30.38 | 88.90 ± 44.17(10d) | ||||||||
| Cont | 84.40 ± 46.14 | 105.22 ± 75.12(10d) | ||||||||||
| GPX | NA | Exp | 0.58 ± 0.93 | 0.55 ± 1.05(10d) | ||||||||
| Cont | 0.40 ± 0.46 | 0.61 ± 1.53(10d) | ||||||||||
| SOD | NA | Exp | 91.55 ± 148.13 | 85.45 ± 122.21(10d) | ||||||||
| Cont | 50.50 ± 56.75 | 61.10 ± 95.67(10d) | ||||||||||
| CAT | NA | Exp | 716.30 ± 342.93 | 532.20 ± 580.85(10d) | ||||||||
| Cont | 667.80 ± 549.23 | 875.70 ± 569.13(10d) | ||||||||||
| CAR | NA | Exp | 116.95 ± 34.85 | 124.30 ± 22.15(10d) | ||||||||
| Cont | 128.55 ± 34.40 | 121.05 ± 27.60(10d) | ||||||||||
| ROS | NA | Exp | 1.02 ± 0.47 | 0.84 ± 0.82(10d) | ||||||||
| Cont | 0.83 ± 0.78 | 0.74 ± 0.86(10d) | ||||||||||
| CEP | IF | NA | Exp | 1.15 ± 0.29 | 1.26 ± 0.16(10d) | |||||||
| Cont | 1.07 ± 0.19 | 1.09 ± 0.26(10d) | ||||||||||
| SII | NA | Exp | 0.28 ± 0.16 | 0.29 ± 0.09(10d) | ||||||||
| Cont | 0.34 ± 0.14 | 0.30 ± 0.17(10d) | ||||||||||
| CSP | NA | Exp | 74.76 ± 16.82 | 70.46 ± 21.35(10d) | ||||||||
| Cont | 67.98 ± 20.94 | 68.69 ± 13.64(10d) | ||||||||||
| Hadizadeh (2017) | VAS | 0–10 | Exp | 50.62 ± 10.19 | 28.62 ± 10.16(IA) | 17 ± 13.59 | ||||||
| Cont | 45.25 ± 16.99 | 33.87 ± 17.54(IA) | 35.25 ± 19.57 | |||||||||
| ROM | NA | Exp | 28.53 ± 7.31 | 32.95 ± 8.05(IA) | 34.74 ± 3.37* | |||||||
| Cont | 30.62 ± 7.28 | 29.24 ± 5.76(IA) | 28.72 ± 5.46 | |||||||||
| Botelho (2018) | VAS | 0–10 | Exp | 5.53 ± 2.28 | 2.6 ± 2.4(12 W)* | |||||||
| Cont | 5.46 ± 2.32 | 4.01 ± 2.58(12 W) | ||||||||||
| B-PCP:S | 0–93 | Exp | 55.85 ± 14.63 | 38.11 ± 19.86(12 W) * | ||||||||
| Cont | 56.33 ± 16.23 | 49.89 ± 14.62(12 W) | ||||||||||
| CEP | MEP | NA | Exp | 2.26 ± 0.52 | 1.84 ± 0.74(12 W) | |||||||
| Cont | 1.75 ± 0.64 | 1.69 ± 0.45(12 W) | ||||||||||
| Brennan (2018) | NPRS | 0–10 | Exp | 2.95 ± 1.52 | 2.62 ± 1.59 | 2.27 ± 1.80(6 W) | 1.92 ± 1.63 | |||||
| Cont | 2.59 ± 1.25 | 2.09 ± 1.07 | 1.71 ± 1.47(6 W) | 1.67 ± 1.45 | ||||||||
| NDI | 0–100 | Exp | 0.17 ± 0.10 | 0.14 ± 0.11 | 0.11 ± 0.09(6 W) | 0.10 ± 0.11 | ||||||
| Cont | 0.14 ± 0.09 | 0.12 ± 0.09 | 0.09 ± 0.10(6 W) | 0.09 ± 0.09 | ||||||||
SD standard deviation, VAS visual analogue scale, MPQ McGill pain questionnaire, ROM range of motion, NA not accessible, Exp. Experimental, Cont. control, W weeks, PPT pain pressure threshold, NDI neck disability index, PB peripheral biomarkers, BDNF brain-derived neurotrophic factor, LDH lactate dehydrogenase, GPx glutathione peroxidase, SOD superoxide dismutase, CAT catalase activity, CAR protein carbonyls, ROS reactive oxygen species, CEP cortical excitability parameters, IF intracortical facilitation, SII short intracortical inhibition, CSP cortical silent period, d days, IA immediately after, B-PCP:S Brazilian profile of chronic pain: screen, MEP motor evoked-potential, NPRS numeric pain rating scale
*Statistically significant difference between two groups (P< 0.05)
Results
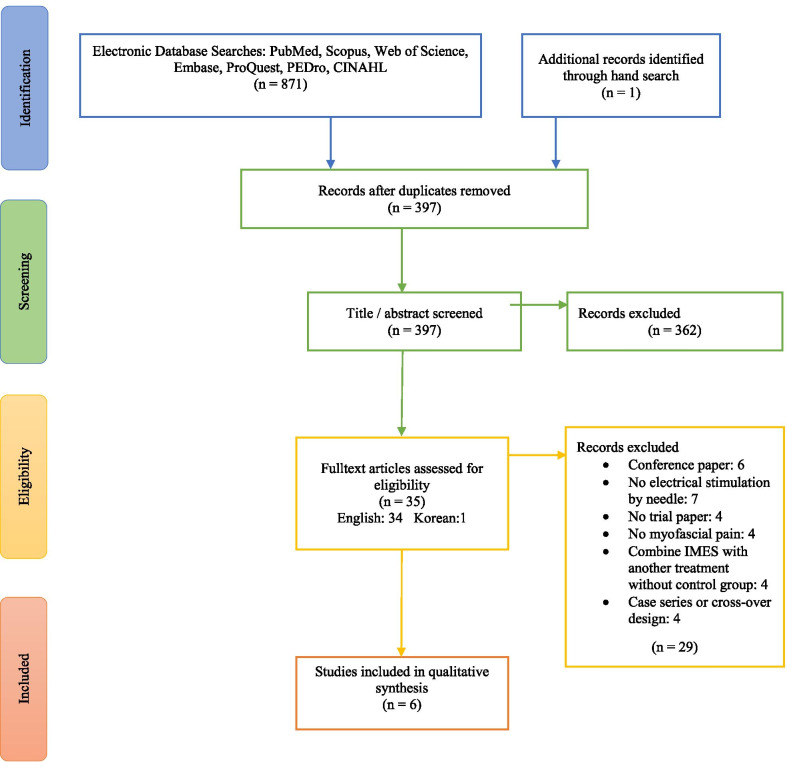
