Heart failure with reduced ejection fraction remains a disease with poor prognosis, particularly when complicated by diastolic dysfunction. A new class of drugs known as myotropes may provide positive inotropy by directly modulating myosin cross‐bridge kinetics, augmenting contractility without significant adrenergic, electrophysiological, or calcium effects. Indeed, the phase 3 trial of omecamtiv mecarbil (OM) improved systolic function in the patient population with heart failure with reduced ejection fraction.1 Surprisingly, these systolic gains did not improve patient mortality. One possible explanation is that OM may exacerbate diastolic dysfunction by prolonging crossbridge attachment, an effect that has been reported in molecular and animal models. Danicamtiv, a second myotrope, has phase 2a clinical trial results that parallel those of OM but with potentially less diastolic impact.2
To provide a detailed comparison of the systolic versus diastolic impacts of OM and danicamtiv, we have measured the acute effects of both myotropes in human engineered heart tissues (EHTs). The resulting data have implications for defining patient subsets for whom these agents may have the greatest therapeutic efficacy. Note that all supporting data not directly included within the article are available upon request.
EHTs were created by seeding human induced pluripotent stem cell–derived cardiomyocytes onto decellularized porcine myocardial slices.3 All EHTs were derived from a single cell line (GM23338, 55‐year‐old man, Coriell Institute). Real‐time mechanical measurements were conducted on EHTs to characterize the effects of OM and danicamtiv on both systolic and diastolic components of force production, using methods previously described.4 During drug infusion, records were periodically collected to ensure attainment of steady‐state behavior at each drug concentration (<30 minutes). Statistical analyses were performed in Prism (GraphPad Software, La Jolla, CA), with P<0.05 as the threshold for significance.
As expected, both myotropes had dose‐dependent positive inotropic effects with associated changes to contraction kinetics (n=6 per group; Figure [A through C]). At its maximally effective dose of 3.16 µmol/L, OM increased systolic force production by an average of 45%, while prolonging time to peak contraction by 13% and time from peak contraction to 50% relaxation by 50%. At its own maximally effective dosage of 10.6 µmol/L, danicamtiv increased systolic force production by an average of 74% while prolonging time to peak contraction 13% and increasing time to 50% relaxation similarly by 50%. We further noted that both OM and danicamtiv had measurable effects on diastolic force production (Figure [D and E]), with OM increasing baseline force by 44% and danicamtiv by 35% at their respective maximally inotropic doses. Beyond 3.16 and 10.6 µmol/L, respectively, OM and danicamtiv actually decreased the active twitch force (data not shown).
Figure 1. Systolic and diastolic comparison between omecamtiv mecarbil (OM) and danicamtiv (DAN).
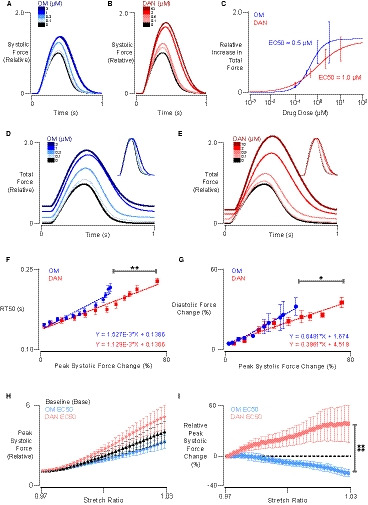
A and B, Representative systolic force responses (normalized to predrug force) in human engineered myocardium under increasing concentrations of OM and DAN. Responses were measured at culture length and 1 Hz stimulation. C, Total force increases (relative to baseline) as a function of drug concentration, fitted with Hill curves to establish half‐maximal concentration (EC50). D and E, Representative total force responses (including both diastolic and systolic components) normalized to predrug levels for OM and DAN, respectively. F, Time to 50% relaxation (RT50) as a function of peak systolic force percentage change in response to increasing concentrations of OM and DAN (ANCOVA). G, Diastolic force change normalized to predrug baseline systolic peak force vs peak systolic force change (ANCOVA). H, Normalized peak systolic forces measured from −3% to 3% stretch at 1‐Hz pacing, before and after EC50 concentrations of OM and DAN. I, Paired peak systolic force percentage change before and after EC50 concentrations of OM and DAN from −3% to 3% stretch at 1‐Hz pacing (2‐way ANOVA with repeated measures). *P<0.05, **P<0.01, ****P<0.0001.
