ABSTRACT
Background
To better inform clinical practice, we summarized the findings from randomized controlled trials (RCTs) of antivirals for COVID-19.
Methods
We systematically searched for literature up to September 2020, and included English-language publications of RCTs among hospitalized COVID-19 patients. We conducted network meta-analysis combining results of both the direct and indirect comparisons of interventions. The efficacy outcomes were clinical progression, all-cause mortality, and viral clearance, and safety outcomes were diarrhea, nausea, and vomiting. We generated treatment rankings (best to worst) and summarized rank probabilities using rankogram.
Results
We included 15 RCTs (14,418 patients) from 7,237 retrieved citations. There was no evidence for efficacy of the assessed antivirals compared with placebo/no treatment or with another antiviral for all efficacy outcomes. Lopinavir (400 mg)/ritonavir (100 mg) significantly increased diarrhea, nausea, and vomiting compared with placebo/no treatment and other antivirals, and was ranked worst for these outcomes, while triazavirin (250 mg), baloxavir marboxil (80 mg), and remdesivir (100 mg – 10 days) ranked best, respectively.
Conclusions and relevance
The available evidence does not support the use of any antiviral drugs for COVID-19. Cautious interpretations of the findings are, however, advised considering the paucity of the evidence. More RCTs are needed for a stronger evidence base.
KEYWORDS: Antiviral drugs, COVID-19, randomized controlled trials, efficacy, safety, systematic review, network meta-analysis
1. Introduction
A huge disease burden is attributable to the coronavirus disease 2019 (COVID-19), a respiratory disease caused by the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). With an estimated reproductive number (transmissibility) of 3.28 [1] and mean serial interval (average transmission time from a primary to a secondary symptomatic infected person) of 3.1 days to 4.9 days [2], SARS-CoV-2 infection quickly spread all over the world leading to a devastating global pandemic, with numerous cases of multi-systemic complications [3–5], and high mortality rates [6,7].
Due to the urgent need for effective treatment options, it has been widely suggested that already approved antiviral drugs for some other diseases may be effective against COVID-19 [8,9]. During the early stage of the COVID-19 pandemic, the World Health Organization (WHO) established a list of preexisting drugs that may aid treatment of the disease, including two antiviral drugs, remdesivir and lopinavir [10]. Remdesivir is a prodrug with a broad antiviral activity spectrum against ribonucleic acid (RNA) viruses, and acts by inhibiting RNA polymerase limiting viral replication [11,12]. Lopinavir is an antiretroviral drug of the protease inhibitor class, often used as a fixed-dose combination with another protease inhibitor, ritonavir, against the human immunodeficiency viruses (HIV) infections [13]. In vivo studies have suggested that remdesivir has therapeutic effects in animal models of SARS-CoV-2 [11], and reduced pulmonary damage in early use on COVID-19 monkeys [14]. Remdesivir has also been credited to reduce time to recovery of hospitalized COVID-19 patients who required supplemental oxygen [15], and may have positive effect on mortality [11]. Further, lopinavir inhibited the Middle East respiratory syndrome coronavirus (MERS-CoV) replication in cell cultures [16].
Following the WHO recommendation of evaluating potential COVID-19 drugs through large multinational randomized controlled trials (RCTs) [17], a multicenter global RCT [15], showed shortened time to recovery in hospitalized patients with remdesivir; leading it to become the first approved drug by the United States (USA) Food and Drug Administration (FDA) for the treatment of severe hospitalized COVID-19 patients [18]. However, an interim report from another multinational RCT [19] in hospitalized COVID-19 patients found that there was no difference in mortality between remdesivir and usual clinical care. Studies aimed at identifying potential inhibitors against SARS-CoV-2 main proteinase (Mpro) explored various FDA-approved drugs such as darunavir, indinavir, saquinavir, tipranavir, raltegravir, velpatasvir, and ledipasvir identified as potential candidates for the treatment of COVID-19 in some previous docking studies involving monomeric SARS-CoV2 Mpro [20]. Saquinavir was identified as a potent inhibitor of dimeric SARS-CoV2 Mpro and may have clinical utility against COVID-19 [20,21]. Studies on other antiviral drugs have revealed largely conflicting findings.
Identifying an efficacious and safe antiviral drug for COVID-19 would be of immense help in mitigating the ravaging impact of the disease. Therefore, we systematically identified, critically appraised and summarized the findings from RCTs of antiviral drugs for the treatment of COVID-19, focusing on clinically relevant outcomes.
2. Methods
We registered a protocol for this systematic review in the International Prospective Register of Systematic Reviews (PROSPERO: CRD42020216817). Details of our methods have been reported in a previous systematic review with meta-analysis and trial sequential analysis of randomized controlled trials of remdesivir for COVID-19 [22]. We conducted this review in accordance with the Methodological Expectations of Cochrane Intervention Reviews (MECIR) guidelines [23]. We reported our findings following the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines for reporting of systematic reviews incorporating network meta-analyses of health-care interventions [24].
2.1. Search strategy
A knowledge synthesis librarian designed the literature search strategy for Embase (Ovid) and this search strategy was peer reviewed by another independent knowledge synthesis librarian using the Peer Review of Electronic Search Strategies (PRESS) checklist [25]. We designed the search strategy to capture all antiviral drugs and applied a randomized controlled trial filter. The revised search strategy for Embase (Appendix Table 1) was adapted by the knowledge synthesis librarian for Web of Science Core Collection (Thomson Reuters), LitCovid [26], the Cochrane COVID-19 study register [27], and the World Health Organization’s Global research on coronavirus disease (WHO COVID-19) online database [28]. In addition, we searched the following websites for links to additional peer reviewed and published literature: ClinicalTrials.gov, the Centers for Disease Control and Prevention (CDC), the Canadian Agency for Drugs and Technologies in Health (CADTH), and the European Center for Disease Prevention and Control (ECDC). We conducted the literature search on 10 September 2020 (September 11 for the CDC, CADTH, and ECDC) and all retrieved literature citations were imported into, and de-duplicated, in the EndNote citation management software, version X9.
