Abstract
Background
Female sex was not included among the high bleeding risk (HBR) criteria by the Academic Research Consortium (ARC) as it remains unclear whether it constitutes an HBR condition after percutaneous coronary intervention. We investigated whether female sex associates with HBR and assessed the performance of ARC HBR criteria separately in women and men.
Methods and Results
Among all consecutive patients undergoing percutaneous coronary intervention between 2009 and 2018, bleeding occurrences up to 1 year were prospectively collected and centrally adjudicated. All but one of the originally defined ARC HBR criteria were assessed, and the ARC HBR score generated accordingly. Among 16 821 patients, 25.6% were women. Compared with men, women were older and had lower creatinine clearance and hemoglobin values. After adjustment, female sex was independently associated with access‐site (adjusted hazard ratio, 2.14; 95% CI, 1.22–3.74; P=0.008) but not with overall or non–access‐site 1‐year Bleeding Academic Research Consortium 3 or 5 bleeding. This association remained consistent when the femoral but not the radial approach was chosen. The ARC HBR score discrimination, using the original criteria, was lower among women than men (c‐index 0.644 versus 0.688; P=0.048), whereas a revised ARC HBR score, in which age, creatinine clearance, and hemoglobin were modeled as continuous rather than dichotomized variables, performed similarly in both sexes.
Conclusions
Female sex is an independent predictor for access‐site bleeding but not for overall bleeding events at 1 year after percutaneous coronary intervention. The ARC HBR framework shows an overall good performance in both sexes, yet is lower in women than men, attributable to dichotomization of age, creatinine clearance, and hemoglobin values, which are differently distributed between sexes.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: NCT02241291.
Keywords: Academic Research Consortium, bleeding, female sex, percutaneous coronary intervention, vascular access
Subject Categories: Percutaneous Coronary Intervention
Nonstandard Abbreviations and Acronyms
- adjHR
adjusted hazard ratio
- ARC
Academic Research Consortium
- BARC
Bleeding Academic Research Consortium
- HBR
high bleeding risk
Clinical Perspective
What Is New?
Female sex per se was associated with greater access‐site bleeding, especially from the femoral artery, but not with overall or non‐access site type 3 or 5 occurrences at 1 year, according to Bleeding Academic Research Consortium classification.
The Academic Research Consortium high bleeding risk framework appears to be a suitable risk stratification tool for bleeding risk assessment in both sexes, provided women are considered at high bleeding risk for access‐site events irrespective of high bleeding risk criteria and age, and creatinine clearance and hemoglobin are counted as continuous instead as dichotomous variables.
What Are the Clinical Implications?
Strategies aiming at mitigating access‐site bleeding risk among women, irrespective of high bleeding risk status and including radial access‐site selection, should be adopted.
The overall and non–access‐site bleeding risk after percutaneous coronary intervention should not be assessed on the basis of sex but on the other validated bleeding predictors included in the Academic Research Consortium high bleeding risk criteria, which need be assessed in a continuous rather than dichotomized fashion.
Bleeding events after percutaneous coronary intervention (PCI) are associated with worse outcomes.1 As a result, the appropriate identification and management of high bleeding risk factors is recommended for all patients undergoing PCI.
Among the clinical factors predisposing to a heightened bleeding risk, the role of sex remains unclear. Evidence suggests that female sex per se confers higher bleeding risks, as it relates to in‐hospital2, 3 and/or overall occurrences.4, 5 On the other hand, several studies did not find a similar association.6, 7, 8 International recommendations on duration of dual antiplatelet therapy reflect this uncertainty, with female sex being included among the bleeding risk factors in the American9 but not the European guidelines.10 Similarly, female sex accrues the predicted hemorrhagic hazard among some risk scores11, 12 but not in others.13, 14
The Academic Research Consortium (ARC) for high bleeding risk (HBR) has recently proposed by consensus a set of clinical and biochemical criteria, among which sex is not included, for the identification of patients with HBR.15 The discriminatory performance of these criteria, as originally described without adaptations, was recently assessed,16 but no independent assessment of this new framework in men and women exists. Inappropriate estimation of the bleeding risk could lead to erroneous therapeutic management among female patients only on the basis of sex.17
We sought therefore to investigate, in a large cohort of consecutive patients undergoing PCI, whether female sex is independently associated with higher bleeding occurrences and whether the recent ARC HBR criteria and definitions provide a consistent risk stratification tool in both men and women.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
All consecutive patients undergoing PCI at Bern University Hospital, Switzerland, between February 2009 and December 2018 were prospectively entered into the Bern PCI Registry (ClinicalTrials.gov: NCT02241291), which complies with the Declaration of Helsinki and was approved by the institutional ethics committee. All patients providing informed consent were included without formal exclusion criteria. Demographic, clinical, and procedural characteristics; laboratory values; hospital outcomes; and bleeding and ischemic events occurring up to 1 year after PCI were prospectively collected. The fulfillment of all but one (planned surgery) ARC HBR criteria were systematically screened for each patient according to the originally proposed definitions without adaptations (Table S1). We excluded patients for whom ≥1 ARC HBR criteria could not be assessed because of missing information (except for planned surgery).
Clinical End Points
The primary end point was the composite of Bleeding Academic Research Consortium (BARC) type 3 or 5, further stratified into non–access‐site and access‐site related. Secondary end points included BARC 2, 3 or 5 bleeding and according to the Thrombolysis in Myocardial Infarction and Global Strategies for Opening Occluded Coronary Arteries scales. Ischemic and fatal outcomes were also reported according to ARC standardized definitions.18, 19
Further details about methods can be found in Data S1.
Statistical Analysis
Baseline and procedural characteristics and medications are shown as means and SDs or counts with percentages; P values for comparisons of women versus men were obtained for continuous variables using t tests, for categorical variables using Fisher's tests (2×2 comparisons) or chi‐square tests. Time‐to‐event analyses and Kaplan‐Meier cumulative event curves were used for clinical outcomes. Univariable and multivariable Weibull time‐to‐event analyses were used to assess the association of sex and other clinical characteristics with the bleeding outcomes specified above. Multiple imputation of missing risk factors (other than ARC HBR criteria) was performed. The 20 generated data sets were used to derive predictors of BARC 3 or 5 bleeding and estimates were combined using Rubin's rule. Predictors with a P value below 0.2 were retained in the final models. Models were robustified by year of PCI. Unadjusted and adjusted rates of BARC 3 or 5 were additionally calculated after stratification of women and men by vascular access. Interaction effects between female sex and each adjusted ARC HBR criterion to predict BARC 3 or 5 bleeding was estimated.
