Abstract
Opioid addiction can produce severe side effects including physical dependence and withdrawal. Perturbations of the gut microbiome have recently been shown to alter opioid-induced side-effects such as addiction, tolerance and dependence. In the present study, we investigated the influence of the gut microbiome on opioid withdrawal by evaluating the effects of fecal microbiota transplantation (FMT), antibiotic and probiotic treatments, and pharmacological inhibition of gut permeability in a mouse model of opioid dependence. Repeated intraperitoneal (i.p.) morphine treatment produced physical dependence that was quantified by measuring somatic signs of withdrawal (i.e. number of jumps) precipitated using the opioid antagonist naloxone. Morphine-dependent mice that received FMT from morphine-treated donor mice exhibited fewer naloxone-precipitated jumps compared to morphine-dependent counterparts receiving FMT from saline-treated donor mice. Microbial contents in the mouse cecum were altered by morphine treatment but were not differentially impacted by FMT. A broad-spectrum antibiotic cocktail regimen reduced the bacterial load and attenuated naloxone-precipitated morphine withdrawal in morphine-dependent mice, whereas commercially available probiotic strains did not reliably alter somatic signs of opioid withdrawal. ML-7, a pharmacological inhibitor of gut permeability, reduced the morphine-induced increase in gut permeability in vivo but did not reliably alter somatic signs of naloxone-precipitated opioid withdrawal. Our results suggest that the gut microbiome impacts the development of physical dependence induced by chronic morphine administration, and that therapeutic manipulations of the gut microbiome may reduce opioid withdrawal.
Keywords: opioid withdrawal, gut microbiome, fecal microbiota transplantation, antibiotic, probiotic
Graphical abstract

Introduction
Monotherapy with opioids such as morphine is an effective tool for the management of moderate to severe pain (Wickham, 2017). Despite their analgesic efficacy, the clinical use of opioids is limited by serious side effects, such as addiction, tolerance, physical dependence and gastrointestinal symptoms (Fields, 2011; Furlan et al., 2006; Jamison and Mao, 2015). Among these side-effects, physical dependence manifests as the need to keep taking drugs to avoid withdrawal symptoms, which occurs when opioid use is discontinued or following exposure to an opioid antagonist, such as naloxone (Kosten and George, 2002). Withdrawal symptoms can lead to compulsive drug use and short-term relapses, thereby increasing the addictive potential of opioids (Koob, 2000). Recent studies have explored the impact of antibiotic-induced changes in gut microbiome on opioid withdrawal following subcutaneous morphine pellet implantation and systemic injection of oxycodone (Kang et al., 2017; Simpson et al., 2020), respectively. However, the impact of gut microbiome manipulations on opioid dependence remains incompletely characterized.
The gut-brain axis is the bidirectional communication that occurs between the gastrointestinal tract (gut) and the central nervous system (brain). This interaction is majorly influenced by the trillions of microorganisms present in the gut, which are collectively referred to as the gut microbiome. The gut microbiome is almost entirely represented by bacteria (Sherwin et al., 2016), and has been implicated recently in numerous physiological and pathophysiological processes mediated by the central nervous system (see reviews in (Heiss and Olofsson, 2019; Principi and Esposito, 2016; Sharon et al., 2016). Crucially, from the standpoint of drug addiction, several studies have highlighted the impact of opioid use on gut microbiome dysbiosis in humans (Acharya et al., 2017; Barengolts et al., 2018; Vincent et al., 2016; Xu et al., 2017) and animals (Banerjee et al., 2016; Kang et al., 2017; Lee et al., 2018; Meng et al., 2013; Wang et al., 2018). Gut dysbiosis is a gut microbial imbalance characterized by a shift towards proinflammatory commensal bacteria and a reduction of microbial diversity, causing a disruption in the homeostasis between the gut microbial community and its host. Opioid-induced gut dysbiosis is associated with a dysregulation of morphine pharmacokinetics leading to a lower bioavailability (Wang et al., 2018), with the development of morphine-induced rewarding or reinforcing behaviors (Lee et al., 2018; Zhang et al., 2021), and with augmentation of morphine-induced analgesic tolerance (Kang et al., 2017; Zhang et al., 2019).
Antibiotic-induced treatment of gut dysbiosis or probiotic-induced restoration of gut homeostasis may diminish opioid-mediated side-effects. Broad-spectrum antibiotic treatment, administered via oral gavage, has been shown to prevent chronic morphine-induced increases in gut permeability, bacterial translocation, colonic and systemic inflammation, and attenuate the development of antinociceptive tolerance to morphine ((Kang et al., 2017; Zhang et al., 2019) but see (Lee et al., 2018)). Conversely, re-colonization of antibiotic-treated drug-naive mice via fecal microbiota transplantation (FMT) with morphine-associated microbiota has been shown to impair reward behavior and produce microglial activation, while transplantation of saline-associated microbiota restores normal reward behavior and leads to a resting microglial state (Lee et al., 2018). Moreover, restoration of gut dysbiosis with probiotics enriched with Bifidobacteria and Lactobacillaeae has been shown to mitigate morphine-induced tolerance, gut epithelial damage, and intestinal and systemic expression of proinflammatory cytokines (Zhang et al., 2019). Finally, inhibitors of myosin light-chain kinase (MLCK) protect against morphineinduced gut epithelial barrier disruption, thereby inhibiting morphine-induced bacterial translocation (Meng et al., 2013). These studies suggest that alterations to the gut microbiome that restore gut homeostasis could abrogate the development of opioid-mediated side effects.
In the present study, we evaluated the impact of gut microbiome alterations on somatic signs of opioid dependence that was quantified by challenging morphine-treated animals with the opioid antagonist naloxone. First, we tested whether FMT from donor mice receiving either repeated (i.p.) morphine or saline treatment would alter the development of morphine dependence in recipient morphine- or saline-treated mice. We quantified the impact of FMT on bacterial communities within the cecum contents of recipient mice using 16S rRNA gene sequencing and short-chain fatty acid (SCFA) analysis. Next, we evaluated the impact of a broad-spectrum antibiotic treatment to deplete gut bacteria as well as probiotic treatments to restore gut dysbiosis on morphine withdrawal in chronic morphine-treated mice. Finally, we examined the ability of ML-7, a MLCK inhibitor known to protect against gut epithelial barrier disruption, on both gut permeability and ability to decrease morphine withdrawal symptoms in mice. Our studies support emerging literature linking the gut microbiome to the unwanted effects of opioids, including opioid dependence.
Methods
Animals
One hundred nineteen adult male WT mice on a C57BL/6J background were purchased from The Jackson Laboratory (Bar Harbor, ME) and used in these studies. All mice weighed 25–30 g and were ~12–14 weeks old when used in this study. Mice were single housed for 7 days before initiating any pharmacological manipulations. All mice were maintained on a 12 h light/dark cycle (lights on from 7 am to 7 pm) in a temperature and humidity-controlled facility and allowed ad libitum access to food and water throughout the experimental period. All experiments used n = 5–8 mice per group. All experiments were approved by the Indiana University Bloomington Animal Care and Use Committee.
Drugs
Morphine sulfate was obtained from the NIDA Drug Supply Program (National Institute on Drug Abuse, Bethesda, MD), dissolved in saline and administered via i.p. injection. Naloxone (Sigma Aldrich, St. Louis, MO) was dissolved in saline. The antibiotic cocktail treatment consisted of vancomycin (Abbot, Abbot Park, IL), neomycin (Invitrogen, Carlsbad, CA), and metronidazole (Actavis, Hafnarfjordur, Iceland) dissolved in sterile water and administered via oral gavage; and ampicillin (Sigma Aldrich, St. Louis, MO) via drinking water ad libitum (Reikvam et al., 2011). The probiotic treatment consisted of Bifidobacterium longum subspecies longum 35624™ (Align) or Lactobacillus rhamnosus GG (Culturelle) in sterile water administered via oral gavage. The MLCK inhibitor, ML-7 (Sigma Aldrich, St. Louis, MO) was diluted in 3% DMSO and saline, and administered via i.p. injection. Drug concentrations and regimens employed are described for each respective experiment below.
