Abstract
Background
Disrupted nighttime sleep has been associated with heart failure (HF). However, the relationship between daytime napping, an important aspect of sleep behavior commonly seen in older adults, and HF remains unclear. We sought to investigate the association of objectively assessed daytime napping and risk of incident HF during follow‐up.
Methods and Results
We studied 1140 older adults (age, 80.7±7.4 [SD] years; female sex, 867 [76.1%]) in the Rush Memory and Aging Project who had no HF at baseline and were followed annually for up to 14 years. Motor activity (ie, actigraphy) was recorded for ≈10 days at baseline. We assessed daytime napping episodes between 9 am and 7 pm objectively from actigraphy using a previously published algorithm for sleep detection. Cox proportional hazards models examined associations of daily napping duration and frequency with incident HF. Eighty‐six participants developed incident HF, and the mean onset time was 5.7 years (SD, 3.4; range, 1–14). Participants who napped longer than 44.4 minutes (ie, the median daily napping duration) showed a 1.73‐fold higher risk of developing incident HF than participants who napped <44.4 minutes. Consistently, participants who napped >1.7 times/day (ie, the median daily napping frequency) showed a 2.20‐fold increase compared with participants who napped <1.7 times/day. These associations persisted after adjustment for covariates, including nighttime sleep, comorbidities, and cardiovascular disease/risk factors.
Conclusions
Longer and more frequent objective napping predicted elevated future risk of developing incident HF. Future studies are needed to establish underlying mechanisms.
Keywords: actigraphy, cardiovascular disease, mobile health, sleep, unobtrusive monitoring, wearables
Subject Categories: Aging, Heart Failure, Risk Factors
Nonstandard Abbreviation and Acronym
- MAP
Memory and Aging Project
Clinical Perspective
What Is New?
Association of daytime napping and future heart failure incidence was examined on the basis of objectively defined daytime napping duration and frequency using actigraphy.
Longer and more frequent objective daytime napping were associated with risk of heart failure independent of nighttime sleep, comorbidities, and cardiovascular disease/risk factors.
What Are the Clinical Implications?
Excessive daytime napping may represent preclinical evidence for heart failure.
The results call for closer attention to monitoring daytime sleep behavior to optimize cardiovascular health in the older population.
Actigraphic daytime sleep monitoring can be valuable as a scalable and easily implemented tool for timely identification of individuals at risk of heart failure.
Adequate nighttime sleep of good quality has increasingly been recognized as beneficial to cardiovascular health.1 Typically defined by short sleep periods during daylight hours, daytime napping is a common practice worldwide.2 It may be a direct consequence of inadequate or poor nighttime sleep, particularly in older adults, leading to daytime sleepiness. Daytime napping may also reflect a covert symptom of undiagnosed disease such as heart failure (HF) and occur because of fatigue associated with HF.3 While the association between HF and excessive daytime sleepiness has been reported in cross‐sectional studies,4 there remains a paucity of prospective studies of daytime napping. Therefore, this longitudinal study was designed to examine the effect of daytime napping behavior on the development of HF.
Many studies have suggested that daytime napping is detrimental to cardiovascular disease.5, 6, 7 For example, longer self‐reported daytime napping saw an increased risk for HF in older men.8 However, others found that daytime napping can be beneficial.2, 9 Lack of consideration for the frequency of napping may explain these inconsistent results. For example, a recent study showed that napping once or twice per week saw a lower risk for incident cardiovascular events, independent of total nap duration.10 Unfortunately, most of these previous studies were exclusively based on self‐report and may be not reliable, particularly in older adults who may fail to report napping habits accurately because of cognitive impairment.11, 12
Traditional sleep assessment in a clinical setting is costly and time consuming. This may explain the lack of large‐scale, community‐based prospective studies with objective sleep measurements. Fortunately, analytical tools developed in the past decade to infer sleep and wake episodes based on ambulatory motor activity recordings have proven invaluable in the assessment of sleep and its changes under pathological conditions.13, 14, 15 A wide range of activity monitors are now available for unobtrusive collection of motor activity without disrupting individuals’ daily behaviors, offering the capacity for monitoring daytime napping patterns in community‐based settings. In this study, we objectively extracted daytime napping events from motor activity recordings in an older cohort.16 We hypothesized that objectively longer and more frequent daytime naps would be associated with increased risk of incident HF.
METHODS
Study Design and Participants
Data were from an ongoing study, namely, the Rush Memory and Aging Project (MAP) that is conducted at the Rush Alzheimer’s Disease Center, which started in 1997. In 2005, a watchlike device (Actical, Philips Respironics, Bend, OR) was introduced to record daily motor activity.16 The protocol of MAP was approved by the Institutional Review Board of the Rush Alzheimer’s Disease Center in accordance with the principles of the Declaration of Helsinki and its later amendments. Written informed consent was obtained, and all participants signed a repository consent to allow their data to be repurposed. The protocol for this current study was approved by the Partners Healthcare Inc. Institutional Review Board. The data that support the findings of this study are available from the corresponding author upon reasonable request. Requests to access the data related to the Rush MAP from qualified researchers trained in human subject confidentiality protocols may be sent to Rush Alzheimer’s Disease Center Research Resource Sharing Hub at https://www.radc.rush.edu/.
Participants with motor activity recordings were included in this work (N=1401; female sex, 1065 (76.5%); age, 81.4±7.5 [mean±SD] years). They were followed annually with cognitive test batteries and questionnaires of medical conditions and medication use. Clinical data censored in January 2020 were used. Participants yet to have follow‐up clinical assessments were excluded (N=115). Subjects who had been diagnosed with HF at analytic baseline (N=64) or had missing HF information at analytic baseline (N=82) were also excluded before further analysis. Therefore, 1140 participants were included in this study.