After searching the databases and removing duplicate items, 397 potentially relevant titles and abstracts were identified. After screening the title and abstracts, 362 articles were excluded. Thirty-five studies were selected for full-text review. Finally, six studies were included in this systematic review based on the inclusion and exclusion criteria. The most frequent reasons for excluding studies were unrelated titles during the initial review, studies without electrical stimulation application via a needle, conference papers, etc. The details of the excluded studies with justification for exclusion are presented in the “Appendix 2: Table 4”. The process of searching and screening is summarized in Fig. 1.
Appendix 4.
Excluded studies with justification for exclusion
| First author (year) |
Title | Reason for exclusion |
|---|---|---|
|
Stalberg 1987 |
Intramuscular Stimulation for The Study of Individual Motor End-Plates and Muscle Fibers | Conference paper |
|
Wright 1991 |
Morphologic and Histochemical Characteristics of Skeletal Muscle After Long-Term Intramuscular Electrical Stimulation | It was not a trial study |
|
Arendt-Nielsen 1998 |
Assessment Of Muscle Pain in Humans: Clinical and Experimental Aspects | Conference paper |
|
Chu 1999 |
The Role of The Monopolar Electromyographic Pin in Myofascial Pain Therapy: Automated Twitch-Obtaining Intramuscular Stimulation (ATOIMS) And Electrical Twitch-Obtaining Intramuscular Stimulation (ETOIMS) | Combine IMES with another treatment without control group |
|
Chu 2000 |
Early Observations in Radiculopathic Pain Control Using Electrodiagnostically Derived New Treatment Techniques: Automated Twitch-Obtaining Intramuscular Stimulation (ATOIMS) And Electrical Twitch-Obtaining Intramuscular Stimulation (ETOIMS) | Combine IMES with another treatment without control group |
|
Gunn 2001 |
Treating Whiplash-Associated Disorders with Intramuscular Stimulation: A Retrospective Review Of 43 Patients with Long-Term Follow-Up | No electrical stimulation by needle |
|
Karakurum 2001 |
The ‘Dry-Needle Technique’: Intramuscular Stimulation in Tension Type Headache | No electrical stimulation by needle |
|
Chu 2002 |
The Efficacy of Automated/Electrical Twitch Obtaining Intramuscular Stimulation (Atoims/Etoims) For Chronic Pain Control: Evaluation with Statistical Process Control Method | Combine IMES with another treatment without control group |
|
Kosek 2003 |
Perceptual Integration of Intramuscular Electrical Stimulation in The Focal and The Referred Pain Area in Healthy Humans | No electrical stimulation by needle |
|
Chu 2004 |
Electrical Twitch-Obtaining Intramuscular Stimulation in Lower Back Pain | It was not a randomized control trial |
|
Ga 2007 |
Intramuscular And Nerve Root Stimulation Vs Lidocaine Injection of Trigger Points in Myofascial Pain Syndrome | No electrical stimulation by needle |
|
Lee 2008 |
Effects Of Needle Electrical Intramuscular Stimulation on Shoulder and Cervical Myofascial Pain Syndrome and Microcirculation | It was not a randomized control trial |
|
Chu 2008 |
Etoims Twitch Relief Method in Chronic Refractory Myofascial Pain (CRMP) | No electrical stimulation by needle |
|
Valeriani 2008 |
Nociceptive Contribution to The Evoked Potentials After Intramuscular Electrical Stimulation | The intervention was not on myofascial pain |
