ABSTRACT
Anakinra, which is an Interleukin-1 (IL-1) receptor antagonist with the advancing disease process, has started to be considered as an alternative treatment for Covid-19 patients with cytokine storms. We evaluated the effect of corticosteroids and IL-1 receptor blockage with anakinra on pregnant patients with Covid-19 at high risk for respiratory distress, ongoing fever, deterioration in their general condition and consequently maternal and fetal complications. Fourteen pregnant women who received anakinra (median dosage: 400 mg) and corticosteroid (methylprednisolone-median dosage: 80 mg) treatment were evaluated retrospectively. Patients were assessed according to the World Health Organization (WHO) scale. The mortality rate of the cohort was 7.1%, the median hospitalization period of the patients was 15 days and 2 patients had premature births. Covid-19 was found to have a similar spectrum of symptoms in pregnant and non-pregnant women, such as dyspnea, cough and fever. Our study was the first to analyze the combined treatment of corticosteroid and anakinra in pregnant patients with pneumonia from Covid-19 based on the WHO scoring system. Due to the obscurity in the treatment process in pregnant patients, studies are ongoing on managing Covid-19 infection in these patients. We presume that the early use of anakinra and corticosteroid treatments in patients severely infected with Covid-19 may have positive effects on disease progression and survival.
KEYWORDS: Anakinra, corticosteroid, Covid-19, cytokine storm, methylprednisolone, pregnant
Introduction
At the end of 2019, a new type of coronavirus identified as the cause of a series of pneumonia cases in Wuhan spread rapidly and caused a pandemic. In February 2020, the virus was identified as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and was identified by the World Health Organization as the cause of Covid-19 (2019 coronavirus) disease.
The virus can trigger a cytokine storm in the body, which can lead to acute respiratory distress syndrome (ARDS) and/or extra pulmonary organ failures in patients. This condition is a major cause of death in Covid-19 infections.
The body may become vulnerable to respiratory pathogens due to physiological and immunological changes such as T lymphocyte immunity, increased oxygen consumption, and decreased functional residual capacity, which varies in the physiological course of pregnancy (Tang, Wang, and Song 2018). Therefore, pneumonia is one of the most common non-obstetric infections experienced during pregnancy. Covid-19 has clinical symptoms, usually with pulmonary involvement, such as fever, shortness of breath and dry cough. Some of the patients experience serious respiratory system problems. Information about the course of Covid-19 during pregnancy is limited. The most common symptoms related to Covid-19 in pregnant women are similar to those experienced by non-pregnant populations; fever and cough, with pneumonia also being common (Sahin et al. 2020). Dexamethasone has been proven to be effective in the treatment of Covid-19 (Group 2020). In addition, randomized controlled drug studies were not carried out for treatment involving pregnant patients (Restrepo, Moorthy, and Preziosi 2020). To manage the diagnosis and treatment process in patients with Covid-19, patients’ cases can be classified as mild, moderate or severe based on the severity of respiratory infection (López et al. 2020). While lopinavir/ritonavir and hydroxychloroquine may be recommended to treat mild-to-moderate severe cases, there is no definitive application regarding the treatment of pregnant women with the same disease state. Treatments using drugs such as methylprednisolone, tocilizumab (recombinant IL-6 receptor monoclonal antibody), anakinra (IL-1 inhibitor) or remdesivir (an RNA polymerase inhibitor) are being investigated (López et al. 2020). Corticosteroids can reduce mortality in patients with ARDS (acute respiratory distress syndrome) and can be considered safe in pregnant women (Meduri et al. 2018). There is no evidence in animal experiments that tocilizumab is teratogenic. There are a limited number of retrospective studies on the use of tocilizumab in pregnancy (Nakajima et al. 2016). In these studies, increased rates of spontaneous abortus or congenital abnormalities were not shown with the use of tocilizumab in pregnant women with rheumatic disease. However, due to the lack of data, there is no definitive opinion on the safety of tocilizumab in pregnancy (Nakajima et al. 2016). There are very little data on anakinra safety related to conception, pregnancy process or breastfeeding (Berger et al. 2009). In a case reported by Ilgen et al., a patient who conceived under anakinra treatment completed the pregnancy process without problems (İlgen and Küçükşahin 2017). Animal experiments did not show a negative effect on fertility or health of the fetus. A multi-centered study of case reports and a retrospective multi-centered study (totaling about 40 cases) showed no increase in malformation or abortion risk with anakinra usage (Berger et al. 2009; Youngstein et al. 2017).