Mouse model of opioid dependence and naloxone-precipitated withdrawal
Morphine dependence was induced in mice by repeated injections for three consecutive days using an escalating dose schedule (112.5 mg/kg i.p. cumulative morphine dose) as described previously (Iyer et al., 2020; Liu et al., 2011) (Fig. 1A). Mice received morphine injections (or saline as a control) twice daily (9 am and 5 pm) for 2 consecutive days (day 1: 7.5 and 15 mg/kg i.p.; day 2: 30 and 30 mg/kg i.p.). On day 3 (48 h following first injection), mice received a final dose of morphine (30 mg/kg i.p.) or saline and were immediately placed into individual Plexiglas observation chambers to allow for habituation. After 30 min, all mice were injected with saline and returned to chambers. Then, 60 min (49 h) after the final morphine dose, all mice were challenged with naloxone (10 mg/kg i.p.) to precipitate a μ-opioid receptor-dependent withdrawal syndrome, as described previously (Iyer et al., 2020; Lichtman et al., 2001; Ramesh et al., 2011). Mice were video recorded continually throughout the observation interval and the number of withdrawal jumps observed over 30 min following the saline challenge and the naloxone challenge was subsequently quantified by a blinded scorer using the BORIS open source software (Friard and Gamba, 2016).
Fig. 1. Morphine FMT attenuates naloxone-precipitated withdrawal in morphine-dependent mice.
(A) The schematic shows the timing and doses of the escalating i.p. morphine dosing schedule used to produce opioid dependence. The grey vertical arrows show the timing of the FMT gavage treatments. After 49 h, naloxone was injected to precipitate opioid withdrawal. (B) Naloxone (10 mg/kg i.p.) produced characteristic jumping behavior consistent with the development of dependence. Morphine-treated mice receiving FMT from morphine-treated donor mice exhibited fewer withdrawal jumps compared to morphine-treated mice receiving FMT from saline-treated donor mice. Withdrawal jumps were induced by morphine but not saline treatments in recipient mice. Withdrawal jumps were higher in the morphine-treated mice receiving saline FMT compared to all other groups. Data are expressed as mean ± S.E.M. (n = 5 per group) ****P < 0.0001 vs. FMT (Sal) - Mor group, ***P < 0.001, two-way ANOVA followed by Bonferroni’s post hoc test.
Fecal microbiota transplantation (FMT)
To study the effect of manipulation of the gut microbiota on morphine dependence, fecal microbiota from donor mice receiving either morphine (morphine FMT) or saline (saline FMT) were transplanted via oral gavage into recipient mice also receiving either morphine or saline (Fig. 1A). Fecal samples were collected directly from donor mice, pooled together and immediately suspended in ice cold sterile water by homogenization with sterile blue pestles at a concentration of 60 mg of feces/ml. After resting on ice for approximately 2 min, 200 μl of supernatant was collected, and delivered to each recipient mouse via oral gavage 30 min before each morphine or saline injection using the FMT protocol described previously (Zhou et al., 2017). Donor and recipient mice received chronic morphine injections concomitantly. To avoid confounding factors due to previous housing situation, we carefully assigned the mice to experimental groups in such a way that animals that previously shared the same cage would not be in the same experimental group. Only recipient mice were tested for naloxone-precipitated opioid withdrawal.
Cecum content dissection
Approximately 30 min following naloxone challenge, cecum contents were dissected from each mouse to assess impact of FMT on gut microbiome. Before cecum content collection, mice were deeply anesthetized with isoflurane, and then transcardially perfused with 0.1 M phosphate-buffered saline containing 0.1% heparin. Cecum content samples were flash frozen and stored at −80°C until analysis.
DNA extraction, 16S rRNA gene amplification, sequencing, and sequence processing
Cecum content samples were analyzed (Microbiome Insights, BC, Canada) to determine bacterial composition. Specimens were placed into a MoBio PowerMag Soil DNA Isolation Bead Plate. DNA was extracted following MoBio’s instructions on a KingFisher robot. Bacterial 16S rRNA genes were PCR-amplified with dual-barcoded primers targeting the V4 region (515F 5’-GTGCCAGCMGCCGCGGTAA-3’, and 806R 5’-GGACTACHVGGGTWTCTAAT-3’), as described previously (Kozich et al., 2013). Amplicons were sequenced with an Illumina MiSeq using the 300-bp paired-end kit (v.3). Sequences were denoised, taxonomically classified using Greengenes (v. 13_8) as the reference database and clustered into 97%-similarity operational taxonomic units (OTUs) with the mothur software package (v. 1.39.5) (Schloss et al., 2009), following the recommended procedure (https://www.mothur.org/wiki/MiSeq_SOP; accessed Nov 2017). The potential for contamination was addressed by co-sequencing DNA amplified the same way as the specimens. Operational taxonomic units were considered putative contaminants and were removed if their mean abundance in controls reached or exceeded 25 % of their mean abundance in specimens.
Short-chain fatty acid (SCFA) concentrations
Cecum content samples were analyzed (Microbiome Insights, BC, Canada) to measure the concentration of short-chain fatty acids. The SCFA extraction procedure was similar to that published previously (Zhao et al., 2006). Material was resuspended in MilliQ-grade water, and homogenized using MP Bio FastPrep, for 1 min at 4.0 m/s. 5M HCl was added to acidify the fecal suspensions to a final pH of 2.0. Acidified fecal suspensions were incubated and centrifuged at 10,000 RPM to separate the supernatant. Fecal supernatants were spiked with 2-Ethylbutyric acid for a final concentration of 1 mM. Extracted SCFA supernatants were stored in 2-ml GC vials, with glass inserts. SCFA were detected using gas chromatography (Thermo Trace 1310), coupled to a flame ionization detector (Thermo Fisher).
Antibiotic treatment
To study the effect of antibiotic treatment on morphine dependence, mice received an antibiotic cocktail as described previously (Reikvam et al., 2011) twice daily (10 ml/kg) via oral gavage (Fig. 4A). The antibiotic cocktail consisted of vancomycin (50 mg/kg), neomycin (100 mg/kg) and metronidazole (100 mg/kg). Ampicillin (1 g/L), also part of the antibiotic cocktail, was administered via drinking water ad libitum for the duration of the experiment (with water replacement at 4-day intervals). Water consumption and body weight were monitored to ensure that mice were not dehydrated and did not show signs of weight loss in response to antibiotic treatment. Antibiotic treatment was initiated (day 1), five days prior to the start of chronic morphine dosing (day 6) and continued until the last day of the experiment (day 8). When administered on the same day as morphine, antibiotic gavage was given 30 min before morphine or saline injection.
Fig. 4. Antibiotic treatment by oral gavage effectively reduces fecal bacterial DNA load and attenuates naloxone-precipitated withdrawal in morphine-dependent mice.
(A) The schematic shows the experimental timeline. The grey vertical arrows show the timing of the antibiotic cocktail (ABX) gavage treatments. The grey horizontal arrow show the duration of the oral ampicillin antibiotic treatment. On day 8, 49 h after the first morphine injection, naloxone was injected to precipitate opioid withdrawal. (B) Antibiotic treatment reduced bacterial load in the Day 8 fecal samples compared to Day 1 samples. (C) Naloxone (10 mg/kg i.p.) produced characteristic jumping behavior consistent with the development of dependence. Withdrawal jumps were significantly lower in the antibiotic-treated mice compared to the saline-treated mice. Impact of antibiotic treatment and three days of escalating morphine treatment on (D) water consumption and (E) body weight in mice. Antibiotic treatment initially reduced water consumption (day 1–4) but did not reliably alter water consumption during morphine treatment (day 5–7) with the exception of the antibiotic-morphine group compared to the water-saline group on Day 7 alone. Data are expressed as mean ± S.E.M. (n = 5–8 per group), # P < 0.05 antibiotic + morphine group vs. water + saline group, ****P < 0.0001 vs. water-morphine - treated group or as indicated, ordinary or repeated measures two-way ANOVA followed by Bonferroni’s post hoc test.
Abundance of bacterial 16S rRNA gene in fecal samples following antibiotic treatment
Fecal pellets were collected directly from mice receiving either the antibiotic cocktail or water, weighted, and immediately flash frozen and stored at −80 °C. Bacterial genomic DNA was extracted with QIAamp Fast DNA Stool Mini Kit (QIAGEN, Germantown, MD) as recommended by the manufacturer (protocol for isolation of DNA for pathogen detection). Bacterial 16S rRNA genes were quantified by real-time PCR using specific primers as described previously (Kang et al., 2017). To determine the number of 16S rRNA copies present in each sample, a standard curve was created by extracting and quantifying DNA from a known number of Escherichia coli.