Harrell's c‐index (concordance statistic) was calculated to assess whether the ARC HBR score predicted BARC 3 or 5 bleeding in the overall population, as well as in women and men separately. The ARC HBR criteria age, hemoglobin, and estimated glomerular filtration rate using creatinine (estimated glomerular filtration rate) were also fitted as continuous risk factors to explore whether some differences between women and men might be attributable to dichotomization of these risk factors.
Analyses were done in Stata Release 16.1 (StataCorp LP, College Station, TX).
RESULTS
Population Characteristics
Between February 2009 and December 2018, 17 339 consecutive patients were included in the Bern PCI Registry. After excluding 518 patients with incomplete hemoglobin (n=459) or creatinine (n=59) data, the final study population comprised 16 821 patients. Follow‐up was complete in 15 708 of 16 821 patients (93.4%); for 531 (3.1%) only information about vital status was available, and 582 (3.5%) were lost to follow‐up. In the final study population, mean age was 67.9 years, 4307 (25.6%) were women, 9503 (56.5%) presented with acute coronary syndrome, and 16.9% had a Killip class >1 at presentation (Table 1).
Table 1.
Baseline Characteristics According to Sex
| All Patients (N=16 821) | Men (n=12 514) | Women (n=4307) | P Value | |
|---|---|---|---|---|
| Age, y | n=16 821, 67.9±12.0 | n=12 514, 66.4±11.8 | n=4307, 72.1±11.4 | <0.001 |
| BMI, kg/m2 | n=16 182, 27.4±4.7 | n=12 066, 27.5±4.4 | n=4116, 26.9±5.5 | <0.001 |
| Current smoker | n=16 575, 4488 (27.1) | n=12 335, 3609 (29.3) | n=4240, 879 (20.7) | <0.001 |
| Hypertension | n=16 694, 11 577 (69.3) | n=12 416, 8330 (67.1) | n=4278, 3247 (75.9) | <0.001 |
| Diabetes mellitus | n=16 757, 3893 (23.2) | n=12 464, 2837 (22.8) | n=4293, 1056 (24.6) | 0.014 |
| Hypercholesterolemia | n=16 665, 10 741 (64.5) | n=12 393, 8028 (64.8) | n=4272, 2713 (63.5) | 0.138 |
| Family history of coronary artery disease | n=16 684, 4323 (25.9) | n=12 404, 3159 (25.5) | n=4280, 1164 (27.2) | 0.027 |
| Previous myocardial infarction | n=16 743, 2976 (17.8) | n=12 453, 2425 (19.5) | n=4290, 551 (12.8) | <0.001 |
| Previous PCI | n=16 741, 3989 (23.8) | n=12 449, 3168 (25.4) | n=4292, 821 (19.1) | <0.001 |
| Previous CABG | n=16 759, 1701 (10.1) | n=12 463, 1435 (11.5) | n=4296, 266 (6.2) | <0.001 |
| Previous TIA or stroke | n=16 750, 1237 (7.4) | n=12 456, 914 (7.3) | n=4294, 323 (7.5) | 0.685 |
| Peripheral arterial disease | n=16 738, 1448 (8.7) | n=12 448, 1064 (8.5) | n=4290, 384 (9.0) | 0.413 |
| History of malignancy | n=16 746, 1810 (10.8) | n=12 455, 1303 (10.5) | n=4291, 507 (11.8) | 0.014 |
| Renal failure* | n=16 098, 3329 (20.7) | n=12 008, 2074 (17.3) | n=4090, 1255 (30.7) | <0.001 |
| History of atrial fibrillation/atrial flutter | n=11 845, 1450 (12.2) | n=8810, 1055 (12.0) | n=3035, 395 (13.0) | 0.131 |
| Chronic obstructive lung disease | n=16 747, 1113 (6.6) | n=12 455, 887 (7.1) | n=4292, 226 (5.3) | <0.001 |
| History of spontaneous bleeding | n=16 819, 729 (4.3) | n=12 513, 539 (4.3) | n=4306, 190 (4.4) | 0.762 |
| History of gastrointestinal bleeding | n=16 744, 368 (2.2) | n=12 452, 274 (2.2) | n=4292, 94 (2.2) | 1.000 |
| Left ventricular function (%) | n=15 122, 52.3±13.7 | n=11 261, 51.9±13.5 | n=3861, 53.5±14.0 | <0.001 |
| Hemoglobin before PCI, g/L | n=14 853, 136.54±18.11 | n=11 059, 139.82±17.49 | n=3794, 127.00±16.44 | <0.001 |
| Hemoglobin nadir, g/L | n=14 003, 126.97±20.64 | n=10 358, 130.42±20.04 | n=3645, 117.16±19.10 | <0.001 |
| ARC HBR score | n=16 821, 0.66±0.83 | n=12 514, 0.60±0.80 | n=4307, 0.82±0.86 | <0.001 |
| Clinical indication for PCI | n=16 821 | n=12 514 | n=4307 | <0.001 |
| Stable CAD | 7318 (43.5) | 5426 (43.4) | 1892 (43.9) | 0.521 |
| Unstable angina | 783 (4.7) | 576 (4.6) | 207 (4.8) | 0.586 |
| NSTEMI | 4218 (25.1) | 3056 (24.4) | 1162 (27.0) | 0.001 |
| STEMI | 4502 (26.8) | 3456 (27.6) | 1046 (24.3) | <0.001 |
| Killip class II, III or IV | n=16 780, 2844 (16.9) | n=12 486, 2042 (16.4) | n=4294, 802 (18.7) | <0.001 |
Data are presented as absolute numbers and percentage or mean±SD. ARC indicates Academic Research Consortium; BMI, body mass index; CABG, coronary artery bypass grafting; CAD, coronary artery disease; HBR, high bleeding risk; NSTEMI, non–ST‐segment–elevation myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST‐segment–elevation myocardial infarction; and TIA, transient ischemic attack.
Renal failure: <60 estimated glomerular filtration rate mL/min/1.73 m2, using the Modification of Diet in Renal Disease formula.
Compared with men, female patients were on average 6 years older (72.1±11.4 versus 66.4±11.8 years); had a lower body mass index and hemoglobin values; were more likely to suffer from hypertension, diabetes mellitus, chronic kidney disease (CKD), and malignancy; and were more likely to have a non–ST‐segment–elevation acute coronary syndrome as well as acute heart failure at presentation. Men were more frequently smokers and more frequently had a history of coronary disease and chronic obstructive lung disease (Table 1). Femoral access was more frequently used among women than men (70.4% versus 65.3%; P<0.001). Women had a lower number of treated coronary lesions, better Thrombolysis in Myocardial Infarction flow before PCI (Thrombolysis in Myocardial Infarction flow 0–1: 21.3% versus 24.1%) and fewer high‐risk features such as in‐stent restenosis, thrombotic lesions, or chronic total occlusion (Table S2). Other procedural and discharge medications are available in Table S3.
