Abstract
Objective:
The objective of this study was to provide an overview of acute disseminating encephalomyelitis, a potential and serious complication of COVID-19.
Methods:
Three primary databases were used, PubMed, LitCovid, and WHO. The final review articles reported acute disseminated encephalomyelitis (ADEM) in COVID-19-positive patients and were full-text, peer-reviewed articles. Articles which did not have patient data such as in vitro studies and articles with unclear inference were excluded.
Results:
Out of 21 cases of ADEM, the diagnosis of severe acute respiratory syndrome-coronavirus 2 was confirmed in 18 and suspected in 3. Among the neurological symptoms, altered consciousness was most common (7/21), followed by anosmia (3), paraplegia (3/21), brain stem involvement (3/21), sphincter involvement (2/21), and quadriplegia (1/21). Raised inflammatory markers were most commonly seen in 9/17. Central nervous system imaging was abnormal in 19 cases and unavailable in 2 cases. Fifteen patients were treated with corticosteroids, 11 patients received intravenous immunoglobulin, while 3 patients received convalescent plasma. Two patients needed surgical intervention. Complications included seizures (1), acute kidney injury and septicemic shock (1), raised intracranial pressure (1), and supraventricular tachycardia secondary to hydroxychloroquine (1). One patient recovered completely and one had good recovery with mild deficits. Thirteen patients had incomplete recovery with residual neurological deficit while three patients died as the consequence of the disease.
Conclusion:
The physicians and neurosurgeons should be diligent while treating the COVID-19 patients with neurological manifestations and include ADEM as a differential diagnosis and stress on early diagnosis and treatment to reduce mortality and achieve satisfactory clinical outcome.
Keywords: Acute disseminating encephalomyelitis, COVID-19, neurological complications, neurological manifestations
Introduction
The coronavirus pandemic began in December 2019 with Wuhan, China, being the epicenter.[1] As per the latest reports on August 8, 2020, there have been 20,806,961 cases worldwide with 747,258 deaths due to COVID-19.[2]
Many reports have indicated extrapulmonary involvement of severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2).[3,4,5,6,7] The common neurological manifestations include headache, anosmia, hypogeusia, and altered consciousness while seizures, encephalitis, meningitis, encephalopathy, demyelination, and stroke are potential serious complications.[8,9]
Acute disseminated encephalomyelitis (ADEM), an acute inflammatory autoimmune demyelinating disease of the central nervous system (CNS),[9,10] is often preceded by a viral infection, but the evidences depicting its occurrence as a complication of SARS-CoV-2 are scarce.[10,11] Our study aims to provide an overview of this potential but serious complication of COVID-19.
Methods
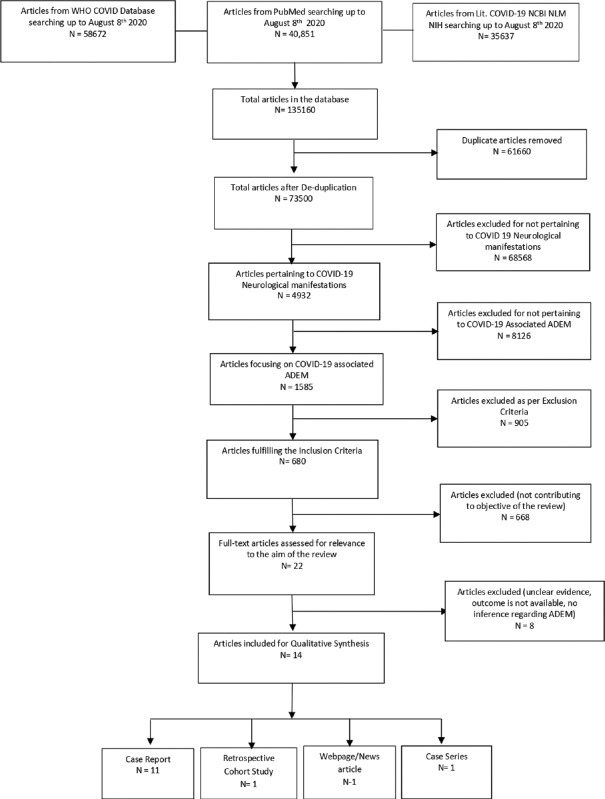
A systematic search was conducted for research articles on COVID-19-associated ADEM. Three primary databases were used, Pub-Med, LitCovid, and WHO. The search strategy used the keywords, corona virus, COVID-19, neurological complications, and acute disseminated encephalomyelitis, and was comprehensive with cross-checking of reference lists from the articles retrieved. Selected articles were independently reviewed by two authors. All disagreements were resolved with discussion between the two authors and mutually agreed upon by the authors. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines were used [Figure 1].[12] The final review articles fulfilled the following criteria:
Figure 1.
PRISMA diagram
Reported ADEM in COVID-19-positive patients
Full-text, peer-reviewed articles (case studies and case series).
Articles which did not have patient data such as in vitro studies and articles with unclear inference were excluded.
This study did not require ethical approval as data were obtained from already available databases and patients were not directly involved.
Risk-of-bias assessment
Risk-of-bias assessments were performed at the outcome measurement level during data collection. The National Institute of Health scale was used for case series and case report studies[13] [Table 1].
Table 1.
