Abstract
Background
Limited data exist on the incremental value of the risk enhancers recommended in the 2018 American Heart Association/American College of Cardiology (ACC/AHA) cholesterol treatment guidelines in addition to the pooled cohort equation.
Methods and Results
Using pooled individual‐level data from 3 epidemiological cohorts involving 22 942 participants (56% women, mean age 59 years), we evaluated the predictive ability of the risk enhancers and coronary artery calcium (CAC) score for atherosclerotic cardiovascular disease, and determined their incremental utility using the C statistic, net reclassification index, and integrated discrimination index. A total of 1960 (8.5%) atherosclerotic cardiovascular disease events were accrued over 10 years. Of the 10 risk enhancers evaluated, only 6 predicted atherosclerotic cardiovascular disease independent of the pooled cohort equation. However, the individual enhancers demonstrated little or no incremental benefit. There was more incremental value from combining the 6 enhancers into an aggregate score (hazard ratio [HR], 1.21; 95% CI, 1.08–1.37 for each additional enhancer), and having ≥3 enhancers represents an optimum threshold for incremental prediction (C statistic, 0.766; net reclassification index, 0.041; integrated discrimination index, 0.010; P≤0.007). On the other hand, CAC was superior to individual enhancers (C statistic, 0.774; net reclassification index, 0.073; integrated discrimination index, 0.010; P<0.001), reliably reclassifies intermediate‐risk participants with <3 risk enhancers (event rate, 3.5% if no CAC and 9.8% if positive CAC), but offered no reclassification among participants with ≥3 enhancers.
Conclusions
The individual risk enhancers evaluated in this study provided no or only marginal incremental information added to the pooled cohort equation. However, the presence of ≥3 risk enhancers reliably identified intermediate‐risk patients that will benefit from statin therapy, and further CAC testing may be considered among those with <3 risk enhancers.
Keywords: cholesterol, guidelines, pooled cohort equation, risk factors
Subject Categories: Primary Prevention
Nonstandard Abbreviations and Acronyms
- ACC
American College of Cardiology
- AHA
American Heart Association
- ARIC
Atherosclerosis Risk in Communities
- CHS
Cardiovascular Health Study
- IDI
integrated discrimination index
- Lp(a)
lipoprotein (a)
- MESA
Multi‐Ethnic Study of Atherosclerosis
- NHLBI
National Heart, Lung, and Blood Institute
- NRI
net reclassification index
- PCE
pooled cohort equation
Clinical Perspective
What Is New?
The individual risk enhancers recommended in the 2018 American College of Cardiology/American Heart Association cholesterol treatment guidelines provided no or only marginal incremental information when used in addition to the pooled cohort equation.
Presence of at least 3 risk enhancers reclassifies patients previously deemed as intermediate risk by the pooled cohort equation to high risk.
What Are the Clinical Implications?
Coronary artery calcium score is a reliable consideration when risk decision remains uncertain for intermediate‐risk patients with <3 risk enhancers, but it is of less utility in those with ≥3 risk enhancers.
The most recent 2018 American College of Cardiology/American Heart Association (ACC/AHA) Multispecialty Society Guideline on the Management of Blood Cholesterol identifies patients with intermediate atherosclerotic cardiovascular disease (ASCVD) risk, defined as pooled cohort equation (PCE)‐based estimated risk of 7.5% to <20%, as one of the groups that will benefit from initiation of statin therapy for primary prevention of ASCVD.1 The PCE was developed from 4 prospective epidemiologic studies using a combination of traditional risk factors to predict the risk of hard cardiovascular events (ie, nonfatal myocardial infarction, coronary heart disease deaths, nonfatal or fatal stroke). However, the PCE may underestimate or overestimate risk in some populations.2, 3, 4, 5 Hence, the guideline recommends a risk discussion to guide initiation of statin therapy for primary ASCVD prevention. The risk discussion should involve evaluation for risk enhancers to supplement the PCE, and when the decision remains uncertain, measuring coronary artery calcium (CAC) score is to be considered for further risk stratification. Although the risk enhancers were selected on the basis of prior studies showing their association with ASCVD, little is known about their incremental value when combined with the PCE.
A prior analysis of the MESA (Multi‐Ethnic Study of Atherosclerosis) study evaluated the utility of 3 risk enhancing factors (ankle‐brachial index [ABI], high‐sensitivity C‐reactive protein, and family history of ASCVD) that were included in the 2013 ACC/AHA cholesterol management guideline.6 However, the most recent 2018 guideline has provided additional risk enhancers that have not been previously evaluated over the PCE. Also, the 2018 guideline has redefined the cutoff for intermediate risk (≥7.5% to <20%), which differs from the 2013 guideline cut point of ≥7.5% 10‐year ASCVD risk without an upper limit. An assessment of the incremental value of the recommended risk enhancers and CAC score in the context of the 2018 guideline may help clinicians and patients in the incorporation of the risk enhancers as part of a risk discussion for initiation of statin therapy. Therefore, we sought to evaluate the incremental value of the 2018 guideline risk enhancers and CAC score when used with the PCE. Also, we evaluated whether having multiple risk enhancers might have a cumulative prognostic impact.