In our article, we wanted to share the information we obtained about anakinra and the clinical course of Covid-19 infection in severely infected pregnant patients undergoing corticosteroid therapy.
Materials and methods
Fourteen pregnant patients over the age of 18 years who were admitted to the Ankara City Hospital Obstetrics and Gynecology Clinic due to Covid-19 were evaluated retrospectively. The study was designed as a retrospective observational study. Ankara City Hospital is one of the leading pandemic centers in Turkey. From the beginning of the pandemic to November 15, 2020, a total of 27794 patients were followed up and treated. Previously, Şahin et al. shared their observations about the clinical course of Covid-19 and its impact on pregnancy in 533 pregnant patients (Sahin et al. 2020). Patients under the age of 18 and patients with active malignancies were excluded from this study. All patients gave written informed consent for off-label use of corticosteroids and anakinra. Ethics committee approvals were obtained from the Ethics Committee of Ankara City Hospital. Hospitalizations, treatment, management and discharge decisions of the patients were made according to guidelines prepared by Turkish Health Ministry.
After receiving written informed consent, demographic, clinical, laboratory, treatment, and outcome data were collected using a standardized case-report form. All data were saved by the same physician (OK). Age, gender, comorbidities, fever at the time of application, SpO2, blood pressure, D-Dimer, fibrinogen, complete blood count, biochemical parameters, CRP, and sedimentation findings were recorded.
In our tertiary medical facility, the patients either were diagnosed with positive polymerase chain reaction (PCR) for Covid-19 or met three of any four of the following clinical criteria: having a fever, respiratory symptoms, contact history with COVID-19 case(s), and decreased lymphocyte count (Tezcan, Doğan Gökçe, and Ozer 2020).
In severely infected pregnant patients who were given anakinra and corticosteroid therapy due to persistent fever and/or accompanying hypoxia (SpO2 < 92%), during follow-up, CRP, fibrinogen and ferritin levels, which increased in treatment despite standard treatment, were taken.
All 14 patients received treatment with methylprednisolone, enoxaparin, lopinavir/ritonavir (1 did not receive lopinavir/ritonavir). Methylprednisolone treatment was given to patients whose disease state progressed with baseline treatment. The median dose of methylprednisolone therapy was started at 80 mg (min 20 mg, max 250 mg). This treatment was applied at a median of 10 days (min. 5, max. 29 days). In addition to corticosteroid treatment, patients started with anakinra treatment at a median dosage of 400 mg/day, following a dose of 2–10 mg/kg indicated in a study by Cavalli et al. (2020). Treatment was started at 600 mg/day due to clinical condition of and weight in 3 patients; 200 mg/day in 1 patient and 100 mg/day in 1 patient were also administered. Anakinra was applied to patients subcutaneously in divided doses every 6 hours. Anakinra treatment was given for a median of 6 days (min 3, max 15 days).
All statistical analysis was done using SPSS software version 22 (IBM Corp., Armonk, NY, USA). Categorical variables were reported as frequencies (percentages). Continuous variables were tested for the normality of the data distribution using the Kolmogorov-Smirnov test and expressed as median (Interquartile range, IQR) values in the case of unusually distributed or continuous data. Categorical variables were reported as frequencies (percentages).
