Abstract
Objectives:
Proprotein convertase subtilisin/kexin type 9 is a central regulator of lipid metabolism and has been implicated in regulating the host response to sepsis. Proprotein convertase subtilisin/kexin type 9 loss-of-function is associated with improved sepsis outcomes in the adult host through increased hepatic bacterial clearance. Thus, there is interest in leveraging proprotein convertase subtilisin/kexin type 9 inhibitors as a therapeutic strategy in adults with sepsis. We sought to validate this association in children with septic shock and in a juvenile murine model of sepsis.
Design:
Prospectively enrolled cohort of children with septic shock; experimental mice.
Setting:
Seventeen participating institutions; research laboratory.
Patients and Subjects:
Five-hundred twenty-two children with septic shock; juvenile (14 d old) and adult (10–14 wk) mice with constitutive proprotein convertase subtilisin/kexin type 9 null and wildtype control mice (C57BL/6).
Interventions:
Proprotein convertase subtilisin/kexin type 9 single-nucleotide polymorphisms, serum proprotein convertase subtilisin/kexin type 9, and lipid profiles in patients. Cecal slurry murine model of sepsis; survival studies in juvenile and adult mice, assessment of lipoprotein fractions, bacterial burden, and inflammation in juvenile mice.
Measurements and Main Results:
PCSK9 loss-of-function genetic variants were independently associated with increased odds of complicated course and mortality in children with septic shock. PCSK9, low-density lipoprotein, and high-density lipoprotein concentrations were lower among patients with complicated course relative to those without. PCSK9 concentrations negatively correlated with proinflammatory cytokine interleukin-8. Proprotein convertase subtilisin/kexin type 9 loss-of-function decreased survival in juvenile mice, but increased survival in adult mice with sepsis. PCSK9 loss-of-function resulted in low lipoproteins and decreased hepatic bacterial burden in juvenile mice.
Conclusions:
In contrast to the adult host, proprotein convertase subtilisin/kexin type 9 loss-of-function is detrimental to the juvenile host with septic shock. PCSK9 loss-of-function, in the context of low lipoproteins, may result in reduced hepatic bacterial clearance in the juvenile host with septic shock. Our data indicate that children should be excluded in sepsis clinical trials involving proprotein convertase subtilisin/kexin type 9 inhibitors.
Keywords: experimental animal model, inflammation, lipoproteins, pediatric septic shock, proprotein convertase subtilisin/kexin type 9, receptors, low-density lipoprotein
Pediatric sepsis is a leading cause of infant and child morbidity and mortality (1). Currently, therapeutic strategies for pediatric sepsis are limited. Further, interventions that have shown promise in adults may not be efficacious in children, and on the contrary, may even be harmful (2). A failure to account for sepsis heterogeneity and to consider the influence of developmental age on the host response to sepsis are likely factors that have contributed to our inability to develop therapies specific to children.
Proprotein convertase subtilisin/kexin type 9 (PCSK9) is involved in clearance of endogenous lipid through its effect on the low-density lipoprotein receptor (LDLR) in hepatocytes. PCSK9 serves as a molecular chaperone that binds the LDLR and promotes its lysosomal degradation (3). Conversely, PCSK9 loss-of-function (LOF) is associated with increased LDLR expression on the hepatocyte surface and results in clearance of low-density lipoproteins (LDLs) from the bloodstream. Several PCSK9 inhibitors are commercially available drugs to treat refractory hypercholesterolemia (4).
PCSK9 was recently identified as a critical regulator of the immune response during sepsis (5). Walley et al (5) hypothesized that PCSK9 may also regulate the clearance of pathogenic lipid moieties associated with bacterial cell wall and modulate the host response to sepsis. In adults with sepsis, those carrying PCSK9 LOF genetic variants had better survival when compared with those with gain-of-function (GOF) variants or those homozygous for the wildtype allele (5). Further, increased serum PCSK9 concentrations were associated with decreased endotoxin clearance and increased risk of organ failure (6). In adult mice, using cecal ligation and puncture (CLP) model of sepsis, PCSK9 inhibition was associated with increased bacterial clearance, a decreased cytokine response, and decreased mortality (5). On the basis of these results, two clinical trials were recently launched to test the safety of PCSK9 inhibitors in adult patients with sepsis (ClinicalTrials.gov: NCT03634293 and NCT03869073).
Serum lipoproteins are thought to exert a protective effect during sepsis. LDL is thought to facilitate bacterial clearance (6, 7), and high-density lipoprotein (HDL) is thought to sequester bacterial pathogens and have anti-inflammatory properties (8). Decrease in LDL, HDL, and total cholesterol (TC) early in sepsis are associated with increased sepsis severity in adult and pediatric patients (9, 10). PCSK9 LOF, through a reduction of lipoproteins, may therefore have a harmful effect during sepsis. This effect may be less dominant in adults, who are sufficiently compensated given age-related increase in lipoproteins (11, 12), but important in the pediatric host with sepsis.
