Abstract
Objectives:
Diagnosis of neuropathic pain is challenging. Recently, scientists developed multiple questionnaires to expedite this diagnosis including the Self-completed Leeds Assessment of Neuropathic Symptoms and Signs (S-LANSS), Douleur Neuropathique 4 questionnaire (DN4), and Neuropathic Pain Questionnaire–Short Form (NPQ-SF).
Materials and Methods:
We conducted a prospective cohort study to compare the psychometric characteristics and accuracy of the three questionnaires. We assessed reliability with the Cronbach's α reliability coefficient and inter-item correlations, and validity with receiver operating characteristic (ROC) and correlation analyses. We assessed agreement between the diagnosis of the questionnaires and the reference clinical diagnosis using Cohen's kappa coefficient.
Results:
188 patients were analyzed: 141 (75%) had “definite neuropathic” and 47 (25%) had “nonneuropathic” pain. The NPQ-SF and S-LANSS questionnaires demonstrated acceptable reliability with Cronbach's α coefficient values of 0.54 (95% CI: 0.41–0.64) and 0.65, (95%CI: 0.57–0.72), respectively. The DN4 questionnaire demonstrated high reliability with Cronbach's α coefficient of 0.74 (95%CI: 0.68–0.79). The NPQ-SF, DN4, and S-LANSS questionnaires demonstrated “excellent” diagnostic ability with an area under the ROC curve of 0.82 (95% CI: 0.75–0.89), 0.89 (95% CI: 0.83–0.95), and 0.83 (95% CI: 0.75–0.90), respectively. Based on their optimal cutoff values, the DN4 had the highest sensitivity and lowest specificity in discriminating between neuropathic and nonneuropathic patients, while the S-LANSS had the lowest sensitivity and highest specificity.
Conclusion:
Both NPQ-SF and S-LANSS demonstrated acceptable reliability, while DN4 demonstrated high reliability. All three demonstrated excellent diagnostic validities; however, it is important to consider the sensitivity and specificity of each.
Keywords: Accuracy, diagnosis, neuropathic pain, questionnaires
Introduction
Chronic pain persists or recurs for more than 3 months, and is traditionally classified into nociceptive or neuropathic.[1] Neuropathic pain is defined as “pain caused by a lesion or disease of the somatosensory nervous system,”[2] and encompasses a variety of peripheral and central nervous system disorders. Peripheral neuropathic pain conditions include post-amputation pain, radiculopathies, trigeminal neuralgia, and postherpetic neuralgia; central neuropathic pain conditions include multiple sclerosis, spinal cord injuries, and central post-stroke pain.[3]
The global prevalence of chronic pain with neuropathic characteristics is between 6.9% and 10%, according to a systematic review by Hecke et al.[4] Studies in that review measured the prevalence of neuropathic pain using multiple neuropathic-pain screening tools including the Self-completed Leeds Assessment of Neuropathic Symptoms and Signs (S-LANSS) questionnaire and the Douleur Neuropathique 4 questionnaire (DN4). Estimates in specific countries were: 8.2% in the UK (using the S-LANSS questionnaire), 6.9% in France (DN4), 8.8% in the United States (S-LANSS), and 10% in Brazil (DN4),[4] as well as 10.6% in Morocco (DN4)[5] and 3.9% in Libya (S-LANSS).[6]
When the underlying pain mechanism is correctly identified, it may be treated with targeted approaches that improve outcomes.[7] This is clinically relevant, as neuropathic pain patients tend to present with more severe pain, higher degrees of impairment in quality of life, poorer outcomes, more comorbidities, and more health-care utilization than those with nociceptive pain.[8,9,10] The accurate diagnosis of neuropathic pain remains a challenging feat, often requiring extensive history taking, clinical examination, and neurological diagnostic evaluation.[11] There is no true “gold standard” of diagnosis, and the disease presents with wide heterogeneity in symptoms, often differing at different stages of disease progression.[11] Work by Boureau et al.[12] (1990) identified several verbal descriptors such as “burning,” “electric shocks,” “itching,” and “tingling” that offered discriminatory function for neuropathic pain, and paved the way for the later development of screening tools aimed at this purpose. Although these pain descriptors are viewed as being suggestive rather than pathognomonic for neuropathic pain, the combination of several descriptors amassed by screening tools offers a high discriminant value for the identification of neuropathic pain, particularly when used by nonspecialists, providing valuable guidance for further assessment.[13]
The clinical evaluation and judgment by an expert physician remain the standard in diagnosing neuropathic pain; however, screening questionnaires can triage patients for a comprehensive diagnostic evaluation.[14] Multiple neuropathic pain questionnaires have been developed including the S-LANSS,[15] DN4,[11] and the Neuropathic Pain Questionnaire–Short Form (NPQ-SF),[16] all of which have been validated and translated into multiple languages.[17,18,19] These questionnaires are recognized as efficient screening tools for neuropathic pain, with high sensitivity and specificity, and cost effectiveness; they are easy to implement and do not require special equipment or investigations.[20]
A few previous studies have compared different neuropathic pain questionnaires (e.g. DN4, S-LANSS, Pain-DETECT, ID-Pain),[21,22,23] but none compared the DN4, NPQ-SF, and S-LANSS questionnaires among the same population. The aim of this study was to compare these three questionnaires in terms of reliability and psychometric validity.