Quality assessment of the included studies
| NIH quality assessment tool for case series/case reports | |||||
|---|---|---|---|---|---|
|
| |||||
| Study name | Was the study question or objective clearly stated? (yes/no) | Was the study population clearly and fully described, including a case definition? (yes/no) | Were the cases consecutive? (yes/no) | Were the subjects comparable? (yes/no) | Was the intervention clearly described? (yes/no) |
| Novi et al. | Yes | Yes | NA | NA | Yes |
| Abdi et al. | Yes | Yes | NA | NA | Yes |
| Utukuri et al. | Yes | Yes | NA | NA | Yes |
| Parsons et al. | Yes | Yes | NA | NA | Yes |
| Reichard et al. | Yes | Yes | NA | NA | Yes |
| Zoghi et al. | Yes | Yes | NA | NA | Yes |
| Zhang et al. | Yes | Yes | NA | NA | Yes |
| Sharma et al. | Yes | Yes | NA | NA | No |
| McCuddy et al. | Yes | Yes | Yes | Yes | Yes |
| Paterson et al. | Yes | Yes | Yes | Yes | Yes |
| Yong et al. | Yes | Yes | NA | NA | Yes |
| Chalil et al. | Yes | Yes | NA | NA | Yes |
| Poyiadji et al. | Yes | Yes | NA | NA | Yes |
| Zanin et al. | Yes | Yes | NA | NA | Yes |
|
| |||||
| NIH quality assessment tool for case series/case reports | |||||
|
| |||||
| Study name | Were the outcome measures clearly defined, valid, reliable, and implemented consistently across all study participants? (yes/no) | Was the length of follow-up? adequate? (yes/no) | Were the statistical methods well described? (yes/no) | Were the results well described? (yes/no) | Quality rating (good, fair, poor) |
|
| |||||
| Novi et al. | Yes | Yes | NA | Yes | Good |
| Abdi et al. | Yes | Yes | NA | Yes | Fair |
| Utukuri et al. | Yes | No | NA | Yes | Fair |
| Parsons et al. | Yes | NA | NA | Yes | Fair |
| Reichard et al. | Yes | Yes | NA | Yes | Good |
| Zoghi et al. | Yes | NA | NA | Yes | Good |
| Zhang et al. | Yes | Yes | NA | Yes | Good |
| Sharma et al. | No | No | NA | Yes | Poor |
| McCuddy et al. | Yes | Yes | NA | Yes | Good |
| Paterson et al. | Yes | Yes | NA | Yes | Good |
| Yong et al. | Yes | NA | NA | Yes | Fair |
| Chalil et al. | Yes | NA | NA | Yes | Good |
| Poyiadji et al. | Yes | Yes | NA | Yes | Fair |
| Zanin et al. | Yes | Yes | NA | Yes | Good |
NIH - National Institute of Health; NA - Not applicable
Results
A total 135,160 articles were found from the database using the abovementioned keywords until August 8, 2020. After de-duplication, 73,500 articles were screened and 4932 were found to be pertaining to neurological manifestations of COVID-19. Out of which, 1585 were focusing on COVID-19-associated acute disseminating encephalomyelitis. After the initial screening, articles were reviewed, out of which 680 articles matched the inclusion criteria. These articles were then screened in the second review. Before the qualitative synthesis, 668 articles were removed from the listed articles as they did not contribute to the manuscript's objective. The final analysis contained 14 articles [Figure 1]. Two independent reviewers reviewed all articles. Out of the 14 articles, 11 articles are case reports, 1 article is a case series, 1 is a retrospective cohort study, and 1 is a webpage/news article.
Novi et al.,[14] Parsons et al.,[15] Zoghi et al.,[16] Zanin et al.,[17] and Zhang et al.[18] reported cases of ADEM following COVID-19 while Abdi et al.[19] reported the ADEM case without prominent clinical pulmonary symptoms. The case reported by Utukuri et al.[20] had no respiratory symptoms of COVID-19 while Reichard et al.[21] described the case where ADEM was confirmed by autopsy. Sharma[22] reported India's first COVID 19-associated ADEM. McCuddy et al.[23] presented a case series of three cases of COVID-19-associated ADEM while Paterson et al.[24] described the retrospective cohort study of neurological manifestations of COVID-19 including ADEM. Yong et al.[25] reported the cases of acute hemorrhagic leukoencephalitis while Poyiadji et al.[26] described acute hemorrhagic necrotizing encephalopathy [Table 2].
Table 2.
Study characteristics
| First author | Type of the study | Number of patients | Age/sex | Comorbidities | Duration of COVID | Symptoms of COVID | SARS-CoV-2 diagnosis | Neurological presentation | CSF routine |
|---|---|---|---|---|---|---|---|---|---|
| Novi et al. | Case report | 1 | 64/female | Hypertension and monoclonal gammopathy | 25 days | Anosmia and ageusia | Serum IgG positive, negative nasopharyngeal swab | Mild behavioral abnormalities (irritability), headache, bilateral relative afferent pupillary defect, ageusia and anosmia, severe visual loss, right abdominal sensory level, and left-sided lower-limb hyperreflexia with the Babinski sign | Total cell count: 22 cells/mm3, Protein 45.2 mg/dL |
| Abdi et al. | Letter to editor/case report | 1 | 58/male | No prior pulmonary/constitutional symptoms | - | Positive nasopharyngeal swab | Decreased level of consciousness, LL weakness, gait disturbance | Normal | |
| Utukuri et al. | Letter to editor/case report | 1 | 44/male | Nil | No prior respiratory symptoms | - | Positive nasopharyngeal swab | Urinary retention, bilateral lower-limb weakness and loss of sensations, inability to walk, bilateral UL ataxia. | Normal |
| Parsons et al. | Letter to editor/case report | 1 | 51/female | Data unavailable | 18 days | Dyspnea, fever, tachycardia, hypoxia | Positive nasopharyngeal swab | Failure to improve and persistent after 18 days on ventilator, GCS 3/15, present brain stem signs, except left dolls eye absent | Normal, bacterial and fungal culture Negative |
| Reichard et al. | Case report | 1 | 71/male | IHD, CAD. double | Contracted infection postoperatively | Breathlessness, increasing oxygen demand | Positive nasopharyngeal swab | Not done | |
| Zoghi et al. | Case report | 1 | 21/male | Nil | 2 weeks | Fever with chills, sore throat, nonproductive cough, loss of appetite, vomiting | Serum IgG positive | Drowsy, progressive worsening paraparesis, also upper extremity weakness urinary retention, fever. Sensory impairment below T8 | Total cell count 150, protein 281 mg/dl, glucose 34/110 mg/dl |
| Zhang et al. | Case report | 1 | 40/female | Hypertension and dyslipidemia | 11 days symptoms but not tested at the time | Headache, myalgia, fever | Data unavailable | Dysphagia, dysarthria, encephalopathy, right gaze preference, mild left facial and bulbar weakness | Normal. Bacterial culture negative |
