ABSTRACT
Introduction: Global emergence of coronavirus disease-19 (COVID-19) has clearly shown variable severity, mortality, and frequency between and within populations worldwide. These striking differences have made many biological variables attractive for future investigations. One of these variables, vitamin D, has been implicated in COVID-19 with rapidly growing scientific evidence.
Areas covered: The review intended to systematically explore the sources, and immunomodulatory role of vitamin D in COVID-19. Search engines and data sources including Google Scholar, PubMed, NCBI, Scopus, and Web of Science were used for data collection. The search terms used were Vitamin D, COVID-19, immune system, and antiviral mechanism. Overall, 232 sources of information were collected and 188 were included in this review.
Expert opinion: Interaction of vitamin D and vitamin D receptor (VDR) triggers the cellular events to modulate the immune system by regulation of many genes. Vitamin D operates as a double-edged sword against COVID-19. First, in macrophages, it promotes the production of antimicrobial and antiviral proteins like β-defensin 2 and cathelicidin, and these proteins inhibit the replication of viral particles and promote the clearance of virus from the cells by autophagy. Second, it suppresses cytokine storm and inflammatory processes in COVID-19.
KEYWORDS: Vitamin D, photosynthesis, immune system, mechanism of action, COVID-19
1. Introduction
Vitamin D is mainly found in two forms, one is cholecalciferol (vitamin D3) and the other is known as ergocalciferol (vitamin D2) [1]. These two forms are either photosynthesized by the human skin cells on exposure to UV radiation or obtained from nutritional sources including red meat, egg yolks, and fatty fish. Ultraviolet radiation has many hazardous effects, according to estimates, UV exposure has been linked to melanoma and non-melanoma cancers that affect more than 1.7 million people annually [2,3]. However, the exposure to adequate UV radiation is essential for the synthesis of vitamin D [4]. A 20–30 minute daily sunlight exposure is sufficient for an adequate synthesis of vitamin D3 in humans; in this context, even physical outdoor activities can help producing sufficient amounts of vitamin D without posing an additional risk for skin cancer [5]. Dietary intake and exposure to UV radiation at 280 nm to 320 nm contribute to the total body requirements of vitamin D. Several factors including the skin pigmentation, solar angle, energy of photons in the incidence light, time of day, application of sunscreens, body concentration of 7-dehydrocholesterol (7‐DHC) are important regulatory factors for the photosynthesis of vitamin D [6–8]. Newly synthesized vitamin D is activated to 1,25-Dihydroxyvitamin D that subsequently interacts with vitamin D receptors (VDRs) where it regulates the expression of many downstream genes. Vitamin D increases calcium absorption in the gut and promotes bone mineralization; it does not decrease mineral deposition [9]. Binding of 1,25-Dihydroxyvitamin D3 to the receptor in the intestinal nuclei provokes the transport of calcium and improves the transcription of genes that code for calcium and phosphorus transport proteins. Hence, vitamin D plays an important role in the calcium-phosphorous homeostasis and bone metabolism [10]. The vitamin has also been reported to have some role in the management of depression and anxiety [11]. Vitamin D plays an established role in boosting the immune system, proper functioning of skeletal muscles, and prevention against diabetes and cancer [12–16]. COVID-19 pandemic has appeared as a threat to human health and life that has affected the world population recently [17]. It has been recently reported that vitamin D deficiency plays an important role in increasing the risk of SARS-CoV-2 infection and COVID-19 severity [18–22]. Vitamin D deficiency has been reported as a significant factor in the transmission and complications of COVID-19 [23,24]. The present review article was aimed at the brief introduction of vitamin D and its role in the immune physiology in general and against COVID-19 in particular.
1.1. Methodology
The present review study was conducted at the digital libraries of King Abdulaziz University, Jeddah, and Princess Nourah Bint Abdulrahman University, Riyadh, Saudi Arabia. There was no need for ethical approval or permission as no animal or human subjects were directly included. The associated literature about the sources, synthesis, activation of vitamin D, and its physiological role in the strengthening of immune system was collected. On the basis of information available in the recent literature, a mechanism of action against SARS-Co-V2 infection was proposed. All the data were collected from online data banks including PubMed, Google Scholars, Yahoo, Web of Science, and other available online sources. For data collection, terms like vitamin D, vitamin D synthesis, vitamin D metabolism, and human immune system and association of COVID-19 and vitamin D and SARS-CoV-2 infection and vitamin D were used. A huge amount of recent data were collected from online search engines, and data comprising peer reviewed research articles published in the reputed journals and webpages of international pharmaceutical companies were included in the further analysis. The data from websites, unpublished articles, and published articles in the non-peer reviewed journals were excluded. The final included data were combined, analyzed, and evaluated. Overall, 232 information sources (published articles, books, and websites) were combined, and 188 were included in the present study. Following PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) flowchart describes the study sequence and details:
PRISMA flow diagram for new systematic reviews, which included searches of databases *Consider, if feasible to do so, reports the number of records identified from each database or register searched (rather than the total number across all databases/registers). **If automation tools were used, indicate how many records were excluded by a human and how many were excluded by automation tools [25].
2. Sources, synthesis, and metabolism of vitamin D
Vitamin D exists in two major forms, cholecalciferol (vitamin D3) and Ergocalciferol (vitamin D2). Vitamin D2 is obtained from the nutritional sources including fungi (mushrooms & yeasts), meat, egg yolks, fatty fish, and some dairy products like yogurt [26]. Vitamin D3 is synthesized endogenously or obtained from diet. By the action of UVB (Ultraviolet radiation B) at 280 nm to 320 nm, 7‐dehydrocholesterol is converted to vitamin D3 [27,28] (Figure 1).
Figure 1.
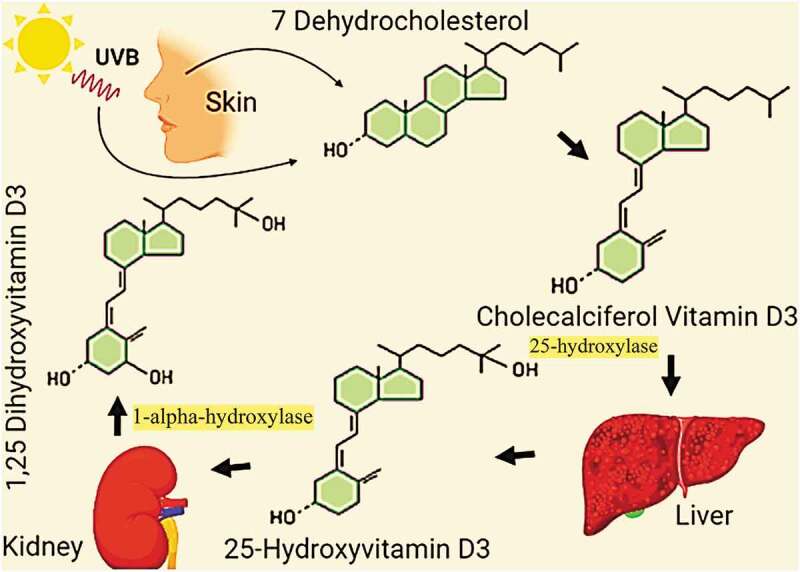
A schematic representation of photosynthesis and activation of vitamin D in the human body
Apparently, the photosynthesis of vitamin D is an enzyme-independent process, but the level of 7‐dehydrocholesterol – the parent compound of vitamin D, is dependent on the 7‐dehydrocholesterol reductase (DHCR7). Lower activity of enzyme results in the higher concentration of 7‐dehydrocholesterol and an increased synthesis of vitamin D [28]. The concentration of 7‐dehydrocholesterol is high in the cells of upper epidermis known as keratinocytes, where the synthesis of vitamin D takes place [29]. Up to 80% of the dark melanin layer is found in the basal epidermis layer and has no significant impact on vitamin D synthesis [30,31]. Hence, the dark-skinned population of Eastern African mostly has higher plasma levels of vitamin D as compared to rest of the world [32]. In general, the daily requirement of vitamin D for an average person is up to 600 IU/day, and the requirement increases in the old individuals above 70 years. The average serum levels greater than 20 ng/mL are normally provided by exposure to sunlight [4]. Geographic location-based Vitamin D deficiency has been reported among populations with mixed ancestry such as 37.3% people with age 60 to 65 years in Mexico (<32° N), 12.1% in greater Toronto (43° N) and 45% in the Netherlands (52° N) [33–35]. Age-dependent variation in the plasma vitamin D levels has been reported, but there is no evidence of significant difference among male and female populations. All kinds of populations living at greater than 35° latitude are at increased risk of vitamin D deficiency due to reduced exposure to sunlight [36]. In addition to limited sun exposure, several other risk factors for vitamin D deficiency include low intake in diet, decreased epidermal levels of 7-dehydrocholesterol, thin epidermis, decreased appetite, overweight/obesity, decreased physical activity, decreased renal synthesis of 1,25(OH)2D and its increased catabolism [37–42]. Despite its proven potential in the vitamin D synthesis, exposure to sunlight during mid-day is not recommended by many international health authorities including the American Cancer Society and World Health Organization [43,44], as the synthesis of vitamin and progression of skin cancer is not dissociable impacts of UV light. Vitamin D3 and D2 are inactive forms, and they are activated in the liver and kidneys to calcidiol, 25(OH)D [45,46]. The activated form that enters the blood stream has a half-life of about 15 days [47]. In the blood stream, 25‐Hydroxyvitamin D is carried to kidneys where it is further hydroxylated to 1,25‐dihydroxyvitamin D (calcitriol) (Figure 1). From kidneys, the finally activated form of vitamin D is transported by vitamin D-binding proteins (DBP) to the organs that have vitamin D receptors (VDRs). The plasma level of calcitriol remains up to 75 pmol/L to 200 pmol/L in the healthy individuals [48].