Clinical Outcomes
At 1‐year follow‐up, 217 (5.5%) overall BARC 3 or 5 bleeding events occurred among women, of which 176 (4.5%) were unrelated and 44 (1.1%) were related to the access site; among men, the corresponding occurrences attained at 458 (4.0%), 417 (3.6%), and 47 (0.4%), respectively. Women as compared with men had an increased unadjusted risk of overall (hazard ratio [HR], 1.41; 95% CI, 1.20–1.66; P<0.001), non–access‐site (HR, 1.25; 95% CI, 1.05–1.49; P=0.013) and access‐site (HR, 2.76; 95% CI, 1.83–4.16; P<0.001) BARC 3 or 5 bleeding (Table 2). Crude event rates of BARC 2, intracranial or coronary artery bypass grafting–related bleeding did not differ between sexes (Table S4). The results remained largely consistent when the Global Strategies for Opening Occluded Coronary Arteries and Thrombolysis in Myocardial Infarction bleeding scales were appraised (Table S4). Other factors associated with BARC 3 or 5 bleeding at unadjusted analyses are shown in Table S5. Mortality and ischemic outcomes are reported in Table 2.
Table 2.
Bleeding and Ischemic Events at 1 Year According to Sex
| Men (n=12 514) | Women (n=4307) | Hazard Ratio (95% CI) | P Value | |
|---|---|---|---|---|
| Bleeding BARC 3 or 5 | 458 (4.0) | 217 (5.5) | 1.41 (1.20–1.66) | <0.001 |
| Access site | 47 (0.4) | 44 (1.1) | 2.76 (1.83–4.16) | <0.001 |
| Non–access site | 417 (3.6) | 176 (4.5) | 1.25 (1.05–1.49) | 0.013 |
| Bleeding BARC 2, 3, or 5 | 724 (6.3) | 296 (7.5) | 1.22 (1.06–1.39) | 0.005 |
| Access site | 53 (0.4) | 51 (1.2) | 2.83 (1.93–4.16) | <0.001 |
| Non–access site | 681 (5.9) | 249 (6.4) | 1.08 (0.94–1.25) | 0.288 |
| Death | 855 (7.2) | 340 (8.4) | 1.17 (1.04–1.33) | 0.012 |
| Cardiac death | 601 (5.1) | 244 (6.1) | 1.20 (1.03–1.39) | 0.018 |
| Vascular (noncardiac) death | 59 (0.5) | 30 (0.8) | 1.50 (0.97–2.33) | 0.070 |
| Noncardiovascular death | 195 (1.7) | 66 (1.7) | 1.00 (0.76–1.33) | 0.977 |
| Myocardial infarction | 514 (4.4) | 187 (4.7) | 1.07 (0.91–1.27) | 0.413 |
| Target vessel myocardial infarction | 377 (3.2) | 142 (3.5) | 1.11 (0.91–1.34) | 0.296 |
| Revascularization (any) | 900 (8.0) | 292 (7.6) | 0.96 (0.84–1.09) | 0.522 |
| Target lesion revascularization | 462 (4.1) | 156 (4.1) | 1.00 (0.83–1.20) | 0.981 |
| Target vessel revascularization | 663 (5.9) | 213 (5.5) | 0.95 (0.81–1.11) | 0.505 |
| Definite stent thrombosis | 153 (1.3) | 42 (1.0) | 0.81 (0.57–1.13) | 0.215 |
| CVE (stroke and TIA) | 221 (1.9) | 82 (2.1) | 1.10 (0.85–1.41) | 0.479 |
| Stroke (any) | 178 (1.6) | 70 (1.8) | 1.16 (0.88–1.53) | 0.288 |
| Stroke (ischemic stroke) | 135 (1.2) | 47 (1.2) | 1.03 (0.74–1.43) | 0.875 |
| Stroke (intracerebral hemorrhage) | 38 (0.3) | 21 (0.6) | 1.64 (0.96–2.79) | 0.070 |
| Stroke (unclear etiology) | 5 (0.0) | 2 (0.1) | 1.18 (0.23–6.08) | 0.844 |
| TIA | 44 (0.4) | 12 (0.3) | 0.80 (0.42–1.52) | 0.504 |
| NACCE | 1662 (13.9) | 675 (16.5) | 1.21 (1.11–1.32) | <0.001 |
Data are presented as absolute numbers and percentage (%). BARC indicates Bleeding Academic Research Consortium; CVE, cerebrovascular event; NACCE, net adverse cardiac and cerebrovascular event; and TIA, transient ischemic attack.
At multivariate analysis, after adjusting for baseline, procedural, and treatment imbalances, female sex was no longer associated with higher risk of overall (adjusted hazard ratio [adjHR], 1.01; 95% CI, 0.81–1.27; P=0.918) or non–access‐site (adjHR, 0.89; 95% CI, 0.73–1.07; P=0.244) BARC 3 or 5 bleeding. However, access‐site–related BARC 3 or 5 events remained 2‐fold higher among women compared with men (adjHR, 2.14; 95% CI, 1.22–3.74; P=0.008) (Figure 1A and Table S6). When the composite of BARC 2, 3, or 5 bleeding was appraised, female sex remained associated with greater access‐site (adjHR 2.15; 95% CI, 1.34–3.43; P=0.001) but not with overall occurrences (adjHR, 0.89; 95% CI, 0.74–1.06; P=0.195), and even appeared protective toward non–access‐site (adjHR, 0.78; 95% CI, 0.65–0.94; P=0.008) events (Figure 1A and Tables S7 and S8).
Figure 1. BARC 3 or 5 bleeding at 1 year and ARC HBR criteria in female (F) vs male patients (M).
