Abstract
Background
Microvascular disease (MVD) is a potential contributor to the pathogenesis of diabetes mellitus–related cardiac dysfunction. However, there is a paucity of data on the link between MVD and incident heart failure (HF) in type 2 diabetes mellitus. We examined the association of MVD with incident HF in adults with type 2 diabetes mellitus.
Methods and Results
A total of 4095 participants with type 2 diabetes mellitus and free of HF were assessed for diabetes mellitus–related MVD including nephropathy, retinopathy, or neuropathy at baseline in the Look AHEAD (Action for Health in Diabetes) study. Incident HF events were prospectively assessed and adjudicated using hospital and death records. Cox models were used to generate hazard ratios and 95% CIs for HF. Of 4095 participants, 34.8% (n=1424) had MVD, defined as the presence of ≥1 of nephropathy, retinopathy, or neuropathy at baseline. Over a median of 9.7 years, there were 117 HF events. After adjusting for relevant confounders, participants with MVD had a 2.5‐fold higher risk of incident HF than those without MVD (hazard ratio, 2.54; 95% CI, 1.73–3.75). This association remained significant after additional adjustment for interval development of coronary artery disease (hazard ratio, 2.42; 95% CI, 1.64–3.57). The hazard ratios for HF by type of MVD were 2.22 (95% CI, 1.51–3.27), 1.30 (95% CI, 0.72–2.36), and 1.33 (95% CI, 0.86–2.07) for nephropathy, retinopathy, and neuropathy, respectively.
CONCLUSIONS
MVD is associated with an excess HF risk in individuals with type 2 diabetes mellitus after adjusting for other known risk factors. Our findings underscore the contribution of MVD to the development of diabetes mellitus–related HF.
REGISTRATION: URL: https://www.clinicaltrials.gov; Unique identifier: NCT00017953.
Keywords: heart failure, microvascular disease, type 2 diabetes mellitus
Subject Categories: Heart Failure, Vascular Disease
Nonstandard Abbreviations and Acronyms
- AHEAD
Action for Health in Diabetes
- MNSI
Michigan Neuropathy Screening Instrument
- MVD
microvascular disease
- T2DM
type 2 diabetes mellitus
Clinical Perspective
What Is New?
Data on the relation of microvascular disease with incident heart failure in diverse cohorts of adults with type 2 diabetes mellitus are scant.
Microvascular disease was highly prevalent (34.8%) among individuals with type 2 diabetes mellitus.
Microvascular disease was associated with increased risk of heart failure, independently of traditional heart failure risk factors including coronary artery disease.
What Are the Clinical Implications?
Our findings highlight the contribution of microvascular disease to the development of heart failure among people with type 2 diabetes mellitus.
Type 2 diabetes mellitus (T2DM) and heart failure (HF) are highly prevalent, and each is associated with a significant burden of morbidity, mortality, and costs.1, 2 T2DM and HF often occur together, and extant evidence suggests a 2‐ to 4‐fold higher risk of HF in adults with T2DM compared with those without T2DM, independently of other cardiovascular risk factors including high blood pressure (BP), hypercholesterolemia, and coronary artery disease (CAD).3, 4 Animal studies have helped to define diabetes mellitus–related cardiac dysfunction,5, 6 and suggested several pathways linking diabetes mellitus to HF, which include microvascular dysfunction. Microvascular disease (MVD) is the hallmark of diabetes mellitus, with retinopathy serving as the basis of its definition.7 MVD’s contribution to HF may be independent of CAD, especially as functional studies have shown an alteration of the myocardial microvasculature among individuals with diabetes mellitus in the absence of CAD.8, 9, 10, 11 Although a few population‐based studies have explored the link between individual microvascular complications of diabetes mellitus and HF risk in T2DM,11, 12 there is overall a paucity of epidemiological data on the relationship between MVD and incident HF in T2DM.
We conducted an analysis of the prospective data from the Look AHEAD (Action of Health in Diabetes) study to evaluate the associations of MVD), assessed in multiple vascular beds, and incident HF in a large sample of individuals with T2DM.
Methods
Study Design
The data used for the analyses are available through the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Central Repository. The Look AHEAD study was a randomized double‐blind clinical trial that enrolled 5145 participants from August 2001 to April 2004 across 16 clinical centers in the United States.13, 14 Participants were randomly assigned to participate in an intensive lifestyle intervention (intervention group) or to receive diabetes mellitus support and education (control group). Eligible participants met the following criteria at baseline: age 45 to 76 years; self‐reported diagnosis of T2DM verified through measured glucose levels, use of antidiabetic medication, or a physician’s report; body mass index of ≥25 kg/m2 (or ≥27 kg/m2 if patients were on insulin); glycosylated hemoglobin ≤11%; systolic BP <160 mm Hg; diastolic BP <100 mm Hg; triglyceride levels <600 mg/dL; the ability to complete a valid maximal exercise test, indicating that it was safe to exercise; as well as an established relationship with a primary care provider.13, 14
For the current analysis, we excluded participants with consent restrictions (n=244), history of HF or atherosclerotic cardiovascular disease, defined as history of prior myocardial infarction or stroke at baseline (n=691), and those with missing data on nephropathy, retinopathy, and/or neuropathy (n=115). After these exclusions, 4095 participants were included in our analyses.
The research protocol was approved by the institutional review board at each participating center, and each participant gave an informed consent.