2.2. Selection criteria
The de-duplicated citations were imported into a specially designed Microsoft Access 2016 database (Microsoft Corporation, Redmond, WA, USA) and screened by two independent reviewers, using a two-stage sifting approach to review the title/abstract and full-text articles of relevant publications in English language. We documented the number of ineligible citations at the title/abstract screening stage, and both the number and reasons for exclusion at the full-text article screening stage. The reviewers resolved any disagreements through discussion or involvement of a third reviewer. We included only RCTs of antiviral drugs compared with placebo, no additional treatment/usual care, a different antiviral drug, or a different antiviral drug regimen, for treatment of laboratory-confirmed (RT-PCR or antigen test) COVID-19 irrespective of the disease severity. We excluded preprint articles superseded by peer reviewed journal publications. The efficacy outcomes were clinical progression measured using the WHO scale [29], all-cause mortality, and viral clearance (determined from testing upper respiratory tract specimens including nasopharyngeal and deep nasal swabs, or throat swabs). We dichotomized the individual scores for clinical progression into ≤5 (hospitalized: moderate disease or ambulatory: mild disease) between intervention and comparator groups. If measured by a scale other than the WHO scale, we re-classified the measurement according to the WHO criteria. The safety outcomes were diarrhea, nausea, and vomiting.
2.3. Data extraction
Two reviewers independently performed data extraction using Microsoft Excel 2016 (Microsoft Corporation, Redmond, WA, USA). Disagreements were resolved through discussion or by a third reviewer. We extracted data such as study information, study population characteristics, information regarding interventions and comparators, outcomes assessed, and study results based on an intention-to-treat (ITT) analysis. We also extracted details relevant to the risk of bias assessment. For outcome data presented at multiple time points, we took the longest period of follow-up.
2.4. Risk of bias assessment
Two reviewers independently assessed the risk of bias in the included studies using the Cochrane risk of bias assessment tool for RCT v2.0.2 [30]. The reviewers resolved disagreements through discussion or by a third reviewer.
2.5. Data synthesis and analysis
We tabulated characteristics of the included studies and the risk of bias assessments. We generated network plots of compared interventions to depict graphically, the available evidence, and the volume of the evidence behind each comparison [31]. We conducted a network meta-analysis [32] using a Bayesian framework and Markov Chain Monte Carlo (MCMC) simulation methods developed in BUGSnet R package [33,34], combining results of all the comparisons in one analysis, exploiting both the direct comparisons within RCTs and the indirect comparisons across RCTs for each outcome [35]. We fitted both random-effects and fixed-effect network meta-analysis models [36], and chose the preferred model (random effects) by comparing the deviance information criteria (DIC) [37]. For all analyses, we assessed model convergence using the Brooks Gelman–Rubin diagnostic tool [38], history plots, autocorrelation, and the form of the posterior density for the between-study heterogeneity. We used vague prior distributions for all parameters, a burn-in period of 50,000 iterations, a sampling period of 100,000 iterations, and 3 chains with varied initial values in all analyses, and we assessed the model goodness of fit by measuring the posterior mean of the residual deviance [39]. We utilized a binomial distribution and logit link function for all outcomes to model the data from the two by two tables directly, and reported risk ratios with the associated 95% credible intervals (CrIs). We evaluated the consistency between the direct and indirect evidence by calculating a Bayesian 2-sided p value for the difference between the direct and indirect estimates using the Bucher method [40]. We considered p < 0.5 to be statistically significant inconsistency. We assessed statistical heterogeneity between the pooled estimates using the I2 statistic [41]. We summarized results by point estimates presented as medians with 95% CrI, and using league table of the relative effect of each treatment compared to each other treatment. We generated treatment rankings (best to worst) and their corresponding probability estimates and summarized rank probabilities using rankogram [42]. Publication bias was not assessed because of small sample sizes (<10 study results contributed to a pooled analysis) [43].
3. Results
We included 15 published RCTs (involving 14,418 patients) from 7,237 retrieved citations (Figure 1) [15,19,44–56]. The main characteristics of these RCTs are summarized in Table 1 and Appendix Table 2. Two of the publications [19,49] were interim study reports, while the rest were final study reports. Of the 15 RCTs, eight (3 from China, 1 from Iran, and 4 multi-countries) [15,19,46,48,52–55] were multicenter trials, whereas seven (6 from China and 1 from Iran) [44,45,47,49–51,56] were single-center RCTs. Ten RCTs were of open-label design [19,44–48,50,51,53,56], three RCTs of double-blinded design [15,54,55], and two RCTs were partially blinded [49,52]. Two RCTs (both open-label) were funded by Gilead Sciences, maker of remdesivir [46,53]. The other RCTs were non-industry funded, with one RCT receiving no funding [51].
Figure 1.

Modified PRISMA flow chart
Table 1.
Main characteristics of the included randomized controlled trials (RCT)
| Study (Report type) | Country (Region) [Centers] |
RCT type (Funding) | COVID-19 severity [Mean or median symptom onset before study] |
No. of patients (% Male) | Mean or median age (SD/ IQR) | Compared interventions | Outcomes (Time to measurement) |
|---|---|---|---|---|---|---|---|
| Beigel 2020 [15] (Final) |
USA, Denmark, UK, Greece, Germany, Korea, Mexico, Spain, Japan, Singapore (Varied regions) [73 centers] |
Double-blinded (Non-Pharma funded) | Mixed severity [9 (IQR 6–12) days] |
1,062 (64.4%) | 58.9 (15) years | Remdesivir (100 mg) [10 days] vs. Placebo | All-cause mortality (29 days); clinical progression [eight-category ordinal scale] (15 days) |
| Cao 2020 [44] (Final) |
China (Wuhan, Hubei Province) [1 center] |
Open-label (Non-Pharma funded) | Severe [13 (IQR 11–16) days] |
199 (60.3%) | 58 (IQR 49–68) years | Lopinavir (400 mg)/Ritonavir (100 mg) [14 days] vs. No treatment | All-cause mortality, nausea, vomiting, diarrhea (28 days); clinical progression [seven-category ordinal scale] (14 days) |
