Abstract
Autosomal dominant polycystic kidney disease is a common inherited renal disorder that results from mutations in either PKD1 or PKD2, encoding polycystin-1 (PC1) and polycystin-2 (PC2), respectively. Downregulation or overexpression of PKD1 or PKD2 in mouse models results in renal cyst formation, suggesting that the quantity of PC1 and PC2 needs to be maintained within a tight functional window to prevent cystogenesis. Here we show that enhanced PC2 expression is a common feature of PKD1 mutant tissues, in part due to an increase in Pkd2 mRNA. However, our data also suggest that more effective protein folding contributes to the augmented levels of PC2. We demonstrate that the unfolded protein response is activated in Pkd1 knockout kidneys and in Pkd1 mutant cells and that this is coupled with increased levels of GRP94, an ER protein that is a member of the HSP90 family of chaperones. GRP94 was found to physically interact with PC2 and depletion or chemical inhibition of GRP94 led to a decrease in PC2, suggesting that GRP94 serves as its chaperone. Moreover, GRP94 is acetylated and binds to HDAC6, a known deacetylase and activator of HSP90 proteins. Inhibition of HDAC6 decreased PC2 suggesting that HDAC6 and GRP94 work together to regulate PC2 levels. Lastly, we showed that inhibition of GRP94 prevents cAMP-induced cyst formation in vitro. Taken together our data uncovered a novel HDAC6-GRP94-related axis that likely participates in maintaining elevated PC2 levels in Pkd1 mutant cells.
Keywords: polycystin-1, polycystin-2, ADPKD, kidney, HSP, chaperone, HSP90, GRP94, HDAC6, UPR, proteasome, autophagy
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is a hereditary disorder affecting 1:500 to 1:1000 individuals worldwide (1). ADPKD is characterized by the formation of fluid-filled cysts that arise from renal tubules and progressively enlarge leading to end-stage renal disease in nearly half of ADPKD patients by the fifth decade of life. ADPKD is a heterogeneous disease that results from mutations in either PKD1 or PKD2, which encode for polycystin-1 (PC1) and polycystin-2 (PC2), respectively (2, 3).
PC1 is a 4302-amino acid protein with a large extracellular N-terminal segment, 11 transmembrane domains and a short C-terminal tail (2, 4). PC2 is a 968-amino acid protein with 6 transmembrane domains (3). PC1 and PC2 interact with each other via C-terminal coiled-coil regions and TOP domains to form a complex (5–7). PC2 is localized in the endoplasmic reticulum (ER), primary cilium, and plasma membrane, and it is thought to function as a non-selective cation channel (8). Although PC1 and PC2 have been studied extensively, their function remains poorly understood and information on how malfunction of either PC1 or PC2 leads to cyst formation remains elusive.
Expression levels of PC1 and PC2 play a critical role in cyst formation. Inactivation of Pkd1 or Pkd2 in mice leads to cystic kidney disease (9, 10). Conversely, overexpression of Pkd1 or Pkd2 in transgenic mice can also result in cyst formation (11, 12). These data suggest that the levels of PC1 and PC2 are tightly regulated within a functional window (13). We show here that PC2 transcription and protein expression are increased in PC1 depleted mouse cells and kidneys. We examined cellular response to increased PC2 expression, and we show that GRP94, a chaperone in the ER plays a role in regulating PC2 levels. There may be also other factors that regulate PC2 expression.
Material and Methods
Isolation of protein from mouse kidneys.
Kidneys were harvested from Pkd1fl/fl;Pax8rtTA;TetO-cre mice at P15 and P20. Pkd1ko mice were injected with doxycycline (20μg/g) at P10 and P11 to inactivate Pkd1. Kidneys were homogenized in lysis buffer (20 mM sodium phosphate, pH 7.2, with 150 mM NaCl, 1 mM EDTA, 10% glycerol, and 1% Triton X-100) supplemented with protease inhibitor mixture (Cat #78429, Thermo Scientific, Waltham, MA). The homogenate was incubated for 1 h on ice and cleared of debris by centrifugation at 17,000 × g for 20 min at 4 °C.
Isolation of primary renal epithelial cells.
Primary epithelial cells were isolated from Pkd1fl/fl;Pax8rtTA;TetO-cre mice. The kidneys were harvested under the sterile conditions from P7 mice, followed by mincing into 1 mm3 cubes. Then, the tissues were transferred into tubes containing a collagenase solution composed of 95% DMEM/F12 (Cat #10565–018, Life Technologies, Carlsbad, CA), 5% FBS (Cat # F0926, Sigma-Aldrich, St. Louis, MO), 50 mg/ml Gentamicin (Cat #15750–060, Life Technologies, Carlsbad, CA), 10 mg/ml insulin (Cat # I9278, Sigma-Aldrich, St. Louis, MO), and 0.1g/ml Collagenase A (Cat # C2139, Sigma-Aldrich, St. Louis, MO). The collagenase solution was placed in a 37 °C, 120 RPM shaker for 10 minutes. Collagenase solution was pipetted up and down (10–15 times) till the mixture becomes opaque. Supernatant was transferred into a BSA (Cat # A9676, Sigma-Aldrich, St. Louis, MO) pre-coated tube, spun for 10 minutes at 1500 rpm and discarded. Pellet was re-suspended in DNase solution ((DMEM/F12+(2U/ml) DNase I (Cat # D4263, Sigma-Aldrich, St. Louis, MO)) and then diluted with DMEM/F12 and spun at 1500 RPM for 10 minutes. Supernatant was discarded and pellet resuspended in DMEM/F12 and filtered through a 70 mm mesh to remove glomeruli. The filtered supernatant was washed in the ice-cold PBS three times to remove collagenase and then spun at 1500 PRM for 3 sec, 3 times. Remaining pellet is the isolated kidney tubule fragments.
Cell culture and Treatments.
mIMCD3 (WT and Pkd1ko) cells generated by CRISPR-Cas9 were provided by Stefan Somlo through the George M. O’Brien Kidney Center at Yale University (New Haven, Connecticut, USA) and were cultured in DMEM/Ham’s F-12 medium supplemented with 10% fetal bovine serum, 100 μg/mL of penicillin/streptomycin and 5 μg/mL of puromycin (Sigma-Aldrich, St. Louis, MO). Primary tubule cells were divided in 2 groups and were grown in DMEM/Ham’s F-12 medium supplemented with 10% fetal bovine serum and 5% penicillin/streptomycin till subconfluent. Then, one group was treated with doxycycline (2 μg/mL) for four days to inactivate Pkd1 and the second group was kept as WT. Cells were treated with CHX (90 μg/mL) (Sigma-Aldrich, St. Louis, MO), MG132 (10 μM/mL) ( Sigma-Aldrich, St. Louis, MO), bafilomycin (50 nM/mL) (Sigma-Aldrich, St. Louis, MO), TSA (50 μM/mL) ( Sigma-Aldrich, St. Louis, MO) or cobalt(II) chloride (300 μM/mL) ( Sigma-Aldrich, St. Louis, MO) as indicated. Cells were transfected with siRNA oligo duplexes with lipofectamine 2000 (Invitrogen) according to manufacturer protocol. siRNA oligo duplexes against HSP90α, HSP90β and GRP94 (SR419647, SR419559, and SR420277, respectively) were purchased from OriGene (Rockville, MD). Cells were harvested at 72 hours post transfection. Pkd1 heterozygous (PH2) and homozygous (PN18) proximal tubule conditionally immortalized cells, provided by Stefan Somlo through the George M. O’Brien Kidney Center at Yale University (New Haven, Connecticut, USA), were cultured as previously described (14).
Quantitative PCR.
Total RNA was isolated from mIMCD3 (WT and Pkd1KO) cells and mouse kidneys with RNeasy Mini Kit (Qiangen, Germantown, MD). SuperScript first strand system for RT-PCR (Invitrogen, Carlsbad, CA) was used to generate complementary DNA. For quantitative PCR, we used iQ SYBR Green Supermix (Bio-Rad, Hercules, CA). Following primers were used for PC2 (GAT GCC ATC TCA GAG AGT CTC and CT CAT CTG TTG ATG CTC ACG), and GAPDH (AGG TCG GTG TGA ACG GAT TTG and TGT AGA CCA TGT AGT TGA GGT CA). PC2 and GAPDH primers were designed based on Mus musculus mRNA sequence.
