Abstract
Objectives:
High prevalence of vitamin D deficiency mandates prescribing an appropriate form of vitamin D that allows attainment of sufficiency in a cost-effective manner. We aimed to compare vitamin D products in Indian market in terms of composition and cost in 2020 with 2013 to understand price dispersion over 7 years.
Methods:
Constituents, formulations, and prices of ‘branded’ and generic vitamin D products were sourced from various drug information compendia and online sources. Price per defined daily dose (DDD), percentage cost variation, and change in prices over 7 years (2020 vs. 2013) was determined.
Results:
There has been a disproportionate increase in the number of brands and cost variation of cholecalciferol and calcitriol in the last 7 years. The percentage cost variation increased almost 10 times for calcitriol and 4.4 times for alfacalcidiol tablets and cholecalciferol granules. An analysis of >1,100 products in 2020 showed that the predominant form was calcitriol which was combined with calcium in >90% of the products with huge cost variation (>3000%). Ergocalciferol and cholecalciferol were available in 22 and 15 different strengths respectively. Median price/unit of cholecalciferol (60,000IU) was lower for tablets/capsules compared to other formulations; but with >1000% cost variation.
Conclusion:
A wide cost variation exists with the use of different vitamin D brands and preparations with conventional cholecalciferol tablets and capsules being a low-priced alternative. Quality control measures and strict enforcements of existing regulations are essential to ensure that competitive prices of branded generics are translated into availability and affordability for the population.
Keywords: Brand, cost analysis, generic drugs, nutritional supplements, treatment, vitamin D deficiency, vitamin D
INTRODUCTION
Vitamin D deficiency has been reported in both developed and developing nations across various age groups, gender and illnesses in both hospital-based studies (50-94%) and community settings (37-99%).[1,2] Fortification of food and vitamin D supplementation are the primary preventive and cost-effective interventions to reduce the burden of vitamin D deficiency.[1,2,3,4,5] Food fortification is an effective population-based strategy that mandates government initiative on a large-scale for effective implementation.[5] Supplementation programs have lowered the incidence rates of rickets in children[6] and shown reduction in falls, fractures; improvement in musculoskeletal health with additional cost-benefits in elderly.[7,8] Despite the cost-effectiveness of this intervention, monitoring their intake for overdose, adherence especially in high-risk groups and mass-outreach are concerns with supplementation.
Globally, with considerable research on vitamin D, high prevalence of vitamin D deficiency, greater prescriber and population awareness, and recommendations for routine supplementation without pre-screening in certain population groups, has led to a considerable rise in the number of prescriptions of vitamin D.[9,10,11,12] It is estimated that the global vitamin D therapy market size is expected to grow at a compound annual growth rate (CAGR) of 11.70% from $2,020.30 million in 2019 to $3,925.67 million by the end of 2025 across the West and Asia Pacific countries including India.[13] Similar data shows that market size in India rose from 2.98 billion (Indian National rupees, INR) in 2014 to 5.38 billion in 2018.[14]
However, variations in dose and duration of vitamin D supplementation as per recommendations put an onus on the prescribing physician to facilitate a rational and cost-effective prescription. An understanding of the various products could aid in an informed decision-making process to prescribe an appropriate cost-effective formulation, in adequate quantity (international unit (IU) per dose and dosing frequency).[10] At present, there are numerous ‘branded generics’ of vitamin D in different forms, strengths and vehicles for delivery in the Indian market that mandates a structured analysis to rationalize prescribing decision.[15,16,17] In low-and middle-income countries (LMICs), low levels of health insurance coverage combined with households spending 50-80% of their total health expenditure on medicines, make high drug prices an important impediment to access and compliance.[15] We aimed to assess the composition and the price variations among various vitamin D supplement products available in the Indian market and estimate price dispersion over 7 years (2013–2020).