Assessment of Microvascular Disease
Urine albumin and creatinine were measured on spot urine. Serum and urine creatinine were assayed by the Jaffa rate method on the Hitachi 917 autoanalyzer.14 The estimated glomerular filtration rate calculated by the Chronic Kidney Disease Epidemiology Collaboration equation.15 Nephropathy was defined as urine albumin–creatinine ratio ≥0.03 and/or estimated glomerular filtration rate <60 mL/min per 1.73 m2.
The presence of neuropathy was assessed using the Michigan Neuropathy Screening Instrument (MNSI) questionnaire administered at baseline.16 The MNSI questionnaire consists of 15 questions, 13 of which have an affirmative response scored as 1 point, and 2 of which have a negative response scored as 1 point, giving a possible maximal score of 15 points. Neuropathy was defined on the basis of a MNSI score ≥4, because this cutoff has been shown to have a good performance at diagnosing peripheral neuropathy.16
The presence of retinopathy was based on a self‐report of a doctor diagnosis, using the question: “Have you ever been told that diabetes mellitus has affected the back of your eye, that is, the retina? (Do not include treatment for cataracts or glaucoma).”
We defined MVD as the presence of at least one of the following: nephropathy, retinopathy, neuropathy.
Ascertainment of Incident Heart Failure Events
Participants were followed from baseline through annual visits and semiannual telephone calls. HF events were classified by an Events Adjudication Committee that reviewed all relevant medical records and death certificates to confirm HF events.13, 14, 17 Each case was classified into one of the following groups: definite or possible acute decompensated HF, chronic stable HF, HF unlikely, or unclassifiable. Incident HF events were defined as the first hospitalization for definite or possible acute decompensated HF.18
Assessment of Covariates
Data on covariates including age, sex, race/ethnicity, duration of diabetes mellitus, history of cardiovascular disease, medication use, current smoking, and alcohol use were obtained from each participant at baseline using standardized questionnaires.13, 14, 17 BP and anthropometric measures were obtained by trained staff using standardized methods.13, 14, 17 Fasting plasma glucose was assayed using the glucokinase method. Glycosylated hemoglobin was measured by a dedicated ion exchange high‐performance liquid chromatography instrument (Variant II; Bio‐Rad Laboratories).13, 14, 17 Total cholesterol and triglyceride were measured enzymatically using methods standardized to the Centers for Disease Control and Prevention reference methods.14, 19 High‐density lipoprotein cholesterol was measured by the treatment of whole plasma with dextran sulfate‐Mg2+ to precipitate all of the apolipoprotein B–containing lipoproteins.20 Low‐density lipoprotein cholesterol concentrations were calculated using the Friedewald equation.21
Statistical Analysis
We compared the baseline characteristics of participants by incident HF status using the t test, Kruskal‐Wallis test, or the χ2 test, as appropriate. The time‐to‐event distributions for incident HF by MVD status were assessed using the Kaplan‐Meier curve and compared using the log‐rank test. Incidence rates per 1000 person‐years were calculated by dividing the cumulative number of events by all at‐risk person‐years during follow‐up. The person‐years were estimated from the baseline evaluation to the date of incident HF event, date of death, or September 14, 2012 (the trial’s termination date), whichever occurred first. We used Cox proportional hazards models to generate hazard ratios (HRs) and 95% CIs relating MVD to the outcome. We evaluated the proportional hazards assumption using formal testing based on Schoenfeld residuals.22 Similar analyses were performed relating the outcome to each individual type of MVD (nephropathy, retinopathy, and neuropathy). We also performed stratified analyses by race/ethnicity. We further explored the effect of neuropathy by evaluating the association of the MNSI score modeled as a continuous variable with incident HF.
We constructed regression models in a hierarchical fashion. Model 1 adjusted for age, sex, race/ethnicity, randomization arm. Model 2 included variables in Model 1 plus current smoking, alcohol drinking, body mass index, systolic BP, use of antihypertensive medications, ratio of total to high‐density lipoprotein cholesterol, glycosylated hemoglobin, and duration of diabetes mellitus. Model 3 included variables in Model 2 plus interval development of CAD during follow‐up.
A 2‐sided P value of <0.05 was considered statistically significant for all analyses. All analyses were performed using Stata 14.2 (StataCorp, College Station, TX).
Results
Baseline Characteristics
Table 1 and Table S1 display the baseline characteristics of participants. The study sample consisted of 4095 participants (mean age, 58.3 years [SD, 6.6 years]; 61.9% women). Of the entire sample, 34.8% of participants had MVD (n=1424), 18.2% had nephropathy (n=745), 6.9% had retinopathy (n=284), and 16.6% had neuropathy (n=681). The participants with MVD were older, more frequently Hispanic, and they had higher body mass index, waist circumference, triglycerides, systolic BP, glycosylated hemoglobin, duration of diabetes mellitus, albumin–creatinine ratio, and MSNI score. They also had a lower estimated glomerular filtration rate, and were more likely to use antihypertensive medications and insulin (Table 1).
Table 1.
