Abstract
Background
Bicuspid aortic valve (BAV) is the most common congenital cardiac malformation, which is often complicated by aortic valve stenosis (AoS). In tricuspid aortic valve (TAV), AoS strongly associates with coronary artery disease (CAD) with common pathophysiological factors. Yet, it remains unclear whether AoS in patients with BAV is also associated with CAD. This study investigated the association between the aortic valve morphological features and the extent of CAD.
Methods and Results
A single‐center study was performed, including all patients who underwent an aortic valve replacement attributable to AoS between 2006 and 2019. Coronary sclerosis was graded on preoperative coronary angiographies using the coronary artery greater even than scoring method, which divides the coronaries in 28 segments and scores nonobstructive (20%–49% sclerosis) and obstructive coronary sclerosis (>49% sclerosis) in each segment. Multivariate analyses were performed, controlling for age, sex, and CAD risk factors. A total of 1296 patients (931 TAV and 365 BAV) were included, resulting in 548 matched patients. Patients with TAV exhibited more CAD risk factors (odds ratio [OR], 2.66; 95% CI, 1.79–3.96; P<0.001). Patients with BAV had lower coronary artery greater even than 20 (1.61±2.35 versus 3.60±2.79) and coronary artery greater even than 50 (1.24±2.43 versus 3.37±3.49) scores (P<0.001), even after correcting for CAD risk factors (P<0.001). Patients with TAV more often needed concomitant coronary revascularization (OR, 3.50; 95% CI, 2.42–5.06; P<0.001).
Conclusions
Patients with BAV who are undergoing surgery for AoS carry a lower cardiovascular risk profile, correlating with less coronary sclerosis and a lower incidence of concomitant coronary revascularization compared with patients with TAV.
Keywords: aortic valve replacement, aortic valve stenosis, bicuspid aortic valve, coronary artery disease
Subject Categories: Cardiovascular Disease, Risk Factors
Nonstandard Abbreviations and Acronyms
- AoS
aortic valve stenosis
- AVR
aortic valve replacement
- BAV
bicuspid aortic valve
- CAGE
coronary artery greater even than
- TAV
tricuspid aortic valve
Clinical Perspective
What Is New?
This study shows, by directly studying the coronary angiographies of all included patients, that patients with bicuspid aortic valve have lower amounts of coronary sclerosis, less concomitant coronary revascularization, and lower coronary artery disease risk factors.
What Are the Clinical Implications?
Patients with bicuspid aortic valve carry a lower cardiovascular risk profile, correlating with less coronary sclerosis and a lower incidence of concomitant coronary revascularization compared with patients with tricuspid aortic valve.
A bicuspid aortic valve (BAV) is the most common congenital cardiac anomaly, with a prevalence of 1% to 2% in the general population.1 BAV is a recognized risk factor for the development of aortic valve and aortic wall alterations, which can result in diseases such as aortic valve stenosis (AoS) or regurgitation, and/or ascending aortic dilation.2 Previous studies showed that a defect in vascular smooth muscle cell differentiation and alterations in extracellular matrix composition play a key role in the development of aortopathy in patients with BAV.3, 4, 5, 6, 7, 8
AoS is thought to reflect a multifaceted process that shares many pathophysiologic and risk factors with coronary artery disease (CAD).9, 10, 11, 12, 13, 14 Common pathophysiologic factors include lipid deposition, inflammatory processes, and calcifications. Age, smoking, hypertension, and dyslipidemia comprise common risk factors of both diseases.
A possible relationship between patients with BAV and CAD has been under debate. Patients with BAV usually develop AoS at a younger age compared with patients with a regular tricuspid aortic valve (TAV).14 Moreover, it has been suggested that AoS in patients with BAV essentially relates to an altered hemodynamic flow pattern rather than to CAD risk factors.15, 16, 17 This altered flow pattern is considered a result of the divergent cusp morphological features, which lead to an increased stress on both the aortic valve cusps and the ascending aorta.4, 15, 16, 17
Although these observations above imply contrasting pathophysiologic backgrounds for AoS in BAV and TAV, conclusions of hitherto conducted studies on this subject are inconsistent18, 19 and need further research.
This study aims to examine the prevalence of CAD in patients with a BAV versus a TAV morphological feature. The coronary angiographies of patients with BAV and TAV who underwent an aortic valve replacement (AVR) between 2006 and 2019 attributable to an AoS were studied, to identify the prevalence, severity, and extent of CAD. Secondly, the presence of CAD risk factors and the need for CAD‐related interventions were scored for both groups.
METHODS
Study Population
This retrospective study was conducted at the Leiden University Medical Center in the Netherlands. Approval for this study was granted by the medical ethics committee of the Leiden University Medical Center (Medisch Ethisch Toetsingscommissie Leiden‐Den Haag‐Delft, case number G19.113), and patient consent was waived. The data that support the findings of this study are available from the corresponding author on reasonable request. The surgical database was searched to identify all patients who underwent an AVR because of an underlying AoS between January 2006 and April 2019. Transcatheter procedures, patients aged <18 years, aortic valve plasty procedures, patients with endocarditis, aortic dissection, or aortic valve regurgitation as the primary problem, and patients with no preoperative coronary angiograms were excluded. Patients with an AVR in the past were also excluded in those cases in which the original aortic valve morphological feature was not retrievable.
