Abstract
Background
Amplitude spectral area (AMSA) predicts termination of fibrillation (TOF) with return of spontaneous circulation (ROSC) and survival in adults but has not been studied in pediatric cardiac arrest. We characterized AMSA during pediatric cardiac arrest from a Pediatric Resuscitation Quality Collaborative and hypothesized that AMSA would be associated with TOF and ROSC.
Methods and Results
Children aged <18 years with cardiac arrest and ventricular fibrillation were studied. AMSA was calculated for 2 seconds before shock and averaged for each subject (AMSA‐avg). TOF was defined as termination of ventricular fibrillation 10 seconds after defibrillation to any non‐ventricular fibrillation rhythm. ROSC was defined as >20 minutes without chest compressions. Univariate and multivariable logistic regression analyses controlling for weight, current, and illness category were performed. Primary end points were TOF and ROSC. Secondary end points were 24‐hour survival and survival to discharge. Between 2015 and 2019, 50 children from 14 hospitals with 111 shocks were identified. In univariate analyses AMSA was not associated with TOF and AMS‐Aavg was not associated with ROSC. Multivariable logistic regression showed no association between AMSA and TOF but controlling for defibrillation average current and illness category, there was a trend to significant association between AMSA‐avg and ROSC (odds ratio, 1.10 [1.00‒1.22] P=0.058). There was no significant association between AMSA‐avg and 24‐hour survival or survival to hospital discharge.
Conclusions
In pediatric patients, AMSA was not associated with TOF, whereas AMSA‐avg had a trend to significance for association in ROSC, but not 24‐hour survival or survival to hospital discharge.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: NCT02708134.
Keywords: cardiopulmonary resuscitation, defibrillation, pediatrics, ventricular fibrillation
Subject Categories: Arrhythmias, Ventricular Fibrillation, Sudden Cardiac Death, Cardiopulmonary Arrest, Cardiopulmonary Resuscitation and Emergency Cardiac Care
Nonstandard Abbreviations and Acronyms
- AMSA
amplitude spectral area
- AMSA‐avg
AMSA‐average
- IHCA
in‐hospital cardiac arrest
- OHCA
out‐of‐hospital cardiac arrest
- pediRES‐Q
The Pediatric Resuscitation Quality Collaborative
- TOF
termination of fibrillation
Clinical Perspective
What Is New?
Unlike adult cardiac arrest studies where amplitude spectral area (AMSA) may predict outcomes of cardiopulmonary arrest, we found no association in pediatric ventricular fibrillation arrest between AMSA and termination of ventricular fibrillation.
Similar to adult out‐of‐hospital cardiac arrest trials, we found a trend towards significance between AMSA‐average and return of spontaneous circulation in pediatric patients.
Unlike adult cardiac arrest studies, we found no association between AMSA‐average and survival outcomes in pediatric ventricular fibrillation arrest.
What Are the Clinical Implications?
Factors specific to pediatric in‐hospital cardiac arrest are vastly different from adult out‐of‐hospital cardiac arrest and may partially explain why we saw no robust association between AMSA and termination of ventricular fibrillation.
Children are more likely to suffer in‐hospital cardiac arrest that is witnessed and monitored, while adults are more likely to experience a sudden cardiac arrest that is out‐of‐hospital, and more often related to coronary artery disease, unwitnessed, and unmonitored which may explain differences in adult and pediatric AMSA findings.
Further study in pediatrics on much larger populations in each of the age categories (infant, child, adolescent) are needed, and will need to be analyzed in subgroups of children with congenital/acquired heart disease versus those without heart disease.
More than 5000 children experience a non‐traumatic pediatric out‐of‐hospital cardiac arrest (OHCA) each year1 and nearly 8000 children in the United States receive in‐hospital cardiopulmonary resuscitation (CPR) each year,2 with a rate of 2% to 6% in pediatric intensive care settings.3, 4 Most of these children do not survive to hospital discharge.5 Although survival to hospital discharge after pediatric in‐hospital cardiac arrest (IHCA) has improved over the last 25 years from 9% to 13.7%6, 7 to 35% (78.1% with a favorable neurological outcome),8 the variability in survival rates suggests potential opportunities for improvement.9, 10 Ventricular fibrillation (VF) and pulseless ventricular tachycardia (pVT) are among the most common causes of cardiac arrest in adults but are much less common causes of cardiac arrest in children. In children, cardiac arrests are more typically the consequence of progressive respiratory failure or shock, with electrocardiographic asystole or pulseless electrical activity, rather than primary arrhythmogenic events. A 2006 multicenter registry report identified VF or pVT in 27% of patients with IHCA.11 Shockable rhythms must be immediately treated with electric shock(s) and high‐quality CPR.5 Although defibrillators are widely used and evidence‐based guidelines exist for adults, defibrillation in children is based on limited evidence and unanswered questions still persist about the optimal treatment of pediatric shockable rhythms.12, 13, 14, 15
Early defibrillation in conjunction with chest compressions can allow for return of spontaneous circulation (ROSC) after cardiac arrest with a shockable rhythm.16, 17 Timing of defibrillation in relationship to chest compressions, however, is a subject of major interest because it is difficult to determine the priority of CPR interventions (ie, defibrillation first or chest compressions first).17 For shockable rhythms the current American Heart Association Pediatric Advanced Life Support Guidelines recommend immediate defibrillation with an initial dose of 2 to 4 J/kg of monophasic or biphasic energy for defibrillation, but for ease of teaching, an initial dose of 2 J/kg may be considered. For refractory VF/pVT, it is recommended to increase the dose to 4 J/kg and for subsequent energy levels a dose of 4 J/kg may be reasonable and higher energy levels may be considered, though not to exceed 10 J/kg or the adult maximum dose.5, 18 Subsequent defibrillations for refractory VF/pVT are recommended to be attempted on a time‐based protocol (ie, after every 2‐minute cycle of chest compressions) without any evaluation of the pathophysiological pattern of the arrested myocardium over time.19, 20, 21, 22 Thus, the current time‐based defibrillation algorithm may lead to futile defibrillation attempts and unnecessary chest compression interruptions, potentially worsening outcome.
Features of the ECG waveform during VF, such as frequency and amplitude‐related information, may help identify when the myocardium can be successfully defibrillated and ultimately lead to a perfusing rhythm sooner.23, 24 Amplitude spectral area (AMSA) describes the amplitude‐weighted mean frequency and reflects the summed product of VF frequency and signal amplitude. AMSA has been shown to correlate with coronary perfusion pressure during chest compressions25, 26 and with myocardial energy phosphate concentrations.27 Furthermore, AMSA has been shown to predict defibrillation success and ROSC in both animal23, 28, 29 and adult cardiac arrest studies,24, 30, 31, 32 as well as survival to hospital discharge.33 In contrast, there have been no studies investigating AMSA and defibrillation success for shockable rhythms in pediatric cardiac arrest to date. Therefore, we sought to characterize defibrillation outcomes and AMSA values during pediatric cardiac arrest from a pediatric resuscitation quality (pediRES‐Q) collaborative consortium. The goal was to test the hypothesis that AMSA is associated with termination of fibrillation (TOF) and ROSC. We also investigated the association of AMSA‐average (AMSA‐avg) values with 24‐hour survival and survival to hospital discharge.
