Abstract
Lyme borreliosis, an infection caused by the tick-borne spirochete Borrelia burgdorferi, is a major health problem for populations in areas of endemicity in the Northern Hemisphere. In the present study we assessed the density of ticks and the prevalence of B. burgdorferi sensu lato among ticks in popular urban recreational areas of Helsinki, Finland. Altogether 1,688 Ixodes ricinus ticks were collected from five areas located within 5 km of the downtown section of Helsinki, and 726 of them (303 nymphs, 189 females, and 234 males) were randomly chosen for laboratory analysis. The midguts of the ticks were divided into three pieces, one for dark-field microscopy, one for cultivation in BSK-II medium, and one for PCR analysis. Ticks were found in all the study areas; their densities varied from 1 to 36 per 100 m along which a cloth was dragged. The rate of tick infection with B. burgdorferi sensu lato varied from 19 to 55%, with the average being 32%. Borellia afzelii was the most predominant genospecies in all the areas, and no B. burgdorferi sensu stricto isolates were detected. Only two ticks were concurrently infected with both B. afzelii and Borrelia garinii. Dark-field microscopy gave more positive results for B. burgdorferi than did cultivation or PCR analysis. However, the agreement between all three methods was fairly good. We conclude that Lyme borreliosis can be contracted even in urban environments not populated with large mammals like deer or elk. The disease should be taken into account in the differential diagnosis of certain symptoms of patients from these areas, and the use of measures to improve the awareness of the general population and health care officials of the risk of contracting the disease is warranted.
Lyme borreliosis is currently the most important vector-borne disease in the developed countries of the Northern Hemisphere. In Europe, the disease is most common in central and eastern territories, where annual incidences of over 300 per 100,000 people have been reported (36). Since 1985, different clinical manifestations of Lyme borreliosis have been reported in Finland, and the number of patients with late Lyme borreliosis reported annually has varied between 300 and 450 (22). Epidemiological studies conducted in the southern archipelago of Finland show an annual incidence of about 200 new cases of Lyme borreliosis per 100,000 people (24).
The disease is caused by a spirochete, Borrelia burgdorferi. B. burgdorferi sensu stricto is the dominating genospecies in North America, whereas several different genospecies of B. burgdorferi sensu lato can be found in Europe (2, 10, 34). In Finland, Borrelia garinii and Borrelia afzelii appear to be the most prevalent species (14).
Ticks of the Ixodes ricinus group are the most important vectors of B. burgdorferi. These ticks have been found in a wide variety of European habitats. In Finland they occur from the south coast, especially in the southwestern archipelago, up to the border of Lapland in the north (23). Ubiquitous small rodents, Clethrionomys glareolus and Apodemus flavicollis, are suspected of being the most important reservoir animals for B. burgdorferi, while large or medium-sized mammals are considered necessary for maintenance of the tick population (12, 13). Most studies of the prevalence of infected ticks have been conducted in rural or suburban settings known to harbor both rodents and larger mammals. In Europe the reported mean rates of unfed I. ricinus ticks infected with B. burgdorferi vary from 0 to 11% (mean, 1.9%) for larvae, from 2 to 43% (mean, 10.8%) for nymphs, and from 3 to 58% (mean, 17.4%) for adults (11). The risk of acquiring borrelia infection in urban environments has generally been neglected, and studies on the rates of infection of ticks in heavily populated urban or suburban areas are limited in number (19, 20, 25–27). However, suitable habitats for ticks are known to exist in cities (6, 9, 15–17, 33).
The main objectives of this study were to estimate the density of I. ricinus ticks in popular recreational areas of Helsinki, Finland, and to estimate the prevalence of borreliae in these ticks. In addition, the occurrence of different B. burgdorferi genospecies in the areas was studied, and three different methods of detecting borreliae in ticks were evaluated.
MATERIALS AND METHODS
Study areas.