| Chen 2020 [45] (Final) |
China (Shanghai) [1 center] |
Open-label (Non-Pharma funded) | Mildly severe [Mean 4 (2–5) days] |
30 (60%) |
47.2 (2.8) years | Darunavir (800 mg)/Cobicistat (150 mg) [5 days] vs. No treatment | Diarrhea, clinical progression [scale type not clear], and all-cause mortality (14 days); viral clearance [oropharyngeal swabs] (5 days) |
| Goldman 2020 [46] (Final) |
United States, Italy, Spain, Germany, Hong Kong, Singapore, South Korea, and Taiwan (Varied regions) [55 centers] |
Open-label (Pharma funded by Gilead Sciences) | Mixed severity [8.5 (IQR 6.4–10.6) days] |
397 (63.7%) | 61.4 (IQR 54.4–68.5) years | Remdesivir (100 mg) [10 days] vs. Remdesivir (100 mg) [5 days] | Clinical progression [seven-category ordinal scale] (14 days); nausea (30 days) |
| Huang 2020 [47] (Final) |
China (Chongqing) [1 center] |
Open-label (Non-Pharma funded) | Mixed severity [Mean 4 (1.5–7) days] | 101 (46%) |
42.5 (11.5) years | Ribavirin (400–600 mg) vs. Lopinavir (400 mg)/Ritonavir (100 mg) [14 days]; Ribavirin (400–600 mg) [14 days] vs. No treatment; Lopinavir (400 mg)/Ritonavir (100 mg) [14 days] vs. No treatment | Viral clearance [nasopharyngeal swabs], all-cause mortality, diarrhea, vomiting (28 days) |
| Hung 2020 [48] (Final) |
China (Hong Kong) [6 centers] |
Open-label (Non-Pharma funded) | Mixed severity [5 (IQR 3–7) days] | 127 (54%) |
52 (IQR 32–62) years | Ribavirin (400 mg)/Interferon β-1b (8 million i-unit) [14 days] vs. No treatment | All-cause mortality, nausea, vomiting (30 days) |
| Li 2020 [49] (Interim) |
China (Guangzhou) [1 center] |
Partially-blinded (Non-Pharma funded) | Mixed severity [4.4 (IQR 3–5.9) days] |
86 (46.5%) |
49.4 (14.7) years | Lopinavir (400 mg)/Ritonavir (100 mg) [7–14 days] vs. No treatment; Umifenovir (200 mg) [7–14 days] vs. No treatment | Viral clearance [mixed swabs], diarrhea, nausea (14 days) |
| Lou 2020 [50] (Final) |
China (Zhejiang) [1 center] |
Open-label (Non-Pharma funded) | Mixed severity [11.7 (SD 4.4) days] | 30 (72.4%) |
52.5 (12.5) years | Baloxavir marboxil (80 mg) [2–3 days] vs. No treatment; Favipiravir (600 mg) [14 days] vs. No treatment | Viral clearance [throat swabs], all-cause mortality, nausea, diarrhea, clinical progression [seven-category ordinal scale] (14 days) |
| Rahmani 2020 [51] (Final) |
Iran (Tehran) [1 center] |
Open-label (Not funded) | Severe [8 (IQR 6.6–9.4) days] | 80 (59.1%) |
60.6 (IQR 52.4–68.8) years | Interferon β-1b (250 mcg) [14 days] vs. No treatment | All-cause mortality, nausea, diarrhea, clinical progression [six-category ordinal scale] (28 days) |
| Sadeghi 2020 [52] (Final) |
Iran (Shariati, Baharloo, Sina, Sayyad, and Shirazi) [4 centers] |
Partially-blinded (Non-Pharma funded) | Mixed severity [1 (IQR 1–2) days] | 66 (52%) |
58 (IQR 43–69) years | Sofosbuvir (400 mg)/Daclatasvir (60 mg) [14 days] vs. No treatment | All-cause mortality, clinical progression [scale type not clear] (14 days) |
| Spinner 2020 [53] (Final) |
USA, Europe, and Asia (Varied regions) [105 centers] |
Open-label (Pharma funded by Gilead Sciences) | Moderately severe [8.4 (IQR 6.6–10) days] |
596 (61.1%) |
Notreported | Remdesivir (100 mg) [10 days] vs. No treatment; Remdesivir (100 mg) [5 days] vs. No treatment | Clinical progression [seven-category ordinal scale], nausea, diarrhea (11 days); all-cause mortality (28 days) |
| Wang 2020 [54] (Final) |
China (Hubei province) [10 centers] |
Double-blinded (Non-Pharma funded) | Severe [Not more than 12 days] |
237 (56%) |
65 (IQR 56–71) years | Remdesivir (100 mg) [10 days] vs. Placebo | All-cause mortality, clinical progression [six-category ordinal scale], nausea, diarrhea, vomiting (28 days) |
| WHO Solidarity TrialConsortium 2020 [19] (Interim) |
30 Countries (Varied regions) [405 centers] |
Open-label (Non-Pharma funded) | Mixed severity [Not reported] |
11,266 (62%) | Not reported | Remdesivir (100 mg) [10 days] vs. No treatment; Lopinavir (400 mg)/Ritonavir (100 mg) [14 days] vs. No treatment; Interferon β-1a (44 µg per 0.5 mL) [6 days] vs. No treatment | All-cause mortality (28 days) |
| Wu 2020 [55] (Final) |
China (Heilongjiang) [10 centers] |
Double-blinded (Non-Pharma funded) | Mixed severity [7 (IQR 5–10) days] | 52 (50%) |
58 (IQR 48–65) years | Triazavirin (250 mg) vs. Placebo [7 days] | Viral clearance [throat swabs], all-cause mortality, diarrhea (28 days) |
| Zheng 2020 [56] (Final) |
China (Changsha) [1 center] |
Open-label (Non-Pharma funded) | Mixed severity [4.2 (IQR 3.1–5.3) days] | 89 (47.2%) |
45.1 (IQR 37.6–52.4) years | Novaferon (20ug) vs. Lopinavir (400 mg)/Ritonavir (100 mg) [7–10 days]; Novaferon (20ug) [7–10 days] vs. No treatment; Lopinavir (400 mg)/Ritonavir (100 mg) [7–10 days] vs. No treatment | Diarrhea, nausea, vomiting, viral clearance [nasopharyngeal swabs] (9 days) |
IQR = interquartile range; SD = standard deviation; vs = versus.
Across the RCTs, the criteria for patients’ inclusion, patients’ COVID-19 severity, definition of severity, and mean/median duration (in days) of patients’ symptom onset before inclusion in the RCTs differed considerably. The overall sample size across the RCTs ranged from 30 to 11,266 patients, with varying proportions of male patients, ranging from 46% to 72.4%. The point mean/median age of patients differed across the RCTs, ranging from 42.5 to 65 years. Antiviral drugs assessed by the RCTs included baloxavir marboxil (80 mg), darunavir (800 mg) with cobicistat (150 mg), favipiravir (600 mg), interferon-β-1b (250mcg), interferon-β-1a (44 µg-per-0.5 mL), lopinavir (400 mg) with ritonavir (100 mg), novaferon (20ug), remdesivir (100 mg – 10 days), remdesivir (100 mg – 5 days), ribavirin (400–600 mg), ribavirin (400 mg) with interferon-β-1b (8million-IU), sofosbuvir (400 mg) with daclatasvir (60 mg), triazavirin (250 mg), and umifenovir (200 mg). Nine RCTs reported on clinical progression; one using an 8-category ordinal scale [15], four using a 7-category ordinal scale [44,46,50,53], two using a 6-category ordinal scale [51,54], and two did not clearly report the scale that was used [45,52]. Twelve RCTs reported on all-cause mortality [15,19,44,45,47,48,50–55], six reported viral clearance [45,47,49,50,55,56], eleven reported on diarrhea [44,45,47–51,53–56], nine reported on nausea [44,46,48–51,53,54,56], and four reported on vomiting [44,47,54,56]. The follow-up time varied across RCTs and by outcome, ranging from 5 to 30 days. However, the dosing duration for each unique antiviral drug was similar.