Probiotic treatment
To study the effect of probiotic treatment on morphine dependence, mice received Bifidobacterium longum subspecies longum 35624™ or Lactobacillus rhamnosus GG via oral gavage (10 ml/kg) 30 min before every morphine injection (Fig. 5A). Probiotics were freshly-prepared by suspending the probiotic capsules in sterile water at a concentration of approximately 106 CFU/ml. Sterile water was administered via oral gavage in control mice.
Fig. 5. Probiotic treatment by oral gavage does not alter naloxone-precipitated withdrawal in morphine-dependent mice.

(A) The schematic shows the experimental timeline. The grey vertical arrows show the timing of the probiotic gavage treatments. After 49 h, naloxone was injected to precipitate opioid withdrawal. (B) Naloxone (10 mg/kg i.p.)-precipitated jumping differed between treatment groups; morphine-treated mice receiving water or Bifidobacterium longum subspecies longum 35624™ (Align) by gavage showed naloxone-precipitated jumping compared to saline-treated mice receiving water by gavage. Naloxone precipitated jumps did not differ reliably in morphine-treated mice receiving Lactobacillus rhamnosus GG (Culturelle) by gavage and saline-treated mice receiving water by gavage. Withdrawal jumps in mice receiving either Bifidobacterium longum subspecies longum 35624™ (Align) or Lactobacillus rhamnosus GG (Culturelle) probiotic treatment by gavage did not differ from each other or from morphine-treated mice receiving water by gavage. Data are expressed as mean ± S.E.M. (n = 6 per group), **P < 0.01, *P < 0.05 as indicated, one-way ANOVA followed by Bonferroni’s post hoc test.
ML-7 treatment
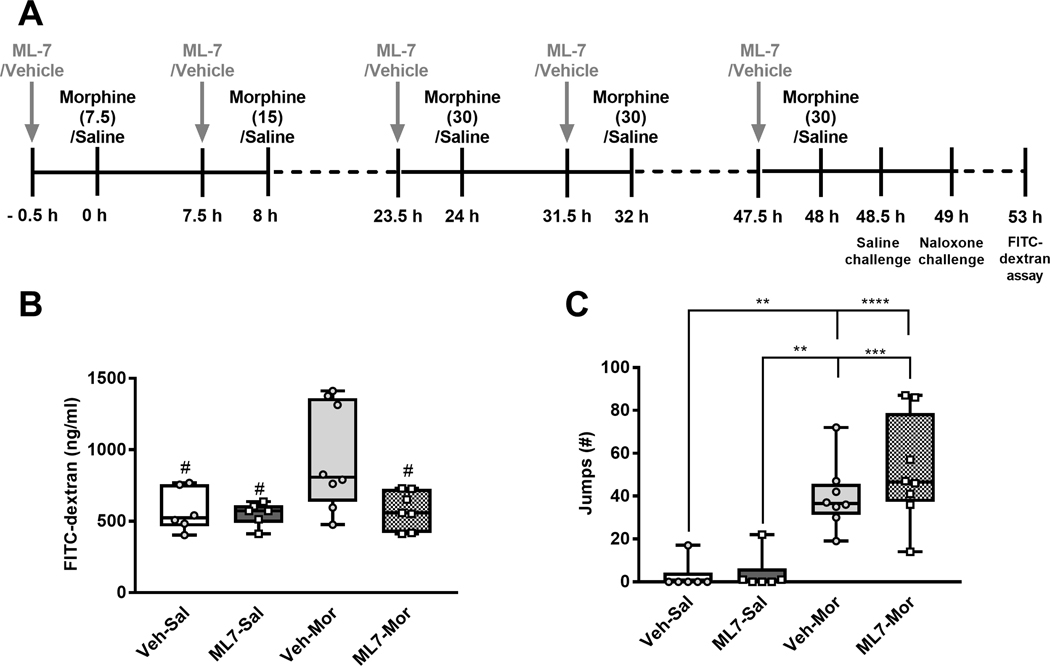
To study the effect of blocking the increase in gut permeability on morphine dependence, mice were treated with the MLCK inhibitor, ML-7 (2 mg/kg, i.p.), administered 30 min before every morphine injection (10 ml/kg) (Fig. 6A). This dose of ML-7 has been previously shown to reliably inhibit MLCK activity and protect the barrier function of endothelial cells in mice (Huppert et al., 2010; Meng et al., 2013).
Fig. 6. ML-7 treatment by gavage does not alter naloxone-precipitated withdrawal in morphine-dependent mice.
(A) The schematic shows the experimental timeline. The grey vertical arrows show the timing of the ML-7 treatments. After 49 h, naloxone was injected to precipitate opioid withdrawal. (B) In the same animals shown in C, morphine treatment increased gut permeability (P = 0.0363) compared to saline-treated mice. ML-7 treatment reduced gut permeability (P = 0.0378) and the interaction approached significance (P = 0.067). Plasma FITC-dextran concentrations were measured to assess impact of morphine and ML-7 treatment on gut permeability. Post hoc analysis confirmed that morphine increased gut permeability, as defined by higher FITC-dextran plasma concentrations, and this increased gut-permeability was blocked by ML-7 treatment. (C) Morphine treatment increased withdrawal jumps precipitated by naloxone (10 mg/kg i.p.) challenge, consistent with the development of dependence. Withdrawal jumps were not significantly altered in ML-7 treated mice compared to vehicle-treated mice and the interaction was not significant. Data are expressed as mean ± S.E.M. (n = 6–8 per group), ****P < 0.0001, ***P < 0.001, **P < 0.01 as indicated, # P < 0.05 vs. the vehicle-morphine -treated group, two-way ANOVA followed by Bonferroni’s post hoc test.
Fluorescein isothiocyanate (FITC)-dextran assay
To measure intestinal permeability in the present mouse model of opioid dependence, FITC-conjugated dextran (Sigma-Aldrich, St. Louis, MO) or PBS was used as described previously (Kang et al., 2017). Following naloxone-precipitated withdrawal, mice received oral gavage of FITC-labeled dextran (44 mg/100 g body weight). After 4 h, whole blood was collected, and plasma was isolated by centrifugation for 15 min at 3000 rpm and 4 °C. FITC concentration was fluorometrically quantified by emission spectrometry (Promega, Madison, WI) at 528 nm, using an excitation wavelength of 485 nm. All concentrations were measured against a standard curve of serially diluted FITC-dextran.
Statistical analysis
Alpha diversity was estimated with the Shannon index on raw OTU abundance tables after filtering out contaminants. The significance of diversity differences was tested with a one-way ANOVA. To estimate beta diversity across samples, OTUs occurring with a count of less than 3 in at least 10 % of the samples were excluded and then computed Bray-Curtis indices. The beta diversity was analyzed, emphasizing differences across samples, using Principal Coordinates Analysis (PCoA) ordination. Variation in community structure was assessed with permutational multivariate analyses of variance (PERMANOVA) with treatment group as the main fixed factor and using 9999 permutations for significance testing. All analyses of beta diversity were conducted in the R environment. All other data was analyzed using GraphPad Prism version 7.05 (GraphPad Software Inc., La Jolla, CA, USA). 2 × 2 ANOVAs were used to assess the impact of FMT, antibiotic and ML-7 treatment on dependent measures (i.e. withdrawal jumps, Shannon diversity index, FITC dextran levels) in morphine- and saline-treated mice. Time course data was analyzed using repeated measures two-way ANOVA. Bonferroni post hoc tests were used, as appropriate, for follow up analyses to explain the source of significant main effects and interactions. Analyses of withdrawal jumps following probiotic treatments were analyzed using a one-way ANOVA followed by Bonferroni post hoc tests. P < 0.05 was considered statistically significant.
