Abstract
Forty-five ovine and caprine nonenterotoxigenic Escherichia coli strains producing F17-related fimbriae were characterized with respect to the fimbrial structural subunit and adhesin subtypes produced. In addition, several characteristics related to the virulence of strains producing F17 fimbriae were studied. Most of the strains (73%) possessed the f17cA structural subunit gene, whereas the f17aA and f17dA genes were detected only on three (6%) and two (4%) strains, respectively. The f17bA gene was not detected. All but one of these strains possessed the f17G genes of the adhesin subfamily II. The only strain having the f17G gene of subfamily I possessed the structural subunit gene f17dA. Sequencing of the f17A and f17G genes of four selected strains confirmed the association of f17cA and f17dA structural subunit genes with the f17G genes of the adhesin subfamily II. These results indicated that adhesins of the subfamily II are prominent among ovine and caprine isolates and that they are indistinctly associated with the F17 structural subunit subtypes on these field strains. CS31A- and CNF2-related genes were not detected. Most of the strains adhered in vitro to ovine intestinal brush borders (36 of 45) and agglutinated the erythrocytes of different species in the presence of d-mannose (39 of 45). F17-positive strains produced colicin V (57%) and were resistant to the bactericidal effect of serum (91%) in significantly higher percentages than F17-negative strains (34% produced colicin V, and 66% were serum resistant). Thus, most of the studied ovine and caprine strains showed phenotypic characteristics of septicemic strains.
Escherichia coli strains producing fimbriae of the F17 family cause intestinal and extraintestinal disease in animals and humans. This family of fimbriae includes the F17a fimbriae, isolated from bovine enterotoxigenic E. coli (ETEC) strains (14), the F17b fimbriae, expressed by E. coli strains isolated from septicemic calves and lambs (11), the F17c fimbriae, formally called 20K and associated with bovine diarrhea or septicemia and with lambs showing nephrosis (3), the F17d fimbriae, previously described on bovine ETEC as F111 fimbriae (2), and the G fimbriae of human uropathogenic E. coli strains (18), which are identical to the F17c fimbriae (17).
The F17 fimbriae mediate binding to N-acetylglucosamine-containing receptors present on intestinal mucosal cells, agglutination of bovine erythrocytes, and in vitro adhesion to bovine intestinal brush borders or to the human Caco-2 cell line (3). Functional F17 fimbriae are composed of two main proteins: the structural fimbrial component and the adhesin recognizing the receptor. The genes encoding the structural subunit (f17A genes) and the adhesin (f17G genes) of the four subtypes of F17 fimbriae of animal origin have been cloned and sequenced, and a high degree of homology has been found (11, 13, 15, 17). The gafA and gafD genes encoding, respectively, structural and adhesin subunits of the human G fimbriae of uropathogenic strains have also been sequenced (17, 20) and are identical to those of the F17c fimbriae subtype (17). The entire gene clusters encoding fimbriae of the F17 family also included the f17C and f17D genes encoding the transmembrane protein and the periplasmic transport protein, respectively (11, 13).
The F17a and F17d subtypes of fimbriae have been mainly associated with ETEC strains. Experimental infections have shown that F17a antibodies in addition to F5 and F41 antibodies are necessary to protect newborn calves against infection with ETEC strains (10). The E. coli strains producing the F17b and F17c antigens have been associated with extraintestinal diseases, and they usually show virulence factors associated with the ability to cause septicemia such as serum resistance, aerobactin production, and colicin V production. The ovine septicemic strain S5, which is the reference strain for F17b fimbriae, carries a transmissible plasmid Vir encoding both the F17b fimbriae and the cytotoxic necrotizing factor 2 (CNF2) (11). This strain also carries colicin V plasmid, associated with the ability to survive in host serum (16). E. coli strains expressing the F17c antigen have been isolated from septicemic and diarrheic calves and lambs and from lambs with nephrosis (3). Most of the F17c bovine strains also express the CS31A antigen, a capsule-like antigen encoded by a large plasmid (12). Septicemia has been experimentally reproduced in gnotobiotic calves with the reference strain CS31A (10).
We have previously reported that nonenterotoxigenic E. coli strains producing F17 fimbriae are frequently isolated from the feces of diarrheic lambs and goat kids in Spain (9). In the present study, we characterized the F17 genotypic subtypes and investigated the adhesive ability and phenotypic characteristics related to the septicemic phenotype among ovine and caprine E. coli strains isolated from diarrheic animals.
MATERIALS AND METHODS
Collection of ovine and caprine F17 field bacterial strains.