3.1. Risk of bias assessment
For the efficacy outcomes, overall, three RCTs were judged to be at a low risk of bias [49,50,54], eight RCTs were judged to be of some concern of risk of bias [15,19,44,47,48,51,55,56], and four RCTs were judged to be at a high risk of bias mainly due to a high risk of bias in the randomization process and/or deviations from the intended treatment [45,46,52,53] (Figure 2). For the safety outcomes, overall, one RCT was judged to be at a low risk of bias [54], eight RCTs were judged to be of some concern of risk of bias [44,47–51,55,56], and four RCTs were judged to be at a high risk of bias mainly due to a high risk of bias in randomization process and/or deviations from the intended treatment [45,46,52,53] (Appendix Figure 1).
Figure 2.

Risk of bias assessment for the efficacy outcomes
3.2. Efficacy outcomes
Nine different interventions involving 2,685 patients were included in the intervention network plot for clinical progression (Figure 3). These included two different treatment durations (10-day and 5-day treatment) for the same dose of remdesivir (100 mg). The forest plots for the direct comparisons between the antiviral drugs and placebo/no treatment are shown in Figure 4, and the league table of all the comparisons is presented in Appendix Figure 2. There was no evidence that any of the antiviral drugs significantly improved clinical progression. Irrespective of the non-significant findings, sofosbuvir (400 mg) with daclatasvir (60 mg) ranked best in terms of the likelihood of improving clinical progression, while baloxavir marboxil (80 mg) ranked worst (Figure 5).
Figure 3.

Network plots of interventions for all outcomes
Bal = Baloxavir marboxil (80 mg); Dar_Cob = Darunavir (800 mg) & Cobicistat (150 mg); Fav = Favipiravir (600 mg); IFb_1b = Interferon-β-1b (250mcg); IFb_1a = Interferon-β-1a (44 µg-per-0.5 mL); Lop_Rit = Lopinavir (400 mg) & Ritonavir (100 mg); Nova = Novaferon (20ug); P_NT = Placebo/No treatment; Rem_10d = Remdesivir (100 mg – 10 days); Rem_5d = Remdesivir (100 mg – 5 days); Rib = Ribavirin (400–600 mg); Rib_IFb_2b = Ribavirin (400 mg) & Interferon-β-1b (8million-iu); Sof_Dac = Sofosbuvir (400 mg) & Daclatasvir (60 mg); Triaz = Triazavirin (250 mg); Umif = Umifenovir (200 mg).
Figure 4.
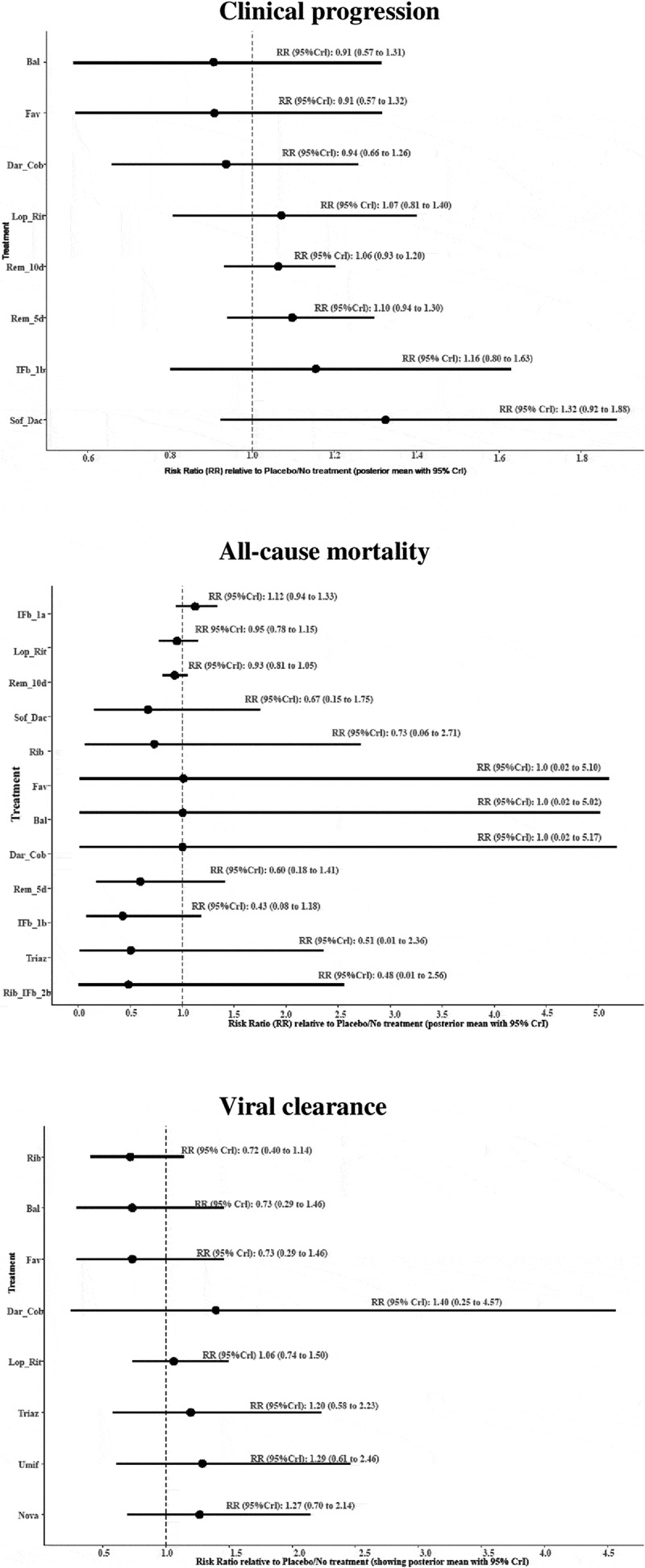
Forest plots of direct comparisons between antiviral drugs and placebo/no treatment for the efficacy outcomes
Bal = Baloxavir marboxil (80 mg); Dar_Cob = Darunavir (800 mg) & Cobicistat (150 mg); Fav = Favipiravir (600 mg); IFb_1b = Interferon-β-1b (250mcg); IFb_1a = Interferon-β-1a (44 µg-per-0.5 mL); Lop_Rit = Lopinavir (400 mg) & Ritonavir (100 mg); Nova = Novaferon (20ug); P_NT = Placebo/No treatment; Rem_10d = Remdesivir (100 mg – 10 days); Rem_5d = Remdesivir (100 mg – 5 days); Rib = Ribavirin (400–600 mg); Rib_IFb_2b = Ribavirin (400 mg) & Interferon-β-1b (8million-iu); Sof_Dac = Sofosbuvir (400 mg) & Daclatasvir (60 mg); Triaz = Triazavirin (250 mg); Umif = Umifenovir (200 mg).
