Abstract
Background
Transcatheter aortic valve replacement (TAVR) has transformed the management of aortic valve stenosis. However, little national data are available characterizing the geographic and demographic dispersion of this disruptive technology relative to surgical aortic valve replacement (SAVR).
Methods and Results
In this US claims‐based study, we analyzed a 100% sample of fee‐for‐service Medicare beneficiaries from 2012 to 2017 and examined national rates of TAVR versus SAVR. Procedure rates were compared across years as a function of age, sex, race, and geography for TAVR and SAVR beneficiaries. There was significant growth in TAVR from 15.4 beneficiaries/100 000 enrollees in 2012 to 90.6 in 2017 (P<0.001). SAVR rates declined from 92.8 beneficiaries/100 000 enrollees in 2012 to 63.5 in 2017 (P<0.001). The growth of TAVR varied as a function of age (P<0.0001). While TAVR was the dominant strategy among beneficiaries ≥85 and 75 to 84 years old, SAVR was more common among beneficiaries 65 to 74 years old. TAVR was also used more frequently than SAVR among women (P<0.001). While TAVR increased among all races, it was less commonly used among non‐White beneficiaries (P<0.001). Contemporary use of TAVR relative to SAVR varied significantly by geographic location, with a TAVR:SAVR ratio in 2017 of 1.24 in the Midwest and 1.68 in the Northeast (P<0.001).
Conclusions
In 2017, the number of Medicare beneficiaries receiving TAVR exceeded SAVR for the first time in the United States. There is significant variation, however, in the geographic expansion of TAVR and in patient demographics relative to SAVR.
Keywords: aortic stenosis, aortic valve replacement, outcomes research, transcatheter aortic valve implantation, utilization
Subject Categories: Aortic Valve Replacement/Transcather Aortic Valve Implantation, Catheter-Based Coronary and Valvular Interventions, Health Services
Transcatheter aortic valve replacement (TAVR) has emerged as a disruptive device technology for the management of patients with severe aortic valve stenosis (AS).1 In November 2011, the US Food and Drug Administration (FDA) provided commercial approval of TAVR for high or prohibitive surgical risk patients with AS, resulting in the rapid use of this procedure nationally.1, 2, 3 Since then, multiple randomized clinical trials in patients with intermediate and low surgical risk have demonstrated non‐inferiority of TAVR versus surgical aortic valve replacement (SAVR).4, 5, 6, 7 The variable extent to which TAVR has outpaced SAVR as a function of geography and patient demographics is unknown.
As the indications for TAVR have continued to expand, now encompassing patients of all surgical risk categories, understanding the differential patterns of TAVR growth relative to SAVR is informative for health policy decisions. Historically, disruptive technologies have shown rapid global adoption, but significant variability when analyzed across specific geographic areas and patient subgroups.8 Recently, the Centers for Medicare and Medicaid Services provided an update to the National Coverage Determination for TAVR.9 Characterizing contemporary utilization of TAVR across the United States compared with SAVR provides a valuable context for these coverage determinations. As such, we performed an analysis of Medicare claims data from 2012 through 2017 to assess the contemporary utilization of TAVR relative to SAVR as a function of beneficiary demographics and geography.
METHODS
This study was reviewed and approved by the Dartmouth‐Hitchcock Institutional Review Board. The requirement to obtain informed consent was waived. The data used in this analysis include restricted Medicare claims data. Therefore, the data cannot be shared directly with other investigators because of the terms of the specified data use agreement. Investigators who wish to obtain the data can do so through submission of an application to the Centers for Medicare and Medicaid Services Research Data and Assistance Center.
We conducted an observational study using a 100% sample of fee‐for‐service Medicare beneficiaries aged 65 and older who were continuously enrolled in Parts A and B for all 12 months of a given calendar year. The 100% Medicare Provider Analysis and Review (MEDPAR) file from 2012 through 2017 was queried to identify all fee‐for‐service Medicare beneficiaries undergoing SAVR (n=165 268) and TAVR (n=98 117). We extracted patients undergoing SAVR using the following International Classification of Diseases, Ninth Revision (ICD‐9) and Tenth Revision (ICD‐10) codes: 3521, 3522, 02RF07Z, 02RF08Z, 02RF0JZ, and 02RF0KZ. For TAVR, we used the following ICD‐9 and ICD‐10 codes: 3505, 3506, 02RF37H, 02RF38H, 02RF3JH, 02RF3KH, 02RF37Z, 02RF38Z, 02RF3JZ, and 02RF3KZ. TAVR and SAVR procedure rates (expressed per 100 000 beneficiaries) were compared across years. We also calculated the ratio of TAVR to SAVR procedure counts, stratified according to geographical location and year, as well as age, sex, and race. Hospital Referral Region template maps were downloaded from the Dartmouth Atlas of Health Care website and choropleth maps were developed utilizing color density to display the ratio of TAVR:SAVR.10 The four geographic regions are based on 2010 US Census data.11 All analyses were performed using SAS 9.4.