Western blotting.
Cells were cultured in 6 well plates till confluency. Cells were harvested and processed as previously described (11). The cell lysates were spun at 10,000xg for 10 min at 4°C to pellet insoluble material, and the supernatants were collected. The supernatants were run on 3–8% Tris-Acetate precast gels or 4–12% Bis-Tris precast gels (#EA03785 and NP0323, respectively, Thermo Scientific, Waltham, MA) before transferring to a polyvinylidene fluoride membrane (Bio-Rad, Hercules, CA) for Western blotting. The membranes were incubated with primary antibodies overnight and then washed with TBS-Tween 20 buffer. An HRP-conjugated secondary anti-mouse (NA934V; 1:5,000), anti-rat (NA935V; 1:3,000) or anti-rabbit (NXA931V; 1:10,000) antibody from GE Healthcare (Chicago, IL) was incubated for 1 h, and then Clarity Western ECL Substrate (Bio-Rad, Hercules, CA) was used for detection on ChemiDoc MP Imaging System (Bio-Rad, Hercules, CA). The membranes were then stripped using Restore Western blot buffer (Pierce, Rockford, IL) and re-probed. The primary antibodies used for immunoblotting included polyclonal antibody against PC2 (provided by Baltimore PKD Core Center, University of Maryland, Baltimore, MD), anti-PC1 (7e12, sc-130554, 1:200) from Santa Cruz Biotechnology (Dallas, TX), anti-HDAC6 antibody (GST-7612S; 1:2,000) from Cell Signaling (Danvers, MA), anti-HSP90α antibody (ab79849; 1:2,000) from Abcam (Cambridge, UK), anti-HSP90β antibody (ab53497; 1:2,000) from Abcam (Cambridge, UK), anti-GRP94 antibody (ab2791; 1:3,000) from Abcam (Cambridge, UK), anti-GRP78 antibody (ab21685; 1:3,000) from Abcam (Cambridge, UK), Acetylated-Lysine antibody (#9441, 1:2,000) from Cell Signaling (Danvers, MA) or (ab190479) from Abcam (Cambridge, UK), anti-PERK antibody (#3192,1:2000) from Cell Signaling (Danvers, MA), XBP1 antibody (ab220783; 1:2,000) from Abcam (Cambridge, UK), IRE1 antibody (#3194,1:2000) from Cell Signaling (Danvers, MA), ATF6 antibody (ab227830; 1:2,000) from Abcam (Cambridge, UK), HIF-1α antibody (ab228649; 1:2,000) from Abcam (Cambridge, UK) mouse anti-β-actin (A5316, 1:10,000) from Sigma-Aldrich (St. Louis, MO).
Immunoprecipitation.
Cells were cultured in 10 cm dishes till confluency. Cells were harvested and processed as described. The anti-PC2 antibody (D3, sc-28331) from Santa Cruz Biotechnology (Dallas, TX), GRP94 antibody (ab2791) from Abcam (Cambridge, UK), or Acetylated-Lysine antibody (#9441) from Cell Signaling (Danvers, MA) was added to each lysate and allowed to incubate overnight; 50 μL of G Sepharose 4 Fast Flow (GE Healthcare, Chicago, IL) were then added, and the mixture was incubated with gentle rocking for 4 hr at 4 °C. Beads were washed four times with lysis buffer and then centrifuged to remove the buffer. The beads were resuspended in 50 μL of Urea sample buffer containing 9M of urea, 50 mM of Tris pH 6.8, 2% SDS, 10% glycerol and 5% mercaptoethanol, vortexed for 1 min, and loaded on 3–8% Tris-Acetate or 4–12% Bis-Tris precast gels as described.
In vitro cystogenesis.
Matrigel bed solution was prepared by adding 500 μl of Matrigel (#354230, Corning, Corning, NY), 5 μl of collagen I (#A10483–01, Gibco, Gaithersburg, MD), 500 μl of MEM (#11095–080, Gibco, Gaithersburg, MD), 20 μl of HEPES (#15630–080, Gibco, Gaithersburg, MD), and 32 μl of sodium bicarbonate (#250080–094, Gibco, Gaithersburg, MD) and mix well. Apply 50 μl of Matrigel bed solution to 96 well plate and allow to solidify for 30 minutes at 37°C. Confluent Pkd1 heterozygous (PH2) and homozygous (PN18) proximal tubule conditionally immortalized cells grown at 33°C were trypsinized and resuspended in a medium without γ-interferon to approximately 2×104/ml. Then 6 μl of cells were mixed with 100 μl of Matrigel and 15 μl of mixture was added to Matrigel bed solution and allowed to solidify for 30 minutes at 37°C, and then 100 μl of medium without γ-interferon was added. Starting day 5, cells were treated with 10 μM of forskolin (#F6886, Sigma-Aldrich, St. Louis, MO) and PU-WS13 (1, 5, 25 or 50 μM) (#508307, Millpore, Burlington, MA) every 72 h. Pictures were taken with a Zeiss Axio microscope on day 16. Cystic areas were analyzed with ImageJ (provided by the NIH).
Statistical Analyses.
Data are presented as means ± SEM. Differences between two groups were assessed by two-tailed Student’s t test. A P value <0.05 was considered statistically significant.
Results
PC2 is increased in Pkd1 knockout kidneys and in Pkd1 mutant cells.
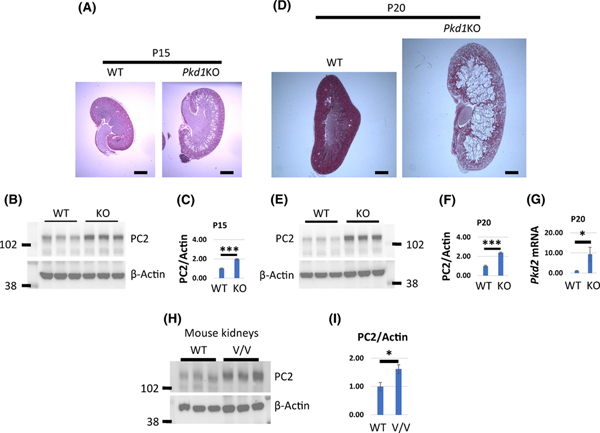
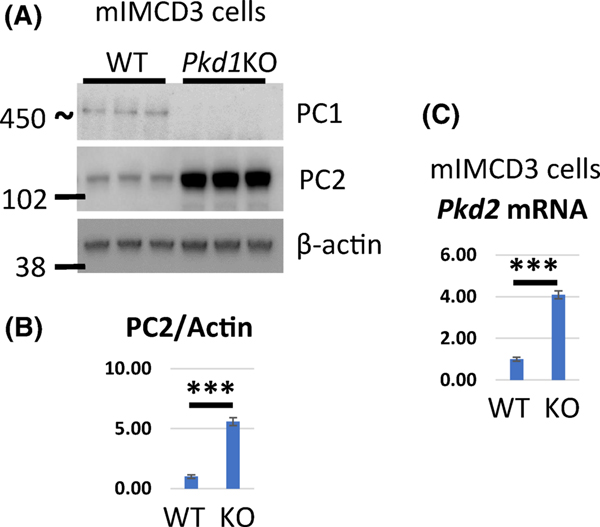
Overexpression of PC1 in MDCK cells leads to downregulation of PC2 whereas PC2 expression is increased in Pkd1 null embryos (15). To test whether PC2 levels are also increased in cystic kidneys we inactivated Pkd1 with doxycycline in Pkd1fl/fl;Pax8rtTA;TetO-cre (Pkd1-CKO) mice at postnatal day 10 (P10) and examined PC2 expression at two time points after inactivation. In this model, mice develop a mild to moderate cystic phenotype at P15 and a severe cystic phenotype by P20 (Fig. 1A and B). We found that the levels of PC2 were increased in renal tissue from mice at P15 (Pkd1-CKO-P15) when an early cystic phenotype was evident (Fig. 1A–C) and also in P20 (Pkd1-CKO-P20) kidneys that exhibit an advanced cystic phenotype (Fig. 1D–F). Likewise, we found that PC2 levels were elevated in renal tissues from Pkd1v/v mice (Fig. 1H and I) that express a non-cleavable form of PC1 and develop cysts at a postnatal stage (16). We confirmed these findings in mIMCD3 cells where Pkd1 was deleted using CRISPR-Cas9 (mIMCD3-Pkd1KO) along with corresponding controls (mIMCD3-WT). PC2 levels were increased in Pkd1 mutant cells in comparison with wild type controls (Fig. 2A and 2B). To determine whether increased transcription of PC2 contributes to higher levels of protein we examined mRNA levels of Pkd2 mouse kidneys and mIMCD3 cells. Indeed, we showed that mRNA levels of PC2 were increased in Pkd1-CKO-P20 kidneys and in mIMCD3-Pkd1KO cells (Figs. 1G and 2C, respectively).