METHODOLOGY
Data source
This was a cross-sectional cost analysis for the assessment of vitamin D products using both online and text sources of drug information. Various text compendia [Current Index of Medical Specialities (CIMS) Reference Guide (July-Sep 2020) and Drug Today (July-October 2020)] and online sources (CIMS website) that detailed products, their medicinal formulations- constituents and dosage, and maximum retail prices (MRP) prices in the Indian Rupee (INR) were utilized.[18,19,20]
The MRP of the product was also considered with its generic price and ceiling price as fixed by the Government of India. Under the Essential Commodities Act, drug prices have been controlled using a series of Drugs Price Control Orders (DPCOs), beginning in 1970.[21] In 1997, India established the National Pharmaceutical Pricing Authority (NPPA), an organization that reviews and fixes pharmaceutical prices using market-based mechanisms.[15] The prevailing DPCO, 2013 had set ceiling prices for essential drugs from the National List of Essential Medicines (NLEM), a list of medications based on the World Health Organization's (WHO) list of essential medicines.[22] Ceiling price are set using price to retailer, which is the price the pharmacist pays for medication, in contrast to MRP that is printed on the medication package.[15] As the NLEM includes only cholecalciferol, the ceiling prices were available only for this product and was used for comparison with MRP.[23] Price of generic products was taken from the official site of the Bureau of Pharma PSUs of India (BPPI) and Government of India that aims to provide quality generic medicines at an affordable price across the country.[24]
Due to differences in nomenclature (both branded and generic), packing sizes, pricing and customary dosages, WHO has developed the Anatomical Therapeutic Chemical (ATC)/Defined Daily Dose (DDD) methodology to facilitate the presentation and comparison of drug consumption statistics at international, national and regional levels. WHO defines a technical unit of measurement DDD as an assumed average maintenance dose per day for a drug for its principal indication in an adult.[25] Cholecalciferol (D3) and ergocalciferol (D2) are the inactive forms of vitamin D from animal and plant source, respectively. Alfacalcidiol and calcitriol are the biologically active forms that have a DDD of 1 mcg. Given that both are available as 0.25 mcg oral strengths, four unit products would be required to attain DDD.
Data analysis
Unit price of each product was calculated by dividing the given price (MRP) with the available pack size of that particular strength. The cost per DDD was calculated by multiplying the number of units needed to attain the DDD with the unit price of the drug of a particular strength. Percentage cost variation was calculated for a particular strength and formulation of vitamin D as follows: [(MRP of most expensive brand – MRP of least expensive brand)/MRP of least expensive brand]*100. Cost ratio was defined as the ratio of cost of the most expensive and least expensive brand product.
Statistical analysis
Data (product details; composition and price) were entered in Microsoft Excel and analyzed using the same software. Descriptive analyses were performed to calculate and compare costs/DDD (minimum and maximum cost), percentage cost variation, and the cost ratio for different products. A comparison was also drawn with our previous analysis of 2013 to understand the price variation of these products over time.[17]
RESULTS
Among a total of 1115 products, vitamin D3 is commonly available as calcitriol (n = 502; 45.02%) and cholecalciferol [Table 1, Figure 1]. Majority of calcitriol preparations were combined with calcium and other micronutrients (94.42%). Cholecalciferol (n = 457; 40.98% of the total products) was available in strength of 60,000IU in 63.47% of cholecalciferol brands and as a single product in 88.81% preparations [Table 1]. Cholecalciferol (DDD-800IU/20 mcg) was available in very few products (5.03%) limiting the estimation of cost per DDD. Among various alfacalcidiol brands, median price per DDD and cost ratio of tablets and capsules was comparable as shown in Table 2. Though the median price per DDD of calcitriol tablet and capsule was comparable, there was a wide variation in calcitriol tablets (3633%) and capsules (729.23%). The median price per unit of 60,000 IU cholecalciferol was comparable in tablets, capsules, and granules; making them economical alternatives for treatment of deficiency. The oral solutions (nano-emulsions) were the most expensive products (unit price range INR 45-260) [Table 2]. The percentage cost variation was more than 1000% for calcitriol tablet and cholecalciferol (60K) tablets and capsules [Figure 2]. Calcium supplements with calcitriol were priced 3 times higher than single ingredient calcitriol tablets.
Table 1.