Methods
This prospective cohort study was conducted between March 2018 and September 2019 in two tertiary care and referral hospitals in Saudi Arabia: King Faisal Specialist Hospital and Research Center (KFSH and RC), in Riyadh and Jeddah. The study protocol was approved by the Institutional Review Board of KFSH and RC (No. 2171175), and informed consent was taken and documented from each patient before any measures were initiated.
Neuropathic pain was defined as any “pain caused by a lesion or disease of the somatosensory nervous system” as per the International Association for the Study of Pain (IASP).[2] We used the revised grading system of “possible,” “probable,” and “definite” neuropathic pain, proposed by the Neuropathic Pain Special Interest Group (NeuPSIG) of the IASP.[13] For the purpose of this study, only patients who fit the criteria of “definite” neuropathic pain were considered to have NP, as follows:
A “history of relevant neurological lesion or disease” and a “neuroanatomically plausible pain distribution”
“Pain associated with sensory signs in the same neuroanatomically plausible distribution”
“Diagnostic test confirming a lesion or disease of the somatosensory nervous system explaining the pain.”[13]
The inclusion criteria consisted of patients aged between 19 and 80 years, having adequate level of understanding of the questionnaire, suffering from persistent or recurrent chronic pain for ≥3 months that is either of nociceptive or “definite” neuropathic pain type, and confirmed by two expert pain physicians. The exclusion criteria comprised patients who refused to participate in the study, were unable to understand the Arabic language, had “possible” or “probable” neuropathic pain, suffered from significant hearing impairment, and/or lacked a testamentary capacity (e.g., psychosis and Alzheimer's disease).
Measures
Numeric pain rating scale (NRS-11)
Composed of an 11-point numerical scale ranging from 0 to 10, it is the simplest and most commonly used scale for measuring perceived pain intensity, where 0 is “no pain at all” and 10 is “worst pain imaginable” at the time of the interview.[24]
Douleur neuropathique 4 questions (DN4)
Initially developed and validated in the French language in 2005, it is a 10-item measure used to assess neuropathic pain.[11] The first seven items of the questionnaire evaluate sensory characteristics (e.g. burning, painful cold, electric shocks, tingling, pins and needles, numbness, and itching), whereas the remaining three items are related to clinical examination (hypoesthesia to touch and prick, and pain caused or increased by brushing).[11] Each item is scored as either 0 (“no” response) or 1 (“yes” response), with a total score calculated by summing of the scores of the 10 items, and a cutoff score of ≥4 suggesting neuropathic pain.[11] The questionnaire demonstrated 83% sensitivity and 90% specificity compared to the clinical diagnosis in the original study. The DN4 has been translated and validated into the Arabic language and showed an excellent diagnostic accuracy with an area under ROC curve (AUC) of 0.88, and a sensitivity and specificity of 88.3% and 74.5%, respectively, at a cutoff value of 4.[17] We used the Arabic validated version from this questionnaire.
Neuropathic pain questionnaire-short form (NPQ-SF)
The original NPQ was developed in 2003 and consisted of 12 items (10 sensory, 2 affect items) evaluating neuropathic pain.[25] A discriminant analysis of the original version yielded a three-item questionnaire assessing tingling pain, numbness, and increased pain to touch, while preserving the original questionnaire's discriminating ability.[16] Each item is scored from 0 (no pain) to 100 (worst pain imaginable), and multiplied by a discriminant function coefficient (tingling: 0.017, numbness: 0.015, increased pain to touch: 0.011). The total sum of these is added to a constant value (−1.302) to yield a final score, with a cutoff of ≥0 suggesting neuropathic pain.[16] The NPQ-SF accurately identified 73% of the pain diagnoses with a sensitivity and specificity of 64.5% and 78.6%, respectively. The questionnaire has also been translated and validated into the Arabic language, and showed an AUC of 0.76, sensitivity of 86%, and specificity of 51% at a cutoff value of 0.[18] However, a cutoff of 0.52 was recommended by the authors who conducted the Arabic translation and validation of the NPQ-SF as a better value in discriminating between patients with and without neuropathic pain.[18] We used the Arabic validated version from this questionnaire.
Self-completed Leeds assessment of neuropathic symptoms and signs (S-LANSS)
The S-LANSS is a seven-item scale used to evaluate pain of predominantly neuropathic origin.[15] It was developed in 2005 and contains five questions querying sensory symptoms (skin sensations, skin appearance, increased sensitivity to touch, sudden bursts of electric shock sensation, and hot/burning skin sensations) and two sensory self-examination items (allodynia and altered pinprick threshold).[15] In addition, the self-completed score was modified over the original to include a body map for pain location, and an 11-point numeric scale querying pain intensity over the previous week. A total score of ≥12 suggested pain of predominantly neuropathic origin.[15] When used in an interview format, the S-LANSS correctly classified 80% of patients to their diagnostic group, with a sensitivity of 74% and specificity of 76% at a cutoff value of 12. The questionnaire has been translated, validated, and culturally adapted for use in Arabic populations; however, no data is available at the time of this study in the literature regarding the sensitivity and specificity of the Arabic version of the S-LANSS.[19] We used the Arabic validated version from this questionnaire.
Study protocol
We explained the purpose of the study to the patients and documented verbal consent. Then two expert pain physicians evaluated and confirmed the diagnosis. The NRS-11, along with Arabic validated versions of the DN4, NPQ-SF, and S-LANSS questionnaires[17,18,19] were administered to patients in an interview at the pain clinic by the same person and during the same setting. The targeted sample was composed of two groups: chronic pain patients with “definite” neuropathic pain and patients with nociceptive type of chronic pain.
