Abstract
Discrepancies in blood sample collection and processing could have a significant impact on levels of metabolites, peptides, and protein biomarkers of inflammation in the blood; thus, sample quality control is critical for successful biomarker identification and validation. In this study, we analyzed the effects of several preanalytical processing conditions, including different storage times and temperatures for blood or plasma samples and different centrifugation forces on the levels of metabolites, peptides, and inflammation biomarkers in human plasma samples using ethylenediaminetetraacetic acid (EDTA) as an anticoagulant. Temperature was found to be the major factor for metabolite variation, and both time and temperature were identified as major factors for peptide variation. For inflammation biomarkers, temperature played different roles depending on the sample type (blood or plasma). Low temperature affected inflammation biomarkers in blood, while room temperature impacted inflammation biomarkers in plasma.
Keywords: preanalytical variability, blood, plasma, metabolites, peptides, inflammation biomarkers, sample quality
Graphical Abstract

1. INTRODUCTION
Human blood is an effective tool for biomedical research such as disease biomarker discovery and validation, as it circulates throughout the body and contains various types of biomolecules, including mRNAs, proteins, peptides, and metabolites, which reflect the physiological and pathological status of the subject. Moreover, millions of human blood samples, including plasma or serum, are available in biobanks or other sources. However, the quality of these existing blood samples may be impacted by discrepancies in preanalytical sample processing and storage time, which may result in erroneous interpretation of the research outcomes.1–4 Therefore, it is essential to evaluate the sample quality and to understand how changes in preanalytical processing can impact the discovery or validation of potential biomarker candidates.
Omics technologies could serve as useful tools to evaluate the sample quality and discover biomarkers related to preanalytical variability, as they can be used to detect and quantify many biomolecules simultaneously. For example, Hebel et al. revealed that different anticoagulants influenced the metabolome, proteome, and to a lesser extent transcriptome; they also observed no systematic influence of time-in-storage in samples stored over a period of 13–17 years.1 Enroth et al. studied the effects of long-term storage on the abundance of 108 proteins in biobank plasma samples collected between 1998 and 2014 using the Proximity Extension Assay and found 18 proteins were affected.2 Aguilar-Mahecha et al. investigated the effects of blood tubes, centrifugal force, and temperature on abundant plasma proteins and a panel of inflammation biomarkers, using a targeted proteomics approach and a bead-based affinity immunoassay. They found that high abundance plasma proteins were highly stable in plasma left unprocessed for up to 6 h, while cytokines were affected depending on collection tubes and the process temperature.3 Kamlage et al. revealed that short-term storage of blood, hemolysis, and short-term storage of noncooled plasma significantly impacted the metabolome based on broad and targeted metabolomics profiling.4
This study examined the effects of common preanalytical confounders in clinical blood sample practice, including centrifugal force to separate plasma from the whole blood, delayed blood sample processing, and storage temperature of blood and plasma on metabolites, peptides, and inflammation biomarkers in human EDTA plasma. Other confounding factors such as hemolysis, icterus, and lipemia are routinely analyzed in clinical laboratories. Therefore, they can be controlled and hence were not considered in this study. Mass spectrometry (MS)-based metabolomics and peptidomics and multiplexed bead-based immunoassay technologies were applied to identify metabolites, peptides, and inflammation biomarkers as potential quality biomarkers that are sensitive to preanalytical variations. This study was also anticipated to provide new insights in metabolite, peptide, and inflammation biomarker stability that could be useful for standardizing blood collection and processing in general, or for specific molecules in biomedical research and other applications.
2. MATERIALS AND METHODS
2.1. Blood Sample Collection and Processing
The study was approved by the U.S. Food and Drug Administration (FDA) Research Involving Human Subjects Committee (RIHSC). Self-reported healthy male and female volunteers were recruited who met the specified subject inclusion/exclusion criteria. The inclusion criteria were age of 18–65 years, overnight fasting, and BMI 18–30 kg/m2, and the exclusion criteria were acute or chronic diseases, anemia, second and third trimester of pregnancy, medication with heparin, nonsteroidal anti-inflammatory drugs (NSAID) or steroidal anti-inflammatory drugs (SAID) within 10 days of study recruitment, medication with antihistamines or selective serotonin reuptake inhibitors within 4 weeks of study recruitment. Informed consent was obtained from all participants.
A total of 20 healthy overnight fasting volunteers, including 9 women and 11 men with an age range of 24 to 64 years and a BMI range of 21.3 to 29.9, were recruited in the study. Six tubes of K2-EDTA blood samples were obtained from each subject. The tubes were randomly assigned to one of six defined preanalytical conditions and each sample was processed to plasma by centrifugation for 15 min at room temperature (RT), between 19 and 22 °C. The six preanalytical conditions include the following: (1) immediately processed to plasma (control), (2) processed to plasma under a centrifugal force of 1300g, (3) blood was kept on wet ice (0 °C) for 6 h and then processed to plasma, (4) blood was kept at RT for 6 h and then processed to plasma, (5) blood was processed to plasma and then stored at 4 °C for 24 h, (6) blood was processed to plasma and then stored at RT for 24 h. Apart from the low centrifugal force (1300g), blood was processed to plasma with a centrifugal force of 2500g for all the other conditions. The collection tubes were stratified over the six blood processing groups to reduce variability between the collection tubes due to physiological changes in the vein during blood draw. The processed plasma under each condition was aliquoted into Eppendorf tubes, snap frozen in liquid nitrogen, and then stored at −80 °C until analysis.
2.2. Metabolite Analysis
Specific sample preparation, LC–MS/MS, and GC–MS measurement for metabolites have been described previously with technical details.4 Briefly, for MxP Broad Profiling, proteins were removed from plasma by precipitation, and then the samples were separated into polar and nonpolar fractions by adding water and a mixture of ethanol and dichloromethane. Both nonpolar and polar fractions were analyzed by LC–MS/MS (Agilent 1100 HPLC system coupled to an Applied Biosystems API4000 MS/MS-System) and GC–MS (Agilent 6890 GC coupled to an Agilent 5973 MS-System) following an additional derivatization step, including derivatization with O-methylhydroxylamine hydrochloride (20 g/L in pyridine, 50 μL) and with a silylating agent [N-methyl-N-(trimethylsilyl)trifluoroacetamide, 50 μL]. MxP Catecholamines and MxP Eicosanoids were measured by solid phase extraction (SPE)–LC–MS/MS (Symbiosis Pharma, Spark, coupled to an Applied Biosystems API4000 MS/MS system). MxP Sphingoids were measured by an Agilent 1290 ultra-HPLC (UHPLC) system coupled to an Applied Biosystems API5500 MS/MS system.4
A pooled sample was prepared from aliquots of all samples and analyzed within each analytical sequence for MxP Broad Profiling and MxP Sphingoids analysis. MxP Broad Profiling and MxP Sphingoids peak data were normalized to the median of the pooled samples to correct for device variability and reported as ratios relative to the pooled samples. MxP Eicosanoids and MxP Catecholamines peak data were normalized to internal standards and expressed as absolute values based on calibration standards.