Study Parameters
The patients' electronic health records were examined to obtain data on the patient demographics, medical history (ie, prior cardiac events, interventions, and surgeries), laboratory findings (lipid and creatinine levels), and echocardiographic characteristics of the aortic valve. CAD risk factors were scored for each patient, including family history (any cardiovascular‐related health issues, such as a myocardial infarction before the age of 65 years), comorbidities (including hypertension and diabetes mellitus), lipid levels, use of tobacco and/or alcohol, and the body mass index.20 In addition, the surgical reports were studied to identify the aortic valve morphological features and classification of the BAV phenotype according to Sievers, the type of procedure, and concomitant CAD‐related procedures (eg, coronary artery bypass grafting [CABG]). Aortic diameters were obtained from preoperative echocardiograms or computed tomographic scans.
Coronary Imaging
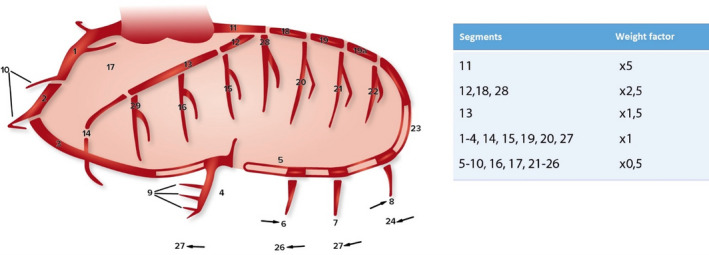
Each patient's last coronary angiography before surgery was studied. Only angiographies performed up to 1 year before the surgery were included. Two independent researchers scored the coronary angiographies of each patient, using the coronary artery greater than or equal to 20 and 50 (CAGE≥20 and CAGE≥50, respectively) method (see below for details). The coronary arteries were divided into 28 segments, as previously described in the Coronary Artery Surgery Study.21, 22, 23 The extension of the CAD was defined as the number of segments with a stenosis of >20% (nonobstructive+obstructive CAD, CAGE≥20) and 50% or greater (obstructive CAD, CAGE≥50). The severity of the CAD was calculated using weight factors per segment, as described earlier by Vlietstra et al (Figure 1).21 Only the native coronary artery system was scored for patients with a previously performed CABG.
Figure 1. Coronary artery segments (according to coronary artery surgery study) and the corresponding weight factors used for the coronary artery greater even than score.22 , 24 .
Statistical Analysis
The current study presents normally distributed continuous variables as mean±SD, whereas continuous variables with a non‐normal distribution are presented as median and interquartile range. Continuous variables were analyzed using a logistic regression. Categorical data are presented as frequencies and percentages and analyzed using the Fisher's exact test. Skewness, kurtosis, and normality tests were performed for all variables. Two strategies were followed to correct for the significant differences in baseline characteristics (especially age and sex) between patients with BAV and TAV. These strategies included an age‐ and sex‐based 1:1 matching and a multivariate analysis on the whole (unmatched) cohort. After univariate analyses, multivariate linear regression analyses were performed to model the dependence of the aortic valve morphological feature (BAV and TAV) on the CAGE≥20 and CAGE≥50 scores, controlling for CAD risk factors (eg, age at surgery, sex, high body mass index, smoking status, alcohol consumption, hypertension, hypercholesterolemia, diabetes mellitus, previous myocardial infarction or angina pectoris, a family history of CAD, and the ascending aortic diameter). P<0.05 was considered to be significant. All statistical analyses were conducted using IBM SPSS for Windows version 25.0.
RESULTS
A total of 3583 AVRs were identified between 2006 and 2019, of which 1296 patients were eventually eligible for inclusion. These included 931 patients with TAV and 365 patients with BAV, resulting in 548 matched patients (274 BAV and 274 TAV). The group of 365 patients with BAV consisted of 30 patients (13%) with a Sievers class 0, 196 (54%) with a Sievers class 1, and 5 (1.4%) with a Sievers class 2 BAV. The Sievers classification was not described for 134 (36.7%) patients. The left‐right positioned raphe was the most common variant (n=151 [41.4%]), followed by right‐noncoronary cusp (n=28 [7.7%]) and left‐noncoronary cusp (n=8 [2.2%]). The raphe position was not described for the remaining 9 (2.5%) patients.
Baseline and Perioperative Characteristics
All baseline characteristics are displayed in Table 1.
Table 1.