METHODS
Participants
All data used in this analysis are publicly available by request from the National Heart, Lung, and Blood Institute (NHLBI) repository. The study was conducted using pooled data from 3 large prospective NHLBI cohorts, namely, the ARIC (Atherosclerosis Risk in Communities) study, CHS (Cardiovascular Health Study), and MESA. Notably, ARIC and CHS were included in the original derivation cohort for the PCE by the risk‐assessment working group for the 2013 ACC/AHA guideline7. Details of the design, rationale, and enrollment for each study have been previously published.8, 9, 10 Briefly, ARIC is a cohort of 15 792 White and Black men and women aged 45–64 years at enrollment who were recruited from 4 field centers (Minneapolis, MN; Washington County, MD; Forsyth County, NC; and Jackson, MS) between 1987 and 1989. The CHS original cohort is an elderly population of 5201 White and Black men and women aged ≥65 years from 4 field centers (Washington County, MD; Forsyth County, NC; Allegheny County, PA; and Sacramento County, CA) who were enrolled between 1988 and 1990. MESA is a more ethnically diverse cohort of 6814 men and women aged 45–84 years from 6 sites (Baltimore City and Baltimore County, MD; Chicago, IL; Forsyth County, NC; Los Angeles County, CA; New York, NY; and St. Paul, MN) and included 38% White, 28% Black, 23% Hispanic, and 11% Chinese participants who were enrolled between 2000 and 2002. The institutional review committee at each participating center approved the study, and all participants provided informed consent. The current analysis was a retrospective analysis of the prospectively collected data and was considered exempt from additional formal review by the University of Iowa Institutional Review Board because it involved deidentified data sets.
Following the methodology used in the development of the PCE, we excluded individuals aged >79 years because complex age interactions were found above this age cutoff during the development of the PCE.7 Older adults aged between 75 and 79 years were included because of evidence of statin benefit in meta‐analyses and recommendation to consider primary prevention statin in this group in the new guideline.1, 11, 12, 13 However, we limited the participants to those free of cardiovascular disease at baseline by excluding those with previous history of myocardial infarction, stroke, congestive heart failure, percutaneous coronary intervention, coronary bypass surgery, or atrial fibrillation. Lastly, we excluded participants with missing information on the key variables required for calculation of the PCE including age, sex, race, treated or untreated systolic blood pressure, total cholesterol, high‐density lipoprotein cholesterol (HDL‐C), current smoking, diabetes mellitus, and statin use (Figure S1).
Covariate Assessment
Extensive baseline examinations, including documentation of sociodemographic details, medical history, and laboratory risk factors of cardiovascular disease, were performed for all 3 cohorts. Scheduled follow‐up visits were performed by trained study personnel at regular intervals for risk factors and events. Follow‐up data for incident cardiovascular disease events were available for >10 years in each study. Details of these measurements are available in prior reports or through the NHLBI website.8, 9, 10
Risk Enhancers
Of the 13 risk enhancers recommended in the guideline, 10 were available for analysis: triglyceride ≥175 mg/dL, low‐density lipoprotein cholesterol ≥160 mg/dL, non–HDL‐C ≥190 mg/dL, chronic kidney disease (CKD; defined as estimated glomerular filtration rate <60 mL/min·1.73 m2), ABI <0.9, high‐sensitivity C‐reactive protein ≥2 mg/L, lipoprotein(a) [Lp(a)] ≥50 mg/dL, apolipoprotein B ≥130 mg/dL, family history of premature ASCVD (men aged <55 years or women aged <65 years), and metabolic syndrome. Metabolic syndrome was defined as the presence of at least 3 of increased waist circumference, elevated triglycerides >150 mg/dL, elevated blood pressure, elevated glucose, and low HDL‐C of <40 mg/dL in men or <50 mg/dL in women. All of the 10 risk enhancers were available in MESA, whereas ARIC and CHS had only 8 (Table S1). None of the cohorts have reliable information on chronic inflammatory conditions, premature menopause, and pregnancy‐associated conditions, or South Asian ancestry; therefore, these were not available for this analysis.
CAC Score
As a comparison with the risk enhancers, we also evaluated the utility of the CAC score, which has now been recommended in the 2018 guideline to supplement the risk enhancers when a risk decision remains uncertain. Of the 3 cohorts, CAC score was only available from the baseline exam of MESA. It was measured by either an electron‐beam or multidetector computed tomography,14 and each participant had 2 scans, which were reconstructed to include a calibration phantom at a central computed tomography reading center (Harbor‐University of California Los Angeles Research and Education Institute, Los Angeles, CA). Calcified plaques were calculated in phantom‐adjusted Agatston score, for which the mean of the 2 scans was computed for each participant.
End Points
The main outcome of the study was the incidence of 10‐year ASCVD events as defined by the ACC/AHA risk‐assessment working group.7 ASCVD is a composite of the first nonfatal myocardial infarction, coronary heart disease death, or nonfatal or fatal stroke over a 10‐year period. Ascertainment of events was adjudicated by a centralized committee using available data from patient contact, next of kin, medical records, or death certificates. For the present analysis, participants were censored when an event occurred or after 10 years of follow‐up (whichever occurred first).
Statistical Analysis
Participant‐level data from the 3 cohorts were pooled together for this study. Subsequent analyses were conducted in accordance with the methodology and recommendations of the ACC/AHA risk‐assessment working group.7 Ten‐year ASCVD risk was estimated for each participant on the basis of the variables included in the PCE (ie, age, sex, race (non‐Hispanic White or Black), treated or untreated systolic blood pressure, total cholesterol, HDL‐C, current smoking, and diabetes mellitus. Risk estimates for Hispanic and Asian American participants were calculated using the equation for non‐Hispanic White participants of the same sex as recommended by the risk‐assessment working group. Baseline characteristics based on the PCE variables were summarized across each cohort into 3 PCE‐based risk categories (<7.5%, 7.5% to <20%, and ≥20%).