|
Hong-You 2009 |
Increased H-Reflex Induced by Intramuscular Electrical Stimulation of Latent Myofascial Trigger Point | It was not a trial study |
|
Rainey 2013 |
The Use of Trigger Point Dry Needling and Intramuscular Electrical Stimulation for A Subject with Chronic Low Back Pain: A Case Report | Combine IMES with another treatment without control group |
|
Jodic 2014 |
Treatment Of Nonspecific Thoracic Spine Pain with Trigger Point Dry Needling and Intramuscular Electrical Stimulation: A Case Series | It was not a randomized control trial |
|
Borg-Stein 2014 |
Myofascial Pain Syndrome Treatments | It was not a trial study |
|
Couto 2014 |
Paraspinal Stimulation Combined with Trigger Point Needling and Needle Rotation for The Treatment of Myofascial Pain: A Randomized Sham-Controlled Clinical Trial | No electrical stimulation by needle |
|
Shin 2014 |
Intramuscular Stimulation of Peri cranial Myofascial Trigger Points in The Treatment of Frequent Episodic Tension-Type Headache | Conference paper |
|
Fogelman 2015 |
Efficacy Of Dry Needling for Treatment of Myofascial Pain Syndrome | No electrical stimulation by needle |
|
Chu 2015 |
Twitch-Obtaining Intramuscular Stimulation: Observations in The Management of Radiculopathic Low Back Pain | The intervention was not on myofascial pain |
|
Calatayud 2016 |
Improvement Of Myofascial Pain in Equine Brachiocephalicus Muscle Using Dry Needling Technique, A Clinical Commentary | Conference paper |
|
Shanmugam 2016 |
Effects Of Intramuscular Electrical Stimulation Using Inversely Placed Electrodes on Myofascial Pain Syndrome in Shoulder—A Case Series | It was not a randomized control trial |
|
Ratmansky 2016 |
Position Statement of The Israeli Society for Musculoskeletal Medicine on Intramuscular Stimulation for Myofascial Pain Syndrome- A Delphi Process | It was not a trial study |
|
Mazloum 2017 |
Comparative Effects of Dry Needling and Intramuscular Electrical Stimulation with And Without Kinesiology Taping in Patients with Non-Specific Chronic Low Back Pain | Conference paper |
|
Graca-Tarrago 2019 |
Intramuscular Electrical Stimulus Potentiates Motor Cortex Modulation Effects on Pain and Descending Inhibitory Systems in Knee Osteoarthritis: A Randomized, Factorial, Sham-Controlled Study | The intervention was not on myofascial pain |
|
Moon 2019 |
Intramuscular Stimulation as A Novel Alternative Method of Pain Management After Thoracic Surgery | The intervention was not on myofascial pain |
|
Kim 2019 |
A New Treatment Modality for The Postoperative Muscular Pain Management in Pylorus Preserving Pancreaticoduodenectomy: A Double-Blind Randomized Control Trial | Conference paper |
Fig. 1.
PRISMA flow diagram
Characteristics of included studies
A summary of the methodological characteristics of the included studies and their results are presented in Table 1. Among the selected studies, five studies were in English [21–25], and one study was in Korean [26]. Two studies used patient and assessor blinding [22, 24], and two studies only used assessor blinding [21, 23]. Two studies did not include any blinding [25, 26].
Table 1.