Baseline Characteristics of the Matched Patients
| Characteristic | Aortic Valve Morphological Feature | OR (95% CI) | P Value | |
|---|---|---|---|---|
| BAV | TAV | |||
| (n=274) | (n=274) | |||
| Men | 182 (66.4) | 182 (66.4) | 1.00 (0.79–1.27) | >.99 |
| Age at surgery, y | 67 (61–71) | 67 (61–71) | 1.00 (0.98–1.02) | >.99 |
| Body mass index, kg/m2 | 26.2 (24.1–28.9) | 27.5 (25.0–30.9) | 1.01 (1.04–1.13) | <0.001 |
| Smoking status | 270/274* | 264/274* | ||
| Never | 120 (43.8) | 125 (45.6) | 1.08 (0.77–1.51) | 0.731 |
| Former | 95 (34.7) | 97 (35.4) | 1.03 (0.73–1.46) | 0.929 |
| Currently | 55 (20.1) | 42 (15.3) | 0.72 (0.46–1.12) | 0.179 |
| Family history of CAD | 32 (11.68) | 39 (14.23) | 1.25 (0.76–2.07) | 0.443 |
| Diabetes mellitus | 40 (14.6) | 94 (34.3) | 3.06 (2.01–4.64) | <0.001 |
| Insulin dependent | 10 (3.6) | 34 (12.4) | 1.70 (0.74–3.90) | 0.233 |
| Hypertension | 142 (51.8) | 187 (68.2) | 2.00 (1.41–2.83) | <0.001 |
| Hypercholesterolemia | 77 (28.1) | 132 (48.2) | 2.38 (1.67–3.39) | <0.001 |
| Total cholesterol, mmol/L | 4.95±1.18 | 4.8 (4.00–5.30) | 0.91 (0.74–1.12) | 0.383 |
| HDL cholesterol, mmol/L | 1.34 (1.1–1.71) | 1.3 (1.07–1.52) | 1.01 (0.73–1.40) | 0.939 |
| LDL cholesterol, mmol/L | 2.82±0.93 | 2.85±1.21 | 1.00 (0.76–1.38) | 0.871 |
| Preoperative creatinine, mmol/L | 82 (72–92.5) | 81 (69–99) | 1.01 (1.001–1.013) | 0.007 |
| Previous CAD | ||||
| Previous MI | 12 (4.4) | 58 (21.2) | 5.86 (3.07–11.2) | <0.001 |
| Previous (i)AP | 12 (4.4) | 21 (7.7) | 1.81 (0.87–3.76) | 0.150 |
| Previous coronary revascularization | ||||
| Previous PCI | 12 (4.4) | 55 (20.1) | 5.48 (2.86–10.50) | <0.001 |
| Previous CABG | 2 (0.7) | 14 (5.1) | 7.32 (1.65–32.54) | 0.004 |
| Previous cardiac surgery | ||||
| CoA correction | 6 (2.2) | 0 | 0.978 (0.96–0.996) | 0.030 |
| AVP | 1 (0.5) | 1 (0.4) | 1.00 (0.62–16.07) | >.99 |
| Aorta surgery | 2 (0.7) | 0 | 0.99 (0.98–1.003) | 0.499 |
Data are presented as number (percentage), mean±SD, or median (interquartile range). (i)AP indicates (instable) angina pectoris; AVP, aortic valve plasty; BAV, bicuspid aortic valve; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CoA, coarctation of the aorta; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; MI, myocardial infarction; OR, odds ratio; PCI, percutaneous coronary intervention; and TAV, tricuspid aortic valve.
Denominator represents number of patients for whom this information was known.
The matched patients were equally divided into 2 groups based on age and sex, with no deviation between the groups. Echocardiographic findings on the aortic valve showed higher mean gradients (45 versus 37 mm Hg; P<0.001) and lower aortic valve areas (0.80 versus 0.90 cm2; P=0.004) in patients with BAV compared with patients with TAV (Table 2).
Table 2.
Echocardiographic Characteristics of the Matched Patients
| Characteristic | Aortic Valve Morphological Feature | OR (95% CI) | P Value | |
|---|---|---|---|---|
| BAV | TAV | |||
| (n=274) | (n=274) | |||
| AVA, cm2 | 0.80 (0.60–1.00) | 0.90 (0.70–1.10) | 2.80 (1.38–5.67) | 0.004 |
| Mean AV gradient, mm Hg | 45 (33–58) | 37 (27–49) | 0.97 (0.96–0.98) | <0.001 |
| Peak AV gradient, mm Hg | 73 (55.3–92.8) | 62 (47–78) | 0.98 (0.98–0.99) | <0.001 |
| Aortic regurgitation (scale, 0–4) | 0 (0–1) | 0 (0–1.5) | 1.00 (0.98–1.01) | 0.483 |
Data are presented as median (interquartile range). AV indicates aortic valve; AVA, AV area; BAV, bicuspid AV; OR, odds ratio; and TAV, tricuspid AV.
Table 3 shows a detailed list of the perioperative characteristics. Patients with TAV were more likely to undergo an isolated AVR (odds ratio [OR], 2.43; 95% CI, 1.71–3.45; P<0.001), whereas patients with BAV more frequently received concomitant aortic replacement procedures (eg, full aortic root replacements [OR, 5.86; 95% CI, 3.68–9.34; P<0.001] or an ascending aortic replacement [OR, 12.10; 95% CI, 6.29–23.23; P<0.001]). Compared with patients with BAV, patients with TAV were more often in need of concomitant surgery of a second valve (OR, 4.62; 95% CI, 2.59–8.25; P<0.001).
Table 3.