A total of 45 E. coli strains expressing F17-related fimbriae, 34 from lambs and 11 from goat kids, were selected from our field strain laboratory collection isolated from different outbreaks of neonatal diarrhea. The F17-positive strains were selected according to their positive reaction with the F17-specific antisera. The strains were isolated from the feces of diarrheic animals from 20 (16 ovine and 4 caprine) different outbreaks, and one strain per animal was selected. None of the strains selected were enterotoxigenic (they did not express the F5 or F41 antigens or elaborate the STa or LT-II enterotoxins), and only one of them was toxigenic in the Vero cell assay (9). The strains selected belong to different serogroups, the most frequent being O11 (14 strains), O44 (3 strains), O77 (2 strains), and O101 (2 strains) (6, 8). E. coli strains not producing F17-related fimbriae were used as negative controls. Bacterial strains were maintained in semisolid nutrient broth (Difco) and plated out on blood agar medium (BioMerieux) as needed. The reference strains 25KHO9st, 111KH86, S5, and CS31A were used as positive controls.
PCR amplification of the f17A- and f17G-related genes.
Bacterial DNA was released by boiling. Bacteria grown on solid media were suspended in 200 μl of sterile water, incubated at 100°C for 10 min, and centrifuged, and the supernatants were used as templates. The primers used for amplification of the f17A- and f17G-related genes were derived from the nucleotide sequence of the f17A structural and f17G adhesin genes previously described (13, 15). They are detailed in Table 1. PCR assays were carried out with reagents and protocols supplied by the manufacturer (Perkin-Elmer). The total reaction mixture volume was 50 μl, containing 10 μl of supernatant from the boiled bacteria, 0.5 μM concentrations of the appropriate oligonucleotide primers, 200 μM (each) deoxynucleotide triphosphate, 5 μl of PCR buffer II (Perkin-Elmer), 3 μl of 25 mM MgCl2 solution, and 2.5 U of AmpliTaq Gold (Perkin-Elmer). All the reaction mixtures included an initial denaturation at 94°C for 10 min and a final cycle of primer extension at 72 or 68°C for 10 min. Thermocycler reaction conditions for primer pairs F17A1 and F17A2, F17A1 and F17A3, and F17G3 and F17G4 were 1 min at 94°C, 1 min at 50°C, and 1 min at 72°C for 30 cycles. PCR conditions for the primer pair F17G1 and F17G2 were 1 min at 94°C, 1 min at 60°C, and 1 min at 72°C. PCR conditions for primers F17A4 and F17A5 and F17G5 and F17G6 were 10 s at 94°C, 30 s at 55°C, and 2 min at 68°C for 30 cycles. PCR-amplified DNA was analyzed on 1% agarose gels by electrophoresis.
TABLE 1.
Oligonucleotide primers used for detection and isolation of f17A- and f17G-related genes
| Primer | Oligonucleotide sequence (5′ to 3′) | Locationa | Size of PCR product (bp) | Gene detected |
|---|---|---|---|---|
| F17A1 | ATGCAGAAAATTCAATTTATCCT | 1–23 | ||
| F17A2 | ATCATTAATAGCAACTGTATC | 421–441 | 441b | f17A |
| F17A3 | GTTTCATACAGGCTCCGT | 600–617 | 618b | f17A |
| F17A4 | CTATGCAGAAAATTCAATTTATC | −2–21 | 542 | f17A |
| F17A5 | CTGATAAGCGATGGTGTAATT | 520–540 | ||
| F17G1 | AGGTCTTTCTGGCTGTATTCAT | 17–38 | 738 | f17G |
| F17G2 | CTGATAGGAAAACTGAAATGT | 729–753 | ||
| F17G3 | ATGAGGCAATAATATGACAA | −13–7 | 1,302 | f17G |
| F17G4 | TTATCCATGCGCTGACGAAGT | 1278–1298 | ||
| F17G5 | GCTGTTCCGGAAAACCATCATGTA | −20–43 | 1,166 | f17G |
| F17G6 | AACGCTGTTATCCAGCTTCAGAAA | 1100–1123 |
Position numbers are from the nucleotide sequence of the f17A cluster from E. coli 25KHO9 (GenBank accession no. AF022140).
Size of PCR product combined with primer F17A1.
PCR assays to identify the four subtypes of structural subunit genes (f17aA, f17bA, f17cA, and f17dA) and the two subfamilies of adhesin genes (subfamily I of the F17a and F17d subtypes and subfamily II of the F17b and F17c subtypes) were done according to the multiplex PCR method described by Bertin et al. (5). Briefly, two primer sets were used: the MXP1 containing six primers which identify the f17 family genes, the f17aA and f17dA subunit genes and f17G subfamily I, and the MXP2 containing five primers which identify the f17b and f17c subunit genes and the f17G subfamily II genes. The PCR mixtures and thermocycler conditions used were described previously (5).
Southern blot hybridization.