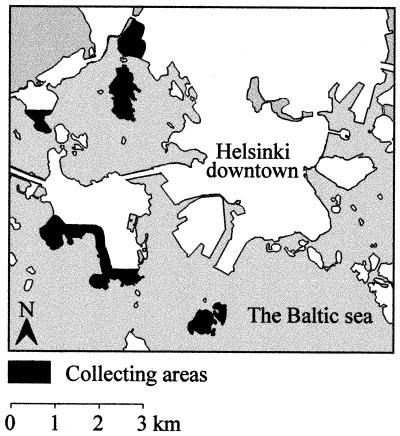
Eighteen of the most popular recreational areas in and around the city of Helsinki were screened in a pilot study during the summer of 1995 by the same sampling method used in the present study. Five areas representing the highest tick densities (Table 1; Fig. 1) were chosen for study during the summer of 1996. All the areas were located within a radius of 5 km from the center of Helsinki, and the estimated number of annual visitors to the areas is 1.4 million (5).
TABLE 1.
Annual number of visitors and tick densities of the five recreational areas of Helsinki studied
| Area | No. of visitors/yra | Total length of drag (m)b | No. of ticks collected | Tick density (no. of ticks/100 m) |
|---|---|---|---|---|
| Meilahti | 700,000 | 1,700 | 119 | 7.0 |
| Seurasaari | 450,000 | 2,550 | 805 | 31.5 |
| Lehtisaari | 10,000 | 1,650 | 590 | 35.7 |
| Lauttasaari | 208,000 | 1,700 | 136 | 8.0 |
| Pihlajasaari | 50,000 | 2,750 | 38 | 1.3 |
| Total | 1,418,000 | 10,350 | 1,688 | 16.3 |
Numbers obtained from the Year Report of 1997 provided by the Sports Department of Helsinki (5).
Ticks were caught by dragging a 1-m2 cotton cloth through the vegetation.
FIG. 1.
The five areas in Helsinki studied, from north to south: Meilahti, Seurasaari, Lehtisaari, Lauttasaari, and Pihlajasaari.
Estimation of tick density.
Ticks were collected during 20 visits to the areas between 17 May and 9 August 1996. They were caught by dragging a 1-m2 cotton cloth through the vegetation. The number of ticks attached to the cloth was counted every 5 m. Dragging was performed along randomly chosen lines with a length of 100 to 200 m per each sampling day and area. The counts of larvae, nymphs, females, and males were recorded, as was the length of the drag. Tick density was expressed as the number of ticks caught per a 100-m drag. Ticks were placed individually in sterile 1.5-ml Eppendorf tubes containing a moist piece of paper and were stored at 4°C until they were prepared for further study.
Tick preparation.
Altogether, 1,688 I. ricinus ticks were collected, and 726 of them (303 nymphs, 189 females, and 234 males) were randomly chosen for the laboratory analysis. No external disinfectants were used during the preparation of the ticks. The ticks were stored for a mean of 25 days (range, 7 to 54 days), and 154 of the ticks died during storage. The midguts of the adults and nymphs were removed under a stereomicroscope and were placed in a drop of BSK-II medium with small sterile forceps, a disposable 28-gauge needle which was used as a scalpel, and sterile insect needles. Each midgut was divided into three equal parts. One portion was examined immediately under a dark-field (DF) microscope, another portion was inoculated in BSK-II medium, and the third was reserved for DNA purification. Because the viability of ticks was suspected to affect the results of all the methods, the vitality of each tick was recorded for further statistical analyses.
DF microscopy.
One of the three parts of the tick midgut was placed on a microscope glass in a drop of BSK-II medium and was covered with a cover glass. The number of spirochetes in the midgut sample was estimated by DF microscopy (Laborlux D; Leitz, Nürnberg, Germany) by examining 100 fields at a magnification of ×400. Typical movement, morphology, and size were used as the identification criteria for the borreliae.
Cultivation.
The samples were inoculated into tubes containing BSK-II medium supplemented with rifampin (100 mg/ml) and phosphomycin (50 mg/ml) and were incubated at 30°C for 8 weeks or until growth was detected. The growth medium was examined by DF microscopy every other week. If growth appeared, the cultures were passaged into new tubes containing BSK-II medium without antibiotics.