Assessment of Daytime Napping
The activity monitor was worn on the nondominant wrist continuously for about 10 days at baseline. The device measures accelerations in 3 directions parallel to the 3 faces of the device with a continuous 32‐Hz sampling frequency and integrates the data into a proprietary count value every 15 seconds. A daytime napping episode was identified as sleep during the common daytime or daily active hours between 9 am and 7 pm using a published and validated sleep scoring algorithm based on wrist activity counts.17, 18
First, the recording for activity counts was integrated every 4 epochs, resulting in a new recording with an epoch length of 1 minute. As a key step to distinguish sleep from “no activity,” the algorithm assigns sleep/wakefulness to an epoch by considering not only the activity count of the current epoch but also activity counts of multiple epochs surrounding the epoch. Specifically, a score was rendered for each epoch based on whether a weighted sum of the current epoch, 4 epochs preceding the current epoch, and 2 epochs following the current one was <1 (sleep) or not (wakefulness). The 7 weights were trained and published in the previous studies.17, 18 Final scores for sleep/wake were obtained from the raw scores followed by 5 rescoring rules to improve specificity.17 We also excluded the segments that were scored as naps for 2 consecutive hours with almost 0 activity counts (ie, sum <10 activity counts) to avoid including periods where participants likely removed their watches. Finally, 2 consecutive nap segments that were within 3 minutes apart were merged as 1 nap episode. Nap duration was calculated as the average nap minutes/day, and nap frequency was calculated as the average number of naps/day during the assessed days.
Annual Clinical Assessment
History of HF is based on annual clinical interviews. During the baseline interview, the participants are asked: “Have you ever been told by a doctor, nurse, or therapist that you had heart failure?” In each follow‐up visit, participants are then asked: “Since your last interview, have you been told by a doctor, nurse, or therapist that you had heart failure?” If so, new incidence was reflected in the data set starting from that visit onward. To verify HF diagnosis, we examined HF‐associated medications taken at the time (or within 1 year) of participants newly reporting incident HF. These included angiotensin‐converting enzyme inhibitors/angiotensin II receptor blockers, beta blockers, cardiac glycosides (eg, digoxin) or diuretics. We then refined HF incidence on the basis of the criterion that at least 2 of these medications were taken, in addition to a positive response from the annual clinical interview for HF.
Assessment of Covariates
We grouped covariates in terms of demographics (age, sex, and education), nighttime sleep (total nighttime sleep duration and sleep fragmentation),19 comorbidities (alcohol consumption, body mass index, frailty, motor function, Parkinsonian signs, mobility disability, depression, medications that may affect sleep [anxiety and insomnia treatments, antipsychotics, analgesics, or anticonvulsants], urinary conditions, thyroid disease, and cognition), and, finally, cardiovascular risk factors/diseases (smoking, hypertension, diabetes mellitus, cholesterol, coronary artery disease, and claudication). More details in assessing each of the covariates are summarized in Data S1.
Statistical Analysis
Descriptive statistics were reported by incident HF. We present frequency and percentages for categorical variables, mean and SD (or median and interquartile range) for continuous variables. Fisher’s exact tests for categorical variables and Student t tests/Wilcoxon rank‐sum tests for continuous variables were used to assess differences in demographic and clinical characteristics between participants with and without HF. Both nap duration and nap frequency were square‐root transformed before further analyses because of right skewness. A series of Cox proportional hazards models were used to assess the relationship between daytime napping (duration or frequency) and incident self‐reported HF. Initial models included demographic factors (age, sex, and years of education) in addition to the nap parameter (models A). Subsequent models further took nighttime sleep factors into consideration (models B). Models C were augmented from models A with adjustment for comorbidities. Cardiovascular diseases and risk factors were adjusted in models D. Finally, 2 full models (models E) were performed by augmenting models A with all covariates considered.
All models were repeated using HF incidences jointly determined by self‐reported records and medications. Sensitivity analyses within subjects who were cognitively intact at baseline were also performed. Since prior studies have reported difference in the association between self‐reported nap duration and cardiovascular outcomes in people having different nighttime sleep durations,2, 7 we examined the association between nighttime sleep duration and incident HF and the interaction effect of nap duration and nighttime sleep duration; we also examined the association between nap duration and incident HF in stratified models by nighttime sleep duration (i.e., <6 or ≥6 hours/night). These additional results were reported in Data S1.
For all Cox models, the Efron approximation was applied to account for ties resulting from grouped survival data. The proportional hazards assumption was assessed and confirmed using a global χ2 test in R (R Foundation for Statistical Computing, Vienna, Austria).20 Statistical significance was determined a priori at an alpha level of 0.05 (2‐sided). All statistical analyses were done using JMP Pro (version 14, SAS Institute, Cary, NC).
RESULTS
Demographic and clinical characteristics of the 1140 participants are shown in Table 1, and of them, 86 participants developed incident HF (7.54% of 1140) during a mean time interval of 5.7 years (SD, 3.4; range, 1–14) after baseline. On average, participants napped for 65.4 minutes (median, 44.4; interquartile range, 67.0), and 2.3 times/day (median, 1.7; interquartile range, 2.4) at baseline (Figure 1); nap duration was positively correlated with nap frequency (Spearman ρ=0.91; P<0.0001). After square‐root transform, both nap duration and nap frequency were positively correlated with age (Pearson r=0.23 and 0.25; both P<0.0001) but not education years (both P>0.1). No sex differences were found in either nap parameter (both P>0.1).
Table 1.
