To the Editor:
Recently, Bohler et al., described the first report of acute macular neuroretinopathy (AMN) following Vaxzevria (formerly ChAdOx1 nCoV-19, Oxford–AstraZeneca, UK) providing a possible causal association with COVID-19 innoculation [1]. Through mid 2021, there are 5 reported cases of AMN following vaccination with Vaxzevria [1–4]. We describe the first AMN case following use of an alternate mRNA vaccine.
A 21-year-old Caucasian female was referred for assessment of ongoing left paracentral scotomas. Her only medication was a combined oral contraceptive (ethinylestradiol 0.02mg-levonorgestrel 0.1mg). Ten weeks previously she had received her first COVID-19 vaccine injection (BNT162b2 mRNA, Pfizer-BioNTech). Three days post-injection, she developed acute chills, myalgia, headache and two left scotomas either side of fixation. Systemic symptoms resolved quickly. The patient reported a gradual decrease in scotoma intensity with time.
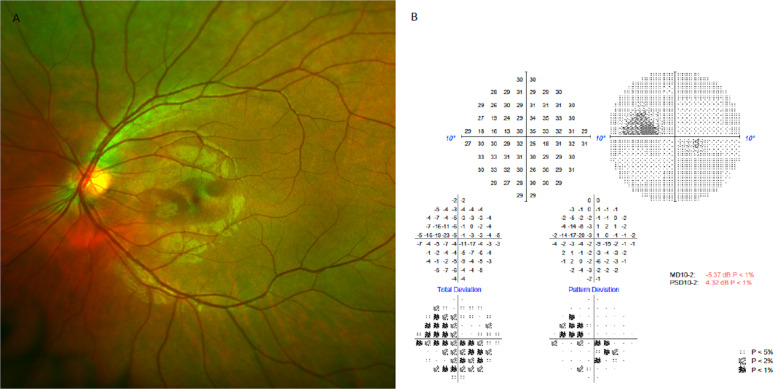
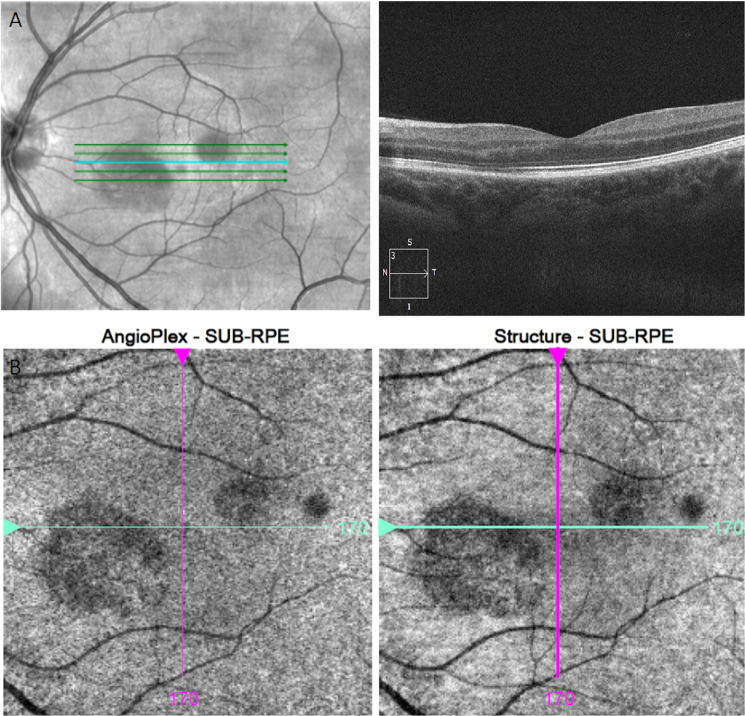
Uncorrected visual acuity was 6/6 bilaterally and right eye examination normal. Left fundus examination demonstrated barely visible oval parafoveal lesions with slight hyper-fluorescence on imaging (Optos, MA, USA). Infrared reflectance imaging (Cirrus, Zeiss, Germany) confirmed two parafoveal oval hypo-reflective lesions. Findings were consistent with left AMN (Figs. 1–2).
Fig. 1. Documented clinical changes at follow up visit.
A (Left) Fundus image of left eye indicating oval parafoveal lesions on autofluorescence imaging. B (Right): Corresponding visual field note parafoveal depressions corresponding to fundus imaging.
Fig. 2. Documented OCT changes at follow up visit.
A (Top): OCT imaging focal areas of paracentral hyper-reflectivity of the outer plexiform layer and outer nuclear layer with disruption of the ellipsoid zone. B (Bottom): oval hypo-reflective lesions located inferonasal and superotemporal to the fovea via Infrared reflectance imaging.
Paracentral scotomas have been described in almost three-quarters of AMN cases (73%), remaining symptomatic at final follow-up in over half of these patients [5]. Decreased acuity remains less common (12–16%) [5]. Exact pathophysiology is unknown but a potential mechanism is ischemia in the deep retinal capillary plexus or choriocapillaris [5]. Dominant associated factors are recent infection, febrile illness and use of oral contraceptives [1].
All reported cases of AMN following Vaxzevria COVID-19 vaccination have noteworthy similarities to our case [1–4]. All cases involved females aged 21 to 28 years taking oral contraceptives. Acute paracentral scotomas developed 2 or 3 days following the initial vaccination. In all but one case, ocular symptoms were coexistent with systemic flu-like symptoms. Our case represents the first following BNT162b2 (Pfizer-BioNTech) injection.
The occurrence of AMN following COVID-19 vaccination is rare; however, with minimal impact on acuity and often subtle retinal signs, it may be under-reported. Our case supports Bohler et al., lending support to the hypothesis that marked systemic inflammation following vaccination may represent a possible risk factor for AMN in young women on oral contraceptives.
Author contributions
SC was responsible for the patient consultation and analysis. SC and CH were responsible for writing the report, extracting and preparation of the images and final review of the correspondence.
Competing interests
The authors declare no competing interests.
Footnotes
Acute macular neuroretinopathy following vaccination with the Pfizer-BioNTech COVID-19 vaccine.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bohler AD, Strom ME, Sandvig KU, Moe MC, Jorstad OK. Acute macular neuroretinopathy following COVID-19 vaccination. Eye. 2021:1–2. 10.1038/s41433-021-01610-1. [DOI] [PMC free article] [PubMed]
- 2.Mambretti M, Huemer J, Torregrossa G, Ullrich M, Findl O, Casalino G. Acute macular neuroretinopathy following coronavirus disease 2019 vaccination. Ocul Immunol Inflamm. 2021:1–4. 10.1080/09273948.2021.1946567. [DOI] [PubMed]
- 3.Book BAJ, Schmidt B, Foerster AMH. Bilateral acute macular neuroretinopathy after vaccination against SARS-CoV-2. JAMA Ophthalmol. 2021;139:e212471. doi: 10.1001/jamaophthalmol.2021.2471. [DOI] [PubMed] [Google Scholar]
- 4.Michel TSN, Gascon P, Dupessey F, Comet A, Attia R, David T, et al. Acute macular neuroretinopathy after COVID-19 vaccine. 2021. J Ophthal Inflamm Infect. 10.21203/rs.3.rs-632137/v1. [DOI] [PMC free article] [PubMed]
- 5.Bhavsar KV, Lin S, Rahimy E, Joseph A, Freund KB, Sarraf D, et al. Acute macular neuroretinopathy: a comprehensive review of the literature. Surv Ophthalmol. 2016;61:538–65. doi: 10.1016/j.survophthal.2016.03.003. [DOI] [PubMed] [Google Scholar]