For the primary analysis, the predictive ability and incremental prediction benefit offered by each risk enhancer over the PCE was evaluated. The risk enhancers were evaluated in categorical form (present or absent) as recommended in the 2018 guideline. The predictive ability of each enhancer for incident 10‐year ASCVD was evaluated using a PCE‐based Cox proportional hazard model that included all the variables in the PCE (above). Also, a variable for statin use at baseline as well as an indicator variable for the different cohorts were included in each model. Age interaction was tested for each PCE variable in the model and was retained if the P value for interaction was statistically significant. The goodness of fit of the PCE‐based model was evaluated using the C statistic (for discrimination) and a calibration χ2 statistic that compares observed with predicted risk. The incremental prediction benefit of adding each risk enhancer to the PCE‐based model was evaluated via C statistics, integrated discrimination index (IDI), and net reclassification index (NRI), as recommended by the risk‐assessment working group.7 Categorical net reclassification was evaluated by cross‐tabulating the participants into 3 PCE‐based risk categories of <7.5%, 7.5% to <20%, and ≥20% before and after the addition of risk enhancer to the model. Also, to evaluate the potential cumulative effect of the presence of multiple risk enhancers in a participant, an aggregate variable that represented the number of risk enhancers that were present was created for each participant. Only risk enhancers that demonstrated significant association with incident ASCVD were considered for the aggregate variable, the number of risk enhancers present. Based on this aggregate variable, the optimum number of risk enhancers for predicting incident ASCVD was determined using the Youden index, which identifies a cut point on the receiver operating characteristic curve that maximizes the sum of the sensitivity+specificity−1.15, 16 Similar to the analysis for the individual risk enhancers, the optimum number of risk enhancers was evaluated for incremental benefit over the PCE. Lastly, the same analyses with CAC score as the variable of interest were performed. Analyses of the optimum number of risk enhancers and the CAC score was limited only to MESA, because it was the only cohort that contained all the 10 risk enhancers and CAC score.
We performed multiple sensitivity analysis. First, we evaluated the individual risk enhancers and CAC score in continuous form (when possible) and compared the results to the categorical form. For the continuous form, normalization of the skewed distribution of high‐sensitivity C‐reactive protein, Lp(a), and CAC+1 was achieved via the natural logarithm function. Second, we restricted the analysis of prognostic utility of risk enhancer to the ARIC cohort.
All analyses were performed using Stata 16 (StataCorp, College Station, TX), and a 2‐tailed P value <0.05 was considered statistically significant.
RESULTS
A total of 22 942 participants free of ASCVD at baseline were included in this study. Baseline demographics were mean age 58.7 years (range, 45–79 years), 55.6% women, 68.3% White, and 22.3% Black (Table 1 and Table S1). When participants in the combined cohort were classified into the 3 risk categories, there were 13 899 (60.6%), 6625 (28.9%), and 2418 (10.5%) in the low/borderline (<7.5% 10‐year ASCVD risk), intermediate (7.5% to <20% 10‐year ASCVD risk), and high‐risk category (≥20% 10‐year ASCVD risk), respectively (Table 1). Across the 3 risk categories, age, male sex, White race, low‐density lipoprotein cholesterol level, blood pressure level, hypertension, smoking, diabetes mellitus, and the prevalence of each risk enhancer increased, whereas HDL‐C level and statin use decreased.
Table 1.
Baseline Characteristics of Study Participants, Stratified by Pooled Cohort Equation‐Based Risk Categories
| Low/Borderline Risk, PCE<7.5%, n=13 899 | Intermediate Risk, PCE 7.5% to <20%, n=6625 | High Risk, PCE≥20%, n=2418 | |
|---|---|---|---|
| No. by cohort | |||
| Age, y, mean (SD) | 54.9 (7.3) | 63.3 (8.2) | 68.3 (8.6) |
| Women, % | 64.9 | 42.8 | 37.4 |
| Race | |||
| White, % | 64.2 | 72.2 | 80.6 |
| Black, % | 22.8 | 22.7 | 18.2 |
| Other, % | 13.0 | 5.1 | 1.1 |
| Total cholesterol, mg/dL, mean (SD) | 203 (37.5) | 214 (41.9) | 218 (45.3) |
| HDL‐C, mg/dL, mean (SD) | 55.4 (16.9) | 48.3 (14.3) | 46.9 (13.4) |
| LDL‐C, mg/dl, mean (SD) | 125 (34.9) | 139 (39.1) | 141 (41.4) |
| Untreated SBP, mm Hg, mean (SD) | 115 (15) | 129 (18) | 145(22) |
| Treated SBP, mm Hg, mean (SD) | 123 (16) | 134 (18) | 149 (21) |
| Blood pressure medications, % | 18 | 39.4 | 57.6 |
| Current smoker, % | 14.6 | 28.7 | 30.5 |
| Diabetes mellitus, % | 3.1 | 14.1 | 46.1 |
| Statin use, % | 5.4 | 4.0 | 1.2 |
| Triglyceride ≥175 mg/dL, % | 13.4 | 24.6 | 32.7 |
| LDL‐C ≥160 mg/dL, % | 15.8 | 27.5 | 29.3 |
| Non–HDL‐C ≥190 mg/dL, % | 13.7 | 26.7 | 29.4 |
| Metabolic syndrome, % | 17.8 | 38.2 | 55.2 |
| CKD, % | 3.5 | 14.2 | 29.1 |
| ABI ≤0.9, % | 2.5 | 5.4 | 12.4 |
| hs‐CRP ≥2 mg/dL, % | 46.0 | 53.7 | 62.1 |
| Lp(a) ≥50 mg/dL, % | 22.0 | 35.5 | 38.5 |
| apoB ≥130 mg/dL, % | 8.9 | 20.2 | 25.1 |
| Family history of premature CVD, % | 13.7 | 15.3 | 14.0 |
| CAC >0 Agatston unit, % | 37.7 | 65.8 | 78.6 |
ABI indicates ankle‐brachial index; apoB, apolipoprotein B; CAC, coronary artery calcium; CKD, chronic kidney disease; CVD, cardiovascular disease; HDL‐C, high‐density lipoprotein cholesterol; hs‐CRP, high‐sensitivity C‐reactive protein; LDL‐C, low‐density lipoprotein cholesterol; Lp(a), lipoprotein (a); PCE, pooled cohort equation; and SBP, systolic blood pressure.