Vitamin D products in the Indian market
| Calcitriol | Cholecalciferol | Alfacalcidiol | Ergocalciferol | Total | |
|---|---|---|---|---|---|
| Total number of products (%)# | 502 (45.02%) | 457 (40.98%) | 124 (11.12%) | 32 (2.86%) | 1115 (100%) |
| Number of strengths available | 2 | 15 | 4 | 22 | 43 |
| Number of constituents (range) | 1-9 | 1-5 | 1-6 | 1-41 | 1-41 |
| Number of products-single ingredient- vitamin D (%)@ | 28 (5.57%) | 421 (92.12%) | 24 (19.35%) | 1 (3.12%) | 474 |
| Number of products with calcium (%)@ | 474 (94.42%) | 17 (3.38%) | 99 (79.83%) | 28 (87.5%) | 618 |
#This percentage is calculated as the selected number of vitamin D products in the numerator and total vitamin D products (1115) in the denominator. For example, in case of calcitriol, 502/1115=45.02% calcitriol products of the total vitamin D products. @ This percentage is calculated as the number of particular vitamin D products in the selected option as numerator and the particular vitamin D product in the denominator. For example, in case of calcitriol, 28/502=5.57% calcitriol products contain single ingredient- vitamin D.
Figure 1.
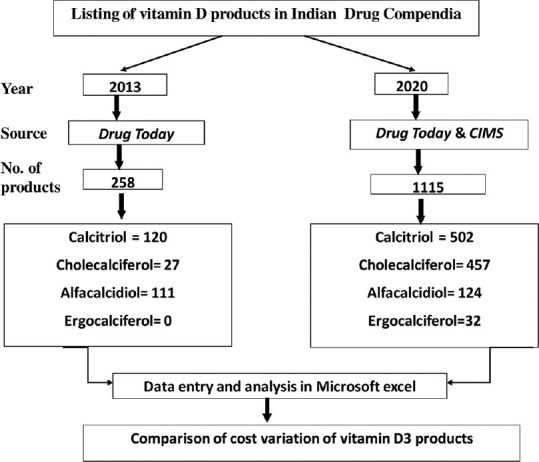
Flowchart for the analysis of vitamin D products in 2013 and 2020
Table 2.
Cost variation among vitamin D3 products (All prices are in INR)
| Drug (DDD) | Generic Price in INR | Strength and Formulation (no of brands) | Median price (per unit) | Median price (per DDD) * | Range price (per unit) | Range price (per DDD) * | Cost Ratio | Cost Variation % |
|---|---|---|---|---|---|---|---|---|
| Calcitriol (1 mcg) | 1.3 | 0.25 mcg Tab. (168) | 10.5 | 42 | 2.25-84 | 327 | 37.33 | 3633 |
| 1.8 | 0.25 mcg Cap.(321) | 12 | 48 | 3.9-32.34 | 113.76 | 8.29 | 729.23 | |
| Alfacalcidiol (1 mcg) | 0.25 mcg Tab. (84) | 6 | 24 | 3.5-17.4 | 55.6 | 4.97 | 397.14 | |
| 2.2 | 0.25 mcg Cap. (31) | 6 | 24 | 3.1-11.7 | 34.4 | 3.77 | 277.41 | |
| Cholecalciferol (800IU) † | 1.8 | 800IU Oral Liquids (Drop, Suspension) (23) | 4.76 | 4.76 | 2.16-8.28 | 6.12 | 3.83 | 283.33 |
| 60K Cap. (92) † | 27.25 | 3.62-62.25 | 17.19 | 1619 | ||||
| 9 | 60K Granules (97) † | 25.5 | 10-51 | 5.1 | 410 | |||
| 60K Tab. (68) † | 25 | 5.77-78.51 | 13.6 | 1260 | ||||
| 60K Solution (18) † | 61 | 45-260 | 5.77 | 477.77 | ||||
| 60K Oral Strip (10) † | 34.5 | 21-68 | 3.23 | 223.8 | ||||
| 60K Inj. (5) † | 29.75 | 29.75-55 | 1.84 | 84.87 |
*DDD= Defined Daily Dose; Tab.= Tablet; Cap.= Capsule; Inj.= Injection; 60K=60,000IU. All prices in INR. † Price per DDD was not estimated for cholecalciferol (60,000 IU); strength commonly used for treatment of vitamin D deficiency.
Figure 2.
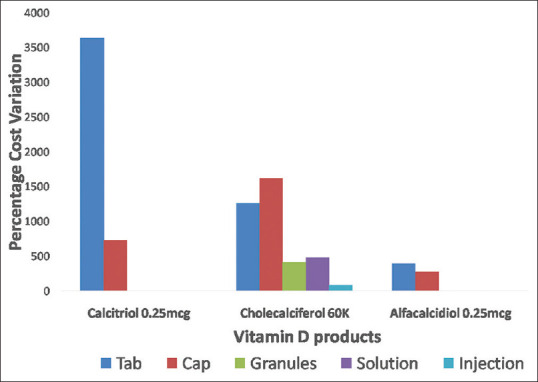
Comparison of mean cost variations of various vitamin D3 products. Legend: Percentage cost variation =[(MRP of most expensive brand – MRP of least expensive brand)/MRP of least expensive brand]*100
A wide gap was observed on comparison of ceiling price fixed by NPPA, and maximum price per unit available in the market. This indicates that market products were priced much higher (nearly 2-3 times for cholecalciferol capsules and tablets respectively) than the ceiling price.