DNA extraction.
DNA was extracted from the processed ticks with the InstaGene DNA extraction matrix (Bio-Rad Laboratories, Hercules, Calif.). A total of 200 μl of the matrix was added to the sample, and the mixture was incubated at 56°C for 30 min. The specimen was then mixed for 30 s, incubated at 100°C for 8 min, mixed briefly, and centrifuged at 13,000 rpm for 3 min (Biofuge 13; Heraeus Instruments GmbH, Haman, Germany). Five microliters of the supernatant was used in the PCR analysis.
PCR amplification.
The nested PCR was carried out by the method described by Schmidt et al. (29), which is based on the flagellin gene. The 50-μl reaction mixture contained 50 mM KCl, 1.5 mM MgCl2, 10 mM Tris-HCl (pH 8.8), 0.1% Triton X-100, each deoxynucleoside triphosphate (Pharmacia Biotech, Espoo, Finland) at a concentration of 200 mM, 20 pmol of the outer or inner primers, and 1 U of DynaZyme DNA polymerase (Finnzymes, Espoo, Finland). In the first PCR, outer primers BBSCH31 and BBSCH42 were used. It consisted of an initial denaturation at 95°C for 1 min, denaturation at 94°C for 30 s, annealing at 55°C for 30 s, and extension at 72°C for 30 s. The procedure was repeated 25 times, and the final extension was done at 72°C for 10 min. Five microliters of the amplicon from the first PCR was used as the template in the second PCR, for which primers FL59 and FL7 were used. The second PCR analysis was carried out for 35 cycles by the same procedure used for the first PCR, except that the annealing temperature was raised to 58°C. The final 277-bp PCR products were visualized by 1.5% agarose gel electrophoresis.
Identification of the positive cultures by PCR.
The spirochetes grown from the ticks were identified to the species level by the PCR method developed by Marconi and Garon (18) and based on amplification of the 16S rRNA gene. DNA was extracted from the cultures by the InstaGene procedure described above. The samples were amplified with four sets of primers. The primer sets were specific for B. burgdorferi sensu lato (LD primers), B. burgdorferi sensu stricto (BB primers), B. afzelii (VS461 primers), and B. garinii (BG primers). The PCR mixtures consisted of the components described above. For the LD primers, 40 cycles of denaturation at 94°C for 1 min, annealing at 47°C for 30 s, and extension at 72°C for 1.5 min were carried out. For the other primers, the PCR procedure was the same, except that the annealing was performed at 42°C. The resulting PCR products were visualized by agarose gel electrophoresis.
Confirmation of double infections by sequencing.
The samples that gave ambiguous results in PCRs specific for B. afzelii and B. garinii were further analyzed by PCR-based sequencing of the flagellin gene. A 277-bp product was obtained by using primers FL7 (biotinylated) and FL59. The biotinylated PCR products were rendered single stranded with streptavidin-coated Dynabeads according to the instructions of the manufacturer (Dynabeads M-280 with streptavidin; Dynal AS, Oslo, Norway). Manual sequencing was performed by Sanger’s dideoxynucleotide chain termination method and with Sequenase 2.0 (United States Biochemical Corp., Cleveland, Ohio) as described previously (31). The sequences that were obtained were compared with the flagellin gene sequences of the type strains B. afzelii Bo23 and B. garinii 387.
Statistical methods.
The student edition of Statistics, version 4.0 (1992; Analytical Software, Torrance, Calif.) was used for all the statistical analyses. A chi-square analysis was used to test the association of borrelia prevalence and subspecies distribution with the sampling area, developmental stage, and viability of the ticks. After the preliminary association tests, log-linear modeling was applied to control the interactions of sex, area, and borrelia infection, as well as the possible confounding effect of the viability of the ticks.
The independence of the occurrence of B. garinii and B. afzelii in ticks was tested by Fisher’s exact test. Furthermore, the expected number of mixed infections was calculated under the null hypothesis that both infections can persist independently of each other in the reservoir animals.