Figure 5.

Ranking of interventions for the efficacy outcomes (rankogram)
Bal = Baloxavir marboxil (80 mg); Dar_Cob = Darunavir (800 mg) & Cobicistat (150 mg); Fav = Favipiravir (600 mg); IFb_1b = Interferon-β-1b (250mcg); IFb_1a = Interferon-β-1a (44 µg-per-0.5 mL); Lop_Rit = Lopinavir (400 mg) & Ritonavir (100 mg); Nova = Novaferon (20ug); P_NT = Placebo/No treatment; Rem_10d = Remdesivir (100 mg – 10 days); Rem_5d = Remdesivir (100 mg – 5 days); Rib = Ribavirin (400–600 mg); Rib_IFb_2b = Ribavirin (400 mg) & Interferon-β-1b (8million-iu); Sof_Dac = Sofosbuvir (400 mg) & Daclatasvir (60 mg); Triaz = Triazavirin (250 mg); Umif = Umifenovir (200 mg).
Thirteen different interventions involving 14,874 patients were included in the intervention network plot for all-cause mortality (Figure 3). These included two different treatment durations (10-day and 5-day treatment) for the same dose of remdesivir (100 mg). The forest plots for the direct comparisons between the antiviral drugs and placebo/no treatment are shown in Figure 4, and the league table of all the comparisons is presented in Appendix Figure 3. There was no evidence that any of the antiviral drugs significantly reduced all-cause mortality. Irrespective of the non-significant findings, ribavirin (400 mg) with interferon-β-1b (8 million-IU) ranked best in terms of the likelihood of reducing all-cause mortality, while interferon-β-1a (44 µg per 0.5 mL) ranked worst (Figure 5).
Nine different interventions involving 384 patients were included in the intervention network plot for viral clearance (Figure 3). The forest plots for the direct comparisons between the antiviral drugs and placebo/no treatment are shown in Figure 4, and the league table of all the comparisons is presented in Appendix Figure 4. There was no evidence that any of the antiviral drugs significantly improved viral clearance. Irrespective of the non-significant findings, darunavir (800 mg) with cobicistat (150 mg) ranked best in terms of the likelihood of improving viral clearance, while baloxavir marboxil (80 mg) ranked worst (Figure 5).
3.3. Safety outcomes
Thirteen different interventions involving 1,615 patients were included in the intervention network plot for diarrhea (Figure 3). These included two different treatment durations (10-day and 5-day treatment) for the same dose of remdesivir (100 mg). The forest plots for the direct comparisons between the antiviral drugs and placebo/no treatment are shown in Appendix Figure 5, and the league table of all the comparisons is presented in Appendix Figure 6. Lopinavir (400 mg) with ritonavir (100 mg) significantly increased diarrhea compared with placebo/no treatment [RR 2.07; 95% Crl 1.39 to 3.03]; compared with triazavirin (250 mg) [RR 81.83; 95% Crl 1.34 to 287.01]; compared with remdesivir (100 mg – 10 days) [RR 2.92; 95% Crl 1.42 to 5.42]; compared with remdesivir (100 mg – 5 days) [RR 2.60; 95% Crl 1.22 to 4.93]; and compared with ribavirin (400 mg) with interferon-β-1b (8 million-IU) [RR 2.43; 95% Crl 1.31 to 4.11]. Triazavirin (250 mg) ranked best in terms of the likelihood of decreased diarrhea, while lopinavir (400 mg) with ritonavir (100 mg) ranked worst (Appendix Figure 7).
Ten different interventions involving 1,884 patients were included in the intervention network plot for nausea (Figure 3). These included two different treatment durations (10-day and 5-day treatment) for the same dose of remdesivir (100 mg). The forest plots for the direct comparisons between the antiviral drugs and placebo/no treatment are shown in Appendix Figure 8, and the league table of all the comparisons is presented in Appendix Figure 9. Lopinavir (400 mg) with ritonavir (100 mg) significantly increased nausea compared with placebo/no treatment [RR 2.92; 95% Crl 1.20 to 6.39]. Remdesivir (100 mg – 10 days) significantly increased nausea compared with placebo/no treatment [RR 2.27; 95% Crl 1.29 to 3.81]. Remdesivir (100 mg – 5 days) significantly increased nausea compared with placebo/no treatment [RR 2.47; 95% Crl 1.37 to 4.22] and compared with ribavirin (400 mg) with interferon-β-1b (8million-IU) [RR 2.47; 95% Crl 1.07 to 4.88]. Baloxavir marboxil (80 mg) ranked best in terms of the likelihood of decreased nausea, while lopinavir (400 mg) with ritonavir (100 mg) ranked worst (Appendix Figure 10).
Five different interventions involving 626 patients were included in the intervention network plot for vomiting (Figure 3). The forest plots for the direct comparisons between the antiviral drugs and placebo/no treatment are shown in Appendix Figure 11, and the league table of all the comparisons is presented in Appendix Figure 12. Lopinavir (400 mg) with ritonavir (100 mg) significantly increased vomiting compared with placebo/no treatment [RR 3.83; 95% Crl 1.90 to 7.32]; compared with ribavirin (400–600 mg) [RR 2.59; 95% Crl 1.05 to 5.54]; and compared with remdesivir (100 mg – 10 days) [RR 7.44; 95% Crl 1.16 to 24.89]. Remdesivir (100 mg – 10 days) ranked best in terms of the likelihood of decreased vomiting, while lopinavir (400 mg) with ritonavir (100 mg) ranked worst (Appendix Figure 13).