3. Vitamin D, genes, and immune system
Human immune system responds to external invaders and infections by a complex mechanism comprising many soluble, mobile signaling molecules such as cytokines, chemokines, and multiple types of cells [49–53]. Vitamin D contributes to regulating the immune response, which was first demonstrated by the presence of VDR in almost all cells of the immune system. Both the innate and adaptive immune systems operate against bacterial and viral infections, especially to the chronic inflammatory conditions by the influence of vitamin D [49,54,55]. Vitamin D implements its genomic impact by using VDRs. Calcitriol interacts with VDRs and results in the downstream regulation of vitamin D response elements, genes coding for cathelicidin, and the active form of vitamin D has a suppressive effect on PTH synthesis. Calcitriol activates a number of signaling systems such as the discharge of Ca2+ from intracellular stores, Ca2+ influx; modulation of phospholipase C, adenylate cyclase, and protein kinases C [56]. It has been demonstrated that the vitamin D/VDR signaling results in the chromatin modeling and significant epigenome modification in the monocytes during perturbation, consequently reducing the release of cytokines and modulation of innate immune response [57]. VDRs are the receptors found in almost all the cardiovascular and digestive systems where they operate to regulate transcription and expression of about 100 genes, directly and indirectly influencing 3% of human genome [58,59]. Some of the important genes regulated by or associated with vitamin D via VDRs have been tabulated (Table 1).
Table 1.
Some examples of genes regulated by vitamin D – VDR interaction. It should be noted that the genes extensively reported in the literature have been included in the table
| Sr. No. | Name of gene | Regulatory impact | Reference |
|---|---|---|---|
| 1 | FGF23 gene | Upregulation | [60,61] |
| 2 | Klotho gene | Upregulation | [62–64] |
| 3 | CYP27B1(1α-hydroxylase) | Downregulation | [65,66] |
| 4 | CYP24 (24-hydroxylase) | Upregulation | [66] |
| 5 | PHEX gene (Phosphate Regulating Endopeptidase Homolog X-Linked) | Downregulation | [67,68] |
| 6 | DMP1 (dentin matrix protein 1) | Downregulation | [68–70] |
| 7 | VDR (vitamin D receptor) | Upregulation | [71,72] |
| 8 | CYP3A4 (25-hydroxylase) | Upregulation | [63–75] |
| 9 | UCP2 (uncoupling protein 2) | Upregulation | [76,77] |
| 10 | ILT3 (immunoglobulin-like transcript 3) | Upregulation | [78,79] |
| 11 | TSLP (Thymic stromal lymphopoietin) | Upregulation | [80,81] |
| 12 | TLR (Toll-like receptor) | Downregulation | [82] |
| 13 | TLR10 (Toll-like receptor 10) | Upregulation | [83] |
| 14 | TNF-α (tumor necrosis factor-α) | Upregulation | [84,85] |
| 15 | NF-κB (nuclear factor kappa B) | Downregulation | [86,87] |
| 16 | Cathelicidin | Upregulation | [88,89] |
| 17 | Beclin‐1 | Upregulation | [90,91] |
| 18 | Defensin | Upregulation | [92–94] |
| 19 | CaBP-D9k (Calbindin-D9k) | Upregulation | [95] |
| 20 | RANKL (Receptor activator of nuclear factor kappa-Β ligand) | Upregulation | [96,97] |
| 21 | SFRP2 (Secreted Frizzled Related Protein 2) | Downregulation | [98] |
| 22 | DKK1 (Dickkopf WNT signaling pathway inhibitor 1) | Downregulation | [98] |
Vitamin D regulates the expression of at least 11 genes involved in the bone homeostasis. Genes associated with ion channels phosphatases or kinases, intestinal calcium absorption, and bone resorption systems are important examples [99,100]. Cathelicidins represent a group of proteins associated with anti-microbial activity, and the synthesis of these proteins is activated by vitamin D. For example, a cathelicidin propeptide hCAP18 is cleaved to an active antimicrobial peptide LL-37 to counter microbial invasions [101]. Most of the cathelicidins are found in the neutrophil granules and released at the infection sites. However, some other types of immune cells such as NK cells, monocytes, and B cells can also produce antimicrobial hCAP18 protein [102]. The activated protein enters the blood stream and is transported to epithelia of digestive tract, cornea, the conjunctiva, skin, and urinary tract [103,104]. In the absence or severe deficiency of active vitamin D, the ability of immune cells to induce cathelicidin is impaired significantly [105]. Immune and bone systems are linked at multiple levels and give rise to the concept of osteoimmunity. Bone marrow is basically the origin of all immune cells including B, T, neutrophils, and macrophages [106]. Hence, the low levels of deficiency of vitamin D have been associated with immune suppression and initiation of many diseases. As, for example, the reduced exposure to sunlight during winters (leading to reduced synthesis of vitamin D) has been positively correlated with the onset of type 1 diabetes mellitus (T1DM) [107]. Application of vitamin D supplements, cod oil, and other forms of dietary intake of vitamin can significantly reduce the chances of T1DM [108]. The use of vitamin D (≥2000 IU/d) in the first year of life can reduce the risk of T1DM up to 80%. On the other hand, the kids with chances of rickets have 3 times increased chances of T1DM. The vitamin also reduces the chances of T1DM by supporting the immune system [109]. In the preclinical studies on mice, vitamin D has been found to have inverse relation with insulin secretion and glucose intolerance. It regulated the glucose homeostasis. The mice with nonfunctional VDRs have shown a decreased level of insulin mRNA levels [110]. The changes in insulin concentrations may be mediated by calcium levels in the pancreatic beta cells [111]. Studies have shown that the deficiency of vitamin D can increase the chances of cardiovascular diseases [112]. The individuals with insufficient plasma levels of vitamin D have higher incidence of peripheral arterial disease, stroke, myocardial infarction, and extended coronary artery calcification [113,114]. VDR activation reduces the risk of various types of cancers via p53 and p21 activation, it also promotes the apoptosis and cell differentiation mechanisms [115,116]. The deficiency of vitamin D has also been associated with many other chronic diseases including asthma [117], inflammatory bowel disease [118], chronic obstructive pulmonary disease (COPD) [119], multiple sclerosis (MS), rheumatoid arthritis (RA), and systemic lupus erythematosus (SLE) [120].
4. Proposed mechanism of vitamin D action against SARS-COV-2
In addition to its role as a mediator of immune system, promoter of antimicrobial activity, vitamin D, represents a potential candidate against viral infections [121]. Application of vitamin D increases the production of cathelicidins that have shown potential antiviral properties in addition to antimicrobial impact [122–124]. In the airy pathways of human respiratory system, vitamin D triggers the production of IkBα (nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha) that serves as an inhibitor of NF-kB (nuclear factor kappa light-chain enhancer of activated B cells), resulting in the reduced expression of virus induced inflammatory genes [125]. Therefore, vitamin D has been found to be effective against influenza [126] and human immunodeficiency viruses (HIV) [127]. The antiviral activity of vitamin D has become an important topic of discussion with reference to the worldwide struggle and fight against COVID-19 (Coronavirus Disease 19). The action of vitamin D against SARS-CoV-2 may involve the mechanism similar to its reported antimicrobial and antiviral activities in the previous studies. These mechanisms include production of cathelicidin and defensins to inhibit the viral entry into the cells and its replication [128] and induction of autophagy represented by the expression of autophagy marker LC3 (light chain 3) [129–131]. Mechanistic target of rapamycin (mTOR) pathway that inhibits autophagy is negatively regulated by vitamin D [132], and vitamin D also promotes the enzymes involved in autophagy including PI3KC3 and Beclin 1 by upregulating the Ca (intracellular calcium and NO (nitric oxide) levels [133–135]. The autophagy-associated impact of vitamin D is closely linked with apoptosis, and both processes promote the antiviral response [136]. Recent in silico studies have reported the involvement of TLRs and TLR4 in particular in the recognition and induction of immune response to SARS‐CoV‐2. TLR4 receptor having the strongest TLR-Spike protein interaction has been reported to play a vital role in the SARS‐CoV‐2 induced inflammatory events in COVID‐19. Hydrogen and hydrophobic interactions are involved in the TLR4-Spike interaction [137]. Based on the above information, a proposed mechanism of vitamin D action against COVID-19 is described (Figure 2). In the case of hypertensive patients, a heat shock protein of 60 kDa molecular weight (HSP60) is produced post-SARS-CoV-2 infection and triggers the inflammatory response by induction of proinflammatory cytokines via cardiac toll-like receptor pathways. This makes the heart vulnerable and enhances thrombosis in the case of acute respiratory condition during COVID-19, which results in unbearable burden to the heart, leading to multiorgan failure and mortality. Hence, HSP60 can be an attractive target in the management of COVID-19 [138] (Jakovac, 2020).