2.3. Free Hemoglobin Measurement
Free hemoglobin was measured by the 2-wavelength (540/680) cyanhemoglobin method.5
2.4. Peptide Identification and Quantitation by LC–MS/MS
Peptides were extracted by ultrafiltration. Plasma (50 μL) was mixed with 150 μL of 40% acetonitrile (ACN) (ThermoFisher Scientific, Fair Lawn, NJ) and 20 μL of iRT standard peptides (Pierce, Rockford, IL), which contains 15 synthetic heavy stable isotope-coded peptides with 50 fmol/μL for each peptide. The mixtures were transferred to a 10 kDa MWCO filter (Millipore, Bedford, MA) for centrifugation at 13 000g for 30 min. The filtrates were vacuum-speed dried and stored at −20 °C. The peptides were resuspended with 50 μL of 0.1% formic acid (FA) (Fluka, Milwaukee, WI) and analyzed on an Orbitrap Elite mass spectrometer (ThermoFisher Scientific, Bremen, Germany) coupled to a nanoAcquity UPLC system (Waters, Milford, MA). Solvent A was 0.1% FA in water, and solvent B was 0.1% FA in ACN. Peptides (4 μL) were loaded into a UPLC Symmetry trap column (180 μm i.d. × 2 cm packed with 5 μm C18 resin, Waters) by 2% solvent B for 8 min at 6 μL/min. Peptides were eluted at 500 nL/min using the following gradient: 5–9% solvent B over 2 min, 9–27% solvent B over 43 min, 27–42% solvent B over 10 min, 42–98% solvent B over 5 min, and holding at 98% solvent B for 5 min. To minimize carry-over and re-equilibrate the column between sample injections, 2 μL of solvent A was injected between each sample (30-min run). The full MS scan was acquired with an m/z range of 300–1800 and a resolution of 120 000 in a centroid mode in the Orbitrap, followed by data-dependent MS/MS scans in the linear ion trap on the 15 most abundant precursor ions. Monoisotopic precursor selection was enabled. Precursor ions subjected to MS/MS were excluded from repeated analysis for 30 s.
MS raw data files were processed and searched by the MaxQuant software (version 1.5.8.3) with the Andromeda search engine against the human Swiss-Prot database (released 2017_04, with 20198 entries) under the following criteria: unspecific digestion mode, precursor tolerance of 20 ppm in the first search for mass calibration and 4.5 ppm in the main search, false discovery rate (FDR) of 0.01 for peptide spectrum matching and 0.05 for protein identification, and variable methionine oxidation. Peptides identified from MaxQuant search and the iRT peptides were imported into Skyline 3.6 to be manually verified and quantified with MS1 peak area as the signal intensity.6 Ten iRT peptides with reproducible signals between samples were used for normalization. First, each iRT peptide was normalized to its median intensity of 118 samples (the plasma processed from the low centrifugal force and the plasma at RT for 24 h from subject #20 were not analyzed due to lack of enough sample), and then for each sample, the peak area of each identified peptide was divided by the mean of the median-normalized values from 10 iRT peptides within the same sample to reduce run-to-run variation.
2.5. Multiplex Immunoassays for Inflammation Biomarkers
Thirty-seven inflammation biomarkers from the TNF super-family proteins, IFN family proteins, Treg cytokines, and MMPs were measured using a commercially available multiplexed bead-based immunoassay kit (Bio-Plex, #171AL001M) on the Luminex 200 system (Bio-Rad Laboratories, Hercules, CA) following the manufacturer’s instruction. Briefly, plasma samples were diluted 1:4 and measured in duplicate. The concentrations of the analytes were calculated on the basis of standard calibration curves using Bio-Plex Manager Software Version 4.0 (Bio-Rad Laboratories, Hercules, CA). Sample measurements were considered reliable if the standards for the calibration curve were within 70–130% of recovery as recommended by the manufacturer and sample concentrations were within the standard range.
2.6. Statistical Analysis
Blood samples for each subject were collected in a sequence of six tubes. The collection tubes were randomly assigned to each of the six processing conditions as shown in Table S1. William’s design, based on replicates of a standard 6 × 6 Latin square, was used to randomly assign sequences of the six preanalytical processing conditions to blood samples for each subject. A repeated measures analysis of variance (ANOVA) profile model was used to evaluate the effects of various preanalytical confounders on blood sample quality. The ANOVA model was preferred because of its robustness to slight deviations from normality due to the large number of analytes with variable scales of measurement. A fold change of 1.2 was considered to be a reasonable exploratory benchmark for metabolites and inflammation biomarkers, while a fold change of 2 was used for peptides. Statistical tests for pairwise group comparison were adjusted for multiplicity testing to control the Type I Error rate. Dunnett’s method was used for comparisons relative to the control. All statistical tests were two-sided, and significance was assessed at the nominal 5% level. The Benjamini and Hochberg method was used to control the false discovery rate (FDR) across the analytes. The SAS System for Windows (v9.3) was used for statistical analysis.
Principal component analysis (PCA) and partial least-squares discriminant analysis (PLS-DA) were performed to cluster and classify samples. For PLS-DA modeling (Figure S1), the data was randomly divided into a training set of 12 subjects and a testing set of 8 subjects, and model performance was evaluated by 50 × 5-fold cross-validation (CV) and prediction of the testing set (test). This process was repeated 50 times and the performance parameters (prediction error rate, sensitivity, and specificity) are presented as mean with standard deviation. The software R 3.3.1 with packages ggplot2, caret, and mixOmics was used for multivariate data analysis, hierarchical clustering analysis, and visualization.7–10
3. RESULTS
The effects of low centrifugal force, storage of blood at 0 °C or RT for 6 h, and storage of plasma at 4 °C or RT for 24 h on metabolites, peptides, and inflammation biomarkers are shown in Figures 1 and 2. Detailed statistical results of metabolites, peptides, and inflammation biomarkers are listed in Tables S2, S3, and S4, respectively.
Figure 1.