METHODS
Design and Setting
The data that support the findings of this study are available from the corresponding author (T.T.R.) upon reasonable request. This was a convenience sample of data collected from the pediRES‐Q Collaborative (ClinicalTrials.gov: NCT02708134). The pediRES‐Q Collaborative is a large, multi‐center international pediatric resuscitation quality improvement network (Appendix). The study was approved by local institutional review boards (United States) or research ethics boards (Europe and Canada) and met criteria for a waiver of consent per code of Federal Regulations 45 CFR 46.116(d) and 45 CFR 46.408(a) https://www.hhs.gov/ohrp/regulations‐and‐policy/regulations/45‐cfr‐46/index.html—46.116. An additional Data Use Agreement was obtained per local institutional regulations. Strict compliance with the Health Insurance Portability and Accountability Act to ensure patient confidentiality was maintained at all times.
Population
This study includes patients with cardiac arrest from hospitals participating in the pediRES‐Q collaborative. A convenience sample of cardiac arrest events (that were entered in the collaborative database) in children aged <18 years with cardiac arrest and VF, and those who had complete CPR quality metric data captured from the bedside defibrillator (ZOLL R‐Series, Chelmsford, MA) were studied. Only the index (initial) cardiac arrest event was analyzed per patient. Events with defibrillation attributable to ventricular tachycardia, inappropriate shocks (ie, sinus rhythm, supraventricular tachycardia, conduction block, asystole, paced rhythm), ECG noise or drift, and unavailable AMSA values were excluded.
ECG Analyses
Electronic waveform data were recorded from the defibrillator pads to the monitor/defibrillator at a sampling rate of 250 samples/s, then downloaded and analyzed with customized software (Matlab Mathworks, Natick, MA) in accordance with previous studies.24 Briefly, the ECG signals were filtered (2 Hz high pass, 48 Hz low pass) to remove noise. AMSA was then computed as the sum of products of individual frequencies and their amplitude, where Ai represents the amplitude at the ith frequency, Fi.24 The analysis was performed on a 512‐point window (≈2 seconds of ECG signal at a sample rate of 250 Hz); a Tukey window was used to reduce edge effects.24 In a majority of cases (98/111 shocks), the AMSA analysis was performed on the ECG signal ≈2.5 seconds before shock, only if there was no CPR artifact in the ECG signal. However, if a CPR‐related noise was present immediately before the shock, then the ECG record was scanned temporally for up to 1 minute before the defibrillation shock to find a 2‐second ECG signal free of CPR artifact which was closest to the shock reference point to calculate the AMSA value (13/111 shocks).
In addition to calculating AMSA for each shock, AMSA was also averaged over all shocks within a subject to calculate AMSA‐avg, as per a previous study.33 The average of AMSA was chosen to allow for an overall representation of the VF waveform as AMSA values can vary over time.33 The post‐shock defibrillation outcome was also analyzed, and assigned binary values of 0 or 1, depending upon the presence of VF or TOF, respectively. Three independent investigators (T.T.R., D.L.A., S.V.P.) reviewed all Zoll defibrillator files and confirmed VF and TOF. TOF was defined as termination of VF 10 seconds after defibrillation to any rhythm other than VF. ROSC was defined as >20 minutes without chest compressions; ROC‐ECMO was defined as the return of circulation by means of ECMO. Delta AMSA (dAMSA) was also calculated in patients with at least 2 shocks and was defined as AMSA at the second shock (AMSA2)—AMSA at the first shock (AMSA1). Prior adult studies have shown in the early phase of VF OHCA, both a high AMSA and an increase in AMSA (dAMSA) indicated a high likelihood of a successful defibrillation.34, 35 Figure 1 shows an ECG example during VF, with accompanying AMSA calculations and the CPR artifact signals. The AMSA value for the VF signal 2.5 seconds before shock was calculated to be 18.37 mV‐Hz in this case. A prior adult OHCA study that calculated AMSA using the same ECG analyses methods used in our work, found that AMSA threshold for all defibrillation success was 15.5 mV‐Hz.36
Figure 1. Shock 1 parameters.
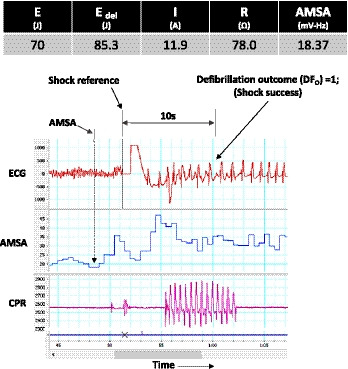
AMSA indicates amplitude spectral area; and CPR, cardiopulmonary resuscitation.
The CPR artifact is seen to be absent before shock, and only present for a few seconds post‐shock. Defibrillation outcome was seen to be 1 (TOF), at 10 seconds post‐shock. Finally, we also recorded all‐shock parameters associated with each defibrillation as shown for this case; these included the shock energy selected on the defibrillator (70 J), the actual energy delivered (85.3 J), the current delivered (11.9 A), and the trans‐thoracic impedance (78 Ω).
Data Collection
A data collection form with 100 data elements was created which aligns with the American Heart Association's Get with the Guidelines‐Resuscitation cardiopulmonary arrest patient management tool (http://www.heart.org/idc/groups/heart‐public/@private/@wcm/@hcm/@gwtg/documents/downloadable/ucm_457481.pdf). Data on each resuscitation event were collected by a site investigator or research staff. Research staff at each site designated to perform data entry were required to complete a 1‐hour training session by the Data Coordinating Center (Children's Hospital of Philadelphia) consisting of a 1‐on‐1 live webinar before database access. Data were entered into and managed using Research Electronic Data Capture electronic data capture tools coordinated and hosted at The Children's Hospital of Philadelphia under an agreement with the software's development consortium, led by Vanderbilt University. Finally, once entered, each event record went through a manual review by the Data Coordinating Center to be approved and added to the data set.
Statistical Analysis
Demographic characteristics and clinical data are summarized using descriptive statistics. Median and interquartile ranges (IQR) were used for reporting continuous variables since AMSA was not normally distributed. Count and percentage were used for categorical variables. Univariate associations were evaluated between TOF and AMSA at all shocks, and between AMSA‐avg value and survival outcomes (ROSC, 24‐hour survival and survival to discharge) by using simple logistic regressions. Primary end points were TOF and ROSC. Secondary end points were 24‐hour survival and survival to hospital discharge. Then multivariable logistic regression models were used to assess the association between AMSA‐avg and ROSC/survival outcomes after adjusting for average defibrillation current and illness category (cardiac versus noncardiac). Generalized estimating equations method with an exchangeable working correlation structure was used in the all‐shock regression analysis to account for the multiple shocks within same patient. Sensitivity analysis of the 98 shocks where the AMSA analysis was performed on the ECG signal ≈2.5 seconds before shock was also calculated. Lastly, an ROC curve analysis using AMSA‐avg to predict ROSC was performed. Sensitivity, specificity, positive predictive value, and negative predictive value were calculated and reported. Data analyses were performed using Excel and the statistical packages SAS version 9.4 (SAS Institute, Inc., Cary, NC).