Figure 2.
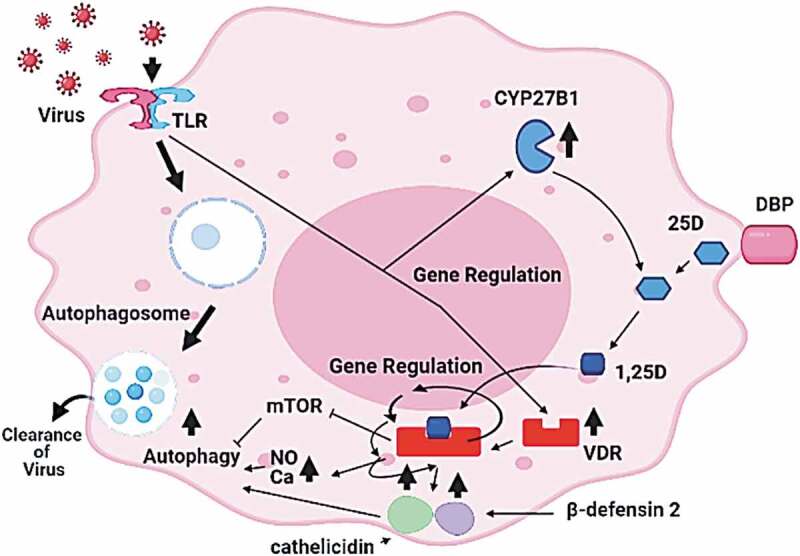
Antiviral role of vitamin D by autophagy. A possible macrophage responses to viral infection involves the induction of VDR and 1α-hydroxylase (CYP27B1). Vitamin D (25-OHD) interacts with the vitamin D binding protein (DBP), enters the cells, activated to 1, 25 (OH)2D, and binds the VDR. Vitamin D-VDR binding results in the expression of genes coding for cathelicidin and β-defensin 2 (indicated by arrows), upregulation of NO and Ca, inhibition of mTOR mechanism, all these processes promote autophagy (adopted and modified from the recent literature [128,132–134]
5. Vitamin D in COVID-19 – preventive and treatment potential
Studies indicating a positive correlation of vitamin D deficiency with the incidence of acute respiratory infections (ARI) naturally raised a question if vitamin D can prevent COVID-19? [139]. Many studies have suggested that adequate vitamin D levels play a vital role in preventing COVID-19 infection and in avoiding mortality, in the case of infection [140]. According to reports, the deficiency of vitamin D can enhance the chances of COVID-19 infection [141]. According to recent findings, the countries with less availability of UVB, consequently an overall vitamin D deficiency, have significantly high rate of COVID-19 infection [142]. Up to 4.4% increase in mortality rate by COVID-19 has been found in the geographic areas with each degree difference from North of 28° latitude, suggesting an indirect role of UVB and vitamin D in the protection against COVID‐19 [143]. COVID-19 is a disease commonly characterized by acute respiratory disease, pneumonia, myocarditis, cytokine storms, and inflammation. Main defense system against COVID-19 infection depends on T regulatory lymphocytes (Tregs), and these are effective immunosuppressive cells with a critical role in the homeostasis of immune response. It has been assumed that the Tregs are responsible for SARS-CoV-2-specific immune tolerance by suppression of inflammation in the patients. Changes in the Tregs of COVID-19 patients with acute respiratory problems can provide targets against COVID-19. Regulatory effect of vitamin D in the activity of Tregs is well established [144–146].
Vitamin D deficiency that mostly occurs among obese and diabetic individuals can enhance COVID-19 fatality [147–149]. To counter COVID-19 infection, a rapid increase in the active vitamin D levels with an initial dose of 10,000 IU/d, followed by 5000 IU/d, has been recommended by the research studies to achieve a final level of 40–60 ng/mL [150]. Acute respiratory distress syndrome (ARDS) is one of the most prominent conditions of COVID-19 that leads to multiple organ damage. Inadequate levels of vitamin D have been associated with cardiovascular diseases (CVDs), diabetes, and hypertension, and these comorbidities significantly increase the severity of COVID-19 events [151]. Vitamin D has been, therefore, suggested to prevent multiple organ failure comorbidity in COVID-19 [152], and a combination of vitamin D and remdesivir (a common antiviral medicine) has been recently recommended for the treatment of disease [153]. SARS-COV-2 uses ACE2 (angiotensin converting enzyme II) receptor for intracellular invasion and pathogenicity. SARS-CoV-2 infection and viral replication are associated with downregulation of ACE2 and play a critical role in the pathogenesis of COVID-19 [154]. Recent findings have suggested that the application of vitamin D can help in the management of COVID-19 by downregulation of the cytokine storm, RAS (rat sarcoma – a family of genes) pathway, and blood pressure and upregulating the ACE2 expression and immune regulatory system [155–157]. The enhanced expression of ACE2 has been positively linked with the production of angiotensin 1–7, molecules with antifibrotic and anti-inflammatory activities. ACE2 has shown protective effects against ARDS (acute respiratory distress syndrome), and angiotensin II has been reported as harmful moiety causing fibrosis and pulmonary edema. Hence, the upregulation of ACE2 gene expression by vitamin D is an important factor in reduced inflammatory response [158].
Vitamin D has an ability to enhance the production of type I interferons (INFs). These are potent antiviral molecules of immune system capable of suppressing the viral replication and rapid virus clearance without extra inflammatory response. Vitamin D also reduces the expression of antithrombin gene. Hence, it can be particularly useful in the case of COVID-19 infection [159]. (Kralj and Jakovac, 2021). Currently, the unavailability of highly targeted procedures and medicine for the treatment of COVID-19 has left us with no choice other than the precautionary measures and improved immunity. Studies have shown that the individuals subjected to vitamin D supplements have less chances of COVID-19 [160], and an Italian study has reported low plasma level of vitamin D among PCR positive cases of SARS‐CoV‐2 [161]. The information accumulated in this article can be used by the front-line fighters against COVID-19. Supplementation of vitamin D up to 10,000 IU has been considered as safe dose. However, the application of vitamin D can cause hypercalcemia among the individuals suffering from sarcoidosis and tuberculosis [162].
6. Expert opinion
COVID-19 pandemic has seriously affected human health, economy and sociology worldwide. Serious efforts have been made, in terms of treatment therapies, vaccine development and understanding the viral pathophysiology. A number of approaches including antiviral medicines, herbal drugs have been successfully used for the management of disease [163–165]. Many minerals and vitamins have also been suggested by the health experts to improve immunity and manage COVID-19 [166,167]. Currently, vitamin D is one of the most widely discussed compounds for the prevention, treatment and management of COVID-19 [168]. Rapid spread, severity and mortality rates of COVID-19 in the Northern hemisphere, especially in the populations with vitamin D deficiency has made it more attractive for the researchers, physicians and general population [169,170]. Vitamin D has been well known for its physiological role in the calcium/phosphorous homeostasis, bone health, structure and physiology, and promotion of immune system. Most important cause of mortality by COVID-19 is due to respiratory failure. Prior to COVID-19, vitamin D has been reported to ameliorate the common repertory conditions [171] including tuberculosis [172], pneumonia [173], asthma [174], and influenza [175]. Recently, numerous investigations have highlighted the role of vitamin D in the prevention and treatment of COVID-19 [176–179]. These findings have suggested a positive association of infection, severity and rate of mortality with the vitamin D deficiency [141], and suggest vitamin D supplementation to improve the efficacy of antiviral medicine. Broad spectrum trials are in progress to determine the exact quantitative effect of vitamin D in the management of COVID-19 and its ability to improve the effect of commonly used drugs. Vitamin D is an essential and non-hazardous biomolecule with well established role in the functioning of immune system. Hence, studies on the dose dependent impact, and combinations of vitamin D with relevant medicines can be safely continued in the near future. There are many studies illustrating the mechanism of action of vitamin D in boosting the fighting ability against viral and bacterial respiratory infections. The nutshell of these studies has advocated that vitamin D operates as an effective, double-edged sword against COVID-19. On one hand, the vitamin D-VDR interaction modulates the expression of up to 100 genes, many of those are associated with immune system, trigger the induction of antimicrobial and antiviral proteins like β-defensin 2 and cathelicidins. These proteins have been reported to inhibit the proliferation of viral particles [180,181]. Further, detailed effect of COVID-19 infection, mechanism, vaccine development and therapy including vitamin D discussed in detail in our previous work [17,182–184]. Vitamin D, promotes the process of virus clearance by autophagy through upregulation of Ca and NO levels and inhibition of mTOR pathway, the latter being a suppressor of autophagy. In case of SARS-CoV-2, ORF3a (open reading frame 3a) protein plays an inhibitory role to autophagy. Hence, this protein can be targeted to improve the viral clearance by the cells. Pharmaceutical compounds acting as modulators and promoters of autophagy have been suggested to play a critical role in the virus clearance, vitamin D is one of the major candidates [185–187]. On the other hand, vitamin D has been found effective in reducing the ‘cytokine storm’ and inflammatory response by the cells during COVID-19. Emerging and remerging respiratory epidemics and pandemics have been a big challenge to the world population in the recent past. Epidemics of influenza (1985), SARS (2003), MERS (2012) and COVID-19 are the respiratory conditions caused by RNA viruses with similar yet specific genetic characteristics and mechanisms of infection pathophysiology. The health experts have sounded alarm about the probability of more robust and deadly episodes of RNA virus based epidemics in the near future. These serious threats have dragged the attention of biomedical researchers to focus in making the new developments and modernizing the existing arsenal against COVID-19 like respiratory conditions. Hence, the recent studies on the prominent role of vitamin D in fighting against COVID-19 can be prolonged to future tactics to prevent and cure such viral diseases. There are many research questions to determine the direct or indirect, qualitative/quantitative effect of vitamin D on the cellular entery, replication, and pathophysiology of SARS‐CoV‐2 or other similar RNA viruses that can infect the human population in the near future. Development and selection of suitable experimental models for mechanistic studies will be prerequisite and it will need a parallel research area. Studies on the vitamin D based regulatory mechanisms for proinflammatory cytokines and consequent suppression of inflammatory response may be required. These studies may not only provide better insights into the mechanistic control of SARS‐CoV‐2 but also help to overcome possible future RNA virus based pandemic in near future.