RESULTS
Nationally, TAVR rates increased from 15.4 beneficiaries/100 000 enrollees in 2012 to 90.6 in 2017 (P<0.001). Concurrently, rates of SAVR decreased from 92.8 beneficiaries/100 000 enrollees in 2012 to 63.5 in 2017 (P<0.001) (Figure 1). There was a significant increase in the ratio of TAVR:SAVR claims throughout the study period, with TAVR surpassing SAVR for the first time in 2017 (Table).
Figure 1. Utilization of surgical aortic valve replacement (SAVR) and transcatheter aortic valve replacement (TAVR) across United States Food and Drug Administration (FDA) approval timeline.
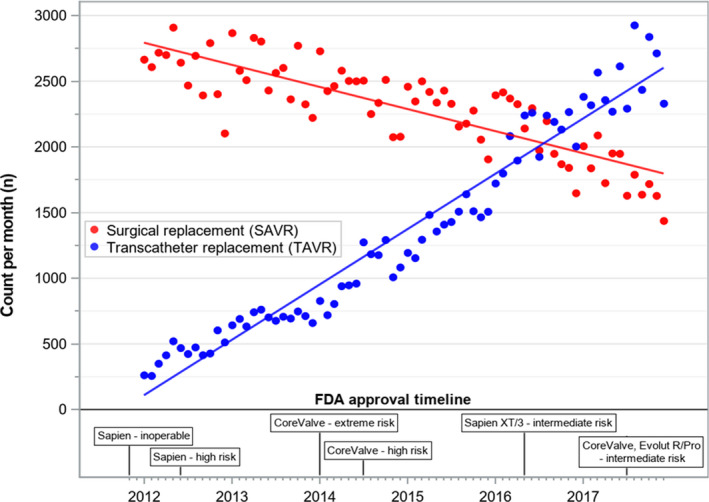
Table 1.
Ratio of TAVR: SAVR Over Time
| 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | P Value | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TAVR n | SAVR n | Ratio | TAVR n | SAVR n | Ratio | TAVR n | SAVR n | Ratio | TAVR n | SAVR n | Ratio | TAVR n | SAVR n | Ratio | TAVR n | SAVR n | Ratio | ||
| Overall | 5154 | 31 117 | (0.17) | 8399 | 30 891 | (0.27) | 12 242 | 28 983 | (0.42) | 16 975 | 27 417 | (0.62) | 24 782 | 25 442 | (0.97) | 30 565 | 21 418 | (1.43) | <0.0001* |
| Age, y | |||||||||||||||||||
| 65–74 | 747 | 12 099 | (0.06) | 1168 | 12 411 | (0.09) | 1775 | 12 404 | (0.14) | 2507 | 12 387 | (0.20) | 4208 | 12 638 | (0.33) | 5542 | 11 418 | (0.49) | |
| 75–84 | 1786 | 14 814 | (0.12) | 2987 | 14 714 | (0.20) | 4544 | 13 564 | (0.34) | 6459 | 12 547 | (0.51) | 9742 | 11 145 | (0.87) | 12 940 | 9001 | (1.44) | |
| ≥85 | 2621 | 4204 | (0.62) | 4244 | 3766 | (1.13) | 5923 | 3015 | (1.96) | 8009 | 2483 | (3.23) | 10 832 | 1659 | (6.53) | 12 083 | 999 | (12.10) | <0.0001† |
| Sex | |||||||||||||||||||
| Male | 2699 | 18 483 | (0.15) | 4182 | 18 654 | (0.22) | 6382 | 17 792 | (0.36) | 8745 | 17 090 | (0.51) | 13 131 | 16 272 | (0.81) | 16 376 | 13 986 | (1.17) | |
| Female | 2455 | 12 634 | (0.19) | 4217 | 12 237 | (0.34) | 5860 | 11 191 | (0.52) | 8230 | 10 327 | (0.80) | 11 651 | 9170 | (1.27) | 14 189 | 7432 | (1.91) | <0.0001† |
| Race/Ethnicity | |||||||||||||||||||
| White | 4860 | 29 009 | (0.17) | 7918 | 28 809 | (0.27) | 11 478 | 26 938 | (0.43) | 15 934 | 25 415 | (0.63) | 23 173 | 23 507 | (0.99) | 28 552 | 19 591 | (1.46) | |
| Black | 167 | 1080 | (0.15) | 268 | 997 | (0.27) | 394 | 979 | (0.40) | 531 | 923 | (0.58) | 788 | 824 | (0.96) | 991 | 743 | (1.33) | |
| Hispanic | 43 | 297 | (0.14) | 65 | 291 | (0.22) | 113 | 262 | (0.43) | 146 | 233 | (0.63) | 217 | 214 | (1.01) | 249 | 182 | (1.37) | |