Figure 1. PC2 protein and mRNA levels are increased in ADPKD Mouse Kidneys.
(A-G) Pkd1fl/fl;Pax8rtTA;TetO-cre mice were injected with doxycycline (20μg/g) at P10 and P11 to inactivate Pkd1 (Pkd1-CKO). Pkd1fl/fl;Pax8rtTA mice were similarly injected and served as controls (WT). (A, D) Representative Hematoxylin and Eosin staining of kidney sections harvested from mice at P15 (A) and P20 (D). (B, C, E, F) Western blots showing PC2 protein levels in kidneys harvested from Pkd1-CKO (KO) and WT mice at P15 (B, C) and P20 (E, F). (C, F) Quantification of PC2 protein levels from B and E respectively. (G) mRNA levels in kidneys harvested from Pkd1-CKO and WT mice at P20. (H) Western blots showing PC2 protein levels in kidneys harvested from Pkd1v/v and WT mice at P20. (I) Quantification of PC2 protein levels from H. PC2 level in WT kidneys was set to 1 in C, F, G and I. The bars represent mean ± SEM. *p < 0.05, ***p < 0.001. Scale bar - 1mm.
Figure 2. PC2 protein and mRNA levels are increased in Pkd1 mutant cells.
(A) Western blot showing protein expression of PC1, PC2 and β-actin in mIMCD3 (WT and Pkd1KO) cells. (B) Quantification of PC2 protein levels in (A). PC2 level in WT kidneys was set to 1. (C) mRNA levels in mIMCD3 (WT and Pkd1KO) cells. PC2 level in WT kidneys was set to 1. The bars represent mean ± SEM. ***p < 0.001.
Depletion of PC1 does not alter the mechanism of PC2 degradation.
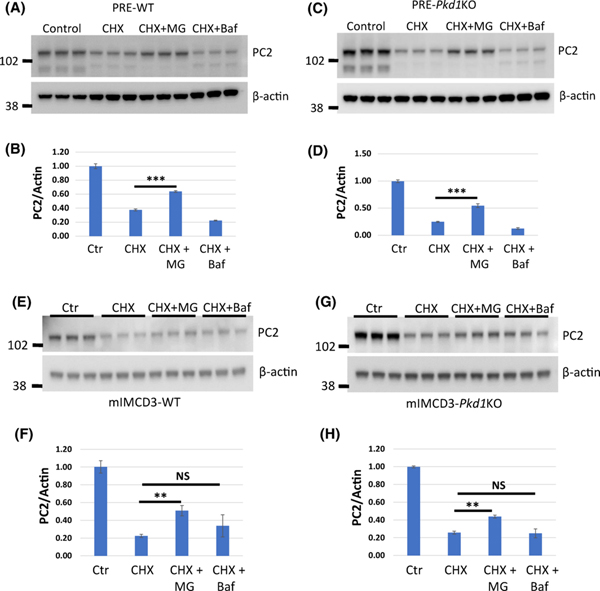
Cytosolic proteins are usually degraded by the proteasome or autophagy also known as lysosomal degradation (17). It has been shown that PC2 is degraded via the proteasome in wild type (WT) MDCK cells (18). However, overexpression of PC1 in MDCK cells decreases PC2 levels and inhibition of autophagy prevents this process suggesting that PC2 is degraded via autophagy in this situation (15). We asked whether an excess of PC2 in Pkd1 mutant cells shifts PC2 degradation from proteasome to autophagy as a compensatory mechanism. To examine the degradation of PC2 in cells lacking PC1, we used mIMCD3-Pkd1KO cells and confirmed the findings in primary renal epithelial cells. For the latter, we isolated primary renal epithelial (PRE) cells from Pkd1fl/fl;Pax8rtTA;TetO-cre mice at P7 and treated them with doxycycline to inactivate Pkd1 (PRE-Pkd1KO cells). The wild type controls were untreated (PRE-WT). We inhibited protein translation with cycloheximide and then blocked proteasomal degradation with MG132 or lysosomal degradation with bafilomycin followed by examination of PC2 level. We showed that MG132 but not bafilomycin rescued PC2 degradation in both Pkd1 mutant and wild type mIMCD3 and PRE-Pkd1KO and WT cells (Fig. 3). We conclude that PC2 is degraded via the proteasome but not autophagy regardless of cell type and genotype.
Figure 3. PC2 is degraded via proteasome in Pkd1 mutant and WT cells.
(A-H) Western blots showing PC2 levels in PRE-WT (A, B), PRE-Pkd1KO (C, D), mIMCD3-WT (E, F) and mIMCD3- Pkd1KO (G, H) cells treated with cycloheximide (CHX), MG-132 (MG) or bafilomycin (Baf) for 24 hours as indicated. CHX inhibits protein translation, MG inhibits proteasomal degradation and Baf inhibits lysosomal degradation. (B, D, F, H) Quantification of corresponding Western Blot data. PC2 level in control/untreated cells was set to 1. The bars represent mean ± SEM. **p<0.01, ***p < 0.001, NS – not significant. MG rescued PC2 degradation in both wild type and Pkd1 mutant cells suggesting that PC2 is mainly degraded by the proteasomes.
PC2 stability is similar in Pkd1 mutant and WT cells.
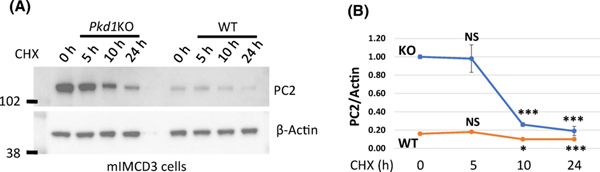
We asked whether increased levels of PC2 affects its degradation. To examine PC2 degradation, we inhibited protein translation with cycloheximide and then examined PC2 levels at different time points. We showed that in this in vitro system, PC2 levels are decreasing at a faster rate in Pkd1 mutant cells compared to WT cells at 5 h and 10 h. However, by 24 hours after treatment with cycloheximide PC2 levels were approximately at 20% from baseline in both KO and WT cells (Fig. S1). At baseline and after treatment with cycloheximide, PC2 levels remained higher in Pkd1 mutant compared to WT cells (Fig. 4). We conclude that PC2 stability is similar in Pkd1 mutant and WT cells, yet steady state levels of PC2 are higher in Pkd1 mutant cells.
Figure 4. PC2 stability is similar in Pkd1 mutant and WT cells.
(A) Western blots showing PC2 levels in mIMCD3 (Pkd1KO and WT) cells treated with CHX as indicated. (B) Quantification of corresponding Western Blot data. PC2 level in control/untreated mIMCD3-Pkd1KO cells was set to 1. The bars represent mean ± SEM. *p<0.05, ***p < 0.001, NS – not significant. This experiment was performed three times.
Expression of Grp94 is elevated in Pkd1 knockout kidneys and Pkd1 mutant cells.