Results
14 pregnant patients were taken into the study. The median age was 30 years [IQR: 6 (min 21, max 38)]. All patients but one patient did not have accompanying comorbidities; one patient had schizoaffective disorder. Patients were diagnosed with Covid-19 at the median 24.5th week of their pregnancy [IQR: 9.25 (min 16-max 37th week)]
The most common complaints were dyspnea (78.6%) and cough (64.3%). 12 patients needed oxygen. Anakinra was administered at a median SpO2 value of 92%. Intensive care was needed in the follow-up of four patients. 2 patients were connected to mechanical ventilators. Fever was observed in 35.7% of patients with 2 patients being treated with anakinra for persistent fever. Post-treatment fever response was observed. Fatigue was seen in 35.7% of patients, while myalgia was observed in 21.4% of patients.
Data from laboratory tests (Figure 1) showed that 8 pregnant women infected with Covid-19 had lymphopenia (<1000/mm3) at the time of application and 1 patient had leukopenia (<4000/mm3) at the time of the application. There was an increase in the number of lymphocytes after treatment. The number of median lymphocytes at discharge was 1230/mm3. The C reactive protein (CRP) level of all patients was high at the time of application [median 48.5 (min: 12, max: 186) Normal <6 mg/L]. After anakinra treatment was started, a decline in CRP values was observed. The median CRP value was 2 mg/L at discharge.
Figure 1.
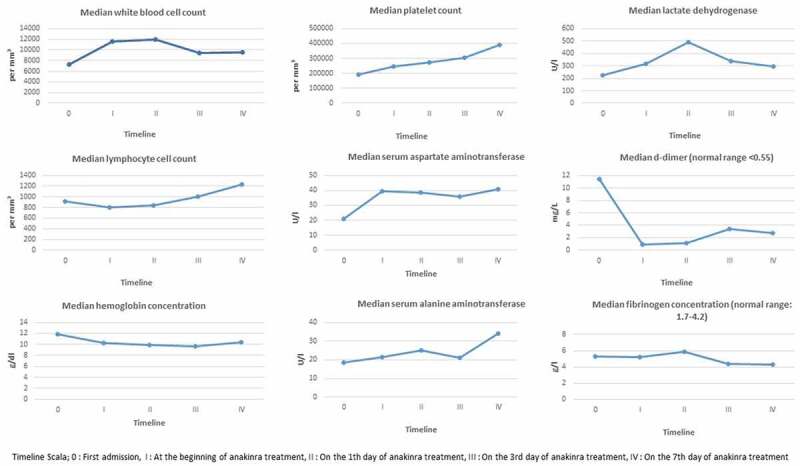
Laboratory findings, fever rate, SpO 2 level and WHO score before and after Anakinra treatment
Figure 1.
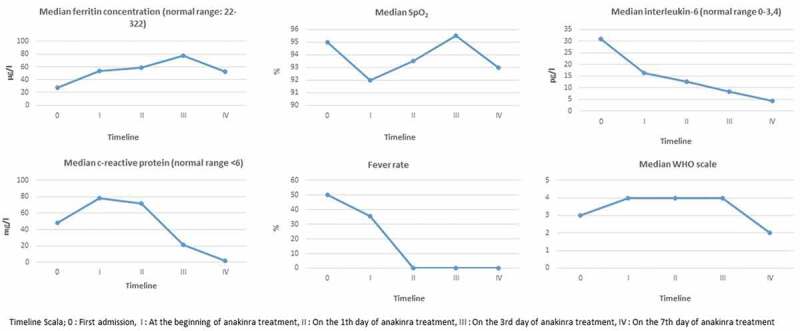
Continued
The median hospitalization period of the patients was 15 days [IQR: 7.5 (min 7 – max 30)]. 12 patients (85.7%) were discharged after complications improved. Treatment of 1 patient (7.1%) is ongoing. 1 patient (7.1%) was died. This patient was diagnosed with Covid-19 at the age of 36 years during her 32nd week of pregnancy. There was a complaint of shortness of breath and SpO2 at time of admission was 60%; high-flow oxygen therapy was started. The patient was intubated due to hypoxia continuation during follow-up. Lymphopenia (520/mm3), LDH (594 U/l) and CRP height (186 mg/L) were detected in the examinations performed at the time of admission. She gave birth in her 32nd week with an emergency C-section (fetus 2100 gr, the 1-minute Apgar score was determined to be 7, and the 5-minute Apgar score was 9). During the treatment process, lopinavir/ritonavir, hydroxychloroquine, initially 250 mg methylprednisolone for 3 days and then 80 mg for a total of 29 days, 600 mg anakinra for 6 days, colchicine, vitamin C, and plasma (convalescent plasma) belonging to recovering patients were given. However, the patient did not respond to any of these treatments.
