Abstract
Background
Perioperative atrial fibrillation (POAF) is common in patients undergoing cardiac surgery. Conflicting evidence exists whether patients with POAF after cardiac surgery have an increased long‐term risk of stroke and other adverse events.
Methods and Results
We prospectively followed for up to 5 years 4624 patients without prior atrial fibrillation who underwent coronary artery bypass grafting in an international study. POAF was defined as atrial fibrillation that occurred during the initial hospitalization for surgery, lasted for ≥5 minutes, and required treatment. Outcomes assessed were a composite of death, nonfatal myocardial infarction or nonfatal stroke, and its individual components. Median age was 67 years, and 778 (16.8%) had an episode of POAF. The incidence of the composite outcome was 6.84 and 4.10 per 100 patient‐years in patients with and without POAF, and the incidence of stroke was 0.75 versus 0.45, respectively. The adjusted hazard ratios (aHRs) were 1.36 (95% CI, 1.16–1.59) for the composite outcome; 1.33 (95% CI, 1.10–1.61) for death; 1.58 (95% CI, 1.23–2.02) for myocardial infarction, and 1.27 (95% CI, 0.81–2.00) for stroke. In a landmark analysis excluding events of the initial hospital admission, the aHRs were 1.26 (95% CI, 1.03–1.54) for the composite outcome, 1.28 (95% CI, 1.03–1.59) for death, 1.70 (95% CI, 0.86–3.36) for myocardial infarction, and 1.07 (95% CI, 0.59–1.93) for stroke. At hospital discharge, 10.7% and 1.4% of patients with and without POAF received oral anticoagulation, respectively.
Conclusions
Patients with POAF after cardiac surgery had an increased long‐term risk of adverse outcomes, mainly death and myocardial infarction. The risk of stroke was low and not increased in patients with POAF.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: NCT00463294.
Keywords: cardiac surgery, coronary artery disease, myocardial infarction, perioperative atrial fibrillation, stroke
Subject Categories: Atrial Fibrillation, Cardiovascular Surgery
Nonstandard Abbreviations and Acronyms
- CORONARY
CABG Off or On Pump Revascularization Study
- POAF
perioperative atrial fibrillation
Clinical Perspective
What Is New?
Patients with perioperative atrial fibrillation after coronary artery bypass grafting had an increased long‐term risk of death and myocardial infarction.
The risk of stroke was low and not increased in patients with perioperative atrial fibrillation.
What Are the Clinical Implications?
Long‐term oral anticoagulation may not have an advantageous risk‐benefit profile in patients with perioperative atrial fibrillation after cardiac surgery.
Different risk mitigation strategies may be needed in this patient population.
Patients undergoing cardiac surgery have a high rate of developing perioperative atrial fibrillation (POAF). Rates of AF range from 20% to 40%1 in patients undergoing coronary artery bypass grafting (CABG) alone, and increase 40% to 50%2 in patients undergoing valve surgery. Given the large number of cardiac surgeries performed every year,3 the prognostic implications of POAF should be clarified. Although some studies have found an increased risk of stroke and death in patients with POAF,4, 5, 6 others have not.7, 8, 9
The CORONARY (CABG Off or On Pump Revascularization Study) study provides a unique opportunity to address some of the limitations identified from previous studies, because it prospectively followed 4752 patients for up to 5 years, with prospective ascertainment of both POAF and adverse outcome events. The main aim of this post hoc CORONARY study was to compare the long‐term risk of death, myocardial infarction, and stroke in patients undergoing CABG with and without perioperative AF, and to evaluate the potential influence of early postoperative events in these associations. A secondary aim of this analysis was to quantify the use of oral anticoagulation during follow‐up in these patients.
Methods
The data, analytical methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
CORONARY was a randomized controlled trial comparing off‐pump versus on‐pump surgery in patients undergoing isolated CABG. Details about the trial design and the main results have been published previously.10, 11, 12, 13 The trial was approved by national regulatory authorities and local ethics committees. All patients provided informed consent.
Eligibility Criteria
Patients were eligible to participate in CORONARY if they required isolated CABG with median sternotomy and had 1 or more of the following risk factors: age ≥70 years, peripheral arterial disease, cerebrovascular disease including a ≥70% carotid artery stenosis, or renal insufficiency. Patients aged 60 to 69 years were eligible if they had at least 1, whereas patients aged 55 to 59 years required at least 2, of the following risk factors: diabetes mellitus requiring treatment with an oral hypoglycemic agent or insulin, acute coronary syndrome with the need for urgent revascularization, a left ventricular ejection fraction <35%, or a history of smoking within 1 year before randomization. For the current analysis, we excluded patients with a history of AF before surgery.
