Abstract
Background
Multiple myeloma is a malignant plasma cell disorder characterised by clonal plasma cells that cause end‐organ damage such as renal failure, lytic bone lesions, hypercalcaemia and/or anaemia. People with multiple myeloma are treated with immunomodulatory agents including lenalidomide, pomalidomide, and thalidomide. Multiple myeloma is associated with an increased risk of thromboembolism, which appears to be further increased in people receiving immunomodulatory agents.
Objectives
(1) To systematically review the evidence for the relative efficacy and safety of aspirin, oral anticoagulants, or parenteral anticoagulants in ambulatory patients with multiple myeloma receiving immunomodulatory agents who otherwise have no standard therapeutic or prophylactic indication for anticoagulation.
(2) To maintain this review as a living systematic review by continually running the searches and incorporating newly identified studies.
Search methods
We conducted a comprehensive literature search that included (1) a major electronic search (14 June 2021) of the following databases: Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library, MEDLINE via Ovid, and Embase via Ovid; (2) hand‐searching of conference proceedings; (3) checking of reference lists of included studies; and (4) a search for ongoing studies in trial registries. As part of the living systematic review approach, we are running continual searches, and we will incorporate new evidence rapidly after it is identified.
Selection criteria
Randomised controlled trials (RCTs) assessing the benefits and harms of oral anticoagulants such as vitamin K antagonist (VKA) and direct oral anticoagulants (DOAC), anti‐platelet agents such as aspirin (ASA), and parenteral anticoagulants such as low molecular weight heparin (LMWH)in ambulatory patients with multiple myeloma receiving immunomodulatory agents.
Data collection and analysis
Using a standardised form, we extracted data in duplicate on study design, participants, interventions, outcomes of interest, and risk of bias. Outcomes of interest included all‐cause mortality, symptomatic deep vein thrombosis (DVT), pulmonary embolism (PE), major bleeding, and minor bleeding. For each outcome we calculated the risk ratio (RR) with its 95% confidence interval (CI) and the risk difference (RD) with its 95% CI. We then assessed the certainty of evidence at the outcome level following the GRADE approach (GRADE Handbook).
Main results
We identified 1015 identified citations and included 11 articles reporting four RCTs that enrolled 1042 participants. The included studies made the following comparisons: ASA versus VKA (one study); ASA versus LMWH (two studies); VKA versus LMWH (one study); and ASA versus DOAC (two studies, one of which was an abstract).
ASA versus VKA
One RCT compared ASA to VKA at six months follow‐up. The data did not confirm or exclude a beneficial or detrimental effect of ASA relative to VKA on all‐cause mortality (RR 3.00, 95% CI 0.12 to 73.24; RD 2 more per 1000, 95% CI 1 fewer to 72 more; very low‐certainty evidence); symptomatic DVT (RR 0.57, 95% CI 0.24 to 1.33; RD 27 fewer per 1000, 95% CI 48 fewer to 21 more; very low‐certainty evidence); PE (RR 1.00, 95% CI 0.25 to 3.95; RD 0 fewer per 1000, 95% CI 14 fewer to 54 more; very low‐certainty evidence); major bleeding (RR 7.00, 95% CI 0.36 to 134.72; RD 6 more per 1000, 95% CI 1 fewer to 134 more; very low‐certainty evidence); and minor bleeding (RR 6.00, 95% CI 0.73 to 49.43; RD 23 more per 1000, 95% CI 1 fewer to 220 more; very low‐certainty evidence).
One RCT compared ASA to VKA at two years follow‐up. The data did not confirm or exclude a beneficial or detrimental effect of ASA relative to VKA on all‐cause mortality (RR 0.50, 95% CI 0.05 to 5.47; RD 5 fewer per 1000, 95% CI 9 fewer to 41 more; very low‐certainty evidence); symptomatic DVT (RR 0.71, 95% CI 0.35 to 1.44; RD 22 fewer per 1000, 95% CI 50 fewer to 34 more; very low‐certainty evidence); and PE (RR 1.00, 95% CI 0.25 to 3.95; RD 0 fewer per 1000, 95% CI 14 fewer to 54 more; very low‐certainty evidence).
ASA versus LMWH
Two RCTs compared ASA to LMWH at six months follow‐up. The pooled data did not confirm or exclude a beneficial or detrimental effect of ASA relative to LMWH on all‐cause mortality (RR 1.00, 95% CI 0.06 to 15.81; RD 0 fewer per 1000, 95% CI 2 fewer to 38 more; very low‐certainty evidence); symptomatic DVT (RR 1.23, 95% CI 0.49 to 3.08; RD 5 more per 1000, 95% CI 11 fewer to 43 more; very low‐certainty evidence); PE (RR 7.71, 95% CI 0.97 to 61.44; RD 7 more per 1000, 95% CI 0 fewer to 60 more; very low‐certainty evidence); major bleeding (RR 6.97, 95% CI 0.36 to 134.11; RD 6 more per 1000, 95% CI 1 fewer to 133 more; very low‐certainty evidence); and minor bleeding (RR 1.42, 95% CI 0.35 to 5.78; RD 4 more per 1000, 95% CI 7 fewer to 50 more; very low‐certainty evidence).
One RCT compared ASA to LMWH at two years follow‐up. The pooled data did not confirm or exclude a beneficial or detrimental effect of ASA relative to LMWH on all‐cause mortality (RR 1.00, 95% CI 0.06 to 15.89; RD 0 fewer per 1000, 95% CI 4 fewer to 68 more; very low‐certainty evidence); symptomatic DVT (RR 1.20, 95% CI 0.53 to 2.72; RD 9 more per 1000, 95% CI 21 fewer to 78 more; very low‐certainty evidence); and PE (RR 9.00, 95% CI 0.49 to 166.17; RD 8 more per 1000, 95% CI 1 fewer to 165 more; very low‐certainty evidence).
VKA versus LMWH
One RCT compared VKA to LMWH at six months follow‐up. The data did not confirm or exclude a beneficial or detrimental effect of VKA relative to LMWH on all‐cause mortality (RR 0.33, 95% CI 0.01 to 8.10; RD 3 fewer per 1000, 95% CI 5 fewer to 32 more; very low‐certainty evidence); symptomatic DVT (RR 2.32, 95% CI 0.91 to 5.93; RD 36 more per 1000, 95% CI 2 fewer to 135 more; very low‐certainty evidence); PE (RR 8.96, 95% CI 0.49 to 165.42; RD 8 more per 1000, 95% CI 1 fewer to 164 more; very low‐certainty evidence); and minor bleeding (RR 0.33, 95% CI 0.03 to 3.17; RD 9 fewer per 1000, 95% CI 13 fewer to 30 more; very low‐certainty evidence). The study reported that no major bleeding occurred in either arm.
One RCT compared VKA to LMWH at two years follow‐up. The data did not confirm or exclude a beneficial or detrimental effect of VKA relative to LMWH on all‐cause mortality (RR 2.00, 95% CI 0.18 to 21.90; RD 5 more per 1000, 95% CI 4 fewer to 95 more; very low‐certainty evidence); symptomatic DVT (RR 1.70, 95% CI 0.80 to 3.63; RD 32 more per 1000, 95% CI 9 fewer to 120 more; very low‐certainty evidence); and PE (RR 9.00, 95% CI 0.49 to 166.17; RD 8 more per 1000, 95% CI 1 fewer to 165 more; very low‐certainty evidence).
ASA versus DOAC
One RCT compared ASA to DOAC at six months follow‐up. The data did not confirm or exclude a beneficial or detrimental effect of ASA relative to DOAC on DVT, PE, and major bleeding and minor bleeding (minor bleeding: RR 5.00, 95% CI 0.31 to 79.94; RD 4 more per 1000, 95% CI 1 fewer to 79 more; very low‐certainty evidence). The study reported that no DVT, PE, or major bleeding events occurred in either arm. These results did not change in a meta‐analysis including the study published as an abstract.
Authors' conclusions
The certainty of the available evidence for the comparative effects of ASA, VKA, LMWH, and DOAC on all‐cause mortality, DVT, PE, or bleeding was either low or very low. People with multiple myeloma considering antithrombotic agents should balance the possible benefits of reduced thromboembolic complications with the possible harms and burden of anticoagulants.
Editorial note: This is a living systematic review. Living systematic reviews offer a new approach to review updating in which the review is continually updated, incorporating relevant new evidence as it becomes available. Please refer to the Cochrane Database of Systematic Reviews for the current status of this review.
Plain language summary
Blood thinners in people with multiple myeloma
Background
The risk of blood clots is high in people with blood cancer, especially in those who have multiple myeloma, cancer that begins in plasma cells, a type of white blood cell. Lenalidomide, pomalidomide, and thalidomide are common treatments for multiple myeloma, which when combined with other chemotherapy agents, have been shown to increase the risk of blood clots.
Study characteristics
We searched the scientific databases for clinical trials looking at the effects of different blood thinners on blood clots in people with multiple myeloma receiving immunomodulatory agents (lenalidomide, pomalidomide, and/or thalidomide). The studies looked at survival, blood clots in the limbs or in the lung, and/or bleeding. The evidence is current to 14 June 2021.
Key results
We included four studies enrolling a total of 1042 people with multiple myeloma. The included studies made the following comparisons: aspirin (oral medication used to prevent blood clots) to vitamin K antagonist (VKA) (oral blood thinner) (one study); aspirin to low molecular weight heparin (LMWH) (injectable blood thinner) (two studies); VKA to LMWH (one study); and aspirin to direct oral anticoagulants (oral blood thinner) (two studies). In people with multiple myeloma receiving thalidomide, the data do not provide a clear answer about the comparative effect of these drugs on all of the studied outcomes (death, blood clots, bleeding).
Certainty of the evidence
When comparing aspirin to VKA, aspirin to LMWH, or VKA to LMWH, the certainty of the evidence was very low for all studied outcomes (death, blood clots in the limbs or the lung, and bleeding). When comparing aspirin to direct oral anticoagulants, the certainty of the evidence was very low for all of the studied outcomes (death, blood clots in the limbs or the lung, and bleeding).
Editorial note: This is a living systematic review. Living systematic reviews offer a new approach to review updating in which the review is continually updated, incorporating relevant new evidence as it becomes available. Please refer to the Cochrane Database of Systematic Reviews for the current status of this review.
Summary of findings
Background
Description of the condition
Multiple myeloma is a malignant plasma cell disorder characterised by neoplastic proliferation of clonal plasma cells producing monoclonal immunoglobulin and causing end‐organ damage such as renal failure, lytic bone lesions, hypercalcaemia and/or anaemia (Rajkumar 2014). Multiple myeloma has an incidence of approximately six cases per 100,000 people per year in the United States (Costa 2017). In the last 10 to 15 years, therapeutic options for people with multiple myeloma have expanded dramatically, contributing to improvements in patients’ five‐ and 10‐year overall survival (Costa 2017). One area of advancement has been the introduction of immunomodulatory agents including thalidomide and its derivatives lenalidomide and pomalidomide. Whilst these agents have contributed to improved patient outcomes, they have also introduced unique toxicities. In particular, immunomodulatory agents appear to increase the risk of thromboembolic events. People with cancer have a four to six fold‐ increased risk of venous thromboembolism compared to the general population; that risk increases up to 28‐fold in people with haematological malignancies (Blom 2005). The baseline incidence of thromboembolic events in people with multiple myeloma not receiving immunomodulatory agents is high, with risk estimates ranging from 4% to 12% of all patients (Rajkumar 2002). Immunomodulatory agents, particularly when given with dexamethasone or with chemotherapy such as anthracycline, appear to increase this baseline risk, with estimates ranging up to 28% of all people (Musallam 2009; Zangari 2001), and up to 59% when given with chemotherapy (Baz 2005). The reason for the increase in thrombosis secondary to the use of Immunomodulatory agents is unknown. However, it has been shown that serum levels of the anticoagulant cofactor thrombomodulin decrease in people treated with thalidomide (Corso 2004). Moreover, extremely high levels of von Willebrand factor antigen and factor VIII, known factors associated with increased risk of thrombosis, have been documented in people with multiple myeloma receiving thalidomide, dexamethasone, and chemotherapy (Minnema 2003).
See Table 8 for a list of abbreviations used throughout this review.
1. Abbreviations.
| Abbreviation | Term |
| ASA | Anti‐platelet agents (acetylsalicylic acid) such as aspirin |
| ASH | American Society of Hematology |
| ASCO | American Society of Clinical Oncology |
| CENTRAL | Cochrane Central Register of Controlled Trials |
| CI | Confidence interval |
| CT | Computed tomography |
| DM | Diabetes Mellitus |
| DOAC | Direct oral anticoagulants |
| DVT | Deep vein thrombosis |
| GRADE | Grading of Recommendations, Assessment, Development and Evaluations |
| IMWG | International Myeloma Working Group |
| ITT | Intention‐to‐treat |
| LMWH | Low molecular weight heparin |
| NCCN | National Comprehensive Cancer Network |
| PE | Pulmonary embolism |
| RCT | Randomised controlled trial |
| RD | Risk Difference |
| RR | Relative risk |
| TEE | Transesophageal echocardiogram |
| VKA | Vitamin K antagonist |
| VTE | Venous thromboembolic events |
| WHO ICTRP | World Health Organization International Clinical Trials Registry Platform |
Description of the intervention
The intervention of interest in this review is prophylactic anticoagulation with vitamin K antagonists (VKA), low molecular weight heparin (LMWH), or direct oral anticoagulants (DOAC) in people with multiple myeloma receiving immunomodulatory agents. VKAs have been the mainstay of oral anticoagulant therapy since the mid‐1950s. Well‐designed clinical trials have shown their effectiveness for the primary and secondary prevention of several venous and arterial thrombotic diseases (Ansell 2008).
LMWHs do not have intrinsic anticoagulant activity but potentiate the activity of antithrombin III in inhibiting activated coagulation factors. These agents constitute indirect anticoagulants, as their activity is mediated by plasma cofactors. LMWHs are not absorbed orally and must be administered parenterally by subcutaneous injections (Hirsh 1993). In recent years, DOACs have become an alternative to LMWH for the treatment of thrombosis, mainly due to their rapid onset of action and convenience of oral administration (Farge 2019).
How the intervention might work
Venous thromboembolic events (VTE) are common in people with multiple myeloma, especially in those receiving immunomodulatory agents. Prophylactic anticoagulants may improve outcomes by reducing the incidence of these events. Moreover, researchers have hypothesised that anticoagulants may improve outcomes in people with cancer through an antitumour effect in addition to its antithrombotic effect (Nagy 2009; Park 2015; Sanford 2014; Smorenburg 2001; Thodiyil 2002). The American Society of Clinical Oncology (ASCO) guidelines recommend prophylaxis with LMWH or aspirin (ASA) in patients receiving thalidomide, lenalidomide, or pomalidomide with chemotherapy or dexamethasone, or both (Lyman 2015). The International Myeloma Working Group (IMWG) and the National Comprehensive Cancer Network (NCCN) recommend prophylaxis with ASA for patients receiving thalidomide or its derivatives with a lower risk of VTE, and LMWH or VKA for patients at higher risk of VTE (Palumbo 2008; Palumbo 2014). Few RCTs have been conducted comparing the safety and effectiveness of these medications in people with multiple myeloma receiving immunomodulatory agents with or without other anticancer medications.
Why it is important to do this review
This is the first systematic review to specifically assess the evidence for primary thromboprophylaxis in people with multiple myeloma.
Living systematic review approach: We will maintain this review as a living systematic review by continually running the searches and incorporating newly identified studies (for more information about the living systematic review approach by Cochrane, see Appendix 1). We consider that a living systematic review approach is appropriate for this review for three reasons. Firstly, the review addresses an important subject for the clinical practice: people with multiple myeloma are at increased risk of developing VTE, especially after starting treatment with immunomodulatory agents (Fradley 2018). Secondly, several trials in this area are still ongoing and might present important new data to incorporate in a timely manner (Louzada 2018 (RithMM)). Thirdly, this living systematic review may be used as part of a living guideline project (Akl 2017).
Objectives
(1)To systematically review the evidence for the relative efficacy and safety of aspirin, oral anticoagulants, or parenteral anticoagulants in ambulatory patients with multiple myeloma receiving immunomodulatory agents who otherwise have no standard therapeutic or prophylactic indication for anticoagulation.
(2) To maintain this review as a living systematic review by continually running the searches and incorporating newly identified studies.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs).
Types of participants
Ambulatory participants of any age (including children) with multiple myeloma and without VTE receiving immunomodulatory agents (e.g. thalidomide, lenalidomide, pomalidomide) with no other standard indication for prophylactic anticoagulation (e.g. for acute illness, for central venous line placement, perioperatively) or for therapeutic anticoagulation (e.g. for the treatment of deep vein thrombosis (DVT) or pulmonary embolism (PE)). Participants might be also receiving any chemotherapy, corticosteroids and/or systemic therapies (e.g. monoclonal antibodies or proteasome inhibitors).
Types of interventions
Intervention: pharmacological thromboprophylaxis with:
oral anticoagulants, e.g. VKA and DOACs;
antiplatelet agents, e.g. ASA;
parenteral anticoagulants, e.g. LMWH.
Comparator: no pharmacological thromboprophylaxis or any of the agents listed above (i.e. as an active comparator).
We excluded studies in which thrombolytic therapy (e.g. streptokinase) was part of the intervention.
Types of outcome measures
Primary outcomes
All‐cause mortality.
Secondary outcomes
Symptomatic DVT: events had to be suspected clinically, and diagnosed using an objective diagnostic test.
PE: events had to be suspected clinically, and diagnosed using an objective diagnostic test.
Major bleeding: we accepted the authors' definitions of major bleeding.
Minor bleeding: we accepted the authors' definitions of minor bleeding.
We considered symptomatic DVT and PE as efficacy outcomes, and major and minor bleeding as safety outcomes.
Search methods for identification of studies
Electronic searches
We conducted a comprehensive search on 14 June 2021. We did not use language restrictions. We searched the following electronic databases: the Cochrane Central Register of Controlled Trials (CENTRAL; 2021, Issue 6) in the Cochrane Library, MEDLINE via Ovid (1946 to 14 June 2021), and Embase via Ovid (1980 to 14 June 2021). The full search strategies for each of the electronic databases are shown in Appendix 2.
Living systematic review approach: We will be updating the searches using auto‐alerts monthly. We will review search methods and strategies approximately annually to ensure that they reflect any terminology changes in the topic area or the databases.
Searching other resources