Statistical analysis
Data were analyzed using IBM-SPSS Statistics for Windows (version 23.0). Counts and percentages were reported for categorical variables and range; median, mean and standard deviation, or 95% confidence interval for continuous/ordinal data as appropriate. A Spearman's rank correlation coefficient was utilized to evaluate associations between continuous scores of the measurements. The Kruskal–Wallis or Wilcoxon rank-sum test was applied accordingly to compare screening-tool scores between neuropathic and nonneuropathic groups.
Reliability
Internal consistency of the DN4, NPQ-SF, and S-LANSS questionnaires were examined using Cronbach's α reliability coefficient, ranging from 0 (no internal consistency; none of the items is correlated with each other) to 1 (perfect internal consistency; all of the items are perfectly correlated with each other).[26,27] Since the size of α is dependent on the number of items in the scale, a larger number of items can result in a larger α, and vice versa.[26,27] Generally, Cronbach's alpha is widely interpreted as excellent if ≥0.9; high if 0.9> α ≤ 0.7, moderate if 0.7> α ≤0.6, acceptable 0.6> α ≤0.5, and low if <0.5.[28]
Inter-item correlations, or “the extent to which items on a scale are assessing the same content,” of the DN4, NPQ-SF, and S-LANSS questionnaires were examined using Spearman's correlation coefficient (r). An average between 0.2–0.4 of inter-item correlation for a set of items typically suggests that the items are reasonably homogenous while containing sufficient variance so as to not be isomorphic with each other.[29]
Validity
The diagnostic validity of the three neuropathic pain questionnaires (DN4, NPQ-SF, and S-LANSS) was assessed using receiver operating characteristic (ROC) analysis. The area under ROC curve (AUC) was used to depict the ability of the test to discriminate between the two diagnostic groups (NP and Non-NP) independent of cutoff points, with a value of ≤0.50 considered as “negative,” 0.51–0.70 as “poor,” 0.71–0.80 as “acceptable,” 0.81–0.90 as “excellent,” and >0.90 as “outstanding” discrimination.[30] The optimum cutoff diagnostic values for each of the three questionnaires were determined by means of the Youden index (sensitivity + specificity − 1).[31] The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were evaluated using the original diagnostic cutoffs for each of the three questionnaires.
The agreement between the diagnosis of the questionnaires and the reference clinical diagnosis was examined using Cohen's kappa coefficient.[32] A kappa value of 0 signified poor agreement, 0.01–0.20 as slight agreement, 0.21–0.40 as fair agreement, 0.41–0.60 as moderate agreement, 0.61–0.80 as substantial agreement, and >0.81 as almost perfect agreement.[27,32]
To establish construct validity or “the extent to which a test measures what it claims to be measuring,” the strength of associations between the DN4, NPQ-SF, and S-LANSS questionnaires, and NRS-11 were evaluated using Spearman's correlation coefficient (r), where r < 0.3 was considered to be poor, fair if 0.3 ≥ r < 0.6, moderate if 0.6 ≥ r < 0.8, very strong if 0.8 ≥ r < 0.9, and perfect if r > 0.9.[27,33] The concurrent validity, or “the extent to which a test correlates with other measures of the same construct that are measured at the same time,” of the questionnaires was examined by investigating their correlations among each other, using Spearman's correlation coefficient (r).
Sample size calculation
The power calculations were done in Power and Sample Size Calculation (PS) software (version 3.1.6). Data from an earlier study of pain assessment tools indicated an inter-rater reliability of 0.30–0.63 as measured by the Intra-class correlation (ICC). The standard deviation was estimated as 0.25. A sample size of 180 was deemed sufficient to achieve 80% power and to detect a difference of 0.10 between the null hypothesis ICC of 0.60 and the alternative hypothesis ICC of 0.70 using a t-test with a significance level of 0.05.
Results
A total of 204 patients were approached and agreed to participate in the study. Sixteen patients were excluded, among which, 4 patients had their responses recorded twice on different occasions, 3 patients had an incomplete recorded data, and the remaining 9 patients had mixed pain with a “probable neuropathic pain” diagnosis (5 with fibromyalgia, 3 with complex regional pain syndrome, and 1 with unknown etiology).
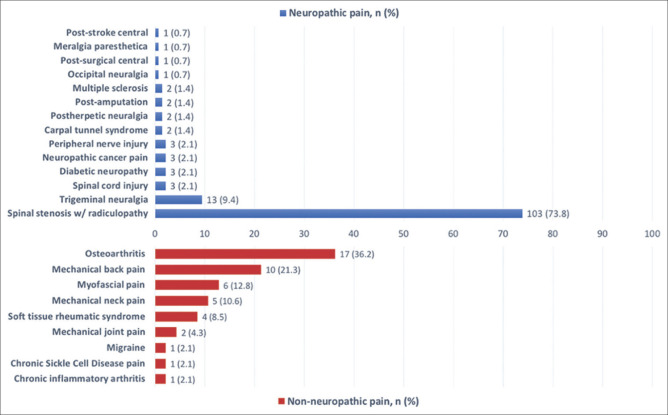
The remaining 188 patients were included in the analysis, of whom 141 (75%) had “definite neuropathic pain” diagnosis and 47 (25%) had nonneuropathic pain. There was no statistically significant difference between the demographic characteristics of the two groups [Table 1]. Figure 1 summarizes the causes of pain in the neuropathic and nonneuropathic pain groups. Neuropathic pain patients had a lower prevalence of comorbidities and a higher mean duration of pain compared to the nonneuropathic group. Spinal stenosis with radiculopathy (73.1%) was the most frequent clinical diagnosis among the neuropathic pain patients, while osteoarthritis (36.2%) was the most common among the nonneuropathic group [Table 1].