RESULTS
Between October 2015 and November 2019, there were 125 children who received 331 shocks from the pediRES‐Q database. Seventy‐five children with 220 shocks were excluded. A total of 111 shocks for VF were analyzed for AMSA in 50 children from 14 hospitals (Figure 2). A breakdown revealed that of the 111 shocks, 39 were shock 1, 21 shock 2, 17 shock 3, 12 shock 4, 7 shock 5, 3 Shock 6, 3 shock 7, 1 shock 8, 2 shock 9, 2 shock 10, 1 shock 11, 1 shock 13, 1 shock 14, and 1 shock 15. IHCA occurred in 47 children and OHCA in 3 children. The patient and resuscitation characteristics of the subjects is depicted in Table 1. The median age was 3.7 years [IQR, 0.6‒13.1], median weight 16.3 kg [IQR, 6.9‒37.2], with 46% male and 73% with a cardiac illness category. Sixty‐four percent of the events had an immediate cause of arrhythmia, 78% of the IHCA events occurred in an intensive care unit or emergency room, and 52% were on continuous vasoactive infusions. We analyzed 111 shocks with a median number of defibrillations of 1.0 [IQR, 1.0‒3.0], median defibrillation energy dose of 3.27 J/kg [IQR, 2.65‒5.01], median defibrillation current of 0.64 A/kg [IQR, 0.38‒0.96], median AMSA of 12.21 [IQR, 7.17‒17.93], and median AMSA‐avg of 14.6 [IQR, 8.6‒19.2]. TOF was achieved in 72 defibrillations (65%), ROSC in 31 (62%), ROC‐ECMO in 11 (22%), 24‐hour survival in 40 (80%), and survival to hospital discharge in 28 (56%). Of the 26 children who survived to hospital discharge, 15 (58%) had favorable neurologic outcome (Pediatric Cerebral Performance Category=1–2). Table 2 depicts univariate predictors of resuscitation outcome and Table 3 multivariate predictors of outcome. Controlling for average defibrillation current and illness category, there was a trend toward significant association between AMSA‐avg and ROSC (odds ratio [OR], 1.10 [1.00‒1.22]; P=0.058). Additionally, defibrillation average current was also significantly associated with ROSC (OR, 1.13 [1.01‒1.26]; P=0.03). There was no significant association between AMSA‐avg and 24‐hour survival or survival to hospital discharge.
Figure 2. Patient selection cohort.
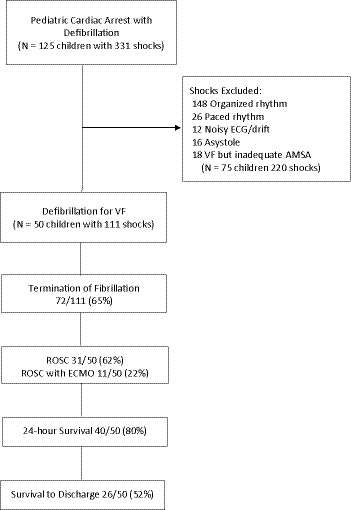
AMSA indicates amplitude spectral area; ECMO, extracorporeal membrane oxygenation; ROC‐ECMO, return of circulation by means of ECMO (extracorporeal membrane oxygenation); ROSC, return of spontaneous circulation; and VF, ventricular fibrillation.
Table 1.
Patient and Resuscitation Characteristics of Trial Subjects (n=50)
| Age, y | 3.7 [0.6‒13.1] |
| Weight, kg | 16.3 [6.9‒37.2] |
| Women | 27 (54) |
| Illness category | |
| Cardiac, medical or surgical | 35 (73) |
| Noncardiac, medical or surgical | 13 (27) |
| Location of arrest | |
| Cardiac ICU | 13 (26) |
| Neonatal ICU | 1 (2) |
| Pediatric ICU | 22 (44) |
| Emergency department | 3 (6) |
| Other | 8 (16) |
| Out‐of‐hospital | 3 (6) |
| Preexisting conditions at time arrest | |
| Cardiac malformation | 27 (54) |
| Noncardiac malformation | 7 (14) |
| Metabolic/electrolyte abnormality | 8 (16) |
| Renal insufficiency | 8 (16) |
| Respiratory insufficiency | 24 (48) |
| Hypotension | 14 (28) |
| Congestive heart failure | 15 (30) |
| Interventions in place at time arrest | |
| Arterial catheter | 19 (38) |
| Continuous vasoactive agent | 26 (52) |
| Continuous anti‐arrhythmic | 6 (12) |
| ECG | 31 (62) |
| Pulse oximeter | 37 (74) |
| Supplemental oxygen | 26 (52) |
| Vascular access | 41 (82) |
| Mechanical ventilation | 23 (46) |
| Immediate cause of arrest | |
| Arrhythmia | 32 (64) |
| Hypotension/hypoperfusion | 8 (16) |
| Hypoxia/respiratory insufficiency | 10 (20) |
| Pharmacologic interventions | |
| Epinephrine | 44 (88) |
| Sodium bicarbonate | 21 (42) |
| Calcium chloride/gluconate | 29 (58) |
| Lidocaine | 11 (22) |
| Atropine | 6 (12) |
| Magnesium sulfate | 14 (28) |
| No. defibrillation attempts | 1 [1‒3] |
| Initial rhythm | |
| Pulseless | 38 (76) |
| Pulse then pulseless | 5 (10) |
| Unknown | 7 (14) |
| Initial pulseless rhythm | |
| Asystole | 4 (8) |
| PEA | 10 (20) |
| VF | 16 (32) |
| pVT | 16 (32) |
| Unknown | 4 (8) |
| Defibrillation dose, J/kg | 3.27 [2.65‒5.01] |
| Defibrillation current, A/kg | 0.64 [0.38‒0.96] |
| AMSA, mV‐Hz | 12.21 [7.17‒17.93] |
| AMSA‐average, mV‐Hz | 14.6 [8.6‒19.2] |
| Total number of shocks | 111 |
| E‐CPR | 18 (36) |
| Daytime (07:00–22:59) | 36 (72) |
| Epinephrine doses | |
| 1 dose | 8 (16) |
| 2–4 doses | 10 (20) |
| ≥5 doses | 26 (52) |
| Duration CPR | |
| <15 min | 14 (28) |
| 15–30 min | 13 (26) |
| >30 min | 20 (40) |
| Unknown | 3 (6) |
| CC metrics* | |
| CC rate, CC/min | 119 (112‒125) |
| CC depth, cm | 3.9 (2.7‒5.8) |
| CC fraction | 84% (76%‒91%) |
| Outcomes | |
| TOF | 72 (65) |
| ROSC | 31 (62) |
| ROC‐ECMO | 11 (22) |
| 24‐h survival | 40 (80) |
| Survival to hospital discharge | 28 (56) |
| Hospital discharge PCPC score | |
| Normal | 8 (31) |
| Mild disability | 7 (27) |
| Moderate disability | 6 (23) |
| Severe disability | 1 (4) |
| Coma/vegetative state | 0 (0) |
| Brain death | 0 (0) |
| Unknown | 4 (15) |
Values are mean±SD, %, median [interquartile range], or n (%), unless otherwise noted. AMSA indicates amplitude spectral area; CC, chest compressions; CPR, cardiopulmonary resuscitation; ECMO, extracorporeal membrane oxygenation; E‐CPR, extracorporeal cardiopulmonary resuscitation; ICU, intensive care unit; PCPC, pediatric cerebral performance category; PEA, pulseless electrical activity; pVT, pulseless ventricular tachycardia; ROC‐ECMO, return of circulation by the means of ECMO (extracorporeal membrane oxygenation); ROSC, return of spontaneous circulation; TOF, termination of fibrillation; and VF, ventricular fibrillation.
n=38 with chest compression metric data.