Summary of the study design, participants characteristics, outcome measurements, assessment time and summary of results of included studies
| First author (year) | Type of disorder | Sample size (F/M) (n) |
Age (Mean ± SD) | Exp. group | Cont. group | Outcome measurement | Time of assessment | Main results |
|---|---|---|---|---|---|---|---|---|
| Byeon [26] (2003) | UT MPS |
20 (8/12) Exp.: 10 Cont.: 10 |
Total participants: 50.7 ± 10.1 |
IMES | DN |
VAS MPQ Neck ROM (lateral flex.) |
Before Three days One week Two weeks |
No significant difference of all outcome measurements between groups in all assessment times |
| Sumen [21] (2015) | MPS |
30 (22/8) Exp.: 15 Cont.: 15 |
Total Participants: 38.6* |
SIMES + Stretching exercise | Home-based stretching exercise twice daily (10 repetition) |
VAS PPT Neck ROM (opposite side lateral Flex.) NDI |
Before After One month |
Significantly VAS decrease & PPT increase in experimental than control groups in all assessment times |
| Medeiros [22] (2016) | MPS |
23 (23/0) Exp.: 11 Cont.: 12 |
Exp.: 49.18 ± 11.63 Cont.: 45.83 ± 9.63 |
Sham-rTMS + IMES | Sham-rTMS + Sham-IMES |
VAS Peripheral biomarkers Cortical excitability parameters |
End of every session Before After |
Significant pain decreases in experimental group than control group There was not any change in all peripheral biomarker’s parameters in both groups |
| Hadizadeh [23] (2017) | UT MPS |
16 (16/0) Exp.: 8 Cont.: 8 |
Exp.: 24.6 ± 6.4 Cont.: 26.7 ± 6.5 |
IMES | Sham-IMES |
VAS Neck ROM |
Before After One week |
Significantly higher ROM in IMES group compared to control group one week after treatment No significant differences of pain in the all assessment times |
| Botelho [24] (2018) | MPS |
24 (24/0) Exp.: 12 Cont.: 12 |
Exp.: 48.36* Cont.: 46* |
IMES | Sham-IMES |
VAS B-PCP:S Cortical excitability parameters taking analgesic during the treatment |
Before After |
Experimental group presented lower pain and disability in comparison to control group significantly Analgesic use was 69.4% in sham group and 30.6% in EIMS |
| Brennan [25] (2020) | MPS |
45(37/8) Exp.: 20 Cont.: 25 |
Exp.: 28 ± 9.99 Cont.: 26.32 ± 8.94 |
IMES | DN |
NPRS NDI |
Before 3th week 6th week 12th week |
At no time did NDI or NPRS differ significantly between groups |
F female, M male, n number, SD standard deviation, Exp. Experimental, Cont. control, UT upper trapezius, MPS myofascial pain syndrome, IMES intramuscular electrical stimulation, DN dry needling, VAS visual analogue scale, MPQ McGill pain questionnaire, ROM range of motion, Flex. Flexion, SIMES sensory intramuscular electrical stimulation, PPT pain pressure threshold, NDI neck disability index, rTMS repetitive transcranial magnetic stimulation, B-PCP:S Brazilian profile of chronic pain: screen, NPRS numeric pain rating scale
*Standard deviation was not reported
Among the included studies, 76 and 82 patients were allocated to IMES and control groups, respectively. Three studies had parallel RCT designs with IMES and control groups [23–25]. Two other studies featured three parallel RCT designs. One compared the effectiveness of low-level laser therapy, IMES, vs. a control group [21]. Another included DN, IMES, and intramuscular stimulation (Gunn-IMS) groups [26]. One study had four groups, including repetitive Transcranial Magnetic Stimulation (rTMS) + IMES, rTMS + sham-IMES, sham- rTMS + IMES, and sham- rTMS + sham-IMES. We considered sham- rTMS + IMES and sham- rTMS + sham-IMES as experimental and control group, respectively in this study [22]. All studies recruited patients with chronic cervical MPS.
In three studies, sham-IMES groups were used as a control group [22–24]. In one study, participants in the control group received prescribed home-based exercises [21], while subjects in another two studies control group received DN [25, 26]. The number of treatment sessions varied from one to ten sessions between studies.
Risk of bias assessment of selected articles
Among six included studies, two had low risk of bias [22, 24] and three of them had moderate risk of bias [21, 23, 26]. The study by Brennan et al. [25] was the only study with high risk of bias due to inappropriate intention to treat analysis. Details of the study quality assessment are presented in Fig. 2. The details of the scoring of each item for the included studies are presented in the Additional file 1.