Tick densities were assumed to follow a Poisson distribution, and pairwise comparisons between the areas were made under this assumption.
The agreement between different methods of detecting borreliae (DF microscopy, culture, and PCR analysis) was expressed as kappa statistics, and the disagreement was evaluated by McNemar’s chi-square test. The sensitivity and predictive values of each method were estimated from the results of the other two methods in parallel, which were the “gold standard.”
The prevalence at which B. burgdorferi sensu stricto could occur in the study area and still have escaped our sampling was estimated by applying the formula described by Cannon and Roe (4).
RESULTS
Tick densities in survey areas.
Altogether, 1,688 I. ricinus ticks were collected from the study sites. The tick densities varied from 1 to 36 ticks/100 m (Table 1). Two of the areas (Seurasaari and Lehtisaari) had significantly higher densities than the others; Pihlajasaari had the lowest density. Nymphs and larvae were more abundant in Seurasaari and Lehtisaari, which partly explains the density differences.
Prevalence of ticks infected with B. burgdorferi.
Of the 1,688 ticks, 726 (303 nymphs, 189 females, and 234 males) were randomly chosen for the laboratory analysis. The infection rate for the ticks varied from 19 to 55% (Table 2); the overall mean was 32% (234 of 726 ticks). The infection rate was significantly associated with area (P < 0.001) but not with the developmental stage or viability of the ticks (P = 0.06 and P = 0.19, respectively). The log-linear analysis supported the results of the simple chi-square tests.
TABLE 2.
Distribution of B. burgdorferi genospecies isolated from the I. ricinus ticks collected from five recreational areas of Helsinki
| Study area | No. of ticks studied | No. (%) of positive ticksa | No. (%) of typeable isolatesb | No. (%) of infections with the following:
|
|||
|---|---|---|---|---|---|---|---|
| B. afzelii | B. garinii | Mixed | B. burgdorferi sensu lato | ||||
| Meilahti | 103 | 20 (19) | 14 (70) | 7 (50) | 6 (43) | 1 (7) | |
| Seurasaari | 238 | 74 (31) | 43 (58) | 27 (63) | 14 (32) | 2 (5) | |
| Lehtisaari | 247 | 78 (32) | 44 (56) | 35 (80) | 7 (16) | 2 (4) | |
| Lauttasaari | 100 | 41 (41) | 26 (63) | 21 (81) | 4 (15) | 1 (4) | |
| Pihlajasaari | 38 | 21 (55) | 15 (71) | 9 (60) | 5 (33) | 1 (7) | |
| Total | 726 | 234 (32) | 142 (61) | 99 (70) | 36 (25) | 2 (1) | 5 (4) |
Numbers of ticks yielding a positive result by either cultivation, DF microscopy, or PCR analysis.
B. burgdorferi isolates grown in BSK-II medium were identified by genospecies-specific PCR methods or PCR-based sequencing of the flagellin gene.
The final model was STAGE + VIABILITY · AREA + AREA · RESULT. The goodness of fit for this model was reduced from a P value of 0.48 to a P value of <0.01 if any term was removed. Furthermore, the addition to or change of any second-order term in the model did not increase the P value over a maximum of 0.50.
Therefore, when the uneven distribution and possible confounding effects of developmental stage (sex) and tick viability are taken into account, the infection rates differed significantly between the areas. Neither developmental stage nor viability affected the infection status of the tick.
Simple chi-square tests indicated that genospecies was independent of both sampling area and developmental stage (P = 0.14 and P = 0.34, respectively). However, because the sampling model resulted in uneven numbers of nymphs, males, and females from each sampling area, the possible interactions between these three factors could not be resolved by two-dimensional chi-square tests. In order to control and evaluate these interactions, we used log-linear modeling. The best-fitting model (P = 0.40) was STAGE · AREA + GENOSPECIES · AREA.
This model indicates that although B. afzelii is predominant in all areas studied, it is significantly more prevalent in some areas than in others. The interaction term of developmental stage and genospecies is not needed. On the contrary, it slightly reduces the goodness of fit if it is added to the model. In other words, simple chi-square analysis could not detect the association between genospecies and area because of the confounding effect of developmental stage.