Perioperative Atrial Fibrillation
In CORONARY, information on POAF was collected on the hospital discharge case report form. We defined POAF as confirmed postoperative atrial fibrillation (AF) that occurred during the index hospitalization, lasted for >5 minutes, and required treatment. Treatment of POAF was not standardized and was left to the discretion of the individual centers.
Outcomes
The main outcomes for this study were a composite of death, nonfatal stroke, and nonfatal myocardial infarction, as well as the individual components of the composite (ie, stroke, myocardial infarction, and death). Stroke was defined as a new acute focal neurological deficit thought to be of vascular origin with signs or symptoms lasting longer than 24 hours. Strokes were confirmed by a neurologist. Myocardial infarction within 72 hours of surgery was defined by any of the following 3 criteria: (1) a creatine kinase‐myocardial band measurement ≥5 times the 99th percentile upper reference limit without new pathological Q waves or new left bundle branch block (ie, non–Q wave myocardial infarction) or with new pathological Q waves or new left bundle branch block (ie, Q wave myocardial infarction), (2) angiographic evidence of new graft or native coronary artery occlusion, or (3) imaging evidence of new loss of viable myocardium. Myocardial infarction >72 hours after surgery was defined as detection of rise and/or fall of cardiac biomarkers with at least 1 value above the 99th percentile of the upper reference limit together with evidence of myocardial ischemia with at least 1 of the following: (1) symptoms of ischemia, (2) ECG changes indicative of new ischemia, (3) development of pathological Q waves in the ECG, or (4) imaging evidence of new loss of viable myocardium or new regional wall motion abnormality. All deaths in the first 30 days were considered to be cardiovascular‐related deaths. All reported outcomes were reviewed by an adjudication committee. Only unrefuted events were used in the statistical analyses.
In‐person or telephone follow‐up with patients or their next of kin (if patients were not available) were conducted by trained study personnel at 30 days, 1 year, and yearly thereafter until the end of the trial. If a patient indicated the occurrence of any outcome event, we obtained source documents about the event.
Statistical Analysis
Baseline characteristics were stratified by the presence or absence of POAF. Continuous variables are reported as median (interquartile range) and compared by Wilcoxon rank sum test between the 2 groups; categorical variables are reported as counts (percentages) and compared by χ2 test. Incidence rates were calculated as the number of events per 100 patient‐years of follow‐up. To compare the risk of adverse outcome events between patients with and without POAF, we calculated hazard ratio (HR) and 95% CI using Cox proportional hazards models. We first ran such models by only including POAF without adjustment. Subsequently, we ran the models adjusted for a predefined set of potential confounders, including age, sex, smoking, history of hypertension, history of diabetes mellitus, history of heart failure, history of myocardial infarction, history of peripheral artery disease, history of stroke, urgent surgery, and the randomized treatment assignment.
To better assess the long‐term risks of adverse outcome events associated with POAF independent of the initial surgery, we performed a landmark analysis consisting of a series of Cox models taking into account only events that occurred after the initial hospital discharge. The same covariates were used for adjustment as in the initial models. Patients who died during their initial hospital stay were not included in these analyses.
The proportional hazard assumption of all Cox models was assessed using plots of log of negative log of the survival function against the log of time, and no major violations were identified. Given the small amount of missing data, we used a complete case analysis in multivariable models without imputation for missing data. All statistical analyses were performed at a 2‐sided significance level of 0.05 using the statistical software SAS version 9.4 (SAS Institute, Cary, NC). A P value <0.05 was considered to indicate statistical significance. No adjustments were made for multiple testing.
Results
Of the 4752 patients enrolled in CORONARY, we excluded 128 (2.7%) with a history of AF before randomization. Of the remaining 4624 patients, 778 (16.8%) had at least 1 episode of POAF. The median age of all included patients was 67 years (interquartile range, 62–72 years), and 81% of participants were men. Median length of the initial hospitalization was 12 days (interquartile range, 9–18 days) versus 9 days (interquartile range, 8–13 days) in patients with and without POAF, respectively (P<0.0001). Baseline characteristics stratified by the presence or absence of POAF are shown in Table 1. Patients with POAF had a higher prevalence of risk factors and comorbidities, with the exception of type 2 diabetes mellitus, which was more common in those without POAF. Cardioprotective medications were more commonly used in patients with POAF. During the initial hospital stay, patients with POAF had more respiratory infections (10.8% versus 2.9%, P<0.0001) and reoperations for bleeding (3.0% versus 1.7%, P=0.02) than patients without POAF.
Table 1.