Characteristics of Participants by Microvascular Disease Status at Baseline in the Look AHEAD Study
| Entire Sample | No Microvascular Disease | Microvascular Disease | P Value | |
|---|---|---|---|---|
| No. | 4095 | 2671 | 1424 | … |
| Age, y | 58.3 (6.6) | 57.9 (6.5) | 59.0 (6.8) | <0.001 |
| Women, % | 62.0 | 62.4 | 61.2 | 0.446 |
| Race/ethnicity, % | 0.021 | |||
| White | 64.7 | 64.7 | 64.5 | |
| Non‐Hispanic Black | 17.1 | 18.1 | 15.2 | |
| Hispanic | 14.8 | 13.8 | 16.5 | |
| Other race/ethnicity | 3.5 | 3.3 | 3.8 | |
| Treatment assignment, % | 0.386 | |||
| Diabetes mellitus support and education | 50.2 | 49.7 | 51.1 | |
| Intensive lifestyle intervention | 49.8 | 50.3 | 48.9 | |
| Body mass index, kg/m2 | 36.0 (5.9) | 35.8 (5.9) | 36.5 (6.0) | <0.001 |
| Waist circumference, cm | 113.6 (14.1) | 112.8 (13.9) | 115.6 (14.2) | <0.001 |
| Current smoking, % | 4.0 | 3.6 | 4.8 | 0.065 |
| Alcohol drinking, % | 32.7 | 33.1 | 32.0 | 0.472 |
| Systolic blood pressure, mm Hg | 129.0 (16.9) | 127.8 (16.2) | 131.3 (17.8) | <0.001 |
| Diastolic blood pressure, mm Hg | 70.4 (9.5) | 70.5 (9.4) | 70.3 (9.6) | 0.554 |
| Hypertension, % | 85.6 | 82.8 | 90.8 | <0.001 |
| Use of antihypertensive medication, % | 70.9 | 66.3 | 79.6 | <0.001 |
| Glycosylated hemoglobin, % | 7.2 (1.2) | 7.2 (1.1) | 7.4 (1.2) | <0.001 |
| Duration of diabetes mellitus, y | 5.0 (2.0–9.0) | 4.0 (2.0–8.0) | 6.0 (3.0–11.0) | <0.001 |
| Use of insulin, % | 13.8 | 10.4 | 20.4 | <0.001 |
| Total cholesterol, mg/dL | 193.1 (36.8) | 192.8 (35.9) | 193.7 (38.3) | 0.480 |
| HDL‐cholesterol, mg/dL | 43.9 (11.9) | 44.2 (11.9) | 43.3 (12.0) | 0.028 |
| LDL‐cholesterol, mg/dL | 114.4 (31.9) | 115.2 (31.4) | 112.9 (32.9) | 0.030 |
| Total/HDL‐cholesterol ratio | 4.7 (1.5) | 4.6 (1.5) | 4.8 (1.6) | 0.005 |
| Triglycerides, mg/dL | 152 (107–218) | 146 (103–207) | 164 (113–237) | <0.001 |
| Albumin–creatinine ratio | 0.008 (0.005–0.017) | 0.007 (0.005–0.011) | 0.018 (0.007–0.057) | <0.001 |
| eGFR, mL/min per 1.73 m2 | 91.1 (15.8) | 92.7 (13.9) | 88.1 (18.5) | <0.001 |
| MNSI score | 1 (0–3) | 1 (0–2) | 3 (1–5) | <0.001 |
Data are presented as mean (standard deviation), median (interquartile range), or proportion (%) as appropriate.
AHEAD indicates Action for Health in Diabetes; eGFR, estimated glomerular filtration rate; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; and MNSI, Michigan Neuropathy Screening Instrument.
Incident Heart Failure by Microvascular Disease Status
During a median follow‐up of 9.7 years (interquartile range, 8.9–10.3 years), 117 participants experienced a HF event (incidence rate, 3.1; 95% CI, 2.6–3.7; over person‐years). In unadjusted comparisons, participants with MVD had higher cumulative risks of developing HF compared with those without MVD (Table 2 and Figure, P value log rank <0.001).
Table 2.
Rates and Hazard Ratios for Incident Heart Failure by Microvascular Disease Status at Baseline in the Look AHEAD Study
| Microvascular Disease | P Value | ||
|---|---|---|---|
| Absent | Present | ||
| No events/no at risk | 44/2671 | 73/1424 | … |
| Person‐years | 24 765.1 | 12 795.6 | … |
| IR (95% CI) per 1000 person‐years | 1.8 (1.3–2.4) | 5.7 (4.5–7.2) | … |
| Model, hazard ratio (95% CI) | |||
| Model 1 | Reference | 3.02 (2.08–4.40) | <0.001 |
| Model 2 | Reference | 2.54 (1.73–3.75) | <0.001 |
| Model 3 | Reference | 2.42 (1.64–3.57) | <0.001 |
Model 1 is adjusted for age, sex, race/ethnicity, and randomization arm. Model 2 includes variables in Model 1 with further adjustment for body mass index, current smoking (yes/no), alcohol drinking (ounces per week), systolic blood pressure, use of antihypertensive medications (yes/no), ratio of total to high‐density lipoprotein cholesterol, glycosylated hemoglobin, and duration of diabetes mellitus. Model 3 includes variables in Model 2 with additional adjustment for interval development of coronary artery disease (as a time‐dependent covariate). AHEAD indicates Action for Health in Diabetes; and IR, incidence rate.
Figure. 1. Cumulative hazards of incident heart failure (HF) by evidence of microvascular disease (MVD) (A), nephropathy (B), retinopathy (C), and neuropathy (D) in the Look AHEAD (Action of Health in Diabetes) study.