Effects of preanalytical variations on metabolites, peptides, and inflammation biomarkers. Colored areas indicate the number of analytes which are stable (cyan), increased (red), and decreased (green) relative to the control. The abbreviations Lowxg, B6h0C, B6hRT, P24h4C, and P24hRT represent a low centrifugation force (1300g), blood incubation at 0 °C for 6 h, blood incubation at RT for 6 h, plasma incubation at 4 °C for 24 h, and plasma incubation at RT for 24 h, respectively.
Figure 2.

Venn diagrams of significantly changed metabolites, peptides, and inflammation biomarkers from preanalytical variations in blood sample processing. Numbers show unique and frequently altered analytes among preanalytical variations. The abbreviations Lowxg, B6h0C, B6hRT, P24h4C, and P24hRT represent a low centrifugation force (1300g), blood incubation at 0 °C for 6 h, blood incubation at RT for 6 h, plasma incubation at 4 °C for 24 h, and plasma incubation at RT for 24 h, respectively.
3.1. Metabolites
Metabolite levels were most significantly affected by storing blood at RT for 6 h and plasma at RT for 24 h, resulting in 47 (19.8% of total) and 26 (11% of total) metabolites changing significantly (p < 0.05, FDR < 0.2, and fold change ⩾1.2), accounting for 82% and 46% of the significantly changed metabolites across all conditions (57, not including free hemoglobin) in this study (Figure 1, Table S2A).
3.1.1. Blood Processing Delay.
As shown in Figure 2, 24 altered metabolites were unique to storage of blood at RT for 6 h, of which 19 metabolites (aspartate, taurine, ornithine, ribose, isocitrate, malate, hexadecanoylcarnitine, hexadecenoylcarnitine, octadecanoylcarnitine, oleoylcarnitine, normetanephrine, allantoin, uridine, nicotinamide, sphingadienine-1-phosphate (d18:2), sphinganine-1-phosphate (d18:0), sphingosine-1-phosphate (d16:1), sphingosine-1-phosphate (d17:1), sphingosine-1-phosphate (d18:1)) were significantly increased, and five metabolites (arginine, fructosamine, glucose-1-phosphate, glucose, mannose) were significantly decreased in concentration (Table S2A).
Nine metabolites (maltose, sphingosine (d18:1), sphingadienine (d18:2), sphingosine (d16:1), 12-hydroxyeicosatetraenoic acid (C20:cis[5,8,10,14]4), pyruvate, serotonin (5-HT), hypoxanthine, and an unknown polar (internal ID# 38102095)) were significantly changed in concentration by blood storage at both 0 °C and RT for 6 h (Figure 2, Table S2A). No metabolites were found to have a specific response to storing blood at 0 °C for 6 h.
3.1.2. Plasma Processing Delay.
Ten metabolites altered were unique to storing plasma at RT for 24 h, including seven metabolites (ceramide (d18:1, C24:0), DAG (C18:1, C18:2), lysophosphatidylcholine (C17:0), lysophosphatidylcholine (C18:0), 8-hydroxyeicosatetraenoic acid (C20:trans[5]cis-[9,11,14]4) (8-HETE), prostaglandin F2 alpha, and an unknown lipid (internal ID# 68100033)) that were significantly increased, and three metabolites (cysteine, cystine, adrenaline) that were significantly decreased in concentration (Figure 2, Table S2A). Eight metabolites (sphingosine (d18:1), sphingadienine (d18:2), sphingosine (d16:1), glycerate, 5-hydroxyeicosatetraenoic acid (C20:trans[6]cis[8,11,14]-4) (5-HETE), threonic acid, 3,4-dihydroxyphenylacetic acid (DOPAC), 3,4-dihydroxyphenylglycol (DOPEG)) were significantly changed after storing plasma at 4 °C for 24 h, and these eight metabolites were also significantly changed after storing blood at RT and/or in other conditions (Figure 2, Table S2A).
3.1.3. Low Centrifugal Force.
Only one metabolite, maltose, was significantly changed under the low centrifugation condition.
3.2. Peptides
In total, 623 peptides were identified from 104 protein groups, and of those, 57 protein groups had more than 2 peptides (Table S5A and S5B). The majority of the peptides identified were from fibrinogen alpha/beta, complement C3, C4A/B, alpha-2-microglobin, transthyretin, apolipoprotein A-IV, inter-alpha-trypsin inhibitor heavy chain, alpha-2-antiplasmin, and kininogen-1.
Forty-three peptides were quantified across all sample processing conditions. As shown in Figure 1, peptides were significantly affected by storing blood at 0 °C or RT for 6 h and plasma at 4 °C or RT for 24 h, resulting in statistically significant changes of 4 (9.3%), 9 (20.9%), 10 (23.3%), 17 (39.5%) peptides, respectively. Significantly changed peptides are listed in Table S3A.
There are considerable overlaps in significantly altered peptides between blood and plasma processing conditions (Figure 2). Sixteen peptides identified from fibrinogen, complement C4B, complement C3, and alpha-2-antiplasmin were significantly increased, while only three peptides identified from fibrinogen, trafficking protein particle complex subunit 2B, and PAB-dependent poly(A)-specific ribonuclease subunit PAN2 were significantly decreased in abundance in at least one of the considered processing conditions (Table S3A).
There were nine significantly changed peptides common to plasma stored at 4 °C and RT for 24 h (Table S3A), and some peptides had a large fold change in plasma stored at 4 °C for 24 h, which was quite different as compared to plasma stored at RT for 24 h and other preanalytical conditions (Table 1). Interestingly, these results were associated with individual subjects (Figure 3), as the peptide levels were significantly higher in subjects 6, 12, 14, and 19 at 4 °C than in other subjects or other conditions.
Table 1.