We sought to determine whether the association between PCSK9 LOF and septic shock outcomes is operative among children and in juvenile mice challenged with sepsis. We hypothesized that PCSK9 LOF would not improve outcomes in the pediatric host with septic shock.
MATERIALS AND METHODS
Patients, Samples, and Data Collection
We used blood samples that were collected as part of an ongoing prospective observational cohort study (13–17) of children admitted with septic shock to one of 17 PICUs between August 2003 and March 2016. The study was approved by the Institutional Review Boards of each of the participating institutions. Informed consent was obtained from parents or legal guardians of children less than 18 years old, meeting pediatric-specific diagnostic criteria for septic shock (18). There were no exclusion criteria other than inability to obtain informed consent. Blood samples were obtained within 24 hours of admission to the PICU. Severity of illness was calculated using the Pediatric Risk of Mortality (PRISM) III scores (19).
The primary outcome was complicated course, defined as the persistence of two or more organ failures at 7 days after meeting criteria for septic shock or death within the 28-day study period (17, 20). Secondary outcomes included all-cause 28-day mortality, maximum numbers of organs failed, PICU length of stay, and PICU-free days.
Genetic Association Study
We performed polymerase chain reactions (TaqMan; Thermo Fisher Scientific, Waltham, MA) to detect single-nucleotide polymorphisms (SNP) from isolated DNA. We tested for the most common PCSK9 missense LOF variants: rs11591147 (R46L), rs11583680 (A53V), and rs562556 (V474I); and the most common GOF variant rs505151 (G670E). We also genotyped a variant of the LDLR gene rs688 that renders it insensitive to changes in PCSK9 (21). These were the same SNPs tested by Walley et al (5) in adults with sepsis.
Serum PCSK9
PCSK9 concentrations were measured on day 1 serum samples by enzyme-linked immunosorbent assay (DPC900; R&D Systems, Minneapolis, MN) according to the manufacturers’ specifications.
Serum Lipid Profiles
Lipid profiles were measured on day 1 serum samples on a Randox RX Daytona clinical analyzer (Crumlin, United Kingdom). LDL and HDL were measured by direct clearance, TC by enzymatic endpoint method, and triglyceride by glycerol phosphate oxidase p-amino phenazone method.
Serum Biomarkers
Serum concentrations of interleukin (IL)-1a, IL-8, granzyme B, heat shock protein 70, C-C chemokine ligand (CCL) 3, CCL4, and matrix metalloproteinase 8 were measured using a multiplex magnetic bead platform (MILLIPLEX MAP by the EMD Millipore Corporation, Billerica, MA) in a Luminex 100/200 System (Luminex Corporation, Austin, TX). These biomarkers have been shown to be associated with mortality risk in children with septic shock (22, 23).
Juvenile Murine Model of Sepsis
Our animal studies complied with the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health (24) and were approved by the Institutional Animals Care and Use Committee. We established breeding colonies of constitutive PCSK9 null mice with C57BL/6 genetic background (Pcsk9−/−; B6;129S6-Pcsk9tm1Jdh/J; Jackson Laboratory, Bar Harbor, ME) and wildtype mice (C57BL/6; Charles River Laboratory, Wilmington, MA). Mice were maintained with standard housing, food, and day/night regulation. Juvenile mice were housed with their mothers, with no separation except for procedures. There were no obvious differences in phenotype between mice strains. We used a cecal slurry model of sepsis, based on those previously described (25, 26) (Appendix, Supplemental Digital Content 1, http://links.lww.com/CCM/F657).
Survival Studies
Sepsis was induced in 14-day-old mixed-sex juvenile mice via a single intraperitoneal injection of cecal slurry (0.8 mg/g body weight) via a 27-gauge needle. Experimental animals received an antibiotic mixture: ceftriaxone (25 μg/g body weight) and metronidazole (12.5 μg/g body weight) mixed in 0.9% saline (50 μL per injection, 10 mL/kg volume per animal), at 8 and 32 hours after injection of cecal slurry. Similar procedures were followed in 10–14 week old adult mice; adult animals received standardized doses of cecal slurry and antibiotic mixture (200 μL per injection, 10 mL/kg volume per animal). Animals were monitored up to 7 days for survival.
Bacterial Burden
Juvenile mice were euthanized at 24 hours after induction of sepsis and specimens of blood, peritoneal fluid, and liver tissue were collected to evaluate bacterial growth as previously described (27). Serial dilutions of specimens were plated onto 5% sheep blood agar plates. All plates were incubated at 37°C for 48 hours, and plated dilutions with 30–300 colonies were used to quantify bacterial burden.