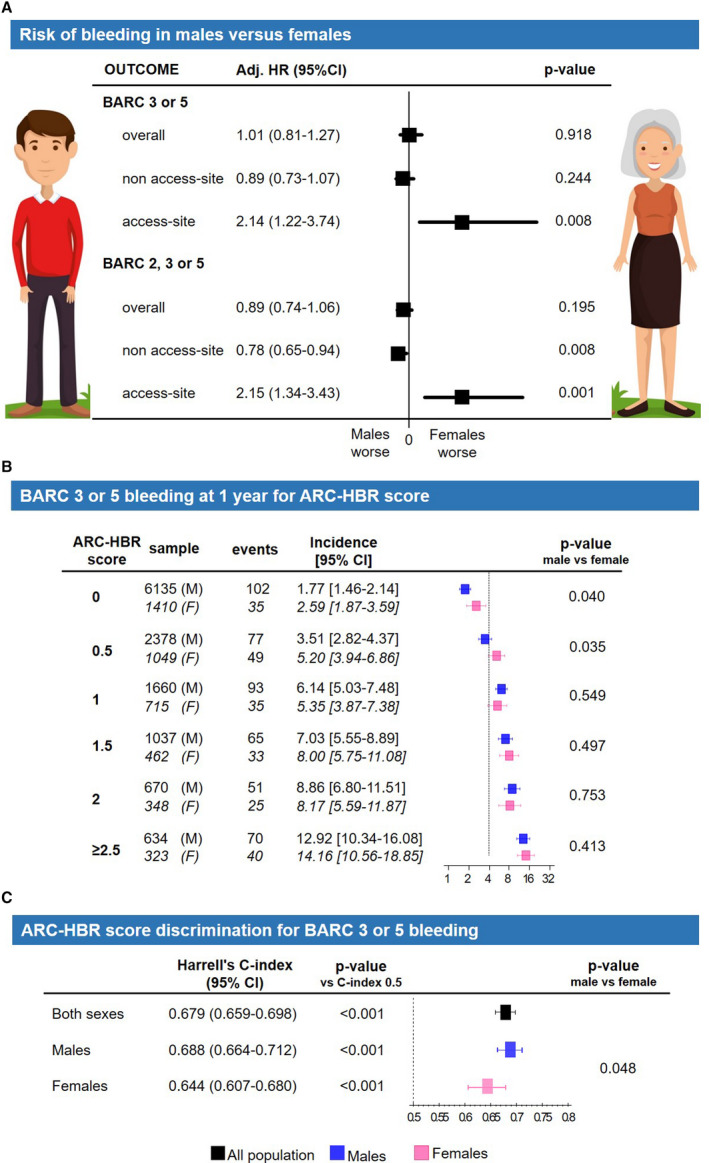
Adjusted (Adj) hazard ratio for overall, non–access‐site and access‐site bleeding (A). ARC HBR criteria modeled as a score (1 point for major criterion, 0.5 for minor): bleeding incidence for score values from 0 to ≥2.5 (B) and score discrimination using Harrell's c‐index (C). The dotted line indicates a 4% incidence of bleeding, expected in presence of a major ARC HBR criterion (definition in Table S1). ARC indicates Academic Research Consortium; BARC, Bleeding Academic Research Consortium; and HBR, high bleeding risk.
Bleeding Outcomes Stratified by Access Site
Among patients in whom the femoral access was used, the crude rate of 1‐year BARC 3 or 5 bleeding in women and men reached 7.3% and 5.1% for overall, 6.2% and 4.7% for non–access‐site, and 1.3% and 0.5% for access‐site occurrences, conferring to women as compared with men an increased unadjusted risk of overall, non–access‐site and access‐site 1‐year BARC 3 or 5 bleeding (Figure S1A). Conversely, when the radial approach was chosen, there were no differences between women and men for overall (crude rate 4.3% versus 4.4%), non–access‐site (3.8% versus 4.1%) or access‐site (0.5% versus 0.3%) 1‐year BARC 3 or 5 bleeding events (Figure S1B).
At adjusted analysis, when the femoral access was attempted, female sex conferred an increased risk of access‐site bleeding very close to statistical significance (adjHR, 1.99; 95% CI, 0.96–4.11; P=0.063) but not of overall and non–access‐site bleeding (Figure S1A). By choosing the radial approach, female sex did not associate with a higher risk of access‐site bleeding complications (adjHR, 1.12; 95% CI, 0.43–2.96; P=0.811) and resulted even protective in regard to overall and non–access‐site occurrences (Figure S1B). These results remained consistent when also BARC 2 bleeding events were taken into account (Figure S1A and S1B).
ARC HBR Criteria Stratified by Sex
Forty‐three percent of women and 32% of men qualified as HBR, defined by the presence of at least 2 minor or 1 major ARC HBR criterion. Advanced age, CKD, anemia, and use of nonsteroidal anti‐inflammatory drugs or corticosteroids were more common among women, while a history of active malignancy was more frequent among men (Figure S2A). The remaining ARC HBR criteria were similarly distributed between sexes. The unadjusted risks of BARC 3 or 5 bleeding for each ARC HBR criterion among men or women are shown in Figure S3. At multivariable analysis, in which each ARC HBR criterion was adjusted for all major or minor criteria but the one considered, no significant interaction was found between sex and each criterion in the likelihood of BARC 3 or 5 bleeding, except for CKD (P interaction for sex=0.035) (Figure 2). When age, creatinine clearance, and hemoglobin were entered into the model as continuous rather than dichotomized variables, as suggested by the ARC HBR, interaction testing between sex and ARC HBR criteria became consistently negative (Figure 3A).
Figure 2. Adjusted (Adj) incidence of BARC 3 or 5 bleedings at 1 year for minor and major (*) ARC HBR criteria in male (M) and female patients (F).
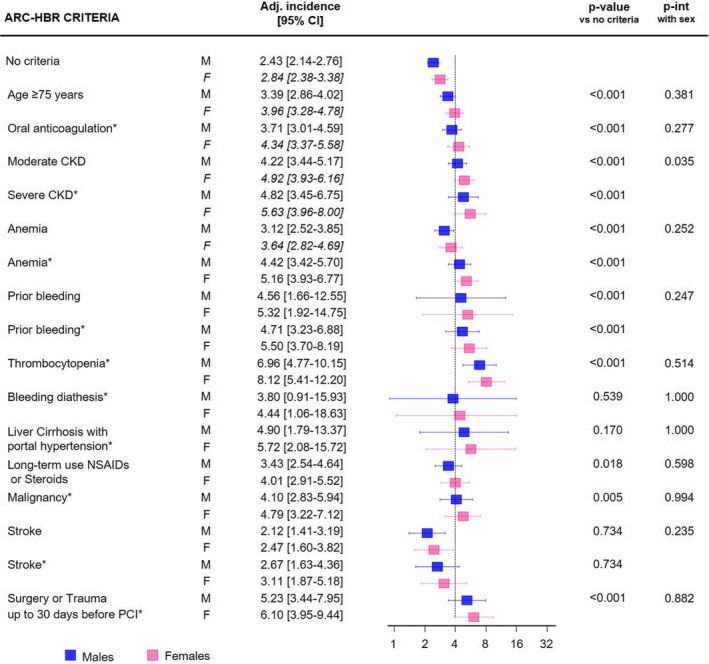
Interaction between sex and each ARC HBR criterion is reported (P interaction [int] with sex). The dotted line indicates a 4% incidence of bleeding, expected in presence of a major ARC HBR criterion (definition in Table S1). ARC indicates Academic Research Consortium; BARC, Bleeding Academic Research Consortium; CKD, chronic kidney disease; HBR, high bleeding risk; and PCI, percutaneous coronary intervention.
