Abstract
Background:
The U.S. military health system (MHS) provides universal health care access to its beneficiaries. However, whether the universal access has translated into improved patient outcome is unknown. This study compared survival of non-small cell lung cancer (NSCLC) patients in the MHS with that in the U.S. general population.
Methods:
The MHS data were obtained from The Department of Defense’s (DoD) Automated Central Tumor Registry (ACTUR) and the U.S. population data were drawn from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program. The study subjects were NSCLC patients diagnosed between January 1, 1987 and December 31, 2012 in ACTUR and a sample of SEER patients who were matched to the ACTUR patients on age group, sex, race, and year of diagnosis group with a matching ratio of 1:4. Patients were followed through December 31, 2013.
Results:
16,257 NSCLC patients were identified from ACTUR and 65,028 matched patients from SEER. Compared with SEER patients, ACTUR patients had significantly better overall survival (Log Rank P<0.001). The better overall survival among the ACTUR patients remained after adjustment for potential confounders (HR=0.78, 95% CI=0.76 to 0.81). The survival advantage of the ACTUR patients was present regardless of cancer stage, grade, age group, sex or race.
Conclusions:
The MHS’s universal care and lung cancer care programs may have translated into improved survival among NSCLC patients.
Impact:
This study supports improved survival outcome among NSCLC patients with universal care access.
Keywords: Military Health System, Universal Health Care Access, Lung cancer, Survival, SEER
Introduction
Lung cancer is the leading cause of cancer death among both men and women accounting for 26.5% of all cancer deaths in the United States. In 2017, it is estimated that there will be 222, 500 new cases of lung cancer and 155,870 deaths(1). Non-small cell lung cancer (NSCLC), comprises 85% to 90% of lung cancers(2). Despite the slight decline in incidence and mortality over the last 20 years, in the U.S. general population, the five-year survival rates of NSCLC remain a dismal 1% for advanced disease(3) Accessibility to health care reflected by health insurance and type of insurance is an important predictor of lung cancer survival(4–7). Research has found that lung cancer patients without health insurance or with Medicaid had a higher mortality than patients with private insurance or Medicare (4–7). These patients were less likely to receive cancer-directed therapies than those with non-Medicaid insurance (4,6). Lack of or low access to health care affects the utilization of screening services, medical visits, receipt of treatments and quality of care delivered, lessening survival of lung cancer patients, as demonstrated in the general population(4–7).
The U.S. military health system (MHS) provides universal access to health care for 9.6 million beneficiaries, including service members of the seven uniformed services, National Guard and Reserve members, retirees, and their family members(8). In the MHS, all beneficiaries have access to health care defined by the access standards in the MHS policies and guidance(8). However, little is known about whether the universal access to health care has translated into improved patient outcomes among the MHS beneficiaries. To the best of our knowledge, there have been no studies comparing the MHS and the U.S. general populations in survival of lung cancer. In this study, we compared NSCLC patients in the MHS with those in the U. S. general population in overall survival and receipt of lung cancer treatments.
Materials and Methods
Data sources
This study was based on data from the Department of Defense’s (DoD) Automated Central Tumor Registry (ACTUR) and the National Cancer Institute’s (NCI) Surveillance, Epidemiology, and End Results (SEER) program. ACTUR was described previously(9,10). Briefly, ACTUR tracks cancer patients who are diagnosed and/or receive cancer treatment at military treatment facilities (MTFs). MTFs are required to report to ACTUR cancer information of DoD beneficiaries, including active-duty members, retirees and their dependents. Data are collected on demographics, tumor characteristics, cancer treatment, and vital status of patients. ACTUR complies with the uniform data standards set by the North American Association of Central Cancer Registries (NAACCR)(11). The SEER program is a nation-wide cancer registry that collects data on patient demographics, primary tumor site, tumor stage, grade, and size at diagnosis, first course of treatment, vital status and other information(12). The population residing within the areas served by SEER cancer registries is comparable to the general population (13). We used SEER 18 (1973– 2012) with catchments for the 18 SEER registries (Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco-Oakland, Seattle-Puget Sound, Utah, Los Angeles, San Jose-Monterey, Rural Georgia, the Alaska Native, Greater California, Greater Georgia, Kentucky, Louisiana, and New Jersey). The SEER records were obtained for this study based on the November 2014 submission public use file(14).
This study was based on non-identifiable ACTUR data approved for our research by the institutional review board of Walter Reed National Military Medical Center and SEER data were de-identified for public use.