Table 1.
Demographics and clinical characteristics of patients in the neuropathic and nonneuropathic pain groups
| NP (n=141) | NNP (n=47) | |
|---|---|---|
| Age (years), mean±SD | 53.3±12.5 | 53.2±13.5 |
| Female gender, n (%) | 82 (58.2) | 26 (55.3) |
| BMI (Kg/m²), mean±SD | 31.7±7.2 | 34.0±6.8 |
| Education (lifetime) ≤9 y, n (%) | 39 (27.7) | 11 (23.4) |
| Occupation, n (%) | ||
| Unemployed | 49 (34.8) | 17 (36.2) |
| Retired | 40 (28.4) | 10 (21.3) |
| Marital status, n (%) | ||
| Single | 12 (8.5) | 3 (6.4) |
| Widowed | 7 (5.0) | 0 (0.0) |
| Associated chronic diseases, n (%) | 98 (69.5) | 38 (80.9) |
| American society of anesthesiologists physical status (ASA score), n (%) | ||
| 4 | 1 (0.7) | 0 (0) |
| 3 | 34 (24.1) | 8 (17.0) |
| 2 | 85 (60.3) | 32 (68.1) |
| 1 | 21 (14.9) | 7 (14.9) |
| Pain-relief Medication, n (%) | 133 (94.3) | 45 (95.7) |
| Symptom duration (m), mean±SD (min-max) | 101.1±95.8 (3-468) | 58.0±59.9 (3-240) |
NP=Neuropathic pain, NNP=Nonneuropathic pain, SD=Standard deviation, n=number of patients, BMI=Body mass index, y=years, m=months
Figure 1.
Etiology of pain in the neuropathic and nonneuropathic pain study groups
Table 2 illustrates the frequency of pain descriptors of the NPQ-SF, DN4, and S-LANSS questionnaires for the neuropathic and nonneuropathic pain groups. The questionnaires’ mean total and item scores were statistically significantly higher for the neuropathic pain group (P < 0.05), with the exception of the “increased pain to touch” item on the NPQ-SF, “itching” and “brushing” items on the DN4, and “autonomic changes” and “pain evoked by light touch” items on the S-LANSS.
Table 2.
Frequency of pain descriptors in the neuropathic and nonneuropathic pain groups
| NP (n=141) | NNP (n=47) | P | |||
|---|---|---|---|---|---|
|
|
|
||||
| n (%) | Mean Score±SD (95% CI) | n (%) | Mean Score±SD (95% CI) | ||
| NPQ-SF items | |||||
| Tingling | 111 (78.7) | 0.90±0.59 (0.81-1.0) | 16 (34.0) | 0.35±0.55 (0.18-0.51) | <0.001 |
| Numbness | 126 (89.4) | 0.84±0.44 (0.76-0.91) | 17 (36.2) | 0.25±0.39 (0.14-0.37) | <0.001 |
| Increased pain to touch | 58 (41.1) | 0.35±0.45 (0.27-0.42) | 16 (34.0) | 0.28±0.41 (0.16-0.40) | 0.32 |
| Total score | 0.79±1.07 (0.61-0.97) | -0.43±0.82 (-0.67-0.19) | <0.001 | ||
| DN4 items | |||||
| Burning | 97 (68.8) | 14 (29.8) | |||
| Painful cold | 35 (24.8) | 6 (12.8) | |||
| Electric shocks | 112 (79.4) | 7 (14.9) | |||
| Tingling | 113 (80.1) | 11 (23.4) | |||
| Pins and needles | 106 (75.2) | 16 (34.0) | |||
| Numbness | 124 (87.9) | 17 (36.2) | |||
| Itching | 36 (25.5) | 7 (14.9) | |||
| Hypoesthesia to touch | 76 (53.9) | 5 (10.6) | |||
| Hypoesthesia to pinprick | 80 (56.7) | 2 (4.3) | |||
| Brushing | 51 (36.2) | 16 (34.0) | |||
| Total score | 5.89±1.96 (5.56-6.21) | 2.15±2.05 (1.55-2.75) | <0.001 | ||
| S-LANSS items | |||||
| Pins and needles, shocks, tingling | 129 (91.5) | 22 (46.8) | |||
| Autonomic changes | 16 (11.3) | 6 (12.8) | |||
| Pain evoked by light touching | 84 (59.6) | 25 (53.2) | |||
| Electric shocks or shooting | 98 (69.5) | 13 (27.7) | |||
| Hot or burning | 94 (66.7) | 14 (29.8) | |||
| Allodynia | 103 (73.0) | 10 (21.3) | |||
| Raised pin prick threshold | 82 (58.2) | 6 (12.8) | |||
| Total score | 14.38±5.41 (13.48-15.28) | 6.87±5.96 (5.12-8.62) | <0.001 | ||
| NRS-11, mean±SD | 6.4±2.2 | 5.9±2.3 | |||
NP=Neuropathic pain, NNP=Nonneuropathic pain, SD=Standard deviation, N=Number of patients
Reliability
The NPQ-SF and S-LANSS questionnaires demonstrated acceptable reliability with Cronbach's α coefficient values of 0.54 (95%CI: 0.41–0.64) and 0.65 (95%CI: 0.57–0.72), respectively, while the DN4 questionnaire had an α coefficient of 0.74 (95%CI: 0.68–0.79), demonstrating high reliability [Table 3].