Baseline Characteristics According to POAF Status
| All Patients, N=4624 | No POAF, n=3846 | POAF, n=778 | P Value | |
|---|---|---|---|---|
| Initial length of stay, d (IQR) | 10 (8–13) | 9 (8–13) | 12 (9–18) | <0.0001 |
| Age, y (IQR) | 67 (62–72) | 66 (62–72) | 71 (65–75) | <0.0001 |
| Men | 3732 (80.7) | 3108 (80.8) | 624 (80.2) | 0.70 |
| History of myocardial infarction | 1592 (34.4) | 1262 (32.8) | 330 (42.4) | <0.0001 |
| History of PCI | 451 (9.8) | 347 (9.0) | 104 (13.4) | 0.0002 |
| History of stroke | 326 (7.1) | 257 (6.7) | 69 (8.9) | 0.03 |
| History of peripheral artery disease | 373 (8.1) | 290 (7.5) | 83 (10.7) | 0.004 |
| History of congestive heart failure | 273 (5.9) | 199 (5.2) | 74 (9.5) | <0.0001 |
| History of diabetes mellitus | 2183 (47.2) | 1861 (48.4) | 322 (41.4) | 0.0004 |
| History of hypertension | 3495 (75.6) | 2848 (74.1) | 647 (83.2) | <0.0001 |
| Smoking | ||||
| Never | 2117 (45.8) | 1829 (47.6) | 288 (37.0) | <0.0001 |
| Current | 1139 (24.6) | 965 (25.1) | 174 (22.4) | 0.11 |
| Former | 1288 (27.9) | 997 (25.9) | 291 (37.4) | <0.0001 |
| Urgent surgery | 1786 (38.6) | 1450 (37.7) | 336 (43.2) | 0.004 |
| History of LVEF <35% | 193 (4.2) | 172 (4.5) | 21 (2.7) | 0.02 |
| Extent of coronary artery disease | ||||
| Left main disease | 963 (21.3) | 776 (20.6) | 187 (25.0) | 0.007 |
| Triple‐vessel disease | 3280 (72.5) | 2711 (71.8) | 569 (75.9) | 0.02 |
| Double‐vessel disease | 962 (21.3) | 832 (22.0) | 130 (17.3) | 0.04 |
| Single‐vessel disease | 148 (3.3) | 131 (3.5) | 17 (2.3) | 0.09 |
| Medications | ||||
| Aspirin | 3208 (69.4) | 2604 (67.7) | 604 (77.6) | <0.0001 |
| Renin–angiotensin blockers | 2856 (61.8) | 2345 (61.0) | 511 (65.7) | 0.01 |
| Lipid‐lowering treatment | 3459 (74.8) | 2846 (74.0) | 613 (78.8) | 0.005 |
| Randomized to on‐pump | 2309 (49.9) | 1930 (50.2) | 379 (48.7) | 0.46 |
Data are median (IQR) or counts (percentages). AF indicates atrial fibrillation; IQR, interquartile range; LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention; and POAF, perioperative atrial fibrillation.
Incidence rates and HRs for all outcomes are shown in Table 2 and the Figure. The incidence of the composite of death, nonfatal myocardial infarction, or nonfatal stroke was 6.68 and 4.01 per 100 patient‐years in those with and without POAF, respectively. After multivariable adjustment, the HR was 1.36 (95% CI, 1.16–1.59; P=0.0001). The incidence of stroke was 0.75 versus 0.45 events per 100 patient‐years, respectively, and the adjusted HR was 1.27 (95% CI, 0.81–2.00; P=0.30).
Table 2.
Risk of Adverse Outcomes in Patients With and Without POAF
| Event | Overall | No POAF, n=3846 | POAF, n=778 | Unadjusted Relative Risk | Adjusted Relative Risk | ||
|---|---|---|---|---|---|---|---|
| Incidence* | Incidence* | n (%) | Incidence* | n (%) | HR; 95% CI; P Value | HR; 95% CI; P Value† | |
| Death, myocardial infarction, stroke | 4.44 | 4.01 | 743 (19) | 6.68 | 239 (31) | 1.70; 95% CI, 1.47–1.96; <0.0001 | 1.36; 95% CI, 1.16–1.59; 0.0001 |
| Death | 2.90 | 2.59 | 480 (12.5) | 4.52 | 162 (20.8) | 1.73; 95% CI, 1.45–2.07; <0.0001 | 1.33; 95% CI, 1.10–1.61; 0.003 |
| Cardiovascular | 2.03 | 1.86 | 344 (8.9) | 2.90 | 104 (13.4) | 1.55; 95% CI, 1.24–1.93; <0.0001 | 1.19; 95% CI, 0.94–1.50; 0.14 |
| Noncardiovascular | 0.88 | 0.73 | 136 (3.5) | 1.62 | 58 (7.5) | 2.21; 95% CI, 1.62–3.00; <0.0001 | 1.68; 95% CI, 1.21–2.33; 0.002 |
| Myocardial infarction | 1.61 | 1.42 | 263 (6.8) | 2.60 | 93 (12) | 1.81; 95% CI, 1.42–2.29; <0.0001 | 1.58; 95% CI, 1.23–2.02; 0.0004 |
| Stroke | 0.50 | 0.45 | 84 (2.2) | 0.75 | 27 (3.5) | 1.63; 95% CI, 1.06–2.52; 0.027 | 1.27; 95% CI, 0.81–2.00; 0.30 |
AF indicates atrial fibrillation; HR, hazard ratio; and POAF, perioperative atrial fibrillation.