Study Subjects
Patients diagnosed with histologically confirmed primary NSCLC between January 1, 1987 and December 31, 2012 and identified from ACTUR and SEER databases were included in this study. This time period was selected to ensure the same diagnosis time frame for patients from both registries. NSCLC was defined with the cancer site codes (C34.0 to C34.3, C34.8, C34.9) and morphology codes (8050–8078, 8083, 8084, 8250–8260, 8480–8490, 8570–8574, 8140, 8211, 8230, 8231, 8323, 8550, 8551, 8576, 8010–8012, 8014–8031, 8035, 8310) of the International Classification of Diseases for Oncology, third edition (ICD-O-3)(15). Data on tumor stage were consolidated and stages were defined as derived stages I–IV according to the Revised International System for Staging Lung Cancer 6th edition(16). Patients with diagnosis from death certificate or autopsy were excluded from the analysis. To reduce differences in age, gender, race, and year of diagnosis, the ACTUR and SEER patients were matched on age group (<50, 50–64, 65–80, >80), sex, race, and year of diagnosis group (1987–1989, 1990–1994, 1995–1999, 2000–2004, 2005–2009, 2010–2012). For each ACTUR patient, four matched patients in the same matching stratum were randomly selected from SEER.
Statistical Analysis
We first compared the distributions of demographic and tumor characteristics the among ACTUR and SEER patients using Chi-square test. We then used Kaplan-Meier curve and log-rank test to compare overall survival between patients from ACTUR and SEER with all-cause death as the outcome. Multivariable Cox proportional hazards model for matched data was then used to estimate HRs and 95% CI for ACTUR compared with SEER. Patients who did not die during follow-up were censored. In multivariable Cox regression modeling, we adjusted for the following variables: region at diagnosis (Northeast, South, Midwest, West and other, as defined by the U.S. Census Bureau), tumor stage, tumor grade, surgery (for stages I and II) and radiation (for stages III and IV). Matching variables were no longer needed to be adjusted in the models. The Cox analysis was further stratified by age group, sex, race, and cancer stage. In addition to survival, we also compared ACTUR and SEER patients in receipt of surgery and radiation treatment. Receipt of surgery was compared for stages I and II patients only and receipt of radiation therapy was compared for stages III and IV patients only according to the recommended treatment guidelines(17,18). Multivariable logistic regression was used to estimate odds ratios (ORs) and 95% confidence intervals (CIs) of treatment with SEER as the reference.
Statistical analyses were conducted using the SAS software version 9.3.0 (SAS Institute, Inc.). All reported P values are two sided, with the significance level set at P<0.05.
Results