Peptides with a Large Fold Change in Plasma Stored at 4 °C for 24 h As Compared to Other Preanalytical Conditions
| peptide | precursor charge | UniProt | protein | changea | fold change | p-value | FDR |
|---|---|---|---|---|---|---|---|
| A: Blood at 0 °C for 6 h | |||||||
| SSSYSKQFTSSTSYNRGDSTFESKS | 4 | P02671 | FIBA | + | 2.31 | 0.000 | 0.006 |
| SSSYSKQFTSSTSYNRGDSTFES | 3 | P02671 | FIBA | + | 2.80 | 0.107 | 0.545 |
| GDSTFESKSYKMA | 3 | P02671 | FIBA | + | 1.58 | 0.674 | 1.000 |
| SSSYSKQFTSSTSYNRGDSTFESKSY | 4 | P02671 | FIBA | + | 2.81 | 0.289 | 1.000 |
| B: Blood at RT for 6 h | |||||||
| SSSYSKQFTSSTSYNRGDSTFESKS | 4 | P02671 | FIBA | + | 2.00 | 0.000 | 0.001 |
| SSSYSKQFTSSTSYNRGDSTFES | 3 | P02671 | FIBA | + | 2.02 | 0.989 | 1.000 |
| GDSTFESKSYKMA | 3 | P02671 | FIBA | + | 2.05 | 1.000 | 1.000 |
| SSSYSKQFTSSTSYNRGDSTFESKSY | 4 | P02671 | FIBA | − | 1.08 | 1.000 | 1.000 |
| C: Low centrifugation force | |||||||
| SSSYSKQFTSSTSYNRGDSTFESKS | 4 | P02671 | FIBA | + | 1.08 | 0.047 | 0.433 |
| SSSYSKQFTSSTSYNRGDSTFES | 3 | P02671 | FIBA | − | 1.87 | 0.925 | 1.000 |
| GDSTFESKSYKMA | 3 | P02671 | FIBA | − | 1.03 | 0.961 | 1.000 |
| SSSYSKQFTSSTSYNRGDSTFESKSY | 4 | P02671 | FIBA | − | 1.86 | 0.128 | 0.735 |
| D: Plasma at 4 °C for 24 h | |||||||
| SSSYSKQFTSSTSYNRGDSTFESKS | 4 | P02671 | FIBA | + | 30.69 | 0.000 | 0.000 |
| SSSYSKQFTSSTSYNRGDSTFES | 3 | P02671 | FIBA | + | 38.19 | 0.001 | 0.004 |
| GDSTFESKSYKMA | 3 | P02671 | FIBA | + | 36.85 | 0.023 | 0.082 |
| SSSYSKQFTSSTSYNRGDSTFESKSY | 4 | P02671 | FIBA | + | 84.20 | 0.015 | 0.059 |
| E: Plasma at RT for 24 h | |||||||
| SSSYSKQFTSSTSYNRGDSTFESKS | 4 | P02671 | FIBA | + | 6.20 | 0.000 | 0.000 |
| SSSYSKQFTSSTSYNRGDSTFES | 3 | P02671 | FIBA | + | 2.06 | 0.016 | 0.042 |
| GDSTFESKSYKMA | 3 | P02671 | FIBA | + | 5.42 | 0.030 | 0.069 |
| SSSYSKQFTSSTSYNRGDSTFESKSY | 4 | P02671 | FIBA | + | 3.70 | 0.053 | 0.111 |
The symbols + and − indicate an increase or decrease of peptide abundance compared to the control group.
Figure 3.

Individual subject responses at peptide levels to preanalytical variations in blood sample processing. Subjects 6, 12, 14, and 19 showed more sensitive response to storing plasma at 4 °C for 24 h than other conditions or subjects in terms of the peptides from fibrinogen alpha chain. The abbreviations Lowxg, B6h0C, B6hRT, P24h4C, and P24hRT represent a low centrifugation force (1300g), blood incubation at 0 °C for 6 h, blood incubation at RT for 6 h, plasma incubation at 4 °C for 24 h, and plasma incubation at RT for 24 h, respectively.
3.3. Inflammation Biomarkers
Inflammation biomarkers were significantly affected by storing blood at 0 °C for 6 h and storing plasma at RT for 24 h, resulting in statistically significant abundance changes of 3 (13.6% of total) and 10 (45.5% of total) inflammation biomarkers (Figure 1). Low centrifugal force had the least effect among the preanalytical variations. Significantly changed inflammation biomarkers are listed in Table S4A.
Seven inflammation biomarkers (APRIL, sCD163, Chitinase 3-like 1, gp130, sIL6, sTNFR1, sTNFR2) were specifically affected in response to storing plasma at RT for 24 h, and one (MMP-2) had a specific response to storing blood at RT for 6 h (Figure 2, Table S4A). Interestingly, pentraxin-3, sCD30, and osteopontin were significantly elevated when blood was stored at 0 °C for 6 h and when plasma was stored at RT for 24 h. In addition, pentraxin-3 was also significantly elevated after prolonged plasma incubation at 4 °C for 24 h (Table S4A).
3.4. PCA and PLS-DA
3.4.1. PCA.
To examine the effects of biological and preanalytical variations on the potential patterns of metabolites, peptides, and inflammation biomarkers, PCA was employed for unpaired multivariate analysis. Analysis of all measured metabolites (Table S2B) revealed that samples from the same subject were clustered (Figure 4A, left), indicating that the variation among individual subjects was greater than between preanalytical conditions. In order to highlight the effect of preanalytical conditions within intraindividual variation, the data from preanalytical variations were paired for each subject and analyzed using a multilevel PCA, which separated the effects of preanalytical confounders from subject variations.11,12 Blood samples stored at RT for 6 h and plasma samples stored at RT for 24 h were well separated from other groups (Figure 4A, right), indicating that temperature is a major factor driving the changes in metabolite concentration. In contrast to metabolites, the change of peptides induced by preanalytical variations could be identified by the PCA. As shown in Figure 4B (left), plasma samples storing at RT for 24 h were clustered, which indicated that storage time and temperature were critical factors driving peptide abundance changes. These changes can be further highlighted with a multilevel PCA (Figure 4B, right), where samples were clustered based on preanalytical variables. Interestingly, subjects 6, 12, 14, and 19 showed a different response to plasma storage at 4 °C for 24 h compared to other subjects. This analysis further demonstrated that preanalytical variation has driven individual specific responses at the peptide levels. For inflammation biomarkers, there were no clear clusters in the PCA (Figure 4C, left). However, blood samples stored at 0 °C for 6 h and plasma samples stored at RT for 24 h were clustered in a multilevel PCA (Figure 4C, right), which indicated that the impact of temperature on inflammation biomarkers is dependent on the sample type, i.e., inflammation biomarkers were affected by low-temperature storage of blood and by RT storage of plasma. The loading plots of PCA and multilevel PCA analyses of metabolites, peptides, and inflammation biomarkers, which provide the contribution of specific molecules for a given component, are presented in Figure S2A–F.
Figure 4.

Score plots of PCA (left) and multilevel PCA (right) of the analyzed metabolites (A), peptides (B), and inflammation biomarkers (C). Explained variances of the first two principal components (PC1 and PC2) are given. The abbreviations Lowxg, B6h0C, B6hRT, P24h4C, and P24hRT represent a low centrifugation force (1300g), blood incubation at 0 °C for 6 h, blood incubation at RT for 6 h, plasma incubation at 4 °C for 24 h, and plasma incubation at RT for 24 h, respectively.