3.4. Ongoing RCTs
We identified in ClinicalTrials.gov website, 21 ongoing RCTs of antiviral drugs compared with placebo or no treatment for treatment of COVID-19. Relevant information regarding these trials is presented in Appendix Table 3.
4. Discussion
None of the assessed antiviral drugs was found to be efficacious for improving clinical progression, reducing all-cause mortality, and viral clearance among hospitalized COVID-19 patients. Lopinavir (400 mg) with ritonavir (100 mg) appeared to be the worst antiviral drug treatment with respect to the risk of diarrhea, nausea, and vomiting. Triazavirin (250 mg), baloxavir marboxil (80 mg), and remdesivir (100 mg – 10 days) ranked best with respect to the risk of diarrhea, nausea, and vomiting, respectively. Generally, there was a paucity of evidence and a substantial risk of bias in most of the evidence; hence, the need for cautious interpretations of these findings.
Noteworthy was the substantial variability in the definitions of COVID-19 severity across the included RCTs, which meant that patients’ severity varied significantly across the RCTs (Appendix Table 2). It was therefore difficult to ascertain the exact levels of COVID-19 severity across the studies and to explore the influence of different levels of severity on treatment efficacy and safety outcomes of the antiviral drugs. Patients’ eligibility criteria also varied considerably across the RCTs, with substantial variability in the number of days from patients’ symptom onset to enrollment in the RCTs and differences in minimum age for enrollment, although most were adult patients. In addition, across studies, it was not clear to what extent the patients differed by comorbidity status and the impact that any differences may have made to our overall findings. Furthermore, there was extensive variability in standards of care across health jurisdictions in which the RCTs were conducted, with many of the RCTs conducted in multiple countries across various continents, with differing health systems and practices. However, all the included RCTs involved laboratory-confirmed hospitalized COVID-19 patients. While the assessed outcomes were evaluated alike across the RCTs, albeit with different but largely similar follow-up times, assessment of clinical progression involved varied scales although the scales were comparable, which allowed us to compare patients between intervention and comparator groups according to whether they were still hospitalized with moderate disease or were ambulatory with mild disease at the end of follow-up, irrespective of the scale used for assessment.
Notwithstanding the variability across the included RCTs, findings from this systematic review show a lack of evidence for the use of any of the assessed antiviral drugs for COVID-19 treatment, including remdesivir, which has been approved for this purpose. Others have also reached similar conclusions to those in this review. A living systematic review and network meta-analysis of drug treatments for COVID-19 found that interferon-beta and remdesivir did not reduce mortality in patients with COVID-19 compared with standard care [57]. However, this review included patients with suspected or probable COVID-19 (not limited to laboratory confirmed patients). Another systematic review and meta-analysis also found that remdesivir did not reduce all-cause mortality and that time to recovery, need for invasive ventilation, and varied pharmacokinetic adverse effect outcomes were similar between remdesivir and the control groups [58]. Similarly, a systematic review found no evidence to support the use of umifenovir for improving patient‐important outcomes in patients with COVID‐19 [59], and an earlier systematic review during the initial stages of the COVID-19 pandemic suggested likely increases in diarrhea, nausea, and vomiting with lopinavir/ritonavir although the conclusions were not based on meta-analysis [60].
Various public health measures such as the use of facial masks, social distancing, and quarantine of suspected or confirmed infected individuals have been implemented all over the world. While these measures have been largely successful in mitigating the spread of COVID-19, vaccination remains the most practical, and the main strategy for prevention of the disease, with vaccines now available in most countries. However, new strains of the SARS-CoV-2 have been identified in various countries [61], and the newly developed vaccines may not be effective or as effective against these new strains. Even if the newly developed vaccines are effective against all strains of SARS-CoV-2, vaccine insufficiency (shortages), unaffordability (financial and storage constraints), and the urgency of need for intervention may make vaccine prevention against COVID-19 suboptimal. In addition, the already infected individuals, particularly, the very severe and those individuals that may be more vulnerable to complications [62] are likely to require immediate treatment. In these scenarios, the use of therapeutic measures such as antiviral drugs becomes of immense importance. Furthermore, antiviral drugs may reduce viral shedding in infected individuals, thus reducing infectivity and making onward transmission from these individuals less likely [63,64].
4.1. Review limitations and merits
First, we did not search Asian, or non-English, bibliographic databases and therefore may have missed potentially eligible RCTs. However, we searched varied COVID-19 curated databases, which represent comprehensive multilingual sources of current up-to-date literature on COVID-19. Secondly, we only included English language publications and may therefore have missed any relevant non-English publication. However, this is unlikely because publications in languages other than English would have also been reported in English, considering that COVID-19 is a global problem and a pandemic. Additionally, it was not clear whether the scales for assessment of clinical progression in the RCTs were validated and to what extent our deduction of clinical progression outcome may have affected our assessment of the outcome.
Notwithstanding the limitations, this review has many merits. The search strategies for literature were developed by a knowledge synthesis librarian and peer reviewed by an independent knowledge synthesis librarian using the PRESS checklist [25]. Appropriate databases and websites were searched for published literature, and known guidelines and standards were adhered to in the conduct and reporting of the review. The review findings answer important clinical questions that inform evidence-based COVID-19 patient management and would be of help to clinicians and policymakers in decision-making regarding treatment of COVID-19.
5. Conclusions
The available evidence does not support the use of any antiviral drugs for COVID-19, despite the FDA approval of remdesivir for COVID-19 treatment. Cautious interpretations of the review findings are, however, advised considering the paucity of the evidence and the substantial risk of bias in most of the evidence. High quality, multicenter RCTs are needed for a stronger evidence base. Until then, antiviral drugs should only be used as experimental drugs for COVID-19.
Supplementary Material
Funding Statement
This study was not funded.
Article highlights
We systematically identified, critically appraised, and summarized the findings from randomized controlled trials (RCTs) of antiviral drugs for the treatment of COVID-19.
This review included 15 RCTs involving 14,418 hospitalized COVID-19 patients.
Most of the RCTs (80%) were of an unclear to high risk of bias.
There was no evidence for efficacy of any of the assessed antiviral drugs for improving clinical progression, reducing all-cause mortality and viral clearance among the patients.
Lopinavir (400 mg) with ritonavir (100 mg) significantly increased diarrhea, nausea, and vomiting compared with placebo/no treatment and other antiviral drugs.