A total of 16,257 patients were identified from ACTUR and 65,028 matched patients from SEER. Demographic and tumor characteristics of patients are shown in Table 1. The ACTUR and SEER patients were well matched on age group, sex, race, and diagnosis year group (P=1.00 for all). However, compared to the SEER patients, the ACTUR patients were more likely to be diagnosed in the South region and less likely to be diagnosed in the Northeast, Midwest or West regions (P<0.0001). In regard to tumor stage, the ACTUR patients were less likely to be diagnosed at stage IV than SEER cases (28.65% vs. 33.39%) and more likely to be diagnosed at earlier stages (P<0.0001). The ACTUR patients had a lower percentage of poorly differentiated tumor than the SEER patients (35.71% vs. 37.14%), and higher percentages of well differentiated tumor (7.50% vs. 5.40%) and moderately differentiated tumor (21.78% vs. 19.80%) (P<0.0001).
Table 1.
Demographic and tumor characteristics of NSCLC Patients diagnosed during 1987 – 2012 from the ACTUR and SEER registries.*
| ACTUR | SEER | P-Value | |||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Age groups | 1.00 | ||||
| <50 | 1202 | 7.39 | 4808 | 7.39 | |
| 50–64 | 7004 | 43.08 | 28016 | 43.08 | |
| 65–80 | 7129 | 43.85 | 28516 | 43.85 | |
| >80 | 922 | 5.67 | 3688 | 5.67 | |
| Sex | 1.00 | ||||
| Male | 10879 | 66.92 | 43516 | 66.92 | |
| Female | 5378 | 33.08 | 21512 | 33.08 | |
| Race | 1.00 | ||||
| White | 13693 | 84.23 | 54772 | 84.23 | |
| Black | 1683 | 10.35 | 6732 | 10.35 | |
| Asian or Pacific Islander | 818 | 5.03 | 3272 | 5.03 | |
| American India/Alaska Native | 19 | 0.12 | 76 | 0.12 | |
| Other | 44 | 0.27 | 176 | 0.27 | |
| Year of Diagnosis | 1.00 | ||||
| 1987–1989 | 2026 | 12.46 | 8104 | 12.46 | |
| 1990–1994 | 4643 | 28.56 | 18572 | 28.56 | |
| 1995–1999 | 3409 | 20.97 | 13636 | 20.97 | |
| 2000–2004 | 2614 | 16.08 | 10456 | 16.08 | |
| 2005–2009 | 2285 | 14.06 | 9140 | 14.06 | |
| 2010–2012 | 1280 | 7.87 | 5120 | 7.87 | |
| Region of Diagnosis & | <0.0001 | ||||
| Northeast | 273 | 1.68 | 10287 | 15.82 | |
| South | 9111 | 56.04 | 9617 | 14.79 | |
| Midwest | 1432 | 8.81 | 14749 | 22.68 | |
| West | 4916 | 30.24 | 30375 | 46.71 | |
| Other | 525 | 3.23 | 0 | 0.00 | |
| Stage | <0.0001 | ||||
| Stage I | 4747 | 29.20 | 16761 | 25.78 | |
| Stage II | 1059 | 6.51 | 3384 | 5.20 | |
| Stage III | 4020 | 24.73 | 15725 | 24.18 | |
| Stage IV | 4657 | 28.65 | 21715 | 33.39 | |
| Unknown | 1774 | 10.91 | 7443 | 11.45 | |
| Grade | <0.0001 | ||||
| Well Differentiated, Grade I | 1220 | 7.50 | 3510 | 5.40 | |
| Moderately Differentiated, Grade II | 3541 | 21.78 | 12875 | 19.80 | |
| Poorly Differentiated, Grade III | 5806 | 35.71 | 24151 | 37.14 | |
| Undifferentiated, Grade IV | 526 | 3.24 | 4002 | 6.15 | |
| Unknown | 5164 | 31.76 | 20490 | 31.51 | |
Patients were matched by age group, sex, race and year of diagnosis group with ACTUR: SEER ratio= 1:4
Defined following the U.S. Census Bureau definition
The median survival times for ACTUR and SEER patients were 15.5 months and 10.0 months, respectively. Kaplan-Meier survival curves showed significantly better survival for ACTUR patients than SEER patients (Log Rank P<0.001) (Figure 1). In Cox proportional hazards analysis, ACTUR patients exhibited significantly better overall survival than did their SEER counterparts (HR=0.78, 95% CI=0.76 to 0.81) (Table 2). The survival advantage of ACTUR patients remained in subgroups stratified by age group, sex, race, tumor stage or tumor grade (Table 2).
Figure 1.
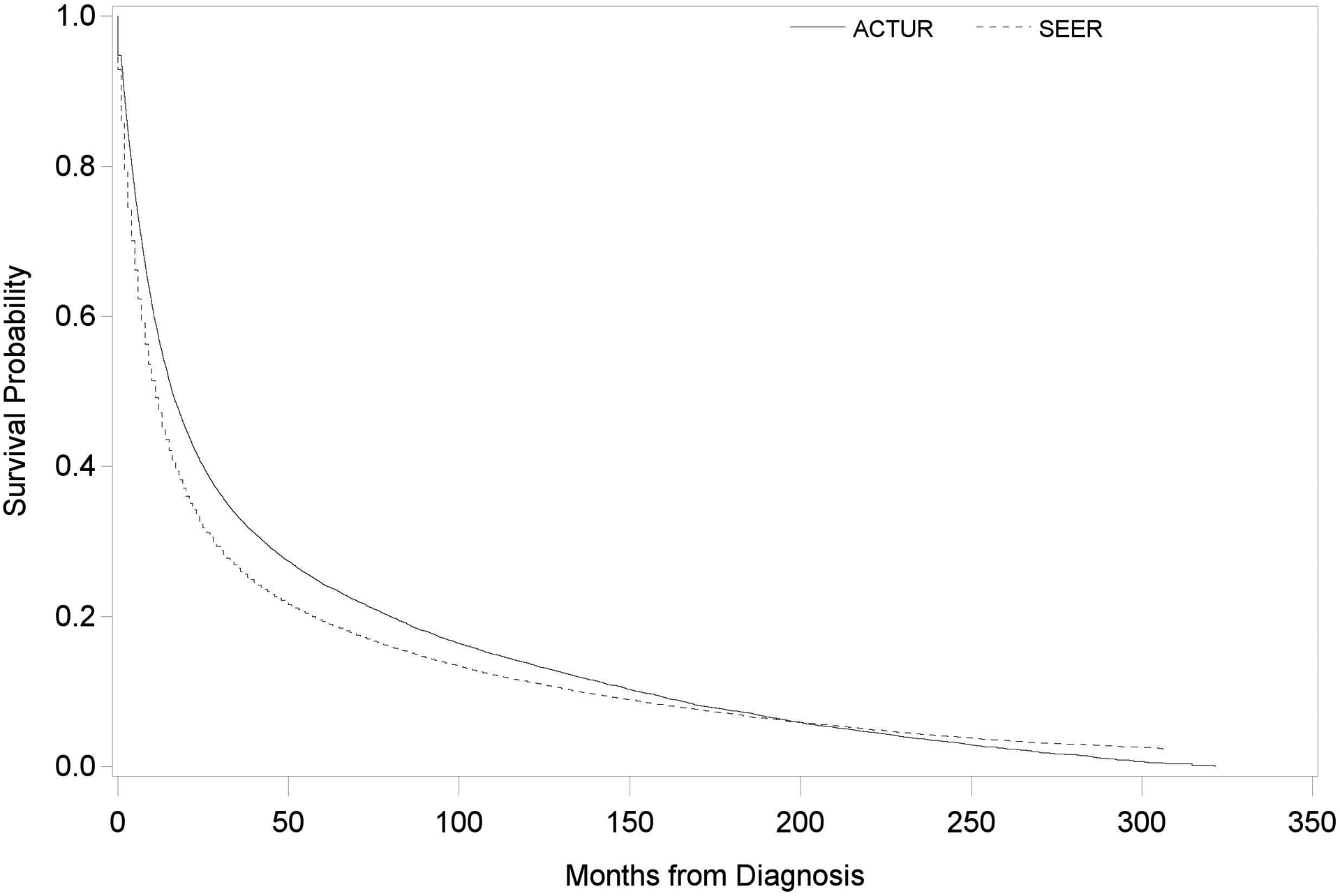
Kaplan-Meier Survival Curves for ACTUR and SEER patients with non-small cell lung cancer (NSCLC).