We hand‐searched the conference proceedings of the ASCO, starting with its first volume, 1982, up to 14 June 2021, and the American Society of Hematology (ASH) starting with its 2003 issue up to 14 June 2021. We also searched the US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov/) and the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (www.who.int/ictrp/search/en/) for ongoing studies up to 14 June 2021. We reviewed the reference lists of papers included in this review and of other relevant systematic reviews. We contacted experts in the field to check for unpublished and ongoing trials.
Living systematic review approach: We will search monthly the conference proceedings of ASCO and ASH soon after their publications; ClinicalTrials.gov; and WHO ICTRP. As an additional step, we will contact the corresponding authors of ongoing studies as they are identified and ask them to advise when results are available. We will continue to review the reference lists for any prospectively identified studies. Furthermore, we will contact the corresponding authors of any newly included studies for information as to other relevant studies.
Data collection and analysis
Selection of studies
Four pairs of review authors (LAK, CFM, MH, MB, IGT, FS, VY) independently screened the titles and abstracts of identified articles for eligibility. We retrieved the full text of articles judged as potentially eligible by at least one review author. The review authors then independently screened the full‐text articles for eligibility using a standardised form with explicit inclusion and exclusion criteria (see Criteria for considering studies for this review), resolving any disagreements by discussion or by consulting a third review author.
Living systematic review approach: For the monthly searches, we will immediately screen any new citations retrieved each month. As the first step of monthly screening, we will apply the machine learning classifier (RCT model) available in the Cochrane Register of Studies (CSR‐Web; Wallace 2017). The machine learning classifier currently has a specificity/recall of 99.987% and assigns a probability (from 0 to 100) to each citation for being a true RCT. For citations assigned a score from 10 to 100, we will screen these in duplicate and independently. Citations that score 9 or less will be screened by Cochrane Crowd (Cochrane Crowd). Any citations that are deemed to be potential RCTs (i.e., scored 10 or above) by Cochrane Crowd will be returned to the review authors for screening.
Data extraction and management
Two review authors (CFM and IGT) independently extracted data from each included study, resolving any disagreements by discussion. We aimed to collect the following data.
Participants
Number of participants randomised to each study arm.
Number of participants followed up in each study arm.
Population characteristics (e.g. age, gender, comorbidities, co‐interventions).
History of VTE.
Stage of multiple myeloma.
Time since multiple myeloma diagnosis.
Multiple myeloma therapy (e.g. immunomodulators such as thalidomide or pomalidomide).
Interventions
Type of pharmacological intervention: oral anticoagulants (e.g. VKA, DOACs); parenteral anticoagulants (e.g. LMWH); antiplatelet agents (e.g. ASA).
Intensity of VKA therapy (international normalised ratio (INR) target) or dose, if applicable.
Type and dosage schedule of LMWH.
Dosage schedule of antiplatelet agents.
Duration of treatment.
Control: no pharmacological thromboprophylaxis or any of the agents listed above (oral anticoagulants, e.g. VKA and DOACs; antiplatelet agents, e.g. ASA; parenteral anticoagulants, e.g. LMWH).
Co‐interventions including corticosteroids, chemotherapy, immunomodulatory agents, target therapy, immunotherapy, or radiotherapy (type and duration).
Outcomes
We attempted to extract both time‐to‐event data (for survival outcome) and categorical data (for all outcomes). However, none of the studies reported time‐to‐event data.
For dichotomous variables, we extracted data needed to conduct a complete‐case analysis as the primary analysis.
We attempted to contact study authors for incompletely reported data. We decided a priori to consider abstracts in the main analysis only if the study authors supplied us with full reports of their methods and results; otherwise, abstracts were included only in the sensitivity analysis.
Other
Source of funding.
Ethical approval.
Conflict of interest.
Whether the intention‐to‐treat (ITT) principle was applied.
Assessment of risk of bias in included studies
We assessed the risk of bias at the study level using Cochrane's risk of bias tool (Higgins 2011). Two review authors (CFM and IGT) independently assessed the methodological quality of each included study, resolving any disagreements by discussion. The risk of bias criteria were as follows:
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other bias (e.g. whether the study was stopped early for benefit).
For information on assessing the risk of bias associated with participants with missing data per outcome and across studies, see Dealing with missing data.
Measures of treatment effect
We collected and analysed risk ratios (RRs) for dichotomous data. None of the outcomes of interest was reported as a continuous variable.
Unit of analysis issues
The unit of analysis was the individual participant.
Dealing with missing data
Identifying participants with missing data
It was not clear whether certain categories of participants (e.g. those described as "withdrew consent" or "experienced adverse events") were actually followed up by the trial authors (versus had missing data) (Akl 2016). To identify participants with missing data, we followed the guidance suggested by Kahale and colleagues (Kahale 2019), described below.
Definitely not missing data: (1) participants explicitly reported as followed up; (2) participants who died during the trial; (3) participants belonging to centers that were excluded.
Definitely missing data: (1) participants explicitly reported as not followed up; (2) participants with unclear follow‐up status and (a) excluded from the denominator of the analysis (i.e. complete‐case analysis); or (b) included in the denominator of the analysis, and their outcomes were explicitly stated to be imputed. However, we did not treat them as missing data unless it was possible to obtain the number of observed/actual events (i.e. excluding imputed events) to avoid double counting.
Potentially missing data: participants with unclear follow‐up status (e.g. included in the denominator of the analysis, and their outcomes were not explicitly stated to be imputed).
Dealing with participants with missing data in the primary meta‐analysis
We used a complete‐case analysis approach in the primary meta‐analysis, that is excluding participants considered to have missing data (Guyatt 2017; Kahale 2020).
For categorical data, we used the following calculations for each study arm:
denominator: (number of participants randomised) − (number of participants definitely with missing data);
numerator: number of participants with observed events (i.e. participants who experienced at least one event for the outcome of interest during their available follow‐up time).
Assessing risk of bias associated with participants with missing data
When the primary meta‐analysis of a specific outcome found a statistically significant effect, we conducted sensitivity meta‐analyses to assess the risk of bias associated with missing outcome data. Those sensitivity meta‐analyses used a priori plausible assumptions about the outcomes of participants considered to have missing data. The assumptions we used in the sensitivity meta‐analyses were increasingly stringent in order to challenge the statistical significance of the results of the primary analysis progressively (Akl 2013; Kahale 2020).
For categorical data and for an RR showing a reduction in effect (RR < 1), we used the following increasingly stringent but plausible assumptions.
For the control arm, relative incidence (RI) amongst those with missing data (lost to follow‐up (LTFU)) compared with those with available data (followed up, FU) in the same arm (RILTFU/FU) = 1; for the intervention arm, RILTFU/FU = 1.5.
For the control arm, RILTFU/FU = 1; for the intervention arm, RILTFU/FU = 2.
For the control arm, RILTFU/FU = 1; for the intervention arm, RILTFU/FU = 3.
For the control arm, RILTFU/FU = 1; for the intervention arm, RILTFU/FU = 5.
For RR showing an increase in effect (RR > 1), we switched the above assumptions between the control and interventions arms (i.e. used RILTFU/FU = 1 for the intervention arm).
Specifically, we used the following calculations for each study arm:
denominator: (number of participants randomised);
numerator: (number of participants with observed events) + (number of participants definitely with missing data with assumed events).
Assumed events are calculated by applying the a priori plausible assumptions to the participants definitely with missing data.
As noted above, none of the outcomes of interest was meta‐analysed as a continuous variable.
Assessment of heterogeneity
We assessed heterogeneity between trials by visual inspection of forest plots, estimation of the percentage heterogeneity between trials that could not be ascribed to sampling variation (I2 statistic; Higgins 2011), and by a formal statistical test of the significance of the heterogeneity (Deeks 2001). If there was evidence of substantial heterogeneity, we investigated and reported the possible reasons for it (see Subgroup analysis and investigation of heterogeneity).
Assessment of reporting biases
We explored whether the study was included in a trial registry and whether a protocol was available. We planned to create funnel plots for outcomes including 10 or more trials.
Data synthesis
For dichotomous data, we calculated the RR separately for each study (Review Manager 2020). As noted earlier, we used a complete‐case analysis approach in the primary meta‐analysis, that is excluding participants considered to have missing data (Guyatt 2017). When analysing data related to participants who were reported as not compliant, we attempted to adhere to the principles of ITT analysis. We approached the issue of non‐compliance independently from that of missing data (Alshurafa 2012). We then pooled the results of the different studies using a random‐effects model.
Living systematic review approach: Whenever new evidence (studies, data, or information) that meets the review inclusion criteria is identified, we will immediately assess the risk of bias and extract the data and incorporate this information in the synthesis, as appropriate. We will not adjust the meta‐analyses to account for multiple testing given that the methods related to frequent updating of meta‐analyses are under development (Simmonds 2017).
Subgroup analysis and investigation of heterogeneity
We planned subgroup analyses based on characteristics of participants (e.g. stage of multiple myeloma, dose of corticosteroids, type of chemotherapy/immunomodulatory agents), but did not conduct these analyses due to insufficient data.
Sensitivity analysis
As described above under sections of 'Data extraction and management and Dealing with missing data', we planned sensitivity analyses to:
assess the risk of bias associated with missing outcome data when the primary meta‐analysis of a specific outcome found a statistically significant effect;
include abstracts without full reports of study methods and results.
Summary of findings and assessment of the certainty of the evidence
We assessed the certainty of evidence at the outcome level using the GRADE approach for each of the following four comparisons (GRADE handbook and Guyatt 2011).
ASA versus VKA.
ASA versus LMWH.
VKA versus LMWH.
ASA versus DOAC.
We followed the guidance developed by the GRADE Working Group to communicate the findings of the systematic review (Santesso 2020).
Results
Description of studies
Results of the search
A study flow diagram is shown in Figure 1. As of 14 June 2021, the search strategy identified a total of 1015 unique citations. Title and abstract screening identified 18 potentially eligible citations. Screening of the full‐text reports identified 11 articles reporting four RCTs that enrolled 1042 participants; three eligible RCTs published as full reports (Larocca 2012; Palumbo 2011; Sayar 2019), and one eligible study published as an abstract but for which we were unable to obtain the necessary data from the authors (Campos‐Cabrera 2018 (abstract)). We identified one ongoing study comparing DOAC to ASA (Louzada 2018 (RithMM)).
1.
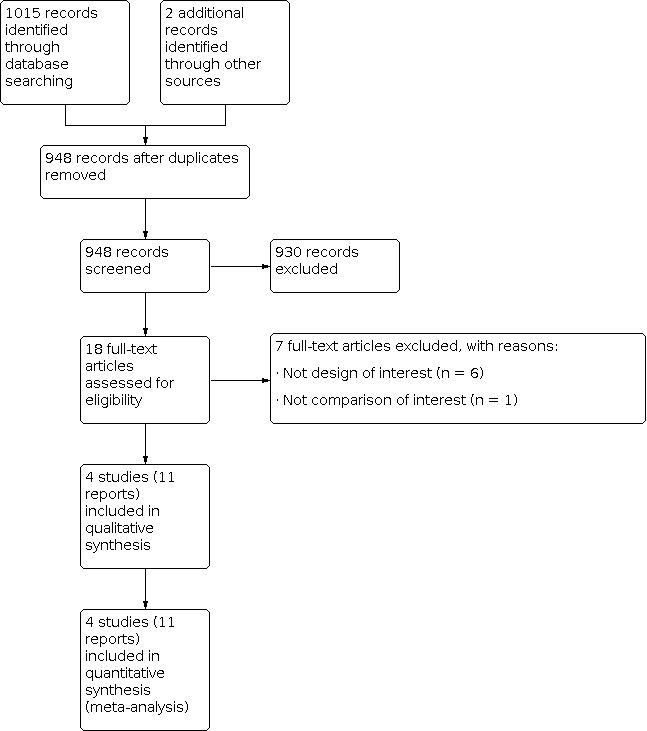
Study flow diagram.
Included studies
For details, see the Characteristics of included studies.
The four included RCTs enrolled a total of 1042 participants. One study compared prophylaxis with ASA versus LMWH (Larocca 2012); one compared prophylaxis with ASA versus VKA versus LMWH (Palumbo 2011); and two compared prophylaxis with ASA versus DOAC (Campos‐Cabrera 2018 (abstract); Sayar 2019).
Larocca and colleagues randomised 342 participants with newly diagnosed multiple myeloma aged between 18 and 65 years (Larocca 2012). Participants were randomised to receive ASA 100 mg/day orally or enoxaparin 40 mg/day subcutaneously. All participants enrolled in the study received induction with lenalidomide plus low‐dose dexamethasone treatment comprising four 28‐day cycles of lenalidomide in combination with dexamethasone (40 mg/day orally on days 1, 8, 15, and 22), followed by cyclophosphamide for stem cell mobilisation and collection before entering the consolidation phase with either melphalan‐prednisone‐lenalidomide or melphalan 200 mg/m2. Prophylaxis was administered during the four cycles of lenalidomide plus low‐dose dexamethasone therapy and the six cycles of melphalan‐prednisone‐lenalidomide consolidation. Participants who were assigned to the melphalan consolidation arm stopped thromboprophylaxis at the end of the induction. Outcomes assessed were a composite primary endpoint defined as the proportion of participants developing a first episode of symptomatic DVT, PE, arterial thrombosis, any cardiovascular event, or sudden death. Secondary endpoints included major and minor bleeding. The study authors did not report on follow‐up data.
Palumbo and colleagues recruited 667 participants with newly diagnosed multiple myeloma (Palumbo 2011). This is a subgroup of two different studies enrolling 991 participants. In one study, participants aged less than 65 years were randomly assigned to bortezomib, thalidomide (200 mg/day), and dexamethasone (320 mg) or thalidomide and dexamethasone in each 21‐day cycle for three cycles as induction therapy before autologous transplantation. In the other study, participants aged more than 65 years were randomly assigned to bortezomib, melphalan, prednisone (60 mg/m2 on days 1 to 4), and thalidomide (50 mg/day) for nine cycles followed by continuous therapy with bortezomib and thalidomide (50 mg/day), or to bortezomib, melphalan, and prednisone for nine cycles without any further continuous treatment. Participants randomly assigned to receive bortezomib, melphalan, and prednisone did not receive any antithrombotic prophylaxis. Participants receiving thalidomide‐based regimens in both trials were eligible for the substudy. Participants receiving thalidomide‐based regimens were randomly assigned to receive one of the following: ASA 100 mg/day orally, VKA (warfarin) 1.25 mg/day orally, or LMWH (enoxaparin) 40 mg/day subcutaneously. The prophylaxis was administered during the three cycles of induction therapy in the younger participants and during the first six cycles of induction therapy in the elderly participants. Outcomes assessed were a composite primary endpoint defined as the proportion of participants developing a first episode of symptomatic DVT, PE, arterial thrombosis, any cardiovascular event, or sudden death. Secondary endpoints included major and minor bleeding. The study authors reported 99% follow‐up.
Sayar and colleagues conducted a randomised, open‐label phase IV feasibility clinical trial to prepare for a multicentre trial at King’s College Hospital and Princess Royal University Hospital (PRUH) and identify any safety concerns with apixaban (Sayar 2019). Participants with newly diagnosed multiple myeloma were randomised to either standard thromboprophylaxis (enoxaparin 40 mg administered as a subcutaneous injection daily if classified as high risk for VTE, and ASA 75 mg orally daily if considered standard risk for VTE according to the Palumbo risk assessment model) or apixaban 2.5 mg twice a day. Ten participants were recruited: two were considered high risk and received apixaban, and eight were considered standard risk, of which four were randomised to ASA and four to apixaban. Amongst the 10 participants, 10% received bortezomib (Velcade)/thalidomide/dexamethasone (VTD); 70% received carfilzomib/cyclophosphamide/dexamethasone (CCD); and 100% received bortezomib (Velcade)/melphalan/prednisolone (VMP). Participants were followed up for six months or until in remission.
Campos‐Cabrera and colleagues randomised 23 participants with multiple myeloma receiving thalidomide and dexamethasone‐based triplet induction therapy to receive either 100 mg ASA (18 participants) or 10 mg rivaroxaban (five participants) (Campos‐Cabrera 2018 (abstract)). Doppler ultrasound was performed every six months or as a medical indication in all participants, and pulmonary computed tomography (CT)scan was performed if PE was suspected. Outcomes assessed were thrombosis and bleeding.
Excluded studies
We excluded seven studies for the following reasons (see Characteristics of excluded studies): not the design of interest (n = 6) or not the comparison of interest (n = 1).
Risk of bias in included studies
Risk of bias judgements are summarised in Figure 2 and Figure 3.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The method of sequence generation as well as allocation concealment was clear and adequate in Larocca 2012and Palumbo 2011 and not reported in Campos‐Cabrera 2018 (abstract) and Sayar 2019
Blinding
Blinding of participants and personnel (performance bias)
Participants and personnel were not blinded in all four studies (Campos‐Cabrera 2018 (abstract); Larocca 2012; Palumbo 2011; Sayar 2019)
Blinding of outcome assessment (detection bias)
Blinding of outcome assessment was not reported in all four studies; however, we judged that knowledge of the assigned intervention would likely not impact the assessment of outcomes of interest (all‐cause mortality, DVT, PE, bleeding, etc.)
Incomplete outcome data
Larocca 2012 and Sayar 2019 did not report on incomplete outcome data. Campos‐Cabrera 2018 (abstract) and Palumbo 2011 reported almost complete follow‐up.
Selective reporting
None of the studies were registered or had a published protocol. Campos‐Cabrera 2018 (abstract), Larocca 2012, and Sayar 2019 reported the outcomes listed in their methods sections. Palumbo 2011 did not report toxicity, which was mentioned in the study's methods section.
Other potential sources of bias
None noted.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7
Summary of findings 1. Aspirin (ASA) compared to vitamin K antagonist (VKA) for ambulatory patients with multiple myeloma receiving immunomodulatory agents: 6 months follow‐up.
| Aspirin (ASA) compared to vitamin K antagonist (VKA) for ambulatory patients with multiple myeloma receiving immunomodulatoryagents: 6 months follow‐up | |||||
| Patient or population: ambulatory patients with multiple myeloma receiving immunomodulatory agents: 6 months follow‐up Setting: outpatient Intervention: aspirin prophylaxis Control: vitamin K antagonist prophylaxis | |||||
| Outcomes | № of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with VKA | Risk difference with ASA | ||||