Figure 3. Modified ARC HBR criteria and BARC 3 or 5 bleedings at 1 year.
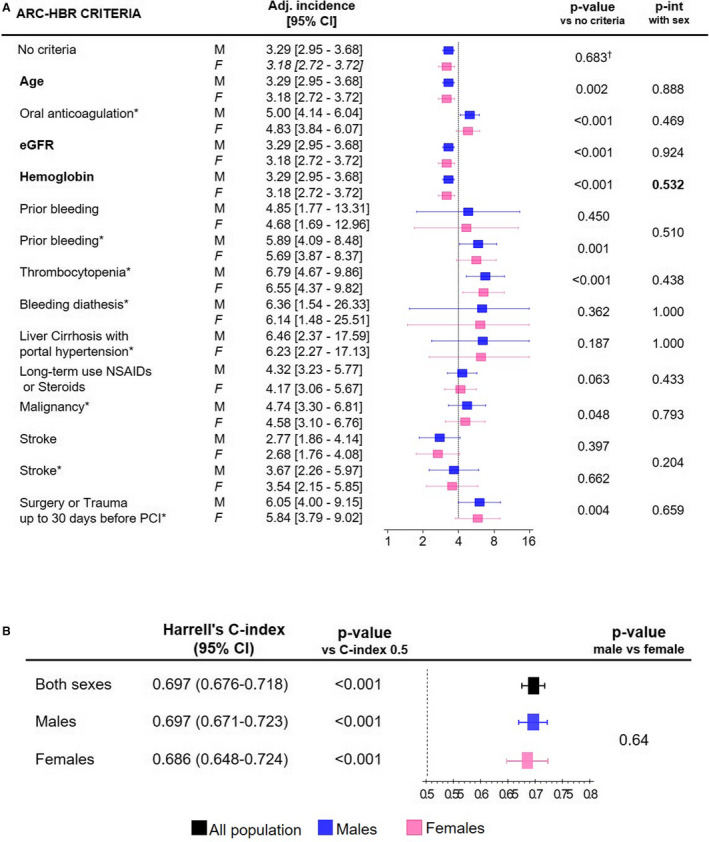
Modified ARC HBR criteria (age, creatinine clearance, and hemoglobin analyzed as continuous variables) to predict BARC 3 or 5 bleedings at 1 year in males (M) and females (F): adjusted (Adj) bleeding incidence for minor and major (*) ARC‐HBR criteria (A) and score discrimination using Harrell's c‐index (B). Bold risk factors: cumulative incidence calculated for the mean of that risk factor. The dotted line indicates a 4% incidence of bleeding, expected in presence of a major ARC HBR criterion (definition in Table S1). ARC indicates Academic Research Consortium; BARC, Bleeding Academic Research Consortium; eGFR, glomerular filtration rate according to Modification of Diet in Renal Disease formula; HBR, high bleeding risk; int, interaction; and PCI, percutaneous coronary intervention. † P value for female vs male sex after adjustment for all other modified ARC‐HBR criteria.
ARC HBR Score Stratified by Sex
The ARC HBR score was higher among women than men (0.82±0.86 versus 0.60±0.80; P<0.001) and differently distributed in the 2 groups (Figure S2B). For each 0.5‐point increase of the ARC HBR score from 0 to ≥2.5, there was a stepwise increase of BARC 3 or 5 bleeding rates at 1 year in both groups, with event rates increasing from 2.89% to 14.16% among women and from 1.77% to 12.92% among men. The incidence of BARC 3 or 5 bleeding was similar in the 2 sexes for each point of the score ≥1. For score values <1, women showed a significantly higher bleeding risk compared with men (Figure 1B), mainly attributable to an excess of access‐site occurrences (Figure S4).
The ARC HBR score discrimination, in terms of Harrell's c‐index with respect to BARC 3 or 5 bleeding, was lower among women compared with men (0.644; 95% CI, 0.607–0.680 versus 0.688; 95% CI, 0.664–0.712; P=0.048) (Figure 1C). When age, creatinine clearance, and hemoglobin were entered into the model as continuous rather than dichotomized variables, as suggested by the ARC HBR, the ARC HBR score discrimination significantly improved among female patients to the extent that it no longer differed between sexes (Figure 3B).
DISCUSSION
We aimed at addressing the conundrum between bleeding risk and female sex in patients undergoing PCI and ascertained whether the ARC HBR definition performs consistently across both sexes in a prospective all‐comer cohort of 16 821 consecutive patients undergoing PCI, who underwent central event adjudication.
Our findings can be summarized as follows:
Female patients had higher crude risk of BARC 3 or 5 bleeding at 1 year compared with male patients; after adjustment, female sex per se remained independently associated with access‐site–related but not overall or non–access‐site–related BARC 3 or 5 bleeding.
These results remained consistent when considering only patients in whom the femoral access was used, while there was no unadjusted and adjusted sex differences in terms of bleeding (overall, not related and related to access site) when the radial approach was chosen.
Compared with men, women were more likely to qualify as HBR according to the ARC HBR criteria and presented higher ARC HBR scores, mainly attributable to more frequent fulfillment of advanced age, CKD, or anemia criteria.
In a multivariate adjusted model, among the ARC HBR criteria, CKD showed a significant interaction with sex, suggesting higher risk of BARC 3 or 5 bleeding among women than men.
The performance of the ARC HBR score in terms of bleeding risk discrimination showed an overall good performance in both sexes, yet lower in women than men.
When age, hemoglobin, and creatinine clearance were forced into the model as continuous rather than dichotomized variables, interaction testing with sex was no longer significant, and the ARC HBR score performed consistently across sexes.