2 patients had to give birth prematurely in the 28th and 32nd weeks (birth weight of premature babies was 1170 and 2000 gr, respectively). The pregnancy of 11 patients continued smoothly. 1 patient had a healthy birth in her 38th week after Covid-19 infection.
The initial characteristics of the patients, relevant biomarkers, and results associated with disease severity, including WHO score, are given in Table 1 and Figure 1.
Table 1.
Demographics and past-history of 14 pregnant patients with severe COVID-19
| Age median, (IQR) years | 30 (6) |
| Any Comorbidity – no. (%) | 1 (7.1) |
| Dyspnea – no. (%) | 11 (78.6) |
| Cough – no. (%) | 9 (64.3) |
| Fever – no. (%) | 5 (35.7) |
| Fatigue – no. (%) | 5 (35.7) |
| Back pain – no. (%) | 3 (21.4) |
| Myalgia – no. (%) | 3 (21.4) |
| Diarrhea – no. (%) | 2 (14.3) |
| Throat ache – no. (%) | 2 (14.3) |
| Abdominal pain – no. (%) | 1 (7.1) |
| Headache – no. (%) | 1 (7.1) |
| Nausea and vomiting – no. (%) | 1 (7.1) |
| Anosmi – no. (%) | 1 (7.1) |
| Taste disturbance – no. (%) | 1 (7.1) |
| Pregnancy week at diagnosis median, (IQR) | 24.5 (9.25) |
| Inpatient duration – median (IQR) days | 15 (7.5) |
| Need for oxygen – no. (%) | 12 (85.7) |
| Intensive care unit – no. (%) | 4 (28.6) |
| Invasive mechanical ventilation – no. (%) | 3 (21.4) |
| Fetal complication – no. (%) | 2 (16.7) |
| Anakinra starting dose median, (IQR) mg | 400 (75) |
| Steroid starting dose median, (IQR) mg | 80 (90.5) |
| Deceased – no. (%) | 1 (7.1) |
Discussion
In our study 14 pregnant women were evaluated retrospectively. Patients were treated with median 80 mg/day methylprednisolone and 2–10 mg/kg/day anakinra treatments. At the end of the follow-up, the mortality rate was determined as 7.1%. The pregnancy of 11 patients continued smoothly, 2 patients had to give birth prematurely and 1 patient had a healthy birth.
A 7-stage scale was developed by the World Health Organization for use in patients with influenza pneumonia. It has also been used in various studies with patients with Covid-19 (Ramiro et al. 2020). In these studies, patients are evaluated with a score of ≥2 on the WHO scale (Ramiro et al. 2020). In our study, we decided to treat patients with anakinra with a median WHO scale of 4. After 7 days of treatment, patients’ WHO scale score was a median of 2 with also a median of 2 units of improvement.
Acute Covid-19 infections are similar to the seasonal flu with symptoms such as fever, headache, shortness of breath, cough, muscle pains and fatigue. In most infected people, the severity of the disease is mild to moderate, and can be treated without the need for hospital treatment. Patients with serious symptoms such as breathing difficulties and chest pain need immediate medical attention and should be monitored under observation in the hospital.