Serum Separation by Gel Filtration Chromatography
Lipoprotein fractions in juvenile mice were assessed by applying serum (100 μl per animal) directly to three Superdex 200 gel filtration columns (10 × 300 mm; GE Healthcare Life Sciences, Chicago, IL) arranged in series on an ÄKTA FPLC system (GE Healthcare, Chicago, IL). Samples were processed at a flow rate of 0.3 mL/min in standard Tris buffer (10 mM Tris, 0.15 M sodium chloride, 1 mM EDTA, and 0.2% sodium azide). Eluate was collected as 47 1.5-mL fractions on a Frac-900 Fraction Collector (GE Healthcare) at 4°C. Apolipoprotein B-containing lipoproteins VLDL and LDL elute as a single peak between fractions 14 and 18 (peak 1); HDL-sized particles elute as a broader peak between fractions 19 and 26 (peak 2) (28, 29). Fractions were assessed for TC by colorimetric kits (Wako, Cape Charles, VA).
Statistics
Data were described using frequencies, percentages, and medians with interquartile ranges. Differences between groups were determined by chi-square test for categorical variables and by Kruskal-Wallis one-way analysis of variance for continuous variables. Multiple comparison tests were used where appropriate. Logistic regression was used to model the effects of PCSK9 genotype on study outcomes. Age, self-identified ancestry, gender, and PRISM III score were considered as independent variables in our model. PCSK9, LDL, and HDL cholesterol concentrations were included as covariates in secondary analyses. Log-rank test was used to compare survival between animal groups. A p value of less than 0.05 was considered to be statistically significant. Statistical analysis were performed using SigmaPlot 14 (Systat Software, San Jose, CA). Figures were created using GraphPad Prism 8 (GraphPad Software, San Diego, CA).
RESULTS
We genotyped 522 children with septic shock. All SNPs tested were in Hardy-Weinberg equilibrium (Supplemental Table 1, Supplemental Digital Content 2, http://links.lww.com/CCM/F658). Patients who carried both LOF and GOF variants of the PCSK9 gene (n = 19) were excluded from analysis. Table 1 shows the demographic data of the remaining cohort (n = 503) according to PCSK9 genotype. Forty-one percent (n = 206) carried at least one LOF variant, 9% (n = 43) carried a GOF variant, and 50% (n = 254) carried neither LOF nor GOF variants. The GOF variant tested was present less frequently among patients with Caucasian ancestry (p < 0.001).
TABLE 1.
Demographic Data According to Proprotein Convertase Subtilisin/Kexin Type 9 Genotype
| Variable | LOF+ | GOF+ | LOF−/GOF− | p |
|---|---|---|---|---|
|
| ||||
| n = 503, n (%) | 206 (41) | 43 (9) | 254 (50) | |
| Age, yr, median (IQR) | 3.1 (1.0–6.7) | 1.9 (0.6–4.6) | 2.9 (1.3–6.3) | 0.19 |
| Male, n (%) | 124 (60) | 26 (60) | 142 (56) | 0.62 |
| Ancestry | < 0.01 | |||
| Caucasian, n (%) | 167 (44) | 17 (5) | 193 (51) | |
| African-American, n (%) | 18 (30) | 19 (32) | 23 (38) | |
| Comorbidity, n (%) | 94 (46) | 21 (49) | 107 (42) | 0.61 |
| Pediatric Risk of Mortality score, median (IQR) | 13 (8–19) | 12 (6–19) | 11 (7–17) | 0.34 |
| Corticosteroids, n (%) | 109 (53) | 22 (51) | 129 (51) | 0.90 |
| Immunosuppression, n (%) | 24 (12) | 4 (9) | 28 (11) | 0.93 |
| Malignancy, n (%) | 24 (12) | 3 (7) | 24 (10) | 0.57 |
| Bone marrow transplantation, n (%) | 9 (4) | 0 (0) | 10 (4) | 0.38 |
GOF = gain-of-function, IQR = interquartile range, LOF = loss-of-function.
Table 2 shows outcomes when comparing those with at least one PCSK9 LOF allele to those with either a GOF allele or homozygous for the wildtype. Children with at least one PCSK9 LOF allele had a higher rate of complicated course, greater 28-day mortality, and a greater number of maximum organ failures when compared with those without. Patients carrying PCSK9 LOF alleles had lower serum PCSK9 concentrations, but similar serum lipid profiles, in comparison to those without such genetic variants.
TABLE 2.