Results
Morphine FMT attenuates naloxone-precipitated withdrawal in morphine-dependent mice
In recipient mice subjected to FMT and our escalating i.p. morphine dosing regimen, naloxone challenge produced characteristic jumping behavior compared to the chronic saline-treated mice (Fig. 1B). Both FMT (F1,16= 18.2, P = 0.0006) and morphine (F1,16= 66.77, P < 0.0001) treatment altered the number of naloxone-precipitated jumps and the interaction between FMT and the morphine treatment was significant (F1,16= 18.2, P = 0.0006). Strikingly, post hoc analyses revealed that morphine-treated mice receiving FMT from morphine-treated donor mice exhibited fewer jumps compared to morphine-treated mice receiving FMT from saline-treated donor mice (P = 0.0001). Post hoc analyses also showed that morphine-treated mice receiving saline FMT exhibited more jumps compared to saline-treated mice receiving either saline FMT or morphine FMT (P < 0.0001 vs. each group). We also compared the FMT groups in our current study to a control group from a different experiment that did not receive oral gavage but underwent the same 3-day escalating morphine dosing regimen followed by identical procedures for precipitating and quantifying naloxone precipitated withdrawal. We compared the number of naloxone precipitated jumps from the FMT(Sal)-Mor (34.4±6.95) and FMT(Mor)-Mor (10.8±10.23) groups to a group of mice that did not receive oral gavage but underwent the same morphine dosing regimen (34.1±21.57). A one-way ANOVA followed by Bonferroni’s post hoc tests indicated that there was a main effect of treatment (F2,17= 3.638, P = 0.0484). Further, post hoc analyses indicated that mice that did not receive oral gavage differed from the FMT (Mor)-Mor (P = 0.0430) but not the FMT(Sal)-Mor group (P > 0.9999). Thus, FMT from the normal gut microbiome was unlikely to impact naloxone-precipitated withdrawal jumps compared to animals receiving the same morphine and naloxone treatment but no oral gavage.
Microbial analysis of cecum content reveals alterations in Firmicutes and Verrucomicrobia phyla in morphine-treated mice
The 16S rRNA gene sequencing of cecum contents showed that all groups of mice shared the same dominant taxa, including the phyla Bacteroidetes, Firmicutes, Verrucomicrobia, Tenericutes, Proteobacteria and Actinobacteria (Fig. 2A). A comparison of the average proportions of each bacterial phylum showed that two of the major phyla, Firmicutes and Verrucomicrobia, were significantly altered in morphine-treated mice compared to saline-treated mice (Fig. 2B). Overall, morphine treatment did not reliably alter phyla proportion (F3,112= 1.503e-011, P > 0.05), although a significant main effect of phyla (F6,112= 253.4, P < 0.0001) was observed, as expected, and the interaction between the phyla and morphine treatment was significant (F18,112= 2.687, P = 0.0008). Post hoc analyses revealed that the average proportion of Firmicutes was higher in the saline donor FMT/saline-injected recipient mice compared to either the saline donor FMT/morphine-injected recipient mice (P = 0.0277) or the morphine donor FMT/morphine-injected recipient mice (P = 0.0345). Further, the average proportion of Firmicutes was higher in the morphine donor FMT/saline-injected recipient mice compared to the saline donor FMT/morphine-injected recipient mice (P = 0.0447). The average proportion of Verrucomicrobia, was lower in the saline donor FMT/saline-injected recipient mice compared to saline donor FMT/morphine-injected recipient mice (P < 0.0001) and morphine donor FMT/morphine-injected recipient mice (P = 0.0136).
Fig. 2. Cecum content analyses reveal alterations in Firmicutes and Verrucomicrobia phyla in morphine-dependent mice.
(A) Gene sequencing of cecum contents showed that all mice shared the same dominant taxa, including the phyla Bacteroidetes, Firmicutes, Verrucomicrobia, Tenericutes, Proteobacteria and Actinobacteria. (B) The Firmicutes and Verrucomicrobia phyla were altered in morphine-treated mice compared to saline-treated mice but no reliable differences between phyla were observed due to FMT treatment. Data are expressed as mean ± S.E.M. (n = 5 per group) ****P < 0.0001, *P < 0.05, # P < 0.05 as indicated, two-way ANOVA followed by Bonferroni’s post hoc test.
Analysis of cecum content from mice receiving either morphine treatment or FMT reveals no significant alterations in microbial diversity or SCFA concentrations
Analysis of the alpha diversity estimation (Fig. 3A) revealed that neither morphine treatment (F1,16= 0.5163, P = 0.4828) nor FMT (F1,16= 0.1127, P = 0.7414) altered the Shannon Diversity index and the interaction between morphine and FMT treatment was not significant (F1,16= 1.205, P = 0.2887). Similarly, analysis of the beta diversity using a PCoA ordination did not reveal any differences between the groups of mice receiving FMT or morphine treatment (Fig. 3B). SCFA concentrations differed as a function of fatty acid type (F4,80= 115.6, P < 0.0001), as expected (Fig. 3C), but no main effect of drug/FMT combination treatment was observed (F3,80= 0.5532, P = 0.6475), and the interaction between SCFA type and drug/FMT treatment was not significant (F12,80= 0.1915, P = 0.9985).
Fig. 3. Alpha and Beta diversity estimations and SCFA concentrations are not impacted by morphine treatment.
(A) Alpha and (B) Beta diversity estimations did not reliably differ among the groups of mice. (C) SCFA concentrations did not reliably differ among the groups of mice. Data are expressed as mean ± S.E.M. (n = 5 per group), two-way ANOVA followed by Bonferroni’s post hoc test.
Antibiotic treatment by gavage effectively reduces fecal bacterial DNA load
Antibiotic-treated mice showed a markedly enlarged ceca, based upon qualitative evaluation, compared to the water-treated mice (data not shown), which is indicative of a germ-free phenotype. A comparison of the bacterial 16S rRNA genes quantified by real-time PCR in fecal pellet samples collected on Day 1 and Day 8 showed the efficacy of antibiotic treatment in reducing the bacterial load (Fig. 4B). As expected, morphine treatment (F3,20= 72.64, P < 0.0001) and the day of the sample collection (F1,20= 384.6, P < 0.0001) impacted 16S rRNA genes and the interaction between morphine treatment and sample collection day was significant (F3,20= 114.3, P < 0.0001). Prior to antibiotic/water treatment, all groups of mice had a similar level of bacterial load on Day 1 (P > 0.05 vs. other groups). Following repeated injections, on Day 8, the antibiotic-morphine and antibiotic-saline -treated mice exhibited a lower bacterial load compared to the water-morphine and water-saline -treated groups (P < 0.0001 vs. each group). Further, the antibiotic-morphine and antibiotic-saline -treated mice exhibited a lower bacterial load on Day 8 compared to Day 1 of repeated injections (P < 0.0001 vs. Day 1).
Antibiotic treatment attenuates naloxone-precipitated withdrawal in morphine-dependent mice
In mice subjected to an escalating i.p. morphine dosing regimen along with antibiotic or vehicle treatment, naloxone challenge produced characteristic jumping behavior (Fig. 4C). Both morphine (F1,23= 32.57, P < 0.0001) and antibiotic (F1,23= 12.95, P < 0.0015) treatment altered the number of naloxone-precipitated jumps and the interaction between morphine and antibiotic status was significant (F1,23= 12.95, P = 0.0015). Naloxone-precipitated jumps were higher in water-morphine -treated mice compared to either water-saline or antibiotic-saline -treated groups (P < 0.0001 vs. each group). Strikingly, naloxone-precipitated jumps were lower in antibiotic-morphine -treated mice compared to water-morphine -treated mice (P < 0.0001) and did not differ from levels observed in either the water-saline or antibiotic-saline -treated groups (P > 0.05 vs. each group). Thus, antibiotic treatment in morphine-treated mice attenuated levels of naloxone-precipitated jumping to levels observed in saline-treated groups that never received morphine.
Impact of antibiotic treatment on water consumption and body weight
Water consumption was monitored in all groups of mice to quantify consumption of ampicillin-treated water and to ensure adequate animal hydration (Fig. 4D). As expected, antibiotic-morphine treatment status (F3,23= 8.719, P = 0.0005) and treatment day (F6,138= 13.44, P < 0.0001) altered daily water consumption and the interaction between the antibiotic treatment and treatment day was significant (F18,138= 2.368, P = 0.0027). Water consumption was initially lower in the antibiotic-treated groups from Day 1 – Day 4, but not during the subsequent morphine treatment phase form Day 5 – Day 7, with one exception (the antibiotic-morphine group exhibited lower water consumption compared to the water-saline group on Day 7; P = 0.0240). The change in body weight as a % of baseline was also monitored in all groups of mice to assess effects of antibiotic treatment on animals’ general health (Fig. 4E). Body weight differed across days (F7,147= 23.29, P < 0.0001) but was not impacted by morphine treatment (F3,21= 1.035, P = 0.3973), and the interaction between the morphine treatment and treatment day (F21,147= 1.424, P = 0.1157) was not significant. However, post hoc analyses failed to reveal any reliable changes in body weight in any groups of mice irrespective of morphine or antibiotic treatment.