| Sharma et al. | Webpage/news report | 1 | 36/male | Data unavailable | Data unavailable | Brought in unconscious state | Positive nasopharyngeal swab | Unconscious | Data unavailable |
| McCuddy et al. | Case series | 3 | 38-70/2 male 1 female | DM | 3 weeks | Fatigue, cold, fever, ARDS | Serum COVID PCR positive | Unresponsiveness postextubation, left gaze preference. Severe diffuse weakness | Raised protein 55-95 mg/dl. Cultures negative |
| Paterson et al. | Retrospective cohort | 6 | 47-61 | Nil | 3-10 days | Fever, cough, dyspnea, myalgia, shortness of breath | Positive in 4, probable in 2 | Decreased consciousness, headache, backache, vomiting, quadriparesis, left weakness numbness | CSF OCB negative, CSF opening pressure raised, CSF protein raised |
| Yong et al. | Letter to editor/case report | 1 | 61/male | HTN, DM, hyperlipidemia | 18 days | Fever, cough, anosmia | Positive nasopharyngeal swab | Decreased consciousness, only grimace, intact brain stem response, quadriplegia | Not performed (raised ICP) |
| Chalil et al. | Case report | 1 | 48/female | Nil | 2 weeks | Fever, myalgia, dry cough, breathlessness | Positive nasopharyngeal swab | Drowsy, absent brain stem reflexes | Total cell count: 76×106, 33×109 RBC |
| Poyiadji et al. | Case report | 1 | Late 50/female | Data unavailable | 3 days | Cough, fever | Positive nasopharyngeal swab | Altered consciousness | Traumatic tap, not tested. Bacterial c/s negative |
| Zanin et al. | Case report | 1 | 54/female | Anterior communicating artery aneurysm operated 20 years back | Data unavailable | Anosmia ageusia | Positive nasopharyngeal swab | Seizure - found unconscious with tongue bite and urinary incontinence, then recovered to GCS 12/15 | Normal |
|
| |||||||||
| First author | CSF SARS-CoV RT-PCR | CSF other viral panel | Autoimmune analysis | Serology | CNS imaging | Final diagnosis | Treatment | Complications | Outcome |
|
| |||||||||
| Novi et al. | Positive | Data unavailable | Negative AQ4 and MOG antibody, IgG OCB mirror pattern | MRI brain - Multiple T1 postcontrast enhancing lesions of the brain with bilateral optic nerve enhancement, a single spinal cord lesion at the T8 level | ADEM | IV methylprednisolone f/b oral prednisone; IVIG | Nil | Partial recovery | |
| Abdi et al. | Negative | Negative | CSF OCB negative | Lymphopenia, Raised ear, CRP, ferritin | MRI brain - Cortex, deep gray and dorsal midbrain FLAIR hyperintensities. No contrast enhancement | ADEM | IV low-dose dexamethasone | Status epilepticus | Death |
| Utukuri et al. | Negative | Negative | Rheumatologic workup negative, inflammatory markers normal. ACE levels normal. CSF oligoclonal bands negative | Normal | Lumbar MRI - Conus expansion, patchy enhancement. Brain and Spine MRI - Cervical and thoracic cord T2 hyperintense lesions. Brain periventricular and juxtacortical T2 hyperintense lesions, enhancement in left parietal juxtacortical lesions. No hemorrhagic lesions | ADEM | IV methylprednisolone f/b IVIG | Nil | Incomplete recovery |
| Parsons et al. | Negative | Negative | Negative ANA ANCA, syphilis, AQ 4 antibodies. OCB in serum and CSF | Data unavailable | MRI brain - Deep hemispheric and juxtacortical T2 signal intensities, nonhemorrhagic with mild contrast enhancement. Intraventricular hemorrhage | ADEM | IV methylprednisolone f/b IVIG | Nil | Incomplete recovery |
| Reichard et al. | Data unavailable | Data unavailable | Data unavailable | Raised CRP IL-6 ferritin | Data unavailable | ADEM like on autopsy | Prone ventilation, Stress dose steroids, vasopressors | AKI secondary to shock, respiratory failure needing CRRT | Death |
| Zoghi et al. | Negative | Negative | Negative | Normal | MRI spine - LETM cervicothoracic MRI brain - FLAIR hyperintensity in CST, peduncles, pons, corpus callosum. No diffusion restriction, no enhancement | ADEM>NMO spectrum | Vancomycin, meropenem, acyclovir | Incomplete recovery | |
| Zhang et al. | Negative | Negative | Data unavailable | Data unavailable | CT brain - Multiple white matter hypoattenuation. MRI brain - Abnormal subcortical and deep gray T2 signal. Patchy contrast enhancement | ADEM | Hydroxychloroquine, ceftriaxone, IVIG | Ongoing recovery | |
| Sharma et al. | Data unavailable | Data unavailable | Data unavailable | Data Unavailable | Data unavailable | ADEM | IVIG | Recovered | |
| McCuddy et al. | Negative | Negative | Negative OCB and IgG | Normal | Diffuse white matter T2 hyperintense lesions. Corpus callosum and brain stem involvement, no hemorrhage. Minimal enhancement on T1C | ADEM | Convalescent plasma and IV steroids (Solu-Medrol for 2 patients, dexamethasone for 1 patient). 2 patients who did not improve with steroids received IVIG | 1 Recovered, Incomplete recovery for 2 | |
| Paterson et al. | CSF RTPCR negative in 3, brain tissue PCR negative in 1 | Negative in 4 | Negative MOG, AQ4, NAMDAR, lg1, GAD | DD 1160 μg/L - 80 000 μg/L in 4, lymphopenia in 2 | MRI brain - Diffuse subcortical, white matter, limbic and insular lobes, deep gray matter T2 hyperintense lesions. Corpus callosum, brain stem involved. Hemorrhagic changes in some lesions and some lesions showed enhancement on postcontrast images. One patient had severe vasogenic edema and midline shift | 5 AHEM, 1 ADEM | IV methylprednisolone, IVIG, antiepileptic, antibiotics, antiviral drugs | 5 Incomplete ongoing recovery; 1 death | |
| Yong et al. | Data unavailable | Negative MOG, AQ4, NAMDAR, lg1, GAD | Lymphopenia, raised serum ferritin CRP, DD, IL-6 | MRI brain - Multifocal cortical subcortical white matter lesions. Lt cortical lesion caused midline shift of 10 mm, lesions hemorrhagic. Patchy incomplete enhancement of few lesions. (thalamic) | AHEM | Remdesivir, enoxaparin, mannitol, IVIG, methylprednisolone | Incomplete recovery, quadriparetic | ||
| Chalil et al. | Negative (external ventricular drain sample) | Data unavailable | CSF IgG ratio 1.35, IgG index 1.05 | Raised Ferritin CRP | CT- B/L parietal occipital intraparenchymal hemorrhages with intraventricular extension, acute hydrocephalus. MRI - T2 Flair hyperintensities surrounding the hemorrhages, enhancement seen | AHEM | Hydroxychloroquine and tocilizumab, EVD for hydrocephalus | SVT and prolonged QT interval | Incomplete recovery, residual neurological deficits |