| Other†† | 84 | 731 | (0.11) | 148 | 794 | (0.19) | 257 | 804 | (0.32) | 364 | 846 | (0.43) | 604 | 897 | (0.67) | 773 | 902 | (0.86) | <0.0001† |
| Geography | |||||||||||||||||||
| South | 1971 | 11 086 | (0.18) | 2980 | 10 906 | (0.27) | 4137 | 10 298 | (0.40) | 5847 | 9885 | (0.59) | 8632 | 9011 | (0.96) | 10 946 | 7709 | (1.42) | |
| Northeast | 1202 | 7245 | (0.17) | 2309 | 7238 | (0.32) | 3311 | 6627 | (0.50) | 4390 | 6202 | (0.71) | 6287 | 5771 | (1.09) | 7510 | 4460 | (1.68) | |
| Midwest | 1082 | 7475 | (0.14) | 1843 | 7390 | (0.25) | 2716 | 7026 | (0.39) | 3707 | 6517 | (0.57) | 5401 | 6109 | (0.88) | 6610 | 5325 | (1.24) | |
| West | 899 | 5311 | (0.17) | 1267 | 5357 | (0.24) | 2078 | 5032 | (0.41) | 3031 | 4813 | (0.63) | 4462 | 4551 | (0.98) | 5499 | 3924 | (1.40) | <0.0001† |
Cochran‐Armitage trend test of TAVR:SAVR ratios.
Chi‐square test of patient characteristic TAVR:SAVR ratios.
Other includes unknown, other, Asian/Pacific Islander, American Indian/Alaska Native
Significant variability existed, however, in the ratio of TAVR:SAVR claims across age, sex, and race strata. While TAVR demonstrated growth across all age groups over time, the degree of utilization across age groups varied (P<0.0001). In 2017, among beneficiaries ≥85 years old, TAVR largely replaced SAVR (ratio TAVR:SAVR 12.10), and among beneficiaries aged 75 to 84, TAVR was also more common (ratio 1.44). However, among beneficiaries 65 to 74 years old, SAVR remained more common in the most contemporary era (ratio 0.49). The utilization of TAVR also varied significantly by sex and was consistently higher among women (P<0.0001). Although TAVR use increased among both women and men over time, by 2017, the ratio of TAVR:SAVR was 1.91 in women and 1.17 in men. The mean age of women undergoing TAVR (82.8 years old) was statistically greater than that of men (81.9 years old) (P<0.001); however, the difference was not clinically significant. The utilization of TAVR relative to SAVR also varied significantly by race (P<0.001). While TAVR utilization increased across all races during the study period, the ratio of TAVR:SAVR in the most contemporary period was highest among White beneficiaries (ratio 1.46).
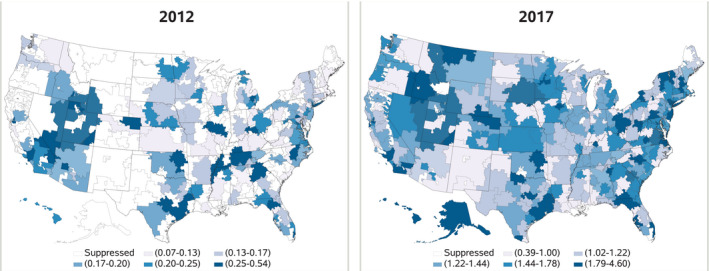
TAVR utilization relative to SAVR also varied significantly as a function of geographic location in the United States (P<0.0001) (Table). While TAVR utilization outpaced SAVR across all geographic locations in 2017, utilization relative to SAVR was highest among beneficiaries in the Northeast (ratio 1.68) and lowest among beneficiaries in the Midwest (ratio 1.24) (Figure 2).