Funding Statement
This research was funded by the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University through the Fast-track Research Funding Program to support publication in the top journal (Grant no.42-FTTJ-20).
Article highlights
Vitamin D is synthesized by the human skin cells under UVB radiation and activated in the liver and kidneys.
Vitamin D deficiency has been positively linked with increased chances of infection, severity, and mortality by respiratory infections including COVID-19.
VRD-Vitamin D interaction results in the regulation of many genes associated with immune system and promotes the innate and adaptive immune response against respiratory infections.
It enhances the production of antibacterial and antiviral proteins including beta-defensins and cathelicidin to inhibit the cellular entry and subsequent proliferation of virus particles.
In the macrophages, vitamin D promotes autophagy and clearance of virus particles by upregulation of calcium/nitric oxide, immunomodulatory proteins, and downregulation of mTOR pathway.
Future studies on anti-inflammatory and antiproliferative mechanisms involving vitamin D an development and application of appropriate animal models are recommended to combat COVID-19 or any upcoming similar pandemic.
Declaration of interest
The author(s) have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers in this manuscript have no relevant financial or other relationships to disclose.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Lips P.Vitamin D physiology. Progress Biophy Mol Biol. 2006;92(1):4–8. [DOI] [PubMed] [Google Scholar]
- 2.Longstreth JD, De Gruijl FR, van der Leun JC, et al. Effects of increased solar ultraviolet radiation on human health. AmBio. 1995;1:24. [Google Scholar]
- 3.Lucas RM, McMichael AJ, Armstrong BK, et al. Estimating the global disease burden due to ultraviolet radiation exposure. Intl J Epidemiol. 2008;37(3):654–667. . [DOI] [PubMed] [Google Scholar]
- 4.Holick MF. Vitamin D deficiency. New Engl J Med. 2007;357(3):266–281. [DOI] [PubMed] [Google Scholar]
- 5.Wolpowitz D, Gilchrest BA. The vitamin D questions: how much do you need and how should you get it? J Am Acad Dermatol. 2006;54(2):301–317. [DOI] [PubMed] [Google Scholar]
- 6.Bonjour JP, Trechsel U, Granzer E, et al. The increase in skin 7-dehydrocholesterol induced by a hypocholesterolemic agent is associated with elevated 25-hydroxyvitamin D3 plasma level. Pflügers Archiv. 1987;410(1–2):165–168. . [DOI] [PubMed] [Google Scholar]
- 7.Matsuoka LY, Wortsman J, Hanifan N, et al. Chronic sunscreen use decreases circulating concentrations of 25-hydroxyvitamin D: a preliminary study. Arch Dermatol. 1988;124(12):1802–1804. . [PubMed] [Google Scholar]
- 8.Chen TC, Chimeh F, Lu Z, et al. Factors that influence the cutaneous synthesis and dietary sources of vitamin D. Arch Biochem Biophys. 2007;460(2):213–217. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goltzman D. Functions of vitamin D in bone. Histoch Cell Biol. 2018;149(4):305–312. [DOI] [PubMed] [Google Scholar]
- 10.DeLuca HF. The metabolism and functions of vitamin D In: Chrousos G.P., Loriaux D.L., Lipsett M.B. (eds) Steroid Hormone Resistance. Advances in Experimental Medicine and Biology, vol 196. Springer, Boston, MA.Check Tagging, 1986. [DOI] [PubMed] [Google Scholar]
- 11.Casseb GA, Kaster MP, Rodrigues AL. Potential role of vitamin D for the management of depression and anxiety. CNS Drugs. 2019;33(7):619–637. [DOI] [PubMed] [Google Scholar]
- 12.Garland CF, Garland FC, Gorham ED, et al. The role of vitamin D in cancer prevention. Am J Public Health. 2006;96(2):252–261. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palomer X, González‐Clemente JM, Blanco‐Vaca F, et al. Role of vitamin D in the pathogenesis of type 2 diabetes mellitus. Diabet Obesity Metabol. 2008;10(3):185–197. . [DOI] [PubMed] [Google Scholar]
- 14.Feldman D, Krishnan AV, Swami S, et al. The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer. 2014;14(5):342–357. . [DOI] [PubMed] [Google Scholar]
- 15.Caprio M, Infante M, Calanchini M, et al. Vitamin D: not just the bone. Evidence for beneficial pleiotropic extraskeletal effects. Eating and Weight Disorders-Studies on Anorexia, Bulimia and Obesity. Eat Weight Disord. 2017;22(1):27–41. . [DOI] [PubMed] [Google Scholar]
- 16.Fabbri A, Infante M, Ricordi C. Editorial-Vitamin D status: a key modulator of innate immunity and natural defense from acute viral respiratory infections. Eur Rev Med Pharmacol Sci. 2020;24(7):4048–4052. [DOI] [PubMed] [Google Scholar]
- 17.Nadeem MS, Zamzami MA, Choudhry H, et al. Origin, potential therapeutic targets and treatment for coronavirus disease (COVID-19). Pathogens. 2020;9(4):307. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This paper summarises origin, potential therapeutic targets and detailed treatment for Coronavirus Disease
- 18.Alzaman NS, Dawson-Hughes B, Nelson J, et al. Vitamin D status of black and white Americans and changes in vitamin D metabolites after varied doses of vitamin D supplementation. Am J Clin Nutrition. 2016;104(1):205–214. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jain A, Chaurasia R, Sengar NS, et al. Analysis of vitamin D level among asymptomatic and critically ill COVID-19 patients and its correlation with inflammatory markers. Sci Rep. 2020;10(1):1–8. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Infante M, Buoso A, Pieri M, et al. Low Vitamin D status at admission as a risk factor for poor survival in hospitalized patients with COVID-19: an Italian retrospective study. J Am College Nutrition. 2021;22:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pereira M, Dantas Damascena A, Galvão Azevedo LM, et al. Vitamin D deficiency aggravates COVID-19: systematic review and meta-analysis. Crit Rev Food Sci Nutrition. 2020;3:1–9. [DOI] [PubMed] [Google Scholar]
- 22.Panfili FM, Roversi M, D’Argenio P, et al. Possible role of vitamin D in Covid-19 infection in pediatric population. J Endocrinol Invest. 2021; 44(1): 27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martineau AR, Jolliffe DA, Hooper RL, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017; 356: i6583.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trovas G, Tournis S. Vitamin d and covid-19. Hormones. 2020;14:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021; 372: n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kiely M, Cashman KD. Summary outcomes of the ODIN project on food fortification for vitamin D deficiency prevention. Intl J Environ Res Public Health. 2018;15(11):2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lichtenstein A, Ferreira-Júnior M, Sales MM, et al. Vitamin D: non-skeletal actions and rational use. Revista Da Associação Médica Brasileira. 2013;59(5):495–506. . [DOI] [PubMed] [Google Scholar]
- 28.Prabhu AV, Luu W, Li D, et al. DHCR7: a vital enzyme switch between cholesterol and vitamin D production. Prog Lipid Res. 2016;64:138–151. [DOI] [PubMed] [Google Scholar]
- 29.Holick MF. The cutaneous photosynthesis of previtamin D3: a unique photoendocrine system. J Invest Dermatol. 1981;77(1):51–58. [DOI] [PubMed] [Google Scholar]
- 30.Hakim OA, Hart K, McCabe P, et al. Vitamin D production in UK Caucasian and South Asian women following UVR exposure. J Steroid Biochem Mol Biol. 2016;164:223–229. [DOI] [PubMed] [Google Scholar]
- 31.Young AR, Morgan KA, Ho TW, et al. Melanin has a small inhibitory effect on cutaneous vitamin D synthesis: a comparison of extreme phenotypes. J Invest Dermatol. 2020;140(7):1418–1426. . [DOI] [PubMed] [Google Scholar]
- 32.Luxwolda MF, Kuipers RS, Kema IP, et al. Traditionally living populations in East Africa have a mean serum 25-hydroxyvitamin D concentration of 115 nmol/l. British J Nutrition. 2012;108(9):1557–1561. . [DOI] [PubMed] [Google Scholar]