B. garinii and B. afzelii were detected concurrently in only two adult (one female and one male) ticks. If these two genospecies were assumed to exist independently in the reservoir population, the expected number of mixed infections would be 5.1. If the number of typeable strains (n = 142) is chosen as a denominator in the calculations, the expected number of mixed infections increases to 25. The observed number is significantly smaller than the expected number in either case when assessed by Fisher’s exact test (P < 0.001).
At least one tick infected with B. burgdorferi sensu stricto would have been detected with a probability of 99% if this genospecies had been present in the study areas at a minimum prevalence of 0.6% (4). It can be concluded at a reasonable level of confidence that B. burgdorferi sensu stricto was missing from the study areas.
Comparison between methods used to detect borreliae.
From the agreement (kappa statistics) between the results of DF microscopy, cultivation, and PCR analysis (Table 3), the kappa values for all three comparisons indicate fairly good agreement. The kappa values for comparisons of cultivation and DF microscopy, cultivation and PCR, and DF microscopy and PCR were 0.59, 0.53, and 0.57, respectively (P values were <0.001, 0.380, and <0.001, respectively, by McNemar’s chi-square test). The discrepancies between culture and PCR methods were numerous (N = 105), but they were symmetrical and therefore not statistically significant (Table 3). DF microscopy gave significantly more positive results than either culture or PCR analysis (P < 0.001 and P < 0.001, respectively).
TABLE 3.
Comparison of cultivation (BSK-II), DF microscopy, and PCR analysis in the detection of B. burgdorferi sensu lato in I. ricinus ticks collected from five recreational areas of Helsinki
| Result by the following methoda
|
Total no. of ticks | ||
|---|---|---|---|
| Cultivation | DF | PCR | |
| − | − | + | 12 |
| − | + | − | 42 |
| + | − | − | 17 |
| − | + | + | 36 |
| + | + | − | 40 |
| + | − | + | 12 |
| + | + | + | 75 |
| 144b | 193b | 135b | 234c |
+, positive finding; −, negative finding.
Number of ticks positive by each method.
Number of ticks positive by any method.
The ticks that showed a positive result by DF microscopy but a negative result by either PCR analysis or culture were found to harbor only a few spirochetes by DF microscopy. Ticks alive at the time of preparation were more often positive than the dead ticks when they were tested by culture (21 versus 14%) or DF microscopy (28 versus 21%), although the difference was not statistically significant at the 95% confidence level (P = 0.051 and P = 0.066, respectively). The PCR results were not affected by the viability of the ticks (P = 0.93).
DISCUSSION
This study shows that a considerable risk of contracting a borrelia infection can be present in an urban environment, and therefore, a large human population can be at risk. Both the density of vector ticks and the prevalence of borreliae in these vectors were higher than expected in light of previously published studies (1, 3, 9, 21, 27, 30, 33). These observations indicate a need to take Lyme borreliosis into account as one possible differential diagnosis even for patients without a history of a visit to rural areas of endemicity. In addition, the predominance of B. afzelii and absence of B. burgdorferi sensu stricto may affect the clinical picture of Lyme borreliosis in this area.
The presence of large mammals has been considered a prerequisite for Lyme borreliosis in an area of endemicity (7, 8, 32). Our observations are in dispute with these presumptions and confirm the suspicions raised by previously published results (13). None of the study areas was known to be populated by deer or elk. The most numerous mammals are small rodents (Apodemus flavicollis, Apodemus sylvaticus, and Peromyscus leucopus) and insectivores (shrews and hedgehogs), with the largest ones being the hare (Lepus timidus). The areas where ticks were most abundant were also the most capable of supporting a permanent hare population.