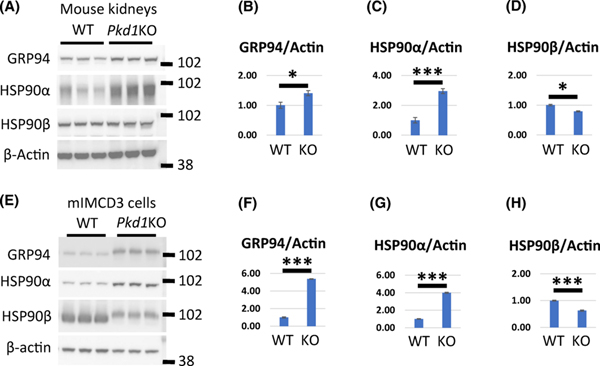
Next, we examined whether increased PC2 levels in Pkd1KO kidneys and cells demands activation of folding chaperones, thereby protecting PC2 from degradation. The HSP90 family consists of highly conserved chaperones that promote the folding and maturation of newly synthesized proteins to prevent their degradation (19, 20). There are two cytosolic HSP90 chaperones, HSP90α and HSP90β and an ER chaperone, GRP94 (20). It was previously shown that HSP90α levels are elevated in epithelial cells lining dilated tubules and cysts in Pkd1 mutant kidneys (21). In addition, several HSP90 clients have been implicated in the pathological changes that are associated with ADPKD and inhibition of HSP90 ameliorates PKD in mouse models (21, 22). Much less is known about GRP94, which is an abundant ER glycoprotein that binds calcium and regulates calcium homeostasis as well as the folding and assembly of membrane and secretory proteins (20). To confirm and extend prior observations, we assayed the expression of HSP90 chaperones in Pkd1 mutant tissues and cells. We found that the levels of HSP90α as well as GRP94 were higher in protein lysates prepared from Pkd1-CKO-P20 kidneys and mIMCD3-Pkd1KO cells compared with wild type controls (Fig. 5). In contrast, HSP90β was decreased in Pkd1 mutants compared with controls. GRP94 levels are also increased in Pkd1v/v kidneys (Fig. S2). In summary, our data show that GRP94 expression is increased in Pkd1 knockout kidneys and Pkd1 mutant cells.
Figure 5. Expression of HSP90 proteins in Pkd1KO and WT mouse kidneys and cells.
(A-D) Western blot expression of GRP94, HSP90α and HSP90β in three WT and three Pkd1-CKO-P20 kidneys. (B-D) Quantification of GRP94, HSP90α and HSP90β expression as indicated. Protein levels in WT cells was set to 1. (E-H) Western blot expression of GRP94, HSP90α, and HSP90β in mIMCD3-WT and mIMCD3-Pkd1KO cells. (F-H) Quantification of GRP94, HSP90α, and HSP90β as indicated. Protein levels in WT cells was set to 1. The bars represent mean ± SEM. *p < 0.05, ***p < 0.001.
HIF-1α does not induce Grp94 expression in Pkd1KO and WT cells
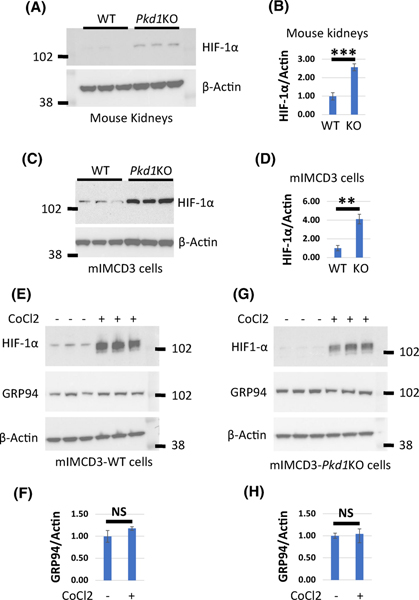
Inducible factor 1 alpha (HIF-1α) is implicated in cyst formation (23) and induces GRP94 expression during hypoxia (23). We showed that baseline HIF-1α expression is increased in Pkd1KO kidneys (Fig. 6A and B) and Pkd1 mutant cells (Fig. 6A and D) compared to respective controls. To determine whether HIF-1α induces GRP94 expression, we treated cells with cobalt (II) chloride (CoCl2) for 6 h (300 μM) to activate HIF-1α and examined GRP94 expression (Fig. 6E–H). We showed that activation of HIF-1α did not induce GRP94 expression in Pkd1 mutant or WT cells.
Figure 6. HIF-1α does not induce GRP94 expression in Pkd1KO and WT cells.
(A) Western blot expression of HIF-1α in three WT and three Pkd1-CKO-P20 kidneys. (B) Quantification of HIF-1α expression in (A). Protein levels in WT cells was set to 1. (C) Western blots showing HIF-1α levels in mIMCD3-WT and mIMCD3-Pkd1KO cells. (D) Quantification of HIF-1α expression in (C). HIF-1α level in WT cells was set to 1. (E and G) Western blots showing HIF-1α and GRP94 levels in mIMCD3-WT and mIMCD3-Pkd1KO cells treated with cobalt(II) chloride (CoCl2) as indicated. (F and H) Quantification of GRP94 in E and G, respectively. GRP94 level in untreated cells was set to 1. The bars represent mean ± SEM. **p < 0.01, ***p < 0.001, NS - not significant.
Unfolded protein response is activated in Pkd1 knockout kidneys and Pkd1 mutant cells.
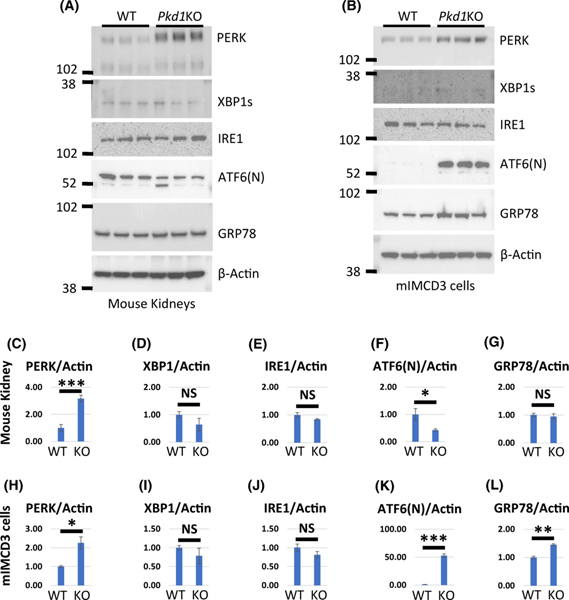
GRP94 together with GRP78 participate in the unfolded protein response (UPR) that is activated by ER stress (23). There are three core elements of the UPR: PERK, ATF6, and IRE1. ATF6 and XBP1 (IRE1 target) are known to activate GRP94 (23). We examined baseline expression of the core elements of the UPR signaling (PERK, ATF6, IRE1), XBP1 and GRP78 in Pkd1KO kidneys and Pkd1 mutant cells compared to controls (Fig. 7). PERK was significantly elevated in both Pkd1KO kidneys and Pkd1 mutant cells, Figs. 7A, B, C, and H, respectively whereas the active forms of XBP1 (XBP1s) and IRE1 were unchanged in both Pkd1KO kidneys and Pkd1 mutant cells Figs. 7A, D, E, B, I and J, respectively. Although full length ATF was undetectable (not shown), the N-terminal fragment, ATF6(N), which moves into the nucleus to activate UPR targets including GRP94, was increased in Pkd1 mutant cells but not in Pkd1KO kidneys, Figs. 7A, B, F and K. GRP78, which is also a target of ATF6 (24), was also increased only in Pkd1 mutant cells, Figs. 7A, G, B, and L. From this we conclude that UPR is activated in Pkd1 knockout kidneys and Pkd1 mutant cells suggesting that GRP94 may be activated by UPR.
Figure 7. Expression of UPR markers in Pkd1KO and WT mouse kidneys and cells.
(A) Western blot expression of PERK, XBP1, IRE1, ATF6(N), and GRP78 in three WT and three Pkd1-CKO-P20 kidneys. (C-G) Quantification of PERK, XBP1, IRE1, ATF6(N), and GRP78 expression as indicated. Protein levels in WT cells was set to 1. (B) Western blot expression of PERK, XBP1, IRE1, ATF6(N), and GRP78 in mIMCD3-WT and mIMCD3-Pkd1KO cells. (H-L) Quantification of PERK, XBP1, IRE1, ATF6(N), and GRP78 as indicated. Protein levels in WT cells was set to 1. The bars represent mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, NS – not significant.