- 33.Ginter JK, Krithika S, Gozdzik A, et al. Vitamin D status of older adults of diverse ancestry living in the greater Toronto area. BMC Geriatr. 2013;13(1):1. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brouwer-Brolsma EM, Vaes AM, van der Zwaluw NL, et al. Relative importance of summer sun exposure, vitamin D intake, and genes to vitamin D status in Dutch older adults: the B-PROOF study. J Steroid Biochem Mol Biol. 2016;164:168–176. [DOI] [PubMed] [Google Scholar]
- 35.Carrillo-Vega MF, García-Peña C, Gutiérrez-Robledo LM, et al. Vitamin D deficiency in older adults and its associated factors: a cross-sectional analysis of the Mexican Health and Aging Study. Arch Osteoporosis. 2017;12(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holick MF. Vitamin D and health: evolution, biologic functions, and recommended dietary intakes for vitamin D. [Google Scholar]
- 37.Sauermann K, Clemann S, Jaspers S, et al. Age related changes of human skin investigated with histometric measurements by confocal laser scanning microscopy in vivo. Skin Res Technol. 2002;8(1):52–56. . [DOI] [PubMed] [Google Scholar]
- 38.Snijder MB, Van Dam RM, Visser M, et al. Adiposity in relation to vitamin D status and parathyroid hormone levels: a population-based study in older men and women. J Clin Endocrinol Metabol. 2005;90(7):4119–4123. . [DOI] [PubMed] [Google Scholar]
- 39.Veldurthy V, Wei R, Oz L, et al. Vitamin D, calcium homeostasis and aging. Bone Res. 2016;4(1):1–7. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hill TR, Granic A, Aspray TJ. Vitamin D and ageing. Subcell Biochem. 2018; 90: 191-220.. [DOI] [PubMed] [Google Scholar]
- 41.Czekalla C, Schönborn KH, Lademann J, et al. Noninvasive determination of epidermal and stratum corneum thickness in vivo using two-photon microscopy and optical coherence tomography: impact of body area, age, and gender. Skin Pharmacol Physiol. 2019;32(3):142–150. . [DOI] [PubMed] [Google Scholar]
- 42.Orces C, Lorenzo C, Guarneros JE. The prevalence and determinants of vitamin D inadequacy among US older adults: national Health and Nutrition Examination Survey 2007-2014. Cureus. 2019;11:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vainio H, Miller AB, Bianchini F. An international evaluation of the cancer-preventive potential of sunscreens - Meeting held at Lyon, France, 10–18, 2000. Intl J Cancer. 2000;88(5):838–842. [DOI] [PubMed] [Google Scholar]
- 44.American Cancer Society Skin Cancer Facts . Available online: http://www.cancer.org/cancer/cancercauses/sunanduvexposure/skin-cancer-facts (accessed on 2021 Feb 5
- 45.Prosser DE, Jones G. Enzymes involved in the activation and inactivation of vitamin D. Trends Biochem Sci. 2004;29(12):664–673. [DOI] [PubMed] [Google Scholar]
- 46.Ohyama Y, Yamasaki T. Eight cytochrome P450s catalyze vitamin D metabolism. Front Biosci. 2004;9(1–3):3007–3018. [DOI] [PubMed] [Google Scholar]
- 47.Jones G. Pharmacokinetics of vitamin D toxicity. Am J Clin Nutrition. 2008;88(2):582S–6S. [DOI] [PubMed] [Google Scholar]
- 48.Levine BS, Singer FR, Bryce GF, et al. Pharmacokinetics and biologic effects of calcitriol in normal humans. J Lab Clin Med. 1985;105(2):234–238. [PubMed] [Google Scholar]
- 49.Hewison M. An update on vitamin D and human immunity. Clin Endocrinol (Oxf). 2012;76(3):315–325. [DOI] [PubMed] [Google Scholar]
- 50.Leaf DE, Croy HE, Abrahams SJ, et al. Cathelicidin antimicrobial protein, vitamin D, and risk of death in critically ill patients. Crit Care. 2015;19(1):1–9. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yegorov S, Bromage S, Boldbaatar N, et al. Effects of vitamin D supplementation and seasonality on circulating cytokines in adolescents: analysis of data from a feasibility trial in mongolia. Front Nutrition. 2019;6:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abdel-Hady H, Yahia S, Megahed A, et al. Mediators in preterm infants with late-onset sepsis: a randomized controlled trial. J Pediatr Gastroenterol Nutr. 2019;68(4):578–584. . [DOI] [PubMed] [Google Scholar]
- 53.Zhou L, Wang J, Li J, et al. 1, 25-Dihydroxyvitamin D3 ameliorates collagen-induced arthritis via suppression of Th17 cells through miR-124 mediated inhibition of IL-6 signaling. Front Immunol. 2019;10:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jiang WL, Gu HB, Zhang YF, et al. Vitamin D supplementation in the treatment of chronic heart failure: a meta‐analysis of randomized controlled trials. Clin Cardiol. 2016;39(1):56–61. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Veldman CM, Cantorna MT, DeLuca HF. Expression of 1, 25-dihydroxyvitamin D3 receptor in the immune system. Arch Biochem Biophys. 2000;374(2):334–338. [DOI] [PubMed] [Google Scholar]
- 56.Lehmann B, Meurer M. Vitamin D metabolism. Dermatol Ther. 2010;23(1):2–12. [DOI] [PubMed] [Google Scholar]
- 57.Carlberg C. Vitamin D signaling in the context of innate immunity: focus on human monocytes. Front Immunol. 2019;10:2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hanel A, Malmberg HR, Carlberg C. Genome-wide effects of chromatin on vitamin D signaling. J Mol Endocrinol. 2020;64(4):R45–56. [DOI] [PubMed] [Google Scholar]
- 59.Koivisto O, Hanel A, Carlberg C. Key vitamin D target genes with functions in the immune system. Nutrients. 2020;12(4):1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bergwitz C, Jüppner H. Regulation of phosphate homeostasis by PTH, vitamin D, and FGF23. Annual Rev Med. 2010;61(1):91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Saini RK, Kaneko I, Jurutka PW, et al. 1, 25-dihydroxyvitamin D 3 regulation of fibroblast growth factor-23 expression in bone cells: evidence for primary and secondary mechanisms modulated by leptin and interleukin-6. Calcified Tissue Intl. 2013;92(4):339–353. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tsujikawa H, Kurotaki Y, Fujimori T, et al. Klotho, a gene related to a syndrome resembling human premature aging, functions in a negative regulatory circuit of vitamin D endocrine system. Mol Endocrinol. 2003;17(12):2393–2403. . [DOI] [PubMed] [Google Scholar]
- 63.Forster RE, Jurutka PW, Hsieh JC, et al. Vitamin D receptor controls expression of the anti-aging klotho gene in mouse and human renal cells. Biochem Biophys Res Commun. 2011;414(3):557–562. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Haussler MR, Whitfield GK, Haussler CA, et al. 1, 25-Dihydroxyvitamin D and Klotho: a tale of two renal hormones coming of age. Vitamins & Hormones. 2016;100:165–230. [DOI] [PubMed] [Google Scholar]
- 65.Turunen MM, Dunlop TW, Carlberg C, et al. Selective use of multiple vitamin D response elements underlies the 1 α, 25-dihydroxyvitamin D3-mediated negative regulation of the human CYP27B1 gene. Nucleic Acids Res. 2007;35(8):2734–2747. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Avila E, Díaz L, Barrera D, et al. Regulation of Vitamin D hydroxylases gene expression by 1, 25-dihydroxyvitamin D3 and cyclic AMP in cultured human syncytiotrophoblasts. J Steroid Biochem Mol Biol. 2007;103(1):90–96. . [DOI] [PubMed] [Google Scholar]
- 67.Hines ER, Kolek OI, Jones MD, et al. 1, 25-dihydroxyvitamin D3 down-regulation of PHEX gene expression is mediated by apparent repression of a 110 kDa transfactor that binds to a polyadenine element in the promoter. J Biol Chem. 2004;279(45):46406–46414. . [DOI] [PubMed] [Google Scholar]
- 68.Rowe PS. Regulation of bone− renal mineral and energy metabolism: the PHEX, FGF23, DMP1, MEPE ASARM pathway. Crit Rev Eukaryotic Gene Express. 2012;22:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nociti FH Jr, Foster BL, Tran AB, et al. Vitamin D represses dentin matrix protein 1 in cementoblasts and osteocytes. J Dental Res. 2014;93(2):148–154. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang L, Tran AB, Nociti FH Jr, et al. PTH and vitamin D repress DMP1 in cementoblasts. J Dental Res. 2015;94(10):1408–1416. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kamei Y, Kawada T, Kazuki R, et al. Vitamin D receptor gene expression is up-regulated by 1, 25-dihydroxyvitamin D3 in 3T3-L1 preadipocytes. Biochem Biophys Res Commun. 1993;193(3):948–955. [DOI] [PubMed] [Google Scholar]