Fig. 2.
Quality assessment for RCT (RoB 2.0)
Wave properties and needle location in IMES group
In four studies, the upper trapezius muscle was treated [21, 23, 25, 26]. Medeiros et al. and Botelho et al. applied IMES to the cervical paraspinal muscles [22, 24]. Only three studies targeted trigger points [21, 23, 25]. The frequencies of the electrical stimulation ranged from 2 to 80 Hz. In two studies, the intensity was increased to the point of contraction [23, 26]. Sumen et al. [21] increased the intensity until the patient sensed the stimulus. Three studies did not report any details about the intensity [22, 24, 25]. Wave properties and IMES technical characteristics are summarized in Table 2.
Table 2.
The properties of applied intramuscular electrical stimulation of included studies
| First author (year) | Location of treatment | Total sessions/session(s) per week/total duration | Wave properties | Needle electrode | Needle in TrP | Reference electrode | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Shape | Freq. (Hz) | Intensity | Duration of treatment | Type | Pole | |||||
|
Byeon (2003) |
Upper trapezius | 6/3/2 w | Biphasic pulse | 10 |
3 times of sensory threshold (contraction level) |
15 m | NR | NR | NR | NR |
|
Sumen (2015) |
Upper trapezius | 10/5/2 | Pulse | 80 |
NR (Sensory level) |
20 m | NR | Yes | NR | NR |
|
Medeiros (2016) |
Dermatomes related to C2-C5 & Paraspinal muscles |
10/NR/NR | Pulse | 2 | NR | 20 m | NR | NR | NR | NR |
| Hadizadeh (2017) | Upper trapezius | 1/1/1 d | Biphasic burst |
Basic freq.: 120 Burst freq.: 2 |
NR (Contraction level) |
10 m | Cathode | Yes | Patch electrode | Anode |
|
Botelho (2018) |
C2-C4 Paraspinal muscle | 10/NR/NR | Biphasic pulse | 2 | NR | 20 m | NR | No | NR | NR |
|
Brennan (2020) |
Upper trapezius | 6/1/6 w | NR | 10 | NR | 10 m | NR | Yes | NR | NR |
TrP trigger point, Freq. frequency, Hz hertz, W weeks, m minute, NR not reported, d day
Outcome measures and summary of results
The visual analog scale (VAS) was the most common pain outcome measure. Three studies evaluated ROM measurements [21, 23, 26]. Other outcome measurements included pain by numeric pain rating scale (NPRS), pain pressure thresholds (PPT), biomarkers such as BDNF, pain or functional ability questionnaires, the neck disability index (NDI) and the McGill pain questionnaire (MPQ), and analgesic drug intake (Table 1). Also, the Details of included studies outcome measurements in assessment times (means with standard deviations) are presented in the “Appendix 1: Table 3”.
Byeon et al. [26] compared the effectiveness of IMES and DN; they showed improvement in pain and cervical lateral flexion ROM in all groups, but there were no significant differences of all outcome measurements in all assessment times in both groups [26]. Sumen et al.'s [21] results present statistically significant VAS decreases and PPT increases in the IMES group vs. the control group. Medeiros et al. [22] showed a significant difference in pain reduction between the IMES and control groups but no change in peripheral biomarkers parameters in the experimental and control groups. Hadizadeh et al. [23] showed that ROM was significantly higher in the IMES group than the control group one week after treatment. There were no significant differences in pain in all assessment times between both groups. Botelho et al. [24] showed a significant improvement in pain and analgesic drugs in the IMES group compared to the control group. Brennan et al. [25] compared the effectiveness of IMES and DN; they showed a significant improvement in pain and disability index in both groups and did not NDI or NPRS differ significantly between groups in any assessment times.
Discussion
The current study is the first systematic review evaluating IMES’s effectiveness in patients with MPS to the best of our knowledge. Six studies with a total of 158 subjects were included in this review. Pain, the most common outcome measurement, was assessed by the VAS and NPRS or the MPQ. The effectiveness of IMES was compared with sham IMES, DN, or no intervention. The number of sessions varied from 1 to 10 sessions. The duration of IMES ranged from 10 to 20 min. The study by Hadizadeh et al. [23] was the only study with a single-session intervention. Three articles reported following the patients from 1 to 6 weeks [21, 23, 25, 26].
In general, studies with a low risk of bias showed a significant improvement in the variables of pain, disability and analgesic use in the IMES group compared to the control group [22, 24]. Also, in studies with moderate risk of bias (Some concerns), reduced pain and improved range of motion have been reported. However, in some cases, there was no significant difference with the control group [21, 23, 26]. In a study with a high risk of bias, no significant difference was reported between the IMES group and the control group in the variables of pain and disability [25].