Table 3.
Analysis of the internal consistency and inter-item correlations of the neuropathic pain questionnaires
| Cronbach’s alpha | Inter-item correlation | Cronbach’s α if item deleted | |
|---|---|---|---|
| NPQ-SF | 0.535 | Mean=0.270 | |
| Tingling | 0.413 | 0.330 | |
| Numbness | 0.540 | 0.121 | |
| Increased pain to touch | 0.143 | 0.691 | |
| DN4 | 0.739 | Mean=0.215 | |
| Burning | 0.326 | 0.730 | |
| Painful cold | 0.125 | 0.753 | |
| Electric shocks | 0.564 | 0.691 | |
| Tingling | 0.657 | 0.677 | |
| Pins and needles | 0.466 | 0.708 | |
| Numbness | 0.574 | 0.693 | |
| Itching | 0.170 | 0.748 | |
| Hypoesthesia to touch | 0.554 | 0.693 | |
| Hypoesthesia to pinprick | 0.585 | 0.687 | |
| Brushing | 0.009 | 0.774 | |
| S-LANSS | 0.650 | Mean=0.241 | |
| Pins and needles, shocks, tingling | 0.436 | 0.589 | |
| Autonomic changes | 0.149 | 0.674 | |
| Pain evoked by light touching | 0.330 | 0.623 | |
| Electric shocks or shooting | 0.406 | 0.615 | |
| Hot or burning | 0.453 | 0.634 | |
| Allodynia | 0.530 | 0.555 | |
| Raised pinprick threshold | 0.496 | 0.575 |
Validity
Diagnostic validity
The NPQ-SF, DN4, and S-LANSS questionnaires demonstrated “excellent” diagnostic ability with an AUC of 0.82 (95% CI: 0.75–0.89), 0.89 (95% CI: 0.83–0.95), and 0.83 (95% CI: 0.75–0.90), respectively [Figure 2]. Further discriminant statistics (sensitivity, specificity, positive and negative predictive values) using the optimum cutoff values of the questionnaires are presented in Table 4. The DN4 had the highest sensitivity and lowest specificity in discriminating between NP and non-NP patients, while the S-LANSS had the lowest sensitivity and highest specificity, compared to the other two questionnaires.
Figure 2.
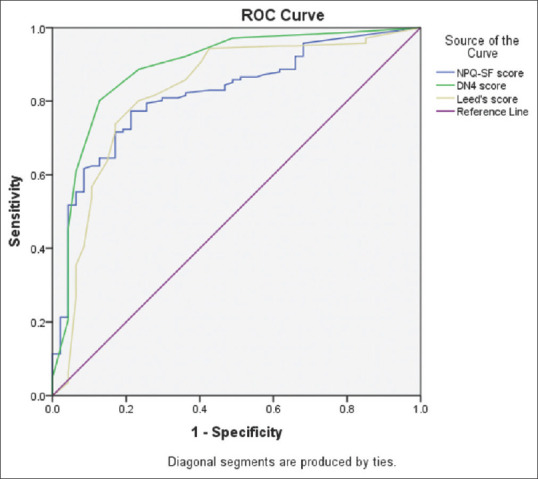
ROC curves of the neuropathic pain questionnaires: Plot of sensitivity versus 1-specificity. ROC = Receiver operating characteristic
Table 4.
Accuracy and diagnostic validity of the neuropathic pain questionnaires
| NPQ-SF | DN4 | S-LANSS | |
|---|---|---|---|
| Area Under Curve | 0.82 (0.75-0.89) | 0.89 (0.83-0.95) | 0.83 (0.75-0.90) |
| Cutoff point | ≥0 | ≥4 | ≥12 |
| Sensitivity | 77% | 89% | 74% |
| Specificity | 79% | 77% | 83% |
| PPV | 92% | 92% | 93% |
| NPV | 54% | 69% | 51% |
| Cohen’s Kappa | 0.48 (0.41-0.58) | 0.64 (0.41-0.66) | 0.47 (0.33-0.58) |
PPV=Positive predictive value, NPV=Negative predictive value
The diagnostic cutoff points for each of the three questionnaires, determined using the Youden index, mirrored the original cutoff values. There was a “substantial” diagnostic concordance between the DN4 questionnaire and the expert-led clinical diagnosis, with a Cohen's kappa coefficient of 0.64 (95% CI: 0.41–0.66). In contrast, the NPQ-SF and S-LANSS questionnaires had kappa coefficients values of 0.48 (0.41–0.55) and 0.48 (0.41–0.58), respectively, demonstrating only “fair” agreement [Table 4].
Construct validity
The construct validity of the NPQ-SF, DN4, and S-LANSS questionnaires was assessed by examining their correlation with the NRS. The NRS score was “fairly” correlated with the total score of the NPQ-SF (r = 0.31, P < 0.001), and “weakly” correlated with the scores of the DN4 (r = 0.23, P < 0.001) and S-LANSS questionnaires (r = 0.26, P < 0.001) [Table 5].
Table 5.