Bacterial DNA was obtained with cetyltrimethylammonium bromide after pretreatment of the bacterial cells with lysozyme (20 mg/ml) for 1 h at 37°C in Tris-EDTA-sucrose (1). Bacterial DNA was then digested with PstI, AseI, and KpnI restriction enzymes (New England Biolabs) as recommended by the manufacturer. Restriction fragments were resolved in 1% agarose gels by electrophoresis and transferred onto nylon membranes (N+; Amersham).
The DNA probes for detection of the f17G- and f17A-related genes were derived from recombinant plasmids. The HindIII-EcoRI fragment, isolated from plasmid pPLHD101, was used as the probe for F17G (13). The HindIII-AflII fragment from pPLHD202 was used as the probe for F17A (15). DNA probes were 32P labeled by using the random oligonucleotide primer system (Amersham). Hybridizations were performed overnight at 60°C in 7% sodium dodecyl sulfate (SDS)–0.5 M sodium phosphate (pH 7.2)–1 mM EDTA. Membranes were washed for 20 min at 60°C in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–0.1% SDS and then 20 min at 60°C in 0.1× SSC–0.1% SDS.
Cloning and sequencing of f17A and f17G genes.
The f17A and f17G genes of the selected strains were amplified by PCR as described above. PCR products were analyzed by electrophoresis on 1% agarose gels, recovered with the Qiaquick gel extraction kit (Qiagen), and ligated into the SmaI site of pUC18 with the SureClone ligation kit (Pharmacia Biotech) or into the EcoRV site of pMOSBlue with the pMOSBlue T-vector kit (Amersham). Ligation mixtures were transformed into competent E. coli strains, and transformants were selected on Luria-Bertani agar containing ampicillin (100 μg/ml) or carbenicillin (100 μg/ml). DNA sequencing was performed by the dideoxy chain-termination method (21) with the Thermosequenase radiolabeled terminator cycle sequencing kit (Amersham) and the 17-mer forward and reverse M13/pUC sequencing primers (Boehringer). Internal deletions were made by digestion with restriction endonucleases when necessary. The nucleotide sequences of the f17A and f17G genes were also determined directly from purified PCR products by automated sequencing performed with a model 373A sequencer (Applied Biosystems). Internal oligonucleotide primers were constructed as required from the obtained sequence data.
PCR for detection of the CNF2 and CS31A genes.
Bacterial DNA for PCR amplification was released as described above. The oligonucleotide primers to detect the CNF2 gene were based on the sequence of the gene, and PCR conditions were as described previously (7). Detection of CS31A-related sequences was carried out with primers clpG1 (5′-GGGCGCTTCTCTCCTTCAAC) and clpG2 (5′-CGCCCTAATTGCTGGCGAC) described by Bertin et al. (4). PCR conditions for amplification of CS31A-related sequences were 2 min at 94°C, 1 min at 55°C, and 2 min at 72°C for 25 cycles. All PCR amplifications included a first cycle at 94°C for 10 min and a final cycle of 72°C for 10 min.
Hemagglutination tests.
Hemagglutination tests were performed by the rocked-tile method with human group A, bovine, ovine, equine, and guinea pig erythrocytes. Erythrocytes were washed twice with phosphate-buffered saline (PBS) (pH 7.4) and diluted to 3% (vol/vol) in the same buffer containing 1% α-methyl-d-mannoside (Sigma) to test for mannose-resistant hemagglutination (MRHA). The bacterial strains grown on Minca-IsoVitalex medium or Penassay broth (Difco) at 37°C for 18 h were suspended in PBS (pH 7.4) to obtain a dense bacterial suspension (approximately 1010 cells/ml). Fifty microliters of bacterial suspension was mixed with 50 μl of erythrocyte suspensions in a tile. The tile was rocked for 5 min at room temperature and 5 min at 4°C.
In vitro adhesion to brush border intestinal cells.
Assay of adhesion to ovine intestinal brush borders was performed as described by Robins-Browne et al. (19). Intestines from 7- to 10-day-old lambs were obtained from freshly killed animals. The small intestine was removed and washed in a solution of 5 mM phosphate buffer containing 5 mM EDTA (pH 7.4). After scraping of the mucosa, the villi were suspended in EDTA buffer and disrupted in a Teflon glass tissue grinder. The brush border cells were separated by centrifugation (180 × g for 20 min) and suspended in PBS (pH 6.8) at approximately 2 × 107 cells/ml. For the adhesion assay the bacteria were grown in Penassay broth (Difco) at 37°C for 18 h. Bacteria cells were washed with PBS (pH 6.8) and suspended in PBS containing 1% d-mannose, at approximately 2 × 109 cells/ml. The assay was performed in round-bottom wells in microtiter plates by mixing equal volumes of brush border suspension and bacterial suspension. Plates were incubated at 37°C for 20 min, and adhesion was examined by phase-contrast microscopy at ×400 magnification.
Serum resistance.