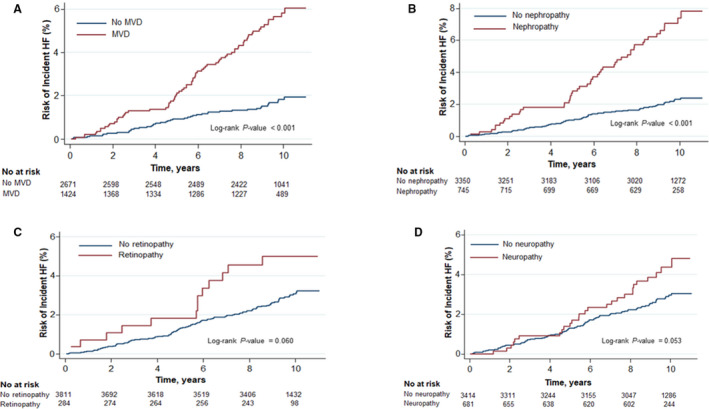
After controlling for age, sex, race/ethnicity, treatment arm, body mass index, current smoking, alcohol consumption, use of antihypertensive medication, systolic BP, ratio of total to high‐density lipoprotein cholesterol, glycosylated hemoglobin, and duration of diabetes mellitus, MVD was associated with increased risk of incident HF (HR, 2.54; 95% CI, 1.73–3.75; P<0.001). Additional adjustment for interval development of CAD did not affect the magnitude and significance of the association significant (HR, 2.42; 95% CI, 1.64–3.57; P<0.001).
Incident Heart Failure by Individual Type of Microvascular Disease
We assessed the risks of HF by type of MVD. For each type of MVD assessed individually, the cumulative risk of developing HF was higher among those with the specific MVD compared with those without it (Figure).
After adjusting for the relevant confounders (Table 3), nephropathy was associated with increased risk of incident HF (HR, 2.21; 95% CI, 1.50–3.26). This association remained significant after adjusting for interval CAD at follow‐up (HR, 2.22; 95% CI, 1.51–3.27).
Table 3.
Rates and Hazard Ratios for Incident Heart Failure by Individual Type of Microvascular Disease at Baseline in the Look AHEAD Study
| Nephropathy | Retinopathy | Neuropathy | ||||
|---|---|---|---|---|---|---|
| Absent | Present | Absent | Present | Absent | Present | |
| No events/no at risk | 69/3350 | 48/745 | 104/3811 | 13/284 | 90/3414 | 27/681 |
| Person‐years | 30905.9 | 6654.9 | 35012.8 | 2547.9 | 31 376.0 | 6184.7 |
| IR (95% CI) per 1000 person‐years | 2.2 (1.8–2.8) | 7.2 (5.4–9.6) | 3.0 (2.5–3.6) | 5.1 (3.0–8.8) | 2.9 (2.3–3.5) | 4.4 (3.0–6.4) |
| Model, hazard ratio (95% CI) | ||||||
| Model 1 | Reference | 2.88 (1.99–4.19)† | Reference | 1.59 (0.89–2.83) | Reference | 1.57 (1.02–2.41)* |
| Model 2 | Reference | 2.21 (1.50–3.26)† | Reference | 1.34 (0.74–2.44) | Reference | 1.46 (0.94–2.25) |
| Model 3 | Reference | 2.22 (1.51–3.27)† | Reference | 1.30 (0.72–2.36) | Reference | 1.33 (0.86–2.07) |
Model 1 adjusted for age, sex, race/ethnicity, and randomization arm. Model 2 includes variables in Model 1 with further adjustment for body mass index, current smoking (yes/no), alcohol drinking (ounces per week), systolic blood pressure, use of antihypertensive medications (yes/no), ratio of total to high‐density lipoprotein cholesterol, glycosylated hemoglobin, and duration of diabetes mellitus. Model 3 includes variables in Model 2 with additional adjustment for interval development of coronary artery disease (as a time‐dependent covariate). AHEAD indicates Action for Health in Diabetes; and IR, incidence rate.
P<0.05.
P<0.001.
The adjusted HR for incident HF associated with the presence of retinopathy was 1.34 (95% CI, 0.74–2.44); the HR was 1.30 (95% CI, 0.72–2.36) after additionally accounting for interval CAD (Table 3).
The adjusted HR for incident HF related to neuropathy was 1.57 (95% CI, 1.02–2.41) in the minimally adjusted model. When evaluated on a continuous scale using the MNSI score (Table 4), the HR for incident HF per 1–standard deviation increment in the MNSI score was 1.23 (95% CI, 1.04–1.44). Upon accounting for interval CAD during follow‐up, the HR for HF was 1.13 (95% CI, 0.96–1.34; Table 4).
Table 4.
Hazard Ratios for Incident Heart Failure According to the Michigan Neuropathy Screening Instrument Score in the Look AHEAD Study
| Hazard Ratio (95% CI) | P Value | |
|---|---|---|
| Model 1 | 1.23 (1.04–1.44) | 0.014 |
| Model 2 | 1.17 (1.00–1.39) | 0.058 |
| Model 3 | 1.13 (0.96–1.34) | 0.154 |
Hazard ratios are reported per 1–standard deviation increment in the Michigan Neuropathy Screening Instrument Score.
Model 1 adjusted for age, sex, race/ethnicity, and randomization arm. Model 2 includes variables in Model 1 with further adjustment for body mass index, current smoking (yes/no), alcohol drinking (ounces per week), systolic blood pressure, use of antihypertensive medications (yes/no), ratio of total to high‐density lipoprotein cholesterol, glycosylated hemoglobin, and duration of diabetes mellitus. Model 3 includes variables in Model 2 with additional adjustment for interval development of coronary artery disease (as a time‐dependent covariate).