P<0.001 for all comparison (except Lp(a), P=0.07; and family history of premature CVD, P=0.02). P value was based on χ2 test for categorical variables and ANOVA or Kruskal‐Wallis test (as appropriate) for continuous variables.
A total of 1960 (8.5%) incident ASCVD events occurred over 10 years of follow‐up (46% myocardial infarction, 19% coronary heart disease death, and 35% nonfatal or fatal stroke). The PCE‐based model demonstrated a good fit, with a C statistic of 0.79, and was well calibrated (P=0.35) in the combined cohort. Only 6 of the 10 risk enhancers independently predicted incident ASCVD when added to the PCE (Figure 1). ABI <0.9 was the strongest predictor (hazard ratio [HR], 1.68; 95% CI, 1.46–1.94; P<0.001). The other significant predictors in increasing order of magnitude of association were high‐sensitivity C‐reactive protein ≥2 mg/L, apolipoprotein B ≥130 mg/dL, CKD, family history of premature cardiovascular disease (CVD), and Lp(a) ≥50 mg/dL. On the other hand, triglyceride ≥175 mg/dL, low‐density lipoprotein cholesterol ≥160 mg/dL, non–HDL‐C ≥190 mg/dL, and metabolic syndrome were not significant predictors.
Figure 1. Strength of association among 2018 guideline‐recommended risk enhancers after adjusting for the pooled cohort equation.
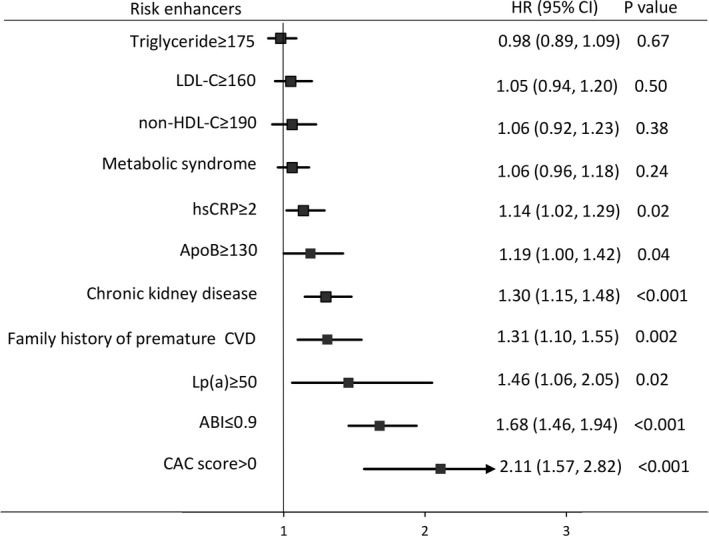
Coronary artery calcium (CAC) score is provided for comparison. ABI indicates ankle‐brachial index; ApoB, apolipoprotein B; CVD, cardiovascular disease; HR, hazard ratio; hsCRP, high‐sensitivity C‐reactive protein; LDL‐C, low‐density lipoprotein cholesterol; Lp(a), lipoprotein (a); and non‐HDL‐C, non–high‐density lipoprotein cholesterol.
None of the 10 risk enhancers, however, significantly improved the C statistic of the model, although the P value was 0.05 for Lp(a) ≥50 mg/dL, apolipoprotein B ≥130 mg/dL, and family history of premature CVD. CKD and ABI marginally improved IDI but worsened category‐free NRI. Lp(a) ≥50 mg/dL and apolipoprotein B ≥130 mg/dL modestly improved NRI across the 3 risk categories, although the IDI and category‐free NRI were not significant (Table 2).
Table 2.
Incremental Utility of Risk Enhancers and CAC Score Over the Pooled Cohort Equation*
| C Statistic | Integrated Discrimination | Net Reclassification | ||||
|---|---|---|---|---|---|---|
| PCE Alone | PCE+Risk Enhancer | P Value for Improvement | IDI (P Value) | NRI Across 7.5%–20% Cut Points (P Value) | Category‐Free NRI (P Value) | |
| Triglyceride ≥175 mg/dL | 0.789 | 0.789 | 0.27 | −0.0001 (0.89) | 0.005 (0.06) | 0.024 (0.23) |
| LDL‐C≥160 mg/dL | 0.789 | 0.789 | 0.51 | −0.0002 (0.90) | 0.002 (0.41) | −0.013 (0.21) |
| Non–HDL‐C≥190 mg/dL | 0.789 | 0.789 | 0.52 | −0.0001 (0.31) | −0.001 (0.71) | −0.026 (0.34) |
| Metabolic syndrome | 0.789 | 0.789 | 0.27 | −0.0001 (21) | 0.006 (0.23) | 0.116 (0.19) |
| CKD | 0.789 | 0.790 | 0.30 | 0.002 (0.04) | 0.002 (0.57) | −0.153 (0.001) |
| ABI ≤0.9 | 0.789 | 0.790 | 0.22 | 0.003 (0.03) | 0.005 (0.35) | −0.374 (0.001) |
| hs‐CRP ≥2 mg/dL | 0.796 | 0.796 | 0.93 | 0.0001 (0.43) | −0.004 (0.61) | 0.181 (0.001) |
| Lp(a) ≥50 mg/dL | 0.796 | 0.798 | 0.05 | 0.0001 (0.82) | 0.024 (0.01) | 0.058 (0.10) |
| apoB ≥130 mg/dL | 0.773 | 0.775 | 0.05 | 0.0001 (0.50) | 0.014 (0.04) | 0.156 (0.07) |
| Family history of premature CVD | 0.773 | 0.776 | 0.05 | −0.0001 (0.76) | −0.004 (0.41) | 0.024 (0.79) |
| No. of risk enhancers ≥3* | 0.762 | 0.766 | 0.10 | 0.010 (0.005) | 0.041 (0.007) | 0.120 (0.01) |
| CAC >0 Agatston units | 0.762 | 0.774 | 0.01 | 0.010 (<0.001) | 0.073 (0.005) | 0.585 (<0.001) |
ABI indicates ankle‐brachial index; apoB, apolipoprotein B; CAC, coronary artery calcium; CKD, chronic kidney disease; CVD, cardiovascular disease; HDL‐C, high‐density lipoprotein cholesterol; hs‐CRP, high‐sensitivity C‐reactive protein; IDI, integrated discrimination index; LDL‐C, low‐density lipoprotein cholesterol; Lp(a), lipoprotein (a); NRI, net reclassification index; and PCE, pooled cohort equation.