Figure 1 shows the comparison of survival probability over time for ACTUR and SEER patients. Patients were diagnosed between 1987 and 2012 and followed through December 31, 2013.
Table 2.
All-cause mortality among NSCLC Patients diagnosed between 1987 and 2012, the ACTUR and SEER registries
| Variables | Numbers | Adjusted HR* | 95% CI | |
|---|---|---|---|---|
| Alive | Dead | |||
| Overall | ||||
| SEER | 7700 | 57328 | 1.00 (Ref.) | 1.00 (Ref.) |
| ACTUR | 1895 | 14362 | 0.78 | 0.76 to 0.81 |
| By Age | ||||
| <50 | ||||
| SEER | 877 | 3931 | 1.00 (Ref.) | 1.00 (Ref.) |
| ACTUR | 272 | 930 | 0.66 | 0.60 to 0.74 |
| 50–64 | ||||
| SEER | 3534 | 24482 | 1.00 (Ref.) | 1.00 (Ref.) |
| ACTUR | 916 | 6088 | 0.78 | 0.75 to 0.81 |
| 65–80 | ||||
| SEER | 2921 | 25595 | 1.00 (Ref.) | 1.00 (Ref.) |
| ACTUR | 645 | 6484 | 0.81 | 0.78 to 0.85 |
| >80 | ||||
| SEER | 368 | 3320 | 1.00 (Ref.) | 1.00 (Ref.) |
| ACTUR | 62 | 860 | 0.74 | 0.66 to 0.82 |
| By Sex | ||||
| Male | ||||
| SEER | 4110 | 39406 | 1.00 (Ref.) | 1.00 (Ref.) |
| ACTUR | 958 | 9921 | 0.79 | 0.76 to 0.82 |
| Female | ||||
| SEER | 3590 | 17922 | 1.00 (Ref.) | 1.00 (Ref.) |
| ACTUR | 937 | 4441 | 0.77 | 0.73 to 0.81 |
| By Race | ||||
| White | ||||
| SEER | 6050 | 48722 | 1.00 (Ref.) | 1.00 (Ref.) |
| ACTUR | 1433 | 12260 | 0.80 | 0.77 to 0.82 |
| Black | ||||
| SEER | 775 | 5957 | 1.00 (Ref.) | 1.00 (Ref.) |
| ACTUR | 238 | 1445 | 0.67 | 0.62 to 0.73 |
| Asian or Pacific Islander | ||||
| SEER | 767 | 2505 | 1.00 (Ref.) | 1.00 (Ref.) |
| ACTUR | 199 | 619 | 0.84 | 0.74 to 0.96 |
| By Stage & | ||||
| Stage I | ||||
| SEER | 4372 | 12389 | 1.00 (Ref.) | 1.00 (Ref.) |
| ACTUR | 1197 | 3550 | 0.87 | 0.80 to 0.95 |
| Stage II | ||||
| SEER | 538 | 2846 | 1.00 (Ref.) | 1.00 (Ref.) |
| ACTUR | 147 | 912 | 1.00 | 0.72 to 1.37 |
| Stage III | ||||
| SEER | 1290 | 14435 | 1.00 (Ref.) | 1.00 (Ref.) |
| ACTUR | 239 | 3781 | 0.76 | 0.70 to 0.83 |
| Stage IV | ||||
| SEER | 930 | 20785 | 1.00 (Ref.) | 1.00 (Ref.) |
| ACTUR | 147 | 4510 | 0.72 | 0.67 to 0.76 |
| Unknown | ||||
| SEER | 570 | 6873 | 1.00 (Ref.) | 1.00 (Ref.) |
| ACTUR | 165 | 1609 | 0.86 | 0.75 to 0.98 |
| By Grade | ||||
| Well Differentiated | ||||
| SEER | 981 | 2529 | 1.00 (Ref.) | 1.00 (Ref.) |
| ACTUR | 379 | 841 | 0.73 | 0.50 to 1.06 |
| Moderately Differentiated | ||||
| SEER | 2287 | 10588 | 1.00 (Ref.) | 1.00 (Ref.) |
| ACTUR | 537 | 3004 | 0.76 | 0.69 to 0.85 |
| Poorly Differentiated | ||||
| SEER | 2237 | 21914 | 1.00 (Ref.) | 1.00 (Ref.) |
| ACTUR | 462 | 5344 | 0.81 | 0.76 to 0.86 |
| Undifferentiated | ||||
| SEER | 213 | 3789 | 1.00 (Ref.) | 1.00 (Ref.) |
| ACTUR | 35 | 491 | 0.81 | 0.55 to 1.21 |
| Unknown | ||||
| SEER | 1982 | 18508 | 1.00 (Ref.) | 1.00 (Ref.) |
| ACTUR | 482 | 4682 | 0.73 | 0.68 to 0.78 |
All HRs were adjusted for tumor stage, tumor grade and region at diagnosis. In stratified analysis, tumor stage was not adjusted in the analysis stratified by tumor stage, and tumor grade was not adjusted in the analysis stratified by tumor grade.