Perioperative Characteristics of the Matched Patients
| Surgery Type | Aortic Valve Morphological Feature | OR (95% CI) | P Value | |
|---|---|---|---|---|
| BAV | TAV | |||
| (n=274) | (n=274) | |||
| Single AVR | 137 (50) | 194 (70.8) | 2.43 (1.71–3.45) | <0.001 |
| Concomitant CABG | 63 (23) | 140 (51.1) | 3.50 (2.42–5.06) | <0.001 |
| No. of distal anastamoses | 2 (1–3) | 2 (1–3.75) | 1.25 (0.99–1.59) | 0.064 |
| Concomitant aortic surgery | ||||
| Root | 107 (39.1) | 27 (9.9) | 5.86 (3.68–9.34) | <0.001 |
| Ascending | 92 (33.6) | 11 (4) | 12.1 (6.29–23.23) | <0.001 |
| (Hemi) arch | 12 (4.4) | 1 (0.4) | 12.5 (1.61–96.84) | 0.003 |
| Other concomitant procedures | ||||
| Rhythm surgery | 20 (7.3) | 21 (7.7) | 1.05 (0.56–1.99) | 1.000 |
| MVP | 13 (4.7) | 34 (12.4) | 2.84 (1.47–5.52) | 0.002 |
| MVR | 3 (1.1) | 22 (8) | 7.89 (2.33–26.67) | <0.001 |
| TVP | 6 (2.2) | 28 (10.2) | 5.08 (2.07–12.49) | <0.001 |
Data are presented as number (percentage) or median (interquartile range). AVR indicates aortic valve replacement; BAV, bicuspid aortic valve; CABG, coronary artery bypass grafting; MVP, mitral valve plasty; MVR, mitral valve replacement; OR, odds ratio; TAV, tricuspid aortic valve; and TVP, tricuspid valve plasty.
Coronary Artery Disease
CAD and CAD risk factors were more common in patients with TAV. A history of CAD (eg, myocardial infarction or instable angina pectoris) was more prevalent in patients with TAV (OR, 4.15; 95% CI, 2.52–6.80; P<0.001), resulting in more coronary revascularization procedures (eg, percutaneous coronary intervention [OR, 5.48; 95% CI, 2.86–10.50; P<0.001] and CABG [OR, 7.32; 95% CI, 1.65–32.54; P=0.004]).
Furthermore, patients with TAV had a higher count of CAD risk factors compared with patients with BAV (eg, hypertension [OR, 2.00; 95% CI, 1.41–2.83; P<0.001], diabetes mellitus [OR, 3.06; 95% CI, 2.01–4.64; P<0.001], and hypercholesterolemia (OR, 2.38; 95% CI, 1.67–3.39; P<0.001]).
Similarly, the CAGE≥20 and CAGE≥50 scores and the number of affected coronary segments were both higher in patients with TAV (all P<0.001; Figure 2 and Table 4). In line with these results, concomitant CABG at the time of valve replacement was more often performed in patients with TAV than patients with BAV (OR, 3.50; 95% CI, 2.42–5.06; P<0.001).
Figure 2. Coronary artery disease (CAD) characteristics of the matched patients with bicuspid aortic valve (BAV) and tricuspid aortic valve (TAV).
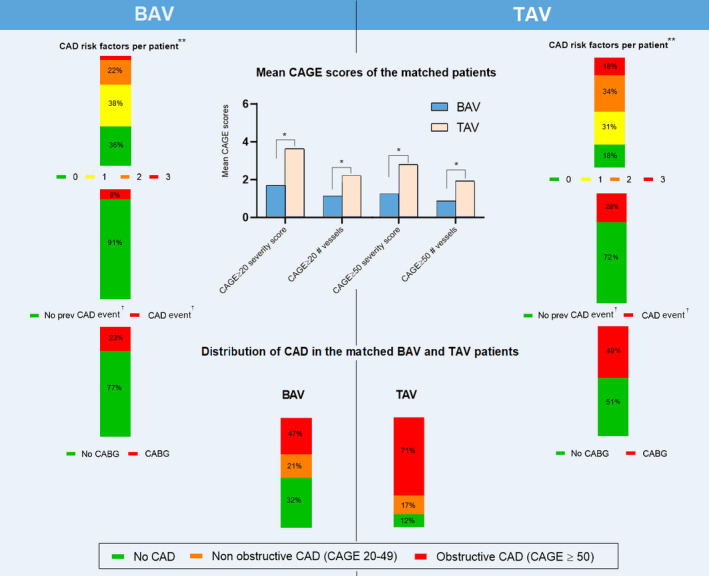
Assessment of the number of CAD risk factors per patient showed higher amounts of CAD risk factors** per patient in patients with TAV (top row bar diagrams) as compared to BAV patients. The medical histories of patients with TAV displayed higher rates of previous (prev) CAD events as compared to patients with BAV (odds ratio [OR], 4.15; 95% CI, 2.52–6.80; P<0.001) (second row bar diagrams). Concomitant coronary artery bypass grafting (CABG) was more often performed in patients with TAV (OR, 3.50; 95% CI, 2.42–5.06; P<0.001) (third row bar diagrams). Preoperative coronary angiographies showed higher rates of coronary sclerosis (both nonobstructive and obstructive) in patients with TAV, graded using the coronary artery greater even than (CAGE) scores (center bar graph). The bottom bar diagrams display the distribution of obstructive and nonobstructive CAD between patients with BAV and TAV, which shows higher rates of obstructive CAD in patients with TAV. *P<0.001; **Diabetes mellitus, hypertension, and/or hypercholesterolemia; †Previous myocardial infarction or instable angina pectoris.
Table 4.