Clinical Variables and Outcomes According to Proprotein Convertase Subtilisin/Kexin Type 9 Genotype
| Variable | Loss-of-Function + | Other Genotype | p |
|---|---|---|---|
|
| |||
| n = 503, n (%) | 206 (41) | 297 (59) | |
| Complicated course, n (%) | 69 (34) | 69 (23) | 0.01 |
| 28-d mortality, n (%) | 27 (13) | 21 (7) | 0.03 |
| Maximum organ failure, median (IQR) | 2 (2–3) | 2 (2–3) | < 0.01 |
| PICU length of stay days, median (IQR) | 7 (3–14) | 8 (3–14) | 0.46 |
| PICU-free days, median (IQR) | 19 (6–25) | 19 (12–24) | 0.54 |
| n = 480 | 199 | 281 | |
| Serum proprotein convertase subtilisin/kexin type 9 level (ng/mL), median (IQR) | 309.2 (201.2–418.1) | 370.4 (264.9–491.5) | < 0.01 |
| n = 421 | 174 | 247 | |
| Low-density lipoprotein cholesterol (mg/dL), median (IQR) | 33.4 (22.6–55.1) | 35.2 (20.9–55.5) | 0.99 |
| High-density lipoprotein cholesterol (mg/dL), median (IQR) | 15.1 (7.6–22.5) | 15.9 (8.5–25.2) | 0.22 |
| Total cholesterol (mg/dL), median (IQR) | 83.4 (61.1–106.1) | 80.2 (63.0–110.3) | 0.97 |
| Triglycerides (mg/dL), median (IQR) | 95.2 (69.1–145.4) | 105.8 (64.7–154.7) | 0.56 |
| Infection type, n (%) | |||
| Culture negative | 87 (42) | 119 (40) | 0.94 |
| Gram negative | 52 (25) | 68 (23) | |
| Gram positive | 46 (22) | 79 (27) | |
| Viral | 16 (8) | 25 (8) | |
| Fungal | 3 (2) | 3 (1) | |
| Mixed | 1 (1) | 2 (1) | |
IQR = interquartile range.
There were no significant differences in type of infection by genotype (Table 2) or outcome (Supplemental Table 2, Supplemental Digital Content 3, http://links.lww.com/CCM/F659). Serum PCSK9 concentrations were negatively correlated with proinflammatory cytokine IL-8 (Supplemental Table 3, Supplemental Digital Content 4, http://links.lww.com/CCM/F660).
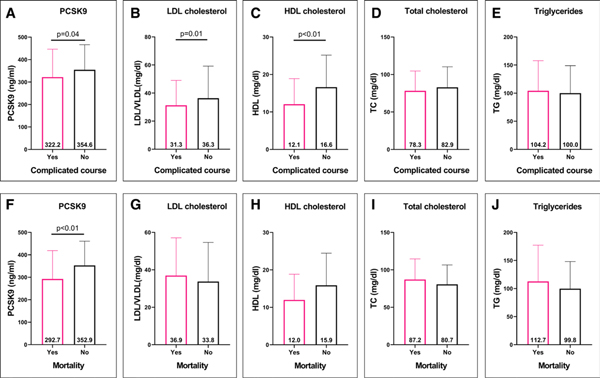
Figure 1 shows serum PCSK9 and lipid profiles in those with complicated course and among nonsurvivors relative to those without these events. Patients with complicated course had lower serum PCSK9, LDL, and HDL cholesterol concentrations compared with those patients without. Nonsurvivors had lower PCSK9 concentrations compared with survivors. However, there were no significant differences in lipid profiles between nonsurvivors and survivors. Age-related differences in serum PCSK9 and lipid profiles were observed, with infants noted to have the lowest PCSK9, LDL, and triglyceride concentrations (Supplemental Table 4, Supplemental Digital Content 5, http://links.lww.com/CCM/F661).
Figure 1.
Serum proprotein convertase subtilisin/kexin type 9 (PCSK9) and lipid profile concentrations in children with septic shock. Correlation of serum PCSK9 and lipid profile concentrations (median [interquartile range]) in pediatric septic shock patients with complicated course (A–E) and in nonsurvivors (F–J) relative to those without these adverse events. HDL = high-density lipoprotein, LDL = low-density lipoprotein, TC = total cholesterol, TG = triglyceride, VLDL = very-low-density lipoprotein.
Table 3 shows the results of the multivariable logistic regression. The presence of at least one LOF variant of the PCSK9 gene in children with septic shock was independently associated with higher odds of complicated course and 28-day mortality. With each additional year in age, there was an 8% decrease in the odds of complicated course. When patients homozygous for the LDLR gene variant were excluded from analyses, the strength of association between PCSK9 LOF genotype and odds of complicated course and 28-day mortality, increased relative to the primary analysis including the patients homozygous for this LDLR variant.
TABLE 3.