Depletion/inactivation of Grp94 chaperone decreases PC2.
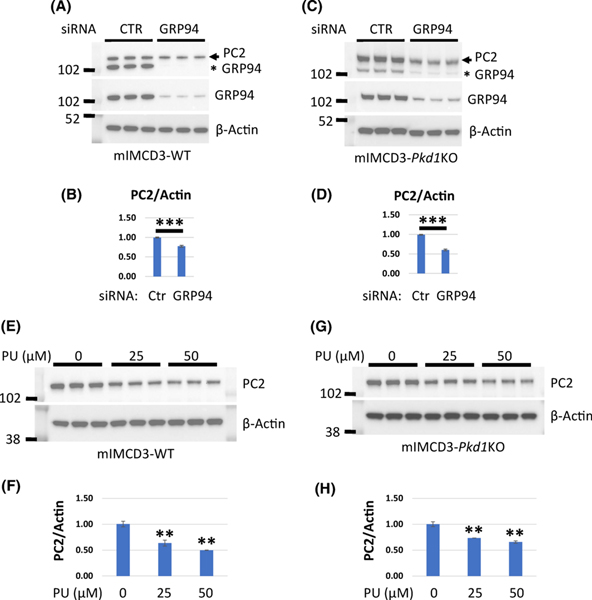
Since GRP94 levels are increased in Pkd1 mutant kidneys and cells and since both GRP94 and PC2 are ER proteins, we considered the possibility that GRP94 might chaperone the folding of PC2. First, we depleted GRP94 in mIMCD3 cells with three independent siRNAs and examined PC2 expression (Fig 8A–D). We found that depletion of GRP94, decreased PC2 levels in both WT and Pkd1 mutant cells. To extend these findings, we treated mIMCD3 cells with PU-WS13, a specific GRP94 inhibitor. Consistent with our siRNA experiments, we observed that PU-WS13 treated cells had decreased PC2 protein levels (Fig. 8E–H).
Figure 8. Depletion/inactivation of GRP94 chaperone decreases levels of PC2.
(A-D) mIMCD3-WT and mIMCD3-Pkd1KO cells were transfected with 3 independent siRNAs to GRP94 along with a scrambled oligonucleotide control and harvested at 72 hours post transfection. (A) Western blot showing expression of PC2 and GRP94 in mIMCD3-WT cells transfected with GRP94-siRNAs. Membrane was probed with GRP94 antibody first (middle panel), then stripped and probed with PC2 antibody (upper panel). GRP94 expression after siRNA was reduced to 15% in comparison to control scrambled siRNA. Despite stripping a GRP94 band is still detectable in the top panel and is indicated by an asterisk. (B) Quantification of PC2 expression in (A). PC2 level in control cells was set to 1. (C) Western blot showing expression of PC2 and GRP94 in mIMCD3-Pkd1KO cells transfected with GRP94-siRNAs. Membrane was probed with GRP94 antibody first (middle panel), then stripped and probed with PC2 antibody (upper panel). GRP94 expression after siRNA was 30%. After stripping a GRP94 band remains detectable in the top panel and is indicated by an asterisk. (D) Quantification of PC2 expression in (C). PC2 level in control cells was set to 1. (E-H) Western blot expression of PC2 and GRP94 in mIMCD3-WT (E, F) and mIMCD3-Pkd1KO (G, H) cells treated with indicated concentrations of the GRP94 inhibitor PU-WS13 (PU) for 24 hours. (F, H) Quantification of PC2 expression from panels E and G, respectively. PC2 level in control cells was set to 1. The bars represent mean ± SEM. **p<0.01, **p<0.01, NS - not significant.
GRP94 interacts with PC2.
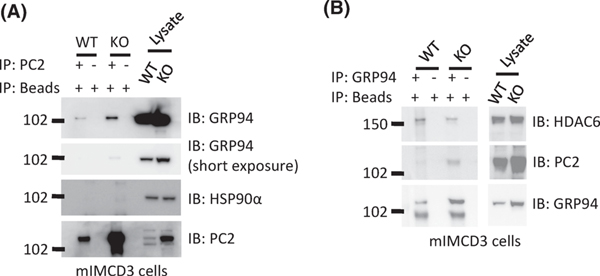
If GRP94 is involved in chaperoning PC2, then these two proteins should interact. To test this, we conducted co-immunoprecipitation (IP) experiments in mIMCD3 (WT and Pkd1KO) cells. We found that anti-PC2 antibodies were able to bring down GRP94 (Fig. 9A), while antibodies against GRP94 were also able to IP PC2 (Fig. 9B). In contrast although HSP90α levels are elevated in epithelial cells lining dilated tubules and cysts in Pkd1 mutant kidneys (21), anti-PC2 antibodies did not bring down HSP90α (Fig. 9A). Taken together these data suggest that GRP94 binds to PC2, presumably to chaperone its folding and prevent its degradation in both WT and Pkd1KO cells.
Figure 9. GRP94 interacts with PC2 and HDAC6 in mIMCD3 cells.
(A) Co-immunoprecipitation assay showing that PC2 associates with GRP94 in mIMCD3-WT and mIMCD3-Pkd1KO cells. Cell lysates were prepared from mIMCD3-WT and mIMCD3-Pkd1KO cells and immunoprecipitated with anti- PC2 or beads alone as a control for non-specific binding. Western blots were probed with antibodies to GRP94, HSP90α, and PC2 as indicated. This experiment was performed three times. (B) Cell lysates were prepared from mIMCD3-WT and mIMCD3-Pkd1KO cells and immunoprecipitated with anti-GRP94 or beads as a negative control. Western blot was probed with the indicated antibodies. This experiment was performed three times.
GRP94 is acetylated and interacts with HDAC6.
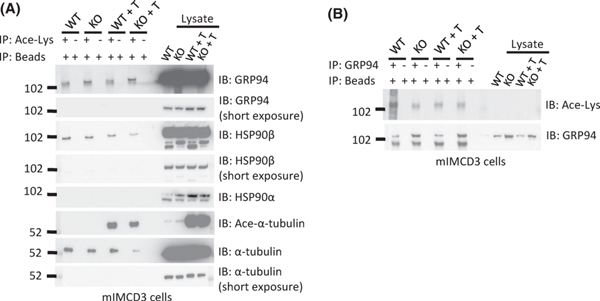
Histone deacetylase 6 (HDAC6) is a class IIb HDAC that is predominantly localized in the cytoplasm where it regulates many biological processes including cellular handling of misfolded and aggregated proteins (17, 25, 26). HDAC6 has an affinity for non-histone proteins and has been shown to de-acetylate HSP90 (27). Inhibition of HDAC6 activity leads to hyperacetylation of HSP90 and loss of its chaperoning activity (19, 28). It is unknown whether HDAC6 similarly regulates GRP94. To determine whether GRP94 is acetylated, we immunoprecipitated (IPed) acetylated-lysine proteins in mIMCD3 (WT and Pkd1KO) cells with antisera against acetylated lysine and we were able to detect GRP94, HSP90β but not HSP90α in the IP products (Fig. 10A). Previous studies that examined the acetylation pattern of HSP90 used antibodies that did not discriminate between HSP90α and HSP90β (28). HSP90β is constitutively expressed and was easily detected at high levels in the mIMCD3 cells used our studies. However, HSP90α is induced during heat shock and was expressed at relatively low basal levels in mIMCD3 cells, which is why we may have failed to detect low levels of acetylated HSP90α. Immunoprecipitation with anti-GRP94 followed by blotting with anti-acetylated lysine confirmed that GRP94 is acetylated. (Fig. 10B).
Figure 10. GRP94 is acetylated.