The generic prices were much lower than the median prices of various brand products. The median price of calcitriol brands was at least 7 times higher than the price of generics in same strength and formulation. In case of alfacalcidiol (0.25 mcg capsules) and cholecalciferol 60K granules, branded products were nearly 3 times more expensive than generic products in the market [Figure 3].
Figure 3.
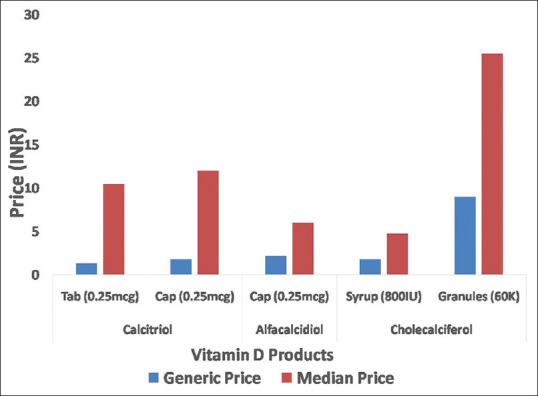
Comparison of generic price and median price of various vitamin D products
The vitamin D3 brands increased 4.19 times in 7 years (18 times in case of cholecalciferol), with increase in the cost variation in most of the products [Figures 1 and 4]. The percentage cost variation increased 4.4 times in case of alfacalcidiol tablets and cholecalciferol granules and nearly 10 times for calcitriol tablets [Figure 4].
Figure 4.
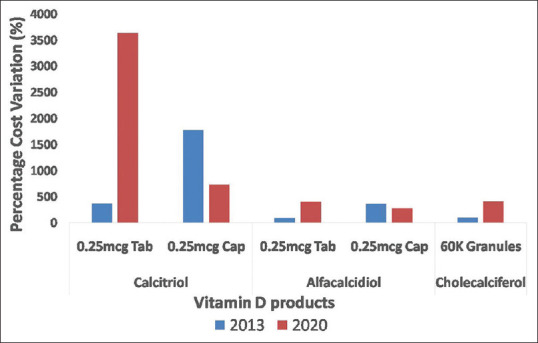
Comparison of percentage cost variation of vitamin D products over 7 years
DISCUSSION
In the Indian market, cholecalciferol is the most readily available, efficacious and cheap form of vitamin D that should be considered for food fortification, supplementation and treatment.[11,26,27] It has demonstrated superior short-term and long-term outcomes such as raising serum 25-hydroxy vitamin D concentrations and mortality reduction over ergocalciferol.[28] Conventional oral fat-soluble supplements offer a simple and low-cost option, but efficacy is limited by lipophilic character and low bioavailability in gastrointestinal tract. Newer hydrophilic micellised formulations have higher bioavailability and greater response to therapy.[29] However, their use is limited by higher cost (nearly 12 times) which escalates the expenditure for treatment of deficiency. Serum vitamin D levels tend to decline within a year in the absence of continued supplementation.[30] Thus, whether this greater bioavailability of expensive nano-emulsion product translates into a better and sustained clinical response needs to be studied further.
The wide cost variation of cholecalciferol tablets and capsules (>1000%) indicates the impact of choice of brand in relation to expense for the treatment of deficiency. Alfacalcidiol and calcitriol are the active and more potent formulations that are reserved for patients with chronic renal and hepatic disease.[26] However, the present study showed that calcitriol was the most commonly available D3 analog which was combined with calcium in >94% of the products. These combined supplements were 3 times more expensive than calcitriol (single-ingredient) tablets and can significantly raise expenditures and risk of adverse reactions; thus impacting compliance when prescribed for long-term use. With regards to pediatric population, cholecalciferol was available in various strengths and formulations like syrups and drops. As milk remains a poor source of vitamin D, the Indian pediatric guidelines recommend daily supplementation of 400-600 IU of vitamin D in infancy and older children and 60,000 IU monthly in adolescence with emphasis on calcium intake.[31] The use of cholecalciferol instead of calcitriol in most pediatric formulations was reassuring. Apart from tablets and capsules, cost-variation of D3 drops was also significant suggesting indirect economic burden which may result in poor compliance to routine supplementation of D3 during infancy.