Incidence per 100 person‐years of follow‐up.
All models are adjusted for age, sex, smoking, history of hypertension, history of diabetes mellitus, history of heart failure, history of myocardial infarction, history of peripheral artery disease, history of stroke, urgent surgery, and randomized treatment assignment. Because of missing covariate data, the multivariable models included 4545 patients (98.3%).
Figure 1. Proportions of various outcomes according to the presence or absence of POAF.
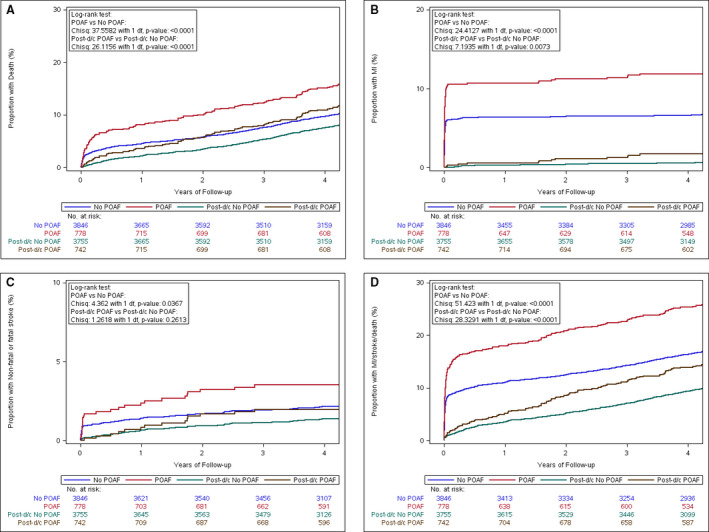
A, Death. B, Myocardial infarction. C, Stroke. D, Composite of death, MI, and stroke. All incident events occurring during follow‐up are illustrated in red (POAF) and blue (No POAF). The landmark analysis analyzing incident events occurring after discharge from the initial hospital admission only is shown in brown (Post‐d/c POAF) and green (Post‐d/c No POAF). Patients who died during the initial hospital admission were excluded from the landmark analysis. Chisq indicates χ2; d/c, discharge; MI, myocardial infarction; and POAF, perioperative atrial fibrillation.
A total of 4497 (97.3%) patients survived the initial hospital admission. In landmark analyses starting at hospital discharge, incidence rates were lower for all events, as shown in Table 3 and the Figure. The incidence per 100 patient‐years of the composite outcome was 4.11 versus 2.52 in patients with and without POAF, respectively (adjusted HR, 1.26; 95% CI, 1.03–1.54; P=0.02). The incidence of stroke after discharge was 0.42 versus 0.30, respectively, with an adjusted HR of 1.07 (95% CI, 0.59–1.93; P=0.83).
Table 3.
Landmark Analysis of Outcomes After Hospital Discharge in Patients With and Without POAF
| Event | Overall | No POAF, n=3755 | POAF, n=742 | Unadjusted Relative Risk | Adjusted Relative Risk | ||
|---|---|---|---|---|---|---|---|
| Incidence* | Incidence* | n (%) | Incidence* | n (%) | HR; 95% CI; P Value | HR; 95% CI; P Value† | |
| Death, myocardial infarction, stroke | 2.78 | 2.52 | 467 (12) | 4.11 | 147 (20) | 1.65; 95% CI, 1.37–1.98; <0.0001 | 1.26; 95% CI, 1.03–1.54; 0.02 |
| Death | 2.33 | 2.10 | 389 (10) | 3.52 | 126 (17) | 1.68; 95% CI, 1.37–2.05; <0.0001 | 1.28; 95% CI, 1.03–1.59; 0.02 |
| Cardiovascular | 1.46 | 1.37 | 253 (6.7) | 1.93 | 69 (9.3) | 1.42; 95% CI, 1.09–1.85; 0.01 | 1.07; 95% CI, 0.81–1.43; 0.63 |
| Noncardiovascular | 0.87 | 0.73 | 136 (3.6) | 1.59 | 57 (7.7) | 2.17; 95% CI, 1.59–2.95; <0.0001 | 1.66; 95% CI, 1.19–2.31; 0.003 |
| Myocardial infarction | 0.20 | 0.17 | 31 (0.8) | 0.39 | 14 (1.9) | 2.32; 95% CI, 1.23–4.36; 0.009 | 1.70; 95% CI, 0.86–3.36; 0.13 |
| Stroke | 0.32 | 0.30 | 56 (1.5) | 0.42 | 15 (2.0) | 1.38; 95% CI, 0.78–2.45; 0.26 | 1.07; 95% CI, 0.59–1.93; 0.83 |
AF indicates atrial fibrillation; HR, hazard ratio; and POAF, perioperative atrial fibrillation.