Mean CAGE Scores of the Matched Patients
| Characteristic | Aortic Valve Morphological Feature | OR (95% CI) | P Value | |
|---|---|---|---|---|
| BAV | TAV | |||
| (n=274) | (n=274) | |||
| CAGE 20 severity score | 1.61±2.35 | 3.60±2.79 | 1.36 (1.26–1.47) | <0.001 |
| CAGE 20 No. of affected vessels | 0.99±1.34 | 2.08±1.52 | 1.71 (1.49–1.95) | <0.001 |
| CAGE 50 severity sore | 1.24±2.43 | 3.37±3.49 | 1.29 (1.20–1.38) | <0.001 |
| CAGE 50 No. of affected vessels | 0.86±1.58 | 2.32±2.28 | 1.49 (1.34–1.65) | <0.001 |
Data are presented as mean±SD. BAV indicates bicuspid aortic valve; CAGE, coronary artery greater even than; OR, odds ratio; and TAV, tricuspid aortic valve.
In the light of the differences in the patient characteristics between patients with BAV and TAV, an additional analysis was performed besides the matched analyses. To control for the effects of CAD risk factors on the CAGE scores, the risk factors were added to a multivariate analysis as confounders or colinear variables. These multivariate analyses were performed on the whole population. After taking the CAD risk factors into account, patients with TAV still had higher CAGE≥20 and CAGE≥50 scores (OR, 1.15; 95% CI, 1.07–1.23; P<0.001 and OR, 1.16; 95% CI, 1.09–1.24; P<0.001, respectively).
DISCUSSION
The primary aim of this study was to identify the prevalence, severity and extent of CAD comparing patients with BAV and TAV, by studying the medical histories and surgical reports and scoring the preoperative coronary angiographies. This study showed a lower rate of CAD in patients with BAV compared with patients with TAV. When assessing the histories of both groups, patients with BAV had lower rates of CAD (eg, myocardial infarction and angina pectoris), coronary artery revascularization (eg, CABG and percutaneous coronary intervention), and CAD risk factors (higher age, hypertension, hypercholesterolemia, and diabetes mellitus). In addition, preoperative coronary angiographies showed lower rates of coronary artery sclerosis in patients with BAV when compared with patients with TAV. Two different strategies were followed to analyze the study population because atherosclerosis is an age‐dependent process and as the BAV population is younger and more often male. First, the patients with BAV and TAV were matched on the basis of age (mean age, 66.5 years) and sex (66.4%), and the differences were analyzed between the groups. However, as this matched population is relatively young and predominantly men, a secondary multivariate analysis was also performed to correct for the differences in baseline characteristics (ie, a potential confounding by indication) on the complete (unmatched) population. Conclusions for both strategies (as above mentioned) were similar, which indicates that the differences between the groups were corrected adequately.
As pointed out previously, AoS is a multifaceted process that shares both the risk factors and the pathophysiological factors of CAD.9, 10, 11, 12, 13, 14 The fact that patients with BAV develop AoS at a younger age,14 while they often carry a lower cardiovascular risk profile compared with patients with TAV, makes this an interesting group to study. This study endorses the results of previous studies that identified a lower cardiovascular risk profile in patients with BAV.9, 18 The manifestation of AoS at a younger age (approximately 7 years earlier), while at the same time carrying fewer CAD risk factors, could indicate different causes of AoS between patients with BAV and TAV. This notion is supported by the lower coronary calcium burden for patients with BAV observed in this study, but a higher aortic valve calcium load in patients with BAV versus patients with TAV.24 In addition, preoperative echocardiographies showed significant differences in aortic valve gradients and aortic valve area between BAV and TAV. These results implicate that CAD risk factors are less contributive to the pathophysiological features of AoS in patients with BAV than in patients with TAV. Instead, higher mechanical stress, which is caused by an abnormal flow pattern that results from the divergent cusp morphological feature in patients with BAV, could be the leading cause of the earlier development of AoS in patients with BAV.4, 25 BAV cusps display a more excessive bending strain during the cardiac cycle, leading to higher shear stresses on the cusps, especially in the raphal area,15 which leads to the thickening and early degeneration of the aortic valve.15, 16, 17 These observations may reflect the fact that AoS in BAV generally relates to a primary valve defect, whereas AoS in TAV more often relates to a secondary defect. More research is warranted to study the possible differences in the cause of AoS in patients with BAV and TAV.