For stages I and II patients, HRs were adjusted for tumor grade, region at diagnosis and surgery. For stages III and IV patients, HRs were adjusted for tumor stage, region at diagnosis and radiation.
HR, Hazard Ratio
CI, Confidence Interval
Table 3 shows the results on receipt of cancer treatments. The ACTUR patients were more likely to receive surgery for early-stage (stages I and II) tumors (OR=1.41, 95% CI =1.28 to 1.55) (Table 3), and radiation therapy for late stage (stages III to IV) tumors (OR=1.09, 95% CI=1.03 to 1.15). When the analysis was stratified by age, sex, race, or cancer stage, the higher likelihood of receiving surgery among early-stage patients in ACTUR compared to SEER remained in all subgroups, except for Asian or Pacific Island population. While the same tendency was observed for radiation receipt, the association was not significant in some subgroups.
Table 3.
Cancer treatments among NSCLC patients diagnosed between 1987 and 2012, the ACTUR and SEER registries
| Variables | Surgery (Stages I and II) | Radiation (Stages III and IV) | ||||
|---|---|---|---|---|---|---|
| Surgery | No Surgery | Adjusted OR (95% CI)* | Radiation | No Radiation | Adjusted OR (95% CI)* | |
| Overall | ||||||
| SEER | 16430 | 3692 | 1.00 (ref) | 21504 | 15154 | 1.00 (ref) |
| ACTUR | 4891 | 876 | 1.41 (1.28 to 1.55) | 5099 | 3217 | 1.09 (1.03 to 1.15) |
| By Age | ||||||
| <50 | ||||||
| SEER | 975 | 91 | 1.00 (ref) | 2111 | 1001 | 1.00 (ref) |
| ACTUR | 297 | 23 | 1.34 (1.14 to 1.58) | 478 | 215 | 1.13 (0.92 to 1.38) |
| 50–64 | ||||||
| SEER | 7071 | 1112 | 1.00 (ref) | 10259 | 6068 | 1.00 (ref) |
| ACTUR | 2096 | 266 | 1.35 (1.15 to 1.58) | 2437 | 1263 | 1.09 (1.01 to 1.19) |
| 65–80 | ||||||
| SEER | 7751 | 1996 | 1.00 (ref) | 8265 | 6897 | 1.00 (ref) |
| ACTUR | 2286 | 443 | 1.46 (1.28 to 1.66) | 1975 | 1482 | 1.08 (0.99 to 1.17) |
| >80 | ||||||
| SEER | 633 | 493 | 1.00 (ref) | 869 | 1188 | 1.00 (ref) |
| ACTUR | 212 | 144 | 1.42 (1.05 to 1.91) | 209 | 257 | 1.03 (0.82 to 1.30) |
| By Sex | ||||||
| Male | ||||||
| SEER | 10214 | 2527 | 1.00 (ref) | 14780 | 10256 | 1.00 (ref) |
| ACTUR | 3064 | 582 | 1.50 (1.34 to 1.69) | 3570 | 2190 | 1.10 (1.03 to 1.18) |
| Female | ||||||
| SEER | 6216 | 1165 | 1.00 (ref) | 6724 | 4898 | 1.00 (ref) |
| ACTUR | 1827 | 294 | 1.24 (1.05 to 1.46) | 1529 | 1027 | 1.05 (0.95 to 1.17) |
| By Race | ||||||
| White | ||||||
| SEER | 14298 | 3080 | 1.00 (ref) | 17820 | 12435 | 1.00 (ref) |
| ACTUR | 4151 | 751 | 1.30 (1.17 to 1.44) | 4229 | 2662 | 1.09 (1.02 to 1.16) |
| Black | ||||||
| SEER | 1277 | 457 | 1.00 (ref) | 2517 | 1711 | 1.00 (ref) |
| ACTUR | 458 | 75 | 2.65 (1.95 to 3.62) | 584 | 351 | 1.04 (0.89 to 1.22) |
| Asian or Pacific Islander | ||||||
| SEER | 813 | 144 | 1.00 (ref) | 1106 | 921 | 1.00 (ref) |
| ACTUR | 262 | 42 | 1.35 (0.86 to 2.13) | 270 | 189 | 1.31 (1.02 to 1.67) |
Adjusted for tumor stage, tumor grade, and region at diagnosis.