Probiotic treatment does not alter naloxone-precipitated withdrawal in morphine-dependent mice
We also examined the impact of probiotic treatments by oral gavage on naloxone-precipitated withdrawal jumps (Fig. 5B). Naloxone-precipitated jumps differed as a function of treatment group (F3, 20= 5.793, P = 0.0051). Morphine-treated mice receiving water (P = 0.0345) or the Bifidobacterium longum subspecies longum 35624™ (Align) probiotic treatment (P = 0.0049) exhibited more naloxone-precipitated jumps compared to saline-treated mice receiving water. Naloxone-precipitated jumping behavior did not reliably differ between morphine-treated mice receiving Lactobacillus rhamnosus GG (Culturelle) probiotic treatment and saline-treated mice receiving water (P = 0.085). The number of naloxone-precipitated jumps did not differ between the morphine-treated mice receiving either water alone or either the Bifidobacterium longum subspecies longum 35624™ (Align) or Lactobacillus rhamnosus GG (Culturelle) probiotic treatment (P > 0.05 vs. each group), indicating that the two commercially available probiotic strains did not reliably alter morphine withdrawal. Further, a comparison of the number of naloxone-precipitated jumps between the morphine-treated mice receiving water alone (Fig. 5B) to the morphine-treated mice receiving FMT from saline-treated donor mice (Fig. 1B) did not show any reliable difference (P = 0.2640) between the groups.
ML-7 treatment blocks morphine-induced gut permeability, but does not attenuate naloxone-precipitated withdrawal in morphine-dependent mice
ML-7 treatment blocked the increases in gut permeability induced by chronic morphine treatment, as revealed by the FITC-dextran assay used to quantify intestinal permeability (Fig. 6B). Morphine treatment reliably increased FITC-dextran concentrations in plasma overall (F1,23= 4.945, P = 0.0363), whereas ML-7 treatment overall reduced FITC-dextran concentrations (F1,23= 4.858, P = 0.0378), and the interaction between the ML-7 treatment and morphine treatment approached significance (F1,23= 3.684, P = 0.0674). Post hoc analyses revealed that morphine-treated mice receiving vehicle treatment had a significantly higher gut permeability compared to saline-treated mice receiving vehicle (P = 0.0407) or ML-7 treatment (P = 0.0251). Further, plasma FITC-dextran levels were lower in ML7-morphine -treated groups compared to saline-morphine -treated groups (P = 0.0311), documenting that ML-7 treatment effectively abrogated morphine-induced increases in gut permeability.
In mice subjected to an escalating i.p. morphine dosing regimen along with ML-7 treatment, naloxone challenge produced characteristic jumping behavior (Fig. 6C). As expected, morphine treatment (F1,24= 44.78, P < 0.0001) increased the number of naloxone-precipitated jumps, but there was no main effect of the ML-7 treatment (F1,24= 1.083, P = 0.3084), and the interaction between ML-7 and morphine treatment was not significant (F1,24= 0.7332, P = 0.4003). Morphine-treated mice receiving either ML-7 (P < 0.0001) or vehicle (P = 0.0023) treatment exhibited more naloxone-precipitated jumps compared to saline-treated mice receiving vehicle alone. Further, morphine-treated mice receiving either ML-7 (P = 0.0001) or vehicle (P = 0.0032) treatment exhibited more naloxone-precipitated jumps compared to ML-7 -treated mice receiving saline alone. Finally, the number of naloxone-precipitated jumps did not differ between the morphine-treated mice receiving the vehicle alone and the morphine-treated mice receiving ML-7 treatment (P = 0.9620), indicating that the ML-7 treatment did not reliably alter levels of somatic signs of morphine withdrawal.
Discussion
Here we evaluate, for the first time, the impact of FMT, probiotics and gut permeability modulation on somatic expression of morphine dependence in mice, as defined by quantification of naloxone-precipitated withdrawal jumps. Our results suggest that FMT, which did not reliably alter the cecum microbiota composition in our analysis, and a broad-spectrum antibiotic treatment, which reduced the gut bacterial load, are effective in attenuating naloxone-precipitated opioid withdrawal. Our studies support an emerging literature implicating a vital role for the gut microbiome in several opioid-induced side effects (see review in (Ren and Lotfipour, 2020)). Our findings suggest a potential role for the gut microbiome in the expression of the somatic signs of morphine withdrawal, which may facilitate identification of better therapeutics for opioid dependence and withdrawal.
We used FMT from chronic morphine-treated mice to study the effects of morphine-induced changes in the gut microbiome and their effects on the expression of morphine withdrawal following dependence. Morphine has been shown to alter the gut microbiome composition as soon as one day following the start of treatment and can also increase microbial communities associated with pathogenic function (Banerjee et al., 2016; Lee et al., 2018; Wang et al., 2018). Control FMT led to the restoration of microbial communities and improvement in gut tissue damage of morphine-treated recipient mice (Banerjee et al., 2016). Intriguingly, in our study, morphine-treated animals receiving FMT from morphine-treated donor mice showed lower levels of naloxone-precipitated opioid withdrawal compared to morphine-treated animals receiving control FMT. In addition, naloxone-precipitated withdrawal jumps in morphine-treated mice receiving FMT from saline-treated donor mice did not differ from that observed in groups receiving identical morphine treatments together with either water by gavage or no gavage.
To investigate the gut microbiome alterations associated with the FMT, we performed a microbial analysis of the cecum contents of the same recipient mice subjected to withdrawal. The 16S rRNA gene sequencing showed that two major phyla, Firmicutes and Verrucomicrobia, changed in response to morphine treatment, but not in response to FMT. All other microbial analyses (overall changes in phyla proportions, alpha and beta diversity, and SCFA) did not differ among groups, suggesting that the morphine dosing paradigm used here did not, itself, reliably alter the gut microbiome composition. Nonetheless, FMT from morphine-treated donor mice abrogated naloxone-precipitated opioid withdrawal in morphine-treated recipient mice.
Several factors could contribute to the lack of significant changes in gut microbiome composition following FMT, including the relatively short morphine dosing paradigm and sample sizes used here. However, it is important to emphasize that FMT from morphine-treated donor mice produced a robust suppression of withdrawal jumps compared to mice receiving FMT from saline-treated donor mice and the same morphine dosing regimen. Limitations in the microbial analyses used in the current study could have precluded the identification of possible gut microbiome changes (for a review of limitations in gut microbiome analysis see (Rezasoltani et al., 2020)). Notably, our analyses were performed in the cecum contents (as opposed to feces) of mice. Both cecum content and fecal samples have been frequently and interchangeably used to analyze the gut microbiome (Banerjee et al., 2016; Kang et al., 2017; Lee et al., 2018). However, cecum and fecal samples may represent the gut microbiome composition very distinctively and lead to different conclusions (Gu et al., 2013; Pang et al., 2012; Tanca et al., 2017). Furthermore, while the 16S rRNA gene sequencing and SCFA analysis are common ways to investigate gut microbiome changes, other abundant components of the feces, such as archaea, viruses, fungi, protists, colonocytes and other metabolites could contribute to changes in the recipient’s biology (Bojanova and Bordenstein, 2016).