| All‐cause mortality follow‐up: 6 months | 440 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 | RR 3.00 (0.12 to 73.24) | Low | |
| 1 per 1000 3 | 2 more per 1000 (1 fewer to 72 more) | ||||
| Symptomatic deep vein thrombosis follow‐up: 6 months | 440 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 2 4 | RR 0.57 (0.24 to 1.33) | Study population | |
| 64 per 1000 | 27 fewer per 1000 (48 fewer to 21 more) | ||||
| Pulmonary embolism follow‐up: 6 months | 440 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 2 5 | RR 1.00 (0.25 to 3.95) | Study population | |
| 18 per 1000 | 0 fewer per 1000 (14 fewer to 54 more) | ||||
| Major bleeding follow‐up: 6 months | 440 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 2 6 | RR 7.00 (0.36 to 134.72) | Low | |
| 1 per 1000 3 | 6 more per 1000 (1 fewer to 134 more) | ||||
| Minor bleeding follow‐up: 6 months | 440 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 2 7 | RR 6.00 (0.73 to 49.43) | Study population | |
| 5 per 1000 | 23 more per 1000 (1 fewer to 220 more) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
1Downgraded by two levels due to concerns about imprecision; 95% CI is consistent with the possibility of no effect (1 per 1000 absolute reduction) and the possibility of important harm (72 per 1000 absolute increase), including one event in total. Given the observed baseline risk of 0%, we used 0.1% to generate an absolute effect and a confidence interval. 2Downgraded by one level due to serious risk of bias. Lack of blinding of participants and personnel and selective reporting (all outcomes listed in the methods section were reported on in the results except for secondary endpoint related to any toxicity that required interruption of study prophylaxis). 3There were zero events in the control arm. 4Downgraded by two levels due to concerns about imprecision; 95% CI is consistent with the possibility of important benefit (48 per 1000 absolute reduction) and the possibility of important harm (21 per 1000 absolute increase), including 22 events in total. 5Downgraded by two levels due to concerns about imprecision; 95% CI is consistent with the possibility of important benefit (14 per 1000 absolute reduction) and the possibility of important harm (54 per 1000 absolute increase), including eight events in total. 6Downgraded by two levels due to concerns about imprecision; 95% CI is consistent with the possibility of no effect (1 per 1000 absolute reduction) and the possibility of important harm (134 per 1000 absolute increase), including three events in total. Given the observed baseline risk of 0%, we used 0.1% to generate an absolute effect and a confidence interval. 7Downgraded by two levels due to concerns about imprecision; 95% CI is consistent with the possibility of no effect (1 per 1000 absolute reduction) and the possibility of important harm (220 per 1000 absolute increase), including seven events in total.
Summary of findings 2. Aspirin (ASA) compared to vitamin K antagonist (VKA) for ambulatory patients with multiple myeloma receiving immunomodulatory agents: 2 years follow‐up.
| Aspirin (ASA) compared to vitamin K antagonist (VKA) for ambulatory patients with multiple myeloma receiving immunomodulatory agents: 2 years follow‐up | |||||
| Population: ambulatory patients with multiple myeloma receiving immunomodulatory agents Setting: outpatient Intervention: ASA prophylaxis Control: VKA prophylaxis | |||||
| Outcomes | № of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with VKA | Risk difference with ASA | ||||
| All‐cause mortality follow‐up: 2 years | 440 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 | RR 0.50 (0.05 to 5.47) | Study population | |
| 9 per 1000 | 5 fewer per 1000 (9 fewer to 41 more) | ||||
| Symptomatic deep vein thrombosis follow‐up: 2 years | 440 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 3 | RR 0.71 (0.35 to 1.44) | Study population | |
| 77 per 1000 | 22 fewer per 1000 (50 fewer to 34 more) | ||||
| Pulmonary embolism follow‐up: 2 years | 440 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 4 | RR 1.00 (0.25 to 3.95) | Study population | |
| 18 per 1000 | 0 fewer per 1000 (14 fewer to 54 more) | ||||
| Major bleeding ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Minor bleeding ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
1Downgraded by one level due to serious risk of bias. Lack of blinding of participants and personnel and selective reporting (all outcomes listed in the methods section were reported on except for secondary endpoint related to any toxicity that required interruption of study prophylaxis). 2Downgraded by two levels due to concerns about imprecision; 95% CI is consistent with the possibility of benefit (9 per 1000 absolute reduction) and the possibility of important harm (41 per 1000 absolute increase), including three events in total. 3Downgraded by two levels due to concerns about imprecision; 95% CI is consistent with the possibility of benefit (21 per 1000 absolute reduction) and the possibility of important harm (78 per 1000 absolute increase), including 22 events in total. 4Downgraded by two levels due to concerns about imprecision; 95% CI is consistent with the possibility of no effect (1 per 1000 absolute reduction) and the possibility of important harm (165 per 1000 absolute increase), including four events in total. Given the observed baseline risk of 0%, we used 0.1% to generate an absolute effect and a confidence interval.
Summary of findings 3. Aspirin (ASA) compared to low molecular weight heparin (LMWH) for ambulatory patients with multiple myeloma receiving immunomodulatory agents: 6 months follow‐up.
| Aspirin (ASA) compared to low molecular weight heparin (LMWH) for ambulatory patients with multiple myeloma receiving immunomodulatory agents: 6 months follow‐up | |||||
| Population: ambulatory patients with multiple myeloma receiving immunomodulatory agents Setting: outpatient Intervention: ASA prophylaxis Control: LMWH prophylaxis | |||||
| Outcomes | № of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with LMWH | Risk difference with ASA | ||||
| All‐cause mortality follow‐up: 6 months | 781 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 | RR 1.00 (0.06 to 15.81) | Study population | |
| 3 per 1000 | 0 fewer per 1000 (2 fewer to 38 more) | ||||
| Symptomatic deep vein thrombosis follow‐up: 6 months | 781 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 3 | RR 1.23 (0.49 to 3.08) | Study population | |
| 21 per 1000 | 5 more per 1000 (11 fewer to 43 more) | ||||
| Pulmonary embolism follow‐up: 6 months | 781 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 4 | RR 7.71 (0.97 to 61.44) | Low | |
| 1 per 1000 5 | 7 more per 1000 (0 fewer to 60 more) | ||||
| Major bleeding follow‐up: 6 months | 781 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 6 | RR 6.97 (0.36 to 134.11) | Low | |
| 1 per 1000 5 | 6 more per 1000 (1 fewer to 133 more) | ||||
| Minor bleeding follow‐up: 6 months | 781 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 7 | RR 1.42 (0.35 to 5.78) | Study population | |
| 10 per 1000 | 4 more per 1000 (7 fewer to 50 more) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
1Downgraded by one level due to serious risk of bias. Lack of blinding of participants and personnel (in Palumbo 2011 and Larocca 2012) and selective reporting (in Palumbo 2011, all outcomes listed in the methods section were reported on except for secondary endpoint related to any toxicity that required interruption of study prophylaxis). 2Downgraded by two levels due to concerns about imprecision; 95% CI is consistent with the possibility of no effect (2 per 1000 absolute reduction) and the possibility of important harm (38 per 1000 absolute increase), including two events in total. 3Downgraded by two levels due to concerns about imprecision; 95% CI is consistent with the possibility of benefit (11 per 1000 absolute reduction) and the possibility of important harm (43 per 1000 absolute increase), including 18 events in total. 4Downgraded by two levels due to concerns about imprecision; 95% CI is consistent with the possibility of no effect (0 per 1000 absolute reduction) and the possibility of important harm (60 per 1000 absolute increase), including seven events in total. Given the observed baseline risk of 0%, we used 0.1% to generate an absolute effect and a confidence interval. 5There were zero events in the control arm. 6Downgraded by two levels due to concerns about imprecision; 95% CI is consistent with the possibility of benefit (1 per 1000 absolute reduction) and the possibility of important harm (133 per 1000 absolute increase), including three events in total. Given the observed baseline risk of 0%, we used 0.1% to generate an absolute effect and a confidence interval. 7Downgraded by two levels due to concerns about imprecision; 95% CI is consistent with the possibility of benefit (7 per 1000 absolute reduction) and the possibility of important harm (50 per 1000 absolute increase), including 10 events in total.
Summary of findings 4. Aspirin (ASA) compared to low molecular weight heparin (LMWH) for ambulatory patients with multiple myeloma receiving immunomodulatory agents: 2 years follow‐up.
| Aspirin (ASA) compared to low molecular weight heparin (LMWH) for ambulatory patients with multiple myeloma receiving immunomodulatory agents: 2 years follow‐up | |||||
| Population: ambulatory patients with multiple myeloma receiving immunomodulatory agents Setting: outpatient Intervention: ASA prophylaxis Control: LMWH prophylaxis | |||||
| Outcomes | № of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with LMWH | Risk difference with ASA | ||||
| All‐cause mortality follow‐up: 2 years | 440 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 | RR 1.00 (0.06 to 15.89) | Study population | |
| 5 per 1000 | 0 fewer per 1000 (4 fewer to 68 more) | ||||
| Symptomatic deep vein thrombosis follow‐up: 2 years | 440 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 3 | RR 1.20 (0.53 to 2.72) | Study population | |
| 45 per 1000 | 9 more per 1000 (21 fewer to 78 more) | ||||
| Pulmonary embolism follow‐up: 2 years | 440 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 4 | RR 9.00 (0.49 to 166.17) | Low | |
| 1 per 1000 5 | 8 more per 1000 (1 fewer to 165 more) | ||||
| Major bleeding ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Minor bleeding ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
1Downgraded by one level due to serious risk of bias. Lack of blinding of participants and personnel and selective reporting (all outcomes listed in the methods section were reported on except for secondary endpoint related to any toxicity that required interruption of study prophylaxis). 2Downgraded by two levels due to concerns about imprecision; 95% CI is consistent with the possibility of no effect (4 per 1000 absolute reduction) and the possibility of important harm (68 per 1000 absolute increase), including four events in total. 3Downgraded by two levels due to concerns about imprecision; 95% CI is consistent with the possibility of benefit (21 per 1000 absolute reduction) and the possibility of important harm (78 per 1000 absolute increase), including 27 events in total. 4Downgraded by two levels due to concerns about imprecision; 95% CI is consistent with the possibility of no effect (1 per 1000 absolute reduction) and the possibility of important harm (165 per 1000 absolute increase), including four events in total. Given the observed baseline risk of 0%, we used 0.1% to generate an absolute effect and a confidence interval. 5There were zero events in the control arm.
Summary of findings 5. Vitamin K antagonist (VKA) compared to low molecular weight heparin (LMWH) for ambulatory patients with multiple myeloma receiving immunomodulatory agents: 6 months follow‐up.
| Vitamin K antagonist (VKA) compared to low molecular weight heparin (LMWH) for ambulatory patients with multiple myeloma receiving immunomodulatory agents: 6 months follow‐up | |||||
| Population: ambulatory patients with multiple myeloma receiving immunomodulatory agents Setting: outpatient Intervention: VKA prophylaxis Control: LMWH prophylaxis | |||||
| Outcomes | № of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with LMWH | Risk difference with VKA | ||||
| All‐cause mortality follow‐up: 6 months | 439 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 | RR 0.33 (0.01 to 8.10) | Study population | |
| 5 per 1000 | 3 fewer per 1000 (5 fewer to 32 more) | ||||
| Symptomatic deep vein thrombosis follow‐up: 6 months | 439 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 3 | RR 2.32 (0.91 to 5.93) | Study population | |
| 27 per 1000 | 36 more per 1000 (2 fewer to 135 more) | ||||
| Pulmonary embolism follow‐up: 6 months | 439 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 4 | RR 8.96 (0.49 to 165.42) | Low | |
| 1 per 1000 5 | 8 more per 1000 (1 fewer to 164 more) | ||||
| Major bleeding ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Minor bleeding follow‐up: 6 months | 439 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 6 | RR 0.33 (0.03 to 3.17) | Study population | |
| 14 per 1000 | 9 fewer per 1000 (13 fewer to 30 more) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
1Downgraded by one level due to serious risk of bias. Lack of blinding of participants and personnel and selective reporting (all outcomes listed in the methods section were reported on except for secondary endpoint related to any toxicity that required interruption of study prophylaxis). 2Downgraded by two levels due to concerns about imprecision; 95% CI is consistent with the possibility of no effect (5 per 1000 absolute reduction) and the possibility of important harm (32 per 1000 absolute increase), including one event in total. 3Downgraded by two levels due to concerns about imprecision; 95% CI is consistent with the possibility of no effect (2 per 1000 absolute reduction) and the possibility of important harm (135 per 1000 absolute increase), including 20 events in total. 4Downgraded by two levels due to concerns about imprecision; 95% CI is consistent with the possibility of no effect (1 per 1000 absolute reduction) and the possibility of important harm (164 per 1000 absolute increase), including four events in total. Given the observed baseline risk of 0%, we used 0.1% to generate an absolute effect and a confidence interval. 5There were zero events in the control arm. 6Downgraded by two levels due to concerns about imprecision; 95% CI is consistent with the possibility of benefit (13 per 1000 absolute reduction) and the possibility of important harm (30 per 1000 absolute increase), including four events in total.
Summary of findings 6. Vitamin K antagonist (VKA) compared to low molecular weight heparin (LMWH) for ambulatory patients with multiple myeloma receiving immunomodulatory agents: 2 years follow‐up.
| Vitamin K antagonist (VKA) compared to low molecular weight heparin (LMWH) for ambulatory patients with multiple myeloma receiving immunomodulatory agents: 2 years follow‐up | |||||
| Population: ambulatory patients with multiple myeloma receiving immunomodulatory agents Setting: outpatient Intervention: VKA prophylaxis Control: LMWH prophylaxis | |||||
| Outcomes | № of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with LMWH | Risk difference with VKA | ||||
| All‐cause mortality follow‐up: 2 years | 440 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 | RR 2.00 (0.18 to 21.90) | Study population | |
| 5 per 1000 | 5 more per 1000 (4 fewer to 95 more) | ||||
| Symptomatic deep vein thrombosis follow‐up: 2 years | 440 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 3 | RR 1.70 (0.80 to 3.63) | Study population | |
| 45 per 1000 | 32 more per 1000 (9 fewer to 120 more) | ||||
| Pulmonary embolism follow‐up: 2 years | 440 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 4 | RR 9.00 (0.49 to 166.17) | Low | |
| 1 per 1000 5 | 8 more per 1000 (1 fewer to 165 more) | ||||
| Major bleeding ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Minor bleeding ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
1Downgraded by one level due to serious risk of bias. Lack of blinding of participants and personnel and selective reporting (all outcomes listed in the methods section were reported on except for secondary endpoint related to any toxicity that required interruption of study prophylaxis). 2Downgraded by two levels due to concerns about imprecision; 95% CI is consistent with the possibility of no effect (4 per 1000 absolute reduction) and the possibility of important harm (95 per 1000 absolute increase), including four events in total. 3Downgraded by two levels due to concerns about imprecision; 95% CI is consistent with the possibility of benefit (9 per 1000 absolute reduction) and the possibility of important harm (120 per 1000 absolute increase), including 27 events in total. 4Downgraded by two levels due to concerns about imprecision; 95% CI is consistent with the possibility of no effect (1 per 1000 absolute reduction) and the possibility of important harm (165 per 1000 absolute increase), including four events in total. Given the observed baseline risk of 0%, we used 0.1% to generate an absolute effect and a confidence interval. 5There were no events in the control arm.
Summary of findings 7. Aspirin (ASA) compared to direct oral anticoagulants (DOAC) for ambulatory patients with multiple myeloma receiving immunomodulatory agents: 6 months follow‐up.
| Aspirin (ASA) compared to direct oral anticoagulants (DOAC) for ambulatory patients with multiple myeloma receiving immunomodulatory agents: 6 months follow‐up | |||||
| Population: ambulatory patients with multiple myeloma receiving immunomodulatory agents Setting: outpatient Intervention: ASA prophylaxis Comparison: DOAC prophylaxis | |||||
| Outcomes | № of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with DOAC | Risk difference with ASA | ||||
| All‐cause mortality ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Symptomatic deep vein thrombosis ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Major bleeding ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ |
| Minor bleeding follow‐up: 6 months | 8 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 | RR 5.00 (0.31 to 79.94) | Low | |
| 1 per 1000 3 | 4 more per 1000 (1 fewer to 79 more) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
1Downgraded by one level due to serious risk of bias. Allocation not concealed and lack of blinding. 2Downgraded by two levels due to very serious risk of bias. Very low number of events and sample size. 3There were zero events in the control arm.
Comparison 1: ASA versus VKA prophylaxis
Six months follow‐up
Palumbo 2011 did not confirm or exclude a beneficial or detrimental effect of ASA relative to VKA on all‐cause mortality at six months (risk ratio (RR) 3.00, 95% confidence interval (CI) 0.12 to 73.24; risk difference (RD) 2 more per 1000, 95% CI 1 fewer to 72 more; very low‐certainty evidence) (Analysis 1.1); symptomatic DVT (RR 0.57, 95% CI 0.24 to 1.33; RD 27 fewer per 1000, 95% CI 48 fewer to 21 more; very low‐certainty evidence) (Analysis 1.2); PE (RR 1.00, 95% CI 0.25 to 3.95; RD 0 fewer per 1000, 95% CI 14 fewer to 54 more; very low‐certainty evidence) (Analysis 1.3); major bleeding (RR 7.00, 95% CI 0.36 to 134.72; RD 6 more per 1000, 95% CI 1 fewer to 134 more; very low‐certainty evidence) (Analysis 1.4); and minor bleeding (RR 6.00, 95% CI 0.73 to 49.43; RD 23 more per 1000, 95% CI 1 fewer to 220 more; very low‐certainty evidence) (Analysis 1.5).
1.1. Analysis.