Blood was obtained from clinically normal adult sheep at an abattoir. Serum was separated, pooled from at least five animals, divided into aliquots, and frozen at −20°C. Bacterial resistance to bactericidal activity of serum was determined by counting the number of viable E. coli cells after 1 h of exposure to serum as described previously (22). Briefly, bacterial strains were grown in 5 ml of brain heart infusion (Difco) at 37°C until the logarithmic growth phase was reached. Bacterial cells were washed once with Veronal buffer (pH 7.35) containing 2 mM MgCl2 and 0.3 mM CaCl2 and resuspended in the same buffer to an optical density at 540 nm of 0.3. Twenty-five microliters of bacterial suspension was added to 2.5 ml of serum, and the inoculated serum was incubated at 37°C in a water bath. Fifty E. coli strains not producing F17 fimbriae were used as negative controls.
Colicin V test.
Production of colicin V was determined by the agar overlay method. Colonies of E. coli strains grown on nutrient agar (Difco) were lysed by chloroform vapor. A second layer of semisolid nutrient agar containing the suitable E. coli indicator strain was added to the plates, and these were further incubated at 37°C overnight. Production of colicins was evidenced by the inhibition of growth of the E. coli-susceptible strain. Colicin V production was evidenced in the same manner by using a colicin V-resistant strain. The colicin-producing strains that did not inhibit the growth of a colicin V-resistant strain were considered to be colicin V producers. Levels of colicin V production were also determined in 145 E. coli strains randomly selected from our collection that did not produce F17-related fimbriae.
Statistical analysis.
A chi-square (χ2) test was used for statistical analysis.
Nucleotide sequence accession numbers.
Nucleotide sequences deposited in the GenBank database and their accession numbers, respectively, are as follows: CL114 f17A, AF055306; CK210 f17A, AF055307; CK377 f17A, AF055308; CL394 f17A, AF055309; CL114 f17G, AF055310; CK210 f17G, AF055311; CK377 f17G, AF055312; and CL394 f17G, AF055313.
RESULTS
Detection of the f17-related genes among ovine and caprine strains.
In all the analyzed strains, the f17A- and f17G-related genes were amplified by PCR with at least one of the primer pairs used. The ovine and caprine strains were classified into five genotypic subtypes according to the different associations that were found between structural subunit gene subtypes and adhesin gene subfamilies I and II (Table 2). In most of the strains (35 of 45) the association observed was between the f17cA fimbrial subunit subtype and subfamily II adhesin genes. Strains of this genotypic subtype belonged to 9 of the 11 different serogroups. Seven ovine strains which amplified with the pairs of primers F17A1 plus F17A2 and F17G1 plus F17G2 did not amplify with primers for the four subtypes of the structural subunits or for the adhesin genes. The total DNA of these seven strains showed a positive reaction with probes specific for the f17A and f17G genes by Southern hybridization. This genotypic subtype was designated F17A plus F17G (Table 2). None of the strains amplified CNF2- or CS31A-related genes by PCR.
TABLE 2.
Classification of E. coli strains producing f17-related genes into genotypic subtypes
| Origin and serogroup (no. of strains analyzed) | No. of strains with genotypic subtypea
|
||||
|---|---|---|---|---|---|
| F17dA + F17GI | F17aA + F17GII | F17cA + F17GII | F17dA + F17GII | F17A + F17Gb | |
| Ovine | |||||
| NT (7) | 1 | 0 | 6 | 0 | 0 |
| O6 (1) | 0 | 0 | 1 | 0 | 0 |
| O8 (3) | 0 | 0 | 3 | 0 | 0 |
| O11 (13) | 0 | 0 | 8 | 0 | 5 |
| O23 (2) | 0 | 0 | 2 | 0 | 0 |
| O26 (1) | 0 | 0 | 1 | 0 | 0 |
| 077 (2) | 0 | 0 | 2 | 0 | 0 |
| O101 (2) | 0 | 0 | 1 | 0 | 1 |
| O116 (1) | 0 | 0 | 1 | 0 | 0 |
| O117 (1) | 0 | 0 | 0 | 1 | 0 |
| O138 (1) | 0 | 0 | 0 | 0 | 1 |
| Caprine | |||||
| NT (6) | 0 | 0 | 6 | 0 | 0 |
| O11 (1) | 0 | 0 | 1 | 0 | 0 |
| O44 (3) | 0 | 3 | 0 | 0 | 0 |
| O116 (1) | 0 | 0 | 1 | 0 | 0 |
| Total | 1 | 3 | 33 | 1 | 7 |
No. of strains positive by the multiplex PCR (5).
No. of strains negative for the multiplex PCR method with a positive amplification by primer pairs F17A1 plus F17A2 (specific for f17A-related genes) and F17G1 plus F17G2 (specific for f17G genes) and with a positive reaction by Southern blot hybridization with probes for F17A and F17G.