Table 2.
Univariate Predictors of Resuscitation Outcome
| Variable | TOF | ROSC | 24‐h Survival | Survival to Discharge |
|---|---|---|---|---|
| AMSA‐shock1 | ||||
| P Value | 0.55 | |||
| OR (95% CI), per mV‐Hz | 1.04 (0.92–1.16) | |||
| AMSA‐all shocks | ||||
| P Value | 0.28 | |||
| OR (95% CI), per mV‐Hz | 1.05 (0.97–1.13) | |||
| AMSA‐average | ||||
| P Value | 0.19 | 0.72 | 0.16 | |
| OR (95% CI), per mV‐Hz | 1.06 (0.97–1.15) | 1.02 (0.93–1.12) | 1.06 (0.98–1.15) | |
AMSA indicates amplitude spectral area; OR, odds ratio; ROSC, return of spontaneous circulation; and TOF, termination of fibrillation.
Table 3.
Multivariate Predictors of Resuscitation Outcome
| Variable | TOF | ROSC | 24‐h Survival | Survival to Discharge |
|---|---|---|---|---|
| AMSA shock1 | ||||
| P Value | 0.49 | |||
| OR (95% CI), per mV‐Hz | 1.04 (0.93–1.18) | |||
| Defibrillation current, Amp | ||||
| P Value | 0.85 | |||
| OR (95% CI) | 1.01 (0.90–1.14) | |||
| Noncardiac vs cardiac | ||||
| P Value | 0.48 | |||
| OR (95% CI) | 0.52 (0.10–2.93) | |||
| AMSA‐all shocks | ||||
| P Value | 0.36 | |||
| OR (95% CI), per mV‐Hz | 1.04 (0.96–1.14) | |||
| Defibrillation current, Amp | ||||
| P Value | 0.92 | |||
| OR (95% CI) | 1.00 (0.93, 1.06) | |||
| Noncardiac vs cardiac | ||||
| P Value | 0.33 | |||
| OR (95% CI) | 0.55 (0.17–1.83) | |||
| AMSA‐average | ||||
| P Value | 0.06 | 0.61 | 0.22 | |
| OR (95% CI), per mV‐Hz | 1.10 (1.00–1.22) | 1.03 (0.92–1.14) | 1.06 (0.97–1.16) | |
| Defibrillation average current, Amp | ||||
| P Value | 0.03 | 0.50 | 0.25 | |
| OR (95% CI) | 1.13 (1.01–1.26) | 1.04 (0.93–1.16) | 0.95 (0.87–1.04) | |
| Noncardiac vs cardiac | ||||
| P Value | 0.39 | 0.61 | 0.09 | |
| OR (95% CI) | 0.54 (0.13–2.20) | 0.67 (0.14–3.22) | 0.30 (0.08–1.21) | |
AMSA indicates amplitude spectral area; OR, odds ratio; ROSC, return of spontaneous circulation; and TOF, termination of fibrillation.
No statistically significant differences in AMSA‐avg were noted between ROSC and ROC‐ECMO groups (Figure 3A). Similarly, no differences were found for median AMSA between patients with TOF and those without TOF when comparing all individual shocks (Figure 3B). Figure 3C depicts the ROC analysis for AMSA‐avg. For ROSC, the area under the curve was 0.61. For a sensitivity of 77% to predict ROSC, the AMSA‐avg threshold was 9.7 mV‐Hz, giving a specificity of 53%, a positive predictive value of 73%, and a negative predictive value of 59%.
Figure 3. Comparison of AMSA and AMSA‐avg in patients with ROSC and TOF compared with those without ROSC and TOF.
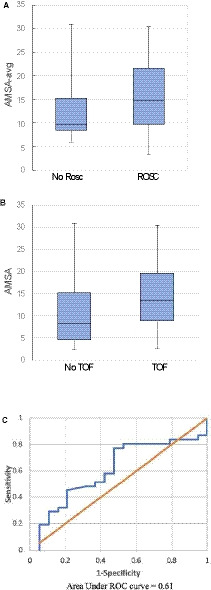
A, Patients with ROSC had a median AMSA‐avg of 14.7 mV‐Hz compared with 9.7 mV‐Hz in patients without ROSC (P=0.19). B, Patients with TOF had a median AMSA of 13.4 V‐Hz compared with 8.2 V‐Hz in patients who did not have TOF (P=0.42). C, For AMSA‐avg in ROSC, the area under the curve was 0.61. For a sensitivity of 77% to predict ROSC, the AMSA‐avg threshold was 9.7 mV‐Hz, giving a specificity of 53%, a positive predictive value of 73%, and a negative predictive value of 59%. AMSA indicates amplitude spectral area; AMSA‐avg, amplitude spectral area‐average; ROC, receiver operator curve; ROSC, return of spontaneous circulation; and TOF, termination of fibrillation.
Figure 4 depicts the change in AMSA (dAMSA) for consecutive shocks during the VF event. Analyses were done for both the first and second shocks as well as consecutive shocks at any time during the VF event. Delta AMSA was calculated by subtracting AMSA shock2 from AMSA shock1 (consecutive shocks, may or may not be first and second shocks). There were no statistically significant differences in dAMSA between patients who achieved TOF versus those who did not achieve TOF in either first or second shock analysis or analysis at any time during the VF event. We also calculated the trans‐thoracic impedance for all VF shock1, and found this to be 75 Ω (IQR, 54‒84) with the distribution of values across the 39 subjects noted in Figure S1.
Figure 4. Delta AMSA (dAMSA) in patients with and without TOF.
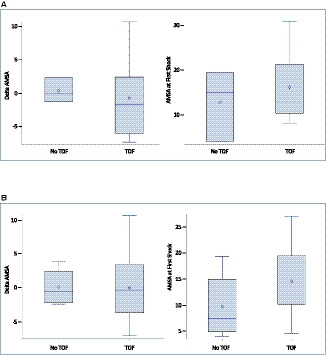
A, The dAMSA in 14 patients with ≥2 shocks (11 TOF, 3 no TOF) calculated by subtracting AMSA shock2 from AMSA shock1. Area under the curve for dAMSA is 0.61 and area under the curve for AMSA shock1 is 0.56. B, dAMSA in 22 patients with ≥2 shocks (16 TOF, 6 without TOF) calculated by subtracting AMSA shock2 from AMSA shock1 (consecutive shocks, may or may not be first and second shocks). area under the curve for dAMSA is 0.51 and area under the curve for AMSA at shock1 is 0.72. The odds ratio of shock success was 1.16 for every 1 mV‐Hz increase in AMSA value at first shock (P=0.14). AMSA indicates amplitude spectral area; and TOF, termination of fibrillation.