Triazavirin (250 mg), baloxavir marboxil (80 mg), and remdesivir (100 mg – 10 days) ranked best with regard to diarrhea, nausea, and vomiting, respectively.
Supplementary material
Supplemental data for this article can be accessed here.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
- 1.Liu Y, Gayle A, Wilder-Smith A, et al. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J Travel Med Journal Translated Name Journal of Travel Medicine. 2020Mar1;27:taaa021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nishiura H, Linton N, Akhmetzhanov A.. Serial interval of novel coronavirus (COVID-19) infections. Int J Infect Dis Journal Translated Name International Journal of Infectious Diseases. 2020April93:284–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiang L, Tang K, Levin M, et al. COVID-19 and multisystem inflammatory syndrome in children and adolescents. Lancet Infect Dis. 2020Nov;20(11):e276–e288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tam H, El Tal T, Go E, et al. Pediatric inflammatory multisystem syndrome temporally associated with COVID-19: a spectrum of diseases with many names. CMAJ. 2020Sep21;192(38):E1093–E1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roberts CM, Levi M, McKee M, et al. COVID-19: a complex multisystem disorder. Br J Anaesth. 2020Sep;125(3):238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark A, Jit M, Warren-Gash C, et al. Global, regional, and national estimates of the population at increased risk of severe COVID-19 due to underlying health conditions in 2020: a modelling study. Lancet Glob Health. 2020;8(8):E1003–E1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. Jama-J Am Med Assoc. 2020May12;323(18):1775–1776. [DOI] [PubMed] [Google Scholar]
- 8.Grein J, Ohmagari N, Shin D, et al. Compassionate use of remdesivir for patients with severe Covid-19. New Engl J Med. 2020Jun11;382(24):2327–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu R, Wang L, Kuo HD, et al. An update on current therapeutic drugs treating COVID-19. Curr Pharmacol Rep. 2020;11:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization . WHO R&D blueprint novel coronavirus (nCov) vaccine prioritization for clinical trials 2020. [cited 2021 Jan 12]. Available from: https://bit.ly/2MTACxX
- 11.Malin JJ, Suarez I, Priesner V, et al. Remdesivir against COVID-19 and Other Viral Diseases. Clin Microbiol Rev. 2020Dec16;34(1):e00162-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saha A, Sharma AR, Bhattacharya M, et al. Probable molecular mechanism of remdesivir for the Treatment of COVID-19: need to Know More. Arch Med Res. 2020Aug;51(6):585–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaplan SS, Hicks CB. Safety and antiviral activity of lopinavir/ritonavir-based therapy in human immunodeficiency virus type 1 (HIV-1) infection. J Antimicrob Chemoth. 2005Aug;56(2):273–276. [DOI] [PubMed] [Google Scholar]
- 14.Williamson BN, Feldmann F, Schwarz B, et al. Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2. Nature. 2020Sep;585(7824):273–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19 - final report. N Engl J Med. 2020Oct;383(19):1813–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Wilde AH, Jochmans D, Posthuma CC, et al. Screening of an FDA-approved compound library identifies four small-molecule inhibitors of middle east respiratory syndrome coronavirus replication in cell culture. Antimicrob Agents Ch. 2014Aug;58(8):4875–4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization . A coordinated global research roadmap: 2019 novel coronavirus2020 [cited 2021 Jan 12]. Available from: https://bit.ly/38DNAbz
- 18.United States Food and Drug Administration . NDA 214787: cross Discipline Team Leader, Division Director and ODE Director Summary Review Maryland, USA: United States Food and Drug Administration; 2020. [cited 2020 Oct 23]. Available from: https://bit.ly/2TiAYOz [Google Scholar]
- 19.WHO Solidarity Trial Consortium, Pan H, Peto R, et al . Repurposed antiviral drugs for Covid-19 - interim WHO solidarity trial results. N Engl J Med. 2020;384(6):497-511. [DOI] [PMC free article] [PubMed]
- 20.Bello M, Martinez-Munoz A, Balbuena-Rebolledo I. Identification of saquinavir as a potent inhibitor of dimeric SARS-CoV2 main protease through MM/GBSA. J Mol Model Journal Translated Name Journal of Molecular Modeling. 2020December;26(12):340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khan A, Ali S, Khan M, et al. Combined drug repurposing and virtual screening strategies with molecular dynamics simulation identified potent inhibitors for SARS-CoV-2 main protease (3CLpro). J Biomol Struct Dyn Journal Translated Name Journal of Biomolecular Structure and Dynamics. 2020; 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okoli GN, Rabbani R, Copstein L, et al. Remdesivir for coronavirus disease 2019 (COVID-19): a systematic review with meta-analysis and trial sequential analysis of randomized controlled trials. Infect Dis (Lond). 2021;53(9):691–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higgins J, Lasserson T, Chandler J, et al. Methodological expectations of Cochrane intervention reviews (MECIR). 2016.