We used an antibiotic cocktail treatment to test the impact of the depletion of the gut microbiome on morphine withdrawal. The broad-spectrum antibiotic treatment used here markedly reduced the bacterial load in mice and produced an enlarged ceca compared to the water-treated animals, a typical phenotype of germ free and antibiotic-treated mice as described previously (Reikvam et al., 2011). Strikingly, our antibiotic treatment regimen also attenuated the expression of naloxone-precipitated opioid withdrawal in morphine-dependent mice. Kang and colleagues previously reported no changes to naloxone-precipitated jumping in morphine-pelleted mice following antibiotic treatment (Kang et al., 2017), which may reflect differences in morphine dosing regimens between studies. A subcutaneous implanted morphine pellet, as used by Kang et al., provides sustained levels of morphine throughout the experiment. In contrast, an escalating i.p. morphine dosing regimen, as used in the current study, provides an intermittent level of morphine and its periodic removal/withdrawal, that potentially allows for a closer reproduction of opioid use commonly seen in people. Intermittent, but not continuous, morphine treatment has been shown to cause microglial activation, hyperalgesia and impaired reward in mice, and it leads to differential effects in the taxa of the gut microbiome (Lee et al., 2018). The concentration of the antibiotics also differed between the two studies, with a 10-fold increase in the dosage of neomycin, vancomycin and metronidazole used in our experiment. The higher dose of antibiotics used here resulted in a greater level of microbial clearance in our study (approximately 1000x less bacterial concentration post antibiotic treatment compared to only 3x less in the study by Kang and colleagues). Another recent study also reported no changes in oxycodone-induced physical withdrawal following antibiotic treatment (Simpson et al., 2020). Other differences exist between the latter and present studies, such as the use of rats instead of mice, the use of oxycodone vs. morphine, and a dramatically lower concentration of antibiotics compared to the present study. Further, the lack of information on the bacterial load prior to and following antibiotic treatment limits interpretation of the level of bacterial depletion (Simpson et al., 2020). Taken together, these factors could potentially account for the efficacy of antibiotic treatment in attenuating morphine withdrawal seen in our study.
Along with gut microbiome changes, antibiotic treatments have been shown to directly alter nervous system structure and functioning. Antibiotic exposure causes a downregulation in μ-opioid receptor (MOR) expression in the frontal cortex of antibiotic-treated mice (Johnson and Burnet, 2020). Moreover, antibiotic treatment induced a widespread neuronal ensemble activation in the central nervous system in rats (Simpson et al., 2020). Although the mechanisms underlying these antibiotic-induced neurobiological changes are still unknown, these studies warrant caution when interpreting the impact of antibiotic-induced gut microbiome manipulations. For this reason, we opted to use multiple ways to manipulate the gut microbiome (i.e. FMT, antibiotic treatment, probiotic treatment and manipulation of gut permeability with ML-7) to investigate its effects on morphine dependence.
We tested the impact of two commercially available probiotic strains, Bifidobacterium longum subspecies longum 35624™ (B. longum) and Lactobacillus rhamnosus GG (L. rhamnosus), on morphine withdrawal. Morphine tolerance has been shown to be associated with a selective depletion of Bifidobacteria and Lactobacillaeae in the gut, and probiotics that contain these bacterial communities can counteract the development of analgesic tolerance in morphine-treated mice (Zhang et al., 2019). Manipulation of the gut microbiome by probiotics, including B. longum and L. rhamnosus, is reported to prevent gut barrier disruption (Bellavia et al., 2014; Bergmann et al., 2013; Isolauri et al., 1993; Shi et al., 2018) and bacterial translocation and neuroinflammation (Ait-Belgnaoui et al., 2012). Probiotics from the Lactobacillus genera are also shown to induce μ-opioid and cannabinoid CB2 receptor expression in the intestinal epithelial cells and mediate analgesic functions in the gut in mice (Rousseaux et al., 2007). Further, B. longum and L. rhamnosus have been shown to be anti-inflammatory in several in vitro, animal and clinical studies (Ganguli et al., 2013; Kandasamy et al., 2011; Nermes et al., 2011; Underwood et al., 2014; Yan et al., 2017). Thus, probiotic therapy during morphine administration may offer an approach to prolong morphine’s efficacy while potentially reducing its side-effects by countering opioid-induced increased gut permeability and neuroinflammation. However, in our study, probiotic treatments did not alter naloxone-precipitated withdrawal behavior. Different probiotics and other dosing regimens should be tested before ruling out this hypothesis. Furthermore, strain isolates, as opposed to commercially available probiotic capsules, may help to obtain exact availability and viability of the strains being tested.
Finally, we tested the impact of the MLCK inhibitor ML-7 on morphine withdrawal. Previous studies showed that morphine increases gut permeability, promoting bacterial translocation and systemic inflammation (Banerjee et al., 2016; Hilburger et al., 1997; Kueppers et al., 1993; Meng et al., 2013; Mora et al., 2012; Runkel et al., 1993). Inactivation of MLCK by ML-7 has been shown to protect barrier function in various endothelial and epithelial cell lines (Huppert et al., 2010; Liu et al., 2013). Importantly, ML-7 has been shown previously to protect against gut epithelial barrier disruption in morphine-pelleted mice and inhibit morphine-induced bacterial translocation (Meng et al., 2013). Our results indicate that the escalating morphine dosing schedule used in the current study increases intestinal permeability in a manner that is blocked by ML-7. However, ML-7 treatment did not alter naloxone-precipitated withdrawal behavior in morphine-dependent mice. These results suggest that gut barrier disruption did not alter expression of morphine withdrawal symptoms. In this regard, other pathways linking the gut microbiome and the morphine withdrawal behavior could be in place, such as the role of the gut microbiome in modulating morphine pharmacokinetics (Wang et al., 2018). Morphine-induced dysbiosis has been claimed to decrease enterohepatic recirculation of morphine into systemic circulation resulting in lower bioavailability of morphine over time (Wang et al., 2018). Most importantly, ML-7 could be involved in mechanisms other than gut permeability modulation and further studies are required to rule out the involvement of gut barrier disruption in opioid withdrawal. Our studies do not preclude the possibility that other signs of opioid withdrawal, not measured herein, could be altered by our experimental treatments.
Opioid withdrawal is one of the most powerful factors driving opioid dependence and addiction. Therefore, efforts to understand the mechanisms underlying these symptoms is crucial to enhancing the safety of opioid use. Clinical and animal studies have recently shown that alterations in gut microbiome composition are strongly associated with chronic opioid use and its side effects (see review in (Ren and Lotfipour, 2020). These findings support a role of the gut microbiome in regulating opioid-induced brain changes. This gut-brain communication could be happening in many ways, one of them being the interaction among the gut bacteria, the immune system and the brain. In pre-clinical studies, opioid treatment can increase intestinal barrier permeability, which ultimately enhances the risk for bacterial translocation to the bloodstream and other tissues (Babrowski et al., 2012; Banerjee et al., 2016; Hilburger et al., 1997; Mora et al., 2012). Bacterial translocation from the gut may activate peripheral and central immune cells, leading to the release of multiple proinflammatory cytokines and the onset of neuroinflammation (Ait-Belgnaoui et al., 2012; Dantzer et al., 2008; Maes et al., 2008). Neuroinflammatory responses are a crucial factor in the development of opioid dependence and withdrawal. Chronic morphine administration, followed by naloxone-precipitated withdrawal, increases levels of proinflammatory cytokines and activates astrocytes and microglia in the brain (Hao et al., 2011; Liu et al., 2011; Pajohanfar et al., 2017). Furthermore, drugs known to suppress glial proinflammatory responses (Hutchinson et al., 2009) downregulate astrocytic expression (Hao et al., 2011), deplete lumbar spinal microglia (Burma et al., 2017), and inhibit the expression of morphine withdrawal symptoms, suggesting the important role of neuroinflammation in the development of morphine dependence. Further investigations of a possible relationship between intestinal barrier impairment, neuroinflammation and opioid withdrawal may reveal a new mechanism underlying the development of opioid dependence and open new therapeutic avenues for pain management.
Our results highlight the impact of multiple experimental approaches used to manipulate the gut microbiome on the somatic expression of morphine withdrawal that is precipitated with the opioid antagonist naloxone. More work is necessary to elucidate the mechanisms underlying these alterations and ascertain the therapeutic implications of these findings.
Acknowledgments
Support: This work is supported by the National Institutes of Health National Institute on Drug Abuse (NIDA) [Grants DA047858, DA041229 and DA042584 (A.G.H.)], a T32 NIDA Predoctoral training grant DA024628 (T.J.W.), the Indiana Addiction Grand Challenge Grant (A.G.H.) and the Harlan Scholars Research Program (V.I.).