OR, Odds Ratio
CI, Confidence Interval
Discussion
This study showed that NSCLC patients in the MHS had better survival than those in the U.S. general population despite age, gender, race, tumor stage or grade. In addition, the ACTUR patients tended to be more likely than the SEER patients to receive surgery for early stage tumor and radiation therapy for late stage tumor. Our results suggest that the access to universal care within the MHS has translated into improved survival, which might partly result from more timely treatment and treatment compliance to the treatment guidelines.
The MHS is one of the largest health care providers in the United States that combines resources to provide ready access to health care for 9.6 million beneficiaries and is dedicated to quality health care and performance improvements(8). Recent evidence showed that MHS outperformed or was equal to national benchmarks in cancer screening and other services(8). There has been a lack of comparison of cancer outcomes between MHS beneficiaries and the U.S. general population. Nevertheless, there were a few studies in the Veterans Health Administration (VHA) health system, which also provides universal access to care for its beneficiaries. But the results are inconsistent. Landrum et al. found that relative to SEER-Medicare patients, VHA patients had earlier stage at diagnosis and higher survival rates of NSCLC with a HR of 0.91 (95% CI=0.88 to 0.95) after adjusting for performance status, comorbidity, smoking history and education. However, the higher survival was not significant after adjusting for stage at diagnosis (19). This suggested that the effects of earlier tumor stage might result from better preventive care in VHA, on survival (19). In a study by Zeliadt et al., veterans with NSCLC, identified from the cancer registry for the Veterans Affairs Pacific Northwest Network, were more likely to be diagnosed with early stage disease than the SEER patients, identified from the Puget Sound Surveillance, Epidemiology, and End Results cancer registry(20), but survival was similar between the two populations among older patients with early-stage tumors(20). However, among younger (younger than 65 years) patients with early-stage disease, veterans had lower age-adjusted survival than SEER cases (20). Nevertheless, factors other than age were not adjusted for in the analysis. Although the reasons for the lower survival among beneficiaries in the VHA are unknown, the authors suggested that the lower surgical rates in younger veterans may be a potential factor (20). In another study in Pennsylvania(21), the survival rates of lung cancer patients were significantly lower among White veterans than White civilians after adjusting for age and stage. Lower socioeconomic status, less patient awareness, worse support systems, lower education and more comorbid illness among VHA patients than civilians may be possible reasons for the worse survival among VHA beneficiaries(21).
To our knowledge, the current study is the first to compare MHS beneficiaries and the U.S. general population in NSCLC survival. In the general population, cancer patients without health insurance have a higher mortality than patients with health insurance (4–7,22). The lower survival of cancer patients without insurance has been attributed to advanced stage at diagnosis(4,6,23), not receiving the same treatment (4–6,22,24,25)and other factors, such as delivery and quality of care received(6). The universal access to health care services of all MHS beneficiaries presumably reduces these disadvantages. In particular, regarding tumor stage at diagnosis, our results showed that the ACTUR patients were more likely to be diagnosed at early stages than the SEER patients. Lung cancer screening was not a standard practice during the time period of the data. The earlier stage at diagnosis in lung cancer observed and the higher utilization of screening in other cancers among MHS beneficiaries (26,27)may suggest the benefit of readily access to diagnosis and care in the MHS.
Our results showed that ACTUR patients had better survival than SEER patients in the overall analysis adjusting for cancer stage and other factors, and in the stratified analysis by stage. In the study conducted by Landrum et al., however, the survival advantage of VHA patients was not observed after adjustment for tumor stage(19). The authors concluded that earlier tumor stage explained much of the survival advantage among VHA patients and that better preventive care provided in VHA resulted in earlier tumor stage and therefore improved patient outcomes(19). In our study, ACTUR patients exhibited better survival than SEER patients after adjustment for tumor stage and the association was observed despite cancer stage, suggesting that the survival advantage may also be explained by factors other than earlier stage at diagnosis. The beneficial roles of cancer care after diagnosis may also play a role in the improved survival for patients of all stages.