Cloning and sequencing of f17A- and f17G-related genes.
To determine the f17G gene subtype and confirm the fimbrial and adhesin gene associations found by PCR, the f17A and f17G genes of four strains were sequenced. Each of the selected strains belonged to a different genotypic subtype as follows: CL114 (F17dA plus F17GII), CK210 (F17aA plus F17GII), CK377 (F17cA plus F17GII), and CL394 (F17dA plus F17GI). The f17A and f17G genes were amplified by PCR with primers located outside the target gene sequence. The f17A genes from strains CL114 and CL394 and strains CK210 and CK377 were amplified with primer pairs F17A1 plus F17A2 and F17A4 plus F17A5, respectively. The f17G genes were amplified from strains CL114, CK377, and CL394 with primer pair F17G3 plus F17G4 and from strain CK210 with primer pair F17G5 plus F17G6.
A single open reading frame (ORF) of 546 bp was identified in the f17A genes of strains CL114, CK377, and CL394, and an ORF of 543 bp was identified in the f17A gene of strain CK210. These ORFs encode proteins of 181 and 180 amino acids, respectively, and both include a signal sequence of 21 residues. The predicted amino acid sequences were compared with the previously described sequences of reference strain proteins F17a-A, F17b-A, F17c-A, and F17d-A. No relevant differences were found between the amino acid sequences of the F17A proteins from these wild-type strains and those of the corresponding F17A protein subtypes from reference strains (99% identity in all cases).
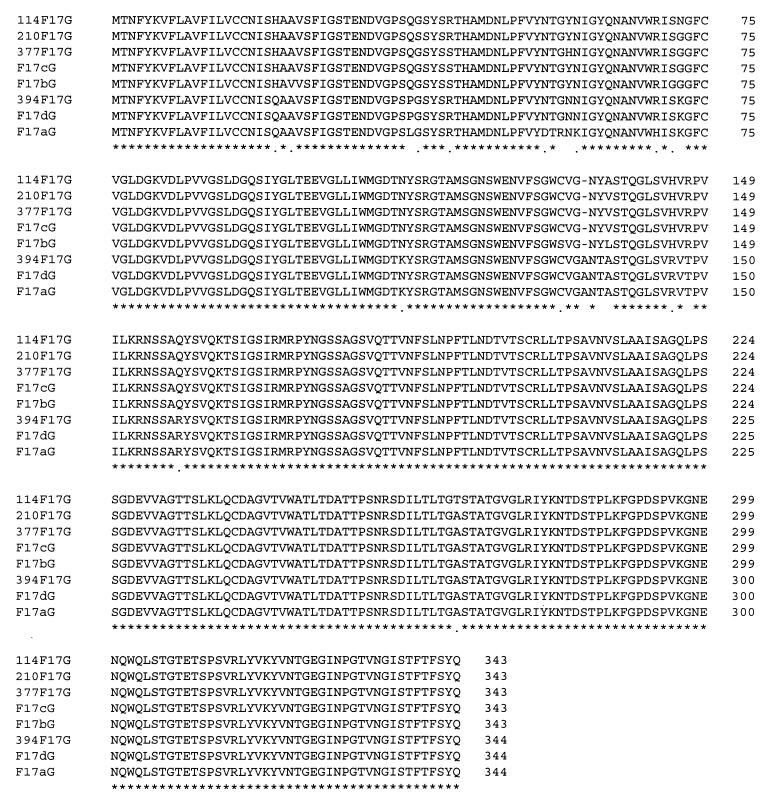
A 1,032-bp ORF encoding a protein of 343 amino acids was identified in the f17G genes of strains CL114, CK210, and CK377, and an ORF of 1,035 bp encoding a protein of 344 amino acids was identified in the f17G gene of strain CL394. The deduced amino acid sequences showed a high degree of identity (93.9%) with the previously described sequences of proteins F17a-G, F17b-G, F17c-G, and F17d-G, although different patterns of identity were found (Fig. 1). The F17G proteins of strains CL114, CK210, and CK377 showed the highest similarity (99% identity at the amino acid level) with the F17c-G protein, whereas the F17G protein of strain CL394 showed the highest similarity (99% identity) with the F17d-G protein (Fig. 1).
FIG. 1.
Comparison of the predicted amino acid sequences of the F17G proteins of E. coli CL114 (114F17G), CK210 (210F17G), CK377 (377F17G), CL394 (394F17G), 25KH09 (F17aG; accession no. AF022140), S5 (F17bG; accession no. L14319), CS31A (F17cG; accession no. L43374), and 111KH86 (F17dA; accession no. L77091). F17G amino acid sequences were aligned by using the PC/Gene program. Amino acid residues which are perfectly conserved are marked by asterisks, and amino acid residues which are well conserved are marked by dots.
Phenotypic characteristics of the strains. (i) Mannose-resistant hemagglutination and adherence to intestinal brush borders.