Incidence per 100 person‐years of follow‐up.
All models are adjusted for age, sex, smoking, history of hypertension, history of diabetes mellitus, history of heart failure, history of myocardial infarction, history of peripheral artery disease, history of stroke, urgent surgery, randomized treatment assignment. Because of missing covariate data, the multivariable models included 4545 patients (98.3%).
Few patients received oral anticoagulation during follow‐up, as shown in Table 4. At hospital discharge, 10.7% and 1.4% of patients with and without POAF received treatment with vitamin K antagonists, respectively. These proportions were similar at the 30‐day visit, and at 1 year declined to 5.1% in those with POAF. After 5 years, 5.8% and 2.4% of patients with and without POAF received vitamin K antagonists, respectively. The use of other cardioprotective medications increased in the early postdischarge period and then decreased over time, with similar patterns in both groups (Table 4).
Table 4.
Use of Vitamin K Antagonists and Other Preventive Medications During Follow‐Up
| All Patients, N=4624 | No POAF, n=3846 | POAF, n=778 | |
|---|---|---|---|
| Vitamin K antagonists | |||
| In‐hospital period | 65 (1.4%) | 25 (0.7%) | 40 (5.1%) |
| Hospital discharge | 136 (2.9%) | 53 (1.4%) | 83 (10.7%) |
| 30‐d follow‐up | 132 (2.9%) | 52 (1.4%) | 80 (10.3%) |
| 1‐y follow‐up | 81 (1.8%) | 41 (1.1%) | 40 (5.1%) |
| 5‐y follow‐up | 137 (3.0%) | 92 (2.4%) | 45 (5.8%) |
| Aspirin | |||
| In‐hospital period | 4195 (90.7%) | 3450 (89.7%) | 745 (95.8%) |
| Hospital discharge | 3971 (85.9%) | 3315 (86.2%) | 656 (84.3%) |
| 30‐d follow‐up | 3747 (81.0%) | 3158 (82.1%) | 589 (75.7%) |
| 1‐y follow‐up | 3681 (79.6%) | 3076 (80.0%) | 605 (77.8%) |
| 5‐y follow‐up | 3125 (67.6%) | 2620 (68.1%) | 505 (64.9%) |
| Renin–angiotensin blockers | |||
| In‐hospital period | 2856 (61.8%) | 2345 (61.0%) | 511 (65.7%) |
| Hospital discharge | 2061 (44.6%) | 1703 (44.3%) | 358 (46.0%) |
| 30‐d follow‐up | 2131 (46.1%) | 1771 (46.0%) | 360 (46.3%) |
| 1‐y follow‐up | 2242 (48.5%) | 1833 (47.7%) | 409 (52.6%) |
| 5‐y follow‐up | 2094 (45.3%) | 1688 (43.9%) | 406 (52.2%) |
| Lipid‐lowering treatment | |||
| Hospital discharge | 3699 (80.0%) | 3053 (79.4%) | 646 (83.0%) |
| 30‐d follow‐up | 3654 (79.0%) | 3050 (79.3%) | 604 (77.6%) |
| 1‐y follow‐up | 3572 (77.2%) | 2961 (77.0%) | 611 (78.5%) |
| 5‐y follow‐up | 3319 (71.8%) | 2778 (72.2%) | 541 (69.5%) |
AF indicates atrial fibrillation.
Discussion
In this large long‐term study of patients undergoing CABG, 16.8% of patients developed POAF that lasted for >5 minutes and required treatment. These stringent criteria to define POAF likely explain the lower incidence compared with previous studies.1 POAF was associated with a significantly increased risk of the composite end point of death, nonfatal myocardial infarction, and nonfatal stroke. Although the absolute risk was attenuated in both groups when events that occurred during the index hospitalization were excluded, POAF remained significantly associated with the composite outcome in this landmark analysis. In contrast, the incidence of stroke was low and not significantly different in patients with and without POAF, despite a low rate of anticoagulation in both groups.