Demographic and Clinical Characteristics of Participants
| Developed HF | Not Developed HF | P Value | |
|---|---|---|---|
| N (%), Mean±SD, or Median [IQR] | N (%), Mean±SD, or Median [IQR] | ||
| Demographics | |||
| Number of participants | 86 | 1054 | |
| Female sex | 66 (76.7) | 801 (76.0) | 1 |
| Age, y | 81.7±6.5 | 80.6±7.5 | 0.2 |
| Education, y | 14.6±2.8 | 15.1±3.0 | 0.09 |
| Daytime napping characteristics | |||
| Nap duration, min | 47.9 [82.7] | 44.0 [66.0] | 0.3 |
| Nap frequency times | 2.1 [2.6] | 1.7 [2.3] | 0.1 |
| Sleep | |||
| Total nighttime sleep duration, h | 5.3±1.7 | 5.7±1.4 | 0.02 |
| Sleep fragmentation index, ×10−2 | 2.9±1.0 | 2.8±0.7 | 0.02 |
| Comorbidities | |||
| Body mass index, kg/m2 | 28.9±5.6 | 27.2±5.3 | 0.004 |
| Alcohol, at least 1 drink per week | 37 (43.0) | 540 (51.3) | 0.1 |
| Frailty, yes | 11 (13.9) | 74 (7.7) | 0.08 |
| Motor function | 0.94±0.22 | 1.02±0.23 | 0.002 |
| Parkinsonian signs | 8.03±6.48 | 6.96±7.15 | 0.2 |
| Mobility disability | 25 (29.4) | 204 (19.4) | 0.03 |
| Depression | 0 [2] | 0 [1] | 0.4 |
| Anxiety medication use | 5 (5.8) | 68 (6.4) | 1 |
| Insomnia medication use | 3 (3.5) | 96 (9.1) | 0.1 |
| Antipsychotics medication use | 2 (2.3) | 14 (1.3) | 0.3 |
| Analgesic medication use | 65 (75.6) | 780 (74.0) | 0.8 |
| Anticonvulsant medication use | 8 (9.3) | 116 (11.0) | 0.7 |
| Urinary conditions | 51 (59.3) | 445 (42.2) | 0.003 |
| Thyroid disease | 23 (26.7) | 317 (30.1) | 0.6 |
| Global cognition | 0.09±0.62 | 0.06±0.64 | 0.7 |
| Cardiovascular risk factors/diseases | |||
| Smoking | 35 (40.7) | 435 (41.4) | 1 |
| Hypertension | 67 (77.9) | 671 (63.7) | 0.007 |
| Cholesterol >200 | 27 (31.4) | 401 (38.0) | 0.2 |
| Diabetes mellitus | 15 (17.4) | 144 (13.7) | 0.3 |
| Coronary artery disease | 11 (12.8) | 88 (8.3) | 0.2 |
| Claudication | 12 (14.0) | 91 (8.6) | 0.1 |
Data expressed as a count (percentage %), mean±SD (if normally distributed), or median [IQR] (if nonnormally distributed). P values for normally distributed continuous variables were from Student’s t‐test, for nonnormally distributed continuous variables were from nonparametric Wilcoxon rank‐sum test, and for categorical variables were from Fisher’s exact test. Motor function was assessed using a composite measure of global motor function covering 10 motor constructs. Depressive symptoms were assessed with a 10‐item version of the Center for Epidemiologic Studies‐Depression Scale. Urinary conditions included urinary incontinence/spasms, benign prostatic hypertrophy, or diuretic use. Smoking included current or former smokers. Participants were considered to have diabetes mellitus, hypertension, or thyroid disease if they were taking medications or endorsed a diagnosis on interview. HF indicates heart failure; and IQR, interquartile range.
Figure 1. Daytime napping in older adults.
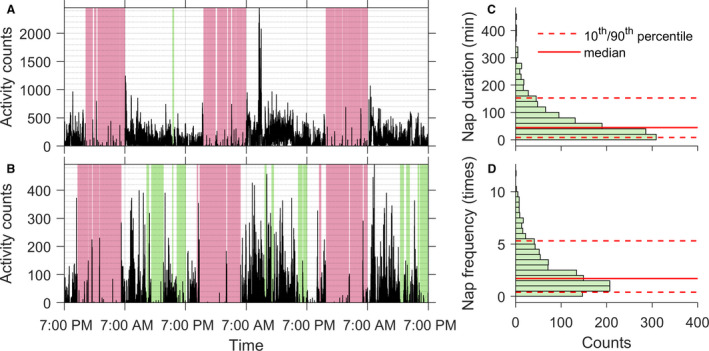
(A and B) Representative 3‐day motor activity recordings of 2 participants. One participant napped shorter/rarely (A) and the other one napped longer/more frequently (B). Green shaded areas indicate identified napping periods, and pink shaded areas indicate identified nighttime sleep episodes. (C) Distribution of daytime napping duration. (D) Distribution of daytime napping frequency.
After adjustment for demographics, the initial models revealed that longer daytime naps were associated with higher risk of HF (Table 2 and Table S1). Specifically, for each SD increase in the square‐root transformed nap duration, the hazard ratio (HR) was 1.38 (95% CI, 1.12–1.69; P=0.003). Similarly, more frequent daytime naps were also associated with increased risk of HF (Table 3 and Table S2), with an HR of 1.47 (95% CI, 1.18–1.81; P=0.0006) for each SD increase in the square‐root transformed nap frequency. To better put these results into context, we dichotomized the nap frequency and nap duration by their corresponding medians (see Table S3). Long nappers (ie, those who napped longer than the cohort median 44.4 minutes) were at 1.73‐fold (95% CI, 1.11–2.69; P=0.014; Figure 2) higher risk of HF than short nappers; frequent nappers (ie, those who napped more than the cohort median frequency 1.7 times) showed a 2.20‐fold (95% CI, 1.41–3.46; P=0.001; Figure 2) higher risk of HF than infrequent nappers. These effects were equivalent to that of being 14.4 and 24.0 years older at baseline, respectively (note that HR for being 1 year older was about 1.04; P<0.05; Tables 2 and 3). Considering a J‐shaped association between nap duration and incident cardiovascular disease in a prior investigation,2 we also refactorized the nap duration into 3 categories (ie, <30 min/d, 30–60 min/d, and >60 min/d). Nap duration still appeared to affect HF risk monotonically in this cohort. Specifically, the HR was 1.68 (95% CI, 0.94–3.00) and 2.11 (95% CI, 1.27–3.48) respectively, in those who napped 30 to 60 minutes and >60 min/d versus those who napped for a shorter time; the HR was 1.26 (95% CI, 0.74–2.14) in those who napped >60 min/d versus those who napped 30 to 60 min/d (results adjusted for age, sex, and education).
Table 2.