Multivariable Regression Analyses of Pediatric Septic Shock Outcomes, Adjusted for Age, Gender, Ancestry, and Illness Severity
| Outcome | Variable | n | OR (95% CI) | p |
|---|---|---|---|---|
|
| ||||
| Complicated course | Allele status | 503 | 1.76 (1.14–2.70) | 0.01 |
| Age | 0.92 (0.86–0.98) | 0.01 | ||
| Gender | 1.30 (0.84–2.01) | 0.23 | ||
| Ancestry | 0.80 (0.49–1.30) | 0.37 | ||
| PRISM III | 1.09 (1.06–1.11) | < 0.01 | ||
| 28-d mortality | Allele status | 503 | 2.07 (1.09–3.96) | 0.03 |
| Age | 0.92 (0.82–1.02) | 0.10 | ||
| Gender | 1.24 (0.64–2.41) | 0.53 | ||
| Ancestry | 1.10 (0.52–2.34) | 0.79 | ||
| PRISM III | 1.09 (1.06–1.13) | < 0.01 | ||
| Patients homozygous for rs688 excluded | ||||
| Complicated course | Allele status | 430 | 2.10 (1.31–3.37) | < 0.01 |
| Age | 0.92 (0.86–0.99) | 0.03 | ||
| Gender | 1.43 (0.89–2.30) | 0.13 | ||
| Ancestry | 0.71 (0.42–1.18) | 0.18 | ||
| PRISM III | 1.09 (1.06–1.14) | < 0.01 | ||
| 28-d mortality | Allele status | 430 | 2.97 (1.42–6.22) | < 0.01 |
| Age | 0.94 (0.84–1.05) | 0.25 | ||
| Gender | 1.35 (0.64–2.83) | 0.42 | ||
| Ancestry | 0.94 (0.42–2.08) | 0.87 | ||
| PRISM III | 1.10 (1.06–1.14) | < 0.01 | ||
OR = odds ratio, PRISM III = Pediatric Risk of Mortality III.
Inclusion of serum PCSK9, LDL, and HDL cholesterol concentrations as covariates in regression models did not affect the association between PCSK9 LOF genotype and outcome. Serum PCSK9 and LDL cholesterol concentrations did not have an independent effect on outcomes. With each 1 mg/dL increase in HDL cholesterol, there was a 3% decrease in odds of complicated course (Supplemental Table 5, Supplemental Digital Content 6, http://links.lww.com/CCM/F662). There was no significant interaction between age and genotype, PCSK9, LDL, or HDL cholesterol concentrations (data not shown).
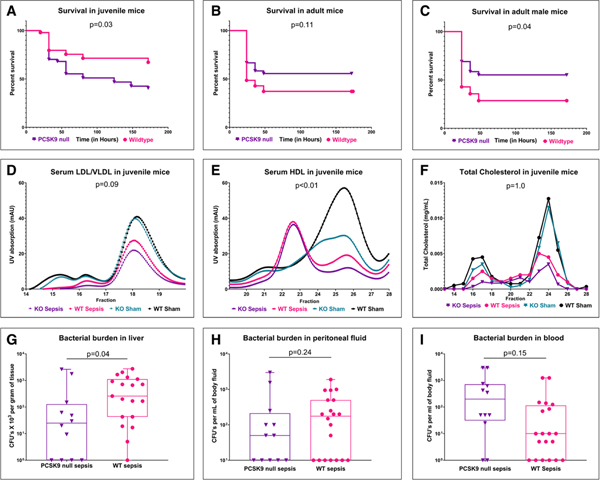
Figure 2 shows survival in juvenile and adult mice, and an assessment of serum lipoproteins and bacterial burden in juvenile mice challenged with sepsis. Consistent with our clinical data, juvenile PCSK9 null mice had significantly decreased survival, relative to wildtype control mice, after induction of sepsis by cecal slurry. In contrast, adult male PCSK9 null mice had increased survival relative to wildtype control mice after induction of sepsis by cecal slurry. This effect was attenuated with inclusion of adult females. Induction of sepsis resulted in a significant reduction in lipoprotein fractions. Juvenile PCSK9 null mice had a trend toward lower LDL/VLDL, significantly lower HDL cholesterol concentrations, and decreased hepatic bacterial burden relative to wildtype control mice after induction of sepsis. There were no other significant difference between the experimental groups with regard to markers of systemic and hepatic inflammation (Appendix, Supplemental Digital Content 1, http://links.lww.com/CCM/F657; Supplemental Fig. 1, Supplemental Digital Content 7, http://links.lww.com/CCM/F663; Supplemental Fig. 2, Supplemental Digital Content 8, http://links.lww.com/CCM/F664; Supplemental Fig. 3, Supplemental Digital Content 9, http://links.lww.com/CCM/F665; and Supplemental Fig. 4, Supplemental Digital Content 10, http://links.lww.com/CCM/F666 [legend, Supplemental Digital Content 11, http://links.lww.com/CCM/F667).