3.4.2. Multilevel PLS-DA.
The significantly changed metabolites, peptides, and inflammation biomarkers identified in this study are considered as potential biomarkers for sample quality control. To assess sample quality using these potential biomarkers in predictive models, the supervised multivariate analysis method multilevel PLS-DA was used to evaluate the significantly changed molecules. A multilevel PLS-DA of 57 significantly altered metabolites (Table S2A) resulted in a clear separation of blood samples stored at RT for 6 h from plasma samples stored at RT for 24 h (Figure 5A). A multilevel PLS-DA of 19 significantly changed peptides (Table S3A) led to clear separation of blood samples stored at RT for 6 h from plasma samples stored at 4 °C or RT for 24 h (Figure 5B), and a multilevel PLS-DA analysis of 11 significantly changed inflammation biomarkers (Table S4A) showed that there was high overlap between groups (Figure 5C). In addition, the heatmap from the hierarchical cluster analysis of these three classes of changed molecules showed strong clustering of samples from each of the following four preanalytical conditions: blood stored at 0 °C or RT for 6 h, and plasma stored at 4 °C or RT for 24 h. However, control samples and the samples from low centrifugal force were basically clustered together with mixed small clusters from each of the two conditions (Figure 5D).
Figure 5.

Multilevel PLS-DA for the significantly changed metabolites (A), peptides (B), and inflammation biomarkers (C), and hierarchical clustering of the changed molecules resulted from preanalytical variations across all the samples (D). The dendrogram on the left represents the clusters of samples processed from variable preanalytical conditions, and the dendrogram on the top represents the clusters of molecules (D). The abbreviations Lowxg, B6h0C, B6hRT, P24h4C, and P24hRT represent a low centrifugation force (1300g), blood incubation at 0 °C for 6 h, blood incubation at RT for 6 h, plasma incubation at 4 °C for 24 h, and plasma incubation at RT for 24 h, respectively.
Multilevel PLS-DA models for metabolites, peptides, and inflammation biomarkers were built and optimized, and their performance was evaluated with repeated 5-fold cross-validation and hold-out test prediction. First, as shown in Figure 6, there was little difference in the balance error rate between cross-validation and the hold-out test, indicating that there was no model overfit issue. Second, the metabolite model had the lowest error rate, while the inflammation biomarker model had the highest error rate. On the basis of error rates, the optimized model parameters were three components and prediction with centroid distance for the metabolite PLS-DA model, four components and prediction with centroid distance for the peptide PLS-DA model, and seven components and prediction with maximum distance for the inflammation biomarker PLS-DA model.
Figure 6.
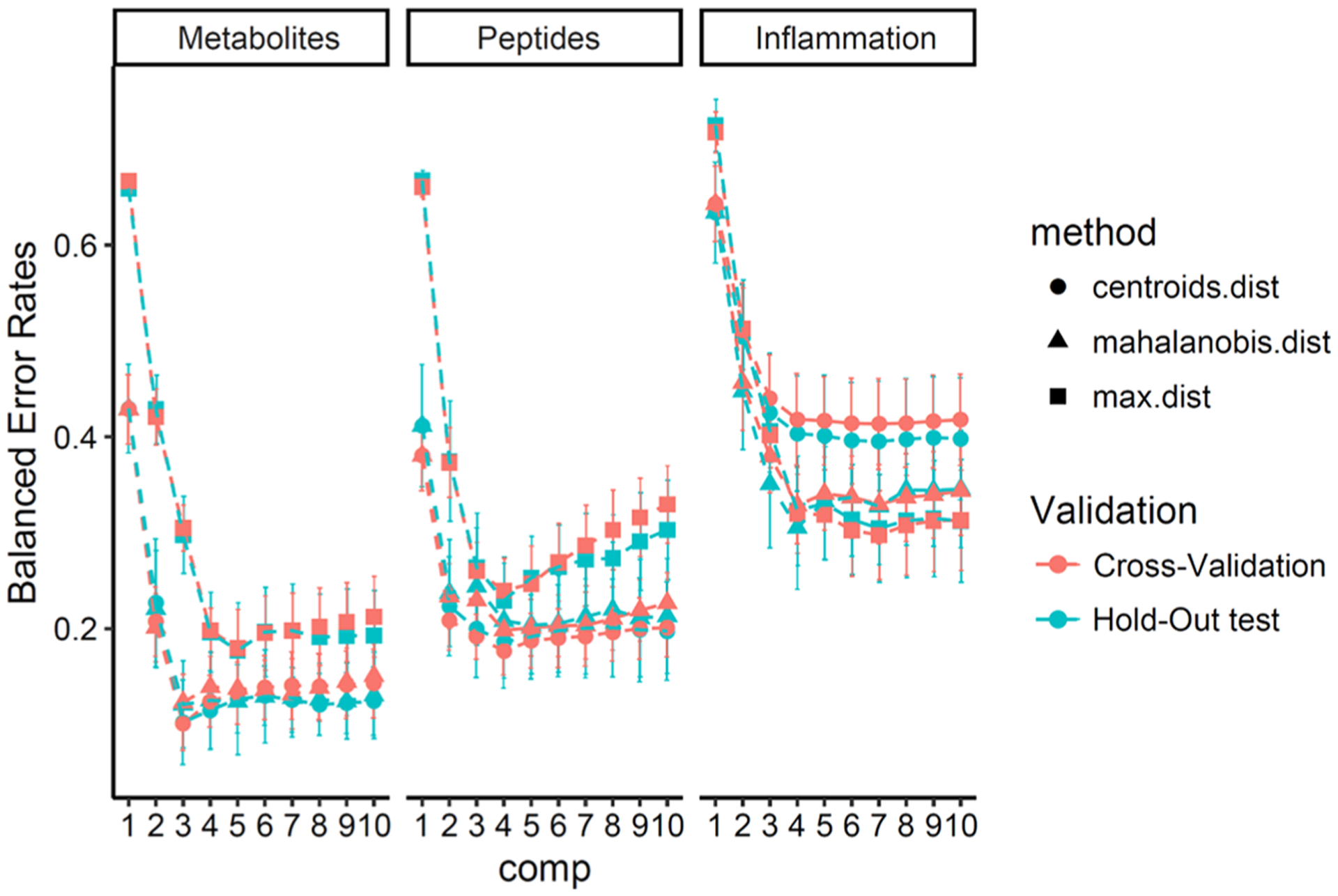
Multilevel PLS-DA model optimization.
The performance of optimized models for classifying hold-out test samples was evaluated by accuracy (the number of correct predictions divide by the total number of test samples), sensitivity, and specificity, as shown in Figure 7. Multilevel PLS-DA models for both metabolites and peptides have high accuracy, sensitivity, and specificity for blood stored at RT for 6 h and plasma stored at RT for 24 h. Moreover, the multilevel PLS-DA model for metabolites also has high accuracy, sensitivity, and specificity for blood stored at 0 °C for 6 h and plasma stored at 4 °C for 24 h.