Daytime Nap Duration, Covariates, and Incident HF
| Variables | Models | ||||
|---|---|---|---|---|---|
| A | B | C | D | E | |
| HR (95% CI) P Value | HR (95% CI) P Value | HR (95% CI) P Value | HR (95% CI) P Value | HR (95% CI) P Value | |
| Age, y* |
1.04 (1.01–1.07) 0.022 |
1.04 (1.01–1.08) 0.013 |
1.04 (1.00–1.09) 0.070 |
1.03 (1.00–1.07) 0.039 |
1.06 (1.01–1.11) 0.028 |
| Sex, female |
0.90 (0.55–1.53) 0.685 |
1.02 (0.62–1.79) 0.930 |
0.85 (0.46–1.63) 0.614 |
0.97 (0.58–1.69) 0.910 |
1.09 (0.55–2.26) 0.811 |
| Education* |
0.94 (0.87–1.01) 0.082 |
0.93 (0.86–1.00) 0.066 |
0.95 (0.87–1.03) 0.204 |
0.95 (0.88–1.03) 0.227 |
0.94 (0.86–1.02) 0.160 |
| Nap duration, square‐root transformed† |
1.38 (1.12–1.69) 0.003 |
1.51 (1.19–1.88) 0.001 |
1.31 (1.02–1.68) 0.037 |
1.34 (1.08–1.65) 0.009 |
1.54 (1.15–2.05) 0.004 |
| Sleep | |||||
| Total night sleep time* | … |
0.92 (0.77–1.09) 0.243 |
… | … |
0.93 (0.75–1.16) 0.498 |
| Sleep fragmentation index† | … |
1.17 (0.95–1.39) 0.141 |
… | … |
1.26 (0.99–1.56) 0.055 |
| Comorbidities | |||||
| Alcohol consumption, ≥ 1 drink/wk | … | … |
0.93 (0.56–1.53) 0.769 |
… |
0.91 (0.53–1.55) 0.730 |
| Body mass index* | … | … |
1.05 (1.01–1.09) 0.029 |
… |
1.04 (1.00–1.09) 0.080 |
| Frailty | … | … |
1.51 (0.59–3.52) 0.374 |
… |
1.72 (0.66–4.06) 0.253 |
| Parkinsonian signs | … | … |
0.96 (0.91–1.01) 0.120 |
… |
0.96 (0.90–1.01) 0.108 |
| Motor function† | … | … |
0.61 (0.41–0.88) 0.009 |
… |
0.63 (0.42–0.94) 0.022 |
| Mobility disability | … | … |
1.11 (0.59–2.04) 0.735 |
… |
1.02 (0.53–1.90) 0.962 |
| Depression, square‐root transformed* | … | … |
1.04 (0.74–1.43) 0.804 |
… |
0.96(0.67–1.35) 0.883 |
| Anxiety | … | … |
1.29 (0.44–3.00) 0.606 |
… |
1.38 (0.47–3.24) 0.523 |
| Insomnia | … | … |
0.35 (0.08–0.95) 0.038 |
… |
0.42 (0.10–1.15) 0.098 |
| Antipsychotic | … | … |
1.49 (0.08–7.56) 0.719 |
… |
1.69 (0.09–9.17) 0.647 |
| Analgesic | … | … |
0.92 (0.54–1.65) 0.778 |
… |
0.78 (0.45–1.42) 0.411 |
| Anticonvulsant | … | … |
1.17 (0.49–2.43) 0.706 |
… |
1.25 (0.52–2.67) 0.595 |
| Urinary conditions | … | … |
1.50 (0.93–2.44) 0.0098 |
… |
1.36 (0.80–2.35) 0.263 |
| Thyroid disease | … | … |
0.69 (0.38–1.19) 0.183 |
… |
0.80 (0.44–1.41) 0.448 |
| Global cognition† | … | … |
1.09 (0.82–1.50) 0.562 |
… |
1.20 (0.89–1.66) 0.237 |
| Cardiovascular risk factors/diseases | |||||
| Smoking | … | … | … |
1.15 (0.74–1.79) 0.527 |
1.54 (0.92–2.56) 0.103 |
| Hypertension | … | … | … |
1.94 (1.17–3.36) 0.009 |
1.38 (0.74–2.69) 0.321 |
| Cholesterol, ≥ 200 | … | … | … |
0.82 (0.50–1.31) 0.405 |
0.85 (0.48–1.47) 0.575 |
| Diabetes mellitus | … | … | … |
1.13 (0.61–2.00) 0.680 |
0.99 (0.47–1.93) 0.970 |
| Coronary artery disease | … | … | … |
1.34 (0.66–2.48) 0.393 |
1.18 (0.52–2.37) 0.674 |
| Claudication | … | … | … |
1.33 (0.68–2.38) 0.385 |
1.15 (0.52–2.28) 0.710 |
Model A is the core model adjusted for age, sex, and years of education. Models B, C, and D all build upon model A by additionally including nighttime sleep factors (B), comorbidities (C), and cardiovascular diseases and risk factors (D), respectively. Model E is the full model with all covariates adjusted.
HF indicates heart failure; and HR, hazard ratio.
Results for 1‐unit increase.
Results for 1‐SD increase.
Table 3.