Model calibration was optimum (P≥0.35) across all models (not shown in the table).
The aggregate risk enhancer variable represents the number of risk enhancers present for each participant. Only the 6 risk enhancers that demonstrated significant predictive ability (ie, hs‐CRP, apoB, CKD, family history of premature CVD, Lp(a), and ABI) were included in this aggregate variable.
The distribution of the number of risk enhancers per participant is shown in Figure S2. Notably, about 79% of the MESA cohort (and 86.7% of the intermediate‐risk category) have at least 1 risk enhancer. In the MESA cohort, we evaluated the prognostic utility of combining only the 6 risk enhancers that significantly predicted incident ASCVD. Hence, each participant had an aggregate score ranging from 0 to 6, representing the number of risk enhancers present. There was a graded increase in the rate of incident ASCVD across the spectrum of the aggregate score (Figure S3). For example, in the full MESA cohort, irrespective of the risk category, participants with none of the 6 significant risk enhancers had 10‐year event rate of 3.4%, which increased in a stepwise fashion with increasing risk enhancer to 23.1% among participants with ≥5 enhancers (HR for each additional risk enhancer, 1.21; 95% CI, 1.08–1.37; P=0.001). A threshold of ≥3 risk enhancers demonstrated the optimum predictive value for incident ASCVD. Participants with ≥3 risk enhancers had an event rate of 8.5% in the full cohort and 12.8% among those with intermediate risk, which is similar to having a CAC score ≥100. (Figure 2A and 2B). Although this optimum threshold did not significantly improve the C statistic of the PCE‐based model (0.762 versus 0.766, P=0.10), it did result in significant net reclassification (Table 2). The reclassification utility was more prominent among those in the intermediate‐risk category, among which a net 9.1% were appropriately reclassified (P=0.007) (Table 3).
Figure 2. Rate of incident atherosclerotic cardiovascular disease (ASCVD) by number of significant risk enhancers (high‐sensitivity C‐reactive protein ≥2 mg/L, apolipoprotein B ≥130 mg/dL, chronic kidney disease, family history of premature cardiovascular disease, lipoprotein (a) ≥50 mg/dL, and ankle‐ brachial index <0.9) and coronary artery calcium (CAC) score.
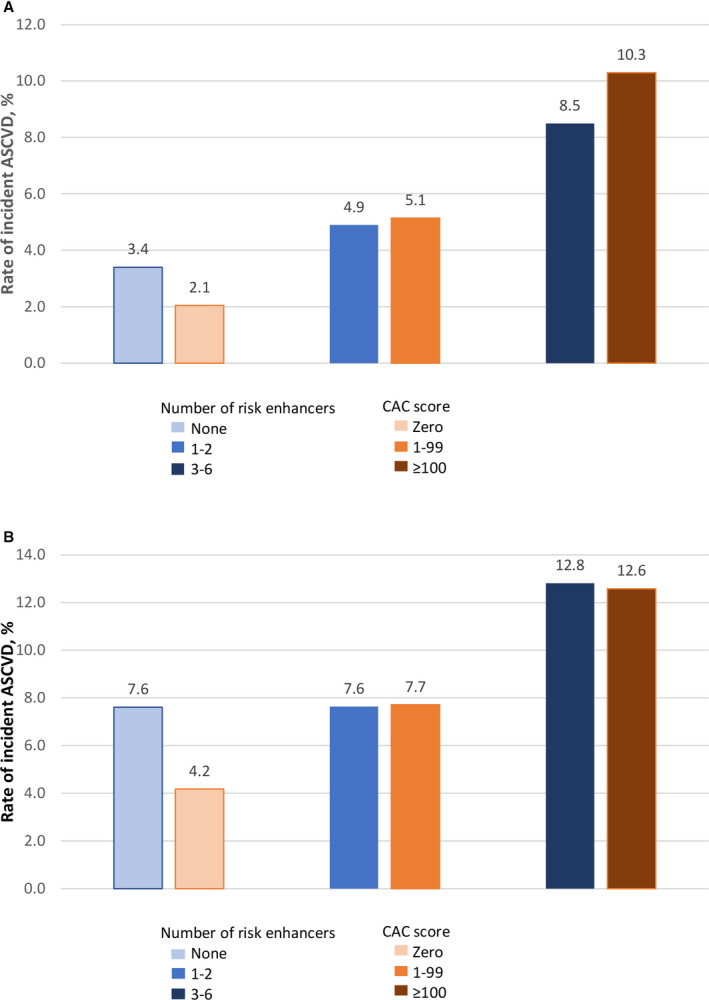
A, Irrespective of risk category. B, Among participants with intermediate risk.
Table 3.