Figure 7.
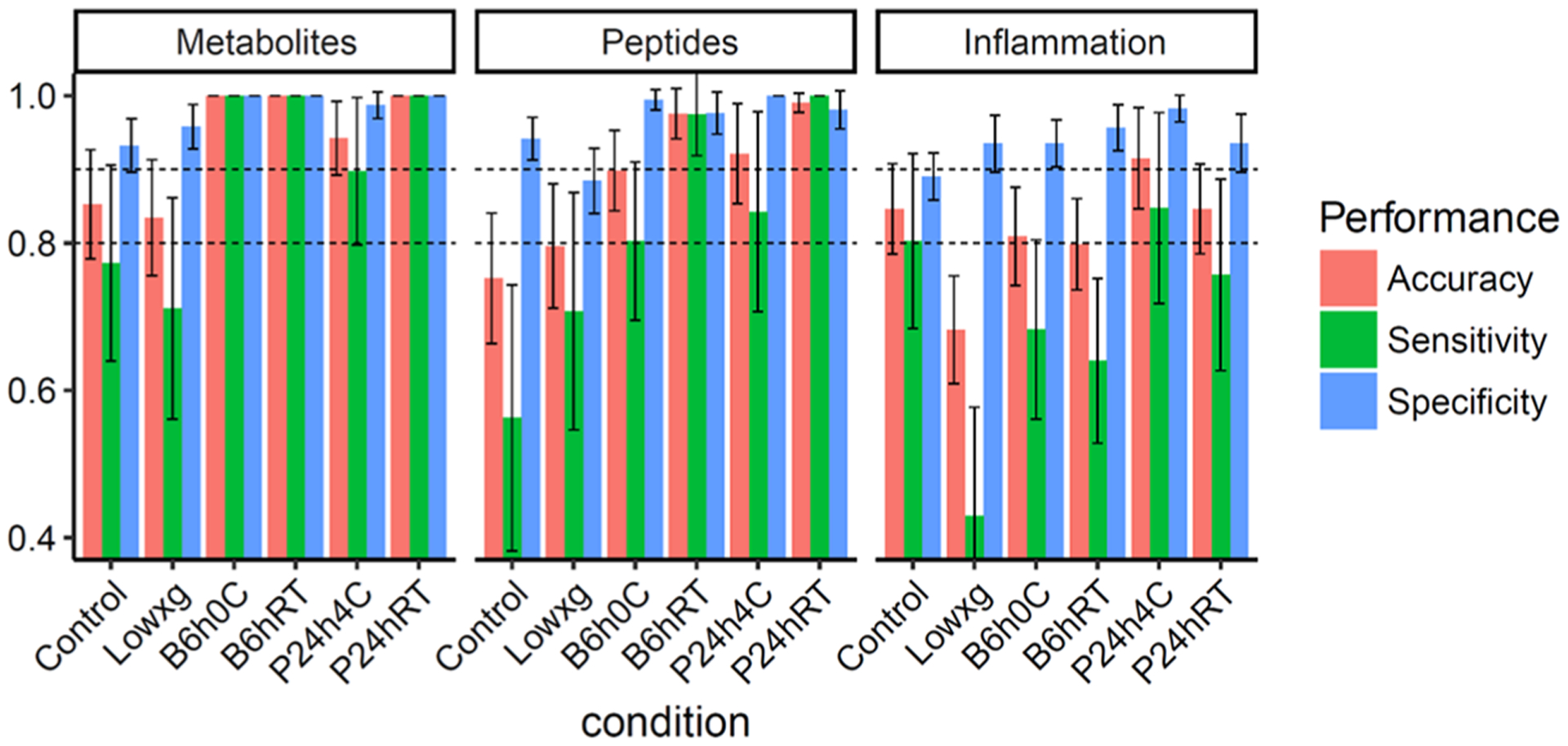
Comparison of accuracy, sensitivity, and specificity of multilevel PLS-DA models for significantly changed metabolites, peptides, and inflammation biomarkers. The abbreviations Lowxg, B6h0C, B6hRT, P24h4C, and P24hRT represent a low centrifugation force (1300g), blood incubation at 0 °C for 6 h, blood incubation at RT for 6 h, plasma incubation at 4 °C for 24 h, and plasma incubation at RT for 24 h, respectively.
4. DISCUSSION
We performed a comprehensive study to evaluate the effects of preanalytical variations on metabolites, peptides, and inflammation biomarkers, and found that significant impacts were seen with delayed sample processing, and sample storage time and temperature. In contrast, the low centrifugal force had little effect on these analytes, based on the number of significantly changed molecules (Figure 1). Identified metabolites, peptides, and inflammation biomarkers that are susceptible to preanalytical variations could be potential biomarkers for sample quality control, although further validation is required.
4.1. Metabolites
Storing blood for 6 h resulted in more significantly changed metabolites at RT than at 0 °C. The altered metabolites included almost all ontological classes, indicating that blood cell metabolism was still very active at RT, while it was reduced at 0 °C.4 Similarly, prolonged plasma processing resulted in more significantly changed metabolites at RT than at 4 °C, as a result of higher enzyme activity and chemical oxidation. Low centrifugal force during processing resulted in elevated maltose levels, indicating that platelets/leukocytes remained in plasma since glycan in platelets/leukocytes can be degraded to maltose by alpha-amylase and results in an increase of maltose in plasma.13,14
4.1.1. Blood Cell Metabolism at RT.
The decrease of simple sugars (glucose, mannose, glucose-1-phosphate, and fructosamine) and the increase of glycolytic intermediates (pyruvate and lactate) as presented in the results section and in Table S2A likely indicates that glycolysis in erythrocytes at RT was not completely blocked by EDTA. The increase of isocitrate and malate likely relate to energy metabolism. The increased levels of hexadecanoylcarnitine, hexadecenoylcarnitine, octadecanoylcarnitine, and oleoylcarnitine (Table S2A) likely relate to energy metabolism and fatty acid oxidation. The increase of ribose and nicotinamine may relate to pentose phosphate cycle and glycohydrolysis.15–17 The increase in ornithine and the decrease in arginine may result from erythrocyte arginase activity.18 The increase of glutamate and aspartate could be the result of glutaminase and asparaginase activities, and the increase of normetanephrine levels may be related to erythrocyte catechol-O-methyltransferase (COMT) activity.19,20 The observed increase in sphingolipid metabolites may be due to release from platelets and erythrocytes or due to lysophospholipase activity of autaxin.21,22 However, Scherer et al. found increases in certain sensitive metabolites, such as lysophosphatidic acid, also in dependence on the extraction method.23 However, this is unlikely to explain our observed results since all samples were treated equally in the metabolite profiling analysis.