- 24.Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015Jun2;162(11):777–784. [DOI] [PubMed] [Google Scholar]
- 25.McGowan J, Sampson M, Salzwedel DM, et al. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol. 2016Jul;75:40–46. [DOI] [PubMed] [Google Scholar]
- 26.Chen Q, Allot A, Lu Z. Keep up with the latest coronavirus research. Nature. 2020;579(7798):193. [DOI] [PubMed] [Google Scholar]
- 27.Cochrane COVID-19 Study Register [Internet]. London, United Kingdom: The Cochrane Collaboration. 2020. [cited 2020 Aug 10]. Available from: https://bit.ly/2TbZssW. [Google Scholar]
- 28.COVID-19: global literature on coronavirus disease [Internet]. Geneva, Switzerland: The World Health Organization. 2020. [cited 2020 Aug 7]. Available from: https://bit.ly/34knI2B. [Google Scholar]
- 29.Marshall JC, Murthy S, Diaz J, et al. A minimal common outcome measure set for COVID-19 clinical research. The Lancet Infectious Diseases. 2020Aug;20(8):E192–E197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sterne JAC, Savovic J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ-Brit Med J. 2019;28:366. [DOI] [PubMed] [Google Scholar]
- 31.Tonin FS, Borba HH, Mendes AM, et al. Description of network meta-analysis geometry: a metrics design study. PLoS One. 201920;14(2):e0212650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salanti G. Indirect and mixed-treatment comparison, network, or multiple-treatments meta-analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Res Synth Methods. 2012Jun;3(2):80–97. [DOI] [PubMed] [Google Scholar]
- 33.Beliveau A, Boyne DJ, Slater J, et al. BUGSnet: an R package to facilitate the conduct and reporting of Bayesian network Meta-analyses. BMC Med Res Methodol. 2019Oct22;19(1):196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Plummer M. JAGS Version 4.3.0 user manual 2017. [cited 2021 Apr 13]. Available from: https://bit.ly/3mOcxqf
- 35.van Valkenhoef G, Lu GB, de Brock B, et al. Automating network meta-analysis. Res Synth Methods. 2012Dec;3(4):285–299. [DOI] [PubMed] [Google Scholar]
- 36.Watt JA, Goodarzi Z, Veroniki AA, et al. Comparative efficacy of interventions for aggressive and agitated behaviors in dementia a systematic review and network meta-analysis. Ann Intern Med. 2019Nov5;171(9):633-642. [DOI] [PubMed] [Google Scholar]
- 37.Spiegelhalter DJ, Best NG, Carlin BR, et al. Bayesian measures of model complexity and fit. J R Stat Soc B. 2002;64(4):583–616. [Google Scholar]
- 38.Brooks SP, Gelman A. General methods for monitoring convergence of iterative simulations. J Comput Graph Stat. 1998Dec;7(4):434–455. [Google Scholar]
- 39.Dias S, Sutton AJ, Welton NJ, et al. Evidence synthesis for decision making 3: heterogeneity subgroups, meta-regression, bias, and bias-adjustment. Med Decis Making. 2013Jul;33(5):618–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bucher HC, Guyatt GH, Griffith LE, et al. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol. 1997Jun;50(6):683–691. [DOI] [PubMed] [Google Scholar]
- 41.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002Jun15;21(11):1539–1558. [DOI] [PubMed] [Google Scholar]
- 42.Salanti G, Ades AE, Ioannidis JPA. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011Feb;64(2):163–171. [DOI] [PubMed] [Google Scholar]
- 43.Sterne JA, Egger M, Smith GD. Systematic reviews in health care: investigating and dealing with publication and other biases in meta-analysis. BMJ. 2001Jul14;323(7304):101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cao B, Wang Y, Wen D, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. New Engl J Med. 2020May7;382(19):1787–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen J, Xia L, Liu L, et al. Antiviral activity and safety of darunavir/cobicistat for the treatment of COVID-19. Open Forum Infect Dis. 2020Jul;7(7):ofaa241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goldman JD, Lye DCB, Hui DS, et al. Remdesivir for 5 or 10 Days in Patients with Severe Covid-19. N Engl J Med. 2020Nov5;383(19):1827–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang YQ, Tang SQ, Xu XL, et al. No statistically apparent difference in antiviral effectiveness observed among ribavirin plus interferon-alpha, lopinavir/ritonavir plus interferon-alpha, and ribavirin plus lopinavir/ritonavir plus interferon-alpha in patients with mild to moderate coronavirus disease 2019: results of a randomized, open-labeled prospective study. Front Pharmacol. 2020;14:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hung IFN, Lung KC, Tso EYK, et al. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020May30;395(10238):1695–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li Y, Xie Z, Lin W, et al. Efficacy and safety of lopinavir/ritonavir or arbidol in adult patients with mild/moderate COVID-19: an exploratory randomized controlled trial. Med (N Y). 2020Dec18;1(1):105–113 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lou Y, Liu L, Yao HP, et al. Clinical outcomes and plasma concentrations of baloxavir marboxil and favipiravir in COVID-19 patients: an exploratory randomized, controlled trial. Eur J Pharm Sci. 2021;1:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rahmani H, Davoudi-Monfared E, Nourian A, et al. Interferon beta-1b in treatment of severe COVID-19: a randomized clinical trial. Int Immunopharmacol. 2020Nov;88:106903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sadeghi A, Ali Asgari A, Norouzi A, et al. Sofosbuvir and daclatasvir compared with standard of care in the treatment of patients admitted to hospital with moderate or severe coronavirus infection (COVID-19): a randomized controlled trial. J Antimicrob Chemother. 2020Nov1;75(11):3379–3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spinner CD, Gottlieb RL, Criner GJ, et al. Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19 A randomized clinical trial. Jama-J Am Med Assoc. 2020Sep15;324(11):1048–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020May16;395(10236):1569–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu X, Yu K, Wang Y, et al. Efficacy and safety of triazavirin therapy for coronavirus disease 2019: a pilot randomized controlled trial. Engineering (Beijing). 2020Oct;6(10):1185–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zheng F, Zhou Y, Zhou Z, et al. SARS-CoV-2 clearance in COVID-19 patients with Novaferon treatment: a randomized, open-label, parallel-group trial. Int J Infect Dis. 2020Oct;99:84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Siemieniuk RAC, Bartoszko JJ, Ge L, et al. Drug treatments for covid-19: living systematic review and network meta-analysis. BMJ-Brit Med J. 2020Jul;370:m2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Piscoya A, Ng-Sueng LF, Parra Del Riego A, et al. Efficacy and harms of remdesivir for the treatment of COVID-19: a systematic review and meta-analysis. PLoS One. 2020;15(12):e0243705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huang D, Yu H, Wang T, et al. Efficacy and safety of umifenovir for coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. J Med Virol. 2021Jan;93(1):481–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu W, Zhou P, Chen K, et al. Efficacy and safety of antiviral treatment for COVID-19 from evidence in studies of SARSCoV-2 and other acute viral infections: a systematic review and meta-analysis. CMAJ. 2020;192(27):E734–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.World Health Organization . Emergencies preparedness, response: SARS-CoV-2 Variants Geneva, Switzerland: The World Health Organization; [cited 2021 Mar 9]. Available from: https://bit.ly/2OEEMLf [Google Scholar]
- 62.Grohskopf LA, Alyanak E, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the advisory committee on immunization practices - United States, 2019-20 Influenza season. MMWR Recomm Rep. 2019Aug23;68(3):1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ng S, Cowling BJ, Fang VJ, et al. Effects of oseltamivir treatment on duration of clinical illness and viral shedding and household transmission of influenza virus. Clin Infect Dis. 2010Mar1;50(5):707–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fry AM, Goswami D, Nahar K, et al. Efficacy of oseltamivir treatment started within 5 days of symptom onset to reduce influenza illness duration and virus shedding in an urban setting in Bangladesh: a randomised placebo-controlled trial. The Lancet Infectious Diseases. 2014Feb;14(2):109–118. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