It has been claimed that the main cause of tissue damage seen in severe Covid-19 infection is the high level of proinflammatory cytokine release and immune irregularity. In a study conducted in patients monitored due to sepsis, it was thought that corticosteroids, especially methylprednisolone, can improve excessive immune response (Lamontagne et al. 2018). A study in patients monitored for Covid-19 pneumonia also found that early, low dose and short-term application of methylprednisolone was associated with better clinical results and that methylprednisolone therapy should be considered before ARDS occurs (Wang et al. 2020). In another randomized controlled trial evaluating Covid-19 patients, the benefits of 250 mg methylprednisolone treatment were shown (Edalatifard et al. 2020). Similarly, in the study of Batırel et al., it was shown that high-dose corticosteroids are more beneficial than standard treatments, without any significant difference between 250 mg methylprednisolone and 6 mg/day dexamethasone equivalents (Batirel et al. 2021). In addition, the American Society of Infectious Diseases made recommendations regarding the routine use of corticosteroids in Covid-19 and the presence of ARDS (Bhimraj et al. 2020).
A retrospective analysis of patients infected with Covid-19 showed increased initial plasma levels of IL-1β and IL-1α in Covid-19 patients (Huang et al. 2020). IL-1 is a proinflammatory mediator that induces both its own production and the synthesis of various secondary inflammatory mediators such as IL-6. The possible role of IL-1/IL-6 blockage in MAS-like conditions makes anakinra application, which successfully inhibits IL-1β and IL-1α, stand out in the treatment of cytokine release syndrome due to Covid-19 infection (Tufan, Güler, and Matucci-Cerinic 2020).
A study using tocilizumab to treat patients with Covid-19 infection found that rapid IL-6 inhibition before the onset of critical illness was associated with reduced mortality from severe Covid-19 disease (Sinha et al. 2020).
In a randomized controlled trial, anakinra was shown to have significant benefits on the health of patients with macrophage activation syndrome, often seen in sepsis patients (Shakoory et al. 2016). Anakinra is a recombinant human IL-1 receptor antagonist with a short half-life, wide therapeutic range and high safety profile. It has been approved for the treatment of rheumatoid arthritis and certain autoinflammatory disorders with daily doses of 1–2 mg/kg/day(2001). Recorded for Covid-19, there are several anakinra cases that test 100 mg of subcutaneous injection daily for 28 days, 400–600 mg/day and intravenously for 5–7 days (Maes et al. 2020).
In the study conducted by Erden et al., the mortality rate in 17 patients who were given anakinra at 100 mg/day subcutaneously for 5 days after 200 mg/day in the first 2 days was 17.8% (Erden et al. 2020).
In a recent study published by Huet et al., when the patient group receiving subcutaneous anakinra treatment was compared with the patient group not given IL inhibitor in severe forms of pneumonia associated with Covid-19, it was stated that the need for mechanical ventilation and mortality were found to be less in the anakinra group (Huet et al. 2020). Another retrospective study; showed that severe Covid-19 patients treated with anakinra had lower mortality compared to the group not taking anakinra (Cavalli et al. 2020).
Based on these findings, we did not give tocilizumab to the pregnant patient population and used corticosteroid and anakinra treatments. We applied anakinra treatment at a median of 400 mg/day dose and median of 6 days, methylprednisolone treatment at a median of 80 mg/day dose and median of 10 days. In our study, the mortality rate was observed to be 7.1% (1 patient). 85.7% of patients were discharged and 7.1% are still being treated.
In a study on the reliability of the use of anakinra in pregnancy, it was stated that secondary infection was not observed in pregnant women or their babies receiving treatment and that all babies were born with the normal APGAR (Appearance, pulse, facial wrinkle, activity and breathing) score (Youngstein et al. 2017). In the case reported by Ilgen et al., similar to this study, the patient who used anakinra during pregnancy, gave birth to a healthy baby with a normal Apgar score.