4.1.2. Blood Cell Metabolism at 0 °C and/or RT.
Cold is a common cause of hemolysis in blood samples.24 The increase of free hemoglobin by storing blood at 0 °C, but not at RT, and the significant increase of sphingadienine d18:2 and sphingosine d16:1 at 0 °C compared to RT indicated slight hemolysis that may occur due to the cooler temperature. The increase of hypoxanthine and the decrease of serotonin by delayed blood processing matched previous findings which may be related to platelet activities.4,25,26 12-Hydroxyeicosatetraenoic acid was decreased by storing blood at 0 °C, while it was increased by storing blood at RT and plasma at RT. These effects are likely related to changes in 5-lipoxygenase activity. The decrease and increase of pyruvate by storing blood at 0 °C and RT are likely related to erythrocyte lactate dehydrogenase activity.
4.1.3. Plasma Processing Delay.
The observation of significant decrease of cysteine, cystine, and adrenaline may be explained by the removal of blood cells in the course of plasma preparation. In particular, the removal of erythrocytes diminishes their antioxidative capacity in plasma, and hence leaves the plasma constituents prone to oxidation, which may well explain the observed decrease in cysteine, cystine, and adrenaline.4
4.2. Peptides
Peptidomic analysis found 19 peptides from 6 proteins were significantly changed by preanalytical variations (Table S3A). Since peptide changes could be the direct consequence of protein abundance changes, we performed mass spectrometry-based multiple reaction monitoring (MRM) analysis of fibrinogen alpha chain, complement C3, and alpha-2-antiplasmin. The analyzed peptide sequences of these three proteins were tryptic peptides and independent from those observed from the peptidomics analysis, thus likely representing the total protein content in the sample. MRM analysis revealed that none of the three proteins changed due to preanalytical sample processing variations (Table S6). Plasma peptides are products of proteolytic digestion by intrinsic endoproteases, such as thrombin, plasmin, and complement proteins, which are then truncated by intrinsic amino-peptidases and carboxypeptidases.27 Storage of either blood or plasma at RT resulted in significantly changed peptide levels as likely a result of peptidase activities. Storage time could be an important factor for peptide variation,28 as more peptides changed significantly after storing plasma for 24 h compared to storing blood for 6 h.
Interestingly, preanalytical variations led to individual specific responses (Figure 3), with peptides such as SSSYSKQFTSSTSYNRGDSTFESKSY, SSSYSKQFTSSTSYNRGDSTFESKS, SSSYSKQFTSSTSYNRGDSTFES, GDSTFESKSYKMA being significantly higher abundance in subject 6, 12, 14, and 19 for storing their plasma at 4 °C for 24 h than in other subjects or under other sample processing conditions. This observation could be explained by either more peptides being generated or fewer peptides being degraded in plasma at 4 °C than at RT within 24 h. Preanalytical individual-driven specific responses to preanalytical variations in tissue and blood have also been reported.4,29
4.3. Inflammation Biomarkers
Prolonged storing blood at 0 °C and storing plasma at RT have significant effects on inflammation biomarkers. Aguilar-Mahecha et al. have shown that low temperatures can elevate cytokine levels in blood samples due to the activation of platelets.3 However, further studies are needed to understand the increased cytokine amounts in plasma at RT.
4.4. PCA and PLS-DA
In this study, PCA analysis revealed interindividual variability of metabolites and inflammation biomarker, which masked the molecular changes caused by preanalytical sample processing conditions. However, by using paired data structure and separating preanalytical variations for each subject from biological variations between subjects, the multilevel PCA revealed the effects of preanalytical variations on metabolome, peptidome, and inflammation biomarkers as described above. Multilevel PLS-DA models based on significantly changed metabolites and peptides from ANOVA statistical analysis showed high accuracy, sensitivity, and specificity for classifying samples subjected to preanalytical variations. Therefore, these significantly altered metabolites and peptides could be potential quality biomarkers. Further validation in a large cohort is needed to qualify these biomarker candidates.
5. CONCLUSIONS
Storage of blood or plasma at RT for 6 or 24 h resulted in the most significant changes in metabolites, peptides, and inflammation biomarkers, while processing from blood to plasma with low centrifugal force had little impact on these biomolecules. Therefore, storage of blood or plasma at different temperatures and for different durations can result in different changes of these biomolecules, depending on the type of molecules to be analyzed. To reduce preanalytical variation, storage of blood or plasma at low temperature or RT should be minimized prior to storage at −80 °C, and practical procedures for blood sample processing should consider the type of molecules to be analyzed. Metabolites, peptides, and inflammation biomarkers identified as susceptible to preanalytical variations could be potential sample quality biomarkers and may also be helpful for standardizing blood collection and processing in general, or for specific molecules in biomedical research and other applications. However, when these molecules are used as biomarker candidates for disease diagnosis or drug efficacy, a thorough validation for robustness with respect to preanalytical variables should be performed.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported with funds (Project# E0755601) from NCTR/FDA (L-R.Y.), Jefferson, Arkansas. The opinions expressed in this manuscript do not necessarily represent those of the U.S. Food and Drug Administration.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.jproteome.8b00903.
Figures S1–S2 (PDF)
The authors declare no competing financial interest.
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the data set identifier PXD011647.