AHEAD indicates Action for Health in Diabetes.
In the analyses stratified by race/ethnicity, among White participants, the presence of MVD, nephropathy, and neuropathy were each associated with higher risks of incident HF; whereas among non‐White participants, only MVD and nephropathy were associated with incident HF in the maximally adjusted model (Table S2).
Sensitivity Analysis
We tested the robustness of our results by performing additional adjustment for use of insulin. This did not affect the magnitude or significance of our results (Table 5).
Table 5.
Hazard Ratios for Incident Heart Failure by Individual Type of Microvascular Disease at Baseline in the Look AHEAD Study
| Nephropathy | Retinopathy | Neuropathy | Microvascular disease | |||||
|---|---|---|---|---|---|---|---|---|
| Absent | Present | Absent | Present | Absent | Present | Absent | Present | |
| Hazard ratio (95% CI) | Reference | 2.21 (1.50–3.26)* | Reference | 1.21 (0.67–2.21) | Reference | 1.30 (0.84–2.01) | Reference | 2.36 (1.59–3.48)* |
Hazard ratio was obtained from multivariable Cox model adjusted for age, sex, race/ethnicity, randomization arm, body mass index, current smoking, alcohol drinking (ounces per week), systolic blood pressure, use of antihypertensive medications (yes/no), ratio of total to high‐density lipoprotein cholesterol, glycosylated hemoglobin, duration of diabetes mellitus, interval development of coronary artery disease (as a time‐dependent covariate), and use of insulin.
AHEAD indicates Action of Health in Diabetes.
P<0.001.
Discussion
This study comprehensively evaluated the association of MVD, assessed in multiple vascular beds, with incident HF in a large sample of individuals with T2DM. We found that overall MVD in each microvascular territory was associated with an increased risk of incident HF, after accounting for the degree of glycemic control, duration of diabetes mellitus, BP, and CAD. The association of MVD and HF was mainly driven by nephropathy, although neuropathy was associated with incident HF among White participants. Our findings point to the importance of accounting for MVD in the assessment of HF risk within the framework of T2DM.
Our study is one of a few to comprehensively assess the effect of MVD in several territories on HF incidence in individuals with T2DM. Previous studies that assessed the influence of MVD on HF occurrence were limited in several ways, because these included the assessment of only one microvascular bed,23, 24, 25, 26, 27 were not focused on people with T2DM,24, 28 or included samples of mainly White individuals.29, 30, 31 Our results are consistent with prior reports from the general population describing an increased HF risk among individuals with nephropathy24, 25, 26, 27 or retinopathy.28 Likewise, our findings corroborate prior studies of people with T2DM, although limited in number, which showed an increased risk of HF associated with retinopathy in a 1021 sample of US adults.23 Similarly, our results are in agreement with prior reports that found that MVD increased the risk for HF hospitalizations in people with T2DM, although these studies were limited by their inclusion of a majority of White29, 30, 31, 32 or Asian individuals.33
Several mechanisms could explain the positive association between MVD and incident HF in people with T2DM. A possible mechanism is related to structural myocardial microvascular changes among individuals with diabetes mellitus.11, 34 High glucose exposure leads to the formation of advanced glycation end‐product from cross‐linked collagen molecules that may deposit in arteriolar walls and endothelial cells.34, 35 Deposition of these products in arterioles have been linked to microvascular remodeling, capillary basement membrane thickening, and microaneurysms formation with ensuing alterations in nitric oxide production.34 Endothelial damage and reduced nitric oxide availability results in endothelial dysfunction, which leads to a lower coronary blood flow reserve and cardiac hypertrophy–diastolic failure.34, 36 Other mechanisms include the activation of protein kinase C (a family of serine/threonine‐related protein kinases) pathways in various tissues, which has been specifically shown to be a driving factor in diabetes mellitus nephropathy or retinopathy, for example.37 Additional mechanisms, include the production of toxic metabolites, such as the advanced glycation end products and redox products, as well as the alteration of osmols and redox potential through activation of the polyol pathway.37 Another potential mechanism might be linked to cardiac autonomic neuropathy. In healthy individuals, sympathetic stimulation results in vasodilation of coronary vessels, which improves left ventricle systole and diastole.34, 35 Cardiac autonomic neuropathy in diabetes mellitus leads to sympathetic denervation, exhaustion of myocardial catecholamine, and impairment in cardiac sympathetic nerve fibers. These processes have been shown to increase the rates of both systolic and diastolic HF.34, 35, 38 Peripheral neuropathy served as a proxy of cardiac autonomic neuropathy in our study, as it tends to track with peripheral neuropathy among individuals with T2DM.39
Our findings of the predominance of the effect of nephropathy in the occurrence of HF could have many explanations. On one hand, chronic kidney disease may be the sign of significantly advanced disease with a higher prevalence of traditional risk factors than subjects with diabetes mellitus with chronic kidney disease (and thus associated with a higher burden of cardiovascular disease) compared with those with other microvascular complications. Chronic kidney disease may reflect generalized endothelial dysfunction and increased vascular permeability or abnormalities in the coagulation and fibrinolytic systems, or may denote the greater severity of end‐organ damage.40 On the other hand, our assessment of other types of MVD was probably less precise than that of chronic kidney disease.