There are limited data in the literature on pregnant women infected with Covid-19. According to a study conducted by Chen et al., the clinical symptoms of Covid-19 in pregnant women were not significantly different from thoses of non-pregnant women such as chest pain, dyspnea and fever (Chen et al. 2020). Sahin et al. in a study of 533 pregnant patients with Covid-19 infection found that patients were diagnosed with the disease at a median of 24.9 weeks into their pregnancies (Sahin et al. 2020). In our study, the median gestational week was determined to be 24.5 at the time of Covid-19 diagnosis. In a study evaluating 1021 patients, mortality rate was determined as 30.4% (Núñez-Gil et al. 2020). The mortality rate was 7.1% in severe pregnant patients in our study. The reason for our low mortality rate may be that corticosteroid and anakinra treatments were started early.
According to a study of 10 infants (1 twin birth) and 9 pregnancies reported by Zhu et al., the clinical appearance of Covid-19 in pregnant patients was similar to that of non-pregnant patients. Intrauterine fetal distress was seen in 6 of the 9 pregnancies, while 6 were born prematurely (Zhu et al. 2020). In our study, although 14 women had severe pregnancy, only 1 had fetal distress and consequential premature birth. Anakinra elicits an effect within 48–72 hours if enacted before potential cytokine storms (Cron et al. 2015). This effect may have prevented fetal distress and therefore premature births in most of the aforementioned patients. The short half-life of anakinra can provide an advantage at critical stages and should be kept in mind as an effective treatment option. Other our causes in the use of anakinra; antivirals such as favipiravir and their limited work due to not being used in pregnancy and the use of tocilizumab due to its longer half-life.
Our study has clear limitations. The study was a single-center retrospective case review. The sample size was too small. Another limitation of this study was the lack of a control group.
Based on data from a limited number of current studies, the treatment of corticosteroid and interleukin 1 inhibitors should be kept in mind, although the fetal effects and exact treatment method for Covid-19 infection in pregnant women are not clear. Further studies are needed to analyze the effect of corticosteroid and interleukin 1 inhibitors in Covid-19 treatment.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Batirel, A., Demirhan R., Eser N., Körlü E., and Tezcan M. E.. 2021. Pulse steroid treatment for hospitalized adults with COVID-19. Turkish Journal of Medical Sciences2021:45. [DOI] [PubMed] [Google Scholar]
- Berger, C., Recher M., Steiner U., and Hauser T.. 2009. A patient’s wish: Anakinra in pregnancy. Annals of the Rheumatic Diseases 68:1794–95. doi: 10.1136/ard.2008.105833. [DOI] [PubMed] [Google Scholar]
- Bhimraj A, RL. Morgan, AH. Shumaker, V. Lavergne, L. Baden, VC. Cheng, KM. Edwards, R. Gandhi, WJ. Muller, JC. O’Horo, et al. 2020. Infectious diseases Society of America guidelines on the treatment and management of patients with COVID-19. Clinical Infectious Diseasesciaa478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalli, G., De Luca G., Campochiaro C., Della-Torre E., Ripa M., Canetti D., Oltolini C., Castiglioni B., Tassan Din C., Boffini N., et al. 2020. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: A retrospective cohort study. The Lancet Rheumatology 2:e325–e331. doi: 10.1016/S2665-9913(20)30127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, H., Guo J., Wang C., Luo F., Yu X., Zhang W., Li J., Zhao D., Xu D., Gong Q., et al. 2020. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: A retrospective review of medical records. The Lancet 395:809–15. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cron, R. Q., Behrens E. M., Shakoory B., Ramanan A. V., and Chatham W. W.. 2015. Does viral hemorrhagic fever represent reactive hemophagocytic syndrome? The Journal of Rheumatology 42 (7):1078–80. doi: 10.3899/jrheum.150108. [DOI] [PubMed] [Google Scholar]