Figure 2.
Impact of proprotein convertase subtilisin/kexin type 9 (PCSK9) loss-of-function in a murine cecal slurry model of sepsis. Seven-day survival in (A) juvenile (14-d old), (B) adult (10–14 wk), (C) adult male (10–14 wk) wildtype (WT) and PCSK9 null mice after induction of sepsis induced by cecal slurry (log-rank test). D, Low-density lipoprotein (LDL)/very-low-density lipoprotein (VLDL), (E) high-density lipoprotein (HDL) concentrations, (F) total cholesterol in juvenile PCSK9 null and WT mice with and without sepsis (analysis of variance [ANOVA]). Bacterial burden (G) in liver (H) peritoneal fluid, and (I) blood, depicted as bacterial colony-forming units (CFUs) in log10 scale (ANOVA). p values shown are for differences between PCSK9 null and WT control mice with sepsis on multiple comparison testing. KO = knockout, UV = ultraviolet.
DISCUSSION
We report a novel association between PCSK9 LOF genetic variants and higher odds of complicated course and 28-day mortality in a large prospectively enrolled cohort of children with septic shock. Our results are in direct contrast to findings in adults with sepsis (5). We found a strengthening of the association between PCSK9 LOF genotype and adverse septic shock outcomes in children when excluding children homozygous for a LDLR gene variant that renders it insensitive to changes in PCSK9. This suggests a biologically plausible mechanism implicating the PCSK9-LDLR pathway in the host response to pediatric sepsis, albeit in a direction of response opposite to that of adults.
Serum PCSK9 concentrations were lower among patients with complicated course and in nonsurvivors in our cohort. These nongenetic and independent data are also opposite to findings in adults with sepsis, wherein higher PCSK9 concentrations were correlated with increased odds of organ failure after sepsis (6). Adult studies that have reported an association between low PCSK9 concentrations and increased risk of sepsis mortality have been confounded by liver disease (30). Only three children in our cohort had liver failure (data not shown). Thus, it is likely that the association between low PCSK9 concentrations and adverse outcomes in our cohort reflects biological differences between adults and children.
Consistent with prior studies (10), serum LDL and HDL cholesterol concentrations were lower among children with septic shock complicated course. However, we did not observe correlation of either with PCSK9 genotype. HDL cholesterol was associated with a modest protective effect in our cohort. Of importance, the median LDL and HDL cholesterol concentrations in our cohort were lower than those previously reported among pediatric septic shock patients (10).
We corroborated our findings in experimental mice and demonstrated the opposing responses in juvenile and adult mice with PCSK9 LOF with a cecal slurry model of sepsis. PCSK9 LOF resulted in lower serum lipoprotein fractions and hepatic bacterial burden in juvenile mice with sepsis. Hepatic bacterial clearance through LDLR is thought to depend on the concentration of lipoproteins (7). We posit that PCSK9 LOF, in the context of low lipoproteins, results in decreased hepatic bacterial clearance in the juvenile host.
Recent research suggests that PCSK9 LOF may have a paradoxical effect during sepsis, due to increased vascular endothelial inflammation and IL-8 production (31). It is possible that this is the dominant effect in the juvenile host during sepsis. Finally, PCSK9 is thought to have pleiotropic effects on cluster differentiation 36 (CD36), LDLR-related protein 1, very very low density lipoprotein receptor, apolipoprotein E receptors, and ATP-binding cassette transporter A1 (32). It is conceivable that such non-LDLR mediated mechanisms may contribute to the detrimental effect in the juvenile host during sepsis.
Several limitations of our study should be considered. Linkage disequilibrium may result in a false positive association between SNPs studied and outcomes. There exists a potential for misclassification by genotype if patients carried less common genetic variants that we did not test for. PCSK9 and lipid profiles were measured at a single time point on day 1 of septic shock. These data should be interpreted with caution as dynamic changes in these markers have been reported throughout the course of sepsis (6, 9, 10). We could not adjust for nutritional status and use of parenteral nutrition/lipids in patients. We used cecal slurry as a murine model of sepsis that differs substantially from CLP or endotoxin-induced sepsis (33), limiting comparisons with other studies. Finally, mice differ significantly from humans with regard to lipoprotein metabolism; mice lack cholesterol ester transfer protein and HDL is the predominant fraction (34). LDL and VLDL were measured together in juvenile mice, which may bias our results toward the null, as VLDL has been shown to increase during sepsis (35).