Figure 2. Geographical dispersion and change in transcatheter aortic valve replacement (TAVR): surgical aortic valve replacement (SAVR) ratio by Hospital Referral Region (HRR) in 2012 and 2017.
DISCUSSION
In this observational study of claims‐based data of US Medicare beneficiaries, we demonstrate that the utilization of TAVR has continued to increase annually, with a corresponding decrease in national SAVR volumes. In 2017, TAVR, for the first time among Medicare beneficiaries, exhibited widespread dispersion across all hospital referral regions and surpassed SAVR in its overall relative use.
The adoption of TAVR has closely paralleled the landmark clinical trial data and subsequent regulatory approvals for populations with lower surgical risk. For instance, publication of the PARTNER‐1A and B (Placement of Aortic Transcatheter Valve Trial) trials for the prohibitive and high surgical risk groups, respectively, served as the foundation for first commercial approval of the Edwards Sapien balloon‐expandable valve in November 2011.12 FDA approval of the self‐expanding Medtronic CoreValve, based on the US Pivotal Trials of patients with extreme and high surgical risk, followed in 2014.13 In 2016 and 2017, respectively, the PARTNER‐2 and SURTAVI (Surgical Replacement and Transcatheter Aortic Valve Implantation) trials showed non‐inferiority of TAVR versus SAVR in the intermediate risk population,4, 5 thus leading to continued commercial expansion.
In our analysis, we demonstrate that over the course of six years, the number of TAVR procedures performed in the United States has now outpaced the number of SAVRs. While SAVR rates were initially constant as TAVR rates increased, we show a progressive rise of TAVR as SAVR has gradually declined in parallel with growing TAVR evidence. Importantly, the relative utilization of TAVR has not occurred at a uniform rate across all demographic and geographic subgroups. In 2017, TAVR was more common in the Northeast and less common in the Midwest compared with SAVR. Even within states with lower TAVR utilization, there was regional variation. Areas in Montana, Idaho, and South Dakota, for example, demonstrated significant within‐state variation. We hypothesize that the demographics of those patient populations, regional resources available, and local practice patterns factor into the differences observed nationally and within states. It will be vital to ensure, however, that these regional differences do not translate into differences in clinical outcomes over time. This finding may be particularly relevant for SAVR, where overall volumes have steadily declined.
We highlight an “age creep” phenomenon in addition to the sex and race differences in TAVR versus SAVR utilization. Beneficiaries aged 75 to 84 and ≥85 years old are now clearly more likely to receive TAVR. This observation is not unexpected, given that these patients are more likely to have higher surgical risk. For beneficiaries 65 to 74 years old, SAVR has remained more common; however, this age group also showed a trend towards growing TAVR use, which will require continued evaluation. With respect to sex differences, female patients were more likely to undergo TAVR than SAVR, and this did not appear to be related to differences in age. This observation may be attributable to several potential factors, including unconscious sex bias, perceptions of relative frailty, or other clinical or social determinant factors not readily capturable in a claims‐based data set.
Importantly, TAVR utilization increased across all races over time, demonstrating that this technology has diffused throughout all patient groups. However, we did observe variability in the relative use of TAVR versus SAVR by race strata, indicating that uniform access to advanced cardiovascular therapies must continue to be monitored. Furthermore, both TAVR and SAVR in Black and Hispanic adults were lower in frequency than they were in White adults across all years of this study. While this could be attributed to differences in disease prevalence, further studies are warranted to understand how these underrepresented minorities are affected by disparities in disease recognition and management of severe aortic stenosis. With regards to limitations, this analysis utilizes an administrative registry based solely on Medicare beneficiaries. While this registry captures a large majority of patients undergoing AVR nationally, there may be a small fraction of younger patients <65 years‐old receiving these therapies who would not be included in this analysis and to whom we cannot extrapolate these findings.
In June 2019, the Centers for Medicare and Medicaid Services enacted an update to the National Coverage Determination for TAVR, which provides detailed specifications about hospital and cardiac surgery volume requirements. In this guideline document, both open heart surgery and aortic valve related procedures are specified, and these guidelines have implications for beginning and maintaining a TAVR program.9 In an era of continually declining SAVR volumes (as evidenced in this study), such regulatory policies with respect to volume mandates will be relevant to both the technological dispersion of TAVR and access‐to‐care. Understanding the primary drivers for the regional and demographic variations in TAVR and SAVR may help guide healthcare policy for future iterations of the National Coverage Determination.