Initially, we aimed to determine what factors would impact the effectiveness of IMES on MPS, such as the frequency of the applied currents, the duration, the exact location of active and reference needles or electrodes, among others, but the limited number of studies and the heterogenicity among studies did not allow for this kind of analysis. The study by Hadizadeh et al. was the only study demonstrating that one session of IMES could effectively reduce pain and increase ROM not immediately but after a one-week follow-up. It can be due to inflammatory processes after needle insertion, which may present as muscle soreness [27]. How many IMES sessions would be sufficient for clinical improvement cannot be deduced from the current research and requires further study.
There are some mechanisms explaining trigger points. One explanation is offered by the integrated hypothesis, which maintains that trigger points result from repetitive low-intensity trauma, leading to sarcoplasmic retinaculum injury, increased calcium concentration, and permanent contraction in the area. This would result in hypoxia and cell damage in the region [28–30]. It seems that surface, motor excitable electrical stimulation can increase the blood flow; therefore, it can decrease regional hypoxia. Commonly, IMES produces muscle contractions. This method can insert electrical stimulation to the depth of muscle with lower resistance against the current. Therefore, IMES seems to be more effective in managing regional hypoxia in TrP zone compared to superficial ES and the use of DN alone [15, 31]. Besides, most studies used low-frequency current; low frequencies may cause the release of endorphins and enkephalins, leading to a reduction in pain [32].
Limitations
Our study has several limitations that should be mentioned. First, we included only primary RCT studies in this systematic review, which reduced the number of studies, limiting the ability to generalize the results of this study. Second limitation of this study is that, because the characteristics of the applied electrical stimulation like intensity, pulse duration, frequency, time, and etc. are not fully mentioned in all studies, it is not possible to make recommendations regarding the appropriate parameters. Third, we included RCTs with various type of interventions due to limitation in original studies. Fourth, the small number of included studies and clinical heterogeneity of included studies such as different fallow up point times, different sessions number, different control groups, and outcome measurements did not allow us to pool data and do a meta-analysis on the results. Further research is recommended to do a meta-analysis on this topic after further randomized controlled trials. Fifth, all of the included studies had a small sample size that can impact the result of the ROB2 tool. Therefore, the results of quality assessment in this study should be accepted with this limitation.
Further studies are needed to overcome these limitations. First, more RCT studies with larger sample sizes are needed to compare this intervention with other routine interventions. Second, studies are needed to investigate the placebo effects of this intervention. Studies with objective variables (like TrP size or stiffness found by radiologic methods) are also needed to evaluate this intervention's effectiveness. Also, future studies should include more detailed parameters of the interventions.
Conclusion
There is preliminary evidence from a few small trials suggesting the efficacy of IMES for the care of myofascial pain syndrome. The data support the conduct of larger trials investigating the efficacy and comparative effectiveness of IMES, and determining the optimal settings and dose of the intervention.
Supplementary Information
Additional file 1. SUP1: The details of the Risk of Bias 2 scoring and rating of each item for the included studies.
Acknowledgements
The present article is part of a Ph.D. thesis and was partially supported by the school of rehabilitation, Shahid Beheshti University of Medical Sciences.
Abbreviations
- MPS
Myofascial pain syndrome
- IMES
Intramuscular electrical stimulation
- RCTs
Randomized controlled trials
- RoB2
Revised cochrane risk-of-bias tool for randomized trials
- TrP
Trigger point
- ROM
Range of motion
- DN
Dry needling
- ES
Electrical stimulation
- MeSH
Medical subject heading
- rTMS
Repetitive transcranial magnetic stimulation
- VAS
Visual analog scale
- NPRS
Numeric pain rating scale
- PPT
Pain pressure thresholds
- NDI
Neck disability index
- MPQ
Mcgill pain questionnaire
Appendix
Authors' contributions
MH carried out the concept of the study and literature search and review, data extraction, prepared the initial draft, coordinated revisions and prepared the final written draft. AR contributed to the concept and design of the study, revising the initial draft and approve the final drafting. MJ contributed to the search strategy and review, data extraction, coordinated the appraisal and contributed to manuscript revisions. MV contributed to the conception of the study and revising the draft and approval the final version. JD contributed to the conception and study design, critically appraising the initial draft of the article and approving the final version of the article to be submitted. All authors read and approved the final manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Availability of data and materials
The search strategy, list of excluded studies by title/abstract or full-text screening are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Monavar Hadizadeh, Email: Hadizade.mahsa1992@yahoo.com.