Spearman rank correlation coefficient between NP questionnaires
| NPQ-SF items | DN4 | S-LANSS | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Tingling | Numbness | Pain to touch | Total | |||
| NPQ-SF items | ||||||
| Numbness | 0.538* | |||||
| Pain to touch | 0.06*** | 0.186** | ||||
| Total | 0.810* | 0.811* | 0.473* | |||
| DN4 | 0.522* | 0.623* | 0.322* | 0.707* | ||
| S-LANSS | 0.518* | 0.608* | 0.348* | 0.712* | 0.690* | |
| NRS | 0.168** | 0.285* | 0.268* | 0.314* | 0.227* | 0.257* |
*P<0.05, **P<0.01, ***P<0.001
The concurrent validity of the NPQ-SF, DN4, and S-LANSS questionnaires was assessed by examining their correlation with one another. The score of NPQ-SF was moderately correlated with those of the DN4 (r = 0.71, P < 0.001) and S-LANSS questionnaires (r = 0.71, P < 0.001, respectively). Similarly, the score of the DN4 was moderately correlated with that of the S-LANSS questionnaire (r = 0.69, P < 0.001) [Table 5].
Discussion
This study demonstrated the reliability, validity, and high sensitivity and specificity of the S-LANSS, DN4, and NPQ-SF questionnaires in discriminating between neuropathic and nonneuropathic types of chronic pain. These findings are consistent with previous reports on the Arabic,[17,18] English,[21,34] Turkish,[22,35] and Chinese[23] versions of the questionnaires. The DN4 scale was revealed to be the most sensitive (identifying neuropathic pain in 89% of the neuropathic patients), while the S-LANSS was the least sensitive (identifying neuropathic pain in 74% of the neuropathic patients). This corroborated the findings in literature of these scales enabling the diagnosis of neuropathic pain in up to 80% of the cases.[21,34]
Our analysis showed that, identical to the original versions, the best cutoff values for discriminating between neuropathic and nonneuropathic patients of the DN4, S-LANSS, and NPQ-SF questionnaires were “4,” “12,” and “0” [Figure 3], respectively. The last cutoff was different than the recommended cutoff score of 0.52 of the Arabic NPQ-SF by Terkawi et al.[18]
Figure 3.
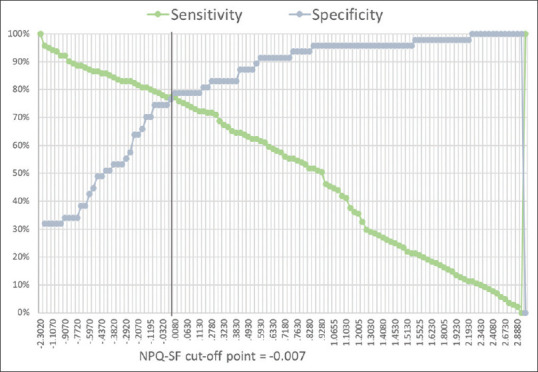
The optimum cutoff point for discriminating between neuropathic and nonneuropathic pain patients (NPQ-SF)
The three questionnaires share similar key verbal descriptors, which likely explains the moderate correlation between them. However, they also have important differences that may explain the higher sensitivity of the DN4 and lower sensitivity of S-LANSS. The DN4 questionnaire included clinical examination items, whereas the S-LANSS and the NPQ-SF did not. In addition, the items on the DN4 are relatively short compared to the S-LANSS, and pain descriptors are distinctively distributed across the questionnaire, unlike those on the S-LANSS, where multiple descriptors are often grouped together on the same item. We believe this might have contributed to the better comprehension of the DN4 question items by our patients, compared to the S-LANSS. Finally, the S-LANSS item number 4 (relating to the feeling of electric shocks) and number 5 (relating to hot or burning sensations) are weighted lower than item number 2 (querying autonomic dysfunction). However, items 4 and 5 were more commonly reported by our neuropathic pain group, whereas item 2 was less commonly reported.
The internal consistency of the S-LANSS (α =0.65), DN4 (α =0.74), and NPQ-SF (α =0.54) were comparable with those revealed by previous work on the Arabic versions of the questionnaires (S-LANSS: α =0.72, DN4: α =0.7, and NPQ-SF: α =0.48).[17,18,19] A low value of α coefficient could be due to a number of reasons including low number of items, poor interrelatedness between items, or items measuring heterogenous constructs.[36] This might explain the lower α coefficient value of the NPQ-SF (0.54) since this questionnaire has just three items, whereas the S-LANSS and DN4 have seven and ten items, respectively.
The mean inter-item correlation among the items of the NPQ-SF was relatively low (0.27), which upon further inspection is believed to be attributed to the poor correlation of the third item (increased pain to touch) with the other two items (tingling and numbness) on the scale [Table 3]. Looking at the inter-item correlation scores of the corresponding items on the DN4 (item #10: Brushing) and S-LANSS (Item #3: Pain evoked by light touch) questionnaires revealed similar findings of lower interrelatedness with the remaining items on the respective scales, suggesting that these three items might be assessing a different construct of neuropathic pain, rather than being attributed to erroneous translation of the questionnaire, or misapprehension by the patients. Likewise, items #2 (painful cold sensation) and #7 (itching sensation) on the DN4 questionnaire, and item #2 (autonomic changes) on the S-LANSS questionnaire also had lower corrected item-total correlations compared to the rest of the items on the respective questionnaires, as shown in Table 3. This highlights the possibility of neuropathic pain having heterogenous components that might not essentially be correlated with one another and/or might be attributed to different underlying mechanisms.