This is one of the first large studies to separate early postoperative events from those occurring during long‐term follow‐up. Several points suggest early events should be handled differently than later events. First, early events are likely connected to the initial surgery and therefore have a different pathophysiological mechanism. Second, these events usually occur around the same time as POAF is diagnosed, and there may not be enough time to start preventative therapies. It may also be difficult to determine whether the POAF occurred before, during, or after the adverse outcome, raising issues about the causal chain of events. For all these reasons, these early events may not be as relevant when the implementation of various long‐term prevention strategies are considered. In an observational study, early anticoagulation for new POAF in patients after CABG was not associated with a lower stroke risk but with a significantly increased bleeding risk at 30 days.14 Adequately powered randomized trials are needed to address this important question.
Rates of oral anticoagulation were low throughout follow‐up. Only up to 10.7% of all patients with POAF were receiving vitamin K antagonists in our study, going down to 5.8% at 5 years of follow‐up. Although the use of oral anticoagulation was higher in patients with POAF, a large majority of all patients did not receive anticoagulation during follow‐up. These data confirm previous studies reporting low anticoagulation rates in patients with POAF after cardiac surgery.9, 15, 16 In addition, they also suggest that the low stroke rate observed in this study (0.50 events per 100 patient‐years) is not explained by the fact that a large proportion of patients with POAF receive oral anticoagulation. Rather, the stroke rate in this patient population seems to be intrinsically lower when compared with patients with POAF after noncardiac surgery and clinical nonoperative AF.17, 18 It is plausible that pathophysiological mechanisms for POAF differ after cardiac surgery, where direct contact with the myocardium during surgery is involved.
Although our study cannot exclude a small increase in long‐term stroke risk among patients with POAF after cardiac surgery given the width of the 95% CIs, the absolute risk difference would remain low. These data are in line with previous studies.4, 5 Although current guidelines call for high‐quality data to determine whether long‐term anticoagulation can prevent strokes in patients with POAF,19 a randomized trial to inform this issue will be difficult to undertake given the low absolute risks. Moreover, it is unlikely that oral anticoagulation has an acceptable benefit‐risk balance in patients undergoing CABG with such a low stroke risk.20
On the other hand, patients with POAF had an increased risk of death and myocardial infarction, with higher absolute risks compared with stroke. Increasing the long‐term use of proven secondary prevention medications (eg, aspirin, renin–angiotensin blockers, lipid‐lowering treatment; Table 4) will help to reduce this risk. However, this would still be an unusual finding for a general AF population. Although patients with POAF have a significantly increased risk of myocardial infarction, the stroke risk is usually higher.21, 22 We observed a similar pattern in our previous analysis among patients with POAF after noncardiac surgery,17 suggesting that the presence of coronary disease in all participants of the current study is not a sufficient explanation for this observation. A higher burden of comorbidities and postoperative complications in patients with POAF may indicate that POAF is a more high‐risk marker of disease, rather than a causal risk factor for adverse events. This hypothesis is further supported by the stronger association of POAF with noncardiovascular death as compared with cardiovascular death, and by the higher risk of noncardiovascular complications in the early postoperative period in these patients. Gaining further insights in these relationships is crucial to identify strategies to prevent POAF and associated outcomes.
Strengths and Limitations
CORONARY was a large long‐term study with near complete follow‐up and prospective ascertainment of POAF and adverse outcome events. Some potential limitations need to be considered in the interpretation of our results. First, CORONARY included patients undergoing CABG only with an increased incidence of preoperative comorbidities, and it is unclear whether our results apply to patients with a different risk profile and undergoing other types of cardiac surgery. Second, this was an observational analysis, and causality cannot be easily established. Third, we cannot exclude an effect of the randomized treatment assignment on the observed associations. However, we consider this unlikely, because we adjusted for this variable in our multivariable analyses, and the intervention did not have a significant effect on either POAF or the outcomes assessed.10, 11, 12 Fourth, although we had POAF recorded as occurring during the initial hospitalization, we did not have the exact date. Because the median length of stay was 10 days, this should not have influenced results up to 5 years of follow‐up. Finally, the duration of the initial POAF episode is unknown, and there may be a subset of patients with prolonged or recurrent AF who may be at high enough risk to benefit from oral anticoagulation.
Conclusions
In patients undergoing CABG, POAF was associated with an increased risk of the composite end point of death, nonfatal myocardial infarction, and nonfatal stroke. This association was maintained when events occurring during the index hospitalization were excluded. On the other hand, stroke is a relatively rare outcome in patients with or without POAF after CABG, suggesting that oral anticoagulation may not have an advantageous risk‐benefit profile in patients with POAF after cardiac surgery. Our findings suggest that different risk mitigation strategies are needed in this patient population.