Finally, we performed a sensitivity analysis removing the 13/111 shocks where the AMSA value was calculated for up to 1‐minute to find a 2‐second window free of any artifact, and which was closest to the shock reference point. This sensitivity analysis resulted in no change from our overall findings.
DISCUSSION
To our knowledge, this is the first study to report on AMSA and defibrillation success for VF pediatric cardiac arrest. Our results show that there is no association between AMSA and TOF in pediatric VF arrest; this is unlike adult VF cardiac arrest where AMSA is predictive for shock success in both the first as well as subsequent shocks.23, 24, 34 We did, however, find a trend towards significance between AMSA‐avg and ROSC in pediatric patients, which is similar to adult OHCA trials.33, 34, 35, 36 Additionally, we found no association between AMSA‐avg and 24‐hour survival or survival to hospital discharge, which is in contrast to findings from adult OHCA VF studies.33, 34, 35, 36
AMSA has been shown to predict successful defibrillation in animals and multiple adult OHCA studies,24, 30, 34, 35, 36, 37, 38 with AMSA values significantly greater in successful defibrillation (defined as restoration of a perfusing rhythm) compared with unsuccessful defibrillation. For example, in analyzing 2447 shocks from 1050 patients in a derivation database (median age, 69.5 years), Ristagno et. al. found that AMSA was significantly higher in a successful defibrillation shock compared with a failed one (13±5 versus 6.8±3.5 mV‐Hz, mean±SD), and area under the curve‐ROC for defibrillation success was 0.86.36 The predictive ability for AMSA has been demonstrated in both initial defibrillation attempts as well as subsequent defibrillation attempts in adults with OHCA VF.24 In our current analysis for pediatric VF cardiac arrest, we found no association between AMSA and TOF, defined as any rhythm other than VF, for either initial defibrillation attempts or subsequent defibrillation attempts. Although not statistically significant, the median values of AMSA in our pediatric cohort did tend to be higher for those associated with TOF (13.4 mV‐Hz) compared with those that did not (8.2 mV‐Hz), similar to the findings in adults. It is difficult to compare the values for AMSA between adults and children, since we report median, whereas adult studies report mean values; further underlying cardiac electrophysiology/ECG may be very different in adult versus pediatric patients. Additionally, most of our cases were IHCA in a critical care location which were likely attended to almost immediately, whereas in patients with OHCA the median emergency medical services arrival time was ≈8 minutes.36
Our definition for TOF, namely any rhythm other than VF meeting criteria for successful defibrillation, was unlike adult studies which have chosen to use complex criteria to determine a “successful defibrillation outcome” or “return of a potentially perusing rhythm”, which was dependent upon a return of an organized rhythm with heart rate=40 beats/min commencing within 60 seconds post‐shock.24 When we looked at post‐shock rhythms in our analyses, we noticed there was usually immediate resumption of CPR post‐shock secondary to Pediatric Advanced Life Support guideline adherence. Further, because of the continuing CPR, it was also not clear if spontaneous return of perfusion had resumed, even if the VF had terminated. We, therefore, reasoned that it would be simpler to try and annotate the rhythm close to the post‐shock phase, ie, at 10 seconds, which is most directly related to defibrillation outcome. The return of perfusion on the other hand might be more complex, and also related to the underlying etiology of VF, which is different in adults (ischemia) compared with children.
An additional factor we considered as potentially influencing AMSA and TOF is the median trans‐thoracic impedance of children, which was 75 Ω in our study. Conversely, in adults during OHCA this value has been reported to be 99 Ω39, 40; so, it is likely that kids may have slightly lower trans‐thoracic impedances. Explanations for this difference in adults and children are likely similar to the reason Hunt et al41 found no significant association for time elapsed from loss of pulse to first defibrillation attempt and survival to hospital discharge in a large pediatric IHCA study, which contradicted that the time to first defibrillation attempt for VF/pVT is associated with survival in large animal laboratory models,42 adult OHCA,43 adult IHCA,44 and recent pediatric OHCA data.45 Factors specific to pediatric IHCA are vastly different from adult OHCA and may partially explain why we saw no robust association between AMSA and TOF. First, 78% of our cardiac arrests occurred in an intensive care unit or emergency room, 82% had vascular access, and the highly monitored status, rapid recognition with nearly immediate CPR, and perfusion of myocardium may attenuate the effects of AMSA on TOF. Second, three quarters of our patients were critically ill, illustrated by the high prearrest rates of hypotension and/or vasopressor administration (68%), respiratory insufficiency (52%), and mechanical ventilation (46%). Finally, the wide age range in our pediatric study consisting of infants, children, and adolescents, as well as the fact most children with VT/VF have an underlying cardiac pathology (underlying congenital heart disease and/or post‐cardiac surgery) are likely contributing to why we did not see the same results with AMSA that adult studies have seen. To draw any meaningful conclusions, much larger populations in each of the age categories (infant, child, adolescent) are needed, and we will need to separate the analysis of children with heart disease versus those without heart disease. These factors differentiate children from adults, who experience a sudden cardiac arrest that is more likely to be out of hospital, and more often related to coronary artery disease, unwitnessed, and unmonitored.
Animal studies have demonstrated that an AMSA–driven approach, compared with guidelines‐driven protocols, were more effective and reduced the shock burden (ie, cumulative number of ineffective shocks) resulting in less post‐resuscitation myocardial dysfunction and better short‐term survival. This has provided support for translational studies in sudden cardiac arrest investigating whether AMSA–driven defibrillation protocols—by timing the defibrillation effort to myocardial readiness for successful defibrillation—could be more effective than time‐fixed guidelines‐driven protocols resulting in less shock burden, less post‐resuscitation myocardial dysfunction, and better survival.46 As such, there is a current adult multi‐center, randomized, controlled study in patients with OHCA underway in Europe to test the hypothesis that real time AMSA analysis during CPR (AMSA‐guided CPR) may predict the success of defibrillation and optimize the timing of defibrillation delivery compared with a standard‐CPR group.47
With respect to secondary outcomes, we did find a trend toward significance between AMSA‐avg and ROSC, which is similar to an adult witnessed OHCA VF study in which AMSA‐avg was associated with pre‐hospital ROSC, hospital admission, and hospital discharge.33 A follow‐up to that study by Indik and colleagues evaluated adult VF OHCA and also found that AMSA‐avg was highly associated not only with survival to hospital discharge, but also survival with good neurologic outcome.37 Additionally, these authors were able to demonstrate that AMSA‐avg could predict resuscitation outcome independent of chest compression quality. Unlike these adult reports we found no association between AMSA‐avg and 24‐hour survival or survival to hospital discharge, nor did we find an association of changes in AMSA (dAMSA) and TOF for the initial shock or subsequent shocks. Because of small numbers and potential additional confounding factors (eg, diverse underlying etiologies and conditions, quality of CPR delivered) and small numbers of patients, we were not surprised and did not power the study to find associations of AMSA with 24‐hour survival or hospital discharge. Factors that could be important to consider include the population subgroup (children), setting (IHCA for the most part), etiology of cardiac arrest in children being different than adults, or simply a small sample size.