Daytime Nap Frequency, Covariates, and Incident HF
| Variables | Models | ||||
|---|---|---|---|---|---|
| A | B | C | D | E | |
|
HR (95% CI) P Value |
HR (95% CI) P Value |
HR (95% CI) P Value |
HR (95% CI) P Value |
HR (95% CI) P Value |
|
| Age, y* |
1.03 (1.00–1.07) 0.032 |
1.04 (1.00–1.09) 0.018 |
1.04 (1.02–1.09) 0.073 |
1.03 (1.00–1.07) 0.051 |
1.06 (1.01–1.11) 0.028 |
| Sex, female |
0.92 (0.57–1.57) 0.757 |
1.06 (0.63–1.85) 0.837 |
0.87 (0.47–1.66) 0.658 |
0.98 (0.59–1.71) 0.955 |
1.11 (0.56–2.29) 0.777 |
| Education* |
0.93 (0.87–1.01) 0.0709 |
0.93 (0.86–1.00) 0.063 |
0.94 (0.87–1.03) 0.184 |
0.95 (0.88–1.03) 0.197 |
0.94 (0.86–1.02) 0.157 |
| Nap frequency, square‐root transformed† |
1.47 (1.18–1.81) 0.0006 |
1.56 (1.24–1.95) 0.0003 |
1.41 (1.09–1.82) 0.010 |
1.41 (1.13–1.74) 0.003 |
1.60 (1.20–2.13) 0.002 |
| Sleep | |||||
| Total night sleep time* | … |
0.90 (0.77–1.07) 0.249 |
… | … |
0.92 (0.75–1.14) 0.428 |
| Sleep fragmentation index† | … |
1.14 (0.92–1.36) 0.213 |
… | … |
1.23 (0.97–1.53) 0.082 |
| Comorbidities | |||||
| Alcohol consumption, ≥1 drink/wk | … | … |
0.96 (0.58–1.59) 0.873 |
… |
0.95 (0.55–1.62) 0.842 |
| Body mass indexa | … | … |
1.05 (1.01–1.10) 0.026 |
… |
1.04 (1.00–1.09) 0.070 |
| Frailty | … | … |
1.54 (0.60–3.58) 0.350 |
… |
1.75 (0.68–4.10) 0.234 |
| Parkinsonian signs | … | … |
0.96 (0.91–1.01) 0.113 |
… |
0.96 (0.90–1.01) 0.105 |
| Motor function† | … | … |
0.61 (0.41–0.88) 0.009 |
… |
0.62 (0.42–0.92) 0.018 |
| Mobility disability | … | … |
1.10 (0.58–2.01) 0.771 |
… |
0.967 |
| Depression, square‐root transformed* | … | … |
1.03 (0.74–1.42) 0.838 |
… |
0.96 (0.66–1.35) 0.802 |
| Anxiety | … | … |
1.34 (0.46–3.13) 0.553 |
… |
1.46 (0.49–3.44) 0.458 |
| Insomnia | … | … |
0.35 (0.09–0.96) 0.040 |
… |
0.43 (0.10–1.18) 0.109 |
| Antipsychotic | … | … |
1.48 (0.08–7.45) 0.722 |
… |
1.63 (0.09–8.76) 0.667 |
| Analgesic | … | … |
0.90 (0.53–1.61) 0.715 |
… |
0.76 (0.44–1.39) 0.363 |
| Anticonvulsant | … | … |
1.12 (0.47–2.34) 0.780 |
… |
1.21 (0.50–2.60) 0.647 |
| Urinary conditions | … | … |
1.50 (0.93–2.44) 0.099 |
… |
1.36 (0.80–2.35) 0.253 |
| Thyroid disease | … | … |
0.70 (0.39–1.21) 0.208 |
… |
0.82 (0.45–1.44) 0.495 |
| Global cognition† | … | … |
1.10 (0.82–1.51) 0.532 |
… |
1.21 (0.90–1.68) 0.219 |
| Cardiovascular risk factors/diseases | |||||
| Smoking | … | … | … |
1.16 (0.74–1.79) 0.516 |
1.53 (0.91–2.54) 0.105 |
| Hypertension | … | … | … |
1.92 (1.16–3.32) 0.011 |
1.37 (0.73–2.67) 0.328 |
| Cholesterol, ≥200 | … | … | … |
0.82 (0.50–1.32) 0.422 |
0.85 (0.48–1.46) 0.552 |
| Diabetes mellitus | … | … | … |
1.12 (0.60–1.98) 0.704 |
0.96 (0.45–1.88) 0.916 |
| Coronary artery disease | … | … | … |
1.31 (0.64–2.42) 0.436 |
1.16 (0.52–2.33) 0.703 |
| Claudication | … | … | … |
1.31 (0.67–2.35) 0.409 |
1.17 (0.53–2.32) 0.683 |
Model A is the core model adjusted for age, sex, and years of education. Models B, C, and D all build upon model A by additionally including nighttime sleep factors (B), comorbidities (C), and cardiovascular diseases and risk factors (D), respectively. Model E is the full model with all covariates adjusted.
HF indicates heart failure; and HR, hazard ratio.
Results for 1‐unit increase.
Results for 1‐SD increase.
Figure 2. Predicted risk over time for heart failure from Cox proportional hazards models for nap duration (left) and nap frequency (right).
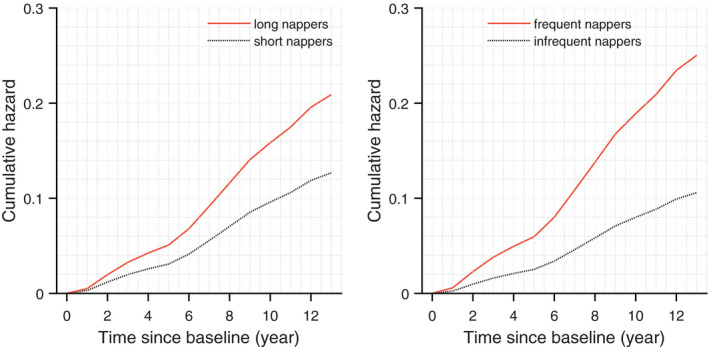
Results obtained from models with dichotomized nap duration and nap frequency by their corresponding medians. Specifically, long nappers napped for >44.4 min/d, while short nappers napped for <44.4 min/d. Similarly, frequent nappers napped >1.7 times/d while infrequent nappers napped <1.7 times/d.
The effects of daytime napping remained after separately adjusting for sleep, comorbidities, and cardiovascular diseases/risk factors (Tables 2 and 3). In fully adjusted models, the associations of nap duration and nap frequency with incident HF still held, specifically, 1‐SD increase in square‐root transformed napping duration and 1‐SD increase in square‐root transformed napping frequency corresponded to a 1.43‐fold (95% CI, 1.10–1.84; P=0.008) and a 1.48‐fold (95% CI, 1.14–1.90; P=0.003) increase in the risk of developing incident HF, respectively.