Sources of Funding
The CORONARY trial was funded by the Canadian Institutes of Health Research. Dr Conen holds a McMaster University Department of Medicine Mid‐Career Research Award. Dr Devereaux holds the McMaster University/Hamilton Health Sciences Chair in Perioperative Care and a Tier 1 Canada Research Chair in Perioperative Medicine. Dr Whitlock holds a Canada Research Chair. Dr McIntyre holds a postdoctoral fellowship award from the Canadian Institutes of Health Research. Dr Whitlock holds a Canada Research Chair. Dr Healey holds the Yusuf Chair in Cardiology and the Connolly Chair in Cardiology Research.
Disclosures
Dr Conen received consulting fees from Roche Diagnostics, Switzerland, outside of the current work. Dr McIntyre received speaker fees from Servier Canada and Bayer Canada outside of the current work. Dr Whitlock has received consultancy fees from Atricure, PhaseBio, and grants from Boehringer Ingelheim, Bayer, and Roche outside of the current work. Dr Devereaux reports grants from Roche‐Diagnostics, Abbott‐Diagnostics, Octopharma, Philips Healthcare, Hoffmann‐La Roche, Siemens, Stryker, Covidien, and Boehringer Ingelheim outside of the submitted work. Dr Healey has received research grants and speaking fees from Abbott, Medtronic, Boston Scientific, BMS/Pfizer, Bayer, Boehringer‐Ingelheim, ARCA Biopharm, Cipher Pharma, Myokardia, and Servier. Dr Yusuf has received grants, travel expenses, and speaking fees from Astra Zeneca and Bayer in the past 5 years. The remaining authors have no disclosures to report.
(J Am Heart Assoc. 2021;10:e020426. DOI: 10.1161/JAHA.120.020426.)
For Sources of Funding and Disclosures, see page 7.
References
- 1.Bessissow A, Khan J, Devereaux PJ, Alvarez‐Garcia J, Alonso‐Coello P. Postoperative atrial fibrillation in non‐cardiac and cardiac surgery: an overview. J Thromb Haemost. 2015;13(suppl 1):S304–S312. DOI: 10.1111/jth.12974. [DOI] [PubMed] [Google Scholar]
- 2.Mariscalco G, Engström KG. Postoperative atrial fibrillation is associated with late mortality after coronary surgery, but not after valvular surgery. Ann Thorac Surg. 2009;88:1871–1876. DOI: 10.1016/j.athoracsur.2009.07.074. [DOI] [PubMed] [Google Scholar]
- 3.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee , et al. Heart disease and stroke statistics‐2019 update: a report from the American Heart Association. Circulation. 2019;139:e56–e528. DOI: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 4.Gialdini G, Nearing K, Bhave PD, Bonuccelli U, Iadecola C, Healey JS, Kamel H. Perioperative atrial fibrillation and the long‐term risk of ischemic stroke. JAMA. 2014;312:616–622. DOI: 10.1001/jama.2014.9143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whitlock R, Healey JS, Connolly SJ, Wang J, Danter MR, Tu JV, Novick R, Fremes S, Teoh K, Khera V, et al. Predictors of early and late stroke following cardiac surgery. CMAJ. 2014;186:905–911. DOI: 10.1503/cmaj.131214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Min JJ, Kim G, Lee JH, Hong KY, Kim WS, Lee YT. Does the type of anesthetic technique affect in‐hospital and one‐year outcomes after off‐pump coronary arterial bypass surgery? PLoS One. 2016;11:e0152060. DOI: 10.1371/journal.pone.0152060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalavrouziotis D, Buth KJ, Ali IS. The impact of new‐onset atrial fibrillation on in‐hospital mortality following cardiac surgery. Chest. 2007;131:833–839. DOI: 10.1378/chest.06-0735. [DOI] [PubMed] [Google Scholar]
- 8.Kernis SJ, Nkomo VT, Messika‐Zeitoun D, Gersh BJ, Sundt TM III, Ballman KV, Scott CG, Schaff HV, Enriquez‐Sarano M. Atrial fibrillation after surgical correction of mitral regurgitation in sinus rhythm: incidence, outcome, and determinants. Circulation. 2004;110:2320–2325. DOI: 10.1161/01.CIR.0000145121.25259.54. [DOI] [PubMed] [Google Scholar]
- 9.Batra G, Ahlsson A, Lindahl B, Lindhagen L, Wickbom A, Oldgren J. Atrial fibrillation in patients undergoing coronary artery surgery is associated with adverse outcome. Ups J Med Sci. 2019;124:70–77. DOI: 10.1080/03009734.2018.1504148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lamy A, Devereaux PJ, Prabhakaran D, Taggart DP, Hu S, Paolasso E, Straka Z, Piegas LS, Akar AR, Jain AR, et al. Off‐pump or on‐pump coronary‐artery bypass grafting at 30 days. N Engl J Med. 2012;366:1489–1497. DOI: 10.1056/NEJMoa1200388. [DOI] [PubMed] [Google Scholar]