Comparison 1: Aspirin versus vitamin K antagonist (6 months), Outcome 1: All‐cause mortality
1.2. Analysis.

Comparison 1: Aspirin versus vitamin K antagonist (6 months), Outcome 2: Symptomatic deep vein thrombosis
1.3. Analysis.

Comparison 1: Aspirin versus vitamin K antagonist (6 months), Outcome 3: Pulmonary embolism
1.4. Analysis.

Comparison 1: Aspirin versus vitamin K antagonist (6 months), Outcome 4: Major bleeding
1.5. Analysis.

Comparison 1: Aspirin versus vitamin K antagonist (6 months), Outcome 5: Minor bleeding
Two years follow‐up
Palumbo 2011 did not confirm or exclude a beneficial or detrimental effect of ASA relative to VKA on all‐cause mortality at two years (RR 0.50, 95% CI 0.05 to 5.47; RD 5 fewer per 1000, 95% CI 9 fewer to 41 more; very low‐certainty evidence) (Analysis 2.1); symptomatic DVT (RR 0.71, 95% CI 0.35 to 1.44; RD 22 fewer per 1000, 95% CI 50 fewer to 34 more; very low‐certainty evidence) (Analysis 2.2); and PE (RR 1.00, 95% CI 0.25 to 3.95; RD 0 fewer per 1000, 95% CI 14 fewer to 54 more; very low‐certainty evidence) (Analysis 2.3). The study did not report on major or minor bleeding outcomes at two years.
2.1. Analysis.

Comparison 2: Aspirin versus vitamin K antagonist (2 years), Outcome 1: All‐cause mortality
2.2. Analysis.

Comparison 2: Aspirin versus vitamin K antagonist (2 years), Outcome 2: Symptomatic deep vein thrombosis
2.3. Analysis.

Comparison 2: Aspirin versus vitamin K antagonist (2 years), Outcome 3: Pulmonary embolism
Comparison 2: ASA versus LMWH prophylaxis
Six months follow‐up
Meta‐analysis of two RCTs including 781 participants did not confirm or exclude a beneficial or detrimental effect of ASA relative to LMWH on all‐cause mortality (RR 1.00, 95% CI 0.06 to 15.81; RD 0 fewer per 1000, 95% CI 2 fewer to 38 more; very low‐certainty evidence) (Analysis 3.1); symptomatic DVT (RR 1.23, 95% CI 0.49 to 3.08; RD 5 more per 1000, 95% CI 11 fewer to 43 more; very low‐certainty evidence) (Analysis 3.2); PE (RR 7.71, 95% CI 0.97 to 61.44; RD 7 more per 1000, 95% CI 0 fewer to 60 more; very low‐certainty evidence) (Analysis 3.3); major bleeding (RR 6.97, 95% CI 0.36 to 134.11; RD 6 more per 1000, 95% CI 1 fewer to 133 more; very low‐certainty evidence) (Analysis 3.4); and minor bleeding (RR 1.42, 95% CI 0.35 to 5.78; RD 4 more per 1000, 95% CI 7 fewer to 50 more; very low‐certainty evidence) (Analysis 3.5) (Larocca 2012; Palumbo 2011).
3.1. Analysis.

Comparison 3: Aspirin versus low molecular weight heparin (6 months), Outcome 1: All‐cause mortality
3.2. Analysis.

Comparison 3: Aspirin versus low molecular weight heparin (6 months), Outcome 2: Symptomatic deep vein thrombosis
3.3. Analysis.

Comparison 3: Aspirin versus low molecular weight heparin (6 months), Outcome 3: Pulmonary embolism
3.4. Analysis.

Comparison 3: Aspirin versus low molecular weight heparin (6 months), Outcome 4: Major bleeding
3.5. Analysis.

Comparison 3: Aspirin versus low molecular weight heparin (6 months), Outcome 5: Minor bleeding
Two years follow‐up
Palumbo 2011 did not confirm or exclude a beneficial or detrimental effect of ASA relative to LMWH on all‐cause mortality (RR 1.00, 95% CI 0.06 to 15.89; RD 0 fewer per 1000, 95% CI 4 fewer to 68 more; very low‐certainty evidence) (Analysis 4.1); symptomatic DVT (RR 1.20, 95% CI 0.53 to 2.72; RD 9 more per 1000, 95% CI 21 fewer to 78 more; very low‐certainty evidence) (Analysis 4.2); and PE (RR 9.00, 95% CI 0.49 to 166.17; RD 8 more per 1000, 95% CI 1 fewer to 165 more; very low‐certainty evidence) (Analysis 4.3). The study did not report on major or minor bleeding outcomes at two years.
4.1. Analysis.

Comparison 4: Aspirin versus low molecular weight heparin (2 years), Outcome 1: All‐cause mortality
4.2. Analysis.

Comparison 4: Aspirin versus low molecular weight heparin (2 years), Outcome 2: Symptomatic deep vein thrombosis
4.3. Analysis.

Comparison 4: Aspirin versus low molecular weight heparin (2 years), Outcome 3: Pulmonary embolism
Comparison 3: VKA versus LMWH prophylaxis
Six months follow‐up
Palumbo 2011 did not confirm or exclude a beneficial or detrimental effect of VKA relative to LMWH on all‐cause mortality (RR 0.33, 95% CI 0.01 to 8.10; RD 3 fewer per 1000, 95% CI 5 fewer to 32 more; very low‐certainty evidence) (Analysis 5.1); symptomatic DVT (RR 2.32, 95% CI 0.91 to 5.93; RD 36 more per 1000, 95% CI 2 fewer to 135 more; very low‐certainty evidence) (Analysis 5.2); PE (RR 8.96, 95% CI 0.49 to 165.42; RD 8 more per 1000, 95% CI 1 fewer to 164 more; very low‐certainty evidence) (Analysis 5.3); and minor bleeding (RR 0.33, 95% CI 0.03 to 3.17; RD 9 fewer per 1000, 95% CI 13 fewer to 30 more; very low‐certainty evidence) (Analysis 5.4). The study reported that no major bleeding occurred in either arm.
5.1. Analysis.

Comparison 5: Vitamin K antagonist versus low molecular weight heparin (6 months), Outcome 1: All‐cause mortality
5.2. Analysis.

Comparison 5: Vitamin K antagonist versus low molecular weight heparin (6 months), Outcome 2: Symptomatic deep vein thrombosis
5.3. Analysis.

Comparison 5: Vitamin K antagonist versus low molecular weight heparin (6 months), Outcome 3: Pulmonary embolism
5.4. Analysis.

Comparison 5: Vitamin K antagonist versus low molecular weight heparin (6 months), Outcome 4: Minor bleeding
Two years follow‐up
Palumbo 2011 did not confirm or exclude a beneficial or detrimental effect of VKA relative to LMWH on all‐cause mortality (RR 2.00, 95% CI 0.18 to 21.90; RD 5 more per 1000, 95% CI 4 fewer to 95 more; very low‐certainty evidence) (Analysis 6.1); symptomatic DVT (RR 1.70, 95% CI 0.80 to 3.63; RD 32 more per 1000, 95% CI 9 fewer to 120 more; very low‐certainty evidence) (Analysis 6.2); and PE (RR 9.00, 95% CI 0.49 to 166.17; RD 8 more per 1000, 95% CI 1 fewer to 165 more; very low‐certainty evidence) (Analysis 6.3). The study did not report on major or minor bleeding outcomes at two years.
6.1. Analysis.

Comparison 6: Vitamin K antagonist versus low molecular weight heparin (2 years), Outcome 1: All‐cause mortality
6.2. Analysis.

Comparison 6: Vitamin K antagonist versus low molecular weight heparin (2 years), Outcome 2: Symptomatic deep vein thrombosis
6.3. Analysis.

Comparison 6: Vitamin K antagonist versus low molecular weight heparin (2 years), Outcome 3: Pulmonary embolism
Comparison 4: ASA versus DOAC prophylaxis
Six months follow‐up
Sayar 2019 did not confirm or exclude a beneficial or detrimental effect of ASA relative to DOAC on DVT, PE, major bleeding, and minor bleeding (minor bleeding: RR 5.00, 95% CI 0.31 to 79.94; RD 4 more per 1000, 95% CI 1 fewer to 79 more; very low‐certainty evidence) (Analysis 7.1). The study reported that no DVT, PE, or major bleeding events occurred in either arm. These results did not change in a meta‐analysis including the study published as an abstract, Campos‐Cabrera 2018 (abstract): DVT (RR 0.95, 95% CI 0.04 to 20.33; RD 0 fewer per 1000, 95% CI 1 fewer to 19 more) (Analysis 8.1); PE (RR was not estimable due to zero number of events in both arms) (Analysis 8.2); and major bleeding (RR 0.95, 95% CI 0.04 to 20.33; RD 0 fewer per 1000, 95% CI 1 fewer to 19 more) (Analysis 8.3). The study did not report on all‐cause mortality outcome at six months.
7.1. Analysis.

Comparison 7: Aspirin versus direct oral anticoagulant (6 months), Outcome 1: Minor bleeding
8.1. Analysis.

Comparison 8: Aspirin versus direct oral anticoagulant (6 months): sensitivity analysis, Outcome 1: Deep vein thrombosis
8.2. Analysis.

Comparison 8: Aspirin versus direct oral anticoagulant (6 months): sensitivity analysis, Outcome 2: Pulmonary embolism
8.3. Analysis.