As shown in our results, early-stage and late-stage ACTUR patients were more likely to receive surgery and radiation treatment than their SEER counterparts, respectively. The higher rates of receiving cancer care in MHS than the general population was also reported in other cancers. A recent study of more than 3,000 MHS cancer patients also found that 74% of breast and 65% of prostate cancer patients received all minimum recommended care over 3 years after diagnosis, and the rates did not vary by age groups(28), while a SEER-Medicare study reported only 57% of breast cancer patients ages 65 or older receive minimum recommended care in 3 years after diagnosis (29). In contrast, the VHA study reported age-adjusted lower surgery rates among younger veterans than civilians (20). Treatment receipt was not compared in the other two studies (19,21) comparing VHA patients and civilians.
Overall, the results of comparison between VHA and general population in terms of survival and receipt of cancer treatment are different from our comparison between MHS and the general population. While it is unclear why the results are different between MHS and VHA when comparing to the general populations, the two systems may differ in population characteristics (e.g. socioeconomic status, education, health behavior, and health status) and cancer treatment and care, which are associated with survival. Further research is warranted.
The strengths of this study include a large number of patients from the ACTUR and SEER registries and matching the patients from the two databases on key demographic characteristics, which reduced confounding by these variables. Previous comparative studies of VHA beneficiaries with the general population did not match on demographics (19–21). However, due to the nature of cancer registry data, the potential effects of comorbidity, life style factors (smoking in particular), performance status, and health care quality on survival differences cannot be assessed. In addition, chemotherapy could not be compared between SEER and ACTUR populations due to the lack of information on chemotherapy in the SEER data. Further, since all-cause death rather than lung cancer-specific death was used as the outcome, we do not exclude potential effects of comorbidities on the results. However, this may not be a major concern in lung cancer because the majority of patients die of the disease. Finally, we also noted a fair amount of unknown tumor grade in both SEER (31.51%) and ACTUR (31.76%). The reasons for the unknown grade with similar extent in both registries warrant further investigation.
In conclusion, our results suggest that survival was better and the likelihood of receiving cancer treatments was higher in the MHS than in the SEER population, implying that universal access to health care within the MHS may have translated into improved patient survival. Future studies are warranted to investigate specific factors that may contribute to the improved survival among NSCLC patients in the MHS.
Acknowledgement
This project was supported by John P. Murtha Cancer Center, Walter Reed National Military Medical Center via the Uniformed Services University of the Health Sciences under the auspices of the Henry M. Jackson Foundation for the Advancement of Military Medicine. The authors thank the Joint Pathology Center (formerly Armed Forces Institute of Pathology) for providing the ACTUR data. The authors declare no conflicts of interest or financial disclosures.
Footnotes
Publisher's Disclaimer: Disclaimer: The contents of this publication are the sole responsibility of the authors and do not necessarily reflect the views, assertions, opinions or policies of the Uniformed Services University of the Health Sciences (USUHS), the Department of Defense (DoD), National Cancer Institute, or the Departments of the Army, Navy, or Air Force. Nothing in the presentation implies any Federal/DoD endorsement.
Conflict of Interest Disclosure: The authors declare no conflict of interest.
References
- 1.American Cancer Society, Cancer Facts and Figures 2017: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2017/estimated-number-of-new-cancer-cases-and-deaths-by-sex-us-2017.pdf.AccessedMarch 27, 2017.
- 2.Types of Lung Cancer: http://www.cancer.org/cancer/lungcancer-non-smallcell/detailedguide/non-small-cell-lung-cancer-what-is-non-small-cell-lung-cancer.American Cancer Society; AccessedMay 6, 2016. [Google Scholar]
- 3.Key Statistics for Lung Cancer: https://www.cancer.org/cancer/non-small-cell-lung-cancer/detection-diagnosis-staging/survival-rates.html.American Cancer Society; AccessedMarch 27, 2017. [Google Scholar]
- 4.Walker GV, Grant SR, Guadagnolo BA, Hoffman KE, Smith BD, Koshy M, et al. Disparities in stage at diagnosis, treatment, and survival in nonelderly adult patients with cancer according to insurance status. J Clin Oncol 2014;32:3118–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradley CJ, Dahman B, Given CW. Treatment and survival differences in older Medicare patients with lung cancer as compared with those who are dually eligible for Medicare and Medicaid. J Clin Oncol 2008;26:5067–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slatore CG, Au DH, Gould MK. An official American Thoracic Society systematic review: insurance status and disparities in lung cancer practices and outcomes. Am J Respir Crit Care Med 2010;182:1195–205 [DOI] [PubMed] [Google Scholar]
- 7.Niu X, Roche LM, Pawlish KS, Henry KA. Cancer survival disparities by health insurance status. Cancer Med 2013;2:403–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Final Report to the Sevretary of Defense: Military Health System Review:http://www.defense.gov/Portals/1/Documents/pubs/140930_MHS_Review_Final_Report_Main_Body.pdf.August29, 2014AccessedFebruary 2, 2017.