- Edalatifard, M., Akhtari M., Salehi M., Naderi Z., Jamshidi A., Mostafaei S., Najafizadeh S. R., Farhadi E., Jalili N., Esfahani M., et al. 2020. Intravenous methylprednisolone pulse as a treatment for hospitalised severe COVID-19 patients: Results from a randomised controlled clinical trial. European Respiratory Journal. 56(6):2002808. doi: 10.1183/13993003.02808-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erden, A., Ozdemir B., Karakas O., Mutlu N. M., Izdes S., et al. 2020. Evaluation of seventeen patients with COVID‐19 pneumonia treated with anakinra according to HScore, SOFA, MuLBSTA and Brescia‐COVID respiratory severity scale (BCRSS) scoring systems. Journal of Medical Virology 93:1532–37. [DOI] [PubMed] [Google Scholar]
- F. Kineret® (anakinra) for injection, for subcutaneous use: Highlights of prescribing information. 2001. Accessed April 10, 2020. https://www.accessdata.fda.gov/drugsatfda.docs/label/2012/103950s5136lbl.pdf
- Group RC . 2020. Dexamethasone in hospitalized patients with covid-19—preliminary report. New England Journal of Medicine 384:693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., et al. 2020. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet 395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huet, T., Beaussier H., Voisin O., Jouveshomme S., Dauriat G., Lazareth I., Sacco E., Naccache J.-M., Bézie Y., Laplanche S., et al. 2020. Anakinra for severe forms of COVID-19: A cohort study. The Lancet Rheumatology 2:e393–e400. doi: 10.1016/S2665-9913(20)30164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- İlgen, U., and Küçükşahin O.. 2017. Anakinra use during pregnancy: Report of a case with familial Mediterranean fever and infertility. European Journal of Rheumatology 4:66. doi: 10.5152/eurjrheum.2017.16075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamontagne, F., Rochwerg B., Lytvyn L., Guyatt G. H., Møller M. H., Annane D., Kho M. E., Adhikari N. K. J., Machado F., Vandvik P. O., et al. 2018. Corticosteroid therapy for sepsis: A clinical practice guideline. bmj 362:k3284. doi: 10.1136/bmj.k3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López, M., Gonce A., Meler E., Plaza A., Hernández S., Martinez-Portilla R., Cobo T., García F., Gómez Roig M., Gratacós E., et al. 2020. Coronavirus disease 2019 in pregnancy: A clinical management protocol and considerations for practice. Fetal Diagnosis and Therapy 47:519–28. doi: 10.1159/000508487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes B, C. Bosteels, E. De Leeuw, J. Declercq, K. Van Damme, A. Delporte, B. Demeyere, S. Vermeersch, M. Vuylsteke, J. Willaert, et al. 2020. Treatment of severely ill COVID-19 patients with anti-interleukin drugs (COV-AID): A structured summary of a study protocol for a randomised controlled trial. Trials 21:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meduri, G. U., Siemieniuk R. A., Ness R. A., and Seyler S. J.. 2018. Prolonged low-dose methylprednisolone treatment is highly effective in reducing duration of mechanical ventilation and mortality in patients with ARDS. Journal of Intensive Care 6:1–7. doi: 10.1186/s40560-018-0321-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima, K., Watanabe O., Mochizuki M., Nakasone A., Ishizuka N., and Murashima A.. 2016. Pregnancy outcomes after exposure to tocilizumab: A retrospective analysis of 61 patients in Japan. Modern Rheumatology 26:667–71. doi: 10.3109/14397595.2016.1147405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Núñez-Gil