Abbas Rahimi, Email: a_rahimi@sbmu.ac.ir.
Mohammad Javaherian, Email: javaherian_m@razi.tums.ac.ir.
Meysam Velayati, Email: Drmeysam.v@gmail.com.
Jan Dommerholt, Email: jan@myopain4u.com.
References
- 1.Hendler N, Kozikowski J. Overlooked physical diagnoses in chronic pain patients involved in litigation. Psychosomatics. 1993;34:494–501. doi: 10.1016/S0033-3182(93)71823-X. [DOI] [PubMed] [Google Scholar]
- 2.Simons D, Travell J, Simons L. Myofascial pain and dysfunction: the trigger point manual. London: Williams & Wilkins; 1999. [Google Scholar]
- 3.Mense S, Simons D. Muscle pain understanding its nature, diagnosis, and treatment. London: Lippincott Williams&Wilkins; 2001. [Google Scholar]
- 4.Mense S, Gerwin D. Muscle pain: understanding the mechanisms. London: Springer; 2010. [Google Scholar]
- 5.Sergienko S, Kalichman L. Myofascial origin of shoulder pain: a literature review. J Bodyw Mov Ther. 2015;19:91–101. doi: 10.1016/j.jbmt.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 6.Celik D, Kaya ME. Clinical implication of latent myofascial trigger point. Curr Pain Headache Rep. 2013;17(353):1–7. doi: 10.1007/s11916-013-353-8. [DOI] [PubMed] [Google Scholar]
- 7.Simons D. Review of enigmatic mtrps as a common cause of enigmatic musculoskeletal pain and dysfunction. J Electromyogr Kinesiol. 2004;14:95–107. doi: 10.1016/j.jelekin.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 8.Vernon H, Schneider M. Chiropractic management of myofascial trigger points and myofascial pain syndrome: a systematic review of the literature. J Manip Physiol Ther. 2009;32:14–24. doi: 10.1016/j.jmpt.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 9.Srbely J. New trends in the treatment and management of myofascial pain syndrome. Curr Pain Headache Rep. 2010;14:346–352. doi: 10.1007/s11916-010-0128-4. [DOI] [PubMed] [Google Scholar]
- 10.Liu L, Huang QM, Liu QG, Ye G, Bo CZ, Chen MJ, Li P. Effectiveness of dry needling for myofascial trigger points associated with neck and shoulder pain: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2015;96:944–955. doi: 10.1016/j.apmr.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 11.Benjaboonyanupap D, Paungmali A, Pirunsan U. Effect of therapeutic sequence of hot pack and ultrasound on physiological response over trigger point of upper trapezius. Asian J Sports Med. 2015;6:57–61. doi: 10.5812/asjsm.23806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mohammadi Kojidi M, Okhovatian F, Rahimi A, Baghban AA, Azimi H. The influence of positional release therapy on the myofascial trigger points of the upper trapezius muscle in computer users. J Bodyw Mov Ther. 2016;20:767–773. doi: 10.1016/j.jbmt.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 13.Liu L, Huang QM, Liu QG, Thitham N, Li LH, Ma YT, Zhao JM. Evidence for dry needling in the management of myofascial trigger points associated with low back pain: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2018;99:144–152. doi: 10.1016/j.apmr.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 14.Sherry J, Oehrlein K, Hegge K, Morgan B. Effect of burst-mode transcutaneous electrical nerve stimulation on peripheral vascular resistance. Phys Ther. 2001;81:1183–1191. doi: 10.1093/ptj/81.6.1183. [DOI] [PubMed] [Google Scholar]
- 15.Yamabata S, Shiraishi H, Munechika M, Fukushima H, Fukuoka Y, Hojo T, Shirayama T, Horii M, Matoba S, Kubo T. Effects of electrical stimulation therapy on the blood flow in chronic critical limb ischemia patients following regenerative therapy. SAGE Open Med. 2016;4:1–10. doi: 10.1177/2050312116660723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chu J, Takehara I, Li TC, Schwartz I. Electrical twitch obtaining intramuscular stimulation (ETOIMS) for myofascial pain syndrome in a football player. Br J Sports Med. 2004;38:E25. doi: 10.1136/bjsm.2003.010306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rainey CE. The use of trigger point dry needling and intramuscular electrical stimulation for a subject with chronic low back pain: a case report. J Orthop Sports Phys Ther. 2013;8:145–161. [PMC free article] [PubMed] [Google Scholar]