With HF incidences jointly determined by self‐report and medication, results were consistent (see Data S1 and Tables S1 and S2). Results were consistent as well from sensitivity analyses including only cognitively intact subjects (see Data S1 and Table S4). We did not find an association between total nighttime sleep duration and incident HF in this cohort (see Table S5). We also did not observe an interaction effect between nap duration and total nighttime sleep duration (see Table S6). In addition, we did not see a difference in the association between nap duration and incident HF between subjects who slept for <6 hours/night (56% of participants) versus those who slept more (44% of participants) (see Table S7).
DISCUSSION
Sleep health has gained increasing prominence as a potentially modifiable risk factor for HF and cardiovascular disease in general. Daytime napping is a common occurrence in the elderly, yet the long‐term links to HF are not well defined. Using ambulatory monitoring of motor activity through a wristwatch, daytime napping patterns were objectively assessed in an older cohort of >1000 participants who were in their 80s on average and free from HF at baseline. We demonstrated that longer and more frequent daytime naps predicted elevated risk of developing incident HF. This was independent from nighttime sleep duration/fragmentation, medical comorbidities, and traditional cardiovascular diseases and risk factors. The objective and ambulatory assessment of daytime napping is a potentially convenient identifier of unique risk for HF.
One potential mechanism linking daytime napping and HF is nighttime sleep disturbances such as multiple arousals that can cause excessive sympathetic activation,21 which, in turn, moderates the renin‐angiotensin system in a maladaptive way.22, 23 Over time, increased sympathetic activation is accompanied by high blood pressure as well as arteriolar and cardiac wall stress.24, 25, 26, 27 The cumulative effects of these frequent arousals from sleep, sympathetic surges, higher blood pressures, and repetitive low oxygen levels, compound to adversely suppress the efficiency and performance of the heart muscles, the failure of which is the defining feature of HF. However, our results refute this hypothesis since nighttime arousals was not associated with incident HF in this cohort (see Data S1 and Fig. S1). In another scenario, those reporting shorter sleep duration increasingly suffer from various sleep disorders as they age, while sleep disorders such as sleep apnea have been established as a risk factor for cardiovascular diseases including HF.28 However, we did not find an association between total nighttime sleep duration and incident HF in this cohort; and the effect of daytime napping remained after accounting for sleep fragmentation, body mass index, and hypertension—major risk factors for sleep apnea. Thus, the observed association between daytime napping and HF risk may indicate additional pathway(s) beyond the maladaptive effects of nighttime sleep disturbances.
An alternative mechanism is that daytime napping may alter the 24‐hour circadian profile of sympathetic/parasympathetic control of the heart and renin‐angiotensin system. Circadian regulation, though coupled to sleep regulation, has different neural circuitry. The circadian system directly impacts autonomic function and its response to external stress,29 and impairs glucose metabolism30 likely via its influence on autonomic control and other cardiovascular risk factors.31 It was thus not surprising that the effect sizes for the impacts of daytime napping on incident HF were slightly reduced after adjustment for cardiovascular risk.
The association between reduced motor function and increased HF risk indicates that poor exercise/movement tolerance associated with fatigue may already be present before HF diagnosis. However, the fact that the association between daytime napping and incident HF remained after adjustment for motor function, Parkinsonism, mobility disability, and frailty strongly suggests that daytime napping is an independent risk factor for HF, with different pathways from overt symptoms like fatigue. Note that we did not directly take physical activity level into consideration (it was inexplicitly included in the assessment of frailty) because of potential collinearity (see Fig. S2); however, we did explore the contribution of napping after adjustment for total daily activity level obtained from actigraphy using dichotomized napping characteristics (see Data S1 and Table S8), which supported an independent role, particularly for napping frequency, on incident HF. Further studies are warranted to better untangle their relationships.
While this is one of the largest studies to date showing a longitudinal association between prior napping behavior and HF, it is by no means conclusive that they are causally related. In fact, we believe this relationship to be more nuanced. Napping as a behavior in the elderly, free‐living community is likely in part a reflection of poorer health that could not be accounted for in all traditional risk factors. The relatively large and robust increase in risk for HF in those who napped longer or more frequently bears attention. Even if not fully causally linked, unobtrusive detection of such a strong predictor up to 14 years prior can alert healthcare providers and reinforce to patients the need to optimize known causal cardiovascular risks. Future studies are needed to examine whether this in turn decreases napping behavior in the elderly or whether direct intervention in sleep health has a bidirectional causal effect on lowering risk.
Strengths of this study include its prospective, long‐term annual follow‐up in a large cohort of community‐dwelling adults. This is, to the best of our knowledge, the first study assessing daytime napping characteristics objectively with nonintrusive measures of daily rest‐activity over relatively long periods of time. The continuous and objective assessments reduced recall bias and other confounds seen in self‐reported sleep measures, minimized the effects of daily variability, and avoided disruption to participants’ sleep environment—a major concern during in‐lab assessment of sleep. Furthermore, we were able to adjust for an extensive set of covariates including comorbidities and medications, as well as cardiac risk factors associated with HF. This study may serve as an early model for characterizing daytime napping in community‐based older adults. It opens up an avenue for collaboration among sleep medicine, cardiology, and clinical trial communities to test whether modifying daytime napping in the elderly community can reduce the risk of HF and its associated morbidity and mortality burden.