Risk Reclassification of MESA Participants by Aggregate Risk Enhancer Score ≥3
| PCE Alone | PCE With Aggregate Risk Enhancer ≥3 | Risk Reclassification | ||||
|---|---|---|---|---|---|---|
| <7.5% | 7.5% to <20% | ≥20% | Row Total | Higher, No. (%) | Lower, No. (%) | |
| Events, n=305 | ||||||
| <7.5% | 154 | 11 | 165 | 11 (6.7) | NA | |
| 7.5%–20% | 3 | 113 | 5 | 121 | 5 (4.1) | 3 (2.5) |
| ≥20% | 2 | 17 | 19 | NA | 2 (10.5) | |
| Total | 157 | 126 | 22 | 305 | 16 (5.2) | 5 (1.6) |
| Nonevent, n=6105 | ||||||
| <7.5% | 5,034 | 54 | 5,088 | 54 (1.1) | NA | |
| 7.5%–20% | 83 | 826 | 14 | 923 | 14 (1.5) | 83 (9.0) |
| ≥20% | 13 | 81 | 94 | NA | 13 (13.8) | |
| Total | 5117 | 893 | 95 | 6,105 | 68 (1.1) | 96 (1.6) |
| Net reclassification improvement | ||||||
| Overall, P=0.007 | 4.1% | … | ||||
| Intermediate group, 7.5%–<20% | 9.1% | … | ||||
MESA indicates Multi‐Ethnic Study of Atherosclerosis; NA, not applicable; and PCE, pooled cohort equation.
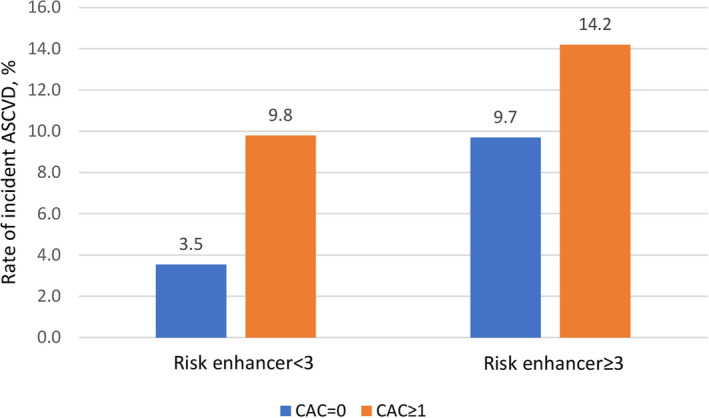
In comparison, a CAC score >0 significantly predicted incident ASCVD (HR, 2.11; 95% CI, 1.57–2.82; P<0.001), improved C statistic of the PCE‐based model (0.762 versus 0.774, P=0.01), IDI (0.010, P<0.001), and resulted in significant net reclassification (Tables 2 and 4). However, this incremental benefit of CAC score was limited only to those with <3 risk enhancers, and it has less utility among those with ≥3 risk enhancers (C statistic, 0.812 versus 0.825, P=0.10; IDI, 0.009, P=0.12; NRI, −0.028, P=0.62) (Table S2). A CAC score of 0 reliably reclassifies intermediate‐risk participants with <3 risk enhancers into low risk (event rate, 3.5%), but participants with a CAC score of 0 and ≥3 risk enhancers remain in elevated risk (event rate, 9.7%) (Figure 3).
Table 4.
Risk Reclassification of MESA Participants by CAC Score
| PCE Alone | PCE With CAC>0 | Risk Reclassification | ||||
|---|---|---|---|---|---|---|
| <7.5% | 7.5% to <20% | ≥20% | Row Total | Higher, No (%) | Lower, No (%) | |
| Events, n=305 | ||||||
| <7.5% | 131 | 34 | 165 | 34 (20.6) | NA | |
| 7.5% to <20% | 16 | 95 | 10 | 121 | 10 (8.3) | 16 (13.2) |
| ≥20% | 2 | 17 | 19 | NA | 2 (10.5) | |
| Total | 147 | 131 | 27 | 305 | 44 (14.4) | 18 (5.9) |
| Nonevent, n=6105 | ||||||
| <7.5% | 4821 | 267 | 5088 | 267 (5.2) | NA | |
| 7.5% to <20% | 192 | 710 | 21 | 923 | 21 (2.3) | 192 (20.8) |
| ≥20% | 21 | 73 | 94 | NA | 21 (22.3) | |
| Total | 5013 | 998 | 94 | 6,105 | 288 (4.7) | 213 (3.5) |
| Net reclassification improvement | ||||||
| Overall, P=0.005 | 7.3% | … | ||||
| Intermediate group (7.5% to <20%) | 13.6% | … | ||||
CAC indicates coronary artery calcium; MESA, Multi‐Ethnic Study of Atherosclerosis; NA, not applicable; and PCE, pooled cohort equation.
Figure 3. Interaction between coronary artery calcium (CAC) score and risk enhancer for rate of incident atherosclerotic cardiovascular disease (ASCVD) among participants with intermediate risk.
Overall, similar results were found in sensitivity analyses as prespecified (Tables S3 through S6).
DISCUSSION
Applying the 2018 ACC/AHA cholesterol treatment guideline to the pooled study population free of ASCVD at baseline showed that an estimated 35.6% of the population would have been eligible for primary prevention statin therapy. This includes 10.5% in the high‐risk category plus 25.1% in the intermediate‐risk category (ie, 86.7% of the intermediate‐risk category who had at least 1 risk enhancer) (class 1 recommendation). However, not all risk enhancers significantly predicted 10‐year incident ASCVD independent of the PCE, and even among those that predicted 10‐year incident ASCVD (ie, high‐sensitivity C‐reactive protein ≥2 mg/L, apolipoprotein B ≥130 mg/dL, CKD, family history of premature CVD, Lp(a) ≥50 mg/dL, and ABI <0.9), none added sufficient information individually to meaningfully reclassify participants to lower or higher risk. Our analysis, therefore, suggests that the individual risk enhancers are of little or no value for modifying the risk of patients up or down when considering statin therapy.