CONCLUSIONS
In a large cohort of children with septic shock, we identified that the presence of PCSK9 LOF genetic variants is independently associated with higher odds of complicated course and mortality. This finding is in contradistinction to what has been reported in adults with sepsis. We further corroborated these findings in experimental mice using the cecal slurry model of sepsis. PCSK9 LOF resulted in low lipoprotein fractions and decreased hepatic bacterial burden in the juvenile host during sepsis, suggestive of decreased bacterial clearance. Our data do not exclude the possibility of pleiotropic effects of PCSK9 that may be specifically detrimental to the juvenile host during sepsis. Taken together, our data provide a strong rationale to exclude children from trials of PCSK9 inhibitors in sepsis.
Supplementary Material
ACKNOWLEDGMENTS
We would like to acknowledge Kelli Harmon, Patrick Lahni, Amy Opoka, Kira Angel, and Hannah Sexmith for their assistance with experiments.
Dr. Atreya received funding from a “Research in Residency” grant awarded by the Cincinnati Children’s Research Foundation. Drs. Cvijanovich, Thomas, Fitzgerald, Allen, Lutfi, and Wong received support for article research from the National Institutes of Health (NIH). Dr. Cvijanovich’s institution received funding from Cincinnati Children’s Hospital Medical Center and Boston Children’s Hospital. Drs. Bigham’s and Thomas’s institutions received funding from a NIH subaward. Drs. Fitzgerald’s, Allen’s, and Lutfi’s institutions received funding from the NIH. Dr. Weiss’s institution received funding from National Institute of General Medical Sciences K23GM110496. Dr. Wong’s institution received funding from NIH R35 GM126943 and R21 HD092896. All authors discuss the off-label product use of proprotein convertase subtilisin/kexin type 9 inhibitors and their use in sepsis.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
This work was performed at Cincinnati Children’s Hospital Medical Center, Cincinnati, OH.
REFERENCES
- 1.Weiss SL, Fitzgerald JC, Pappachan J, et al. ; Sepsis Prevalence, Outcomes, and Therapies (SPROUT) Study Investigators and Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network: Global epidemiology of pediatric severe sepsis: The sepsis prevalence, outcomes, and therapies study. Am J Respir Crit Care Med 2015; 191:1147–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nadel S, Goldstein B, Williams MD, et al. ; REsearching severe Sepsis and Organ dysfunction in children: a gLobal perspective (RESOLVE) study group: Drotrecogin alfa (activated) in children with severe sepsis: A multicentre phase III randomised controlled trial. Lancet 2007; 369:836–843 [DOI] [PubMed] [Google Scholar]
- 3.Lambert G, Sjouke B, Choque B, et al. : The PCSK9 decade. J Lipid Res 2012; 53:2515–2524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenson RS, Hegele RA, Fazio S, et al. : The evolving future of PCSK9 inhibitors. J Am Coll Cardiol 2018; 72:314–329 [DOI] [PubMed] [Google Scholar]
- 5.Walley KR, Thain KR, Russell JA, et al. : PCSK9 is a critical regulator of the innate immune response and septic shock outcome. Sci Transl Med 2014; 6:258ra143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyd JH, Fjell CD, Russell JA, et al. : Increased plasma PCSK9 levels are associated with reduced endotoxin clearance and the development of acute organ failures during sepsis. J Innate Immun 2016; 8:211–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grin PM, Dwivedi DJ, Chathely KM, et al. : Low-density lipoprotein (LDL)-dependent uptake of Gram-positive lipoteichoic acid and Gram-negative lipopolysaccharide occurs through LDL receptor. Sci Rep 2018; 8:10496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morin EE, Guo L, Schwendeman A, et al. : HDL in sepsis - risk factor and therapeutic approach. Front Pharmacol 2015; 6:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Golucci APBS, Marson FAL, Ribeiro AF, et al. : Lipid profile associated with the systemic inflammatory response syndrome and sepsis in critically ill patients. Nutrition 2018; 55–56:7–14 [DOI] [PubMed] [Google Scholar]
- 10.Bermudes ACG, de Carvalho WB, Zamberlan P, et al. : Changes in lipid metabolism in pediatric patients with severe sepsis and septic shock. Nutrition 2018; 47:104–109 [DOI] [PubMed] [Google Scholar]
- 11.Hrachovec JP, Rockstein M: Age changes in lipid metabolism and their medical implications. Gerontologia 1959; 3:305–326 [DOI] [PubMed] [Google Scholar]
- 12.Kritchevsky D: Age-related changes in lipid metabolism. Proc Soc Exp Biol Med 1980; 165:193–199 [DOI] [PubMed] [Google Scholar]