To our knowledge, the current study, which included a total of 1296 patients, is the largest clinical study yet to examine the relationship between CAD and the aortic valve morphological feature by directly studying the coronary angiographies of each patient. Hitherto conducted studies on the relationship of the aortic valve morphological feature and the prevalence of CAD have not resulted in consensus. Poggio et al performed a meta‐analysis to identify this relationship.18 This study indicated a higher incidence of CAD in patients with TAV, but no significant differences remained between the 2 groups after correcting for CAD risk factors. However, it is important to point out that none of the included studies in this meta‐analysis directly investigated coronary sclerosis by examining the patients’ coronary imaging. Instead, the results found by Poggio et al are based on anamnestic or clinical outcomes (eg, concomitant coronary revascularization), thus only looking at significant coronary sclerosis. Because stenoses of <70% are clinically not always revascularized, studying only the clinical outcomes means studying solely the tip of the iceberg.26 On the other hand, another study that explored the associations between AoS and CAD in patients who were planned for an AVR showed a higher incidence of concomitant CABG in patients with TAV than patients with BAV (62.2% and 26.3%, respectively).9
Until now, it is unclear what the mechanism behind the lower rates of CAD in patients with BAV is. A recent review even hypothesized that patients with BAV are more at risk of developing CAD, by providing an overview of several molecular mechanisms that may promote CAD in patients with BAV.27 These included dyslipidemia, which is not in line with the lower cardiovascular risk profile of BAV, as found in this study and other previous studies,9, 18 and the activation of proinflammatory pathways. To our knowledge, there has not been a histopathological study that has directly assessed the relationship between CAD and the aortic valve morphological features. Yet, one could formulate several potential mechanisms that might lead to lower rates of CAD in patients with BAV based on other studies. For example, a thinner tunica intima has been observed in patients with BAV.8 This might be one of the reasons why these patients have lower rates of CAD, because CAD is a disease that primarily develops in this layer of the vessels. Other studies suggested that ascending aortic dilation might have a protective effect on CAD.28, 29, 30 Because ascending aortic dilation is a common problem in patients with BAV, developing in at least 50% of the population with BAV,31, 32 this might also contribute to lower CAD in patients with BAV. Another mechanism that might lead to differences of CAD between patients with BAV and TAV are the inflammatory pathways, which play a role in the development of CAD.33, 34 Yet, hitherto conducted studies show contradictory conclusions on this subject. A study from our laboratory showed lower inflammatory components in the aortic walls of patients with BAV,8 whereas other studies showed the same degree of inflammation between BAV and TAV35 or even more activated inflammatory pathways in patients with BAV.27
To draw conclusions about the complete patients with BAV, patients with lower cardiovascular risk profiles need to be studied as well. Future histopathological studies could provide insight into the possible mechanisms underlying this effect.
Limitations
As with all retrospective and observational studies, this study is subject to some limitations because of the research design. This study only focused on the surgical AVR, excluding those patients who underwent a transcatheter AVR. This could have led to an inclusion bias, because these patients are often older and carry more comorbidities compared with the surgical AVR group. To study the patients with the highest cardiovascular risk profile, the study population only included patients with AoS, which makes it unfit to draw conclusion about the general population, including patients without valvular diseases. Despite matching on age and sex, patients with TAV still displayed a higher number of confounders (eg, a higher cardiovascular risk profile) than patients with BAV. Yet, these differences in cardiovascular risk profiles could be the result of 2 different causes of AoS. Patients with BAV who develop AoS at a much younger age while carrying lower amounts of CAD risk factors than patients with TAV indicate different pathophysiological mechanisms, leading to a similar disease between these 2 groups.
CONCLUSIONS
Patients with BAV had significantly lower CAGE scores, resulting in lower rates of concomitant CABG. The patients’ medical histories revealed that patients with BAV showed lower amounts of CAD and coronary revascularization in the past. In addition, patients with BAV also had lower CAD risk factors at the time of surgery compared with patients with TAV. The differences in the cardiovascular risk profile between BAV and TAV suggest different pathophysiological mechanisms of AoS between the 2 patient groups. Future histopathological studies are mandatory to unravel the possible different mechanisms underlying this effect.
Sources of Funding
None.
Disclosures
None.
Acknowledgments
Author Contributions: Dr Dolmaci contributed to data gathering and scoring, analysis, and manuscript writing; Dr Legué contributed to coronary angiography reviewing; Dr Lindeman contributed to data reviewing and manuscript reviewing; Dr Driessen contributed to manuscript reviewing; Dr Klautz contributed to manuscript and analysis reviewing; Dr Van Brakel contributed to manuscript and analysis reviewing; Dr Siebelink contributed to manuscript reviewing; Dr Mertens contributed to manuscript and analysis reviewing; Dr Poelmann contributed to manuscript reviewing; Dr Gittenberger‐de Groot contributed to manuscript reviewing; Dr Grewal contributed to patient selection and analysis and manuscript reviewing.
(J Am Heart Assoc. 2021;10:e020080. DOI: 10.1161/JAHA.120.020080.)
For Sources of Funding and Disclosures, see page 8.