Limitations
We recognize important limitations of this study. First, the study was retrospective and is of a small sample size; nevertheless, even obtaining this data set (50 patients, 111 shocks) has taken 4 years, indicating the challenge of collecting sufficient data in a reasonable timeframe for pediatric cardiac arrest. We have grouped together all pediatric patients, although the cardiac electrophysiology/ECG and thus, VF characteristics might vary with age as well. Analyses were restricted to patients with analyzable VF data and known shock outcome. Given the known and not fully avoidable limitations of all retrospective studies, to minimize biases and to improve accuracy of our data, all VF cases with a defibrillation attempt were included in the study, and computation of VF parameters was performed by one of the co‐authors (S.V.P.) who was unaware of clinical outcomes, which were adjudicated concurrently by 2 experienced physicians (T.T.R., D.L.A.) on the basis of a predefined criterion for defibrillation outcome. Second, AMSA was calculated only during the pre‐defibrillation hands‐off time and not in real time during chest compressions. A majority of AMSA calculations (≈88% or 98/111 shocks) were made on ECG strips within 2.5 seconds before shock. For 13 shocks, we had to go back in time to get a CPR artifact‐free ECG signal; the average of the time durations for when AMSA was calculated for these 13 shocks before the shock was 16.5±13.18 seconds (mean±SD). This was done because the number of analyzable shocks/patients were small, so we attempted to get additional data. Sensitivity analysis removing these 13 shocks revealed no differences in our results. The definition of defibrillation success used was TOF defined as any rhythm other than VF and included asystole and pulseless electrical activity because while they may be considered successful termination of VF, they are not associated with ROSC. Finally, we did not analyze the role of well performed chest compressions, drug therapy during resuscitation, and their effects upon the waveform and resuscitation outcome, or consider post‐resuscitation interventions, such as therapeutic hypothermia.
CONCLUSIONS
In this population, the first to date (to our knowledge), of largely in‐hospital pediatric cardiac arrest with ventricular fibrillation, AMSA‐avg had a trend to significance with ROSC, but not TOF or survival outcomes at 24 hours or hospital discharge. Future pediatric studies should investigate whether the VF waveform AMSA can add an independent predictive value in a larger cohort of pediatric patients suffering cardiac arrest with shockable rhythms, in addition to other important determinants such as chest compression rate, depth, chest compression fraction, and release velocity.
Sources of Funding
The concept and development of the resuscitation clinical learning laboratory collaboration would not have been possible without the generous and unrestricted educational grant support of Laerdal Medical (2006–2013) and ZOLL Medical (2014–2017). The Children's Hospital of Philadelphia received support from an unrestricted research grant from ZOLL Medical for this collaborative.
Disclosures
Niles and Nadkarni disclose that The Children's Hospital of Philadelphia receives support from an unrestricted research grant from ZOLL Medical. Niles and Nadkarni disclose that The Children's Hospital of Philadelphia receives funding from an unrestricted research grant from The American Heart Association. Silver and Pandit are employees of ZOLL Medical Corporation. The remaining authors have no disclosures to report.
Supporting information
Appendix S1. The pediRES‐Q Collaborative Investigators
Figure S1
Acknowledgments
We would like to thank the patients, families, bedside providers, and teams of clinicians and quality improvement/research staff who invested time, effort, and enthusiasm in the collaborative effort to assess and benchmark these data.
(J Am Heart Assoc. 2021;10:e020353. DOI: 10.1161/JAHA.120.020353.)
This manuscript was sent to N.A. Mark Estes III, MD, Guest Editor, for review by expert referees, editorial decision, and final disposition.
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.020353
For Sources of Funding and Disclosures, see page 12.
REFERENCES
- 1.Atkins DL, Everson‐Stewart S, Sears GK, Daya M, Osmond MH, Warden CR, Berg RA; Resuscitation Outcomes Consortium Investigators . Epidemiology and outcomes from out‐of‐hospital cardiac arrest in children: the Resuscitation Outcomes Consortium Epistry‐Cardiac Arrest. Circulation. 2009;119:1484–1491. DOI: 10.1161/CIRCULATIONAHA.108.802678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holmberg MJ, Ross CE, Fitzmaurice GM, Chan PS, Duval‐Arnould J, Grossestreuer AV, Yankama T, Donnino MW, Andersen LW; for the American Heart Association’s Get With The Guidelines–Resuscitation Investigators . Annual incidence of adult and pediatric in‐hospital cardiac arrest in the United States. Circ Cardiovasc Qual Outcomes. 2019;12:e005580. DOI: 10.1161/CIRCOUTCOMES.119.005580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moler FW, Meert K, Donaldson AE, Nadkarni V, Brilli RJ, Dalton HJ, Clark RSB, Shaffner DH, Schleien CL, Statler K; for the Pediatric Emergency Care Applied Research Network . In‐hospital versus out‐of‐hospital pediatric cardiac arrest: a multicenter cohort study. Crit Care Med. 2009;37:2259–2267. DOI: 10.1097/CCM.0b013e3181a00a6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reis AG, Nadkarni V, Perondi MB, Grisi S, Berg RA. A prospective investigation into the epidemiology of in‐hospital pediatric cardiopulmonary resuscitation using the international Utstein reporting style. Pediatrics. 2002;109:200–209. DOI: 10.1542/peds.109.2.200. [DOI] [PubMed] [Google Scholar]
- 5.de Caen AR, Berg MD, Chameides L, Gooden CK, Hickey RW, Scott HF, Sutton RM, Tijssen JA, Topjian A, van der Jagt ÉW, et al. 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care: part 12: pediatric advanced life support. Circulation. 2015;132:S526–S542. DOI: 10.1161/CIR.0000000000000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slonim AD, Patel KM, Ruttimann UE, Pollack MM. Cardiopulmonary resuscitation in pediatric intensive care units. Crit Care Med. 1997;25:1951–1955. DOI: 10.1097/00003246-199712000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Zaritsky A, Nadkarni V, Getson P, Kuehl K. CPR in children. Ann Emerg Med. 1987;16:1107–1111. DOI: 10.1016/S0196-0644(87)80465-1. [DOI] [PubMed] [Google Scholar]