In the last decade, there has been a progressive increase in the prescriptions of vitamin D supplements.[6,9,10,11,12] In 2017, vitamin D expenditure in Italy was reported to be €180 million, positioning it at the first place for consumption in DDD.[10] Physicians may be unaware of the price of the prescribed drugs and may inadvertently prescribe a more expensive product.[32] In addition, most of the patients are usually hesitant to admit their unaffordability to purchase medicines. In such a case, it is believed that patients usually forego the use of ‘non-essential’ drugs or the ones they perceive to be of least value compared to the essential ones used in the management of chronic conditions.[33] A patient is likely to discontinue vitamin supplements which can have serious consequences in vulnerable groups like infants and elderly. The assessment of cost-effectiveness of supplementation in older adults highlighted that reduction of health and social care expenditure was from avoidance of long-term care following a fall in those aged > 70 years. It is known that generic drug competition is the principal method to contain the rapid growth in pharmaceutical expenditures. However, our analysis indicates that despite an expansion of ‘branded generics’ market over time, a wide price gap remains in the majority of products. This gap has increased nearly ten times in case of cholecalciferol, the recommended agent for supplementation. This is a cause for concern as cholecalciferol is the only D3 analog included in NLEM and DPCO to ensure affordability for the masses. This undermines the purpose of these regulations that ensure access to affordable medicines. It is noteworthy to understand why a causal relationship between price and the number of drug competitors is difficult to establish in the Indian market. The market is dominated by “branded generics” wherein manufacturers of generic drugs compete on brand name as opposed to price. Lack of adequately conducted bioequivalence studies for most generics, ineflective enforcement of good manufacturing practices and rules and regulations has led to a proliferation of ‘substandard’ medications that are usually cheaper than their high-priced counterparts. In contrast, certain products establish a reputation for quality with patients and physicians and are preferred in spite of their rising prices. Thus, despite being one of the world's most competitive generics market, certain segment of population like cost-conscious consumers are ready to pay for a higher price ‘branded generic’ even though they are largely paying for medications out-of-pocket.[15]
The present scenario highlights the need for an integrated approach to ensure rational and cost-effective prescribing of vitamin D supplements. Though physicians in India are encouraged to prescribe generics, patients end up purchasing ‘branded generics’ at the pharmacy due to their non-availability at many centres. At the prescriber level, the prescriptions should clearly indicate the product (cholecalciferol), its formulation, strength and frequency of administration. Prescribing can achieve its goal only if cost awareness is linked to therapeutic reasoning. Our study serves as a scaffold to support training of future prescribers such that they are able to consolidate their knowledge in selection of most appropriate and affordable drug for a patient.[34,35] Understanding the needs for adjustments for purchasing power of a patient can enable access to affordable medicines. The pharmacist should ensure that the prescribed and dispensed drug product match as per prescription. At the regulatory level, implementation and enforcement of existing rules and regulations with curbing of unscrupulous manufacturing practices and pharmaceutical quality assurance is essential to create access to safe and effective generics for affordable healthcare.
This study provides a comparative analysis of single drug/fixed-drug vitamin D and calcium preparations to help a physician prescribe an appropriate and cost-effective product. However, the present study does not cover any information on bioavailability of different brands, which were shown to be highly variable in a previous in-vitro study.[16] The rationality of vitamin D prescriptions across different age-groups and indications was outside the purview of the present study. The total out-of-pocket expenditure was also not assessed as consumption practices of consumers were not recorded.
To summarize, the present study highlights a disproportionate increase in the number of brands and cost variation of cholecalciferol and calcitriol products in the last 7 years. Quality standards of drugs have to be the primary focus area to ensure that the large number of “generic brands” can translate into affordability and accessibility for the masses. As a physician, one should be aware of the available products, their indication and cost to ensure rational and cost-effective treatment.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
We would like to thank Mr. Gulshan, Mrs. Romi Kumari and Ms. Anjali Parmar for their help in data analysis.