- 72.Janik S, Nowak U, Łaszkiewicz A, et al. Diverse regulation of vitamin D receptor gene expression by 1, 25-dihydroxyvitamin D and ATRA in murine and human blood cells at early stages of their differentiation. Intl J Mol Sci. 2017;18(6):1323. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Drocourt L, Ourlin JC, Pascussi JM, et al. Expression of cyp3a4, cyp2b6, and cyp2c9 is regulated by the vitamin d receptor pathway in primary human hepatocytes. J Biol Chem. 2002;277(28):25125–25132. . [DOI] [PubMed] [Google Scholar]
- 74.Pavek P, Pospechova K, Svecova L, et al. Intestinal cell-specific vitamin D receptor (VDR)-mediated transcriptional regulation of CYP3A4 gene. Biochem Pharmacol. 2010;79(2):277–287. . [DOI] [PubMed] [Google Scholar]
- 75.Qin X, Wang X. Role of vitamin D receptor in the regulation of CYP3A gene expression. Acta Pharm Sin B. 2019;9(6):1087–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cândido FG, Bressan J. Vitamin D: link between osteoporosis, obesity, and diabetes? Int J Mol Sci. 2014; 15(4): 6569-6591. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhu X, Wu S, Guo H. Active vita Pavek min D and vitamin D receptor help prevent high glucose induced oxidative stress of renal tubular cells via AKT/UCP2 signaling pathway. BioMed Res Intl. 2019;28:2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Penna G, Roncari A, Amuchastegui S, et al. Expression of the inhibitory receptor ILT3 on dendritic cells is dispensable for induction of CD4+ Foxp3+ regulatory T cells by 1, 25-dihydroxyvitamin D3. Blood. 2005;106(10):3490–3497. . [DOI] [PubMed] [Google Scholar]
- 79.Barragan M, Good M, Kolls JK. Regulation of dendritic cell function by vitamin D. Nutrients. 2015;7(9):8127–8151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xie Y, Takai T, Chen X, et al. Long TSLP transcript expression and release of TSLP induced by TLR ligands and cytokines in human keratinocytes. J Dermatol Sci. 2012;66(3):233–237. . [DOI] [PubMed] [Google Scholar]
- 81.Usategui A, Criado G, Del Rey MJ, et al. Topical vitamin D analogue calcipotriol reduces skin fibrosis in experimental scleroderma. Arch Dermatol Res. 2014;306(8):757–761. . [DOI] [PubMed] [Google Scholar]
- 82.Sadeghi K, Wessner B, Laggner U, et al. Vitamin D3 down-regulates monocyte TLR expression and triggers hyporesponsiveness to pathogen-associated molecular patterns. Eur J Immunol. 2006;36(2):361–370. . [DOI] [PubMed] [Google Scholar]
- 83.Verma R, Jung JH, Kim JY. 1, 25-Dihydroxyvitamin D3 up-regulates TLR10 while down-regulating TLR2, 4, and 5 in human monocyte THP-1. J Steroid Biochem Mol Biol. 2014;141:1–6. [DOI] [PubMed] [Google Scholar]
- 84.Hakim I, Bar‐Shavit Z. Modulation of TNF‐α expression in bone marrow macrophages: involvement of vitamin D response element. J Cell Biochem. 2003;88(5):986–998. [DOI] [PubMed] [Google Scholar]
- 85.Golovko O, Nazarova N, Tuohimaa P. Vitamin D–induced up–regulation of tumour necrosis factor alpha (TNF–α) in prostate cancer cells. Life Sci. 2005;77(5):562–577. [DOI] [PubMed] [Google Scholar]
- 86.Wu S, Liao AP, Xia Y, et al. Vitamin D receptor negatively regulates bacterial-stimulated NF-κB activity in intestine. Am J Pathol. 2010;177(2):686–697. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Szeto FL, Sun J, Kong J, et al. Involvement of the vitamin D receptor in the regulation of NF-κB activity in fibroblasts. J Steroid Biochem Mol Biol. 2007;103(3–5):563–566. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311(5768):1770–1773. . [DOI] [PubMed] [Google Scholar]
- 89.Chung C, Silwal P, Kim I, et al. Vitamin D-cathelicidin axis: at the crossroads between protective immunity and pathological inflammation during infection. Immune Netw. 2020;311(5768):1770–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Trocoli A, Mathieu J, Priault M, et al. ATRA-induced upregulation of Beclin 1 prolongs the life span of differentiated acute promyelocytic leukemia cells. Autophagy. 2011;7(10):1108–1114. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang J. Beclin 1 bridges autophagy, apoptosis and differentiation. Autophagy. 2008;4(7):947–948. [DOI] [PubMed] [Google Scholar]
- 92.Merriman KE, Kweh MF, Powell JL, et al. Multiple β-defensin genes are upregulated by the vitamin D pathway in cattle. J Steroid Biochem Mol Biol. 2015;154:120–129. [DOI] [PubMed] [Google Scholar]
- 93.Lu L, Li S, Zhang L, et al. Expression of β-defensins in intestines of chickens injected with vitamin D. Genetics Mol Res. 2015;14(2):3330–3337. . [DOI] [PubMed] [Google Scholar]
- 94.Nelson CD, Reinhardt TA, Lippolis JD, et al. Vitamin D signaling in the bovine immune system: a model for understanding human vitamin D requirements. Nutrients. 2012;4(3):181–196. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li YC, Bolt MJ, Cao LP, et al. Effects of vitamin D receptor inactivation on the expression of calbindins and calcium metabolism. Am J Physiol-Endocrinol Metabol. 2001;281(3):E558–64. . [DOI] [PubMed] [Google Scholar]
- 96.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423(6937):337–342. [DOI] [PubMed] [Google Scholar]
- 97.Nakamichi Y, Udagawa N, Suda T, et al. Mechanisms involved in bone resorption regulated by vitamin D. J Steroid Biochem Mol Biol. 2018;177:70–76. [DOI] [PubMed] [Google Scholar]
- 98.Cianferotti L, Demay MB. VDR‐mediated inhibition of DKK1 and SFRP2 suppresses adipogenic differentiation of murine bone marrow stromal cells. J Cell Biochem. 2007;101(1):80–88. [DOI] [PubMed] [Google Scholar]
- 99.Milat F, Ng KW. Is Wnt signalling the final common pathway leading to bone formation? Mol Cell Endocrinol. 2009;310(1–2):52–62. [DOI] [PubMed] [Google Scholar]
- 100.Haussler MR, Jurutka PW, Mizwicki M, et al. Vitamin D receptor (VDR)-mediated actions of 1α, 25 (OH) 2vitamin D3: genomic and non-genomic mechanisms. Best Practice Res Clin Endocrinol Metabol. 2011;25(4):543–559. . [DOI] [PubMed] [Google Scholar]
- 101.Sørensen OE, Follin P, Johnsen AH, et al. Human cathelicidin, hCAP-18, is processed to the antimicrobial peptide LL-37 by extracellular cleavage with proteinase 3. Blood, J Am Soci Hematol. 2001;97(12):3951–3959. [DOI] [PubMed] [Google Scholar]
- 102.Agerberth B, Charo J, Werr J, et al. The human antimicrobial and chemotactic peptides LL-37 and α-defensins are expressed by specific lymphocyte and monocyte populations. Blood, J Am Soci Hematol. 2000;96(9):3086–3093. [PubMed] [Google Scholar]
- 103.Chromek M, Slamová Z, Bergman P, et al. The antimicrobial peptide cathelicidin protects the urinary tract against invasive bacterial infection. Nature Med. 2006;12(6):636–641. . [DOI] [PubMed] [Google Scholar]
- 104.Zanetti M. The role of cathelicidins in the innate host defenses of mammals. Current Issues Mol Biol. 2005;7(2):179–196. [PubMed] [Google Scholar]
- 105.Schauber J, Dorschner RA, Coda AB, et al. Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D–dependent mechanism. J Clin Investigation. 2007;117(3):803–811. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hanel A, Carlberg C. Vitamin D and evolution: pharmacologic implications. Biochem Pharmacol. 2020;173:113595. [DOI] [PubMed] [Google Scholar]
- 107.Ponsonby AL, Pezic A, Ellis J, et al. Variation in associations between allelic variants of the vitamin D receptor gene and onset of type 1 diabetes mellitus by ambient winter ultraviolet radiation levels: a meta-regression analysis. Am J Epidemiol. 2008;168(4):358–365. . [DOI] [PubMed] [Google Scholar]
- 108.Stene LC, Joner G. Use of cod liver oil during the first year of life is associated with lower risk of childhood-onset type 1 diabetes: a large, population-based, case-control study. Altern Med Rev. 2004;9(1):99–100. [DOI] [PubMed] [Google Scholar]
- 109.Hyppönen E, Läärä E, Reunanen A, et al. Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet. 2001;358(9292):1500–1503. . [DOI] [PubMed] [Google Scholar]
- 110.Zeitz U, Weber K, Soegiarto DW, et al. Impaired insulin secretory capacity in mice lacking a functional vitamin D receptor. FASEB J. 2003;17(3):1–4. . [DOI] [PubMed] [Google Scholar]