- 8.Girotra S, Spertus JA, Li Y, Berg RA, Nadkarni VM, Chan PS. Survival trends in pediatric in‐hospital cardiac arrests. Circ Cardiovasc Qual Outcomes. 2013;6:42. DOI: 10.1161/CIRCOUTCOMES.112.967968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jayaram N, Spertus JA, Nadkarni V, Berg RA, Tang F, Raymond T, Guerguerian AM, Chan PS; American Heart Association's Get with the Guidelines‐Resuscitation Investigators . Hospital variation in survival after pediatric in‐hospital cardiac arrest. Circ Cardiovasc Qual Outcomes. 2014;7:517–523. DOI: 10.1161/CIRCOUTCOMES.113.000691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan PS, Berg RA, Spertus JA, Schwamm LH, Bhatt DL, Fonarow GC, Heidenreich PA, Nallamothu BK, Tang F, Merchant RM; AHA GWTG‐Resuscitation Investigators . Risk‐standardizing survival for in‐hospital cardiac arrest to facilitate hospital comparisons. J Am Coll Cardiol. 2013;62:601–609. DOI: 10.1016/j.jacc.2013.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Samson RA, Nadkarni VM, Meaney PA, Carey SM, Berg MD, Berg RA; for the American Heart Association National Registry of CPR Investigators . Outcomes of in‐hospital ventricular fibrillation in children. N Engl J Med. 2006;354:2328–2339. DOI: 10.1056/NEJMoa052917. [DOI] [PubMed] [Google Scholar]
- 12.Meaney PA, Bobrow BJ, Mancini ME, Christenson J, de Caen AR, Bhanji F, Abella BS, Kleinman ME, Edelson DP, Berg RA, et al.; for the CPR Quality Summit Investigators, the American Heart Association Emergency Cardiovascular Care Committee, and the Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation . Cardiopulmonary resuscitation quality: improving cardiac resuscitation outcomes both inside and outside the hospital: a consensus statement from the American Heart Association. Circulation. 2013;128:417–435. DOI: 10.1161/CIR.0b013e31829d8654. [DOI] [PubMed] [Google Scholar]
- 13.Rodríguez‐Núñez A, López‐Herce J, García C, Domínguez P, Carrillo A, Bellón JM; Spanish Study Group of Cardiopulmonary Arrest in Children . Pediatric defibrillation after cardiac arrest: initial response and outcome. Crit Care. 2006;10:R113.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haskell SE, Atkins DL. Defibrillation in children. J Emerg Trauma Shock. 2010;3:261–266.10. DOI: 10.4103/0974-2700.66526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tibballs J, Carter B, Kiraly NJ, Ragg P, Clifford M. External and internal biphasic direct current shock doses for pediatric ventricular fibrillation and pulseless ventricular tachycardia. Pediatr Crit Care Med. 2011;12:14–20. DOI: 10.1097/PCC.0b013e3181dbb4fc. [DOI] [PubMed] [Google Scholar]
- 16.Valenzuela TD, Roe DJ, Nichol G, Clark LL, Spaite DW, Hardman RG. Outcomes of rapid defibrillation by security officers after cardiac arrest in casinos. N Engl J Med. 2000;343:1206–1209. DOI: 10.1056/NEJM200010263431701. [DOI] [PubMed] [Google Scholar]
- 17.Wik L, Hansen TB, Fylling F, Steen T, Vaagenes P, Auestad BH, Steen PA. Delaying defibrillation to give basic cardiopulmonary resuscitation to patients with out‐of‐hospital ventricular fibrillation: a randomized trial. JAMA. 2003;289:1389–1395. DOI: 10.1001/jama.289.11.1389. [DOI] [PubMed] [Google Scholar]
- 18.Link MS, Atkins DL, Passman RS, Halperin HR, Samson RA, White RD, Cudnik MT, Berg MD, Kudenchuk PJ, Kerber RE. Part 6: electrical therapies: automated external defibrillators, defibrillation, cardioversion, and pacing: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122:S706–S719. DOI: 10.1161/CIRCULATIONAHA.110.970954. [DOI] [PubMed] [Google Scholar]
- 19.Xie J, Weil MH, Sun S, Tang W, Sato Y, Jin X, Bisera J. High‐energy defibrillation increases the severity of post resuscitation myocardial dysfunction. Circulation. 1997;96:683–688. DOI: 10.1161/01.CIR.96.2.683. [DOI] [PubMed] [Google Scholar]
- 20.Cheskes S, Schmicker RH, Christenson J, Salcido DD, Rea T, Powell J, Edelson DP, Sell R, May S, Menegazzi JJ; for the Resuscitation Outcomes Consortium (ROC) Investigators . Perishock pause: an independent predictor of survival from out‐of‐hospital shockable cardiac arrest. Circulation. 2011;124:58–66. DOI: 10.1161/CIRCULATIONAHA.110.010736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steen S, Liao Q, Pierre L, Paskevicius A, Sjöberg T. The critical importance of minimal delay between chest compressions and subsequent defibrillation: a haemodynamic explanation. Resuscitation. 2003;58:249–258. DOI: 10.1016/S0300-9572(03)00265-X. [DOI] [PubMed] [Google Scholar]
- 22.Yu T, Weil MH, Tang W, Sun S, Klouche K, Povoas H, Bisera J. Adverse outcomes of interrupted precordial compression during automated defibrillation. Circulation. 2002;106:368–372. DOI: 10.1161/01.CIR.0000021429.22005.2E. [DOI] [PubMed] [Google Scholar]
- 23.Marn‐Pernat A, Weil MH, Tang W, Pernat A, Bisera J. Optimizing timing of ventricular defibrillation. Crit Care Med. 2001;29:2360–2365. DOI: 10.1097/00003246-200112000-00019. [DOI] [PubMed] [Google Scholar]
- 24.Ristagno G, Li Y, Fumagalli F, Finzi A, Quan W. Amplitude spectral area to guide resuscitation‐a retrospective analysis during out‐of‐hospital cardiopulmonary resuscitation in 609 patients with ventricular fibrillation cardiac arrest. Resuscitation. 2013;84:1697–1703. DOI: 10.1016/j.resuscitation.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Ristagno G, Bisera J, Tang W, Deng Q, Weil MH. Electrocardiogram waveforms for monitoring effectiveness of chest compression during cardiopulmonary resuscitation. Crit Care Med. 2008;36:211–215. DOI: 10.1097/01.CCM.0000295594.93345.A2. [DOI] [PubMed] [Google Scholar]
- 26.Indik JH, Allen D, Shanmugasundaram M, Zuercher M, Hilwig RQ, Berg RA, Kern KB. Predictors of resuscitation in a swine model of ischemic and nonischemic ventricular fibrillation cardiac arrest: superiority of amplitude spectral area and slope to predict a return of spontaneous circulation when resuscitation efforts are prolonged. Crit Care Med. 2010;38:2352–2357. DOI: 10.1097/CCM.0b013e3181fa01ee. [DOI] [PubMed] [Google Scholar]
- 27.Salcido DD, Menegazzi JJ, Suffoletto BP, Logue ES, Sherman LD. Association of intramyocardial high energy phosphate concentrations with quantitative measures of the ventricular fibrillation electrocardiogram waveform. Resuscitation. 2009;80:946–950. DOI: 10.1016/j.resuscitation.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 28.Indik JH, Allen D, Gura M, Dameff C, Hilwig RW, Kern KB. Utility of the ventricular fibrillation waveform to predict a return of spontaneous circulation and distinguish acute from post myocardial infarction or normal swine in ventricular fibrillation cardiac arrest. Circ Arrhythm Electrophysiol. 2011;4:337–343. DOI: 10.1161/CIRCEP.110.960419. [DOI] [PubMed] [Google Scholar]