- 11.Lamy A, Devereaux PJ, Prabhakaran D, Taggart DP, Hu S, Paolasso E, Straka Z, Piegas LS, Akar AR, Jain AR, et al. Effects of off‐pump and on‐pump coronary‐artery bypass grafting at 1 year. N Engl J Med. 2013;368:1179–1188. DOI: 10.1056/NEJMoa1301228. [DOI] [PubMed] [Google Scholar]
- 12.Lamy A, Devereaux PJ, Prabhakaran D, Taggart DP, Hu S, Straka Z, Piegas LS, Avezum A, Akar AR, Lanas Zanetti F, et al. Five‐year outcomes after off‐pump or on‐pump coronary‐artery bypass grafting. N Engl J Med. 2016;375:2359–2368. DOI: 10.1056/NEJMoa1601564. [DOI] [PubMed] [Google Scholar]
- 13.Lamy A, Devereaux PJ, Prabhakaran D, Hu S, Piegas LS, Straka Z, Paolasso E, Taggart D, Lanas F, Akar AR, et al. Rationale and design of the coronary artery bypass grafting surgery off or on pump revascularization study: a large international randomized trial in cardiac surgery. Am Heart J. 2012;163:1–6. DOI: 10.1016/j.ahj.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 14.Matos JD, McIlvaine S, Grau‐Sepulveda M, Jawitz OK, Brennan JM, Khabbaz KR, Sellke FW, Yeh R, Zimetbaum P. Anticoagulation and amiodarone for new atrial fibrillation after coronary artery bypass grafting: prescription patterns and 30‐day outcomes in the United States and Canada. J Thorac Cardiovasc Surg. 2020:S0022‐5223(20)30408‐6. DOI: 10.1016/j.jtcvs.2020.01.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horwich P, Buth KJ, Legare JF. New onset postoperative atrial fibrillation is associated with a long‐term risk for stroke and death following cardiac surgery. J Card Surg. 2013;28:8–13. DOI: 10.1111/jocs.12033. [DOI] [PubMed] [Google Scholar]
- 16.Philip F, Becker M, Galla J, Blackstone E, Kapadia SR. Transient post‐operative atrial fibrillation predicts short and long term adverse events following CABG. Cardiovasc Diagn Ther. 2014;4:365–372. DOI: 10.3978/j.issn.2223-3652.2014.09.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conen D, Alonso‐Coello P, Douketis J, Chan MTV, Kurz A, Sigamani A, Parlow JL, Wang CY, Villar JC, Srinathan SK, et al. Risk of stroke and other adverse outcomes in patients with perioperative atrial fibrillation 1 year after non‐cardiac surgery. Eur Heart J. 2020;41:645–651. DOI: 10.1093/eurheartj/ehz431. [DOI] [PubMed] [Google Scholar]
- 18.Healey JS, Hart RG, Pogue J, Pfeffer MA, Hohnloser SH, De Caterina R, Flaker G, Yusuf S, Connolly SJ. Risks and benefits of oral anticoagulation compared with clopidogrel plus aspirin in patients with atrial fibrillation according to stroke risk: the atrial fibrillation clopidogrel trial with irbesartan for prevention of vascular events (ACTIVE‐W). Stroke. 2008;39:1482–1486. DOI: 10.1161/STROKEAHA.107.500199. [DOI] [PubMed] [Google Scholar]
- 19.Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener H‐C, Heidbuchel H, Hendriks J, ESC Scientific Document Group , et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962. DOI: 10.1093/eurheartj/ehw210. [DOI] [PubMed] [Google Scholar]
- 20.Homma S, Thompson JLP, Pullicino PM, Levin B, Freudenberger RS, Teerlink JR, Ammon SE, Graham S, Sacco RL, Mann DL, et al. Warfarin and aspirin in patients with heart failure and sinus rhythm. N Engl J Med. 2012;366:1859–1869. DOI: 10.1056/NEJMoa1202299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Conen D, Chae CU, Glynn RJ, Tedrow UB, Everett BM, Buring JE, Albert CM. Risk of death and cardiovascular events in initially healthy women with new‐onset atrial fibrillation. JAMA. 2011;305:2080–2087. DOI: 10.1001/jama.2011.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soliman EZ, Safford MM, Muntner P, Khodneva Y, Dawood FZ, Zakai NA, Thacker EL, Judd S, Howard VJ, Howard G, et al. Atrial fibrillation and the risk of myocardial infarction. JAMA Intern Med. 2014;174:107–114. DOI: 10.1001/jamainternmed.2013.11912. [DOI] [PMC free article] [PubMed] [Google Scholar]