The implications of our findings are manifold for people with T2DM. Our observations suggest the potential relevance of diabetes mellitus–related microvascular complications in the pathogenesis of diabetes mellitus–related cardiac dysfunction. Our findings point to the need to further examine the additive predictive value of MVD in HF risk stratification among individuals with diabetes mellitus. Prior data on cardiovascular disease risk estimation in diabetes mellitus indicate that the inclusion of retinopathy and/or neuropathy improves outcome discrimination.41, 42 Such an exploration of the incremental predictive value of MVD remains to be conducted specifically for the risk of HF.
The limitations of our study should be acknowledged. First, diabetic retinopathy was assessed by self‐report of doctor diagnosis; thus, we might have underestimated the extent of the frequency of retinopathy in our sample, which may have led to an underestimation of the true association between retinopathy and HF.43 Second, we did not assess the microvasculature in the coronary bed using cardiac positron emission tomography, for example, as was done by Taqueti et al in a small and short study (including only 201 participants observed over 4.1 years) that did not specifically focus on individuals with diabetes mellitus.44 Such an approach would provide a more direct form of evidence on the impact of myocardial microvascular dysfunction on cardiac remodeling. Third, we did not assess the subtypes of HF, which may be differentially associated with MVD.33 Our study has several strengths. First, we used a prospective design and a large multiethnic/racial sample of participants. Second, we assessed MVD in multiple vascular territories in contrast to previous studies.23, 24, 25, 26, 27, 28 Third, the presence of neuropathy was assessed using the MNSI, a standardized instrument that has been previously shown to have good performance at detecting peripheral neuropathy in diabetes mellitus.16 Finally, the adjudication of HF events was standardized, and we conducted robust adjustments for relevant cofounders.
In conclusion, in a large sample of adults with T2DM, we observed that MVD, assessed in multiple vascular beds, is associated with higher risk of incident HF after adjusting of traditional risk factors such as BP or coronary heart disease. Our findings support the notion that microangiopathy is involved in the pathogenesis of HF in T2DM.
Sources of Funding
Dr Echouffo‐Tcheugui was supported by National Institutes of Health/National Heart, Lung, and Blood Institute (NIH/NHLBI) grant K23 HL153774.
Disclosures
None.
Supporting information
Tables S1–S2
Acknowledgments
The authors wish to thank the staff and participants of the Look AHEAD study for their valuable contributions. Look AHEAD was conducted by the Look AHEAD Research Group and supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK); the National Heart, Lung, and Blood Institute (NHLBI); the National Institute of Nursing Research (NINR); the National Institute of Minority Health and Health Disparities (NIMHD); the Office of Research on Women’s Health (ORWH); and the Centers for Disease Control and Prevention (CDC). The data from Look AHEAD were supplied by the NIDDK Central Repository. This manuscript was not prepared under the auspices of the Look AHEAD and does not represent analyses or conclusions of the Look AHEAD Research Group, the NIDDK Central Repositories, NIDDK or the NIH.
(J Am Heart Assoc. 2021;10:e018998. DOI: 10.1161/JAHA.120.018998.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.018998.
For Sources of Funding and Disclosures, see page 7.
References
- 1.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, et al. Heart disease and stroke statistics‐2020 update: a report from the American Heart Association. Circulation. 2020;141:e139–e596. 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 2.Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988–2012. JAMA. 2015;314:1021–1029. DOI: 10.1001/jama.2015.10029. [DOI] [PubMed] [Google Scholar]
- 3.Ohkuma T, Komorita Y, Peters SAE, Woodward M. Diabetes as a risk factor for heart failure in women and men: a systematic review and meta‐analysis of 47 cohorts including 12 million individuals. Diabetologia. 2019;62:1550–1560. DOI: 10.1007/s00125-019-4926-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aune D, Schlesinger S, Neuenschwander M, Feng T, Janszky I, Norat T, Riboli E. Diabetes mellitus, blood glucose and the risk of heart failure: a systematic review and meta‐analysis of prospective studies. Nutr Metab Cardiovasc Dis. 2018;28:1081–1091. DOI: 10.1016/j.numecd.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 5.Bugger H, Abel ED. Molecular mechanisms of diabetic cardiomyopathy. Diabetologia. 2014;57:660–671. DOI: 10.1007/s00125-014-3171-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bugger H, Abel ED. Rodent models of diabetic cardiomyopathy. Dis Model Mech. 2009;2:454–466. DOI: 10.1242/dmm.001941. [DOI] [PubMed] [Google Scholar]