- 9.Lin J, Zahm SH, Shriver CD, Purdue M, McGlynn KA, Zhu K. Survival among Black and White patients with renal cell carcinoma in an equal-access health care system. Cancer Causes Control 2015;26:1019–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng L, Enewold L, Zahm SH, Shriver CD, Zhou J, Marrogi A, et al. Lung cancer survival among black and white patients in an equal access health system. Cancer Epidemiol Biomarkers Prev 2012;21:1841–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tryon J User’s Guide For ACTUR Cancer Registry Software System Abstracting Module. 2007.
- 12.Surveillance, Epidemiology, and End Results Program: http://seer.cancer.gov/.AccessedMay 10, 2016
- 13.Edwards BK, Noone AM, Mariotto AB, Simard EP, Boscoe FP, Henley SJ, et al. Annual Report to the Nation on the status of cancer, 1975–2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer 2014;120:1290–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.SEER*Stat Databases: Survival, Case Listing, and Frequency Sessions: http://seer.cancer.gov/data/seerstat/nov2014/.Surveillance, Epidemiology, and End Results Program; AccessedMay 10, 2016.
- 15.Fritz A, Percy C, Lack A, et al. International Classification of Diseases for Oncology. 3rd ed. Geneva: World Health Organization; 2000. [Google Scholar]
- 16.Greene FL, Page DL, I.D. F, Fritz AG, Balch CM, Haller DG, et al. AJCC Cancer Staging Manual, 6th Edition. Pringer-Verlag, New York; 2002. [Google Scholar]
- 17.Pisters KM, Evans WK, Azzoli CG, Kris MG, Smith CA, Desch CE, et al. Cancer Care Ontario and American Society of Clinical Oncology adjuvant chemotherapy and adjuvant radiation therapy for stages I-IIIA resectable non small-cell lung cancer guideline. J Clin Oncol 2007;25:5506–18 [DOI] [PubMed] [Google Scholar]
- 18.Masters GA, Temin S, Azzoli CG, Giaccone G, Baker S Jr., Brahmer JR, et al. Systemic Therapy for Stage IV Non-Small-Cell Lung Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol 2015;33:3488–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Landrum MB, Keating NL, Lamont EB, Bozeman SR, Krasnow SH, Shulman L, et al. Survival of older patients with cancer in the Veterans Health Administration versus fee-for-service Medicare. J Clin Oncol 2012;30:1072–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zeliadt SB, Sekaran NK, Hu EY, Slatore CC, Au DH, Backhus L, et al. Comparison of demographic characteristics, surgical resection patterns, and survival outcomes for veterans and nonveterans with non-small cell lung cancer in the Pacific Northwest. J Thorac Oncol 2011;6:1726–32 [DOI] [PubMed] [Google Scholar]
- 21.Campling BG, Hwang WT, Zhang J, Thompson S, Litzky LA, Vachani A, et al. A population-based study of lung carcinoma in Pennsylvania: comparison of Veterans Administration and civilian populations. Cancer 2005;104:833–40 [DOI] [PubMed] [Google Scholar]
- 22.Parikh AA, Robinson J, Zaydfudim VM, Penson D, Whiteside MA. The effect of health insurance status on the treatment and outcomes of patients with colorectal cancer. J Surg Oncol 2014;110:227–32 [DOI] [PubMed] [Google Scholar]
- 23.Halpern MT, Ward EM, Pavluck AL, Schrag NM, Bian J, Chen AY. Association of insurance status and ethnicity with cancer stage at diagnosis for 12 cancer sites: a retrospective analysis. Lancet Oncol 2008;9:222–31 [DOI] [PubMed] [Google Scholar]
- 24.Coburn N, Fulton J, Pearlman DN, Law C, DiPaolo B, Cady B. Treatment variation by insurance status for breast cancer patients. Breast J 2008;14:128–34 [DOI] [PubMed] [Google Scholar]
- 25.Virgo KS, Little AG, Fedewa SA, Chen AY, Flanders WD, Ward EM. Safety-net burden hospitals and likelihood of curative-intent surgery for non-small cell lung cancer. J Am Coll Surg 2011;213:633–43 [DOI] [PubMed] [Google Scholar]
- 26.Brown DS, Kurlantzick VG, McCall NT, Williams TV, Gantt CJ, Granger E. Use of six clinical preventive services in TRICARE Prime compared to insured, managed care, and all U.S. populations and Healthy People 2010. Preventive medicine 2009;48:389–91 [DOI] [PubMed] [Google Scholar]
- 27.Debarros M, Steele SR. Colorectal cancer screening in an equal access healthcare system. Journal of Cancer 2013;4:270–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fox JP, Jeffery DD, Williams TV, Gross CP. Quality of cancer survivorship care in the military health system (TRICARE). Cancer J 2013;19:1–9 [DOI] [PubMed] [Google Scholar]
- 29.Keating NL, Landrum MB, Guadagnoli E, Winer EP, Ayanian JZ. Factors related to underuse of surveillance mammography among breast cancer survivors. J Clin Oncol 2006;24:85–94 [DOI] [PubMed] [Google Scholar]