| Poyiadji et al. | could not be done | Negative | Data unavailable | Data unavailable | CT brain - Hypoattenuation in both thalami, MRI - Hemorrhagic rim enhancing lesions in thalami, medial temporal and sub insular region | ANHLE | IVIG | Unknown | |
| Zanin et al. | Negative | Negative | Data unavailable | Lymphopenia, raised CRP fibrinogen | Brain and spine MRI - periventricular confluent white matter lesions and high signal cord lesions from bulb medullary junction to T6 level; no contrast enhancement | ADEM | High-dose dexamethasone, antiepileptics, mechanical ventilation | Clinical deterioration. Hypoxic, required intubation ventilation | Complete recovery discharged after 1 month since admission |
IHD - Ischemic heart disease; CAD - Coronary artery disease; CABG - Coronary artery bypass grafting; HTN - Hypertension; DM - Diabetes mellitus; SARS-CoV-2 - Severe acute respiratory syndrome-coronavirus 2; COVID-19 - Coronavirus disease 2019; ARDS - Acute respiratory distress syndrome; RT-PCR - Reverse transcriptase-polymerase chain reaction; CSF - Cerebrospinal fluid; GCS - Glasgow Coma Scale; OCB - Oligoclonal band; ICP - Intracranial pressure; RBC - Red blood cell; MOG - Myelin oligodendrocyte glycoprotein; ACE - Angiotensin-converting enzyme; ANA - Anti neutrophil antibody; ANCA - Ant neutrophil cytoplasmic antibody; GAD - Generalized anxiety disorder; CNS - Central nervous system; CRP - C-reactive protein; FLAIR - Fluid-attenuated inversion recovery; IL-6 - Interleukin-6; MRI - Magnetic resonance imaging; LTEM - Longitudinally extensive transverse myelitis; CST - Corticospinal tract; ADEM - Acute disseminated encephalomyelitis; IV - Intravenous; IVIG - IV immunoglobulin G; NMO - Neuromyelitis optica; ANHLE - Acute necrotizing hemorrhagic leukoencephalopathy; CRRT - Continuous renal replacement therapy; SVT - Supraventricular tachycardia; AHEM - Acute hemorrhagic encephalomyelitis; AKI - Acute kidney injury; AQ4 - Aquaporin-4 antibody; DD - D-dimer; EVD - External ventricular drain; f/b - Followed by; h/o - History of; IgG - Immunoglobulin G
Demographic data
From the 21 total cases, 10 (47%) were male patients and 11 (53%) were female patients. Female:Male ratio is calculated to be 0.9:1. The age range of the patients was 21–71 years with a mean age of 51.36 years. Diabetes mellitus was the most common comorbidity seen in four patients, followed by hypertension seen in three patients. Other comorbidities were ischemic heart disease (1) and dyslipidemia (1). One patient had a past history of anterior communicating artery aneurysm clipping 20 years ago. Four patients had no prior illnesses, and details of 10/21 patients were not mentioned in the studies.
History of COVID-19
Out of 21 cases of ADEM, the diagnosis of SARS-CoV-2 was confirmed in 18 and suspected in 3. Out of 18 confirmed cases, 14 patients had positive oropharyngeal swab SARS-CoV-2 reverse transcriptase–polymerase chain reaction (RT-PCR), 1 patient had positive serum SARS-CoV-2 RT-PCR, 1 patient had positive cerebrospinal fluid (CSF) SARS-CoV-2 RT-PCR, and 2 patients tested positive for serum SARS-CoV-2-immunoglobulin G (IgG) with a negative oropharyngeal swab.
Only 15 patients presented with prior symptoms of SARS-CoV-2 infection. One patient contracted the infection in postoperative period while they were unspecified in three patients. Fever was the most common presenting symptom (9), followed by cough (6), fatigue, and myalgia (3).
Symptoms/clinical presentation
The duration of COVID-19 symptoms ranged from 3 days to 25 days before onset of neurological symptoms. Among the neurological symptoms, altered consciousness was most common (7), followed by anosmia (3), paraplegia (3), brain stem involvement (3), sphincter involvement (2), and quadriplegia (1). Only two patients directly presented with neurological symptoms.
Laboratory investigations
Blood investigations and CSF analysis were done in 17 patients. Raised inflammatory markers were most commonly seen in nine (ferritin raised in four, C-reactive protein in five, and D-dimer in five), and lymphopenia was seen in four patients.
CSF analysis was not reported in four patients. The reasons for the same were traumatic tap (1), raised intracranial pressure (ICP) (1), pandemic reason (1), and unknown reason (1).
Eight patients had normal CSF analysis on routine microscopy. Out of nine abnormal reports, increased CSF protein level was the most commonly reported abnormality in six, followed by lymphocytic pleocytosis in four patients. Meningitis was ruled out by negative CSF bacterial cultures in 5 and viral panel in 15 out of 17 patients. One CSF sample grew Staphylococcus capitis which was probably contaminated.
Although there was neurological involvement in all cases, CSF SARS-CoV-2 PCR was positive only in 1 patient out of 13 who were tested. One patient had brain tissue CoV-2 PCR negative on autopsy.
CSF autoimmune analysis was done in 17 patients. Only one patient had a CSF-IgG ratio of 1.35, one patient had four oligoclonal bands (OCBs), while one patient had mirror pattern of serum and CSF IgG. Antibodies for myelin oligodendrocyte glycoprotein (MOG) and aquaporin-4 were negative in nine patients.
Radiological investigations
CNS imaging was abnormal in 19 patients and unavailable in 2 patients. Diffuse white matter changes, occasionally involving the deep gray matter, were the most common finding. Out of 21 cases of ADEM spectrum, 13 were ADEM, 7 were AHEM (acute hemorrhagic encephalomyelitis [AHEM]), while 1 was acute necrotizing hemorrhagic leukoencephalopathy (ANHLE).
Treatment
Fifteen patients were treated with corticosteroids, 11 patients received IVIg, while 3 patients received convalescent plasma. Two patients needed surgical intervention: one patient underwent decompressive craniectomy (ADEM with hemorrhage) and the other one underwent external ventricular drainage.
Complications
Complications included seizures (1), acute kidney injury (AKI) and septicemic shock (1), raised ICP (1), and supraventricular tachycardia (SVT) secondary to hydroxychloroquine (HCQ) (1).
Outcome
One patient recovered completely and one had good recovery with mild deficits. Thirteen patients had incomplete recovery while three patients died as the consequence of the disease. The cause of death in these three patients was status epilepticus, septicemic shock with multiorgan failure and severe necrotizing encephalitis respectively.