Women have an apparent biological protection from coronary artery disease, which translates to a decade‐long delay in the onset of coronary artery disease and a different presentation profile and comorbid burden as compared with men. It remains unclear whether female sex per se affects the bleeding risk after PCI. Our analysis addresses this important knowledge gap by showing that after extensive adjustments for several characteristics that differ between women and men, like age, prevalence of CKD or anemia, female sex does not confer a higher risk of overall or non–access‐site major bleeding events at 1 year, but remains associated with higher prevalence of major access‐site complications, especially if the femoral access was used. These findings are consistent with previous studies, showing that use of radial access reduces significantly the rate of bleeding in both sexes,20, 21, 22, 23 with a possible greater benefit among women.21, 22
A smaller reference diameter of the common femoral artery have been investigated as possible cause of increased predisposition to access‐site bleeding in women in whom the femoral approach was chosen.24
Moreover, we observed that when any actionable bleeding events were appraised (ie, BARC 2, 3, or 5), female sex emerged as a protective factor for non–access‐site–related occurrences, consistent with prior findings.25, 26
In 8 of 20 contemporary studies reporting an adjusted sex‐related risk of bleeding at >6 months after PCI (Table S9), female sex emerged as an independent predictor of bleeding. Yet a sensitivity analysis excluding periprocedural bleeding was performed in only 1 study,4 which demonstrated that female sex was no longer a predictor of bleeding at 1 year when only postdischarge major bleeding was appraised. Furthermore, in 2 other studies,27, 28 the increased bleeding risk among female patients was largely driven by nonactionable occurrences (BARC 1), and in some other studies,29 the low number of bleeding events may have hampered extensive multivariable adjustments.
Our results are supported by the observation that female sex appears in scores conceived to predict in‐hospital or short‐term bleedings (ie, ACUITY [Acute Catheterization and Urgent Intervention Triage Strategy]11 or CRUSADE [Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes with Early Implementation of the ACC/AHA Guidelines] score12), but not across scores developed to predict only spontaneous (eg, PRECISE‐DAPT [Predicting Bleeding Complications in Patients Undergoing Stent Implantation and Subsequent Dual AntiPlatelet Therapy]14) or long‐term bleeding occurrences (eg, PARIS [Patterns of Non‐Adherence to Anti‐Platelet Regimen in Stented Patients]13).
The recently proposed ARC HBR states that sex differences in bleeding risk should be considered in trial design and the interpretation of study outcomes but did not include female sex among risk factors for HBR.15 No study has so far assessed this new framework separately in both male and female patients. Our analysis supports the ARC consensus decision not to include sex as an independent criterion for HBR and suggests that this novel HBR definition allows a good prediction of bleeding risk in both sexes. Nevertheless, we observed a lower performance of ARC HBR score among female as compared with male patients attributable to increased age and lower creatinine clearance and hemoglobin values among women. A revised HBR model, in which age, renal function, and hemoglobin values are analyzed as continuous instead of dichotomous variables, provides a more consistent bleeding risk prediction in both sexes.
Our results support the adoption of strategies aiming at mitigating access‐site bleeding risk among women, irrespective of HBR status, including radial access‐site selection.21
Strengths and Limitations
To our knowledge, this is the first study assessing the risk of bleeding at 1 year in female versus male patients stratified by the originally proposed ARC HBR criteria without adaptations. Our results have several limitations. First, they arise from a single‐center albeit large cohort study and, as such, may suffer from limited generalizability. Yet the distribution of baseline characteristics between sexes as well as the incidence of bleeding and ischemic outcomes are consistent with those of previous multicenter cohorts.3, 4, 5 Eight ARC HBR criteria were retrospectively adjudicated, which may have led to imprecision and underestimation of their true prevalence in our PCI population, but it is reassuring that after independent readjudication of ARC HBR criteria by a different observer in a random sample of 597 patients, the agreement with the first observer attained 98%.
The ARC HBR criteria were not originally conceived as a point‐based score. Nevertheless, the creation of an ARC HBR score allowed a more precise bleeding risk stratification.
Finally, a single major ARC HBR criterion, namely, nondeferrable major surgery was not assessed.
Conclusions
Female sex after PCI is an independent risk factor for access‐site but not for overall or non–access‐site–related (ie, spontaneous) bleeding at 1 year when the femoral approach is adopted. The ARC HBR framework shows a slightly lower discrimination performance in female as compared with male patients but appears a suitable risk stratification tool for bleeding risk assessment in both sexes, provided female patients are considered at HBR for access‐site events, and greater age and lower creatinine clearance and hemoglobin values are accounted for.
Sources of Funding
This study was supported by the Department of Cardiology at Bern University Hospital, Bern, Switzerland.
Disclosures
Dr Gargiulo reports personal fees from Daiichi Sankyo. Dr Praz reports personal fees from Edwards Lifesciences. Dr Stortecky reports grants from Edwards Lifesciences, Medtronic, Abbott Vascular, and Boston Scientific; and personal fees from Boston Scientific/British Technology Group and Teleflex. Dr Pilgrim reports grants and personal fees from Biotronik and Boston Scientific, and personal fees from HighLife Société par actions simplifiée (SAS; English: simplified joint‐stock company). Dr Capodanno reports personal fees from AstraZeneca, Bayer, Daiichi Sankyo, Sanofi, and Boehringer. Dr Urban reports other fees from Cardiovascular European Research Center (CRO [Contract Research Organization] in Massy, France) and MedAlliance, and personal fees from Edwards Lifesciences and Biosensors. Dr Mehran reports grants from Abbott Laboratories, Abiomed, Applied Therapeutics, AstraZeneca, Bayer, Beth Israel Deaconess, Bristol‐Myers Squibb, CERC, Chiesi, Concept Medical, CSL Behring, DSI, Medtronic, Novartis Pharmaceuticals, and OrbusNeich; personal fees from Abbott Laboratories, Boston Scientific, CardiaWave, Janssen Scientific Affairs, Medscape/WebMD, Roivant Services, Sanofi, Siemens Medical Solutions, Medtelligence (Janssen Scientific Affairs), American College of Cardiology, American Medical Association, and Chiesi; nonfinancial support and other from Idorsia Pharmaceuticals Ltd. and Regeneron Pharmaceuticals; and other from Abbott Laboratories, Abiomed, Bristol‐Myers Squibb, Merck, The Medicines Company, Spectranetics/Philips/Volcano Corp, Watermark Research Partners, Claret Medical, and Elixir Medical. Dr Windecker reports research and educational grants to the institution from Abbott, Amgen, Bristol Myers Squibb, Bayer, Boston Scientific, Biotronik, Cardinal Health, CardioValve, CSL Behring, Daiichi Sankyo, Edwards Lifesciences, Johnson & Johnson, Medtronic, Querbet, Polares, Sanofi, Terumo, and Sinomed; Dr Windecker serves as an unpaid member of the steering/excecutive group of trials funded by Abbott, Abiomed, Amgen, BMS, Boston Scientific, Biotronik, Cardiovalve, Edwards Lifesciences, MedAlliance, Medtronic, Polares, Sinomed, V‐Wave, and Xeltis but has not received personal payments by any pharmaceutical company or device manufacturer. He is also member of the steering/excecutive committee group of several investigated‐initiated trials that receive funding by industry without impact on his personal remuneration. Dr Räber reports grants and personal fees from Abbott and Sanofi Aventis; personal fees from Amgen, Astra Zeneca, Biotronik, and CSL Behring. Dr Valgimigli reports personal fees from Astra Zeneca, Alvimedica/CID, Abbott Vascular, Daiichi Sankyo, Opsens, Bayer, CoreFLOW, Idorsia Pharmaceuticals Ltd, Universität Basel | Dept. Klinische Forschung, Vifor, Bristol Myers Squib SA, iVascular, and Medscape; and grants and personal fees from Terumo. All the declared financial disclosures occurred outside the submitted work. The remaining authors have no disclosures to report.