Thirty-nine of the strains agglutinated erythrocytes of different species in the presence of d-mannose. Six different patterns of MRHA were found (Table 3). A correlation between MRHA pattern and genotypic subtype was not found, since strains belonging to the F17cA plus F17GII genotypic subtype were distributed among all six different patterns of MRHA. Most of the strains (36 of 45) also adhered in vitro to ovine intestinal brush borders (Table 3). Five of the 9 strains which did not adhere to brush borders agglutinated bovine and human type A erythrocytes, one strain agglutinated human and ovine erythrocytes, and three strains did not agglutinate the erythrocytes of any of the species used in the presence of d-mannose. Five of the nonadherent strains were of the F17A plus F17G genotypic subtype.
TABLE 3.
Mannose-resistant hemagglutination and adherence to intestinal brush borders of ovine and caprine strains producing F17 fimbriae
| Pattern | MRHA with indicated erythrocyte typea
|
No. of strains studied/no. showing adherence to brush borders | ||||
|---|---|---|---|---|---|---|
| Bov | Hum | Ov | Eq | Gp | ||
| I | + | + | + | − | − | 13/13 |
| II | + | + | − | − | − | 10/5 |
| III | + | − | − | − | − | 4/4 |
| IV | − | + | + | − | − | 10/9 |
| V | − | + | − | − | − | 2/2 |
| VI | − | − | − | − | − | 6/3 |
| No. of strains | 27 | 35 | 23 | 0 | 0 | 45/36 |
Bov, bovine; Hum, human; Ov, ovine; Eq, equine; Gp, guinea pig.
(ii) Colicin V production and serum resistance.
Production of colicin V and serum resistance were frequent characteristics in ovine and caprine strains: 57% of the F17-positive strains and 34% of the F17-negative strains produced colicin V, and 91% of the F17-positive strains and 66% of the F17-negative strains were resistant to the bactericidal effect of serum. The percentages of strains producing colicin V and resistant to the bactericidal effect of serum were significantly higher in the F17-positive strains than in the F17-negative strains (P < 0.05).
DISCUSSION
In this study we characterized 45 non-ETEC strains expressing F17 fimbriae isolated from diarrheic lambs and goat kids. Our results indicate that the most frequent subtype of F17 fimbriae produced by ovine and caprine strains (33 of 45) is F17c. It has been reported that E. coli strains producing this subtype represent a high percentage of isolates from diarrheic and septicemic calves in European countries and from lambs with nephrosis (3). Most of the bovine septicemic strains producing subtype F17c also produced the plasmid-encoded antigen CS31A (3, 5). However, the CS31A antigen seems to be very infrequent among ovine and caprine isolates, since none of the analyzed strains amplified CS31A-related sequences. These results are in agreement with those previously reported by Bertin et al. (5). None of the strains produced the F17b subtype of fimbriae or the CNF2 toxin, which are virulence factors associated with ovine septicemic strains (11).
Other genotypic subtypes were found among ovine and caprine isolates. Three strains amplified the f17A structural gene of the F17a subtype, and two strains amplified the F17d subtype. Only on one of the strains was the f17dA gene associated with the adhesin of subfamily I. The other strains amplifying the f17dA gene (one strain) or the f17aA gene (three strains) produced adhesins of the subfamily II. This low number of strains did not allow us to establish whether these associations are representative of ovine and caprine strains. However, the global results indicate that the adhesins most frequently expressed by ovine and caprine diarrheic isolates belong to subfamily II. Bertin et al. (5) also found that the f17aA gene was associated with the subfamily II adhesins on most of the fecal E. coli isolates from lambs with nephropathy and that most of the ovine strains produced adhesins of the subfamily II. These researchers suggested that the ovine receptors could contain an exposed oligosaccharide sequence strongly recognized by subfamily II adhesins. According to our results, a similar hypothesis could be proposed for caprine isolates.
Seven strains did not amplify f17-related genes by the multiplex PCR method, but f17-related genes were detected by PCR with F17-specific primers and confirmed by Southern hybridization with F17-specific probes. These results indicate that these strains harbored f17-related genes differing from those described, at least in the target sequence for the multiplex PCR primers. These E. coli strains might produce a new F17 subtype fimbriae, but slight variations in the sequence of the genes could also account for negative results by the multiplex PCR.