We found more incremental benefit in combining the risk enhancers into an aggregate score. The aggregate score demonstrated a graded increase in 10‐year rate of incident ASCVD that may provide more information to restratify patients within the intermediate‐risk category and more informatively supplement the clinician–patient risk discussion. Patients are more likely to participate and be satisfied with the outcome of shared decision making when an estimate of their absolute risk is provided.17 For example, a patient with intermediate risk might give different weights to an absolute 10‐year risk of 7.5% for 0 to 1 risk enhancers, compared with an upwardly revised 33% 10‐year ASCVD risk for 5 or more risk enhancers, during the risk discussion. Also, the absolute risk estimate may provide a useful clue for intensification of statin therapy among those who are already on a low‐ to moderate‐intensity statin. Notably, we found that having ≥3 risk enhancers represent an optimum threshold for initiation or intensification of statin therapy because it resulted in significant net reclassification, particularly among those in the intermediate‐risk category. CAC score was also confirmed to significantly reclassify intermediate‐risk individuals, and the rates of events by the level of CAC score paralleled those by the aggregate risk‐enhancer score. These data suggest that either multiple risk enhancers or CAC should be considered when risk decision remains uncertain, because both were shown to reclassify a clinically meaningful proportion (9%–13%) of intermediate‐risk individuals.
It is not surprising that CAC score and, to some extent ABI, were the strongest predictors of events, because they both represent subclinical markers of disease. This finding is consistent with a prior analysis of the utility of risk markers over the pooled cohort equation that showed that CAC score, ABI, and family history of premature CVD independently predicted ASCVD event, but only CAC score showed modest reclassification benefit when used alone.6 The finding of the incremental value of CAC scoring is also in agreement with prior studies, where the presence of CAC has been shown to predict future ASCVD events and provide better incremental benefit when compared with selected traditional risk factors.6, 18, 19, 20 Extending the findings from these prior studies, our analysis supports the reclassification value of CAC based on the new risk categories in the 2018 guideline. Importantly, we also showed that the presence of ≥3 enhancers performed close to CAC score for identifying participants with elevated risk. Although similar utility of combining multiple biomarker risk markers had been reported in the literature,21, 22 our current analysis is the first to evaluate the combination of the guideline‐recommended risk enhancers. In our analysis, having ≥3 of the 6 risk enhancers we identified as being independently predictive (high‐sensitivity C‐reactive protein ≥2 mg/L, apolipoprotein B≥130 mg/dL, CKD, family history of premature CVD, Lp(a)≥50 mg/dL, and ABI<0.9) is associated with similar rate of event as a CAC score ≥100, and CAC scoring did not show meaningful incremental value over the PCE among participants with ≥3 risk enhancers. However, CAC scoring demonstrated significant reclassification benefit among participants with <3 risk enhancers, for which a CAC score of 0 identified those with low risk. Hence, patients with ≥3 risk enhancers should be considered as having sufficient risk to commence statin therapy without the need for further downstream testing for CAC score. On the other hand, CAC scoring may be considered for further risk stratification in patients with <3 risk enhancers, because a 0 calcium score reliably identified the true low‐risk participants in this subgroup.
Potential limitations of our study should also be considered. First, our analysis included 10 out of the recommended 13 risk enhancers. None of the cohorts has reliable information on chronic inflammatory conditions, premature menopause, and pregnancy‐associated conditions, or South Asian ancestry. Hence, these enhancers were not included in our analysis and we cannot comment on their prognostic utility. Also, ARIC and CHS have data on only 8 risk enhancers, and therefore, part of the analysis did not involve the total participants in the combined cohort. Second, our study included Hispanic and Asian participants who were not included in the derivation of the PCE. However, this is an inherent limitation of the PCE, and we followed the risk‐assessment working group recommendation to approximate these population risk estimates using PCE for White participants of the same sex.7
In conclusion, the individual risk enhancers evaluated in this study provided no or only marginal incremental information added to the PCE. This probably reflects the potential drawbacks of using a single risk modifier (other than CAC score) to supplement a moderately robust risk estimation model such as the pooled cohort equation, which was developed on the basis of a combination of multiple strong risk factors. However, we found a novel utility in combining the risk enhancers, because the presence of multiple risk enhancers of at least 3 had prognostic utility in identifying intermediate‐risk participants with elevated risk. Lastly, CAC score is a reliable consideration when risk decision remains uncertain for intermediate‐risk participants with <3 risk enhancers, but it is of less utility in those with ≥3 risk enhancers.
Sources of Funding
None.
Disclosures
None.
Supporting information
Tables S1–S6
Figures S1–S3
Acknowledgments
This article was prepared using the ARIC, CHS, and MESA research materials obtained from the NHLBI Biologic Specimen and Data Repository Information Coordinating Center and does not necessarily reflect the opinions or views of ARIC, CHS, MESA, or the NHLBI. The authors are solely responsible for the design and conduct of this study, all study analyses, and drafting and editing of the article.
(J Am Heart Assoc. 2021;10:e019589. DOI: 10.1161/JAHA.120.019589.)
This article was sent to Daniel Edmundowicz, MD, Guest Editor, for review by expert referees, editorial decision, and final disposition.
Part of these results were presented as an oral presentation at the American College of Cardiology/World Congress of Cardiology Scientific Sessions, March 28–30, 2020.
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.019589.
For Sources of Funding and Disclosures, see page 11.