Supporting information
Data S1
Tables S1–S9
Figures S1–S4
Acknowledgments
The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
(J Am Heart Assoc. 2021;10:e021965. DOI: 10.1161/JAHA.121.021965.)
For Sources of Funding and Disclosures, see page 10.
References
- 1.Valgimigli M, Costa F, Lokhnygina Y, Clare RM, Wallentin L, Moliterno DJ, Armstrong PW, White HD, Held C, Aylward PE, et al. Trade‐off of myocardial infarction vs. bleeding types on mortality after acute coronary syndrome: lessons from the thrombin receptor antagonist for clinical event reduction in acute coronary syndrome (TRACER) randomized trial. Eur Heart J. 2017;38:804–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehran R, Chandrasekhar J, Urban P, Lang IM, Windhoevel U, Spaulding C, Copt S, Stoll HP, Morice MC; Investigators LF . Sex‐based outcomes in patients with a high bleeding risk after percutaneous coronary intervention and 1‐month dual antiplatelet therapy: a secondary analysis of the LEADERS FREE randomized clinical trial. JAMA Cardiol. 2020;5:939–947. DOI: 10.1001/jamacardio.2020.0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Potts J, Sirker A, Martinez SC, Gulati M, Alasnag M, Rashid M, Kwok CS, Ensor J, Burke DL, Riley RD, et al. Persistent sex disparities in clinical outcomes with percutaneous coronary intervention: insights from 6.6 million PCI procedures in the United States. PLoS One. 2018;13:e0203325. DOI: 10.1371/journal.pone.0203325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hess CN, McCoy LA, Duggirala HJ, Tavris DR, O'Callaghan K, Douglas PS, Peterson ED, Wang TY. Sex‐based differences in outcomes after percutaneous coronary intervention for acute myocardial infarction: a report from TRANSLATE‐ACS. J Am Heart Assoc. 2014;3:e000523. DOI: 10.1161/JAHA.113.000523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu J, Mehran R, Grinfeld L, Xu KE, Nikolsky E, Brodie BR, Witzenbichler B, Kornowski R, Dangas GD, Lansky AJ, et al. Sex‐based differences in bleeding and long term adverse events after percutaneous coronary intervention for acute myocardial infarction: three year results from the HORIZONS‐AMI trial. Catheter Cardiovasc Interv. 2015;85:359–368. DOI: 10.1002/ccd.25630. [DOI] [PubMed] [Google Scholar]
- 6.Anderson ML, Peterson ED, Brennan JM, Rao SV, Dai D, Anstrom KJ, Piana R, Popescu A, Sedrakyan A, Messenger JC, et al. Short‐ and long‐term outcomes of coronary stenting in women versus men: results from the National Cardiovascular Data Registry Centers for Medicare & Medicaid services cohort. Circulation. 2012;126:2190–2199. [DOI] [PubMed] [Google Scholar]
- 7.Chandiramani R, Cao D, Claessen BE, Sorrentino S, Guedeney P, Blum M, Goel R, Roumeliotis A, Krucoff M, Kozuma K, et al. Sex‐related differences in patients at high bleeding risk undergoing percutaneous coronary intervention: a patient‐level pooled analysis from 4 postapproval studies. J Am Heart Assoc. 2020;9:e014611. DOI: 10.1161/JAHA.119.014611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Husted S, James SK, Bach RG, Becker RC, Budaj A, Heras M, Himmelmann A, Horrow J, Katus HA, Lassila R, et al. The efficacy of ticagrelor is maintained in women with acute coronary syndromes participating in the prospective, randomized, platelet inhibition and patient outcomes (PLATO) trial. Eur Heart J. 2014;35:1541–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levine GN, Bates ER, Bittl JA, Brindis RG, Fihn SD, Fleisher LA, Granger CB, Lange RA, Mack MJ, Mauri L, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol. 2016;68:1082–1115. DOI: 10.1016/j.jacc.2016.03.513. [DOI] [PubMed] [Google Scholar]
- 10.Valgimigli M, Bueno H, Byrne RA, Collet JP, Costa F, Jeppsson A, Juni P, Kastrati A, Kolh P, Mauri L, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio‐Thoracic Surgery (EACTS). Eur Heart J. 2018;39:213–260. [DOI] [PubMed] [Google Scholar]
- 11.Palmerini T, Genereux P, Caixeta A, Cristea E, Lansky A, Mehran R, Della Riva D, Fahy M, Xu K, Stone GW. A new score for risk stratification of patients with acute coronary syndromes undergoing percutaneous coronary intervention: the ACUITY‐PCI (acute catheterization and urgent intervention triage strategy‐percutaneous coronary intervention) risk score. JACC Cardiovasc Interv. 2012;5:1108–1116. [DOI] [PubMed] [Google Scholar]
- 12.Subherwal S, Bach RG, Chen AY, Gage BF, Rao SV, Newby LK, Wang TY, Gibler WB, Ohman EM, Roe MT, et al. Baseline risk of major bleeding in non–ST‐segment–elevation myocardial infarction: the CRUSADE (can rapid risk stratification of unstable angina patients suppress adverse outcomes with early implementation of the ACC/AHA guidelines) bleeding score. Circulation. 2009;119:1873–1882. DOI: 10.1161/CIRCULATIONAHA.108.828541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baber U, Mehran R, Giustino G, Cohen DJ, Henry TD, Sartori S, Ariti C, Litherland C, Dangas G, Gibson CM, et al. Coronary thrombosis and major bleeding after PCI with drug‐eluting stents: risk scores from Paris. J Am Coll Cardiol. 2016;67:2224–2234. [DOI] [PubMed] [Google Scholar]
- 14.Costa F, van Klaveren D, James S, Heg D, Raber L, Feres F, Pilgrim T, Hong MK, Kim HS, Colombo A, et al. Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE‐DAPT) score: a pooled analysis of individual‐patient datasets from clinical trials. Lancet. 2017;389:1025–1034. [DOI] [PubMed] [Google Scholar]