Official statistics of the National Public Health Institute of Finland show that the incidence of late manifestations of Lyme borreliosis is twice as high in the district of Helsinki as in the surrounding, more rural areas (13 versus 6.6 cases per 100,000 inhabitants, respectively) (22). These incidence figures may be biased. However, if a bias existed, it should affect Helsinki and its surroundings in a similar manner and should therefore not explain the observed difference. This difference suggests that our findings also have relevance for the occurrence of Lyme borreliosis in the area.
The observed distribution of genospecies is in agreement with the results of earlier studies performed in Finland and Russia (14, 28). B. burgdorferi sensu stricto has been detected only in the southwestern parts of Finland, and the prevalence of B. garinii seems to increase toward the eastern border. The untypeable spirochetes could possibly represent new genospecies, but further studies are needed to confirm their identities.
If all reservoir animals were equally and nonexclusively favorable hosts for both B. afzelii and B. garinii, the expected number of mixed infections would be significantly higher than the number observed in this study. The low number of concurrent infections suggests that these two genospecies favor two distinct reservoir animal populations. Because these genospecies did, however, coexist in two adult ticks, significant competition between them in culture media or tick tissues is unlikely. The total lack of B. burgdorferi sensu stricto may indicate that there are no suitable reservoirs for this genospecies in the areas studied.
DF microscopy, culture, and PCR analysis have all been used in studies on the borrelia infestation rates of ticks (35). However, extensive comparisons between these methods have not been available. According to the present study, DF microscopy seems to be the method of choice in surveillance studies, with culture and PCR analysis being complementary in nature.
We conclude that dense populations of I. ricinus ticks heavily infested with B. burgdorferi can exist even in urban environments not populated with large mammals like deer or elk. Inhabitants and health care officials of cities should be made more aware of the risk of contracting Lyme borreliosis in parks or other recreational areas harboring infected ticks.
ACKNOWLEDGMENTS
We thank Marja-Leena Helin, Ulla Toivonen, Leena Palmunen, and Risto Holma for excellent technical assistance. We also acknowledge funding for this work by the Clinical Research Institute of the University Central Hospital of Helsinki, the Orion Research Foundation, and the Academy of Finland.
The language of the manuscript was revised by Georgianna Oja.
REFERENCES
- 1.Alpert B, Esin J, Sivak S L, Wormser G P. Incidence and prevalence of Lyme disease in a suburban Westchester County community. N Y State J Med. 1992;92:5–8. [PubMed] [Google Scholar]
- 2.Baranton G, Postic D, Saint Girons I, Boerlin P, Piffaretti J C, Assous M, Grimont P A. Delineation of Borrelia burgdorferi sensu stricto, Borrelia garinii sp. nov., and group VS461 associated with Lyme borreliosis. Int J Syst Bacteriol. 1992;42:378–383. doi: 10.1099/00207713-42-3-378. [DOI] [PubMed] [Google Scholar]
- 3.Bauch R J. Faunistic-ecological studies of Ixodes ricinus in the urban area of Leipzig. Appl Parasitol. 1993;34:203–214. [PubMed] [Google Scholar]
- 4.Cannon R M, Roe R T. Livestock disease surveys: field manual for veterinarians. Canberra: Australian Bureau of Animal Health; 1982. [Google Scholar]
- 5.City of Helsinki. Year report. Helsinki, Finland: Sports Department, City of Helsinki; 1997. [Google Scholar]