(A) An antibody against acetylated-lysine was used to immunoprecipitate lysates from mIMCD3-WT and mIMCD3-Pkd1KO cells with and without tubastatin-A (T) treatment. Western blots were probed with antibodies to GRP94, HSP90β or HSP90α, α-tubulin and acetylated-α-tubulin. GRP94 and HSP90β are acetylated. HSP90α acetylation was not detected. A marked increase in tubulin acetylation upon treatment with tubastatin-A was appreciated only with antisera directed at the acetylated for of α-tubulin. This experiment was performed three times. (B) An antibody to GRP94 was used for immunoprecipitation of lysates prepared from mIMCD3-WT and mIMCD3-Pkd1KO cells with and without tubastatin-A treatment. The Western blot was probed with an antibody to acetylated lysine. GRP94 is acetylated in both cells. This experiment was performed two times.
To determine whether HDAC6 might serve as a deacetylase for GRP94, we first IPed GRP94 from mIMCD3 (WT and Pkd1KO) cells and found HDAC6 in the IP products suggesting that these two proteins can be found in a complex (Fig. 9B). Next, we treated mIMCD3 (WT and Pkd1KO) cells with tubastatin-A, a specific HDAC6 inhibitor, which would be expected to yield hyperacetylation of target proteins. This assay did not conclusively show increased levels of GRP94 acetylation (Fig. 10A). Similarly, we were unable to detect hyperacetylation of HSP90α, HSP90β or α-tubulin with antibodies that were not specific for acetylated forms of these proteins. However, when we used an antibody directed at the acetylated form of α-tubulin we detected hyperacetylation of this protein upon treatment with tubastatin-A. It is possible that an antibody specific for the acetylated form of GRP94 might have increased the sensitivity of this assay. In summary our data shows that HDAC6 binds to GRP94, and leaves open the possibility that it may serve as a de-acetylase for this protein.
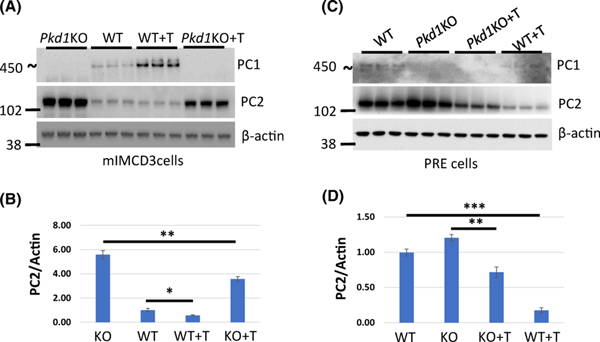
Inhibition of HDAC6 activity decreases levels of PC2.
If HDAC6 and GRP94 work together to induce proper folding of PC2, then we expect that inhibition of HDAC6 in Pkd1 mutant cells should yield reduced levels of PC2. We treated mIMDC3 and PRE (WT and Pkd1KO) cells with the HDAC-6 inhibitor tubastatin-A and found reduced PC2 levels in the treated cells (Fig. 11). Therefore, based on these findings, HDAC6 and GRP94 likely work together to regulate protein levels of PC2.
Figure 11. Inhibition of HDAC6 activity decreases levels of PC2.
(A and C) mIMCD3 (WT and Pkd1KO) and PRE (WT and Pkd1KO) cells were treated with Tubastatin-A (T) for 18 hours and proteins were isolated for Western Blot analysis with the indicated antibodies. (B) Quantification of PC2 expression in (A). PC2 level in untreated WT cells was set to 1. (D) Quantification of PC2 expression in (C). PC2 level in untreated WT cells was set to 1. The bars represent mean ± SEM. *p<0.05, **p<0.01, ***p < 0.001.
Inhibition of Grp94 activity prevents cAMP-induced in vitro cyst formation.
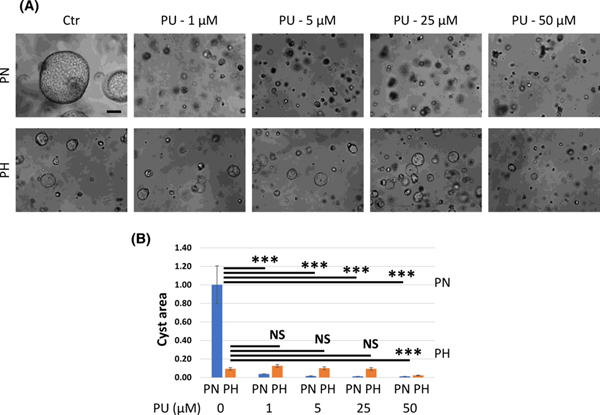
It is known that cyclic AMP (cAMP) plays a major role in cyst growth in ADPKD (29, 30). To test whether GRP94 plays a role in cAMP-induced in vitro cystogenesis, we used PN18 and PH2 cells, which are murine derived, Pkd1 null and heterozygous proximal tubule conditionally immortalized cell lines that spontaneously develop cysts when grown in three-dimensional (3D) culture (31). We grew PH2 and PN18 cells in 3D Matrigel supplemented with cAMP and then inhibited GRP94 activity with PU-WS13 (Fig. 12). We found that cAMP induced large cysts in PN18 cells and small cysts in PH2 cells. PU-WS13 reduced cyst formation in PN18 cells at very low concentration (1 μM), whereas in PH2 cells cystogenesis was inhibited only at high concentration (50 μM), suggesting that inhibition of GRP94 activity slows cAMP-induced in vitro cystogenesis.
Figure 12. Inhibition of Grp94 activity prevents cAMP-induced in vitro cyst formation.
(A) PH2 and PN18 cells grown in Matrigel for 16 days. Starting day 5, cells were treated with forskolin (10 μM) and PU-W13 (PU) (0, 1, 5, 25 or 50 μM) every 72 hours as indicated. (B) Summary data for in vitro cystogenesis experiment. We used Image J to measure cyst size in 6 different fields from 3 separate experiments. Cyst area in PN18 (untreated) cells was set to 1. The bars represent mean ± SEM. ***p < 0.001, NS - not significant. Scale bar – 100 μm.
Discussion
Upregulation of PC2 is a consistent finding in PKD1 knock out tissues and cells. We have shown previously that Pkd1 knock out embryos have increased levels of PC2 protein (15). Here we show that kidneys and cells where Pkd1 has been conditionally inactivated similarly have elevated levels of polycystin-2 in comparison with wild type controls. We also found increased Pkd2 mRNA levels in Pkd1 mutant kidneys and mIMCD3 cells suggesting that increased transcription is in part responsible for higher PC2 levels.
A second process that might also contribute to maintaining elevated PC2 levels is increased or more effective protein folding. HSP90 constitutes a family of highly conserved chaperones that promote folding and maturation of proteins (20) and has been previously implicated in ADPKD pathogenesis (21, 22). In a survey of HSP90 protein expression, we found that GRP94 was upregulated in Pkd1 mutant kidneys and renal epithelial cells. This is particularly relevant because GRP94 is a chaperone for secretory/membrane proteins (20) and resides primarily in the ER where PC2 is also found in abundant quantities (32). This prompted us to test whether GRP94 might be a chaperone for PC2. Here we show that GRP94 interacts with both PC2 and HDAC6. HDAC6 is a known de-acetylase and activator of other HSP90 family members such as HSP90α, which has been implicated in ADPKD. Although GRP94 and HDAC6 are primarily located in different cellular compartments, GRP94 has been shown to interact with other cytosolic proteins (23) and HDAC6 has been detected in the ER (33). Moreover, genetic and/or chemical depletion of either GRP94 (siRNA and PU-WS13) or HDAC6 (tubastatin-A) in both wild type and Pkd1 mutant cells decrease PC2 protein levels. We previously showed that inhibition of HDAC6 with tubastatin-A slows cyst growth in an in vitro 3D cell culture system and also in an in vivo ADPKD mouse model (34). Here we demonstrate that specific inhibition of GRP94 by PU-WS13, which presumably lowers PC2 levels, reduces cAMP induced cyst formation in Pkd1 mutant cells grown in 3D culture. Whether tubastatin-A and PU-WS13 mitigate cyst formation by lowering PC2 levels versus through effects on other client proteins that are chaperoned by GRP94/HSP90 remains to be determined. Taken together the data suggests that upregulation of GRP94 levels in Pkd1 knock out tissues may make PC2 folding more efficient thereby resulting in elevated PC2 levels and possibly worsening cyst formation though this needs to be investigated further using genetic approaches in an in vivo model.