Research corroborates that neuropathic pain is a heterogenous syndrome comprised of several independent dimensions, namely, spontaneous paroxysmal/ongoing pain, evoked pain, and paresthesia/dysesthesia.[37,38] Tingling, numbness, and itching sensations are frequently grouped together under the paresthesia/dysesthesia dimension of neuropathic pain.[37,38] In contrast, allodynia or increased pain to mechanical or thermal stimuli falls under the evoked pain dimension and is generally less reported compared to the other neuropathic pain symptoms.[38] Other infrequently reported neuropathic pain symptoms include cold (spontaneous) and itchy (paresthesia/dysesthesia) sensations.[38,39] These findings mirrored those of our study, where “increased pain to touch” was the least reported item by the neuropathic pain group on the NPQ-SF (41%). The “painful cold” (25%) and “itchy” (26%) items of the DN4, and the “autonomic changes” (11%) item of the S-LANSS questionnaire also shared the same state [Table 2]. Expectedly, when deleting the implicated items from their respective questionnaires, the α coefficient value increased, demonstrating better reliability, as shown in Table 3.
In summary, our results indicated an acceptable reliability for the S-LANSS and NPQ-SF, high reliability for the DN4, and excellent diagnostic ability for all three questionnaires. We found the best diagnostic cutoffs for NPQ-SF, DN4, S-LANSS are ≥0, ≥4, and ≥12, respectively, which are identical with the original questionnaires’ validations. Based on these cutoff values, the DN4 had the highest sensitivity and lowest specificity in discriminating between NP and non-NP patients, while the S-LANSS had the lowest sensitivity and highest specificity. It is important to consider the levels of sensitivity and specificity of the questionnaire that would justify its intended use for clinical or research purposes.
Disclosure
Dr Terkawi received an NIH T32 grant (Number DA 035165).
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We would like to thank: Dr. Kristin Sainani, PhD, Professor with Health Research and Policy at Stanford University for reviewing this manuscript.
References
- 1.Treede R-D, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, et al. A classification of chronic pain for ICD-11. Pain. 2015;156:1003–7. doi: 10.1097/j.pain.0000000000000160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.International Association for the Study of Pain (IASP) terminology. Iasp. 2017 [Google Scholar]
- 3.Costigan M, Scholz J, Woolf CJ. Neuropathic pain: A maladaptive response of the nervous system to damage. Annu Rev Neurosci. 2009;32:1–32. doi: 10.1146/annurev.neuro.051508.135531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Hecke O, Austin SK, Khan RA, Smith BH, Torrance N. Neuropathic pain in the general population: A systematic review of epidemiological studies. Pain. 2014;155:654–62. doi: 10.1016/j.pain.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 5.Harifi G, Amine M, Ait Ouazar M, Boujemaoui A, Ouilki I, Rekkab I, et al. Prevalence of chronic pain with neuropathic characteristics in the moroccan general population: A national survey. Pain Med. 2013;14:287–92. doi: 10.1111/pme.12009. [DOI] [PubMed] [Google Scholar]
- 6.Elzahaf RA, Johnson MI, Tashani OA. The epidemiology of chronic pain in Libya: A cross-sectional telephone survey. BMC Public Health. 2016;16:776. doi: 10.1186/s12889-016-3349-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woolf CJ. Pain: Moving from symptom control toward mechanism-specific pharmacologic management. Ann Intern Med. 2004;140:441–51. doi: 10.7326/0003-4819-140-8-200404200-00010. [DOI] [PubMed] [Google Scholar]
- 8.Smith BH, Torrance N. Epidemiology of neuropathic pain and its impact on quality of life. Curr Pain Headache Rep. 2012;16:191–8. doi: 10.1007/s11916-012-0256-0. [DOI] [PubMed] [Google Scholar]
- 9.Torrance N, Smith BH, Bennett MI, Lee AJ. The epidemiology of chronic pain of predominantly neuropathic origin. Results from a general population survey. J Pain. 2006;7:281–9. doi: 10.1016/j.jpain.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 10.Attal N, Lanteri-Minet M, Laurent B, Fermanian J, Bouhassira D. The specific disease burden of neuropathic pain: Results of a French nationwide survey. Pain. 2011;152:2836–43. doi: 10.1016/j.pain.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 11.Bouhassira D, Attal N, Alchaar H, Boureau F, Brochet B, Bruxelle J, et al. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4) Pain. 2005;114:29–36. doi: 10.1016/j.pain.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 12.Boureau F, Doubrère JF, Luu M. Study of verbal description in neuropathic pain. Pain. 1990;42:145–52. doi: 10.1016/0304-3959(90)91158-F. [DOI] [PubMed] [Google Scholar]
- 13.Finnerup NB, Haroutounian S, Kamerman P, Baron R, Bennett DL, Bouhassira D, et al. Neuropathic pain: An updated grading system for research and clinical practice. Pain. 2016;157:1599–606. doi: 10.1097/j.pain.0000000000000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bouhassira D, Attal N. Diagnosis and assessment of neuropathic pain: The saga of clinical tools. Pain. 2011;152(3 Suppl):S74–83. doi: 10.1016/j.pain.2010.11.027. [DOI] [PubMed] [Google Scholar]