Comparison 8: Aspirin versus direct oral anticoagulant (6 months): sensitivity analysis, Outcome 3: Major bleeding
Discussion
Summary of main results
The certainty of the available evidence for the comparative effects of ASA, VKA, LMWH, or DOAC on all‐cause mortality, symptomatic DVT, or bleeding events was either low or very low. People with multiple myeloma considering antithrombotic therapies should balance the possible benefits of reduced thromboembolic complications with the possible harms and burden of anticoagulants.
Overall completeness and applicability of evidence
The included studies recruited people with newly diagnosed multiple myeloma, which may limit the applicability of the results.
Whilst the absence of statistically significant results might reflect a true absence of difference between the studied drugs, this could also be related to insufficient power to detect important differences between drugs. Another potential explanation is the relatively low baseline risks for the different outcomes.
Quality of the evidence
When comparing ASA to VKA (Table 1; Table 2), ASA to LMWH (Table 3; Table 4), and VKA to LMWH (Table 5; Table 6), we judged the certainty of evidence to be very low for all studied outcomes due to very serious imprecision. The wide confidence interval in the results, in addition to the low number of events and the small number of studies reporting on these events, contributed to our decision to downgrade by two levels. When comparing ASA to DOAC (Table 7), we judged the certainty of evidence to be very low due to very serious imprecision and serious risk of bias.
Potential biases in the review process
Our systematic approach to searching, study selection, and data extraction should have minimised the likelihood of our missing relevant studies or data.
One limitation of this review is that the 'no difference' findings could be related to the relatively small number of RCTs, small numbers of participants and events, as well as the absence of a true effect. Another limitation related to the small number of RCTs was our inability to conduct subgroup analyses exploring the impact on the treatment effect of the characteristics of participants, outcomes (symptomatic versus screening‐detected DVT, early versus late DVTs), and methodological quality criteria.
Agreements and disagreements with other studies or reviews
A recent systematic review by Rutjes and colleagues assessed the efficacy of primary VTE thromboprophylaxis in people with multiple myeloma (Rutjes 2020). When compared with our findings, Rutjes and colleagues included the same major trials Larocca 2012; Palumbo 2011 included in our review and showed comparable results in regard to bleeding outcomes and some differences in regard to VTE outcomes. The review Rutjes 2020 concluded that LMWH resulted in lower symptomatic VTE compared with VKA (high‐certainty evidence), whilst LMWH probably lowers symptomatic VTE more than ASA (moderate‐certainty evidence).
The difference might be explained by the fact that they assessed symptomatic VTE, whereas we assessed symptomatic DVT separately from PE. In addition, we downgraded the certainty of the evidence for this outcome three levels lower for the comparison VKA vs LMWH and two levels lower for the comparison LMWH vs ASA compared to the Rutjes and colleagues rating probably due to different judgments on risk of bias and imprecision.
We identified another systematic review discussing thromboprophylaxis in people with multiple myeloma (Al‐Ani 2016). That review reported on the incidence of VTE in people with multiple myeloma receiving the same antithrombotic medications and different multiple myeloma treatments but did not compare different antithrombotic medications. For example, the authors compared the incidence of VTE in people receiving immunomodulatory agents, high‐dose steroids, and aspirin versus people receiving immunomodulatory agents, low‐dose steroids, and aspirin.
Authors' conclusions
Implications for practice.
The currently available evidence regarding the comparative effects of aspirin, vitamin K antagonist, low molecular weight heparin, or direct oral anticoagulants on all‐cause mortality, symptomatic deep vein thrombosis, or bleeding events is inconclusive. The choice of antithrombotic therapy in people with multiple myeloma in the absence of a standard therapeutic or prophylactic indication should balance the benefits and harms and integrate patients' values and preferences for outcomes and management options (Haynes 2002). In different practice settings, and considering patient preference, prophylaxis with aspirin, vitamin K antagonist, or direct oral anticoagulants may be a reasonable option.
Implications for research.
More data from randomised controlled trials are needed to answer our research question regarding thromboprophylaxis management and which agent to use in people with multiple myeloma, both with regard to newly diagnosed and relapsed/refractory disease. Studies should adhere to high methodological quality and be adequately powered to assess participant‐important outcomes such as all‐cause mortality, the incidence of symptomatic deep vein thrombosis, and bleeding outcomes. Studies should also aim to use standardised definitions for major and minor bleeding. Researchers should consider making the raw data of randomised controlled trials available for individual participant data meta‐analysis. In addition, as is recognised by Cochrane, addressing all important outcomes including harm is of great importance in making evidence‐based healthcare decisions.
What's new
| Date | Event | Description |
|---|---|---|
| 21 December 2022 | Amended | This is a Living Systematic Review. Searches are run and screened monthly. Last search date 14 December 2022 (no new studies found). As such, results of all included studies identified have been incorporated. The conclusions of this Cochrane Review are therefore considered up to date. |
History
Review first published: Issue 9, 2021
| Date | Event | Description |
|---|---|---|
| 24 October 2022 | Amended | This is a Living Systematic Review. Searches are run and screened monthly. Last search date 14 October 2022 (no new studies found). As such, results of all included studies identified have been incorporated. The conclusions of this Cochrane Review are therefore considered up to date. |
| 13 June 2022 | Amended | This is a Living Systematic Review. Searches are run and screened monthly. Last search date 14 May 2022 (no new studies found). As such, results of all included studies identified have been incorporated. The conclusions of this Cochrane Review are therefore considered up to date. |
| 29 December 2021 | Amended | This is a Living Systematic Review. Searches are run and screened monthly. Last search date 14 December 2021 (no new studies found). As such, results of all included studies identified have been incorporated. The conclusions of this Cochrane Review are therefore considered up to date. |
| 29 December 2021 | Amended | Search updated to 14 December 2014 |
| 22 September 2021 | Amended | Edits to text. |
| 8 September 2021 | Amended | Updated affliliation details |
Acknowledgements
This review is published jointly by the Cochrane Gynaecological, Neuro‐oncology and Orphan Cancers Group and the Cochrane Haematology Group.
We would like to thank Lise Estcourt, Co‐ordinating Editor for the Cochrane Haematology Group, for clinical advice, and Vanessa Piechotta, Managing Editor for the Cochrane Haematology Group, for editorial assistance. We would also like to thank Jo Morrison, Co‐ordinating Editor for the Cochrane Gynaecological, Neuro‐oncology and Orphan Cancers Group (CGNOG). We also thank Gail Quinn, Managing Editor of CGNOC, for her exceptional support, as well as Joanne Platt, the Information Specialist for CGNOG, for setting up and managing the monthly search alerts.
This review was supported in part by the American Society of Hematology (ASH) to inform ASH guidelines on the topic. We thank the ASH guideline panel for prioritising questions and for critically reviewing our work, including Drs Pablo Alonso, Waleed Alhazanni, Marc Carrier, Cihan Ay, Marcello DiNisio, Alok Khorana, Andrew Leavitt, Agnes Lee, Gary Lyman, Fergus Macbeth, Rebecca Morgan, Simon Noble, and David Stenehjem, and patient representatives Jackie Cook and Elizabeth Sexton. Their input was valuable in validating some of the review‐related decisions such as eligibility of included studies and the analytical approach.
The authors and CGNOG team are grateful to the following peer reviewers for their time and comments: Faiz Anwer, Rajshekhar Chakraborty, Kristen Sanfilippo, and Tari Turner.
Appendices
Appendix 1. Living systematic review
The methods outlined below are specific to maintaining this review as a living systematic review on the Cochrane Library (Brooker 2019; Synnot 2017). Core review methods, such as the criteria for considering studies in the review and risk of bias assessment, are unchanged. As such, we outline below only those areas of the methods for which additional or different activities are planned or rules apply.
Search methods for identification of studies
We will re‐run the majority of searches monthly. For electronic databases and other electronic sources (CENTRAL, MEDLINE, Embase), we have set up auto‐alerts to deliver a monthly search yield by email. We will search the remaining resources (conference proceedings of the American Society of Clinical Oncology (ASCO), the American Society of Hematology (ASH), and ClinicalTrials.gov) on a monthly basis. For that purpose, we will note when these conference proceedings are published.
As additional steps to inform the living systematic review, we will contact corresponding authors of ongoing studies as they are identified and ask them to advise when results are available, and to share early or unpublished data. We will contact the corresponding authors of any newly included studies for information as to other relevant studies. We will conduct citation tracking of included studies in the Web of Science Core Collection on an ongoing basis. For that purpose, we have set up citation alerts in the Web of Science Core Collection. We will manually screen the reference lists of any newly included studies and identified relevant guidelines and systematic reviews. In addition, we will use the 'related citation' feature in PubMed to identify additional articles.
We will review search methods and strategies approximately yearly to ensure that they reflect any terminology changes in the topic area or in the databases.
Selection of studies
We will immediately screen any new citations retrieved by the monthly searches. As the first step of monthly screening, we will apply the machine learning classifier (RCT model) available in the Cochrane Register of Studies (CSR‐Web; Wallace 2017). The machine learning classifier currently has a specificity/recall of 99.987% and assigns a probability (from 0 to 100) to each citation for being a true RCT. For citations assigned a score from 10 to 100, we will screen these in duplicate and independently. Citations that score 9 or less will be screened by Cochrane Crowd (Cochrane Crowd). Any citations that are deemed to be potential RCTs (i.e., scored 10 or above) by Cochrane Crowd will be returned to the authors for screening.
Data synthesis
Whenever new evidence (studies, data, or information) that meets the review inclusion criteria is identified, we will immediately assess the risk of bias and extract the data and incorporate this information in the synthesis, as appropriate. We will not adjust the meta‐analyses to account for multiple testing given the methods related to frequent updating of meta‐analyses are under development (Simmonds 2017).
Other
We will review the review scope and methods approximately yearly, or more frequently if appropriate, given potential changes in the topic area, or the evidence being included in the review (e.g. additional comparisons, interventions, or outcomes, or new review methods available).
Appendix 2. Full search strategies for the electronic databases ‐ 2019
| Database | Strategy | |
| CENTRAL (the Cochrane Library, latest issue) | #1 MeSH descriptor: [Anticoagulants] explode all trees #2 anticoagulant* or anti‐coagulant* #3 Heparin or Adomiparin or alpha‐Heparin or Arteven or "AVE‐5026" or CY 222 or "Depo‐Heparin" or "EINECS 232‐681‐7" or Fluxum or "Hed‐heparin" or Hepathrom or HSDB 3094 or KB 101 or "Lipo‐hepin" or M 118 or "M 118REH" or M118 or Octaparin or OP 386 or OP 622 or Pabyrin or Pularin or Subeparin or Sublingula or Thromboliquine or Triofiban or "UNII‐1K5KDI46KZ" or "UNII‐4QW4AN84NQ" or "UNII‐5R0L1D739E" or "UNII‐7UQ7X4Y489" or "UNII‐9816XA9004" or "UNII‐E47C0NF7LV" or "UNII‐M316WT19D8" or "UNII‐P776JQ4R2F" or "UNII‐S79O08V79F" or "UNII‐T2410KM04A" or "UNII‐V72OT3K19I" or "UNII‐VL0L558GCB" or Vetren or Vitrum AB or enoxaparin* or klexane or lovenox or fragmin* or normiflo or logiparin or innohep or danaproid or danaparoid or orgaran or antixarin or hibor or zibor or ivor or badyket or lohepa or lowhepa or seleparin* or tedelgliparin or lomoparan or orgaran or sulodexide or zivor or embolex or xaparin or fondaparinux or Arixtra or UFH or Hepalean or Calcilean or Calciparine or "Hep‐lock" or enoxaparin* or klexane or lovenox or fragmin* or normiflo or logiparin or innohep or danaproid or danaparoid or orgaran or antixarin or hibor or zibor or ivor or badyket or lohepa or lowhepa or seleparin* or tedelgliparin or lomoparan or orgaran or sulodexide or zivor or embolex or xaparin or fondaparinux or Arixtra or UFH or Hepalean or Panheprin #4 FR 860 or FR860 or PK‐10,169 or PK 10,169 or PK10,169 or PK‐10169 or PK 10169 or PK10169 or EMT‐967 or EMT 967 or EMT967 or EMT‐966 or EMT 966 or EMT966 or CY 216 or CY‐216 or CY216 or LMF CY‐216 or LMF CY 216 or LMF CY216 #5 MeSH descriptor: [Coumarins] explode all trees #6 coumarin* or chromonar or coumestrol or esculin or isocoumarin* or psoralens or pyranocoumarins or umbelliferones #7 "4‐Hydroxycoumarin*" or warfarin* or acenocoumarol or nicoumalone or sinthrome or Sintrom or phenindione or dicoumarol or coumadin or phenprocoumon or phepromaron or ethyl‐biscoumacetate or phenindione or Diphenadione or Tioclomarol or Racumi or Marcoumar or Marcumar or Falithrom or Jantoven or vitamin* K antagonist* or VKA or fluindione or difenacoum or coumatetralyl or coumadin* or warfant or marevan or aldocumar #8 Dermatan Sulfate or (Chondroitin Sulfate near B) or Dermatan Sulfphate or DS 435 or MF‐701 or OP‐370 or b‐Heparin or Mistral or Venorix #9 thrombin near inhibitor* #10 factor Xa inhibitor* or antithrombin* or anti‐thrombin* or anti‐coagul* or anticoagul* #11 rivaroxaban or Xarelto or apixaban or Eliquis or dabigatran etexilate or Edoxaban or Savaysa or Betrixaban or ximelagatran or pradaxa or lixiana or exanta or Darexaban or Otamixaban* or Razaxaban or Bivalirudin or Desirudin or Lepirudin or Melagatran or YM 150 or Iprivask or argatrovan or pradax* or Xarelto or "BIBR‐953" or BIBR953 or "BIBR‐953ZW" or BIBR953ZW or "BAY 59‐7939" or "BMS‐562247" or BMS562247 or "DU‐176" or DU176 or "DU‐176b" or DU176B #12 MeSH descriptor: [Rivaroxaban] this term only #13. MeSH descriptor: [Dabigatran] this term only #14 target specific oral anticoagulant* or target‐specific oral anticoagulant* or TSOAC* or new oral anticoagulant* or novel oral anticoagulant* or NOAC* or direct‐acting oral anticoagulant* or direct acting oral anticoagulant* or direct oral anticoagulant* or DOAC* #15 Aspirin* or Acuprin* or Anacin* or Ascriptin* or Aspergum* or Aspidrox* or "Aspir‐Mox" or Aspirtab* or "Aspir‐trin" or Bayer* or Bufferin* or Buffex* or Easprin* or Ecotrin* or Empirin* or Entaprin* or Entercote* or Fasprin* or Genacote* or "Gennin‐FC" or Genprin* or Halfprin* or Magnaprin* or Miniprin* or Minitabs* or Ridiprin* or Sloprin* or "Uni‐Buff" or "Uni‐Tren" or Valomag* or Zorprin* or benzoic acid* or Carboxyphenyl acetate or ASA or AC 5230 or Acenterine or Acesal or Aceticyl or Acetilsalicilico or Acetilum acidulatum or Acetisal or Acetol* or Acetonyl or Acetophen or Acetosal* or Acetylin or Acetylsal* or Acide acetylsalicylique or Acido acetilsalicilico or "Acido O‐acetil‐benzoico" or Acidum or acetylsalicylicum or Acimetten or Acisal or Acylpyrin or Adiro or "AI3‐02956" or Asagran or Asaphen or Aspec or Aspergum or Aspirdrops or Aspro* or Asteric or Bay E4465 or Benaspir or Benzoic acid* or "Bi‐prin" or Bialpirina or Bialpirinia or "BRN 0779271" or Bufferin or Caprin or "CCRIS 3243" or Cemirit or Claradin or Clariprin or Colfarit or Contrheuma retard or Coricidin* or Decaten or Delgesic or Dolean pH 8 or Duramax or Durlaza or Durlaza ER or Easprin or "EC 200‐064‐1" or ECM or Ecolen or Ecotrin or Empirin or Endydol or Entericin or Enterophen or Enterosarein or Enterosarine or Entrophen or Extren or Globentyl or Globoid or Helicon or HSDB 652 or Idragin or Istopirin or Kapsazal or Kyselina* or Levius or Measurin or Medisyl or Micristin or Neuronika or Novid or NSC 27223 or Pharmacin or Pirseal or Polopiryna or Premaspin or Rheumin tabletten or Rheumintabletten or Rhodine or Rhonal or Ronal or Salacetin or Salcetogen or Saletin or Salicylic acid* or acetate or Solfrin* or Solpyron or SP 189 or "Spira‐Dine" or Temperal or "Triple‐sal" or Xaxa or Yasta or ZORprin or "EINECS 200‐064‐1" or "o‐Acetoxybenzoic acid" or "O‐Acetylsalicylic acid" or "o‐Carboxyphenyl acetate" or "S‐211" or "UNII‐R16CO5Y76E" or "O‐Acetylsalicylic acid" #16. MeSH descriptor: [Platelet Aggregation Inhibitors] explode all trees #17 (antiplatelet or anti‐platelet or anti platelet) near/5 (agent* or drug* or med*) #18. (platelet*) near/5 (inhibit* or antagon* or antiaggregant* or anti‐aggregant* or anti aggregant*) #19 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 #20 MeSH descriptor: [Multiple Myeloma] explode all trees #21 myelom* #22 MeSH descriptor: [Plasmacytoma] explode all trees #23 plasm?cytom* or plasm?zytom* or plasma cytoma* #24 plasma* near/3 neoplas* #25 plasma cell near/1 (leukaem* or leukem* or tumor* or tumour*) #26 ((plasmacytic* or plasmocytic* or plasmocyte*) near/1 (leukem* or leukaem*)) #27 kahler* #28 #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 #29 #19 and #28 | |