GRP94 and GRP78 upregulation are hallmarks of the unfolded protein response (UPR) which is activated when the accumulation of unfolded proteins in the ER results in ER stress. In this work we sought to determine whether the core elements of UPR transcription pathway such as ATF6, PERK/eIF2a, and IRE1/XBP1 were similarly upregulated in PKD (23, 24). We assayed members of the UPR pathway and found that GRP78, total PERK (but not phosphorylated PERK, data not shown) and the N-terminal fragment of ATF6 were increased in Pkd1 mutant mIMCD3 cells and/or Pkd1 mutant mouse kidneys. ATF6 is known to induce activation of the GRP94 promoter and this might further upregulate GRP94 (23). Our findings are consistent with those of Yanda et al who showed that CHOP/GADD153, a target of PERK, is upregulated in Pkd1 mutant mouse kidneys (35). We note that the increases in GRP78 and N-terminal ATF were only detected in Pkd1 mutant cells but not in ADPKD kidneys. One possible explanation for this difference is that in the conditional mouse model we used, Pkd1 is deleted only in renal tubular epithelial cells and therefore Western Blots prepared from whole kidney might mask smaller cell-specific differences in protein levels.
Our data raises several intriguing questions. It is well accepted that PC1 and PC2 form a complex, which is at the core of the polycystin signaling pathway (5, 36). In addition, the direct interaction of PC1 and PC2 is required for the complex to traffic to the primary cilium where it is thought to play a critical role in averting cyst formation (36–38). Even under wild type conditions, PC2 protein levels far exceed those of PC1, which is rate limiting for the maintenance of tubular architecture (39). In this work we show that PC2 abundance is further increased in PKD1 mutant tissues and cells. Others have also reported that upregulation of PC2 is a ubiquitous response of wild type tissues/organs to a variety of cellular stressors, including renal ischemia reperfusion (40). We assume that excess PC2 under wild type conditions is physiologic while the biological significance of further PC2 upregulation may be context dependent. Brill et al demonstrated that Pkd2 depleted LLC-PK1 cells are more prone to cell death in response to serum starvation or tunicamycin, leading these authors to conclude that upregulation of PC2 is a protective stress response (41). In contrast transgenic overexpression of PC2 in mice can result in cyst formation depending on the dosage (12, 42). Whether extra PC2 in the in vivo Pkd1 null state is protective, detrimental or has a neutral effect remains to be resolved.
The excess levels of PC2 that are observed in Pkd1 null tissues and cells and under conditions of cellular stress also raise the question of whether PC2 has a function that is distinct from that of the PC1-PC2 complex. In the PKD1 null state, at least, PC2 presumably remains in the ER since there is no PC1 present to assist in trafficking to the primary cilium. It is interesting that an ER chaperone, GRP94, is also upregulated in PKD1 mutant cells, presumably increasing the level of properly folded and possibly functional PC2.
In summary, we have shown that depletion of PC1 leads to increased levels of PC2 via upregulation of the UPR. We have uncovered a GRP94-related mechanism that participates in keeping PC2 levels elevated in Pkd1 mutant cells and raises the possibility that manipulation of GRP94 expression or activity could have an effect on cystogenesis in ADPKD.
Supplementary Material
Acknowledgments:
We are grateful to S. Somlo for kindly providing mIMCD3-WT, mIMCD3-Pkd1ko, PH2, and PN18 cell though the George M. O’Brien Kidney Center at Yale University, NIH grant P30 DK079310.
Funding information
This work was supported by a National Institutes of Health Grant K08DK103078 (to V.C.), Pilot and Feasibility Award from P30 DK090868 (to V.C.) and R01DK111611 (to F. Q.). These studies utilized resources provided by the NIDDK-sponsored Baltimore Polycystic Kidney Disease Research and Clinical Core Center, P30 DK090868. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations:
- ADPKD
autosomal dominant polycystic kidney disease
- PKD1
polycystic kidney disease 1 gene
- PKD2
polycystic kidney disease 2 gene
- PC1
polycystin-1
- PC2
polycystin-2
- HDAC6
histone deacetylase 6
- Hsp90
90-kDa heat shock protein
- Grp94
94-kDA glucose-regulated protein
- HIF-1α
hypoxia inducible factor 1
- UPR
unfolded protein response
Footnotes
Conflict of interest: The authors declare that they have no conflicts of interest with the contents of this article.
References
- 1.Torres VE, Harris PC, and Pirson Y. (2007) Autosomal dominant polycystic kidney disease. Lancet 369, 1287–1301 [DOI] [PubMed] [Google Scholar]
- 2.Hughes J, Ward CJ, Peral B, Aspinwall R, Clark K, San Millan JL, Gamble V, and Harris PC (1995) The polycystic kidney disease 1 (PKD1) gene encodes a novel protein with multiple cell recognition domains. Nature genetics 10, 151–160 [DOI] [PubMed] [Google Scholar]
- 3.Mochizuki T, Wu G, Hayashi T, Xenophontos SL, Veldhuisen B, Saris JJ, Reynolds DM, Cai Y, Gabow PA, Pierides A, Kimberling WJ, Breuning MH, Deltas CC, Peters DJ, and Somlo S. (1996) PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science 272, 1339–1342 [DOI] [PubMed] [Google Scholar]
- 4.Nims N, Vassmer D, and Maser RL (2003) Transmembrane domain analysis of polycystin-1, the product of the polycystic kidney disease-1 (PKD1) gene: evidence for 11 membrane-spanning domains. Biochemistry 42, 13035–13048 [DOI] [PubMed] [Google Scholar]
- 5.Qian F, Germino FJ, Cai Y, Zhang X, Somlo S, and Germino GG (1997) PKD1 interacts with PKD2 through a probable coiled-coil domain. Nature genetics 16, 179–183 [DOI] [PubMed] [Google Scholar]
- 6.Casuscelli J, Schmidt S, DeGray B, Petri ET, Celic A, Folta-Stogniew E, Ehrlich BE, and Boggon TJ (2009) Analysis of the cytoplasmic interaction between polycystin-1 and polycystin-2. Am J Physiol Renal Physiol 297, F1310–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Su Q, Hu F, Ge X, Lei J, Yu S, Wang T, Zhou Q, Mei C, and Shi Y. (2018) Structure of the human PKD1-PKD2 complex. Science 361 [DOI] [PubMed] [Google Scholar]
- 8.Hanaoka K, Qian F, Boletta A, Bhunia AK, Piontek K, Tsiokas L, Sukhatme VP, Guggino WB, and Germino GG (2000) Co-assembly of polycystin-1 and −2 produces unique cation-permeable currents. Nature 408, 990–994 [DOI] [PubMed] [Google Scholar]
- 9.Lu W, Peissel B, Babakhanlou H, Pavlova A, Geng L, Fan X, Larson C, Brent G, and Zhou J. (1997) Perinatal lethality with kidney and pancreas defects in mice with a targetted Pkd1 mutation. Nature genetics 17, 179–181 [DOI] [PubMed] [Google Scholar]
- 10.Wu G, Markowitz GS, Li L, D’Agati VD, Factor SM, Geng L, Tibara S, Tuchman J, Cai Y, Park JH, van Adelsberg J, Hou H Jr., Kucherlapati R, Edelmann W, and Somlo S. (2000) Cardiac defects and renal failure in mice with targeted mutations in Pkd2. Nature genetics 24, 75–78 [DOI] [PubMed] [Google Scholar]