REFERENCES
- 1.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella‐Tommasino J, Forman DE, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation. 2019;139:e1082–e1143. DOI: 10.1161/CIR.0000000000000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robinson JG. 2013 ACC/AHA cholesterol guideline for reducing cardiovascular risk: what is so controversial? Curr Atheroscler Rep. 2014;16:413. DOI: 10.1007/s11883-014-0413-5. [DOI] [PubMed] [Google Scholar]
- 3.Rana JS, Tabada GH, Solomon MD, Lo JC, Jaffe MG, Sung SH, Ballantyne CM, Go AS. Accuracy of the atherosclerotic cardiovascular risk equation in a large contemporary, multiethnic population. J Am Coll Cardiol. 2016;67:2118–2130. DOI: 10.1016/j.jacc.2016.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stone NJ. Preventing atherosclerotic cardiovascular disease using American College of Cardiology and American Heart Association prevention guidelines: some good news, but caveats remain. J Am Heart Assoc. 2016;5:e004197. DOI: 10.1161/JAHA.116.004197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeFilippis AP, Young R, McEvoy JW, Michos ED, Sandfort V, Kronmal RA, McClelland RL, Blaha MJ. Risk score overestimation: the impact of individual cardiovascular risk factors and preventive therapies on the performance of the American Heart Association‐American College of Cardiology‐Atherosclerotic Cardiovascular Disease risk score in a modern multi‐ethnic cohort. Eur Heart J. 2017;38:598–608. DOI: 10.1093/eurheartj/ehw301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yeboah J, Young R, McClelland RL, Delaney JC, Polonsky TS, Dawood FZ, Blaha MJ, Miedema MD, Sibley CT, Carr JJ, et al. Utility of nontraditional risk markers in atherosclerotic cardiovascular disease risk assessment. J Am Coll Cardiol. 2016;67:139–147. DOI: 10.1016/j.jacc.2015.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goff DC, Lloyd‐Jones DM, Bennett G, Coady S, D'Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O'Donnell CJ, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation. 2014;129:S49–S73. DOI: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- 8.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A, et al. The cardiovascular health study: design and rationale. Ann Epidemiol. 1991;1:263–276. DOI: 10.1016/1047-2797(91)90005-W. [DOI] [PubMed] [Google Scholar]
- 9.The ARIC Investigators . The atherosclerosis risk in communities (ARIC) study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 10.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR Jr, Kronmal R, Liu K, et al. Multi‐ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. DOI: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 11.Savarese G, Gotto AM, Paolillo S, D'Amore C, Losco T, Musella F, Scala O, Marciano C, Ruggiero D, Marsico F, et al. Benefits of statins in elderly subjects without established cardiovascular disease: a meta‐analysis. J Am Coll Cardiol. 2013;62:2090–2099. DOI: 10.1016/j.jacc.2013.07.069. [DOI] [PubMed] [Google Scholar]
- 12.Orkaby AR, Gaziano JM, Djousse L, Driver JA. Statins for primary prevention of cardiovascular events and mortality in older men. J Am Geriatr Soc. 2017;65:2362–2368. DOI: 10.1111/jgs.14993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karmali KN, Goff DC Jr, Ning H, Lloyd‐Jones DM. A systematic examination of the 2013 ACC/AHA pooled cohort risk assessment tool for atherosclerotic cardiovascular disease. J Am Coll Cardiol. 2014;64:959–968. DOI: 10.1016/j.jacc.2014.06.1186. [DOI] [PubMed] [Google Scholar]
- 14.Carr JJ, Nelson JC, Wong ND, McNitt‐Gray M, Arad Y, Jacobs DR Jr, Sidney S, Bild DE, Williams OD, Detrano RC. Calcified coronary artery plaque measurement with cardiac CT in population‐based studies: standardized protocol of multi‐ethnic study of atherosclerosis (MESA) and coronary artery risk development in young adults (CARDIA) study. Radiol. 2005;234:35–43. DOI: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 15.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–35. DOI: . [DOI] [PubMed] [Google Scholar]
- 16.Perkins NJ, Schisterman EF. The inconsistency of "optimal" cutpoints obtained using two criteria based on the receiver operating characteristic curve. Am J Epidemiol. 2006;163:670–675. DOI: 10.1093/aje/kwj063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krones T, Keller H, Sonnichsen A, Sadowski EM, Baum E, Wegscheider K, Rochon J, Donner‐Banzhoff N. Absolute cardiovascular disease risk and shared decision making in primary care: a randomized controlled trial. Ann Fam Med. 2008;6:218–227. DOI: 10.1370/afm.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kavousi M, Elias‐Smale S, Rutten JHW, Leening MJG, Vliegenthart R, Verwoert GC, Krestin GP, Oudkerk M, de Maat MPM, Leebeek FWG, et al. Evaluation of newer risk markers for coronary heart disease risk classification: a cohort study. Ann Intern Med. 2012;156:438–444. DOI: 10.7326/0003-4819-156-6-201203200-00006. [DOI] [PubMed] [Google Scholar]
- 19.Zhao Y, Evans MA, Allison MA, Bertoni AG, Budoff MJ, Criqui MH, Malik S, Ouyang P, Polak JF, Wong ND. Multisite atherosclerosis in subjects with metabolic syndrome and diabetes and relation to cardiovascular events: the multi‐ethnic study of atherosclerosis. Atherosclerosis. 2019;282:202–209. DOI: 10.1016/j.atherosclerosis.2018.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elkeles RS, Godsland IF, Feher MD, Rubens MB, Roughton M, Nugara F, Humphries SE, Richmond W, Flather MD, Group PS . Coronary calcium measurement improves prediction of cardiovascular events in asymptomatic patients with type 2 diabetes: the PREDICT study. Eur Heart J. 2008;29:2244–2251. DOI: 10.1093/eurheartj/ehn279. [DOI] [PubMed] [Google Scholar]
- 21.Akintoye E, Briasoulis A, Afonso L. Biochemical risk markers and 10‐year incidence of atherosclerotic cardiovascular disease: independent predictors, improvement in pooled cohort equation, and risk reclassification. Am Heart J. 2017;193:95–103. DOI: 10.1016/j.ahj.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Saeed A, Nambi V, Sun W, Virani SS, Taffet GE, Deswal A, Selvin E, Matsushita K, Wagenknecht LE, Hoogeveen R, et al. Short‐term global cardiovascular disease risk prediction in older adults. J Am Coll Cardiol. 2018;71:2527–2536. DOI: 10.1016/j.jacc.2018.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S6
Figures S1–S3