References
- 1.Ward C. Clinical significance of the bicuspid aortic valve. Heart. 2000;83:81–85. DOI: 10.1136/heart.83.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siu SC, Silversides CK. Bicuspid aortic valve disease. J Am Coll Cardiol. 2010;55:2789–2800. DOI: 10.1016/j.jacc.2009.12.068. [DOI] [PubMed] [Google Scholar]
- 3.Andreassi MG, Della CA. Genetics of bicuspid aortic valve aortopathy. Curr Opin Cardiol. 2016;31:585–592. DOI: 10.1097/HCO.0000000000000328. [DOI] [PubMed] [Google Scholar]
- 4.Mordi I, Tzemos N. Bicuspid aortic valve disease: a comprehensive review. Cardiol Res Pract. 2012;2012:196037. DOI: 10.1155/2012/196037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braverman AC, Guven H, Beardslee MA, Makan M, Kates AM, Moon MR. The bicuspid aortic valve. Curr Probl Cardiol. 2005;30:470–522. DOI: 10.1016/j.cpcardiol.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Fedak PW, Verma S, David TE, Leask RL, Weisel RD, Butany J. Clinical and pathophysiological implications of a bicuspid aortic valve. Circulation. 2002;106:900–904. DOI: 10.1161/01.CIR.0000027905.26586.E8. [DOI] [PubMed] [Google Scholar]
- 7.Nataatmadja M, West M, West J, Summers K, Walker P, Nagata M, Watanabe T. Abnormal extracellular matrix protein transport associated with increased apoptosis of vascular smooth muscle cells in Marfan syndrome and bicuspid aortic valve thoracic aortic aneurysm. Circulation. 2003;108:329II–334II. DOI: 10.1161/01.cir.0000087660.82721.15. [DOI] [PubMed] [Google Scholar]
- 8.Grewal N, Gittenberger‐de Groot AC, Poelmann RE, Klautz RJ, Lindeman JH, Goumans MJ, Palmen M, Mohamed SA, Sievers HH, Bogers AJJC, et al. Ascending aorta dilation in association with bicuspid aortic valve: a maturation defect of the aortic wall. J Thorac Cardiovasc Surg. 2014;148:1583–1590. DOI: 10.1016/j.jtcvs.2014.01.027. [DOI] [PubMed] [Google Scholar]
- 9.Boudoulas KD, Wolfe B, Ravi Y, Lilly S, Nagaraja HN, Sai‐Sudhakar CB. The aortic stenosis complex: aortic valve, atherosclerosis, aortopathy. J Cardiol. 2015;65:377–382. DOI: 10.1016/j.jjcc.2014.12.021. [DOI] [PubMed] [Google Scholar]
- 10.Otto CM, Kuusisto J, Reichenbach DD, Gown AM, O'Brien KD. Characterization of the early lesion of “degenerative” valvular aortic stenosis: histological and immunohistochemical studies. Circulation. 1994;90:844–853. DOI: 10.1161/01.CIR.90.2.844. [DOI] [PubMed] [Google Scholar]
- 11.Capoulade R, Clavel MA, Dumesnil JG, Chan KL, Teo KK, Tam JW, Cote N, Mathieu P, Despres JP, Pibarot P, et al. Impact of metabolic syndrome on progression of aortic stenosis: influence of age and statin therapy. J Am Coll Cardiol. 2012;60:216–223. DOI: 10.1016/j.jacc.2012.03.052. [DOI] [PubMed] [Google Scholar]
- 12.Gotoh T, Kuroda T, Yamasawa M, Nishinaga M, Mitsuhashi T, Seino Y, Nagoh N, Kayaba K, Yamada S, Matsuo H, et al. Correlation between lipoprotein(a) and aortic valve sclerosis assessed by echocardiography (the JMS cardiac echo and cohort study). Am J Cardiol. 1995;76:928–932. DOI: 10.1016/S0002-9149(99)80263-X. [DOI] [PubMed] [Google Scholar]
- 13.Stewart BF, Siscovick D, Lind BK, Gardin JM, Gottdiener JS, Smith VE, Kitzman DW, Otto CM. Clinical factors associated with calcific aortic valve disease: cardiovascular health study. J Am Coll Cardiol. 1997;29:630–634. DOI: 10.1016/s0735-1097(96)00563-3. [DOI] [PubMed] [Google Scholar]
- 14.Otto CM. Calcification of bicuspid aortic valves. Heart. 2002;88:321–322. DOI: 10.1136/heart.88.4.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conti CA, Della Corte A, Votta E, Del Viscovo L, Bancone C, De Santo LS, Redaelli A. Biomechanical implications of the congenital bicuspid aortic valve: a finite element study of aortic root function from in vivo data. J Thorac Cardiovasc Surg. 2010;140:890–896. DOI: 10.1016/j.jtcvs.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 16.Robicsek F, Thubrikar MJ, Cook JW, Fowler B. The congenitally bicuspid aortic valve: how does it function? Why does it fail? Ann Thorac Surg. 2004;77:177–185. DOI: 10.1016/S0003-4975(03)01249-9. [DOI] [PubMed] [Google Scholar]
- 17.Katayama S, Umetani N, Hisada T, Sugiura S. Bicuspid aortic valves undergo excessive strain during opening: a simulation study. J Thorac Cardiovasc Surg. 2013;145:1570–1576. DOI: 10.1016/j.jtcvs.2012.05.032. [DOI] [PubMed] [Google Scholar]
- 18.Poggio P, Cavallotti L, Songia P, Di Minno A, Ambrosino P, Mammana L, Parolari A, Alamanni F, Tremoli E, Di Minno MND. Impact of valve morphology on the prevalence of coronary artery disease: a systematic review and meta‐analysis. J Am Heart Assoc. 2016;5:e003200. DOI: 10.1161/JAHA.116.003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boudoulas KD, Vlachopoulos C, Raman SV, Sparks EA, Triposciadis F, Stefanadis C, Boudoulas H. Aortic function: from the research laboratory to the clinic. Cardiol. 2012;121:31–42. DOI: 10.1159/000336147. [DOI] [PubMed] [Google Scholar]