Sequencing of the f17A and f17G genes of four strains, each of a different subtype, confirmed the association of f17 structural genes of the F17a and F17d subtypes with the f17G genes of subfamily II. The structural protein subunit encoded by the f17A genes of strains CL114 and CL394 showed 99% identity at the amino acid level with protein F17A of the F17d subtype, whereas protein F17A of strains CK210 and CK377 showed 99% identity with protein F17A of subtypes F17a and F17c, respectively. However, adhesins encoded by the f17G genes of strains CL114, CK210, and CK377 showed 99% identity with the F17G adhesin of subtype F17c, and only protein F17G of strain CL394 showed 99% identity with protein F17d-G. Strains CL114 and CK210, harboring adhesin genes of subtype F17c and structural genes of subtypes F17d and F17a, respectively, seemed to produce functional fimbriae, since they adhere in vitro to ovine brush borders in a glucosamine-sensitive manner, showing that adhesion is mediated by F17-related adhesins. F17-related gene clusters showed high homology in the structural subunit and adhesin genes, and genetic analysis has demonstrated that gene products encoding the pilins and the adhesins are exchangeable and able to form functional fimbriae (17). The origin of these associations in field isolates remains to be determined. Our results suggest that different structural subtypes in field isolates may be associated with adhesins showing higher affinity for receptors from different animal species.
Analysis of ovine and caprine strains expressing F17 fimbriae showed that most were adherent to brush borders in vitro (80%) and exhibited MRHA with erythrocytes of different animal species (87%). A correlation between MRHA patterns and F17 fimbriae subtype was not found, since most of the strains expressed the F17c subtype and were distributed among the six MRHA patterns found. This finding indicates differences in adherence abilities among these isolates. It has been shown that F17-related fimbriae recognize glucosamine-containing receptors but that each subtype probably recognizes different oligosaccharide sequences (3). The CS31A reference strain for the F17c antigen agglutinated in the presence of d-mannose erythrocytes of bovine and human origin but did not agglutinate sheep erythrocytes (3). The presence of other adhesins cannot be excluded, since 13 of the strains studied exhibited MRHA for ovine erythrocytes.
E. coli strains producing F17 fimbriae constitute a heterogeneous group of strains according to their clinical origin, virulence properties, and serogroups. The ovine and caprine strains expressing F17 fimbriae analyzed in this study produced colicin V and were serum resistant in a significantly higher percentage than F17-negative strains. Both characteristics were also investigated in F17-negative strains, since we previously found that these were very common among ovine and caprine E. coli isolates. Colicin V, aerobactin production, and serum resistance are characteristics associated with the ability to cause septicemia. The ovine septicemic strain S5 and the bovine strain CS31A carry virulence plasmids encoding these characteristics, and septicemia has been experimentally reproduced with both strains. Our results indicate that ovine and caprine strains producing f17-related genes could be associated with septicemic diseases, since they were nonenterotoxigenic and most of them expressed the F17c subtype and exhibited the phenotypic characteristics of septicemic strains.
ACKNOWLEDGMENT
This study was supported by CICYT (grant AGF 95-0834).
REFERENCES
- 1.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: John Wiley and Sons; 1991. [Google Scholar]
- 2.Bertels A, Pohl P, Schlicker C, Van Driessche E, Charlier G, De Greve H, Lintermans P. Isolation of the F111 fimbrial antigen on the surface of a bovine Escherichia coli strain isolated out of calf diarrhea: characterization and discussion for the need to adapt recent vaccines against neonatal calf diarrhea. Vlaams Diergeneeskd Tijdschr. 1989;58:118–122. [Google Scholar]
- 3.Bertin Y, Girardeau J P, Darfeuille-Michaud A, Contrepois M. Characterization of 20K fimbria, a new adhesin of septicemic and diarrhea-associated Escherichia coli strains, that belongs to a family of adhesins with N-acetyl-d-glucosamine recognition. Infect Immun. 1996;64:332–342. doi: 10.1128/iai.64.1.332-342.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bertin Y, Martin C, Girardeau J P, Pohl P, Contrepois M. Association of genes encoding P fimbriae, CS31A antigen and EAST1 toxin among CNF1-producing Escherichia coli strains from cattle with septicemia and diarrhea. FEMS Microbiol Lett. 1998;162:235–239. doi: 10.1111/j.1574-6968.1998.tb13004.x. [DOI] [PubMed] [Google Scholar]