- 6.Daniels T J, Falco R C, Schwartz I, Varde S, Robbins R G. Deer ticks (Ixodes scapularis) and the agents of Lyme disease and human granulocytic ehrlichiosis in a New York City park. Emerg Infect Dis. 1997;3:353–355. doi: 10.3201/eid0303.970312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daniels T J, Fish D, Schwartz I. Reduced abundance of Ixodes scapularis (Acari: Ixodidae) and Lyme disease risk by deer exclusion. J Med Entomol. 1993;30:1043–1049. doi: 10.1093/jmedent/30.6.1043. [DOI] [PubMed] [Google Scholar]
- 8.Gray J S, Kahl O, Janetzki C, Stein J. Studies on the ecology of Lyme disease in a deer forest in County Galway, Ireland. J Med Entomol. 1992;29:915–920. doi: 10.1093/jmedent/29.6.915. [DOI] [PubMed] [Google Scholar]
- 9.Guy E C, Farquhar R G. Borrelia burgdorferi in urban parks. Lancet. 1991;338:253. doi: 10.1016/0140-6736(91)90392-3. [DOI] [PubMed] [Google Scholar]
- 10.Hubalek Z, Halouzka J. Distribution of Borrelia burgdorferi sensu lato genomic groups in Europe, a review. Eur J Epidemiol. 1997;13:951–957. doi: 10.1023/a:1007426304900. [DOI] [PubMed] [Google Scholar]
- 11.Hubalek Z, Halouzka J. Prevalence rates of Borrelia burgdorferi sensu lato in host seeking Ixodes ricinus ticks in Europe. Parasitol Res. 1998;84:167–172. doi: 10.1007/s004360050378. [DOI] [PubMed] [Google Scholar]
- 12.Jaenson T G, Talleklint L. Incompetence of roe deer as reservoirs of the Lyme borreliosis spirochete. J Med Entomol. 1992;29:813–817. doi: 10.1093/jmedent/29.5.813. [DOI] [PubMed] [Google Scholar]
- 13.Jaenson T G, Talleklint L. Lyme borreliosis spirochetes in Ixodes ricinus (Acari:Ixodidae) and the varying hare on isolated islands in the Baltic Sea. J Med Entomol. 1996;33:339–343. doi: 10.1093/jmedent/33.3.339. [DOI] [PubMed] [Google Scholar]
- 14.Junttila J, Tanskanen R, Tuomi J. Prevalence of Borrelia burgdorferi in selected tick populations in Finland. Scand J Infect Dis. 1994;26:349–355. doi: 10.3109/00365549409011805. [DOI] [PubMed] [Google Scholar]
- 15.Kahl O, Schmidt K, Schonberg A, Laukamm Josten U, Knulle W, Bienzle U. Prevalence of Borrelia burgdorferi in Ixodes ricinus ticks in Berlin (West) Zentbl Bakteriol Parasitenkd Infektionskr Hyg Abt 1 Orig Reihe A. 1989;270:434–440. doi: 10.1016/s0176-6724(89)80013-6. [DOI] [PubMed] [Google Scholar]
- 16.Lane R S. Risk of human exposure to vector ticks (Acari: Ixodidae) in a heavily used recreational area in northern California. Am J Trop Med Hyg. 1996;55:165–173. [PubMed] [Google Scholar]
- 17.Magnarelli L A, Denicola A, Stafford III K C, Anderson J F. Borrelia burgdorferi in an urban environment: white-tailed deer with infected ticks and antibodies. J Clin Microbiol. 1995;33:541–544. doi: 10.1128/jcm.33.3.541-544.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marconi R T, Garon C F. Development of polymerase chain reaction primer sets for diagnosis of Lyme disease and for species-specific identification of Lyme disease isolates by 16S rRNA signature nucleotide analysis. J Clin Microbiol. 1992;30:2830–2834. doi: 10.1128/jcm.30.11.2830-2834.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matuschka F R, Endepols S, Richter D, Ohlenbusch A, Eiffert H, Spielman A. Risk of urban Lyme disease enhanced by the presence of rats. J Infect Dis. 1996;174:1108–1111. doi: 10.1093/infdis/174.5.1108. [DOI] [PubMed] [Google Scholar]
- 20.Matuschka F R, Endepols S, Richter D, Spielman A. Competence of urban rats as reservoir hosts for Lyme disease spirochetes. J Med Entomol. 1997;34:489–493. doi: 10.1093/jmedent/34.4.489. [DOI] [PubMed] [Google Scholar]
- 21.Moteiunas L I, Shadzhene A R, Tumosene S V, Kalinin M I, Dauetas S V, Regalene G K. Lyme borreliosis in Lithuania: its prevalence, clinical picture and treatment efficacy. Zh Mikrobiol Epidemiol Immunobiol. 1990;7:19–23. [PubMed] [Google Scholar]
- 22.National Public Health Institute of Finland. Infectious diseases in Finland in 1997. Helsinki: National Public Health Institute of Finland; 1997. [Google Scholar]
- 23.Öhman C. The geographical and topographical distribution of Ixodes ricinus in Finland. Acta Soc Fauna Flora Fenn. 1961;76:1–37. [Google Scholar]
- 24.Oksi, J. Personal communication.