Discussion
The clinical spectrum of COVID-19 is commonly represented by the respiratory system, but it also has myriad extrapulmonary manifestations, and neurological involvement is not unknown. Similar to other coronaviruses, SARS-CoV-2 also has neurotropic potential and the neurological manifestations of SARS-CoV-2 include anosmia, headache, seizures, cerebrovascular accidents, meningitis, encephalitis, ADEM, Guillain–Barre syndrome, and cerebral venous thrombosis.[27]
SARS-CoV-2 may directly invade the CNS, or it may cause a parainfectious autoimmune disease. ADEM is one such parainfectious disease affecting the CNS. It is commonly seen in pediatric population with few cases reported in adults. The pathogenesis of ADEM is still unclear. The most likely mechanism is autoimmunity triggered by cross-reaction of viral antigens to myelin proteins. AHEM or ANHLE are variants of ADEM which have a fulminant course.[9,10]
Recent literature on COVID-19 suggests that it also can have neurological manifestations with mechanisms similar to other members of its family. Yeh et al. reported the first case of ADEM associated with coronavirus OC43 in 2003 where the virus could be isolated from the CSF.[28] Arabi et al. reported a case of ADEM following a Middle East respiratory syndrome infection in 2015.[29] The routes of spread to the CNS can be hematogenous or retrograde via the olfactory nerves. The virus then uses angiotensin-converting enzyme 2 receptors on endothelial cells to enter the brain.[5,6,7,30] In an autopsy study, Paniz-Mondolfi et al. confirmed the presence of SARS-CoV-2 virus particles in the brain. They observed these viral particles in the endothelial cells of the frontal lobe of the brain.[31]
We had 18 confirmed cases of SARS-CoV-2, out of which only one patient had positive CSF SARS-CoV-2 PCR.
ADEM is a diagnosis of exclusion and needs high level of suspicion. The medical fraternity is overwhelmed with the current situation of the pandemic, and rare diagnoses may not seem very obvious unless specifically considered. According to a study by Pohl et al. and Silvia Tenembaum et al., ADEM is characterized by prodromal symptoms, followed by acute-onset encephalopathy along with multifocal neurological deficits.[20,21,32,33,34] We studied 21 patients of ADEM in SARS-CoV-2 infection and found that 71.4% (15) patients had a viral prodrome before the onset of neurological symptoms. Fever (9) was the most common presenting symptom, followed by cough (6), fatigue, and myalgia (3). These may not always be present in every case of ADEM and some patients may directly present with the neurological symptoms. Lin et al. studied 50 cases of ADEM and concluded that previous history of upper respiratory tract infection was seen only in 50% of the cases.[35] In our study, Abdi et al.[33] and Utukuri et al.[20] described one case each where the patient directly presented with neurological symptoms while Reichard et al.[21] described a case where an elderly patient contracted the infection postoperatively.
Wender et al [10] in their study mentioned that the presenting clinical features of ADEM were altered sensorium, seizures with focal neurological deficits like speech difficulty and limb weakness. Our findings were parallel to the literature and that altered sensorium was the most common neurological presentation seen in seven patients. Brain stem involvement indicates severe disease and was seen in three patients; all were elderly patients aged 48, 51, and 59 years.[15,24,36] Other neurological symptoms in our study were anosmia, limb weakness, and sphincter involvement. Less common symptoms were limb ataxia described by Utukuri et al.[20] in one case and vision impairment described by Novi et al.[14] in one case. Zhang et al. in their case report described a case with seventh and lower cranial nerve involvement with gaze abnormality.[18] Gaze abnormality was also seen in another case described by McCuddy et al.[23]
Blood investigations were mentioned in 17 patients. We found that the inflammatory markers were raised in 9/17 patients. It indicates pro-inflammatory state likely from the preceding infection. Four patients had lymphopenia suggestive of a viral infection. CSF analysis in suspected cases of ADEM is done to rule out infection or CNS pathologies. While CSF picture in ADEM is characteristic for its lack of abnormality, it may be abnormal in almost half of the patients.[37,38,39,40,41] The abnormal CSF findings were mild lymphocytic pleocytosis and elevated proteins which were seen in 30-40% of the patients.[32,34,42,43,44,45] In our study, 17 patients underwent CSF analysis which was normal in eight patients (47%). Out of the nine abnormal CSF analyses, raised CSF proteins were seen in six patients and lymphocytic pleocytosis was seen in four patients.
Bacterial meningitis was ruled out categorically in 5 out of 17 patients while viral panel was negative in 15 out of 17 patients. SARS-CoV-2 virus could not be isolated from the CSF samples. PCR was performed in 13 patients out of 21 and it was positive only in 1 patient reported by Novi et al.[14] Paterson et al. reported one case of ADEM with hemorrhage who needed decompression craniectomy where they obtained brain tissue for examination. They too could not isolate the virus in the brain tissue.[24]
Only one autopsy study by Paniz-Mondolfi et al. provides evidence for the presence of SARS-CoV-2 virus particles in the brain. They observed viral particles in the endothelial cells of the frontal lobe of the brain.[31]
CSF and Autoimmune analysis is an important part of investigations in ADEM cases. OCBs are inconsistently found in CSF in patients of ADEM. They have been reported only in 10%–50% of cases.[37,38,39,46] The presence of OCBs is suggestive of evolution to multiple sclerosis (MS).[47] In our study, CSF autoimmune workup was available in 17 patients. Parsons et al.[15] described one patient of ADEM who had 4°CBs in CSF.
Mirror pattern of OCB suggests that they are found in CSF as well as the serum. It is not specific for the diagnosis of ADEM. It only indicates the same autoimmune process in the serum as well as the CSF. Novi et al.[14] described another patient of ADEM with positive mirror pattern of OCB. Among the serum autoimmune studies to diagnose ADEM, high serum anti-MOG antibodies are found in almost half of the patients with ADEM, although they too are not specific to ADEM.[10,48]
The diagnosis of ADEM is based on multifocal CNS involvement, alteration of sensorium (encephalopathy), and brain and spine magnetic resonance imaging (MRI) consistent with demyelinating lesions. Imaging is an important pillar in the diagnosis of ADEM. The MRI findings of ADEM in the studies by Pohl et al [32] and TENEMBAUM et al [34] were multiple asymmetric bilateral hyperintensities, involving subcortical and deep white matter, grey white matter junction, deep grey nuclei, corpus callosum, brain stem, cerebellum and in one third of the cases even long segments in spinal cord. Bilateral thalamic involvement is seen in ANHLE which is a fulminant variant of ADEM. It is rapidly progressive and has high mortality.[49]
Brain lesions may be small or large confluent. They are usually hyperintense on T2-weighted and fluid-attenuated inversion recovery sequences and at times may show minimal enhancement. We found that almost all patients had bilateral asymmetric extensive lesions involving the subcortical and deep white matter. Novi et al.[14] described one patient who had optic nerve enhancement in postcontrast MRI. It occurs due to breakdown of the blood–brain barrier. We found that contrast-enhancing lesions were seen in 7 out of 13 patients of ADEM, 5 out of 7 patients of AHEM, and 1 patient of ANHLE. Contrast enhancement is thus suggestive of a severe disease as seen in AHEM and ANHLE. Four patients also had features of myelitis on imaging involving long segments.