- 111.Danescu LG, Levy S, Levy J. Vitamin D and diabetes mellitus. Endocrine. 2009;35(1):11–17. [DOI] [PubMed] [Google Scholar]
- 112.Lee JH, O’Keefe JH, Bell D, et al. Vitamin D deficiency: an important, common, and easily treatable cardiovascular risk factor? J Am College Cardiol. 2008;52(24):1949–1956. . [DOI] [PubMed] [Google Scholar]
- 113.Poole KE, Loveridge N, Barker PJ, et al. Reduced vitamin D in acute stroke. Stroke. 2006;37(1):243–245. . [DOI] [PubMed] [Google Scholar]
- 114.Melamed ML, Muntner P, Michos ED, et al. Serum 25-hydroxyvitamin D levels and the prevalence of peripheral arterial disease: results from NHANES 2001 to 2004. Arteriosclerosis, Thrombosis, Vascular Biol. 2008;28(6):1179–1185. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Audo I, Darjatmoko SR, Schlamp CL, et al. Vitamin D analogues increase p53, p21, and apoptosis in a xenograft model of human retinoblastoma. Investigative Ophthalmol Visual Sci. 2003;44(10):4192–4199. . [DOI] [PubMed] [Google Scholar]
- 116.Egan JB, Thompson PA, Vitanov MV, et al. Vitamin D receptor ligands, adenomatous polyposis coli, and the vitamin D receptor FokI polymorphism collectively modulate β‐catenin activity in colon cancer cells. Mol Carcinogen: Published in cooperation with the University of Texas MD Anderson Cancer Center. 2010;49(4):337–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bener A, Ehlayel MS, Tulic MK, et al. Vitamin D deficiency as a strong predictor of asthma in children. Int Arch Allergy Immunol. 2012;157(2):168–175. . [DOI] [PubMed] [Google Scholar]
- 118.Fakhoury HM, Kvietys PR, AlKattan W, et al. Vitamin D and intestinal homeostasis: barrier, microbiota, and immune modulation. J Steroid Siochem Mol Biol. 2020;200:105663. [DOI] [PubMed] [Google Scholar]
- 119.Zhu M, Wang T, Wang C, et al. The association between vitamin D and COPD risk, severity, and exacerbation: an updated systematic review and meta-analysis. Intl J Chronic Obstructive Pulmonary Dis. 2016;11:2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dall’Ara F, Cutolo M, Andreoli L, et al. Vitamin D and systemic lupus erythematosus: a review of immunological and clinical aspects. Clin Exp Rheumatol. 2017;36(1):153–162. [PubMed] [Google Scholar]
- 121.Bikle DD. Vitamin D and immune function: understanding common pathways. Current Osteoporosis Rep. 2009;7(2):58–63. [DOI] [PubMed] [Google Scholar]
- 122.Hansdottir S, Monick MM, Hinde SL, et al. Respiratory epithelial cells convert inactive vitamin D to its active form: potential effects on host defense. J Immunol. 2008;181(10):7090–7099. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Steinstraesser L, Tippler B, Mertens J, et al. Inhibition of early steps in the lentiviral replication cycle by cathelicidin host defense peptides. Retrovirol. 2005;2(1):1–2. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bergman P, Walter-Jallow L, Broliden K, et al. The antimicrobial peptide LL-37 inhibits HIV-1 replication. Current HIV Res. 2007;5(4):410–415. . [DOI] [PubMed] [Google Scholar]
- 125.Hansdottir S, Monick MM, Lovan N, et al. Vitamin D decreases respiratory syncytial virus induction of NF-κB–linked chemokines and cytokines in airway epithelium while maintaining the antiviral state. J Immunol. 2010;184(2):965–974. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Urashima M, Segawa T, Okazaki M, et al. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am J Clin Nutrition. 2010;91(5):1255–1260. . [DOI] [PubMed] [Google Scholar]
- 127.Sánchez de la Torre M, Torres C, Nieto G, et al. Vitamin D receptor gene haplotypes and susceptibility to HIV-1 infection in injection drug users. J Infect Dis. 2008;197(3):405–410. . [DOI] [PubMed] [Google Scholar]
- 128.Ahmed A, Siman-Tov G, Hall G, et al. Human antimicrobial peptides as therapeutics for viral infections. Viruses. 2019;11(8):704. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Campbell GR, Spector SA. Autophagy induction by vitamin D inhibits both Mycobacterium tuberculosis and human immunodeficiency virus type 1. Autophagy. 2012a;8(10):1523–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Campbell GR, Spector SA, Deretic V. Vitamin D inhibits human immunodeficiency virus type 1 and Mycobacterium tuberculosis infection in macrophages through the induction of autophagy. PLoS Pathog. 2012b;8(5):e1002689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mushegian AA. Autophagy and vitamin D. Sci Signalling. 2017;10:471. [DOI] [PubMed] [Google Scholar]
- 132.Jang W, Kim HJ, Li H, et al. 1, 25-Dihydroxyvitamin D3 attenuates rotenone-induced neurotoxicity in SH-SY5Y cells through induction of autophagy. Biochem Biophys Res Communi. 2014;451(1):142–147. . [DOI] [PubMed] [Google Scholar]
- 133.Uberti F, Lattuada D, Morsanuto V, et al. Vitamin D protects human endothelial cells from oxidative stress through the autophagic and survival pathways. J Clin Endocrinol Metabol. 2014;99(4):1367–1374. . [DOI] [PubMed] [Google Scholar]
- 134.Bilezikian JP, Bikle D, Hewison M, et al. Mechanisms in endocrinology: vitamin D and COVID-19. Eur J Endocrinol. 2020;183(5):R133–47. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Teymoori‐Rad M, Shokri F, Salimi V, et al. The interplay between vitamin D and viral infections. Rev Med Virol. 2019;29(2):e2032. . [DOI] [PubMed] [Google Scholar]
- 136.Høyer-Hansen M, Nordbrandt SP, Jäättelä M. Autophagy as a basis for the health-promoting effects of vitamin D. Trends Mol Med. 2010;16(7):295–302. [DOI] [PubMed] [Google Scholar]
- 137.Choudhury A, Mukherjee S. In silico studies on the comparative characterization of the interactions of SARS-CoV-2 spike glycoprotein with ACE-2 receptor homologs and human TLRs. J Med Virol. 2020;92(10):2105–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jakovac H. COVID-19 and hypertension: is the HSP60 culprit for the severe course and worse outcome? Am J Physiol-Heart Circulat Physiol. 2020;319(4):H793–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bergman P. The link between vitamin D and Covid‐19: distinguishing facts from fiction. J Intern Med. 2020;289(1):131–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Razdan K, Singh K, Singh D. Vitamin D levels and COVID-19 susceptibility: is there any correlation? Med Drug Discovery. 2020;7:100051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Meltzer DO, Best TJ, Zhang H, et al. Association of vitamin D status and other clinical characteristics with COVID-19 test results. JAMA Network Open. 2020Sep1;3(9):e2019722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Laird E, Rhodes J, Kenny RA. Vitamin D and inflammation: potential implications for severity of Covid-19. Ir Med J. 2020;113(5):81. [PubMed] [Google Scholar]
- 143.Bernard JJ, Gallo RL, Krutmann J. Photoimmunology: how ultraviolet radiation affects the immune system. Nature Reviews Immunol. 2019;19(11):688–701. [DOI] [PubMed] [Google Scholar]
- 144.Weir EK, Thenappan T, Bhargava M, et al. Does vitamin D deficiency increase the severity of COVID-19? Clin Med. 2020;20(4):e107. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wang Y, Zheng J, Islam MS, et al. The role of CD4+ FoxP3+ regulatory T cells in the immunopathogenesis of COVID-19: implications for treatment. Int J Biol Sci. 2021;17(6):1507–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Fang F, Chai Y, Wei H, et al. Vitamin D deficiency is associated with thyroid autoimmunity: results from an epidemiological survey in Tianjin, China. Endocrine. 2021Mar;23:1–8. [DOI] [PubMed] [Google Scholar]
- 147.Munshi R, Hussein MH, Toraih EA, et al. Vitamin D insufficiency as a potential culprit in critical COVID‐19 patients. J Med Virol. 2021;93(2):733–740. . [DOI] [PubMed] [Google Scholar]
- 148.Speeckaert MM, Delanghe JR. Association between low vitamin D and COVID-19: don’t forget the vitamin D binding protein. Aging Clin Exp Res. 2020;32(7):1207–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Grant WB, Lahore H, McDonnell SL, et al. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. 2020;12(4):988. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Biesalski HK. Vitamin D deficiency and co-morbidities in COVID-19 patients - A fatal relationship? Nfs J. 2020;20:10. [Google Scholar]