- 7.McCance DR, Hanson RL, Charles MA, Jacobsson LT, Pettitt DJ, Bennett PH, Knowler WC. Comparison of tests for glycated haemoglobin and fasting and two hour plasma glucose concentrations as diagnostic methods for diabetes. BMJ. 1994;308:1323–1328. DOI: 10.1136/bmj.308.6940.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yokoyama I, Momomura S, Ohtake T, Yonekura K, Nishikawa J, Sasaki Y, Omata M. Reduced myocardial flow reserve in non‐insulin‐dependent diabetes mellitus. J Am Coll Cardiol. 1997;30:1472–1477. DOI: 10.1016/S0735-1097(97)00327-6. [DOI] [PubMed] [Google Scholar]
- 9.Dunlay SM, Givertz MM, Aguilar D, Allen LA, Chan M, Desai AS, Deswal A, Dickson VV, Kosiborod MN, Lekavich CL, et al. Type 2 diabetes mellitus and heart failure: a scientific statement from the American Heart Association and the Heart Failure Society of America: this statement does not represent an update of the 2017 ACC/AHA/HFSA heart failure guideline update. Circulation. 2019;140:e294–e324. DOI: 10.1161/CIR.0000000000000691. [DOI] [PubMed] [Google Scholar]
- 10.Kenny HC, Abel ED. Heart failure in type 2 diabetes mellitus. Circ Res. 2019;124:121–141. DOI: 10.1161/CIRCRESAHA.118.311371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laakso M. Heart in diabetes: a microvascular disease. Diabetes Care. 2011;34(Suppl 2):S145–S149. DOI: 10.2337/dc11-s209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheung N, Bluemke DA, Klein R, Sharrett AR, Islam FMA, Cotch MF, Klein BEK, Criqui MH, Wong TY. Retinal arteriolar narrowing and left ventricular remodeling: the multi‐ethnic study of atherosclerosis. J Am Coll Cardiol. 2007;50:48–55. DOI: 10.1016/j.jacc.2007.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ryan DH, Espeland MA, Foster GD, Haffner SM, Hubbard VS, Johnson KC, Kahn SE, Knowler WC, Yanovski SZ, Group LAR. Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials. 2003;24:610–628. DOI: 10.1016/S0197-2456(03)00064-3. [DOI] [PubMed] [Google Scholar]
- 14.Group LAR, Bray G, Gregg E, Haffner S, Pi‐Sunyer XF, WagenKnecht LE, Walkup M, Wing R. Baseline characteristics of the randomised cohort from the Look AHEAD (Action for Health in Diabetes) study. Diabetes Vasc Dis Res. 2006;3:202–215. DOI: 10.3132/dvdr.2006.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levey AS, Stevens LA, Schmid CH, Zhang Y(, Castro AF, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. DOI: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herman WH, Pop‐Busui R, Braffett BH, Martin CL, Cleary PA, Albers JW, Feldman EL. Use of the Michigan Neuropathy Screening Instrument as a measure of distal symmetrical peripheral neuropathy in Type 1 diabetes: results from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications. Diabet Med. 2012;29:937–944. 10.1111/j.1464-5491.2012.03644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Group LAR, Wing RR, Bolin P, Brancati FL, Bray GA, Clark JM, Coday M, Crow RS, Curtis JM, Egan CM, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369:145–154. 10.1056/NEJMoa1212914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pandey A, Patel KV, Bahnson JL, Gaussoin SA, Martin CK, Balasubramanyam A, Johnson KC, McGuire DK, Bertoni AG, Kitzman D, et al. Association of Intensive lifestyle intervention, fitness and body mass index with risk of heart failure in overweight or obese adults with type 2 diabetes mellitus: an analysis from the look AHEAD trial. Circulation. 2020;141:1295–1306. DOI: 10.1161/CIRCULATIONAHA.119.044865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Warnick GR. Enzymatic methods for quantification of lipoprotein lipids. Methods Enzymol. 1986;129:101–123. [DOI] [PubMed] [Google Scholar]
- 20.Warnick GR, Benderson J, Albers JJ. Dextran sulfate‐Mg2+ precipitation procedure for quantitation of high‐density‐lipoprotein cholesterol. Clin Chem. 1982;28:1379–1388. DOI: 10.1093/clinchem/28.6.1379. [DOI] [PubMed] [Google Scholar]
- 21.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low‐density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. DOI: 10.1093/clinchem/18.6.499. [DOI] [PubMed] [Google Scholar]
- 22.Schoenfeld D. Partial residuals for the proportional hazards regression model. Biometrika. 1982;69:239–241. DOI: 10.1093/biomet/69.1.239. [DOI] [Google Scholar]
- 23.Cheung N, Wang JJ, Rogers SL, Brancati F, Klein R, Sharrett AR, Wong TY. Diabetic retinopathy and risk of heart failure. J Am Coll Cardiol. 2008;51:1573–1578. DOI: 10.1016/j.jacc.2007.11.076. [DOI] [PubMed] [Google Scholar]
- 24.Dhingra R, Gaziano JM, Djoussé L. Chronic kidney disease and the risk of heart failure in men. Circ Heart Fail. 2011;4:138–144. DOI: 10.1161/CIRCHEARTFAILURE.109.899070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu C. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. DOI: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 26.Chae CU, Albert CM, Glynn RJ, Guralnik JM, Curhan GC. Mild renal insufficiency and risk of congestive heart failure in men and women > or =70 years of age. Am J Cardiol. 2003;92:682–686. 10.1016/s0002-9149(03)00822-1. [DOI] [PubMed] [Google Scholar]