MS is a differential diagnosis of ADEM. Although there are no absolute criteria, it is important to differentiate ADEM from MS. Characteristics Multiple Sclerosis lesions are periventricular in location, solitary, perpendicular to corpus callosum and 'black hole appearance' on T1.[32] Gray matter involvement and absence of CSF OCB also help in ruling out MS. History is also important, and MS does not follow a viral illness or vaccine. Other differentials to consider are MOG antibody-associated disease, neuromyelitis optica spectrum disorder, Progressive multifocal leukoencephalopathy–John Cunningham virus, and sarcoidosis.[50]
To date, there have been no randomized controlled trials and treatment of ADEM is not standardized. High-dose steroids are the first line of treatment, followed by intravenous Ig (IVIG) and plasmapheresis in nonresponders. High-dose steroids consist of intravenous methylprednisolone administered at 30 mg/kg/d (maximum 1 g/day for 5 days), followed by oral taper for 4–6 weeks that has shown to be effective in 60%–80% of the cases.[32,34,51,52]
In our study, eight patients received combination therapy with IV methylprednisolone as well as IVIg. There was no mortality in any of these patients, and all of them showed partial recovery. Four patients received only IVIg, while other four patients received only IV methylprednisolone and two patients received convalescent plasma. Acyclovir was given in two, remdesivir in one, HCQ in two, and antibiotics in four patients. Treatment details of one patient were unavailable. Literature shows that supportive empirical treatment with acyclovir is given in some cases of encephalitis till infective cause can be ruled out. The same, however, does not apply for empirical use of antibiotics.[53]
We found that four patients developed complications. They were status epilepticus (1), AKI and septicemic shock (1), raised ICP with mass effect and midline shift (1), and acute hydrocephalus and SVT (1). Out of these, AKI and septicemic shock were secondary to SARS-CoV-2 infection in a case described by Reichard et al.[21] They describe a 71-year-old male patient who contracted SARS-CoV-2 postoperatively and subsequently developed ADEM. His course was complicated by respiratory failure and AKI secondary to septicemic shock. He required continuous renal replacement therapy but eventually succumbed.
Abdi et al.[33] describe a case of a 58-year-old male patient diagnosed as ADEM, who had status epilepticus and finally succumbed. Paterson et al.[24] describe a case with AHLE who developed raised ICP and required decompressive hemicraniectomy. Another patient of AHLE developed SVT secondary to HCQ treatment which could be managed medically. The same patient had also intraventricular hemorrhage and obstructive hydrocephalus for which he needed an external ventricular drain.[36]
ADEM has a favorable outcome in 10%–50% of patients who may show complete recovery.[37,38,40] We observed that 14% (3) patients had good outcome at the end of treatment while 66.6% (14) patients had partial ongoing recovery including all those with AHLE, some patients may be left with residual neurological deficits or may even have a relapse. In case of a relapse, the likelihood of MS should be kept in mind.
The mortality rate of ADEM is reported as 5%–12%[37,38] while another study reported it to be 25%.[54] Adults have a more serious course of ADEM as compared to children. The rapidly progressive cases such as ANHLE or AHEM can be fatal. In our study, mortality was 14% (3) while the outcome of one patient remains unknown at the time of writing this article.
Our study proves to be an important evidence in the literature where data on ADEM as a rare complication of COVID-19 are scarce. However, these findings could be confirmed by larger scale studies. Another limitation of our study is inclusion of data from webpages/news report article[22] as the official case reports of few cases were unavailable at present. This could reflect poor quality of the included study.
Conclusion
In the current situation with very few studies available focusing on this rare complication, our study proves to be an important link in the evidence and literature. The importance of knowledge about this less known but important clinical entity cannot be understated. The physicians and neurosurgeons should be diligent while treating the COVID-19 patients with neurological manifestations and include ADEM as a differential diagnosis and stress on early diagnosis and treatment to reduce mortality and achieve satisfactory clinical outcome.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.WHO. Global Update on Coronavirus Disease. Global Update on Coronavirus Disease. WHO; 2020. [Last accessed on 2020 Aug 13]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov . [Google Scholar]
- 2.Worldometers. COVID-19 Coronavirus Pandemic. 2020. [Last accessed on 2020 Aug 13]. Available from: https://www.worldometers.info/coronavirus/
- 3.Behzad S, Aghaghazvini L, Radmard AR, Gholamrezanezhad A. Extrapulmonary manifestations of COVID-19: Radiologic and clinical overview. Clin Imaging. 2020;66:35–41. doi: 10.1016/j.clinimag.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, et al. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26:1017–32. doi: 10.1038/s41591-020-0968-3. [DOI] [PubMed] [Google Scholar]