- 151.Aygun H. Vitamin D can prevent COVID-19 infection-induced multiple organ damage. Naunyn-schmiedeberg’s Arch Pharmacol. 2020;393(7):1157–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Arya A, Dwivedi VD. Synergistic effect of Vitamin D and Remdesivir can fight COVID-19. J Biomol Structure Dynamics. 2020;8:1–2. [DOI] [PubMed] [Google Scholar]
- 153.Vaduganathan M, Vardeny O, Michel T, et al. Renin–angiotensin–aldosterone system inhibitors in patients with Covid-19. New Eng J Med. 2020;382(17):1653–1659. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hanff TC, Harhay MO, Brown TS, et al. Is there an association between COVID-19 mortality and the renin-angiotensin system? A call for epidemiologic investigations. Clin Infect Dis. 2020;71(15):870–874. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Cui C, Xu P, Li G, et al. Vitamin D receptor activation regulates microglia polarization and oxidative stress in spontaneously hypertensive rats and angiotensin II-exposed microglial cells: role of renin-angiotensin system. Redox Biol. 2019;26:101295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Gombart AF, Pierre A, Maggini S. A review of micronutrients and the immune system–working in harmony to reduce the risk of infection. Nutrients. 2020;12(1):236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Fasano A, Cereda E, Barichella M, et al. COVID‐19 in Parkinson’s Disease Patients Living in Lombardy, Italy. Mov Disord. 2020;35(7):1089–1093. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Jakovac H. COVID-19: is the ACE2 just a foe? Am J Physiol-Lung Cell Mol Physiol. 2020;318(5):L1025–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kralj M, Jakovac H. Vitamin D and COVID‐19 in an immunocompromised patient with multiple comorbidities—A Case Report. Clinical Case Reports. 2021; 9(4): 2269-2275.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metabol. 2011;96(7):1911–1930. . [DOI] [PubMed] [Google Scholar]
- 161.D’Avolio A, Avataneo V, Manca A, et al. 25-Hydroxyvitamin D concentrations are lower in patients with positive PCR for SARS-CoV-2. Nutrients. 2020;12(5):1359. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Tebben PJ, Singh RJ, Kumar R. Vitamin D-mediated hypercalcemia: mechanisms, diagnosis, and treatment. Endocr Rev. 2016;37(5):521–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Theoharides TC, Conti P. Dexamethasone for COVID-19? Not so fast. J Biol Regul Homeost Agents. 2020;34(3):10–23812. [DOI] [PubMed] [Google Scholar]
- 164.Panyod S, Ho CT, Sheen LY. Dietary therapy and herbal medicine for COVID-19 prevention: a review and perspective. J Traditional Complement Med. 2020;10(4):420–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lurie N, Saville M, Hatchett R, et al. Developing Covid-19 vaccines at pandemic speed. New Eng J Med. 2020;382(21):1969–1973. . [DOI] [PubMed] [Google Scholar]
- 166.Dhok A, Butola LK, Anjankar A, et al. Role of vitamins and minerals in improving immunity during Covid-19 pandemic-A review. J Evolution Med Dental Sci. 2020;9(32):2296–2300. . [Google Scholar]
- 167.De Faria Coelho-Ravagnani C, Corgosinho FC, Sanches FL, et al. Dietary recommendations during the COVID-19 pandemic. Nutrition Rev. 2021;79(4):382–393. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Annweiler C, Hanotte B, De L’eprevier CG, et al. Vitamin D and survival in COVID-19 patients: a quasi-experimental study. J Steroid Biochem Mole Biol. 2020;204:105771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Mitchell F. Vitamin-D and COVID-19: do deficient risk a poorer outcome? Lancet Diabet Endocrinol. 2020;8(7):570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Israel A, Cicurel AA, Feldhamer I, et al. The link between vitamin D deficiency and COVID-19 in a large population. In MedRxiv. 2020. [Google Scholar]
- 171.Jolliffe DA, Camargo CA Jr, Sluyter JD, et al. Vitamin D supplementation to prevent acute respiratory infections: a systematic review and meta-analysis of aggregate data from randomised controlled trials. Lancet Diabetes Endocrinol. 2021Mar30;9(5):276–292. [DOI] [PubMed] [Google Scholar]
- 172.Brighenti S, Bergman P, Martineau AR. Vitamin D and tuberculosis: where next? J Intern Med. 2018;284(2):145–162. [DOI] [PubMed] [Google Scholar]
- 173.Oktaria V, Danchin M, Triasih R, et al. The incidence of acute respiratory infection in Indonesian infants and association with vitamin D deficiency. PloS One. 2021;16(3):e0248722. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Jat KR, Goel N, Gupta N, et al. Efficacy of vitamin D supplementation in asthmatic children with vitamin D deficiency: a randomized controlled trial (ESDAC trial). Pediatr Allergy Immunol. 2021;32(3):479–488. . [DOI] [PubMed] [Google Scholar]
- 175. IGrant WB, Baggerly CA, Lahore H. Reply:“Vitamin D supplementation in Influenza and COVID-19 Infections. Comment on: evidence that vitamin D supplementation could reduce risk of Influenza and COVID-19 infections and deaths nutrients 2020, 12 (4), 988.” Nutrients. 2020;12(6):1620. doi: 10.3390/nu12061620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Mohan M, Cherian JJ, Sharma A. Exploring links between vitamin D deficiency and COVID-19. PLoS Pathog. 2020;16(9):e1008874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Jakovac H. COVID-19 and vitamin D—Is there a link and an opportunity for intervention? Am J Physiol Endocrinol Metab. 2020;318(5):E589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ilie PC, Stefanescu S, Smith L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin Exp Res. 2020Jul;32(7):1195–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Brenner H. Vitamin D supplementation to prevent COVID-19 infections and deaths—accumulating evidence from epidemiological and intervention studies calls for immediate action. Nutrients. 2021Feb;13(2):411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Huang J, Qi Y, Wang A, et al. Porcine β-defensin 2 inhibits proliferation of pseudorabies virus in vitro and in transgenic mice. Virol J. 2020;17(1):1–7. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Peng L, Du W, Balhuizen MD, et al. Antiviral activity of chicken cathelicidin B1 against influenza A virus. Front Microbiol. 2020;11:426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Shahid Nadeem M, Ali A, Al-Ghamdi MA, et al. COVID-19: prospective Challenges and Potential Vaccines. Altern Ther Health Med. 2020;26(S2):72–78. [PubMed] [Google Scholar]; • This paper summarises the Challenges and potential Vaccines available for the treatment of COVID-19 .
- 183.Saad Alharbi K, Al-Abbasi FA, Prasad Agrawal G, et al. Impact of COVID-19 on Nephrology Patients: a Mechanistic Outlook for Pathogenesis of Acute Kidney Injury. Altern Ther Health Med. 2020;26(S2):66–71. [PubMed] [Google Scholar]; •• This paper gives detailed mechanistic idea about impact of COVID-19 on kidney
- 184.Singh Y, Gupta G, Kazmi I, et al. SARS CoV-2 aggravates cellular metabolism mediated complications in COVID-19 infection. Dermatol Ther. 2020;33(6):e13871. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This article gives detailed idea regarding SARS CoV-2 cellular metabolism mediated complications in COVID-19 infection
- 185.Zhang Y, Sun H, Pei R, et al. The SARS-CoV-2 protein ORF3a inhibits fusion of autophagosomes with lysosomes. Cell Discov. 2021;7(1):1–2. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.da Silva Pereira GJ, Leão AHFF, Erustes AG, et al. Pharmacological modulators of autophagy as a potential strategy for the treatment of COVID-19. Intl J Mol Sci. 2021;22(8):4067. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Wu S, Sun J. Vitamin D, vitamin D receptor, and macroautophagy in inflammation and infection. Discovery Med. 2011;11:325. [PMC free article] [PubMed] [Google Scholar]