REFERENCES
- 1.Aparna P, Muthathal S, Nongkynrih B, Gupta SK. Vitamin D deficiency in India. J Family Med Prim Care. 2018;7:324–30. doi: 10.4103/jfmpc.jfmpc_78_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Selvarajan S, Gunaseelan V, Anandabaskar N, Xavier AS, Srinivasamurthy S, Kamalanathan SK, et al. Systematic review on vitamin D level in apparently healthy Indian population and analysis of its associated factors. Indian J Endocrinol Metab. 2017;21:765–75. doi: 10.4103/ijem.IJEM_168_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garg S, Dasgupta A, Paul B, Maharana SP. Vitamin D insufficiency risk score for screening for vitamin D insufficiency. Indian J Endocrinol Metab. 2019;23:552–6. doi: 10.4103/ijem.IJEM_539_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lhamo Y, Chugh PK, Gautam SR, Tripathi CD. Epidemic of vitamin D deficiency and its management: Awareness among Indian medical undergraduates. J Environ Public Health 2017. 2017 doi: 10.1155/2017/2517207. 2517207. doi: 10.1155/2017/2517207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Black LJ, Seamans KM, Cashman KD, Kiely M. An updated systematic review and meta-analysis of the efficacy of vitamin D food fortification. J Nutr. 2012;142:1102–8. doi: 10.3945/jn.112.158014. [DOI] [PubMed] [Google Scholar]
- 6.Floreskul V, Juma FZ, Daniel AB, Zamir I, Rawdin A, Stevenson M, et al. Cost-Effectiveness of vitamin D supplementation in pregnant woman and young children in preventing rickets: A modeling study. Front Public Health. 2020;8:439. doi: 10.3389/fpubh.2020.00439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee RH, Weber T, Colón-Emeric C. Comparison of cost-effectiveness of vitamin D screening with that of universal supplementation in preventing falls in community-dwelling older adults. J Am Geriatr Soc. 2013;61:707–14. doi: 10.1111/jgs.12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weaver CM, Bischoff-Ferrari HA, Shanahan CJ. Cost-benefit analysis of calcium and vitamin D supplements. Arch Osteoporos. 2019;14:50. doi: 10.1007/s11657-019-0589-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams J, Williams C. Responsibility for vitamin D supplementation of elderly care home residents in England: falling through the gap between medicine and food. BMJ Nutr Prev Health. 2020;3:256–62. doi: 10.1136/bmjnph-2020-000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanò M, Dutto P, D’Anna S, Rognoni C. Can a different formulation of vitamin D3 allow savings? An analysis from an Italian regional perspective. Health Serv Res Manag Epidemiol. 2019;6 doi: 10.1177/2333392819861881. 2333392819861881. doi: 10.1177/2333392819861881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, et al. The 2011 report on dietary reference intakes for calcium and Vitamin D from the institute of medicine: What clinicians need to know. J Clin Endocrinol Metab. 2011;96:53–8. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–30. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 13.Vitamin D Therapy Market Research Report by Route of Administration (Oral Route of Administration and Parenteral Route of Administration), by Purchasing Pattern (Over-The-Counter Drugs and Prescription Drugs), by Age Group, by Indication-Global Forecast to 2025 - Cumulative Impact of COVID-19. Available from: https://reports.valuates.com/market-reports/360I-Auto-2F379/vitamin-d-therapy .
- 14.Market for vitamin D likely to touch $2.5 billion by 2020, says report. [Last accessed on 2020 Dec 5]. https://www.business-standard.com/article/companies/market-for-vitamin-d-likely-to-touch-2-5-billion-by-2020-saysreport-118091300915_1.html .
- 15.Dean EB. Who Benefits from Pharmaceutical Price Controls? Evidence from India. CGD Working Paper 509. Washington, DC: Center for Global Development. 2019. p. 71. Available from: https://www.cgdev.org/publication/who-benefits-pharmaceutical-pricecontrols-evidence-india .
- 16.Khadgawat R, Ramot R, Chacko KM, Marwaha RK. Disparity in cholecalciferol content of commercial preparations available in India. Indian J Endocrinol Metab. 2013;17:1100–03. doi: 10.4103/2230-8210.122638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lhamo Y, Chugh PK, Tripathi CD. Vitamin D Supplements in the Indian Market. Indian J Pharm Sci. 2016;78:41–7. doi: 10.4103/0250-474x.180248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.CIMS Prescriber's Handbook. July-Sept 2020. CIMS Medica India Pvt.Ltd [Google Scholar]
- 19.Drug Today. Volume 109, No. 2, July-October 2020 [Google Scholar]
- 20.Drug Updates | CIMS India. [Last accessed on 2020 Oct 1]. Available from: https://www.mims.com/india/latestupdate/list .