| MEDLINE | 1. exp Anticoagulants/ 2. (anticoagulant* or anti‐coagulant*).tw. 3. (Heparin or Adomiparin or alpha‐Heparin or Arteven or "AVE‐5026" or CY 222 or "Depo‐Heparin" or "EINECS 232‐681‐7" or Fluxum or "Hed‐heparin" or Hepathrom or HSDB 3094 or KB 101 or "Lipo‐hepin" or M 118 or "M 118REH" or M118 or Octaparin or OP 386 or OP 622 or Pabyrin or Pularin or Subeparin or Sublingula or Thromboliquine or Triofiban or "UNII‐1K5KDI46KZ" or "UNII‐4QW4AN84NQ" or "UNII‐5R0L1D739E" or "UNII‐7UQ7X4Y489" or "UNII‐9816XA9004" or "UNII‐E47C0NF7LV" or "UNII‐M316WT19D8" or "UNII‐P776JQ4R2F" or "UNII‐S79O08V79F" or "UNII‐T2410KM04A" or "UNII‐V72OT3K19I" or "UNII‐VL0L558GCB" or Vetren or Vitrum AB or enoxaparin* or klexane or lovenox or fragmin* or normiflo or logiparin or innohep or danaproid or danaparoid or orgaran or antixarin or hibor or zibor or ivor or badyket or lohepa or lowhepa or seleparin* or tedelgliparin or lomoparan or orgaran or sulodexide or zivor or embolex or xaparin or fondaparinux or Arixtra or UFH or Hepalean or Calcilean or Calciparine or "Hep‐lock" or enoxaparin* or klexane or lovenox or fragmin* or normiflo or logiparin or innohep or danaproid or danaparoid or orgaran or antixarin or hibor or zibor or ivor or badyket or lohepa or lowhepa or seleparin* or tedelgliparin or lomoparan or orgaran or sulodexide or zivor or embolex or xaparin or fondaparinux or Arixtra or UFH or Hepalean or Panheprin).mp. 4. (FR 860 or FR860 or PK‐10,169 or PK 10,169 or PK10,169 or PK‐10169 or PK 10169 or PK10169 or EMT‐967 or EMT 967 or EMT967 or EMT‐966 or EMT 966 or EMT966 or CY 216 or CY‐216 or CY216 or LMF CY‐216 or LMF CY 216 or LMF CY216).mp. 5. exp Coumarins/ 6. (coumarin* or chromonar or coumestrol or esculin or isocoumarin* or psoralens or pyranocoumarins or umbelliferones).tw. 7. (4‐Hydroxycoumarin* or warfarin* or acenocoumarol or nicoumalone or sinthrome or Sintrom or phenindione or dicoumarol or coumadin or phenprocoumon or phepromaron or ethyl‐biscoumacetate or phenindione or Diphenadione or Tioclomarol or Racumi or Marcoumar or Marcumar or Falithrom or Jantoven or vitamin* K antagonist* or VKA or fluindione or difenacoum or coumatetralyl or coumadin* or warfant or marevan or aldocumar).mp. 8. (Dermatan Sulfate or (Chondroitin Sulfate adj B) or Dermatan Sulfphate or DS 435 or MF‐701 or OP‐370 or b‐Heparin or Mistral or Venorix).mp. 9. (thrombin adj inhibitor*).mp. 10. (factor Xa inhibitor* or antithrombin* or anti‐thrombin* or anti‐coagul* or anticoagul*).mp. 11. (rivaroxaban or Xarelto or apixaban or Eliquis or dabigatran etexilate or Edoxaban or Savaysa or Betrixaban or ximelagatran or pradaxa or lixiana or exanta or Darexaban or Otamixaban* or Razaxaban or Bivalirudin or Desirudin or Lepirudin or Melagatran or YM 150 or Iprivask or argatrovan or pradax* or Xarelto or BIBR‐953 or BIBR953 or BIBR‐953ZW or BIBR953ZW or BAY 59‐7939 or BMS‐562247 or BMS562247 or DU‐176 or DU176 or DU‐176b or DU176B).mp. 12. RIVAROXABAN/ 13. DABIGATRAN/ 14. (target specific oral anticoagulant* or target‐specific oral anticoagulant* or TSOAC* or new oral anticoagulant* or novel oral anticoagulant* or NOAC* or direct‐acting oral anticoagulant* or direct acting oral anticoagulant* or direct oral anticoagulant* or DOAC*).ti,ab,kw. 15. (Aspirin* or Acuprin* or Anacin* or Ascriptin* or Aspergum* or Aspidrox* or Aspir‐Mox or Aspirtab* or Aspir‐trin or Bayer* or Bufferin* or Buffex* or Easprin* or Ecotrin* or Empirin* or Entaprin* or Entercote* or Fasprin* or Genacote* or Gennin‐FC or Genprin* or Halfprin* or Magnaprin* or Miniprin* or Minitabs* or Ridiprin* or Sloprin* or Uni‐Buff or Uni‐Tren or Valomag* or Zorprin* or benzoic acid* or Carboxyphenyl acetate or ASA or AC 5230 or Acenterine or Acesal or Aceticyl or Acetilsalicilico or Acetilum acidulatum or Acetisal or Acetol* or Acetonyl or Acetophen or Acetosal* or Acetylin or Acetylsal* or Acide acetylsalicylique or Acido acetilsalicilico or Acido O‐acetil‐benzoico or Acidum or acetylsalicylicum or Acimetten or Acisal or Acylpyrin or Adiro or AI3‐02956 or Asagran or Asaphen or Aspec or Aspergum or Aspirdrops or Aspro* or Asteric or Bay E4465 or Benaspir or Benzoic acid* or Bi‐prin or Bialpirina or Bialpirinia or "BRN 0779271" or Bufferin or Caprin or CCRIS 3243 or Cemirit or Claradin or Clariprin or Colfarit or Contrheuma retard or Coricidin* or Decaten or Delgesic or Dolean pH 8 or Duramax or Durlaza or Durlaza ER or Easprin or EC 200‐064‐1 or ECM or Ecolen or Ecotrin or Empirin or Endydol or Entericin or Enterophen or Enterosarein or Enterosarine or Entrophen or Extren or Globentyl or Globoid or Helicon or HSDB 652 or Idragin or Istopirin or Kapsazal or Kyselina* or Levius or Measurin or Medisyl or Micristin or Neuronika or Novid or NSC 27223 or Pharmacin or Pirseal or Polopiryna or Premaspin or Rheumin tabletten or Rheumintabletten or Rhodine or Rhonal or Ronal or Salacetin or Salcetogen or Saletin or Salicylic acid* or acetate or Solfrin* or Solpyron or SP 189 or Spira‐Dine or Temperal or Triple‐sal or Xaxa or Yasta or ZORprin or EINECS 200‐064‐1 or o‐Acetoxybenzoic acid or O‐Acetylsalicylic acid or o‐Carboxyphenyl acetate or S‐211 or UNII‐R16CO5Y76E or O‐Acetylsalicylic acid).mp. 16. exp Platelet Aggregation Inhibitors/ 17. ((antiplatelet or anti‐platelet or anti platelet) adj5 (agent* or drug* or med*)).mp. 18. (platelet* adj5 (inhibit* or antagon* or antiaggregant* or anti‐aggregant* or anti aggregant*)).mp. 19. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 20. exp MULTIPLE MYELOMA/ 21. myelom*.tw,kf. 22. exp PLASMACYTOMA/ 23. (plasm?cytom* or plasm?zytom* or plasma cytoma*).tw,kf. 24. (plasma* adj3 neoplas*).tw,kf. 25. (plasma cell adj1 (leukaem* or leukem* or tumor* or tumour*)).tw,kf. 26. ((plasmacytic* or plasmocytic* or plasmocyte*) adj1 (leukem* or leukaem*)).tw,kw. 27. kahler*.tw,kf. 28. 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 29. 19 and 28 30. randomized controlled trial.pt. 31. controlled clinical trial.pt. 32. randomized.ab. 33. placebo.ab. 34. clinical trials as topic.sh. 35. randomly.ab. 36. trial.ti. 37. clinical trial, phase iii/ 38. ("Phase 3" or "phase3" or "phase III" or P3 or "PIII").ti,ab,kw. 39. 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 40. 29 and 39 | |
| Embase | 1. exp anticoagulant agent/ 2. (anticoagulant* or anti‐coagulant*).tw. 3. (Heparin or Adomiparin or alpha‐Heparin or Arteven or "AVE‐5026" or CY 222 or "Depo‐Heparin" or "EINECS 232‐681‐7" or Fluxum or "Hed‐heparin" or Hepathrom or HSDB 3094 or KB 101 or "Lipo‐hepin" or M 118 or "M 118REH" or M118 or Octaparin or OP 386 or OP 622 or Pabyrin or Pularin or Subeparin or Sublingula or Thromboliquine or Triofiban or "UNII‐1K5KDI46KZ" or "UNII‐4QW4AN84NQ" or "UNII‐5R0L1D739E" or "UNII‐7UQ7X4Y489" or "UNII‐9816XA9004" or "UNII‐E47C0NF7LV" or "UNII‐M316WT19D8" or "UNII‐P776JQ4R2F" or "UNII‐S79O08V79F" or "UNII‐T2410KM04A" or "UNII‐V72OT3K19I" or "UNII‐VL0L558GCB" or Vetren or Vitrum AB or enoxaparin* or klexane or lovenox or fragmin* or normiflo or logiparin or innohep or danaproid or danaparoid or orgaran or antixarin or hibor or zibor or ivor or badyket or lohepa or lowhepa or seleparin* or tedelgliparin or lomoparan or orgaran or sulodexide or zivor or embolex or xaparin or fondaparinux or Arixtra or UFH or Hepalean or Calcilean or Calciparine or "Hep‐lock" or enoxaparin* or klexane or lovenox or fragmin* or normiflo or logiparin or innohep or danaproid or danaparoid or orgaran or antixarin or hibor or zibor or ivor or badyket or lohepa or lowhepa or seleparin* or tedelgliparin or lomoparan or orgaran or sulodexide or zivor or embolex or xaparin or fondaparinux or Arixtra or UFH or Hepalean or Panheprin).mp. 4. (FR 860 or FR860 or PK‐10,169 or PK 10,169 or PK10,169 or PK‐10169 or PK 10169 or PK10169 or EMT‐967 or EMT 967 or EMT967 or EMT‐966 or EMT 966 or EMT966 or CY 216 or CY‐216 or CY216 or LMF CY‐216 or LMF CY 216 or LMF CY216).mp. 5. exp coumarin derivative/ 6. (coumarin* or chromonar or coumestrol or esculin or isocoumarin* or psoralens or pyranocoumarins or umbelliferones).tw. 7. (4‐Hydroxycoumarin* or warfarin* or acenocoumarol or nicoumalone or sinthrome or Sintrom or phenindione or dicoumarol or coumadin or phenprocoumon or phepromaron or ethyl‐biscoumacetate or phenindione or Diphenadione or Tioclomarol or Racumi or Marcoumar or Marcumar or Falithrom or Jantoven or vitamin* K antagonist* or VKA or fluindione or difenacoum or coumatetralyl or coumadin* or warfant or marevan or aldocumar).mp. 8. (Dermatan Sulfate or (Chondroitin Sulfate adj B) or Dermatan Sulfphate or DS 435 or MF‐701 or OP‐370 or b‐Heparin or Mistral or Venorix).mp. 9. (thrombin adj inhibitor*).mp. 10. (factor Xa inhibitor* or antithrombin* or anti‐thrombin* or anti‐coagul* or anticoagul*).mp. 11. (rivaroxaban or Xarelto or apixaban or Eliquis or dabigatran etexilate or Edoxaban or Savaysa or Betrixaban or ximelagatran or pradaxa or lixiana or exanta or Darexaban or Otamixaban* or Razaxaban or Bivalirudin or Desirudin or Lepirudin or Melagatran or YM 150 or Iprivask or argatrovan or pradax* or Xarelto or BIBR‐953 or BIBR953 or BIBR‐953ZW or BIBR953ZW or BAY 59‐7939 or BMS‐562247 or BMS562247 or DU‐176 or DU176 or DU‐176b or DU176B).mp. 12. rivaroxaban/ 13. dabigatran/ 14. (target specific oral anticoagulant* or target‐specific oral anticoagulant* or TSOAC* or new oral anticoagulant* or novel oral anticoagulant* or NOAC* or direct‐acting oral anticoagulant* or direct acting oral anticoagulant* or direct oral anticoagulant* or DOAC*).ti,ab,kw. 15. (Aspirin* or Acuprin* or Anacin* or Ascriptin* or Aspergum* or Aspidrox* or Aspir‐Mox or Aspirtab* or Aspir‐trin or Bayer* or Bufferin* or Buffex* or Easprin* or Ecotrin* or Empirin* or Entaprin* or Entercote* or Fasprin* or Genacote* or Gennin‐FC or Genprin* or Halfprin* or Magnaprin* or Miniprin* or Minitabs* or Ridiprin* or Sloprin* or Uni‐Buff or Uni‐Tren or Valomag* or Zorprin* or benzoic acid* or Carboxyphenyl acetate or ASA or AC 5230 or Acenterine or Acesal or Aceticyl or Acetilsalicilico or Acetilum acidulatum or Acetisal or Acetol* or Acetonyl or Acetophen or Acetosal* or Acetylin or Acetylsal* or Acide acetylsalicylique or Acido acetilsalicilico or Acido O‐acetil‐benzoico or Acidum or acetylsalicylicum or Acimetten or Acisal or Acylpyrin or Adiro or AI3‐02956 or Asagran or Asaphen or Aspec or Aspergum or Aspirdrops or Aspro* or Asteric or Bay E4465 or Benaspir or Benzoic acid* or Bi‐prin or Bialpirina or Bialpirinia or "BRN 0779271" or Bufferin or Caprin or CCRIS 3243 or Cemirit or Claradin or Clariprin or Colfarit or Contrheuma retard or Coricidin* or Decaten or Delgesic or Dolean pH 8 or Duramax or Durlaza or Durlaza ER or Easprin or EC 200‐064‐1 or ECM or Ecolen or Ecotrin or Empirin or Endydol or Entericin or Enterophen or Enterosarein or Enterosarine or Entrophen or Extren or Globentyl or Globoid or Helicon or HSDB 652 or Idragin or Istopirin or Kapsazal or Kyselina* or Levius or Measurin or Medisyl or Micristin or Neuronika or Novid or NSC 27223 or Pharmacin or Pirseal or Polopiryna or Premaspin or Rheumin tabletten or Rheumintabletten or Rhodine or Rhonal or Ronal or Salacetin or Salcetogen or Saletin or Salicylic acid* or acetate or Solfrin* or Solpyron or SP 189 or Spira‐Dine or Temperal or Triple‐sal or Xaxa or Yasta or ZORprin or EINECS 200‐064‐1 or o‐Acetoxybenzoic acid or O‐Acetylsalicylic acid or o‐Carboxyphenyl acetate or S‐211 or UNII‐R16CO5Y76E or O‐Acetylsalicylic acid).mp. 16. exp antithrombocytic agent/ 17. ((antiplatelet or anti‐platelet or anti platelet) adj5 (agent* or drug* or med*)).mp. 18. (platelet* adj5 (inhibit* or antagon* or antiaggregant* or anti‐aggregant* or anti aggregant*)).mp. 19. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 20. exp multiple myeloma/ 21. myelom*.tw. 22. exp plasmacytoma/ 23. (plasm?cytom* or plasm?zytom* or plasma cytoma*).tw. 24. (plasma* adj3 neoplas*).tw. 25. (plasma cell adj1 (leukaem* or leukem* or tumor* or tumour*)).tw. 26. ((plasmacytic* or plasmocytic* or plasmocyte*) adj1 (leukem* or leukaem*)).tw,kw. 27. kahler*.tw. 28. 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 29. crossover procedure/ 30. double‐blind procedure/ 31. randomized controlled trial/ 32. single‐blind procedure/ 33. random*.mp. 34. factorial*.mp. 35. (crossover* or cross over* or cross‐over*).mp. 36. placebo*.mp. 37. (double* adj blind*).mp. 38. (singl* adj blind*).mp. 39. assign*.mp. 40. allocat*.mp. 41. volunteer*.mp. 42. ("Phase 3" or "phase3" or "phase III" or P3 or "PIII").ti,ab,kw. 43. 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 44. 19 and 28 and 43 |
Data and analyses
Comparison 1. Aspirin versus vitamin K antagonist (6 months).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 All‐cause mortality | 1 | 440 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.12, 73.24] |
| 1.2 Symptomatic deep vein thrombosis | 1 | 440 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.24, 1.33] |
| 1.3 Pulmonary embolism | 1 | 440 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.25, 3.95] |
| 1.4 Major bleeding | 1 | 440 | Risk Ratio (M‐H, Random, 95% CI) | 7.00 [0.36, 134.72] |
| 1.5 Minor bleeding | 1 | 440 | Risk Ratio (M‐H, Random, 95% CI) | 6.00 [0.73, 49.43] |
Comparison 2. Aspirin versus vitamin K antagonist (2 years).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 All‐cause mortality | 1 | 440 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.05, 5.47] |
| 2.2 Symptomatic deep vein thrombosis | 1 | 440 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.35, 1.44] |
| 2.3 Pulmonary embolism | 1 | 440 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.25, 3.95] |
Comparison 3. Aspirin versus low molecular weight heparin (6 months).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 All‐cause mortality | 2 | 781 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.06, 15.81] |
| 3.2 Symptomatic deep vein thrombosis | 2 | 781 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.49, 3.08] |
| 3.3 Pulmonary embolism | 2 | 781 | Risk Ratio (M‐H, Random, 95% CI) | 7.71 [0.97, 61.44] |
| 3.4 Major bleeding | 2 | 781 | Risk Ratio (M‐H, Random, 95% CI) | 6.97 [0.36, 134.11] |