Among several limitations of the current study, this is an observational study that prevents firm conclusions regarding causality. There may still be unmeasured comorbidities that may affect both predictor and outcome including sleep disorders. For example, one major confounder is sleep apnea, a common medical condition that is highly associated with cardiovascular diseases, including HF.27, 32 The MAP has only been collecting sleep apnea risk scores (Berlin Questionnaire) since 2013, thus only few MAP participants had this score available at the time of analysis (ie, <300 participants). We have taken body mass index into consideration, which is a major risk factor for sleep apnea, and hopefully the potential confounding effect of sleep apnea on our observed association between daytime napping and HF can be alleviated this way. It is also possible that those who napped longer or more frequently may have underlying conditions, particularly, preclinical illnesses that are not yet apparent, that in themselves increase the risk of HF. In addition, there is a reliance on self‐reported HF and other covariates, as per the design of the MAP study. Ideally, follow‐up studies should include quantitative measurements of HF via echocardiograms and biochemistry (eg, brain natriuretic peptide), as well as definitive HF outcomes to determine the pathways linking daytime napping and incident HF. Methodologically, we used 9 am and 7 pm as the start and end times to maximize our confidence that we primarily counted daytime nap events. The reason is 2‐fold: First, our sample consists of older participants who are known to have phase advancement (earlier bedtimes); and second, we wanted to avoid potential morning and evening transition periods (7 am to 9 am and 7 pm to 9 pm). Ideally, both sleep diary data and actigraphy data should be included in future work to define individuals’ habitual sleep duration and sleep/wake times more accurately, such that all nap episodes outside of the main sleep period can be included. Besides, timing of naps relative to the main sleep period and regularity/irregularity of napping events would also be of interest should we be able to incorporate habitual sleep times. Adding these aspects in future work may help refine the napping risk exposure.
Sources of Funding
This work was supported by the NIH (RF1AG064312, RF1AG059867, R01AG56352, R01AG17917, T32GM007592, and R03AG067985), and the BrightFocus Foundation Alzheimer’s Research Program (A2020886S).
Disclosures
None.
Supporting information
Data S1. Supplemental Methods and Results
Tables S1–S8
Figures S1–S2
References 33–37
Acknowledgments
The authors thank the participants and staff of the Rush Memory and Aging Project and the Rush Alzheimer’s Disease Center.
(J Am Heart Assoc. 2021;10:e019037. DOI: 10.1161/JAHA.120.019037.)
L. Gao and K. Hu contributed equally.
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.019037
Contributor Information
Peng Li, Email: pli9@bwh.harvard.edu.
Kun Hu, Email: khu1@bwh.harvard.edu.
References
- 1.Malhotra A, Loscalzo J. Sleep and cardiovascular disease: an overview. Prog Cardiovasc Dis. 2009;51:279–284. DOI: 10.1016/j.pcad.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yamada T, Hara K, Shojima N, Yamauchi T, Kadowaki T. Daytime napping and the risk of cardiovascular disease and all‐cause mortality: a prospective study and dose‐response meta‐analysis. Sleep. 2015;38:1945–1953. DOI: 10.5665/sleep.5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evangelista LS, Moser DK, Westlake C, Pike N, Ter‐Galstanyan A, Dracup K. Correlates of fatigue in patients with heart failure. Prog Cardiovasc Nurs. 2008;23:12–17. DOI: 10.1111/j.1751-7117.2008.07275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riegel B, Ratcliffe SJ, Sayers SL, Potashnik S, Buck H, Jurkovitz C, Fontana S, Weaver TE, Weintraub WS, Goldberg LR. Determinants of excessive daytime sleepiness and fatigue in adults with heart failure. Clin Nurs Res. 2012;21:271–293. DOI: 10.1177/1054773811419842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stang A, Dragano N, Moebus S, Möhlenkamp S, Schmermund A, Kälsch H, Erbel R, Jöckel K‐H. Midday naps and the risk of coronary artery disease: results of the Heinz Nixdorf recall study. Sleep. 2012;35:1705–1712. DOI: 10.5665/sleep.2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang L, Yang H, He M, Pan AN, Li X, Min X, Zhang CE, Xu C, Zhu X, Yuan J, et al. Longer sleep duration and midday napping are associated with a higher risk of CHD incidence in middle‐aged and older Chinese: the Dongfeng‐Tongji cohort study. Sleep. 2016;39:645–652. DOI: 10.5665/sleep.5544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang C, Bangdiwala SI, Rangarajan S, Lear SA, AlHabib KF, Mohan V, Teo K, Poirier P, Tse LA, Liu Z, et al. Association of estimated sleep duration and naps with mortality and cardiovascular events: a study of 116 632 people from 21 countries. Eur Heart J. 2019;40:1620–1629. DOI: 10.1093/eurheartj/ehy695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wannamethee SG, Papacosta O, Lennon L, Whincup PH. Self‐reported sleep duration, napping, and incident heart failure: prospective associations in the British Regional Heart Study. J Am Geriatr Soc. 2016;64:1845–1850. DOI: 10.1111/jgs.14255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naska A, Oikonomou E, Trichopoulou A, Psaltopoulou T, Trichopoulos D. Siesta in healthy adults and coronary mortality in the general population. Arch Intern Med. 2007;167:296–301. DOI: 10.1001/archinte.167.3.296. [DOI] [PubMed] [Google Scholar]
- 10.Häusler N, Haba‐Rubio J, Heinzer R, Marques‐Vidal P. Association of napping with incident cardiovascular events in a prospective cohort study. Heart. 2019;105:1793–1798. DOI: 10.1136/heartjnl-2019-314999. [DOI] [PubMed] [Google Scholar]