CONCLUSIONS
TAVR is a disruptive device technology that has continued to disseminate widely and surpass SAVR volumes in the United States. The diffusion of TAVR, however, has not been uniform, with variation in its use being a function of patient demographics and geographic location. Given the significant variation of TAVR versus SAVR utilization, a Heart Team strategy (leveraging the expertise of surgeons and cardiologists) remains critically important to ensure that optimal clinical outcomes are preserved. Moving forward, greater consideration should be given to the stringency of volume mandates, as the use of TAVR and SAVR continue to diverge.
Sources of Funding
This research was supported by an American College of Cardiology Presidential Career Development Award.
Disclosures
None.
Acknowledgments
The authors acknowledge Prezley Duncan for her assistance in organization of the article for publication.
(J Am Heart Assoc. 2021;10:e019588. DOI: 10.1161/JAHA.120.019588.)
For Sources of Funding and Disclosures, see page 5.
REFERENCES
- 1.Boskovski MT, Nguyen TC, McCabe JM, Kaneko T. Outcomes of transcatheter aortic valve replacement in patients with severe aortic stenosis: a review of a disruptive technology in aortic valve surgery. JAMA Surg. 2019. Nov 27 [epub ahead of print]. 155:69–77. DOI: 10.1001/jamasurg.2019.4449. [DOI] [PubMed] [Google Scholar]
- 2.Young MN, Elmariah S, Kennedy KF, Inglessis I, Yeh RW. Utilization and outcomes of transcatheter aortic valve replacement in the United States shortly after device approval. Catheter Cardiovasc Interv. 2017;90:830–838. DOI: 10.1002/ccd.27018. [DOI] [PubMed] [Google Scholar]
- 3.Kundi H, Faridi KF, Wang Y, Wadhera RK, Valsdottir LR, Popma JJ, Kramer DB, Yeh RW. Geographic patterns of growth for transcatheter aortic valve replacement in the United States. Circulation. 2019;140:969–971. DOI: 10.1161/CIRCULATIONAHA.119.040788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, Thourani VH, Tuzcu EM, Miller DC, Herrmann HC, et al. Transcatheter or surgical aortic‐valve replacement in intermediate‐risk patients. N Engl J Med. 2016;374:1609–1620. DOI: 10.1056/NEJMoa1514616. [DOI] [PubMed] [Google Scholar]
- 5.Reardon MJ, Van Mieghem NM, Popma JJ, Kleiman NS, Søndergaard L, Mumtaz M, Adams DH, Deeb GM, Maini B, Gada H, et al. Surgical or transcatheter aortic‐valve replacement in intermediate‐risk patients. N Engl J Med. 2017;376:1321–1331. DOI: 10.1056/NEJMoa1700456. [DOI] [PubMed] [Google Scholar]
- 6.Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M, Kapadia SR, Malaisrie SC, Cohen DJ, Pibarot P, et al. Transcatheter aortic‐valve replacement with a balloon‐expandable valve in low‐risk patients. N Engl J Med. 2019;380:1695–1705. DOI: 10.1056/NEJMoa1814052. [DOI] [PubMed] [Google Scholar]
- 7.Popma JJ, Deeb GM, Yakubov SJ, Mumtaz M, Gada H, O’Hair D, Bajwa T, Heiser JC, Merhi W, Kleiman NS, et al. Transcatheter aortic‐valve replacement with a self‐expanding valve in low‐risk patients. N Engl J Med. 2019;380:1706–1715. DOI: 10.1056/NEJMoa1816885. [DOI] [PubMed] [Google Scholar]
- 8.Rogers E. Diffusion of Innovations. 5th ed. New York, NY: Simon and Schuster; 2003. [Google Scholar]
- 9.Decision memo for transcatheter aortic valve replacement (TAVR). Available at: https://www.cms.gov/medicare‐coverage‐database/details/nca‐decision‐memo.aspx?NCAId=293. Accessed April 14, 2020.
- 10.Research Methods. Dartmouth Atlas of Health Care. Available at: https://www.dartmouthatlas.org/research‐methods/. Accessed April 14, 2020.
- 11.US Census Bureau . 2010 Census Regions and Divisions of the United States. The United States Census Bureau. Available at: https://www.census.gov/geographies/reference‐maps/2010/geo/2010‐census‐regions‐and‐divisions‐of‐the‐united‐states.html. Accessed April 14, 2021. [Google Scholar]
- 12.Premarket Approval (PMA) . Edwards Sapien transcatheter heart valve and accessories. Available at: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P100041. Accessed April 14, 2020.
- 13.Premarket Approval (PMA) . Medtronic Corevalve System. Available at: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P130021. Accessed April 14, 2020.