| 3.5 Minor bleeding | 2 | 781 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [0.35, 5.78] |
Comparison 4. Aspirin versus low molecular weight heparin (2 years).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 All‐cause mortality | 1 | 440 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.06, 15.89] |
| 4.2 Symptomatic deep vein thrombosis | 1 | 440 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.53, 2.72] |
| 4.3 Pulmonary embolism | 1 | 440 | Risk Ratio (M‐H, Random, 95% CI) | 9.00 [0.49, 166.17] |
Comparison 5. Vitamin K antagonist versus low molecular weight heparin (6 months).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 All‐cause mortality | 1 | 439 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 8.10] |
| 5.2 Symptomatic deep vein thrombosis | 1 | 439 | Risk Ratio (M‐H, Random, 95% CI) | 2.32 [0.91, 5.93] |
| 5.3 Pulmonary embolism | 1 | 439 | Risk Ratio (M‐H, Random, 95% CI) | 8.96 [0.49, 165.42] |
| 5.4 Minor bleeding | 1 | 439 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.03, 3.17] |
Comparison 6. Vitamin K antagonist versus low molecular weight heparin (2 years).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 All‐cause mortality | 1 | 440 | Risk Ratio (M‐H, Random, 95% CI) | 2.00 [0.18, 21.90] |
| 6.2 Symptomatic deep vein thrombosis | 1 | 440 | Risk Ratio (M‐H, Random, 95% CI) | 1.70 [0.80, 3.63] |
| 6.3 Pulmonary embolism | 1 | 440 | Risk Ratio (M‐H, Random, 95% CI) | 9.00 [0.49, 166.17] |
Comparison 7. Aspirin versus direct oral anticoagulant (6 months).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Minor bleeding | 1 | 8 | Risk Ratio (M‐H, Random, 95% CI) | 5.00 [0.31, 79.94] |
Comparison 8. Aspirin versus direct oral anticoagulant (6 months): sensitivity analysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Deep vein thrombosis | 2 | 31 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.04, 20.33] |
| 8.2 Pulmonary embolism | 2 | 31 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 8.3 Major bleeding | 2 | 31 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.04, 20.33] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Campos‐Cabrera 2018 (abstract).
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | 105 participants with multiple myeloma received thalidomide‐ and dexamethasone‐based triplet induction therapy, maintenance with thalidomide and creatinine clearance > 30 mL/min. 23 (21.9%) participants had only an additional risk factor. 5 participants received rivaroxaban, 3 males and 2 females, median age of 67.5 years; additional factors were obesity in 4 and DM in one. Aspirin was received by 18 patients, 10 males an 8 females, median age 66.8 years; additional factors were obesity in 10, diabetes mellitus in 5, erythropoietin in 3. |
|
| Interventions |
Intervention 1: 100 mg aspirin Intervention 2: 10 mg rivaroxaban |
|
| Outcomes | Duration of follow‐up for the following outcomes: 6 months
|
|
| Notes |
Funding: not reported Conflict of interest: none reported ITT: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomised 5:1" Comment: probably randomised |
| Allocation concealment (selection bias) | High risk | Not reported Comment: probably not |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported Comment: probably not blinded; knowledge of the assigned intervention would likely lead to differential behaviours across intervention groups (e.g. differential dropout, differential cross‐over to an alternative intervention, or differential administration of co‐interventions) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported Comment: probably not blinded; knowledge of the assigned intervention would likelynot impact the assessment of the physiological outcomes (all‐cause mortality, deep vein thrombosis, pulmonary embolism, bleeding, etc.) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up |
| Selective reporting (reporting bias) | Low risk | Current study not registered. All outcomes listed in the methods section were reported in the results section. |
| Other bias | Low risk | No other bias suspected. |
Larocca 2012.
| Study characteristics | ||
| Methods | Open‐label, phase III, randomised study, multicentre | |
| Participants | Previously untreated patients with myeloma who received lenalidomide‐containing regimens 402 participants had been assigned to thalidomide‐containing regimens, of which 342 were enrolled into the substudy. Median age 57 in ASA group and 58 in LMWH group |
|
| Interventions |
Intervention 1: aspirin given at 100 mg/d orally during four 28‐day cycles of lenalidomide and dexamethasone Intervention 2: LMWH (enoxaparin) given at 40 mg/d subcutaneously during four 28‐day cycles of lenalidomide and dexamethasone Co‐intervention: participants in both arms assigned to Mel 200 (melphalan) consolidation stopped prophylaxis at this point (after the four 28‐cycles). Participants assigned to MPR (melphalan‐prednisone‐lenalidomide) consolidation continued prophylaxis for the six 28‐day cycles of MPR. The median duration of prophylaxis was 3.6 and 3.5 months in the ASA and LMWH groups, respectively. Screening/diagnostic testing for DVT/PE: not reported |
|
| Outcomes | Duration of follow‐up for the following outcomes: 6 months
|
|
| Notes |
Funding: the study RV‐MM‐PI209 was supported by Fondazione Neoplasie angue Onlus. Conflict of interest: personal fees (consultancy, advisory role, honoraria), research funding ITT: all efficacy and safety analyses were performed according to the intention‐to‐treat principle. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a simple randomization sequence run by a central computer, which generated an automated assignment procedure that was concealed from the investigators in each study center." |
| Allocation concealment (selection bias) | Low risk | Quote: "a simple randomization sequence run by a central computer, which generated an automated assignment procedure that was concealed from the investigators in each study center." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study Comment: definitely not blinded; knowledge of the assigned intervention would likelylead to differential behaviours across intervention groups (e.g. differential dropout, differential cross‐over to an alternative intervention, or differential administration of co‐interventions) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported Comment: probably not blinded; knowledge of the assigned intervention would likelynot impact the assessment of the physiological outcomes (all‐cause mortality, DVT, PE, bleeding, etc.) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study does not report on incomplete outcome data. |
| Selective reporting (reporting bias) | Low risk | Current substudy not registered. Substudy of another registered study. All outcomes listed in the methods section were reported in the results section. |
| Other bias | Low risk | Study not reported as stopped early for benefit. No other bias suspected. |
Palumbo 2011.
| Study characteristics | ||
| Methods | Open‐label, phase III, randomised study, multicentre | |
| Participants | Previously untreated patients with myeloma who received thalidomide‐containing regimens in 2 other studies: 734 participants had been randomly assigned to thalidomide‐containing regimens, of which 667 were enrolled into the substudy ("of whom 659 received at least one dose of the study treatment and were included in the efficacy and safety analyses") Median age 61 in ASA group, 60 in VKA group, and 62 in LMWH group |
|
| Interventions |
Intervention 1: aspirin given at 100 mg/d orally for 3 cycles of induction chemotherapy in younger participants and 6 cycles for older participants Intervention 2: VKA (warfarin) given at 1.25 mg/d orally for 3 cycles of induction chemotherapy in younger participants and 6 cycles for older participants Intervention 3: LMWH (enoxaparin) given at 40 mg/d subcutaneously for 3 cycles of induction chemotherapy in younger participants and 6 cycles for older participants The median durations of prophylaxis were 2.6 months in the ASA group, 2.4 months in the VKA group, and 2.6 months in the LMWH group. Co‐intervention: chemotherapy incorporating thalidomide Screening testing for DVT/PE: none Diagnostic testing for DVT: ultrasonography, ascending contrast venography, CT scan Diagnostic testing for PE: high‐probability lung scan; intermediate‐probability lung scan in the presence of objectively confirmed DVT; diagnostic spiral CT scan; diagnostic pulmonary angiography; or diagnostic TEE |
|
| Outcomes | Duration of follow‐up for the following outcomes: 6 months
Duration of follow‐up for the following outcomes: median 24.9 months
|
|
| Notes |
Funding: not reported Conflict of interest: personal fees (consultancy, advisory role, honoraria), research funding ITT: all efficacy and safety analyses were performed according to the intention‐to‐treat principle. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A simple random assignment sequence was generated by a centralized computer." |
| Allocation concealment (selection bias) | Low risk | Quote: "After registration in a centralized database through the Internet and validation of eligibility, patients were randomly allocated to treatments using an automated assignment procedure concealed to the investigators." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study Comment: definitely not blinded; knowledge of the assigned intervention would likely lead to differential behaviours across intervention groups (e.g. differential dropout, differential cross‐over to an alternative intervention, or differential administration of co‐interventions) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported Comment: probably not blinded; knowledge of the assigned intervention would likelynot impact the assessment of the physiological outcomes (all‐cause mortality, DVT, PE, bleeding, etc.) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: judgement based on comparison between missing datarate (ASA 4/224 = 1.7%; VKA 2/222 = 0.9%; LMWH 2/221 = 0.9%) and event rate (all‐cause mortality: ASA arm 1/220 = 0.4%; VKA arm 0%; LMWH arm 1/219 = 0.4%) |
| Selective reporting (reporting bias) | High risk | Current substudy not registered. Substudy of another registered study. All outcomes listed in the methods section were reported on in the results except for secondary endpoint related to any toxicity that required interruption of study prophylaxis. Comment: probably not free of selective reporting, since toxicity is expected to have been reported for such a study |
| Other bias | Low risk | Quote: "The data analysis was performed by the investigators in conjunction with an independent statistical office." Study not reported as stopped early for benefit. No other bias suspected. |
Sayar 2019.
| Study characteristics | ||
| Methods | Randomised, open‐label phase IV feasibility clinical trial | |
| Participants | 10 participants with newly diagnosed multiple myeloma, 8 with standard risk of VTE according to the Palumbo risk assessment model, and 2 with high risk of VTE
|
|
| Interventions |
Intervention 1: aspirin (75 mg orally daily if considered standard risk of VTE): 4 participants with standard risk of VTE, 0 participants with high risk of VTE Intervention 2: apixaban (2.5 mg twice a day): 4 participants with standard risk of VTE, 2 participants with high risk of VTE Co‐intervention: chemotherapy |
|
| Outcomes | Duration of follow‐up for the following outcomes: 6 months
|
|
| Notes |
Funding: funded by the National Institute for Health Research under its Research for Patient Benefit Programme (Grant Reference Number PB‐PG‐0614‐33101) Ethical approval: approved by the London Central Research Ethics Committee and the Medicines and Healthcare Products Regulatory Agency Conflict of interest: personal fees (consultancy, advisory role, honoraria), research funding ITT: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Block randomisation was conducted following risk stratification" |
| Allocation concealment (selection bias) | High risk | Not reported Comment: probably not conducted |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study Comment: definitely not blinded; knowledge of the assigned intervention would likely lead to differential behaviours across intervention groups (e.g. differential dropout, differential cross‐over to an alternative intervention, or differential administration of co‐interventions) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported Comment: probably not blinded; knowledge of the assigned intervention would likelynot impact the assessment of the physiological outcomes (all‐cause mortality, DVT, PE, bleeding, etc.) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up |
| Selective reporting (reporting bias) | Low risk | Current study not registered. All outcomes listed in the methods section were reported in the results section. |
| Other bias | Low risk | No other bias suspected. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Cornell 2019 | Not design of interest: single arm and protocol |
| Lokhorst 2010 | Not comparison of interest: two arms with different chemotherapy regimens, one arm receiving LMWH |
| Minnemma 2004 | Not design of interest: letter to the editor |
| Pegourie 2019 | Not design of interest: single arm |
| Swan 2018 | Not design of interest: review |
| Zangari 2004 | Not design of interest for the comparison of interest |
LMWH: low molecular weight heparin
Characteristics of ongoing studies [ordered by study ID]
Louzada 2018 (RithMM).
| Study name | ASA vs. Rivaroxaban in Newly Diagnosed or Relapsed and Refractory Multiple Myeloma Patients Treated With Len‐Dex Combination Therapy (RithMM) |
| Methods | Multicentre, open‐label randomised controlled clinical trial |
| Participants | Adult patients with Newly diagnosed Multiple Myeloma or Relapsed/Refractory Multiple Myelomavenous or arterial thromboembolism [ Time Frame: 6 months ] |
| Interventions |
Intervention: rivaroxaban (10 mg) daily Control: ASA 81 mg daily |
| Outcomes |
|
| Starting date | March 2018 |
| Contact information | Martha Louzada, MD MSc (Epid) Martha.Louzada@lhsc.on.ca |
| Notes |
NCT03428373 Status as of 14 June 2021: recruiting |
ASA: aspirin
Differences between protocol and review
There is no published protocol for this review.
Contributions of authors
LAK: searching for trials, screening, full‐text retrieval, data extraction, manuscript drafting, review coordination. CFM: screening, full‐text retrieval, data extraction, manuscript drafting, review coordination. IGT: screening, data extraction, data analysis, manuscript drafting, review coordination. MBH: screening, full‐text retrieval, data extraction, manuscript drafting. VY: screening, full‐text retrieval. IT: screening, data extraction. FS: screening, data extraction. MB: screening, full‐text retrieval, data extraction. LKH: manuscript drafting. HS: protocol development, data interpretation, methodological expertise. EAA: protocol development, data analysis, manuscript drafting, methodological expertise, review coordination.
Sources of support
Internal sources
No sources of support provided
External sources
-
American Society of Hematology, USA
This project was supported by the American Society of Hematology
Declarations of interest
LAK: declares no conflicts of interest. CFM: declares no conflicts of interest. IGT: declares no conflicts of interest. MBH: declares no conflicts of interest. VY: declares no conflicts of interest. IT: declares no conflicts of interest. FS: declares conflicts of interest. MB: declares conflicts of interest. LKH: panel member of the ASH VTE in Cancer patients and chair of the ASH Committee on quality. HS: panel member of the ASH VTE in Cancer patients and vice‐chair of the ASH VTE guidelines, and has had various leadership roles from 1999 until 2014 with ACCP VTE guidelines. EAA: served on the executive committee of the ACCP Antithrombotic Therapy Guidelines published in 2016.
Edited (no change to conclusions)
References
References to studies included in this review
Campos‐Cabrera 2018 (abstract) {published data only}