- 11.Thivierge C, Kurbegovic A, Couillard M, Guillaume R, Cote O, and Trudel M. (2006) Overexpression of PKD1 causes polycystic kidney disease. Mol Cell Biol 26, 1538–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park EY, Sung YH, Yang MH, Noh JY, Park SY, Lee TY, Yook YJ, Yoo KH, Roh KJ, Kim I, Hwang YH, Oh GT, Seong JK, Ahn C, Lee HW, and Park JH (2009) Cyst formation in kidney via B-Raf signaling in the PKD2 transgenic mice. J Biol Chem 284, 7214–7222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma M, Gallagher AR, and Somlo S. (2017) Ciliary Mechanisms of Cyst Formation in Polycystic Kidney Disease. Cold Spring Harb Perspect Biol 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shibazaki S, Yu Z, Nishio S, Tian X, Thomson RB, Mitobe M, Louvi A, Velazquez H, Ishibe S, Cantley LG, Igarashi P, and Somlo S. (2008) Cyst formation and activation of the extracellular regulated kinase pathway after kidney specific inactivation of Pkd1. Human molecular genetics 17, 1505–1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cebotaru V, Cebotaru L, Kim H, Chiaravalli M, Boletta A, Qian F, and Guggino WB (2014) Polycystin-1 negatively regulates Polycystin-2 expression via the aggresome/autophagosome pathway. J Biol Chem 289, 6404–6414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu S, Hackmann K, Gao J, He X, Piontek K, Garcia-Gonzalez MA, Menezes LF, Xu H, Germino GG, Zuo J, and Qian F. (2007) Essential role of cleavage of Polycystin-1 at G protein-coupled receptor proteolytic site for kidney tubular structure. Proc Natl Acad Sci U S A 104, 18688–18693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodriguez-Gonzalez A, Lin T, Ikeda AK, Simms-Waldrip T, Fu C, and Sakamoto KM (2008) Role of the aggresome pathway in cancer: targeting histone deacetylase 6-dependent protein degradation. Cancer Res 68, 2557–2560 [DOI] [PubMed] [Google Scholar]
- 18.Liang G, Li Q, Tang Y, Kokame K, Kikuchi T, Wu G, and Chen XZ (2008) Polycystin-2 is regulated by endoplasmic reticulum-associated degradation. Human molecular genetics 17, 1109–1119 [DOI] [PubMed] [Google Scholar]
- 19.Schopf FH, Biebl MM, and Buchner J. (2017) The HSP90 chaperone machinery. Nat Rev Mol Cell Biol 18, 345–360 [DOI] [PubMed] [Google Scholar]
- 20.Hoter A, El-Sabban ME, and Naim HY (2018) The HSP90 Family: Structure, Regulation, Function, and Implications in Health and Disease. Int J Mol Sci 19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seeger-Nukpezah T, Proia DA, Egleston BL, Nikonova AS, Kent T, Cai KQ, Hensley HH, Ying W, Chimmanamada D, Serebriiskii IG, and Golemis EA (2013) Inhibiting the HSP90 chaperone slows cyst growth in a mouse model of autosomal dominant polycystic kidney disease. Proc Natl Acad Sci U S A 110, 12786–12791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nikonova AS, Deneka AY, Kiseleva AA, Korobeynikov V, Gaponova A, Serebriiskii IG, Kopp MC, Hensley HH, Seeger-Nukpezah TN, Somlo S, Proia DA, and Golemis EA (2018) Ganetespib limits ciliation and cystogenesis in autosomal-dominant polycystic kidney disease (ADPKD). FASEB J 32, 2735–2746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marzec M, Eletto D, and Argon Y. (2012) GRP94: An HSP90-like protein specialized for protein folding and quality control in the endoplasmic reticulum. Biochim Biophys Acta 1823, 774–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walter P, and Ron D. (2011) The unfolded protein response: from stress pathway to homeostatic regulation. Science 334, 1081–1086 [DOI] [PubMed] [Google Scholar]
- 25.Boyault C, Gilquin B, Zhang Y, Rybin V, Garman E, Meyer-Klaucke W, Matthias P, Muller CW, and Khochbin S. (2006) HDAC6-p97/VCP controlled polyubiquitin chain turnover. EMBO J 25, 3357–3366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boyault C, Zhang Y, Fritah S, Caron C, Gilquin B, Kwon SH, Garrido C, Yao TP, Vourc’h C, Matthias P, and Khochbin S. (2007) HDAC6 controls major cell response pathways to cytotoxic accumulation of protein aggregates. Genes Dev 21, 2172–2181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y, Shin D, and Kwon SH (2013) Histone deacetylase 6 plays a role as a distinct regulator of diverse cellular processes. The FEBS journal 280, 775–793 [DOI] [PubMed] [Google Scholar]
- 28.Kovacs JJ, Murphy PJ, Gaillard S, Zhao X, Wu JT, Nicchitta CV, Yoshida M, Toft DO, Pratt WB, and Yao TP (2005) HDAC6 regulates Hsp90 acetylation and chaperone-dependent activation of glucocorticoid receptor. Mol Cell 18, 601–607 [DOI] [PubMed] [Google Scholar]
- 29.Gattone VH 2nd, Wang X, Harris PC, and Torres VE (2003) Inhibition of renal cystic disease development and progression by a vasopressin V2 receptor antagonist. Nat Med 9, 1323–1326 [DOI] [PubMed] [Google Scholar]
- 30.Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, Perrone RD, Krasa HB, Ouyang J, Czerwiec FS, and Investigators TT (2012) Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med 367, 2407–2418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wei F, Karihaloo A, Yu Z, Marlier A, Seth P, Shibazaki S, Wang T, Sukhatme VP, Somlo S, and Cantley LG (2008) Neutrophil gelatinase-associated lipocalin suppresses cyst growth by Pkd1 null cells in vitro and in vivo. Kidney Int 74, 1310–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koulen P, Cai Y, Geng L, Maeda Y, Nishimura S, Witzgall R, Ehrlich BE, and Somlo S. (2002) Polycystin-2 is an intracellular calcium release channel. Nat Cell Biol 4, 191–197 [DOI] [PubMed] [Google Scholar]
- 33.Kahali S, Sarcar B, Prabhu A, Seto E, and Chinnaiyan P. (2012) Class I histone deacetylases localize to the endoplasmic reticulum and modulate the unfolded protein response. FASEB J 26, 2437–2445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cebotaru L, Liu Q, Yanda MK, Boinot C, Outeda P, Huso DL, Watnick T, Guggino WB, and Cebotaru V. (2016) Inhibition of histone deacetylase 6 activity reduces cyst growth in polycystic kidney disease. Kidney Int 90, 90–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yanda MK, Liu Q, and Cebotaru L. (2018) A potential strategy for reducing cysts in autosomal dominant polycystic kidney disease with a CFTR corrector. J Biol Chem 293, 11513–11526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim H, Xu H, Yao Q, Li W, Huang Q, Outeda P, Cebotaru V, Chiaravalli M, Boletta A, Piontek K, Germino GG, Weinman EJ, Watnick T, and Qian F. (2014) Ciliary membrane proteins traffic through the Golgi via a Rabep1/GGA1/Arl3-dependent mechanism. Nat Commun 5, 5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pazour GJ (2004) Intraflagellar transport and cilia-dependent renal disease: the ciliary hypothesis of polycystic kidney disease. Journal of the American Society of Nephrology : JASN 15, 2528–2536 [DOI] [PubMed] [Google Scholar]
- 38.Chapin HC, Rajendran V, and Caplan MJ (2010) Polycystin-1 surface localization is stimulated by polycystin-2 and cleavage at the G protein-coupled receptor proteolytic site. Mol Biol Cell 21, 4338–4348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fedeles SV, Tian X, Gallagher AR, Mitobe M, Nishio S, Lee SH, Cai Y, Geng L, Crews CM, and Somlo S. (2011) A genetic interaction network of five genes for human polycystic kidney and liver diseases defines polycystin-1 as the central determinant of cyst formation. Nature genetics 43, 639–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kurbegovic A, and Trudel M. (2016) Acute kidney injury induces hallmarks of polycystic kidney disease. Am J Physiol Renal Physiol 311, F740–F751 [DOI] [PubMed] [Google Scholar]
- 41.Brill AL, Fischer TT, Walters JM, Marlier A, Sewanan LR, Wilson PC, Johnson EK, Moeckel G, Cantley LG, Campbell SG, Nerbonne JM, Chung HJ, Robert ME, and Ehrlich BE (2020) Polycystin 2 is increased in disease to protect against stress-induced cell death. Sci Rep 10, 386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li A, Tian X, Zhang X, Huang S, Ma Y, Wu D, Moeckel G, Somlo S, and Wu G. (2015) Human polycystin-2 transgene dose-dependently rescues ADPKD phenotypes in Pkd2 mutant mice. Am J Pathol 185, 2843–2860 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.