IJ, C. Fernández-Pérez, V. Estrada, VM. Becerra-Muñoz, I. El-Battrawy, A. Uribarri, I. Fernández-Rozas, G. Feltes, MC. Viana-Llamas, D. Trabattoni, et al. 2020. Mortality risk assessment in Spain and Italy, insights of the HOPE COVID-19 registry. Internal and Emergency Medicine 16:957–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramiro, S., Mostard R. L., Magro-Checa C., Van Dongen C. M., Dormans T., Buijs J., Gronenschild M., de Kruif M. D., van Haren E. H. J., van Kraaij T., et al. 2020. Historically controlled comparison of glucocorticoids with or without tocilizumab versus supportive care only in patients with COVID-19-associated cytokine storm syndrome: Results of the CHIC study. Annals of the Rheumatic Diseases 79:1143–51. doi: 10.1136/annrheumdis-2020-218479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restrepo, A., Moorthy V., and Preziosi M.. 2020. Public health emergency SOLIDARITY trial of treatments for COVID-19 infection in hospitalized patients. ISRCTN 384:497–511. [Google Scholar]
- Sahin, D., Tanacan A., Erol S. A., Anuk A. T., Yetiskin F. D., et al. 2020. Updated experience of a tertiary pandemic center on 533 pregnant women with COVID‐19 infection: A prospective cohort study from Turkey. International Journal of Gynecology & Obstetrics. doi: 10.1002/ijgo.13318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin D, A. Tanacan, SA. Erol, AT. Anuk, FDY. Yetiskin, HL. Keskin, N. Ozcan, AS. Ozgu-Erdinc, EGY. Eyi, A. Yucel, et al. 2016. Interleukin-1 receptor blockade is associated with reduced mortality in sepsis patients with features of the macrophage activation syndrome: Re-analysis of a prior Phase III trial. Critical Care Medicine 44:275. doi: 10.1097/CCM.0000000000001402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha, P., Mostaghim A., Bielick C. G., McLaughlin A., Hamer D. H., Wetzler L. M., Bhadelia N., Fagan M. A., Linas B. P., Assoumou S. A., et al. 2020. Early administration of Interleukin-6 inhibitors for patients with severe Covid-19 disease is associated with decreased intubation, reduced mortality, and increased discharge. International Journal of Infectious Diseases 99:28–33. doi: 10.1016/j.ijid.2020.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, P., Wang J., and Song Y.. 2018. Characteristics and pregnancy outcomes of patients with severe pneumonia complicating pregnancy: A retrospective study of 12 cases and a literature review. BMC Pregnancy and Childbirth 18:1–6. doi: 10.1186/s12884-018-2070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezcan, M. E., Doğan Gökçe G., and Ozer R. S.. 2020. Laboratory abnormalities related to prolonged hospitalization in COVID-19. Infectious Diseases 52:666–68. doi: 10.1080/23744235.2020.1776381. [DOI] [PubMed] [Google Scholar]
- Tufan, A., Güler A. A., and Matucci-Cerinic M.. 2020. COVID-19, immune system response, hyperinflammation and repurposing antirheumatic drugs. Turkish Journal of Medical Sciences 50:620–32. doi: 10.3906/sag-2004-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, W. Jiang, Q. He, C. Wang, B. Wang, P. Zhou, N. Dong, Q. Tong. 2020. A retrospective cohort study of methylprednisolone therapy in severe patients with COVID-19 pneumonia. Signal Transduction and Targeted Therapy 5:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngstein, T., Hoffmann P., Gül A., Lane T., Williams R., Rowczenio D. M., Ozdogan H., Ugurlu S., Ryan J., Harty L., et al. 2017. International multi-centre study of pregnancy outcomes with interleukin-1 inhibitors. Rheumatology 56:2102–08. doi: 10.1093/rheumatology/kex305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, H., Wang L., Fang C., Peng S., Zhang L., Chang G., Xia S., and Zhou W.. 2020. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Translational Pediatrics 9:51. doi: 10.21037/tp.2020.02.06. [DOI] [PMC free article] [PubMed] [Google Scholar]