- Campos-Cabrera G, Mendez-Garcia E, Campos-Cabrera S, Campos-Villagomez JL, Campos-Cabrera V. Rivaroxaban or Aspirin As Thromboprophylaxis in Multiple Myeloma. In: Blood. Vol. 132(Supplement 1). 2018 Nov 29:5068.
Larocca 2012 {published data only}
- Larocca A, Cavallo F, Bringhen S, Di Raimondo F, Falanga A, Evangelista et al. Aspirin or enoxaparin thromboprophylaxis for patients with newly diagnosed multiple myeloma treated with lenalidomide. Blood 2012;119(4):933-939. [DOI] [PubMed] [Google Scholar]
Palumbo 2011 {published data only}
- Cavo M, Palumbo A, Bringhen S, Di Raimondo F, Patriarca F, Rossi D et al. Phase Iii Study Of Enoxaparin Versus Aspirin Versus Low-Dose Warfarin As Thromboprophylaxis For Patients With Newly Diagnosed Multiple Myeloma Treated Upfront With Thalidomide-Containing Regimens. Haematologica 2010;95:391. [Google Scholar]
- Cavo M, Palumbo A, Bringhen S, Falcone A, Musto P, Ciceri F et al. A phase III study of enoxaparin versus low-dose warfarin versus aspirin as thromboprophylaxis for patients with newly diagnosed multiple myeloma treated up-front with thalidomide-containing regimens.. Blood 2008;112(11):3017. [Google Scholar]
- Cavo M, Palumbo A, Bringhen S, Falcone A, Musto P, Ciceri F et al. A Phase III Study of Enoxaparin Versus Low-Dose Warfarin Versus Aspirin as Thromboprophylaxis for Patients with Newly Diagnosed Multiple Myeloma Treated up-Front with Thalidomide-Containing Regimens.. Haematologica 2009;94:s4. [Google Scholar]
- Magarotto V, Brioli A, Patriarca F, Rossi D, Petrucci MT, Nozzoli C et al. Enoxaparin, aspirin, or warfarin for the thromboprophylaxis in newly diagnosed myeloma patients receiving thalidomide: a randomized controlled trial.. XI Congress Of The Italian Society Of Experimental Hematology 2010;95:S1-S162. [Google Scholar]
- Palumbo A, Cavo M, Bringhen S, Zaccaria A, Spadano A, Palmieri S et al. Enoxaparin versus aspirin versus low-fixed-dose of warfarin in newly diagnosed myeloma patients treated with thalidomide-containing regimens: a randomized, controlled trial [Abstract No. 0910]. Haematologica 2008;93:362. [Google Scholar]
- Palumbo A, Cavo M, Bringhen S, Zamagni E, Romano A, Patriarca F et al. Aspirin, warfarin, or enoxaparin thromboprophylaxis in patients with multiple myeloma treated with thalidomide: a phase III, open-label, randomized trial.. Journal of Clinical Oncology 2011;29:986-993. [DOI] [PubMed] [Google Scholar]
Sayar 2019 {published data only}
- Sayar Z, Czuprynska J, Patel JP, Benjamin R, Roberts LN, Patel RK et al. What are the difficulties in conducting randomised controlled trials of thromboprophylaxis in myeloma patients and how can we address these? Lessons from apixaban versus LMWH or aspirin as thromboprophylaxis in newly diagnosed multiple myeloma (TiMM) feasibility clinical trial. Journal of Thrombosis and Thrombolysis. 2019 Aug 15;48(2):315-22. 2019;48(2):315-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Cornell 2019 {published data only}
- Cornell RF, Goldhaber SZ, Engelhardt BG, Moslehi J, Jagasia M, Patton D et al. Apixaban for primary prevention of venous thromboembolism in patients with multiple myeloma receiving immunomodulatory therapy. Frontiers in Oncology 2019;9:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornell RF, Goldhaber SZ, Engelhardt BG, Moslehi J, Jagasia M, Patton D et al. Prospective study of apixaban for primary prevention of venous thromboembolism in patients with multiple myeloma receiving immunomodulatory therapy. In: Blood. Vol. 132(Supplement 1):1233. 2018 Nov 29. [DOI] [PMC free article] [PubMed]
Lokhorst 2010 {published data only}
- Lokhorst HM, Holt B, Zweegman S, Vellenga E, Croockewit S, van Oers MH et al. A randomized phase 3 study on the effect of thalidomide combined with adriamycin, dexamethasone, and high-dose melphalan, followed by thalidomide maintenance in patients with multiple myeloma. Blood, The Journal of the American Society of Hematology 2010;115(6):1113-20. [DOI] [PubMed] [Google Scholar]
Minnemma 2004 {published data only}
- Minnema MC, Breitkreutz I, Auwerda JJ, Van der Holt B, Cremer FW, Van Marion AM et al. Prevention of venous thromboembolism with low molecular-weight heparin in patients with multiple myeloma treated with thalidomide and chemotherapy. Leukemia 2004;18(12):2044-6. [DOI] [PubMed] [Google Scholar]
Pegourie 2019 {published data only}
- Pegourie B, Karlin L, Benboubker L, Orsini‐Piocelle F, Tiab M, Auger‐Quittet S et al. Apixaban for the prevention of thromboembolism in immunomodulatory‐treated myeloma patients: Myelaxat, a phase 2 pilot study. American Journal of Hematology 2019;94(6):635-40. [DOI] [PubMed] [Google Scholar]
Swan 2018 {published data only}
- Swan D, Rocci A, Bradbury C, Thachil J. Venous thromboembolism in multiple myeloma–choice of prophylaxis, role of direct oral anticoagulants and special considerations. British Journal of Haematology 2018;183(4):538-56. [DOI] [PubMed] [Google Scholar]
Zangari 2004 {published data only}
- Zangari M, Barlogie B, Anaissie E, Saghafifar F, Eddlemon P, Jacobson J et al. Deep vein thrombosis in patients with multiple myeloma treated with thalidomide and chemotherapy: effects of prophylactic and therapeutic anticoagulation. British Journal of Haematology 2004;126(5):715-21. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Louzada 2018 (RithMM) {published data only}
- ASA vs. Rivaroxaban in Newly Diagnosed or Relapsed and Refractory Multiple Myeloma Patients Treated With Len-Dex Combination Therapy (RithMM). Ongoing study. March 2018. Contact author for more information.
Additional references
Akl 2013
- Akl EA, Johnston BC, Alonso-Coello P, Neumann I, Ebrahim S, Briel M, et al. Addressing dichotomous data for participants excluded from trial analysis: a guide for systematic reviewers. PloS One 2013;8(2):e57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Akl 2016
- Akl EA, Kahale LA, Ebrahim S, Alonso-Coello P, Schünemann HJ, Guyatt GH. Three challenges described for identifying participants with missing data in trials reports, and potential solutions suggested to systematic reviewers. Journal of Clinical Epidemiology 2016;76:147-54. [DOI] [PubMed] [Google Scholar]
Akl 2017
- Akl, EA, Meerpohl, JJ, Elliott, J, Kahale, LA, Schunemann, HJ, on behalf of the Living Systematic Review Network. Living systematic reviews: 4. Living guideline recommendations. Journal of Clinical Epidemiology 2017;91:47-53. [DOI] [PubMed] [Google Scholar]
Al‐Ani 2016
- Al-Ani, F, Bermejo, JMB, Mateos, MV and Louzada, M. Thromboprophylaxis in multiple myeloma patients treated with lenalidomide–A systematic review.. Thrombosis Research 2016;141:84-90. [DOI] [PubMed] [Google Scholar]
Alshurafa 2012
- Alshurafa M, Briel M, Akl EA, Haines T, Moayyedi P, Gentles SJ, et al. Inconsistent definitions for intention-to-treat in relation to missing outcome data: systematic review of the methods literature. PLOS One 2012;7(11):e49163. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ansell 2008
- Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians evidence-based clinical practice guidelines. Chest Journal 2008;133(6):160s-198s. [DOI] [PubMed] [Google Scholar]
Baz 2005
- Baz R, Li L, Kottke-Marchant K, Srkalovic G, McGowan B, Yinnaki E, et al. The role of aspirin in the prevention of thrombotic complications of thalidomide and anthracyclin-based chemotherapy for multiple myeloma. Mayo Clinic Proceedings 2005;80(12):1568. [DOI] [PubMed] [Google Scholar]
Blom 2005
- Blom JW, Doggen CJ, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis.. JAMA 2005;293(6):715-722. [DOI] [PubMed] [Google Scholar]
Brooker 2019
- Brooker, J, Synnot, A, McDonald, S, Elliott, J, Turner, T, Hodder, R, et al and the Living Evidence Network. Guidance for the production and publication of Cochrane living systematic reviews: Cochrane Reviews in living mode. Version December 2019. https://community.cochrane.org/review-production/production-resources/living-systematic-reviews#guidance.
Cochrane Crowd
- Cochrane Crowd. crowd.cochrane.org. (accessed 3 November 2017).
Corso 2004
- Corso A, Lorenzi A, Terilla V, Airo F, Varettoni M, Mangiacavalli S et al. Modification of thrombomodulin plasma levels in refrectaory myeloma patients during treatment with thalidomide and dexamethasone. Annals of Hematology 2004;83(9):588. [DOI] [PubMed] [Google Scholar]
Costa 2017
- Costa LJ, Brill IK, Omel J, Godby K, Kumar SK, Brown EE. Recent trends in multiple myeloma incidence and survival by age, race, and ethnicity in the United States.. Blood Advances 2017;1(4):282-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
CSR‐Web
- CSR-Web. CRS (Cochrane Register of Studies). community.cochrane.org/tools/data-management-tools/crs (accessed 3 November 2017).
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis. In: Egger M, Davey Smith G, Altman DG, editors(s). Systematic Reviews in Health Care: Meta-Analysis in Context. 2nd edition. BMJ Publication Group, 2001. [Google Scholar]
Farge 2019
- Farge D, Frere C. Recent advances in the treatment and prevention of venous thromboembolism in cancer patients: Role of the direct oral anticoagulants and their unique challenges. F1000Research 2019;8:-. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fradley 2018
- Fradley MG, Groarke JD, Laubach J, Alsina M, Lenihan DJ, Cornell RF et al. Recurrent cardiotoxicity potentiated by the interaction of proteasome inhibitor and immunomodulatory therapy for the treatment of multiple myeloma. British Journal of Haematology 2018;180:271–5. [DOI] [PubMed] [Google Scholar]
GRADE handbook
- Schünemann H, Brożek J, Guyatt G, Oxman A. GRADE Handbook, Updated October 2013. gdt.guidelinedevelopment.org/app/handbook/handbook.html (accessed prior to 3 November 2017).
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J et al. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables.. Journal of Clinical Epidemiology 2011;64(4):383-394. [DOI] [PubMed] [Google Scholar]
Guyatt 2017
- Guyatt GH, Ebrahim S, Alonso-Coello P, Johnston BC, Mathioudakis AG, Briel M, et al. GRADE guidelines 17: assessing the risk of bias associated with missing participant outcome data in a body of evidence. Journal of Clinical Epidemiology 2017;87:14-22. [DOI] [PubMed] [Google Scholar]
Haynes 2002
- Haynes RB, Devereaux PJ, Guyatt GH. Clinical expertise in the era of evidence-based medicine and patient choice. Vox Sanguinis 2002;83(suppl 1):383-6. [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbookfor Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration. Available from www.cochrane-handbook.org., updated March 2011. [Google Scholar]
Hirsh 1993
- Hirsh, J. Low molecular weight heparin. Thrombosis and Haemostasis 1993;70(1):204-207. [PubMed] [Google Scholar]
Kahale 2019
- Kahale LA, Guyatt GH, Agoritsas T, Briel M, Busse JW, Carrasco-Labra A et al. A guidance was developed to identify participants with missing outcome data in randomized controlled trials. Journal of Clinical Epidemiology 2019 Nov 1;115:55-63. [DOI] [PubMed] [Google Scholar]
Kahale 2020
- Kahale LA, Khamis AM, Diab B, Chang Y, Lopes LC, Agarwal A, Li L et al. Potential impact of missing outcome data on treatment effects in systematic reviews: imputation study.. BMJ 2020;370:m2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lyman 2015
- Lyman GH, Bohlke K, Khorana AA, Kuderer NM, Lee AY, Arcelus JI et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update 2014. Journal of Clinical Oncology 2015;33(6):654. [DOI] [PMC free article] [PubMed] [Google Scholar]
Minnema 2003
- Minnema MC, Fijnheer R, De Groot PG, Lokhorst HM. Extremely high levels of van Willebrand factor antigen and of procoagulant factor VIII found in multiple myeloma patients are associated with activity but not with thalidomide treatment. Journal of Thrombosis and Haemostasis 2003;1(3):445. [DOI] [PubMed] [Google Scholar]
Musallam 2009
- Musallam KM, Dahdaleh FS, Shamseddine AI, Taher AT. Incidence and prophylaxis of venous thromboembolic events in multiple myeloma patients receiving immunomodulatory therapy.. Thrombosis Research 2009;123(5):679-686. [DOI] [PubMed] [Google Scholar]
Nagy 2009
- Nagy Z, Turcsik V, Blaskó G. The effect of LMWH (Nadroparin) on tumor progression. Pathology & Oncology Research 2009;15(4):689. [DOI] [PubMed] [Google Scholar]
Palumbo 2008
- Palumbo A, Rajkumar SV, Dimopoulos MA, Richardson PG, San Miguel J, Barlogie B et al. Prevention of thalidomide-and lenalidomide-associated thrombosis in myeloma. Leukemia 2008;22(2):414. [DOI] [PubMed] [Google Scholar]
Palumbo 2014
- Palumbo A, Rajkumar SV, San Miguel J F, Larocca A, Niesvizky R. International Myeloma Working Group Consensus Statement for the Management, Treatment, and Supportive Care of Patients With Myeloma Not Eligible for Standard Autologous Stem-Cell Transplantation. Journal of Clinical Oncology 2014;32(6):587-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Park 2015
- Park JC, Pratz CF, Tesfaye A, Brodsky RA, Antonarakis ES. The effect of therapeutic anticoagulation on overall survival in men receiving first-line docetaxel chemotherapy for metastatic castration-resistant prostate cancer.. Clinical Genitourinary Cancer 2015;13(1):32-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rajkumar 2002
- Rajkumar SV, Hayman S, Gertz MA, Dispenzieri A, Lacy MQ, , Greipp PR et al. Combination therapy with thalidomide plus dexamethasone for newly diagnosed myeloma. Journal of Clinical Oncology 2002;20(21):4319-4323. [DOI] [PubMed] [Google Scholar]
Rajkumar 2014
- Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos MV et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. The Lancet Oncology 2014 Nov 1;15(12):e538-48. [DOI] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.4. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Rutjes 2020
- Rutjes AW, Porreca E, Candeloro M, Valeriani E, Di Nisio M. Primary prophylaxis for venous thromboembolism in ambulatory cancer patients receiving chemotherapy. Cochrane Database of Systematic Reviews 2020, Issue 12. Art. No: CD008500. [DOI: 10.1002/14651858.CD008500.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sanford 2014
- Sanford D, Naidu A, Alizadeh N, Lazo-Langner A. The effect of low molecular weight heparin on survival in cancer patients: an updated systematic review and meta-analysis of randomized trials. Journal of Thrombosis and Haemostasis 2014;12(7):1076-1085. [DOI] [PubMed] [Google Scholar]
Santesso 2020
- Santesso N, Glenton C, Dahm P, Garner P, Akl EA, Alper B et al. GRADE guidelines 26: informative statements to communicate the findings of systematic reviews of interventions. Journal of Clinical Epidemiology 2020 March 1;119:126-35. [DOI] [PubMed] [Google Scholar]
Simmonds 2017
- Simmonds ME, Salanti G, Higgins JE, McKenzie J, Elliott JE on behalf of the Living Systematic Review Network. Living Systematic Reviews: 3. Statistical methods for updating meta-analyses. Journal of Clinical Epidemiology 2017 September 11;91:38-46. [DOI] [PubMed] [Google Scholar]
Smorenburg 2001
- Smorenburg SM, Van Noorden CJ. The complex effects of heparins on cancer progression and metastasis in experimental studies. Pharmacological Review 2001;53(1):93-105. [PubMed] [Google Scholar]
Synnot 2017
- Synnot A, Turner T, Elliott J, Akl E, MacLehose H and the Living Systematic Review Network. Cochrane Living Systematic Reviews Interim guidance for pilots. community.cochrane.org/review-production/production-resources/living-systematic-reviews (accessed prior to 3 November 2017);Version 0.3(21 April 2017).
Thodiyil 2002
- Thodiyil PA, Kakkar AK. Variation in relative risk of venous thromboembolism in different cancers.. Thrombosis and Haemostasis 2002;88(6):1076-7. [PubMed] [Google Scholar]
Wallace 2017
- Wallace BC, Noel-Storr A, Marshall IJ, Cohen AM, Smalheiser NR, Thomas J. Identifying reports of randomized controlled trials (RCTs) via a hybrid machine learning and crowdsourcing approach. Journal of the American Medical Informatics Association 2017;0(0):1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zangari 2001
- Zangari M, Anaissie E, Barlogie B, Badros A, Desikan R, Gopal AV et al. Increased risk of deep-vein thrombosis in patients with multiple myeloma receiving thalidomide and chemotherapy. Blood 2001;98(5):1614-1615. [DOI] [PubMed] [Google Scholar]