- 11.Harada CN, Natelson Love MC, Triebel K. Normal cognitive aging. Clin Geriatr Med. 2013;29:737–752. DOI: 10.1016/j.cger.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murman DL. The impact of age on cognition. Semin Hear. 2015;36:111–121. DOI: 10.1055/s-0035-1555115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ancoli‐Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26:342–392. DOI: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 14.Morgenthaler T, Alessi C, Friedman L, Owens J, Kapur V, Boehlecke B, Brown T, Chesson A, Coleman J, Lee‐Chiong T, et al. Practice parameters for the use of actigraphy in the assessment of sleep and sleep disorders: an update for 2007. Sleep. 2007;30:519–529. DOI: 10.1093/sleep/30.4.519. [DOI] [PubMed] [Google Scholar]
- 15.Smith MT, McCrae CS, Cheung J, Martin JL, Harrod CG, Heald JL, Carden KA. Use of actigraphy for the evaluation of sleep disorders and circadian rhythm sleep‐wake disorders: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2018;14:1231–1237. DOI: 10.5664/jcsm.7230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bennett DA, Schneider JA, Buchman AS, Barnes LL, Boyle PA, Wilson RS. Overview and findings from the Rush Memory and Aging Project. Curr Alzheimer Res. 2012;9:646–663. DOI: 10.2174/156720512801322663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cole RJ, Kripke DF, Gruen W, Mullaney DJ, Gillin JC. Automatic sleep/wake identification from wrist activity. Sleep. 1992;15:461–469. DOI: 10.1093/sleep/15.5.461. [DOI] [PubMed] [Google Scholar]
- 18.Jean‐Louis G, Kripke DF, Mason WJ, Elliott JA, Youngstedt SD. Sleep estimation from wrist movement quantified by different actigraphic modalities. J Neurosci Methods. 2001;105:185–191. DOI: 10.1016/S0165-0270(00)00364-2. [DOI] [PubMed] [Google Scholar]
- 19.Gao L, Lim ASP, Wong PM, Gaba A, Cui L, Yu L, Buchman AS, Bennett DA, Hu K, Li P. Fragmentation of rest/activity patterns in community‐based elderly individuals predicts incident heart failure. Nat Sci Sleep. 2020;12:299–307. DOI: 10.2147/NSS.S253757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526. DOI: 10.1093/biomet/81.3.515. [DOI] [Google Scholar]
- 21.Solin P, Kaye DM, Little PJ, Bergin P, Richardson M, Naughton MT. Impact of sleep apnea on sympathetic nervous system activity in heart failure. Chest. 2003;123:1119–1126. DOI: 10.1378/chest.123.4.1119. [DOI] [PubMed] [Google Scholar]
- 22.Mansfield D, Kaye DM, Brunner La Rocca H, Solin P, Esler MD, Naughton MT. Raised sympathetic nerve activity in heart failure and central sleep apnea is due to heart failure severity. Circulation. 2003;107:1396–1400. DOI: 10.1161/01.CIR.0000056520.17353.4F. [DOI] [PubMed] [Google Scholar]
- 23.Bisogni V, Pengo MF, Maiolino G, Rossi GP. The sympathetic nervous system and catecholamines metabolism in obstructive sleep apnoea. J Thorac Dis. 2016;8:243–254. DOI: 10.3978/j.issn.2072-1439.2015.11.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kougias P, Weakley SM, Yao Q, Lin PH, Chen C. Arterial baroreceptors in the management of systemic hypertension. Med Sci Monit. 2010;16:RA1‐8. [PMC free article] [PubMed] [Google Scholar]
- 25.Lambert EA, Chatzivlastou K, Schlaich M, Lambert G, Head GA. Morning surge in blood pressure is associated with reactivity of the sympathetic nervous system. Am J Hypertens. 2014;27:783–792. DOI: 10.1093/ajh/hpt273. [DOI] [PubMed] [Google Scholar]
- 26.Kario K. Morning surge in blood pressure and cardiovascular risk: evidence and perspectives. Hypertension. 2010;56:765–773. DOI: 10.1161/HYPERTENSIONAHA.110.157149. [DOI] [PubMed] [Google Scholar]
- 27.Parish JM, Somers VK. Obstructive sleep apnea and cardiovascular disease. Mayo Clin Proc. 2004;79:1036–1046. DOI: 10.4065/79.8.1036. [DOI] [PubMed] [Google Scholar]
- 28.Somers VK, White DP, Amin R, Abraham WT, Costa F, Culebras A, Daniels S, Floras JS, Hunt CE, Olson LJ, et al. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation scientific statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing in collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health). J Am Coll Cardiol. 2008;52:686–717. DOI: 10.1016/j.jacc.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 29.Hu K, Scheer FAJL, Laker M, Smales C, Shea SA. Endogenous circadian rhythm in vasovagal response to head‐up tilt. Circulation. 2011;123:961–U85. DOI: 10.1161/CIRCULATIONAHA.110.943019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qian J, Scheer FAJL. Circadian system and glucose metabolism: implications for physiology and disease. Trends Endocrinol Metab. 2016;27:282–293. DOI: 10.1016/j.tem.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morris CJ, Purvis TE, Hu K, Scheer FAJL. Circadian misalignment increases cardiovascular disease risk factors in humans. PNAS. 2016;113:E1402–E1411. DOI: 10.1073/pnas.1516953113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drager LF, McEvoy RD, Barbe F, Lorenzi‐Filho G, Redline S. Sleep apnea and cardiovascular disease. Circulation. 2017;136:1840–1850. DOI: 10.1161/CIRCULATIONAHA.117.029400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lim ASP, Yu L, Costa MD, Buchman AS, Bennett DA, Leurgans SE, Saper CB. Quantification of the fragmentation of rest‐activity patterns in elderly individuals using a state transition analysis. Sleep. 2011;34:1569–1581. DOI: 10.5665/sleep.1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buchman AS, Schneider JA, Leurgans S, Bennett DA. Physical frailty in older persons is associated with Alzheimer disease pathology. Neurology. 2008;71:499–504. DOI: 10.1212/01.wnl.0000324864.81179.6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosow I, Breslau N. A Guttman health scale for the aged. J Gerontol. 1966;21:556–559. DOI: 10.1093/geronj/21.4.556. [DOI] [PubMed] [Google Scholar]
- 36.Buchman AS, Wilson RS, Leurgans SE, Bennett DA, Barnes LL. Change in motor function and adverse health outcomes in older African‐Americans. Exp Gerontol. 2015;70:71–77. DOI: 10.1016/j.exger.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buchman AS, Yu L, Boyle PA, Shah RC, Bennett DA. Total daily physical activity and longevity in old age. Arch Intern Med. 2012;172:444–446. DOI: 10.1001/archinternmed.2011.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supplemental Methods and Results
Tables S1–S8
Figures S1–S2
References 33–37