- 20.Hajar R. Risk factors for coronary artery disease: historical perspectives. Heart Views. 2017;18:109–114. DOI: 10.4103/HEARTVIEWS.HEARTVIEWS_106_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vlietstra RE, Kronmal RA, Frye RL, Seth AK, Tristani FE, Killip T 3rd. Factors affecting the extent and severity of coronary artery disease in patients enrolled in the coronary artery surgery study. Arteriosclerosis. 1982;2:208–215. DOI: 10.1161/01.ATV.2.3.208. [DOI] [PubMed] [Google Scholar]
- 22.Emond M, Mock MB, Davis KB, Fisher LD, Holmes DR, Chaitman BR, Kaiser GC, Alderman E, Killip T. Long‐term survival of medically treated patients in the Coronary Artery Surgery Study (CASS) Registry. Circulation. 1994;90:2645–2657. DOI: 10.1161/01.CIR.90.6.2645. [DOI] [PubMed] [Google Scholar]
- 23.Scanlon PJ, Faxon DP, Audet A‐M, Carabello B, Dehmer GJ, Eagle KA, Legako RD, Leon DF, Murray JA, Nissen SE, et al. ACC/AHA guidelines for coronary angiography: executive summary and recommendations. Circulation. 1999;33:1756–1824. DOI: 10.1161/01.CIR.99.17.2345. [DOI] [Google Scholar]
- 24.van Rosendael PJ, Kamperidis V, Kong WK, van Rosendael AR, Marsan NA, Bax JJ, Delgado V. Comparison of quantity of calcific deposits by multidetector computed tomography in the aortic valve and coronary arteries. Am J Cardiol. 2016;118:1533–1538. DOI: 10.1016/j.amjcard.2016.08.021. [DOI] [PubMed] [Google Scholar]
- 25.Michelena HI, Prakash SK, Della Corte A, Bissell MM, Anavekar N, Mathieu P, Bossé Y, Limongelli G, Bossone E, Benson DW, et al. Bicuspid aortic valve: identifying knowledge gaps and rising to the challenge from the International Bicuspid Aortic Valve Consortium (BAVCon). Circulation. 2014;129:2691–2704. DOI: 10.1161/CIRCULATIONAHA.113.007851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eagle KA, Guyton RA, Davidoff R, Ewy GA, Fonger J, Gardner TJ, Gott JP, Herrmann HC, Marlow RA, Nugent W, et al. ACC/AHA guidelines for coronary artery bypass graft surgery: executive summary and recommendations: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (committee to revise the 1991 guidelines for coronary artery bypass graft surgery). Circulation. 1999;100:1464–1480. DOI: 10.1161/01.CIR.100.13.1464. [DOI] [PubMed] [Google Scholar]
- 27.Magni P. Bicuspid aortic valve, atherosclerosis and changes of lipid metabolism: are there pathological molecular links? J Mol Cell Cardiol. 2019;129:231–235. DOI: 10.1016/j.yjmcc.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 28.Chau K, Elefteriades JA. Ascending thoracic aortic aneurysms protect against myocardial infarctions. Int J Angiol. 2014;23:177–182. DOI: 10.1055/s-0034-1382288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Achneck H, Modi B, Shaw C, Rizzo J, Albornoz G, Fusco D, Elefteriades J. Ascending thoracic aneurysms are associated with decreased systemic atherosclerosis. Chest. 2005;128:1580–1586. DOI: 10.1378/chest.128.3.1580. [DOI] [PubMed] [Google Scholar]
- 30.Hung A, Zafar M, Mukherjee S, Tranquilli M, Scoutt LM, Elefteriades JA. Carotid intima‐media thickness provides evidence that ascending aortic aneurysm protects against systemic atherosclerosis. Cardiol. 2012;123:71–77. DOI: 10.1159/000341234. [DOI] [PubMed] [Google Scholar]
- 31.Nistri S, Sorbo MD, Marin M, Palisi M, Scognamiglio R, Thiene G. Aortic root dilatation in young men with normally functioning bicuspid aortic valves. Heart. 1999;82:19–22. DOI: 10.1136/hrt.82.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tadros TM, Klein MD, Shapira OM. Ascending aortic dilatation associated with bicuspid aortic valve. Circulation. 2009;119:880–890. DOI: 10.1161/CIRCULATIONAHA.108.795401. [DOI] [PubMed] [Google Scholar]
- 33.Ali M, Girgis S, Hassan A, Rudick S, Becker RC. Inflammation and coronary artery disease: from pathophysiology to Canakinumab Anti‐Inflammatory Thrombosis Outcomes Study (CANTOS). Coron Artery Dis. 2018;29:429–437. DOI: 10.1097/MCA.0000000000000625. [DOI] [PubMed] [Google Scholar]
- 34.Koenig W. Inflammation and coronary heart disease: an overview. Cardiol Rev. 2001;9:31–35. DOI: 10.1097/00045415-200101000-00007. [DOI] [PubMed] [Google Scholar]
- 35.Wallby L, Janerot‐Sjöberg B, Steffensen T, Broqvist M. T lymphocyte infiltration in non‐rheumatic aortic stenosis: a comparative descriptive study between tricuspid and bicuspid aortic valves. Heart. 2002;88:348–351. DOI: 10.1136/heart.88.4.348. [DOI] [PMC free article] [PubMed] [Google Scholar]