- 13.Wong HR, Shanley TP, Sakthivel B, et al. ; Genomics of Pediatric SIRS/Septic Shock Investigators: Genome-level expression profiles in pediatric septic shock indicate a role for altered zinc homeostasis in poor outcome. Physiol Genomics 2007; 30:146–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cvijanovich N, Shanley TP, Lin R, et al. ; Genomics of Pediatric SIRS/Septic Shock Investigators: Validating the genomic signature of pediatric septic shock. Physiol Genomics 2008; 34:127–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong HR, Cvijanovich N, Lin R, et al. : Identification of pediatric septic shock subclasses based on genome-wide expression profiling. BMC Med 2009; 7:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong HR, Cvijanovich NZ, Allen GL, et al. : Validation of a gene expression-based subclassification strategy for pediatric septic shock. Crit Care Med 2011; 39:2511–2517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong HR, Cvijanovich NZ, Anas N, et al. : Developing a clinically feasible personalized medicine approach to pediatric septic shock. Am J Respir Crit Care Med 2015; 191:309–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldstein B, Giroir B, Randolph A; International Consensus Conference on Pediatric Sepsis: International pediatric sepsis consensus conference: Definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med 2005; 6:2–8 [DOI] [PubMed] [Google Scholar]
- 19.Pollack MM, Patel KM, Ruttimann UE: The Pediatric Risk of Mortality III–Acute Physiology Score (PRISM III-APS): A method of assessing physiologic instability for pediatric intensive care unit patients. J Pediatr 1997; 131:575–581 [DOI] [PubMed] [Google Scholar]
- 20.Abulebda K, Cvijanovich NZ, Thomas NJ, et al. : Post-ICU admission fluid balance and pediatric septic shock outcomes: A risk-stratified analysis. Crit Care Med 2014; 42:397–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao F, Ihn HE, Medina MW, et al. : A common polymorphism in the LDL receptor gene has multiple effects on LDL receptor function. Hum Mol Genet 2013; 22:1424–1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong HR, Cvijanovich NZ, Anas N, et al. : Improved risk stratification in pediatric septic shock using both protein and mRNA biomarkers. PERSEVERE-XP. Am J Respir Crit Care Med 2017; 196:494–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong HR, Caldwell JT, Cvijanovich NZ, et al. : Prospective clinical testing and experimental validation of the pediatric sepsis biomarker risk model. Sci Transl Med 2019; 11:eaax9000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals: Guide for the Care and Use of Laboratory Animals. Eighth Edition. Washington, DC, National Academies Press (US), 2011 [Google Scholar]
- 25.Brook B, Amenyogbe N, Ben-Othman R, et al. : A controlled mouse model for neonatal polymicrobial sepsis. J Vis Exp 2019; (143). doi: 10.3791/58574 [DOI] [PubMed] [Google Scholar]
- 26.Starr ME, Steele AM, Saito M, et al. : A new cecal slurry preparation protocol with improved long-term reproducibility for animal models of sepsis. PLoS One 2014; 9:e115705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ganatra HA, Varisco BM, Harmon K, et al. : Zinc supplementation leads to immune modulation and improved survival in a juvenile model of murine sepsis. Innate Immun 2017; 23:67–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gordon SM, Deng J, Lu LJ, et al. : Proteomic characterization of human plasma high density lipoprotein fractionated by gel filtration chromatography. J Proteome Res 2010; 9:5239–5249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gordon SM, Li H, Zhu X, et al. : Impact of genetic deletion of platform apolipoproteins on the size distribution of the murine lipoproteome. J Proteomics 2016; 146:184–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rannikko J, Jacome Sanz D, Ortutay Z, et al. : Reduced plasma PCSK9 response in patients with bacteraemia is associated with mortality. J Intern Med 2019; 286:553–561 [DOI] [PubMed] [Google Scholar]
- 31.Modulation of the Vascular Endothelial Inflammatory Response by Proprotein Convertase Subtilisin-Kexin Type 9 (PCSK9). C33. Sepsis: Cellular Studies. Available at: 10.1164/ajrccm-conference.2018.197.1_MeetingAbstracts.A4748.Accessed March 13, 2020 [DOI] [Google Scholar]
- 32.Filippatos TD, Christopoulou EC, Elisaf MS: Pleiotropic effects of proprotein convertase subtilisin/kexin type 9 inhibitors? Curr Opin Lipidol 2018; 29:333–339 [DOI] [PubMed] [Google Scholar]
- 33.Gentile LF, Nacionales DC, Lopez MC, et al. : Host responses to sepsis vary in different low-lethality murine models. PLoS One 2014; 9:e94404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yin W, Carballo-Jane E, McLaren DG, et al. : Plasma lipid profiling across species for the identification of optimal animal models of human dyslipidemia. J Lipid Res 2012; 53:51–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bartolomé N, Aspichueta P, Martínez MJ, et al. : Biphasic adaptative responses in VLDL metabolism and lipoprotein homeostasis during Gram-negative endotoxemia. Innate Immun 2012; 18:89–99 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.