- 29.Povoas HP, Weil MH, Tang W, Bisera J, Klouche K, Barbatsis A. Predicting the success of defibrillation by electrocardiographic analysis. Resuscitation. 2002;53:77–82. DOI: 10.1016/S0300-9572(01)00488-9. [DOI] [PubMed] [Google Scholar]
- 30.Ristagno G, Gullo A, Berlot G, Lucangelo U, Geheb E, Bisera J. Prediction of successful defibrillation in human victims of out‐of‐hospital cardiac arrest: a retrospective electrocardiographic analysis. Anaesth Intensive Care. 2008;36:46–55. DOI: 10.1177/0310057X0803600108. [DOI] [PubMed] [Google Scholar]
- 31.Neurauter A, Eftestøl T, Kramer‐Johansen J, Abella BS, Sunde K, Wenzel V, Lindner KH, Eilevstjønn J, Myklebust H, Steen PA, et al. Prediction of countershock success using single features from multiple ventricular fibrillation frequency bands and feature combinations using neural networks. Resuscitation. 2007;73:253–263. DOI: 10.1016/j.resuscitation.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 32.Shanmugasundaram M, Valles A, Kellum MJ, Ewy GA, Indik JH. Analysis of amplitude spectral area and slope to predict defibrillation in out of hospital cardiac arrest due to ventricular fibrillation (VF) according to VF type: recurrent versus shock‐resistant. Resuscitation. 2012;83:1242–1247. DOI: 10.1016/j.resuscitation.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 33.Indik JH, Conover Z, McGovern M, Silver A, Spaite DW, Bobrow KK. Association of amplitude spectral area of the ventricular fibrillation waveform with survival of out‐of‐hospital ventricular fibrillation cardiac arrest. J Am Coll Cardiol. 2014;64:1362–1369. DOI: 10.1016/j.jacc.2014.06.1196. [DOI] [PubMed] [Google Scholar]
- 34.Thannhauser J, Nas J, van Grunsven PM, Meinsma G, Zwart HJ, de Boer MJ, van Royen N, Bonnes JL, Brouwer MA. The ventricular fibrillation waveform in relation to shock success in early vs. late phases of out‐of‐hospital resuscitation. Resuscitation. 2019;139:99–105. DOI: 10.1016/j.resuscitation.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 35.Schoene P, Coult J, Murphy L, Fahrenbruch C, Blackwood J, Kudenchuk P, Sherman L, Rea T. Course of quantitative ventricular fibrillation waveform measure and outcome following out‐of‐hospital cardiac arrest. Heart Rhythm. 2014;11:230–236. DOI: 10.1016/j.hrthm.2013.10.049. [DOI] [PubMed] [Google Scholar]
- 36.Ristagno G, Mauri T, Cesana G, Li Y, Finzi A, Fumagalli F, Rossi G, Grieco N, Migliori M, Andreassi A; Azienda Regionale Emergenza Urgenza Research Group . Amplitude spectrum area to guide defibrillation: a validation on 1617 patients with ventricular fibrillation. Circulation. 2015;131:478–487. DOI: 10.1161/CIRCULATIONAHA.114.010989. [DOI] [PubMed] [Google Scholar]
- 37.Indik JH, Conover Z, McGovern M, Silver AE, Spaite DW, Bobrow BJ, Kern KB. Amplitude‐spectral area and chest compression release velocity independently predict hospital discharge and good neurological outcome in ventricular fibrillation out‐of‐hospital cardiac arrest. Resuscitation. 2015;92:122–128. DOI: 10.1016/j.resuscitation.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 38.Young C, Bisera J, Gehman S, Snyder D, Tang W, Weil MH. Amplitude spectrum area: measuring the probability of successful defibrillation as applied to human data. Crit Care Med. 2004;32:S356. DOI: 10.1097/01.CCM.0000134353.55378.88. [DOI] [PubMed] [Google Scholar]
- 39.Li Y, Ristagno G, Yu T, Bisera J, Weil MH, Tang W. A comparison of defibrillation efficacy between different impedance compensation techniques in high impedance porcine model. Resuscitation. 2009;80:1312–1317. DOI: 10.1016/j.resuscitation.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 40.Morrison LJ, Dorian P, Long J, Vermeulen M, Schwartz B, Sawadsky B, Frank J, Cameron B, Burgess R, Shield J; for the Steering Committee, Central Validation Committee, Safety and Efficacy Committee . Out of hospital cardiac arrest rectilinear biphasic to monophasic damped sine defibrillation waveforms with advanced life support interventional trial (ORBIT). Resuscitation. 2005;66:149–157. DOI: 10.1016/j.resuscitation.2004.11.031. [DOI] [PubMed] [Google Scholar]
- 41.Hunt EA, Duval‐Arnould JM, Bembea MM, Raymond T, Calhoun A, Atkins DL, Berg RA, Nadkarni VM, Donnino M, Andersen LW. Association between time to defibrillation and survival in pediatric in‐hospital cardiac arrest with a first documented shockable rhythm; American Heart Association’s Get With The Guidelines‐Resuscitation Investigators. JAMA Netw Open. 2018;1:e182643. DOI: 10.1001/jamanetworkopen.2018.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yakaitis RW, Ewy GA, Otto CW, Taren DL, Moon TE. Influence of time and therapy on ventricular defibrillation in dogs. Crit Care Med. 1980;8:157–163. DOI: 10.1097/00003246-198003000-00014. [DOI] [PubMed] [Google Scholar]
- 43.Valenzuela TD, Roe DJ, Cretin S, Spaite DW, Larsen MP. Estimating effectiveness of cardiac arrest interventions: a logistic regression survival model. Circulation. 1997;96:3308–3313. DOI: 10.1161/01.CIR.96.10.3308. [DOI] [PubMed] [Google Scholar]
- 44.Chan PS, Krumholz HM, Nichol G, Nallamothu BK; for the American Heart Association National Registry of Cardiopulmonary Resuscitation Investigators . Delayed time to defibrillation after in‐hospital cardiac arrest. N Engl J Med. 2008;358:9–17. DOI: 10.1056/NEJMoa0706467. [DOI] [PubMed] [Google Scholar]
- 45.Mitani Y, Ohta K, Yodoya N, Otsuki S, Ohashi H, Sawada H, Nagashima M, Sumitomo N, Komada Y. Public access defibrillation improved the outcome after out‐of‐hospital cardiac arrest in school‐age children: a nationwide, population‐based, Utstein registry study in Japan. Europace. 2013;15:1259–1266. DOI: 10.1093/europace/eut053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aiello S, Perez M, Cogan C, Baetiong A, Miller SA, Radhakrishnan J, Kaufman CL, Gazmuri RJ. Real‐time ventricular fibrillation amplitude‐spectral area analysis to guide timing of shock delivery improves defibrillation efficacy during cardiopulmonary resuscitation in swine. J Am Heart Assoc. 2017;6:e006749. DOI: 10.1161/JAHA.117.006749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ristagno G, Latini R. Real time amplitude spectrum area to guide defibrillation. [Internet]. Identifier: NCT03237910; August 3, 2017. Available at: ClinicalTrials.govhttps://ClinicalTrials.gov/show/NCT03237910. Accessed April 12, 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. The pediRES‐Q Collaborative Investigators
Figure S1