- 18.Balk E, Chung M, Chen M, Trikalinos T, Kong W. Assessing the accuracy of google translate to allow data extraction from trials published in non-english languages. AHRQ. 2013, Rockville. [PubMed]
- 19.Cruccu G, Pennisi E, Truini A, Iannetti GD, Romaniello A, Le Pera D, De Armas L, Leandri M, Manfredi M, Valeriani M. Unmyelinated trigeminal pathways as assessed by laser stimuli in humans. Brain. 2003;126:2246–2256. doi: 10.1093/brain/awg227. [DOI] [PubMed] [Google Scholar]
- 20.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng H-Y, Corbett MS, Eldridge SM, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT. RoB 2: a revised tool for assessing risk of bias in randomized trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 21.Sumen A, Sarsan A, Alkan H, Yildiz N, Ardic F. Efficacy of low level laser therapy and intramuscular electrical stimulation on myofascial pain syndrome. J Back Musculoskelet Rehabil. 2015;28:153–158. doi: 10.3233/BMR-140503. [DOI] [PubMed] [Google Scholar]
- 22.Medeiros LF, Caumo W, Dussán-Sarria J, Deitos A, Brietzke A, Laste G, Campos-Carraro C, de Souza A, Scarabelot VL, Cioato SG, Vercelino R. Effect of deep intramuscular stimulation and transcranial magnetic stimulation on neurophysiological biomarkers in chronic myofascial pain syndrome. Pain Med. 2016;17:122–135. doi: 10.1111/pme.12919. [DOI] [PubMed] [Google Scholar]
- 23.Hadizadeh M, Tajali SB, Moghadam BA, Jalaie S, Bazzaz M. Effects of Intramuscular Electrical Stimulation on Symptoms Following Trigger Points; A Controlled Pilot Study. J. Mod. Rehabil. 2017; 31–6. 10.18869/nirp.jmr.11.1.31.
- 24.Botelho L, Angoleri L, Zortea M, Deitos A, Brietzke A, Torres IL, Fregni F, Caumo W. Insights about the neuroplasticity state on the effect of intramuscular electrical stimulation in pain and disability associated with chronic myofascial pain syndrome (MPS): a double-blind, randomized, sham-controlled trial. Front Hum Neurosci. 2018;12:388. doi: 10.3389/fnhum.2018.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brennan K, Elifritz KM, Comire MM, Jupiter DC. Rate and maintenance of improvement of myofascial pain with dry needling alone vs dry needling with intramuscular electrical stimulation: a randomized controlled trial. J Man Manip Ther. 2020;30:1–1. doi: 10.1080/10669817.2020.1824469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Byeon HT, Park SH, Ko MH, Seo JH. Effects of intramuscular stimulation in myofascial pain syndrome of upper trapezius muscle. J Korean Acad Rehabil Med. 2003;27:753–756. [Google Scholar]
- 27.Martín-Pintado-Zugasti A, del Moral OM, Gerwin RD, Fernández-Carnero J. Post-needling soreness after myofascial trigger point dry needling: current status and future research. J Bodyw Mov Ther. 2018;22:941–946. doi: 10.1016/j.jbmt.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 28.Dommerholt J, Fernandez-de-las-penas C. Trigger point dry needling. Beijing: Elsevier; 2013. [Google Scholar]
- 29.Gerwin R, Dommerholt J, Shah J. An expansion of Simons’ integrated hypothesis of trigger point formation. Curr Pain Headache Rep. 2004;8:468–475. doi: 10.1007/s11916-004-0069-x. [DOI] [PubMed] [Google Scholar]
- 30.Gerwin R. The taut band and other mysteries of the trigger point: an examination of the mechanisms relevant to the development and maintenance of the trigger point. J Musculoskelet Pain. 2008;16:115–121. doi: 10.1080/10582450801960081. [DOI] [Google Scholar]
- 31.Faghri P, Meerdervort H, Glaser R, Figoni S. Electrical stimulation-induced contraction to reduce blood stasis during arthroplasty. IEEE Trans Rehabil Eng. 1997;5:62–69. doi: 10.1109/86.559350. [DOI] [PubMed] [Google Scholar]
- 32.Lundeberg T, Stener-Victorin E. Is there a physiological basis for the use of acupuncture in pain? Int Congr Ser. 2002;1238:3–10. doi: 10.1016/S0531-5131(02)00416-8. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. SUP1: The details of the Risk of Bias 2 scoring and rating of each item for the included studies.
Data Availability Statement
The search strategy, list of excluded studies by title/abstract or full-text screening are available from the corresponding author on reasonable request.