- 15.Urban P, Mehran R, Colleran R, Angiolillo DJ, Byrne RA, Capodanno D, Cuisset T, Cutlip D, Eerdmans P, Eikelboom J, et al. Defining high bleeding risk in patients undergoing percutaneous coronary intervention. Circulation. 2019;140:240–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corpataux N, Spirito A, Gragnano F, Vaisnora L, Galea R, Svab S, Gargiulo G, Zanchin T, Zanchin C, Siontis GCM, et al. Validation of high bleeding risk criteria and definition as proposed by the Academic Research Consortium for high bleeding risk. Eur Heart J. 2020;41:3743–3749. DOI: 10.1093/eurheartj/ehaa671. [DOI] [PubMed] [Google Scholar]
- 17.Redfors B, Angerås O, Råmunddal T, Petursson P, Haraldsson I, Dworeck C, Odenstedt J, Ioaness D, Ravn‐Fischer A, Wellin P, et al. Trends in gender differences in cardiac care and outcome after acute myocardial infarction in Western Sweden: a report from the Swedish web system for enhancement of evidence‐based care in heart disease evaluated according to recommended therapies (SWEDEHEART). J Am Heart Assoc. 2015;4:e001995. DOI: 10.1161/JAHA.115.001995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es G‐A, Gabriel Steg P, Morel MA, Mauri L, Vranckx P, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344–2351. DOI: 10.1161/CIRCULATIONAHA.106.685313. [DOI] [PubMed] [Google Scholar]
- 19.Garcia‐Garcia HM, McFadden EP, Farb A, Mehran R, Stone GW, Spertus J, Onuma Y, Morel M‐A, van Es G‐A, Zuckerman B, et al. Standardized end point definitions for coronary intervention trials: the Academic Research Consortium‐2 consensus document. Circulation. 2018;137:2635–2650. DOI: 10.1161/CIRCULATIONAHA.117.029289. [DOI] [PubMed] [Google Scholar]
- 20.Chester RC, Mina SA, Lewis B, Zhang N, Butterfield R, Yang EH. Radial artery access is under‐utilized in women undergoing PCI despite potential benefits: Mayo Clinic PCI Registry. Catheter Cardiovasc Interv. 2020;95:675–683. DOI: 10.1002/ccd.28341. [DOI] [PubMed] [Google Scholar]
- 21.Gargiulo G, Ariotti S, Vranckx P, Leonardi S, Frigoli E, Ciociano N, Tumscitz C, Tomassini F, Calabrò P, Garducci S, et al. Impact of sex on comparative outcomes of radial versus femoral access in patients with acute coronary syndromes undergoing invasive management: data from the randomized MATRIX‐Access trial. JACC Cardiovasc Interv. 2018;11:36–50. DOI: 10.1016/j.jcin.2017.09.014. [DOI] [PubMed] [Google Scholar]
- 22.Pandie S, Mehta SR, Cantor WJ, Cheema AN, Gao P, Madan M, Niemela K, Rao SV, Schwalm JD, Valentin V, et al. Radial versus femoral access for coronary angiography/intervention in women with acute coronary syndromes: insights from the RIVAL trial (radial vs femoral access for coronary intervention). JACC Cardiovasc Interv. 2015;8:505–512. [DOI] [PubMed] [Google Scholar]
- 23.Rao SV, Hess CN, Barham B, Aberle LH, Anstrom KJ, Patel TB, Jorgensen JP, Mazzaferri EL Jr, Jolly SS, Jacobs A, et al. A registry‐based randomized trial comparing radial and femoral approaches in women undergoing percutaneous coronary intervention: the SAFE‐PCI for women (study of access site for enhancement of PCI for women) trial. JACC Cardiovasc Interv. 2014;7:857–867. DOI: 10.1016/j.jcin.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 24.Ahmed B, Lischke S, De Sarno M, Holterman LA, Straight F, Dauerman HL. Gender related differences in predictors of vascular complications: role of vessel size and BMI. J Thromb Thrombolysis. 2013;36:84–90. DOI: 10.1007/s11239-012-0847-y. [DOI] [PubMed] [Google Scholar]
- 25.Clemmensen P, Roe MT, Hochman JS, Cyr DD, Neely ML, McGuire DK, Cornel JH, Huber K, Zamoryakhin D, White HD, et al. Long‐term outcomes for women versus men with unstable angina/non–ST‐segment elevation myocardial infarction managed medically without revascularization: insights from the targeted platelet inhibition to clarify the optimal strategy to medically manage acute coronary syndromes trial. Am Heart J. 2015;170:695–705.e695. DOI: 10.1016/j.ahj.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 26.Ko DT, Yun L, Wijeysundera HC, Jackevicius CA, Rao SV, Austin PC, Marquis JF, Tu JV. Incidence, predictors, and prognostic implications of hospitalization for late bleeding after percutaneous coronary intervention for patients older than 65 years. Circ Cardiovasc Interv. 2010;3:140–147. [DOI] [PubMed] [Google Scholar]
- 27.Xanthopoulou I, Davlouros P, Deftereos S, Hamilos M, Sitafidis G, Kanakakis I, Vavouranakis M, Goudevenos J, Lekakis J, Alexopoulos D. Gender‐related differences in antiplatelet treatment patterns and outcome: insights from the GReekAntiPlatElet Registry. Cardiovasc Ther. 2017;35:e12270. DOI: 10.1111/1755-5922.12270. [DOI] [PubMed] [Google Scholar]
- 28.Xu N, Tang XF, Zhao XY, Chen J, Gao Z, Qiao SB, Yang YJ, Gao RL, Xu B, Yuan JQ. Sex‐based differences in bleeding and long‐term adverse events after percutaneous coronary intervention in older patients with coronary artery disease. J Interv Cardiol. 2018;31:345–352. DOI: 10.1111/joic.12500. [DOI] [PubMed] [Google Scholar]
- 29.Shin J‐S, Tahk S‐J, Yang H‐M, Yoon M‐H, Choi S‐Y, Choi B‐J, Lim H‐S, Lee Y‐H, Seo K‐W, Park S‐J, et al. Impact of female gender on bleeding complications after transradial coronary intervention (from the Korean Transradial Coronary Intervention registry). Am J Cardiol. 2014;113:2002–2006. DOI: 10.1016/j.amjcard.2014.03.042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Tables S1–S9
Figures S1–S4