- 5.Bohmwald K, Gálvez NM, Ríos M, Kalergis AM. Neurologic alterations due to respiratory virus infections. Front Cell Neurosci. 2018;12:386. doi: 10.3389/fncel.2018.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arbour N, Day R, Newcombe J, Talbot PJ. Neuroinvasion by human respiratory coronaviruses. J Virol. 2000;74:8913–21. doi: 10.1128/jvi.74.19.8913-8921.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Desforges M, Le Coupanec A, Dubeau P, Bourgouin A, Lajoie L, Dubé M, et al. Human coronaviruses and other respiratory viruses: Underestimated opportunistic pathogens of the central nervous system? Viruses. 2019;12:14. doi: 10.3390/v12010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Montalvan V, Lee J, Bueso T, De Toledo J, Rivas K. Neurological manifestations of COVID19 and other coronavirus infections: A systematic review. Clin Neurol Neurosurg. 2020;194:1–7. doi: 10.1016/j.clineuro.2020.105921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garg RK. Acute disseminated encephalomyelitis. Postgrad Med J. 2003;79:11–7. doi: 10.1136/pmj.79.927.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wender M. Acute disseminated encephalomyelitis (ADEM) J Neuroimmunol. 2011;231:92–9. doi: 10.1016/j.jneuroim.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 11.Garg RK. Spectrum of neurological manifestations in COVID-19: A review. Neurol India. 2020;68:560–72. doi: 10.4103/0028-3886.289000. [DOI] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, Altman DG, Group TP. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma LL, Wang YY, Yang ZH, Huang D, Weng H, Zeng XT. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: What are they and which is better? Mil Med Res. 2020;7:7. doi: 10.1186/s40779-020-00238-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Novi G, Rossi T, Pedemonte E, Saitta L, Rolla C, Roccatagliata L, et al. Acute disseminated encephalomyelitis after SARS-CoV-2 infection. Neurol Neuroimmunol Neuroinflamm. 2020;7:e797. doi: 10.1212/NXI.0000000000000797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parsons T, Banks S, Bae C, Gelber J, Alahmadi H, Tichauer M. COVID-19-associated acute disseminated encephalomyelitis (ADEM) J Neurol. 2020;267:2799–802. doi: 10.1007/s00415-020-09951-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zoghi A, Ramezani M, Roozbeh M, Darazam IA, Sahraian MA. A case of possible atypical demyelinating event of the central nervous system following COVID19. Mult Scler Relat Disord. 2020;44:1–4. doi: 10.1016/j.msard.2020.102324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zanin L, Saraceno G, Panciani PP, Renisi G, Signorini L, Migliorati K, et al. SARS-CoV-2 can induce brain and spine demyelinating lesions. Acta Neurochir (Wien) 2020;162:1491–4. doi: 10.1007/s00701-020-04374-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang T, Hirsh E, Zandieh S. COVID-19-Associated Acute Multi-infarct Encephalopathy in an Asymptomatic CADASIL Patient. Neurocrit Care. 2021;34:1099–1102. doi: 10.1007/s12028-020-01119-7. https://doi.org/10.1007/s12028-020-01119-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alimohammadi E, Bagheri SR, Hadidi H, Rizevandi P, Abdi A. Carpal tunnel surgery: Predictors of clinical outcomes and patients' satisfaction. BMC Musculoskelet Disord. 2020;21:51. doi: 10.1186/s12891-020-3082-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Utukuri PS, Bautista A, Lignelli A, Moonis G. Possible acute disseminated encephalomyelitis related to severe acute respiratory syndrome coronavirus 2 infection. Am J Neuroradiol. 2020;41:E82–3. doi: 10.3174/ajnr.A6714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reichard RR, Kashani KB, Boire NA, Constantopoulos E, Guo Y, Lucchinetti CF. Neuropathology of COVID-19: A spectrum of vascular and acute disseminated encephalomyelitis (ADEM)-like pathology. Acta Neuropathol. 2020;140:1–6. doi: 10.1007/s00401-020-02166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharma N. India's First COVID Associated ADEM Case Cured in Gurgaon Hospital; June 24, 2020. [Last accessed on 2020 Aug 16]. Available from: https://economictimes.indiatimes.com/news/politics-and-nation/indias-first-covid-associated-adem-case-cured-in-gurgaon-hospital/articleshow/76542162.cms .
- 23.McCuddy M, Kelkar P, Zhao Y, Wicklund D. Acute Demyelinating Encephalomyelitis (ADEM) in COVID19 infection: A case series. medRxiv. 2020;68(5):1192–1195. doi: 10.4103/0028-3886.299174. doi: 10.1101/2020.07.15.20126730. [DOI] [PubMed] [Google Scholar]
- 24.Paterson RW, Brown RL, Benjamin L, Nortley R, Wiethoff S, Bharucha T, et al. The emerging spectrum of COVID-19 neurology: Clinical, radiological and laboratory findings. Brain. 2020;143:3104–20. doi: 10.1093/brain/awaa240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yong MH, Chan YF, Liu J, Sanamandra SK, Kheok SW, Lim KC, et al. A rare case of acute hemorrhagic leukoencephalitis in a COVID19 patient. J Neurol Sci. 2020;416:1–3. doi: 10.1016/j.jns.2020.117035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poyiadji N, Shahin G, Noujaim D, Stone M, Patel S, Griffith B. COVID-19–associated acute hemorrhagic necrotizing encephalopathy: Imaging features. Radiology. 2020;296:E119–20. doi: 10.1148/radiol.2020201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Munhoz RP, Pedroso JL, Nascimento FA, Almeida SM, Barsottini OG, Cardoso FE, et al. Neurological complications in patients with SARS-CoV-2 infection: A systematic review. Arq Neuropsiquiatr. 2020;78:290–300. doi: 10.1590/0004-282x20200051. [DOI] [PubMed] [Google Scholar]
- 28.Yeh EA, Collins A, Cohen ME, Duffner PK, Faden H. Detection of coronavirus in the central nervous system of a child with acute disseminated encephalomyelitis. Pediatrics. 2004;113:e73–6. doi: 10.1542/peds.113.1.e73. [DOI] [PubMed] [Google Scholar]
- 29.Arabi YM, Harthi A, Hussein J, Bouchama A, Johani S, Hajeer AH, et al. Severe neurologic syndrome associated with Middle East respiratory syndrome corona virus (MERS-CoV) Infection. 2015;43:495–501. doi: 10.1007/s15010-015-0720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li MY, Li L, Zhang Y, Wang XS. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty. 2020;9:45. doi: 10.1186/s40249-020-00662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paniz-Mondolfi A, Bryce C, Grimes Z, Gordon RE, Reidy J, Lednicky J, et al. Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) J Med Virol. 2020;92:699–702. doi: 10.1002/jmv.25915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pohl D, Alper G, Van Haren K, Kornberg AJ, Lucchinetti CF, Tenembaum S, Belman AL. Acute disseminated encephalomyelitis: Updates on an inflammatory CNS syndrome. Neurology. 2016 Aug;30(9 Supplement 2):S38–45. doi: 10.1212/WNL.0000000000002825. 87. doi: 10.1212/WNL.0000000000002825. PMID: 27572859. [DOI] [PubMed] [Google Scholar]