- 15.Bennett MI, Smith BH, Torrance N, Potter J. LANSS score for identifying pain of predominantly neuropathic origin: Validation for use in clinical and postal research. J Pain. 2005;6:149–58. doi: 10.1016/j.jpain.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 16.Backonja MM, Krause SJ. Neuropathic pain questionnaire-Short form. Clin J Pain. 2003;19:315–6. doi: 10.1097/00002508-200309000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Terkawi AS, Abolkhair A, Didier B, Alzhahrani T, Alsohaibani M, Terkawi YS, et al. Development and validation of Arabic version of the douleur neuropathique 4 questionnaire. Saudi J Anaesth. 2017;11(Suppl 1):S31–9. doi: 10.4103/sja.SJA_97_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Terkawi AS, Backonja MM, Abolkhair A, Almaharbi S, Joy J, Foula F, et al. Development and validation of Arabic version of the neuropathic pain questionnaire-short form. Saudi J Anaesth. 2017;11(Suppl 1):S31–9. doi: 10.4103/sja.SJA_86_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elzahaf RA, Tashani OA, Unsworth BA, Johnson MI. Translation and linguistic validation of the Self-completed leeds assessment of neuropathic symptoms and signs (S-LANSS) scale for use in a libyan population. Pain Pract. 2013;13:198–205. doi: 10.1111/j.1533-2500.2012.00576.x. [DOI] [PubMed] [Google Scholar]
- 20.Magrinelli F, Zanette G, Tamburin S. Neuropathic pain: Diagnosis and treatment. Pract Neurol. 2013;13:292–307. doi: 10.1136/practneurol-2013-000536. [DOI] [PubMed] [Google Scholar]
- 21.Bisaga W, Dorazil M, Dobrogowski J, Wordliczek J. A comparison of the usefulness of selected neuropathic pain scales in patients with chronic pain syndromes: A short communication. Adv Pall Med. 2010;9:117–22. [Google Scholar]
- 22.Unal-Cevik I, Sarioglu-Ay S, Evcik D. A comparison of the DN4 and LANSS questionnaires in the assessment of neuropathic pain: Validity and reliability of the turkish version of DN4. J Pain. 2010;11:1129–35. doi: 10.1016/j.jpain.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 23.Li J, Feng Y, Han J, Fan B, Wu D, Zhang D, et al. Linguistic adaptation, validation and comparison of 3 routinely used neuropathic pain questionnaires. Pain Physician. 2012;15:179–86. [PubMed] [Google Scholar]
- 24.Jensen MP, Karoly P. Self-Report Scales and Procedures for Assessing Pain in Adults. Handbook of Pain Assessment. 2010 [Google Scholar]
- 25.Krause SJ, Backonja MM. Development of a neuropathic pain questionnaire. Clin J Pain. 2003;19:306–14. doi: 10.1097/00002508-200309000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Gliem JA, Gliem RR. Calculating, interpreting, and reporting Cronbach's alpha reliability coefficient for Likert. Type scales. 2003 Midwest Res to Pract Conf Adult, Contin Community Educ. 2003 [Google Scholar]
- 27.Tsang S, Royse CF, Terkawi AS. Guidelines for developing, translating, and validating a questionnaire in perioperative and pain medicine. Saudi J Anaesth. 2017;11(Suppl 1):S80–9. doi: 10.4103/sja.SJA_203_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taber KS. The use of Cronbach's alpha when developing and reporting research instruments in science education. Res Sci Educ. 2018;48:1273–96. [Google Scholar]
- 29.Piedmont RL. Inter-item correlations. Encyclopedia of Quality of Life and Well-Being Research. 2014 [Google Scholar]
- 30.Scott AJ, Hosmer DW, Lemeshow S. Applied logistic regression. Biometrics. (3rd ed) 1991:177. [Google Scholar]
- 31.Fluss R, Faraggi D, Reiser B. Estimation of the Youden index and its associated cutoff point. Biom J. 2005;47:458–72. doi: 10.1002/bimj.200410135. [DOI] [PubMed] [Google Scholar]
- 32.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–74. [PubMed] [Google Scholar]
- 33.Akoglu H. User's guide to correlation coefficients. Turk J Emerg Med. 2018;18:91–3. doi: 10.1016/j.tjem.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bennett MI, Attal N, Backonja MM, Baron R, Bouhassira D, Freynhagen R, et al. Using screening tools to identify neuropathic pain. Pain. 2007;127:199–203. doi: 10.1016/j.pain.2006.10.034. [DOI] [PubMed] [Google Scholar]
- 35.Yurdakul OV, Rezvani A, Küçükakkaş O, Tolu S, Kılıçoğlu MS, Aydın T. Neuropathic pain questionnaire and neuropathic pain questionnaire-short form: Translation, reliability, and validation study of the Turkish version. Turk Neurosurg. 2019;29:683–8. doi: 10.5137/1019-5149.JTN.25466-18.1. [DOI] [PubMed] [Google Scholar]
- 36.Tavakol M, Dennick R. Making sense of Cronbach's alpha. Int J Med Educ. 2011;2:53–5. doi: 10.5116/ijme.4dfb.8dfd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Colloca L, Ludman T, Bouhassira D, Baron R, Dickenson AH, Yarnitsky D, et al. Neuropathic pain. Nat Rev Dis Prim. 2017;3:17002. doi: 10.1038/nrdp.2017.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bouhassira D, Attal N, Fermanian J, Alchaar H, Gautron M, Masquelier E, et al. Development and validation of the neuropathic pain symptom inventory. Pain. 2004;108:248–57. doi: 10.1016/j.pain.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 39.Backonja MM, Stacey B. Neuropathic pain symptoms relative to overall pain rating. J Pain. 2004;5:491–7. doi: 10.1016/j.jpain.2004.09.001. [DOI] [PubMed] [Google Scholar]