- 5.Bertin Y, Martin C, Oswald E, Girardeau J P. Rapid and specific detection of F17-related pilin and adhesin genes in diarrheic and septicemic Escherichia coli strains by multiplex PCR. J Clin Microbiol. 1996;34:2921–2928. doi: 10.1128/jcm.34.12.2921-2928.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blanco J, Cid D, Blanco J E, Blanco M, Ruiz-Santa-Quiteria J A, de la Fuente R. Serogroups, toxins and antibiotic resistance of Escherichia coli strains isolated from diarrhoeic lambs in Spain. Vet Microbiol. 1996;49:209–217. doi: 10.1016/0378-1135(95)00188-3. [DOI] [PubMed] [Google Scholar]
- 7.Blanco M, Blanco J E, Blanco J, Alonso M P, Balsalobre C, Mouriño M, Madrid C, Juarez A. Polymerase chain reaction for detection of Escherichia coli strains producing cytotoxic necrotizing factor type 1 and type 2 (CNF1 and CNF2) J Microbiol Methods. 1996;26:95–101. [Google Scholar]
- 8.Cid D, Blanco M, Blanco J E, Ruiz-Santa-Quiteria J A, de la Fuente R, Blanco J. Serogroups, toxins and antibiotic resistance of Escherichia coli strains isolated from diarrhoeic goat kids in Spain. Vet Microbiol. 1996;53:349–354. doi: 10.1016/s0378-1135(96)01222-9. [DOI] [PubMed] [Google Scholar]
- 9.Cid D, Ruiz-Santa-Quiteria J A, de la Fuente R. F17 fimbriae in Escherichia coli from lambs and kids. Vet Rec. 1993;132:251. doi: 10.1136/vr.132.10.251. [DOI] [PubMed] [Google Scholar]
- 10.Contrepois M, Dubourguier H C, Parodi A L, Girardeau J P, Ollier J L. Septicaemic Escherichia coli and experimental infection of calves. Vet Microbiol. 1986;12:109–118. doi: 10.1016/0378-1135(86)90073-8. [DOI] [PubMed] [Google Scholar]
- 11.El Mazouari K, Oswald E, Hernalsteens J P, Lintermans P, De Greve H. F17-like fimbriae from an invasive Escherichia coli strain producing cytotoxic necrotizing factor type 2 toxin. Infect Immun. 1994;62:2633–2638. doi: 10.1128/iai.62.6.2633-2638.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Girardeau J P, Der Vartanian M, Ollier J L, Contrepois M. CS31A, a new K88-related fimbrial antigen on bovine enterotoxigenic and septicemic Escherichia coli strains. Infect Immun. 1988;56:2180–2188. doi: 10.1128/iai.56.8.2180-2188.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lintermans P F, Bertels A, Schlicker C, Deboeck F, Charlier G, Pohl P, Norgrem M, Normark S, Van Montagu M, De Greve H. Identification, characterization, and nucleotide sequence of the F17G gene, which determines receptor binding of Escherichia coli F17 fimbriae. J Bacteriol. 1991;173:3366–3377. doi: 10.1128/jb.173.11.3366-3373.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lintermans P F, Pohl P, Bertels A, Charlier G, Vandekerckhove J, Vandamme J, Schoup J, Schlicker C, Korhonen T, De Greve H, Van Montagu M. Characterization and purification of the F17 adhesin on the surface of bovine enteropathogenic and septicemic Escherichia coli. Am J Vet Res. 1988;49:1794–1799. [PubMed] [Google Scholar]
- 15.Lintermans P F, Pohl P, Deboeck F, Bertels A, Schlicker C, Vandekerckhove J, Vandamme J, Van Montagu M, De Greve H. The isolation and nucleotide sequence of the F17-A gene encoding the structural protein of the F17 fimbriae in bovine enterotoxigenic E. coli. Infect Immun. 1988;56:1475–1484. doi: 10.1128/iai.56.6.1475-1484.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lopez-Alvarez J, Gyles C L. Occurrence of the Vir plasmid among animal and human strains of invasive Escherichia coli. Am J Vet Res. 1980;41:769–774. [PubMed] [Google Scholar]
- 17.Martin C, Rousset E, De Greve H. Human uropathogenic and bovine septicaemic Escherichia coli strains carry an identical F17-related adhesin. Res Microbiol. 1997;148:55–64. doi: 10.1016/S0923-2508(97)81900-6. [DOI] [PubMed] [Google Scholar]
- 18.Rhen M, Klem P, Korhonen T K. Identification of two new hemagglutinins of Escherichia coli, N-acetyl-d-glucosamine-specific fimbriae and a blood group M-specific agglutinin, by cloning the corresponding genes in Escherichia coli K-12. J Bacteriol. 1986;168:1234–1242. doi: 10.1128/jb.168.3.1234-1242.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robins-Browne R M, Tokhi A M, Adams L M, Bennett-Wood V, Moisidis A V, Krejany E O, O’Gorman L E. Adherence characteristics of attaching and effacing strains of Escherichia coli from rabbits. Infect Immun. 1994;62:1584–1592. doi: 10.1128/iai.62.5.1584-1592.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saarela S, Taira S, Nurmiaho-Lassila E L, Makkonen A, Rhen M. The Escherichia coli G-fimbrial lectin protein participates both in fimbrial biogenesis and in recognition of the receptor N-acetyl-d-glucosamine. J Bacteriol. 1995;177:1477–1484. doi: 10.1128/jb.177.6.1477-1484.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanger J, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taylor P W. Measurement of the bactericidal activity of serum. In: Sussman M, editor. The virulence of Escherichia coli. London, England: Academic Press; 1985. pp. 445–456. [Google Scholar]