- 21.DPCO- National Pharmaceutical pricing authority, 2020. Available from: https://www.nppaindia.nic.in/wp-content/uploads/2020/03/1-1.pdf .
- 22.National List of Essential Medicines of India - 2015. Available from: https://mohfw.gov.in/sites/default/files/NLEM%2C%202015.pdf .
- 23.NPPA has fixed the prices Antidiabetic and Cardiovascular in respect of 108 non-scheduled formulation packs under Paragraph 19 of DPCO, 2013 | Official Website of National Pharmaceutical Pricing Authority, Ministry of Chemicals and Fertilizers, Government of India. Available from: http://www.nppaindia.nic.in/en/14/8090/23/18/01/26/2019/
- 24.Bureau of Pharma PSUs of India (BPPI), Government of India. [Last accessed on 2021 Feb 18]. Available from: http://janaushadhi.gov.in/ProductList.aspx .
- 25.WHO-Defined Daily Dose (DDD). WHO. Available from: http://www.who.int/medicines/regulation/medicines-safety/toolkit_ddd/en/
- 26.Meeta M, Harinarayan CV, Marwah R, Sahay R, Kalra S, Babhulkar S. Clinical practice guidelines on postmenopausal osteoporosis: *An executive summary and recommendations - Update 2019-2020. J Midlife Health. 2020;11:96–112. doi: 10.4103/jmh.JMH_143_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vieth R. Vitamin D supplementation: Cholecalciferol, calcifediol, and calcitriol. Eur J Clin Nutr. 2020;74:1493–97. doi: 10.1038/s41430-020-0697-1. [DOI] [PubMed] [Google Scholar]
- 28.Tripkovic L, Lambert H, Hart K, Smith CP, Bucca G, Penson S, et al. Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: A systematic review and meta-analysis. Am J Clin Nutr. 2012;95:1357–64. doi: 10.3945/ajcn.111.031070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marwaha RK, Dabas A. Bioavailability of nanoemulsion formulations vs conventional fat soluble preparations of cholecalciferol (D3) - An overview. J Clin Orthop Trauma. 2019;10:1094–6. doi: 10.1016/j.jcot.2019.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goswami R, Gupta N, Ray D, Singh N, Tomar N. Pattern of 25-hydroxy vitamin D response at short (2 month) and long (1 year) interval after 8 weeks of oral supplementation with cholecalciferol in Asian Indians with chronic hypovitaminosis D. Br J Nutr. 2008;100:526–9. doi: 10.1017/S0007114508921711. [DOI] [PubMed] [Google Scholar]
- 31.Khadilkar A, Khadilkar V, Chinnappa J, Rathi N, Khadgawat R, et al. From Indian Academy of Pediatrics ‘Guideline for Vitamin D and Calcium in Children’ Committee. Prevention and treatment of Vitamin D and calcium deficiency in children and adolescents: Indian academy of pediatrics (IAP) guidelines. Indian Pediatr. 2017;54:567–73. doi: 10.1007/s13312-017-1070-x. [DOI] [PubMed] [Google Scholar]
- 32.Allan GM, Lexchin J, Wiebe N. Physician awareness of drug cost: A systematic review. PLoS Med. 2007;4:e283. doi: 10.1371/journal.pmed.0040283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gemmill MC, Thomson S, Mossialos E. What impact do prescription drug charges have on efficiency and equity? Evidence from high-income countries. Int J Equity Health. 2008;7:12. doi: 10.1186/1475-9276-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chugh PK, Tripathi C. Spaced education and student learning: Results from a medical school. Clin Teach. 2020;17:655–60. doi: 10.1111/tct.13180. [DOI] [PubMed] [Google Scholar]
- 35.de Vries TPGM, Henning RH, Hogerzeil HV, Fresle DA. WHO Guide to Good Prescribing – A Practical Manual. Geneva. 1994. Available from: https://apps.who.int/iris/bitstream/handle/10665/59001/WHO_DAP_94.11.pdf .