Footnotes
Conflict of Interest: None of the authors report any conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acharya C, Betrapally NS, Gillevet PM, Sterling RK, Akbarali H, White MB, Ganapathy D, Fagan A, Sikaroodi M, Bajaj JS, 2017. Chronic opioid use is associated with altered gut microbiota and predicts readmissions in patients with cirrhosis. Aliment Pharmacol Ther 45, 319–331. [DOI] [PubMed] [Google Scholar]
- Ait-Belgnaoui A, Durand H, Cartier C, Chaumaz G, Eutamene H, Ferrier L, Houdeau E, Fioramonti J, Bueno L, Theodorou V, 2012. Prevention of gut leakiness by a probiotic treatment leads to attenuated HPA response to an acute psychological stress in rats. Psychoneuroendocrinology 37, 1885–1895. [DOI] [PubMed] [Google Scholar]
- Babrowski T, Holbrook C, Moss J, Gottlieb L, Valuckaite V, Zaborin A, Poroyko V, Liu DC, Zaborina O, Alverdy JC, 2012. Pseudomonas aeruginosa virulence expression is directly activated by morphine and is capable of causing lethal gut-derived sepsis in mice during chronic morphine administration. Ann Surg 255, 386–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S, Sindberg G, Wang F, Meng J, Sharma U, Zhang L, Dauer P, Chen C, Dalluge J, Johnson T, Roy S, 2016. Opioid-induced gut microbial disruption and bile dysregulation leads to gut barrier compromise and sustained systemic inflammation. Mucosal Immunol 9, 1418–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barengolts E, Green SJ, Eisenberg Y, Akbar A, Reddivari B, Layden BT, Dugas L, Chlipala G, 2018. Gut microbiota varies by opioid use, circulating leptin and oxytocin in African American men with diabetes and high burden of chronic disease. PLoS One 13, e0194171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellavia M, Rappa F, Lo Bello M, Brecchia G, Tomasello G, Leone A, Spatola G, Uzzo ML, Bonaventura G, David S, Damiani P, Hajj Hussein I, Zeenny MN, Jurjus A, Schembri-Wismayer P, Cocchi M, Zummo G, Farina F, Gerbino A, Cappello F, Traina G, 2014. Lactobacillus casei and bifidobacterium lactis supplementation reduces tissue damage of intestinal mucosa and liver after 2,4,6-trinitrobenzenesulfonic acid treatment in mice. Journal of biological regulators and homeostatic agents 28, 251–261. [PubMed] [Google Scholar]
- Bergmann KR, Liu SX, Tian R, Kushnir A, Turner JR, Li HL, Chou PM, Weber CR, De Plaen IG, 2013. Bifidobacteria stabilize claudins at tight junctions and prevent intestinal barrier dysfunction in mouse necrotizing enterocolitis. Am J Pathol 182, 1595–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bojanova DP, Bordenstein SR, 2016. Fecal Transplants: What Is Being Transferred? PLoS Biol 14, e1002503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burma NE, Bonin RP, Leduc-Pessah H, Baimel C, Cairncross ZF, Mousseau M, Shankara JV, Stemkowski PL, Baimoukhametova D, Bains JS, Antle MC, Zamponi GW, Cahill CM, Borgland SL, De Koninck Y, Trang T, 2017. Blocking microglial pannexin-1 channels alleviates morphine withdrawal in rodents. Nat Med 23, 355–360. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW, 2008. From inflammation to sickness and depression: when the immune system subjugates the brain. Nature reviews. Neuroscience 9, 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields HL, 2011. The doctor’s dilemma: opiate analgesics and chronic pain. Neuron 69, 591–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friard O, Gamba M, 2016. BORIS: a free, versatile open-source event-logging software for video/audio coding and live observations. Methods in Ecology and Evolution 7, 1325–1330. [Google Scholar]
- Furlan AD, Sandoval JA, Mailis-Gagnon A, Tunks E, 2006. Opioids for chronic noncancer pain: a meta-analysis of effectiveness and side effects. CMAJ 174, 1589–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguli K, Meng D, Rautava S, Lu L, Walker WA, Nanthakumar N, 2013. Probiotics prevent necrotizing enterocolitis by modulating enterocyte genes that regulate innate immune-mediated inflammation. Am J Physiol Gastrointest Liver Physiol 304, G132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu S, Chen D, Zhang JN, Lv X, Wang K, Duan LP, Nie Y, Wu XL, 2013. Bacterial community mapping of the mouse gastrointestinal tract. PLoS One 8, e74957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao S, Liu S, Zheng X, Zheng W, Ouyang H, Mata M, Fink DJ, 2011. The role of TNFalpha in the periaqueductal gray during naloxone-precipitated morphine withdrawal in rats. Neuropsychopharmacology 36, 664–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiss CN, Olofsson LE, 2019. The role of the gut microbiota in development, function and disorders of the central nervous system and the enteric nervous system. J Neuroendocrinol 31, e12684. [DOI] [PubMed] [Google Scholar]
- Hilburger ME, Adler MW, Truant AL, Meissler JJ Jr., Satishchandran V, Rogers TJ, Eisenstein TK, 1997. Morphine induces sepsis in mice. The Journal of infectious diseases 176, 183–188. [DOI] [PubMed] [Google Scholar]
- Huppert J, Closhen D, Croxford A, White R, Kulig P, Pietrowski E, Bechmann I, Becher B, Luhmann HJ, Waisman A, Kuhlmann CR, 2010. Cellular mechanisms of IL-17-induced blood-brain barrier disruption. FASEB J 24, 1023–1034. [DOI] [PubMed] [Google Scholar]
- Hutchinson MR, Lewis SS, Coats BD, Skyba DA, Crysdale NY, Berkelhammer DL, Brzeski A, Northcutt A, Vietz CM, Judd CM, Maier SF, Watkins LR, Johnson KW, 2009. Reduction of opioid withdrawal and potentiation of acute opioid analgesia by systemic AV411 (ibudilast). Brain Behav Immun 23, 240–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isolauri E, Majamaa H, Arvola T, Rantala I, Virtanen E, Arvilommi H, 1993. Lactobacillus casei strain GG reverses increased intestinal permeability induced by cow milk in suckling rats. Gastroenterology 105, 1643–1650. [DOI] [PubMed] [Google Scholar]
- Iyer V, Slivicki RA, Thomaz AC, Crystal JD, Mackie K, Hohmann AG, 2020. The cannabinoid CB2 receptor agonist LY2828360 synergizes with morphine to suppress neuropathic nociception and attenuates morphine reward and physical dependence. Eur J Pharmacol 886, 173544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamison RN, Mao J, 2015. Opioid Analgesics. Mayo Clinic proceedings 90, 957–968. [DOI] [PubMed] [Google Scholar]
- Johnson KVA, Burnet PWJ, 2020. Opposing effects of antibiotics and germ-free status on neuropeptide systems involved in social behaviour and pain regulation. BMC Neurosci 21, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandasamy M, Selvakumari Jayasurya A, Moochhala S, Huat Bay B, Kun Lee Y, Mahendran R, 2011. Lactobacillus rhamnosus GG secreting an antigen and Interleukin-2 translocates across the gastrointestinal tract and induces an antigen specific immune response. Microbiol Immunol 55, 704–714. [DOI] [PubMed] [Google Scholar]
- Kang M, Mischel RA, Bhave S, Komla E, Cho A, Huang C, Dewey WL, Akbarali HI, 2017. The effect of gut microbiome on tolerance to morphine mediated antinociception in mice. Sci Rep 7, 42658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, 2000. Neurobiology of addiction. Toward the development of new therapies. Annals of the New York Academy of Sciences 909, 170–185. [DOI] [PubMed] [Google Scholar]
- Kosten TR, George TP, 2002. The neurobiology of opioid dependence: implications for treatment. Sci Pract Perspect 1, 13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozich JJ, Westcott SL, Baxter NT, Highlander SK, Schloss PD, 2013. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl Environ Microbiol 79, 5112–5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kueppers PM, Miller TA, Chen CY, Smith GS, Rodriguez LF, Moody FG, 1993. Effect of total parenteral nutrition plus morphine on bacterial translocation in rats. Ann Surg 217, 286–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Vuong HE, Nusbaum DJ, Hsiao EY, Evans CJ, Taylor AMW, 2018. The gut microbiota mediates reward and sensory responses associated with regimen-selective morphine dependence. Neuropsychopharmacology 43, 2606–2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman AH, Sheikh SM, Loh HH, Martin BR, 2001. Opioid and cannabinoid modulation of precipitated withdrawal in delta(9)-tetrahydrocannabinol and morphine-dependent mice. J Pharmacol Exp Ther 298, 1007–1014. [PubMed] [Google Scholar]