Despite similarities in these results, when the lusitropic effects of these compounds are plotted as a function of inotropic effects, it was apparent that OM incurred a significantly steeper lusitropic cost for a similar gain in systolic contractile function (Figure [F]; ANCOVA, P<0.01). Worsening relaxation effects of OM at ≈35% systolic force change may reflect its complex molecular action.5 Diastolic force was also greater in OM‐treated tissues for the same increase in systolic force, compared with danicamtiv (Figure [G]; ANCOVA, P<0.05).
Additionally, for the dose‐dependent effect on force production at a given preload, we sought to understand whether these myotropes would affect length‐dependent force regulation, given the critical importance of Frank‐Starling behavior in normal physiology. In new experiments (n=5 per group), length‐dependent force production was studied in OM‐ and danicamtiv‐treated EHTs at their respective half‐maximal dosages (Figure [C]; 0.5 and 1 µmol/L, respectively). The 2 compounds exhibited markedly different effects on the relationship between tissue stretch and isometric twitch force (Figure [H and I]). Specifically, danicamtiv resulted in more robust Frank‐Starling slope than OM. OM actually caused a decrease in length sensitivity compared with baseline conditions (Figure [I]; 2‐way ANOVA, P<0.0001).
We present here for the first time a detailed comparison of the effects of 2 novel myotropes on contractile mechanics in a preclinical model of human engineered myocardium. Compared with OM, danicamtiv may have a wider therapeutic index, achieve a significantly larger augmentation of systolic contraction at a smaller lusitropic cost, and enhance Frank‐Starling behavior. All of these point toward a fundamentally different mode of molecular action and distinct clinical profile between the 2 compounds.
These findings could drive further analysis of existing trial data or help guide investigations going forward. For example, it may be worth conducting a post hoc analysis of GALACTIC‐HF (Global Approach to Lowering Adverse Cardiac Outcomes Through Improving Contractility in Heart Failure) to examine responder effect by Tajik diastolic function class. The data could also guide prospective trial design and even molecular screening in the preclinical phase. Additional mechanistic investigation (once molecular targeting of danicamtiv is disclosed) and testing of compounds in EHTs derived from >1 individual should be considered in future studies. In summary, mechanistic analysis using this EHT model highlights specific differences between OM and danicamtiv that shed new light on clinical trial findings and underscore complex, multifaceted effects of both compounds on contractile function.
Sources of Funding
This work was supported in part by National Institutes of Health grant R01 HL136590 and National Science Foundation grant 1653160 (both to Campbell). Dr Sewanan was supported by a P.D. Soros Fellowship for New Americans, National Institute of Health/National Institute of General Medical Sciences Medical Scientist Training Program Grant (T32GM007205), and an American Heart Association Predoctoral Fellowship.
Disclosures
Dr Campbell has equity ownership in Propria LLC, which has licensed technology used in the research reported in this publication. The remaining authors have no disclosures to report.
(J Am Heart Assoc. 2021;10:e020860. DOI: 10.1161/JAHA.121.020860.)
For Sources of Funding and Disclosures, see page 3.
References
- 1.Teerlink JR, Diaz R, Felker GM, McMurray JJV, Metra M, Solomon SD, Adams KF, Anand I, Arias‐Mendoza A, Biering‐Sørensen T, et al. Cardiac myosin activation with omecamtiv mecarbil in systolic heart failure. N Engl J Med. 2020;384:105–116. DOI: 10.1056/NEJMoa2025797. [DOI] [PubMed] [Google Scholar]
- 2.Voors AA, Tamby J‐F, Cleland JG, Koren M, Forgosh LB, Gupta D, Lund LH, Camacho A, Karra R, Swart HP, et al. Effects of danicamtiv, a novel cardiac myosin activator, in heart failure with reduced ejection fraction: experimental data and clinical results from a phase 2a trial. Eur J Heart Fail. 2020;22:1649–1658. DOI: 10.1002/ejhf.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwan J, Kwaczala AT, Ryan TJ, Bartulos O, Ren Y, Sewanan LR, Morris AH, Jacoby DL, Qyang Y, Campbell SG. Anisotropic engineered heart tissue made from laser‐cut decellularized myocardium. Sci Rep. 2016;6:32068. DOI: 10.1038/srep32068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sewanan LR, Schwan J, Kluger J, Park J, Jacoby DL, Qyang Y, Campbell SG. Extracellular matrix from hypertrophic myocardium provokes impaired twitch dynamics in healthy cardiomyocytes. JACC Basic Transl Sci. 2019;4:495–505. DOI: 10.1016/j.jacbts.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woody MS, Greenberg MJ, Barua B, Winkelmann DA, Goldman YE, Ostap EM. Positive cardiac inotrope omecamtiv mecarbil activates muscle despite suppressing the myosin working stroke. Nat Commun. 2018;9:3838. DOI: 10.1038/s41467-018-06193-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