- 25.Oteo J A, Martinez de Artola V, Casas J, Lozano A, Fernandez Calvo J L, Grandival R. Epidemiology and prevalence of seropositivity against Borrelia burgdorferi antigen in La Rioja, Spain. Rev Epidemiol Sante Publique. 1992;40:85–92. [PubMed] [Google Scholar]
- 26.Peavey C A, Lane R S, Kleinjan J E. Role of small mammals in the ecology of Borrelia burgdorferi in a peri-urban park in north coastal California. Exp Appl Acarol. 1997;21:569–584. doi: 10.1023/a:1018448416618. [DOI] [PubMed] [Google Scholar]
- 27.Rees D H, Axford J S. Evidence for Lyme disease in urban park workers: a potential new health hazard for city inhabitants. Br J Rheumatol. 1994;33:123–128. doi: 10.1093/rheumatology/33.2.123. [DOI] [PubMed] [Google Scholar]
- 28.Sato Y, Miyamoto K, Iwaki A, Masuzawa T, Yanagihara Y, Korenberg E I, Gorelova N B, Volkov V I, Ivanov L I, Liberova R N. Prevalence of Lyme disease spirochetes in Ixodes persulcatus and wild rodents in far eastern Russia. Appl Environ Microbiol. 1996;62:3887–3889. doi: 10.1128/aem.62.10.3887-3889.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmidt B, Muellegger R R, Stockenhuber C, Soyer H P, Hoedl S, Luger A, Kerl H. Detection of Borrelia burgdorferi-specific DNA in urine specimens from patients with erythema migrans before and after antibiotic therapy. J Clin Microbiol. 1996;34:1359–1363. doi: 10.1128/jcm.34.6.1359-1363.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwartz B S, Hofmeister E, Glass G E, Arthur R R, Childs J E, Cranfield M R. Lyme borreliosis in an inner-city park in Baltimore. Am J Public Health. 1991;81:803–804. doi: 10.2105/ajph.81.6.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soini H, Böttger E C, Viljanen M K. Identification of mycobacteria by PCR-based sequence determination of the 32-kilodalton protein gene. J Clin Microbiol. 1994;32:2944–2947. doi: 10.1128/jcm.32.12.2944-2947.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stafford K C. Reduced abundance of Ixodes scapularis (Acari: Ixodidae) with exclusion of deer by electric fencing. J Med Entomol. 1993;30:986–996. doi: 10.1093/jmedent/30.6.986. [DOI] [PubMed] [Google Scholar]
- 33.Steere A C. Lyme disease: a growing threat to urban populations. Proc Natl Acad Sci USA. 1994;91:2378–2383. doi: 10.1073/pnas.91.7.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilske B, Preac Mursic V, Schierz G, Busch K V. Immunochemical and immunological analysis of European Borrelia burgdorferi strains. Zentbl Bakteriol Mikrobiol Hyg A. 1986;263:92–102. doi: 10.1016/s0176-6724(86)80108-0. [DOI] [PubMed] [Google Scholar]
- 35.Wittenbrink M M, Thiele D, Krauss H. Comparison of dark-field microscopy, culture and polymerase chain reaction (PCR) for detection of Borrelia burgdorferi in field-collected Ixodes ricinus ticks. Zentbl Bakteriol Mikrobiol Hyg A. 1994;281:183–191. doi: 10.1016/s0934-8840(11)80568-2. [DOI] [PubMed] [Google Scholar]
- 36.World Health Organization. WHO Workshop on Lyme Borreliosis Diagnosis and Surveillance. Warsaw, Poland: World Health Organization; 1995. Country reports. [Google Scholar]