- Liu L, Coller JK, Watkins LR, Somogyi AA, Hutchinson MR, 2011. Naloxone-precipitated morphine withdrawal behavior and brain IL-1beta expression: comparison of different mouse strains. Brain Behav Immun 25, 1223–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Xu J, Mei Q, Han L, Huang J, 2013. Myosin light chain kinase inhibitor inhibits dextran sulfate sodium-induced colitis in mice. Dig Dis Sci 58, 107–114. [DOI] [PubMed] [Google Scholar]
- Maes M, Kubera M, Leunis JC, 2008. The gut-brain barrier in major depression: intestinal mucosal dysfunction with an increased translocation of LPS from gram negative enterobacteria (leaky gut) plays a role in the inflammatory pathophysiology of depression. Neuro endocrinology letters 29, 117–124. [PubMed] [Google Scholar]
- Meng J, Yu H, Ma J, Wang J, Banerjee S, Charboneau R, Barke RA, Roy S, 2013. Morphine induces bacterial translocation in mice by compromising intestinal barrier function in a TLR-dependent manner. PLoS One 8, e54040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora AL, Salazar M, Pablo-Caeiro J, Frost CP, Yadav Y, DuPont HL, Garey KW, 2012. Moderate to high use of opioid analgesics are associated with an increased risk of Clostridium difficile infection. The American journal of the medical sciences 343, 277–280. [DOI] [PubMed] [Google Scholar]
- Nermes M, Kantele JM, Atosuo TJ, Salminen S, Isolauri E, 2011. Interaction of orally administered Lactobacillus rhamnosus GG with skin and gut microbiota and humoral immunity in infants with atopic dermatitis. Clin Exp Allergy 41, 370–377. [DOI] [PubMed] [Google Scholar]
- Pajohanfar NS, Mohebbi E, Hosseini-Bandegharaei A, Amin M, Vaseghi G, Amin B, 2017. Simvastatin prevents morphine-induced tolerance and dependence in mice. Biomed Pharmacother 93, 406–411. [DOI] [PubMed] [Google Scholar]
- Pang W, Vogensen FK, Nielsen DS, Hansen AK, 2012. Faecal and caecal microbiota profiles of mice do not cluster in the same way. Lab Anim 46, 231–236. [DOI] [PubMed] [Google Scholar]
- Principi N, Esposito S, 2016. Gut microbiota and central nervous system development. J Infect 73, 536–546. [DOI] [PubMed] [Google Scholar]
- Ramesh D, Ross GR, Schlosburg JE, Owens RA, Abdullah RA, Kinsey SG, Long JZ, Nomura DK, Sim-Selley LJ, Cravatt BF, Akbarali HI, Lichtman AH, 2011. Blockade of endocannabinoid hydrolytic enzymes attenuates precipitated opioid withdrawal symptoms in mice. J Pharmacol Exp Ther 339, 173–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reikvam DH, Erofeev A, Sandvik A, Grcic V, Jahnsen FL, Gaustad P, McCoy KD, Macpherson AJ, Meza-Zepeda LA, Johansen FE, 2011. Depletion of murine intestinal microbiota: effects on gut mucosa and epithelial gene expression. PLoS One 6, e17996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren M, Lotfipour S, 2020. The role of the gut microbiome in opioid use. Behav Pharmacol 31, 113–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezasoltani S, Ahmadi Bashirzadeh D, Nazemalhosseini Mojarad E, Asadzadeh Aghdaei H, Norouzinia M, Shahrokh S, 2020. Signature of Gut Microbiome by Conventional and Advanced Analysis Techniques: Advantages and Disadvantages. Middle East J Dig Dis 12, 5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseaux C, Thuru X, Gelot A, Barnich N, Neut C, Dubuquoy L, Dubuquoy C, Merour E, Geboes K, Chamaillard M, Ouwehand A, Leyer G, Carcano D, Colombel JF, Ardid D, Desreumaux P, 2007. Lactobacillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors. Nat Med 13, 35–37. [DOI] [PubMed] [Google Scholar]
- Runkel NS, Moody FG, Smith GS, Rodriguez LF, Chen Y, Larocco MT, Miller TA, 1993. Alterations in rat intestinal transit by morphine promote bacterial translocation. Dig Dis Sci 38, 1530–1536. [DOI] [PubMed] [Google Scholar]
- Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B, Thallinger GG, Van Horn DJ, Weber CF, 2009. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75, 7537–7541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon G, Sampson TR, Geschwind DH, Mazmanian SK, 2016. The Central Nervous System and the Gut Microbiome. Cell 167, 915–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin E, Rea K, Dinan TG, Cryan JF, 2016. A gut (microbiome) feeling about the brain. Curr Opin Gastroenterol 32, 96–102. [DOI] [PubMed] [Google Scholar]
- Shi Y, Zhao X, Zhao J, Zhang H, Zhai Q, Narbad A, Chen W, 2018. A mixture of Lactobacillus species isolated from traditional fermented foods promote recovery from antibiotic-induced intestinal disruption in mice. J Appl Microbiol 124, 842–854. [DOI] [PubMed] [Google Scholar]
- Simpson S, Kimbrough A, Boomhower B, McLellan R, Hughes M, Shankar K, de Guglielmo G, George O, 2020. Depletion of the Microbiome Alters the Recruitment of Neuronal Ensembles of Oxycodone Intoxication and Withdrawal. eNeuro 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanca A, Manghina V, Fraumene C, Palomba A, Abbondio M, Deligios M, Silverman M, Uzzau S, 2017. Metaproteogenomics Reveals Taxonomic and Functional Changes between Cecal and Fecal Microbiota in Mouse. Front Microbiol 8, 391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood MA, Arriola J, Gerber CW, Kaveti A, Kalanetra KM, Kananurak A, Bevins CL, Mills DA, Dvorak B, 2014. Bifidobacterium longum subsp. infantis in experimental necrotizing enterocolitis: alterations in inflammation, innate immune response, and the microbiota. Pediatr Res 76, 326–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent C, Miller MA, Edens TJ, Mehrotra S, Dewar K, Manges AR, 2016. Bloom and bust: intestinal microbiota dynamics in response to hospital exposures and Clostridium difficile colonization or infection. Microbiome 4, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Meng J, Zhang L, Johnson T, Chen C, Roy S, 2018. Morphine induces changes in the gut microbiome and metabolome in a morphine dependence model. Sci Rep 8, 3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham RJ, 2017. Cancer Pain Management: Opioid Analgesics, Part 2. J Adv Pract Oncol 8, 588–607. [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Xie Z, Wang H, Shen Z, Guo Y, Gao Y, Chen X, Wu Q, Li X, Wang K, 2017. Bacterial Diversity of Intestinal Microbiota in Patients with Substance Use Disorders Revealed by 16S rRNA Gene Deep Sequencing. Sci Rep 7, 3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan F, Liu L, Cao H, Moore DJ, Washington MK, Wang B, Peek RM, Acra SA, Polk DB, 2017. Neonatal colonization of mice with LGG promotes intestinal development and decreases susceptibility to colitis in adulthood. Mucosal Immunol 10, 117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Deji C, Fan J, Chang L, Miao X, Xiao Y, Zhu Y, Li S, 2021. Differential alteration in gut microbiome profiles during acquisition, extinction and reinstatement of morphine-induced CPP. Prog Neuropsychopharmacol Biol Psychiatry 104, 110058. [DOI] [PubMed] [Google Scholar]
- Zhang L, Meng J, Ban Y, Jalodia R, Chupikova I, Fernandez I, Brito N, Sharma U, Abreu MT, Ramakrishnan S, Roy S, 2019. Morphine tolerance is attenuated in germfree mice and reversed by probiotics, implicating the role of gut microbiome. Proc Natl Acad Sci U S A 116, 13523–13532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G, Nyman M, Jonsson JA, 2006. Rapid determination of short-chain fatty acids in colonic contents and faeces of humans and rats by acidified water-extraction and direct-injection gas chromatography. Biomed Chromatogr 20, 674–682. [DOI] [PubMed] [Google Scholar]
- Zhou D, Pan Q, Shen F, Cao HX, Ding WJ, Chen YW, Fan JG, 2017. Total fecal microbiota transplantation alleviates high-fat diet-induced steatohepatitis in mice via beneficial regulation of gut microbiota. Sci Rep 7, 1529. [DOI] [PMC free article] [PubMed] [Google Scholar]