REFERENCES
- (1).Hebels DG; Georgiadis P; Keun HC; Athersuch TJ; Vineis P; Vermeulen R; Portengen L; Bergdahl IA; Hallmans G; Palli D; Bendinelli B; Krogh V; Tumino R; Sacerdote C; Panico S; Kleinjans JC; de Kok TM; Smith MT; Kyrtopoulos SA; EnviroGenomarkers Project C Performance in omics analyses of blood samples in long-term storage: opportunities for the exploitation of existing biobanks in environmental health research. Environ. Health Perspect 2013, 121 (4), 480–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Enroth S; Hallmans G; Grankvist K; Gyllensten U Effects of Long-Term Storage Time and Original Sampling Month on Biobank Plasma Protein Concentrations. EBioMedicine 2016, 12, 309–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Aguilar-Mahecha A; Kuzyk MA; Domanski D; Borchers CH; Basik M The effect of pre-analytical variability on the measurement of MRM-MS-based mid-to high-abundance plasma protein biomarkers and a panel of cytokines. PLoS One 2012, 7 (6), No. e38290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Kamlage B; Maldonado SG; Bethan B; Peter E; Schmitz O; Liebenberg V; Schatz P Quality markers addressing preanalytical variations of blood and plasma processing identified by broad and targeted metabolite profiling. Clin. Chem 2014, 60 (2), 399–412. [DOI] [PubMed] [Google Scholar]
- (5).Tapernon K; Zander R; Niehoff D; Sibrowski W Quality control of hemolysis rate of erythrocyte concentrates: a proficiency test for determination of free hemoglobin. Anasthesiol Intensivmed Notfallmed Schmerzther 2001, 36 (Suppl 1), S45–S50. [DOI] [PubMed] [Google Scholar]
- (6).MacLean B; Tomazela DM; Shulman N; Chambers M; Finney GL; Frewen B; Kern R; Tabb DL; Liebler DC; MacCoss MJ Skyline: an open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics 2010, 26 (7), 966–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).R Core Team R: A Language and Environment for Statistical Computing, v. 3.3.1; R Foundation for Statistical Computing, 2016. [Google Scholar]
- (8).Wickham H ggplot2: Elegant Graphics for Data Analysis; Springer-Verlag: New York, 2016. [Google Scholar]
- (9).Kuhn M Building Predictive Models in R Using the caret Package. J. Stat. Software 2008, 28 (5), 26. [Google Scholar]
- (10).Rohart F; Gautier B; Singh A; Leê Cao K-A mixOmics: An R package for ‘omics feature selection and multiple data integration. PLoS Comput. Biol 2017, 13 (11), No. e1005752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Liquet B; Le Cao KA; Hocini H; Thiebaut R A novel approach for biomarker selection and the integration of repeated measures experiments from two assays. BMC Bioinf. 2012, 13, 325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Westerhuis JA; van Velzen EJ; Hoefsloot HC; Smilde AK Multivariate paired data analysis: multilevel PLSDA versus OPLSDA. Metabolomics 2010, 6 (1), 119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Guilbault GG; Rietz EB Enzymatic, fluorometric assay of alpha-amylase in serum. Clin Chem. 1976, 22 (10), 1702–1704. [PubMed] [Google Scholar]
- (14).Olsson I; Dahlqvist A; Norden A Glycogen Content of Leukocytes and Platelets. Acta Med. Scand 1963, 174, 123–7. [DOI] [PubMed] [Google Scholar]
- (15).Torti M; Bertoni A; Canobbio I; Sinigaglia F; Balduini C Hydrolysis of NADP+ by platelet CD38 in the absence of synthesis and degradation of cyclic ADP-ribose 2’-phosphate. FEBS Lett. 1999, 455 (3), 359–63. [DOI] [PubMed] [Google Scholar]
- (16).Ramaschi G; Torti M; Festetics ET; Sinigaglia F; Malavasi F; Balduini C Expression of cyclic ADP-ribose-synthetizing CD38 molecule on human platelet membrane. Blood 1996, 87 (6), 2308–2313. [PubMed] [Google Scholar]
- (17).Lee LT; Janney FA; Deas JE; Howe C Comparative immunochemical study of human erythrocyte glycoproteins. Exp. Biol. Med 1978, 158 (4), 530–6. [DOI] [PubMed] [Google Scholar]
- (18).Yang J; Gonon AT; Sjoquist PO; Lundberg JO; Pernow J Arginase regulates red blood cell nitric oxide synthase and export of cardioprotective nitric oxide bioactivity. Proc. Natl. Acad. Sci. U. S. A 2013, 110 (37), 15049–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Ellingson T; Duddempudi S; Greenberg BD; Hooper D; Eisenhofer G Determination of differential activities of soluble and membrane-bound catechol-O-methyltransferase in tissues and erythrocytes. J. Chromatogr., Biomed. Appl 1999, 729 (1–2), 347–53. [DOI] [PubMed] [Google Scholar]
- (20).Assicot M; Bohuon C Presence of two distinct catechol -O-methyltransferase activities in red blood cells. Biochimie 1971, 53 (8), 871–4. [DOI] [PubMed] [Google Scholar]
- (21).Kihara A; Igarashi Y Production and release of sphingosine 1-phosphate and the phosphorylated form of the immunomodulator FTY720. Biochim. Biophys. Acta, Mol. Cell Biol. Lipids 2008, 1781 (9), 496–502. [DOI] [PubMed] [Google Scholar]
- (22).van Meeteren LA; Moolenaar WH Regulation and biological activities of the autotaxin-LPA axis. Prog. Lipid Res 2007, 46 (2), 145–60. [DOI] [PubMed] [Google Scholar]
- (23).Scherer M; Schmitz G; Liebisch G High-throughput analysis of sphingosine 1-phosphate, sphinganine 1-phosphate, and lysophosphatidic acid in plasma samples by liquid chromatography-tandem mass spectrometry. Clin. Chem 2009, 55 (6), 1218–22. [DOI] [PubMed] [Google Scholar]
- (24).Lippi G; Blanckaert N; Bonini P; Green S; Kitchen S; Palicka V; Vassault AJ; Plebani M Haemolysis: an overview of the leading cause of unsuitable specimens in clinical laboratories. Clin. Chem. Lab. Med 2008, 46 (6), 764–772. [DOI] [PubMed] [Google Scholar]
- (25).Holmsen H; Day HJ; Setkowsky CA Secretory mechanisms. Behaviour of adenine nucleotides during the platelet release reaction induced by adenosine diphosphate and adrenaline. Biochem. J 1972, 129 (1), 67–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Bond PA; Cundall RL Properties of monoamine oxidase (MAO) in human blood platelets, plasma, lymphocytes and granulocytes. Clin. Chim. Acta 1977, 80 (2), 317–26. [DOI] [PubMed] [Google Scholar]
- (27).Koomen JM; Li D; Xiao LC; Liu TC; Coombes KR; Abbruzzese J; Kobayashi R Direct tandem mass spectrometry reveals limitations in protein profiling experiments for plasma biomarker discovery. J. Proteome Res 2005, 4 (3), 972–81. [DOI] [PubMed] [Google Scholar]
- (28).Yi J; Liu Z; Craft D; O’Mullan P; Ju G; Gelfand CA Intrinsic peptidase activity causes a sequential multi-step reaction (SMSR) in digestion of human plasma peptides. J. Proteome Res 2008, 7 (12), 5112–8. [DOI] [PubMed] [Google Scholar]
- (29).Gundisch S; Hauck S; Sarioglu H; Schott C; Viertler C; Kap M; Schuster T; Reischauer B; Rosenberg R; Verhoef C; Mischinger HJ; Riegman P; Zatloukal K; Becker KF Variability of protein and phosphoprotein levels in clinical tissue specimens during the preanalytical phase. J. Proteome Res 2012, 11 (12), 5748–62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