- 33.Abdi S, Ghorbani A, Fatehi F. The association of SARSCoV2 infection and acute disseminated encephalomyelitis without prominent clinical pulmonary symptoms. J Neurol Sci. 2020;416:1–2. doi: 10.1016/j.jns.2020.117001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tenembaum S, Chitnis T, Ness J, Hahn JS International Pediatric MS Study Group. Acute disseminated encephalomyelitis. Neurology. 2007;68:S23–36. doi: 10.1212/01.wnl.0000259404.51352.7f. [DOI] [PubMed] [Google Scholar]
- 35.Lin CH, Jeng JS, Hsieh ST, Yip PK, Wu RM. Acute disseminated encephalomyelitis: A follow-up study in Taiwan. J Neurol Neurosurg Psychiatry. 2007;78:162–7. doi: 10.1136/jnnp.2005.084194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chalil A, Baker CS, Johnston RB, Just C, Debicki DB, Mayich MS, Bosma KJ, Steven DA. Acute Hemorrhagic Encephalitis Related to COVID-19. Neurol Clin Pract. 2021 Apr;11(2):e147–e151. doi: 10.1212/CPJ.0000000000000900. doi: 10.1212/CPJ.0000000000000900. PMID: 33842083. PMCID: PMC8032421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwarz S, Mohr A, Knauth M, Wildemann B, Storch-Hagenlocher B. Acute disseminated encephalomyelitis: A follow-up study of 40 adult patients. Neurology. 2001;56:1313–8. doi: 10.1212/wnl.56.10.1313. [DOI] [PubMed] [Google Scholar]
- 38.Ketelslegers IA, Visser IE, Neuteboom RF, Boon M, Catsman-Berrevoets CE, Hintzen RQ. Disease course and outcome of acute disseminated encephalomyelitis is more severe in adults than in children. Mult Scler. 2011;17:441–8. doi: 10.1177/1352458510390068. [DOI] [PubMed] [Google Scholar]
- 39.Koelman DLH, Chahin S, Mar SS, Venkatesan A, Hoganson GM, Yeshokumar AK, et al. Acute disseminated encephalomyelitis in 228 patients: A retrospective, multicenter US study. Neurology. 2016;86:2085–93. doi: 10.1212/WNL.0000000000002723. [DOI] [PubMed] [Google Scholar]
- 40.Höllinger P, Sturzenegger M, Mathis J, Schroth G, Hess CW. Acute disseminated encephalomyelitis in adults: A reappraisal of clinical, CSF, EEG, and MRI findings. J Neurol. 2002;249:320–9. doi: 10.1007/s004150200012. [DOI] [PubMed] [Google Scholar]
- 41.Marchioni E, Ravaglia S, Piccolo G, Furione M, Zardini E, Franciotta D, et al. Postinfectious inflammatory disorders: Subgroups based on prospective follow-up. Neurology. 2005;65:1057–65. doi: 10.1212/01.wnl.0000179302.93960.ad. [DOI] [PubMed] [Google Scholar]
- 42.Torisu H, Kira R, Ishizaki Y, Sanefuji M, Yamaguchi Y, Yasumoto S, et al. Clinical study of childhood acute disseminated encephalomyelitis, multiple sclerosis, and acute transverse myelitis in Fukuoka Prefecture, Japan. Brain Dev. 2010;32:454–62. doi: 10.1016/j.braindev.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 43.Pavone P, Pettoello-Mantovano M, Le Pira A, Giardino I, Pulvirenti A, Giugno R, et al. Acute disseminated encephalomyelitis: A long-term prospective study and meta-analysis. Neuropediatrics. 2010;41:246–55. doi: 10.1055/s-0031-1271656. [DOI] [PubMed] [Google Scholar]
- 44.Erol I, Ozkale Y, Alkan O, Alehan F. Acute disseminated encephalomyelitis in children and adolescents: A single center experience. Pediatr Neurol. 2013;49:266–73. doi: 10.1016/j.pediatrneurol.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hung PC, Wang HS, Chou ML, Lin KL, Hsieh MY, Wong AM. Acute disseminated encephalomyelitis in children: A single institution experience of 28 patients. Neuropediatrics. 2012;43:64–71. doi: 10.1055/s-0032-1309309. [DOI] [PubMed] [Google Scholar]
- 46.Sonneville R, Demeret S, Klein I, Bouadma L, Mourvillier B, Audibert J, et al. Acute disseminated encephalomyelitisin the intensive care unit: Clinical features and outcome of 20 adults. Intensive Care Med. 2008;34:528–32. doi: 10.1007/s00134-007-0926-2. [DOI] [PubMed] [Google Scholar]
- 47.Arrambide G, Tintore M, Espejo C, Auger C, Castillo M, Río J, et al. The value of oligoclonal bands in the multiple sclerosis diagnostic criteria. Brain. 2018;141:1075–84. doi: 10.1093/brain/awy006. [DOI] [PubMed] [Google Scholar]
- 48.Brilot F, Dale RC, Selter RC, Grummel V, Kalluri SR, Aslam M, et al. Antibodies to native myelin oligodendrocyte glycoprotein in children with inflammatory demyelinating central nervous system disease. Ann Neurol. 2009;66:833–42. doi: 10.1002/ana.21916. [DOI] [PubMed] [Google Scholar]
- 49.Hart MN, Earle KM. Haemorrhagic and perivenous encephalitis: A clinical-pathological review of 38 cases. J Neurol Neurosurg Psychiatry. 1975;38:585–91. doi: 10.1136/jnnp.38.6.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Borisow N, Mori M, Kuwabara S, Scheel M, Paul F. Diagnosis and treatment of NMO spectrum disorder and MOG-encephalomyelitis. Front Neurol. 2018;9:888. doi: 10.3389/fneur.2018.00888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dale RC, de Sousa C, Chong WK, Cox TC, Harding B, Neville BG. Acute disseminated encephalomyelitis, multiphasic disseminated encephalomyelitis and multiple sclerosis in children. Brain. 2000;123(12):2407–22. doi: 10.1093/brain/123.12.2407. [DOI] [PubMed] [Google Scholar]
- 52.Hynson JL, Kornberg AJ, Coleman LT, Shield L, Harvey AS, Kean MJ. Clinical and neuroradiologic features of acute disseminated encephalomyelitis in children. Neurology. 2001;56:1308–12. doi: 10.1212/wnl.56.10.1308. [DOI] [PubMed] [Google Scholar]
- 53.Ellul M, Solomon T. Acute encephalitis – Diagnosis and management. Clin Med (Lond) 2018;18:155–9. doi: 10.7861/clinmedicine.18-2-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marchioni E, Marinou-Aktipi K, Uggetti C, Bottanelli M, Pichiecchio A, Soragna D, et al. Effectiveness of intravenous immunoglobulin treatment in adult patients with steroid-resistant monophasic or recurrent acute disseminated encephalomyelitis. J Neurol. 2002;249:100–4. doi: 10.1007/pl00007836. [DOI] [PubMed] [Google Scholar]