- 27.Gottdiener JS, Arnold AM, Aurigemma GP, Polak JF, Tracy RP, Kitzman DW, Gardin JM, Rutledge JE, Boineau RC. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol. 2000;35:1628–1637. DOI: 10.1016/S0735-1097(00)00582-9. [DOI] [PubMed] [Google Scholar]
- 28.Wong TY, Rosamond W, Chang PP, Couper DJ, Sharrett AR, Hubbard LD, Folsom AR, Klein R. Retinopathy and risk of congestive heart failure. JAMA. 2005;293:63–69. DOI: 10.1001/jama.293.1.63. [DOI] [PubMed] [Google Scholar]
- 29.Brownrigg JRW, Hughes CO, Burleigh D, Karthikesalingam A, Patterson BO, Holt PJ, Thompson MM, de Lusignan S, Ray KK, Hinchliffe RJ. Microvascular disease and risk of cardiovascular events among individuals with type 2 diabetes: a population‐level cohort study. Lancet Diabetes Endocrinol. 2016;4:588–597. DOI: 10.1016/S2213-8587(16)30057-2. [DOI] [PubMed] [Google Scholar]
- 30.Verma S, Wanner C, Zwiener I, Ofstad AP, George JT, Fitchett D, Zinman B. Influence of microvascular disease on cardiovascular events in type 2 diabetes. J Am Coll Cardiol. 2019;73:2780–2782. 10.1016/j.jacc.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 31.Mordi IR, Tee A, Palmer CN, McCrimmon RJ, Doney ASF, Lang CC. Microvascular disease and heart failure with reduced and preserved ejection fraction in type 2 diabetes. ESC Hear Fail. 2020;7:1168–1177. DOI: 10.1002/ehf2.12669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seferovic JP, Bentley‐Lewis R, Claggett B, Diaz R, Gerstein HC, Køber LV, Lawson FC, Lewis EF, Maggioni AP, McMurray JJV, et al. Retinopathy, neuropathy, and subsequent cardiovascular events in patients with type 2 diabetes and acute coronary syndrome in the ELIXA: the importance of disease duration. J Diabetes Res. 2018;2018:1631263. DOI: 10.1155/2018/1631263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tromp J, Lim SL, Tay WT, Teng T‐H, Chandramouli C, Ouwerkerk W, Wander GS, Sawhney JPS, Yap J, MacDonald MR, et al. Microvascular disease in patients with diabetes with heart failure and reduced ejection versus preserved ejection fraction. Diabetes Care. 2019;42:1792–1799. DOI: 10.2337/dc18-2515. [DOI] [PubMed] [Google Scholar]
- 34.Low Wang CC, Hess CN, Hiatt WR, Goldfine AB. Clinical update: cardiovascular disease in diabetes mellitus: atherosclerotic cardiovascular disease and heart failure in Type 2 diabetes mellitus ‐ mechanisms, management, and clinical considerations. Circulation. 2016;133:2459–2502. DOI: 10.1161/CIRCULATIONAHA.116.022194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Falcão‐Pires I, Leite‐Moreira AF. Diabetic cardiomyopathy: understanding the molecular and cellular basis to progress in diagnosis and treatment. Heart Fail Rev. 2012;17:325–344. DOI: 10.1007/s10741-011-9257-z. [DOI] [PubMed] [Google Scholar]
- 36.Seddon M, Looi YH, Shah AM. Oxidative stress and redox signalling in cardiac hypertrophy and heart failure. Heart. 2007;93:903–907. DOI: 10.1136/hrt.2005.068270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barrett EJ, Liu Z, Khamaisi M, King GL, Klein R, Klein BEK, Hughes TM, Craft S, Freedman BI, Bowden DW, et al. Diabetic microvascular disease: an Endocrine Society scientific statement. J Clin Endocrinol Metab. 2017;102:4343–4410. DOI: 10.1210/jc.2017-01922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Givertz MM, Sawyer DB, Colucci WS. Antioxidants and myocardial contractility: illuminating the “Dark Side” of beta‐adrenergic receptor activation? Circulation. 2001;103:782–783. DOI: 10.1161/01.CIR.103.6.782. [DOI] [PubMed] [Google Scholar]
- 39.Tentolouris N, Pagoni S, Tzonou A, Katsilambros N. Peripheral neuropathy does not invariably coexist with autonomic neuropathy in diabetes mellitus. Eur J Intern Med. 2001;12:20–27. DOI: 10.1016/S0953-6205(00)00128-X. [DOI] [PubMed] [Google Scholar]
- 40.Stehouwer CD, Lambert J, Donker AJ, van Hinsbergh VW. Endothelial dysfunction and pathogenesis of diabetic angiopathy. Cardiovasc Res. 1997;34:55–68. DOI: 10.1016/S0008-6363(96)00272-6. [DOI] [PubMed] [Google Scholar]
- 41.Kengne AP, Patel A, Marre M, Travert F, Lievre M, Zoungas S, Chalmers J, Colagiuri S, Grobbee DE, Hamet P, et al. Contemporary model for cardiovascular risk prediction in people with type 2 diabetes. Eur J Cardiovasc Prev Rehabil. 2011;18:393–398. DOI: 10.1177/1741826710394270. [DOI] [PubMed] [Google Scholar]
- 42.Woodward M, Hirakawa Y, Kengne A‐P, Matthews DR, Zoungas S, Patel A, Poulter N, Grobbee R, Cooper M, Jardine M, et al. Prediction of 10‐year vascular risk in patients with diabetes: the AD‐ON risk score. Diabetes Obes Metab. 2016;18:289–294. DOI: 10.1111/dom.12614. [DOI] [PubMed] [Google Scholar]
- 43.Jurek AM, Greenland S, Maldonado G, Church TR. Proper interpretation of non‐differential misclassification effects: expectations vs observations. Int J Epidemiol. 2005;34:680–687. DOI: 10.1093/ije/dyi060. [DOI] [PubMed] [Google Scholar]
- 44.Taqueti VR, Solomon SD, Shah AM, Desai AS, Groarke JD, Osborne MT, Hainer J, Bibbo CF, Dorbala S, Blankstein R, et al. Coronary microvascular dysfunction and future risk of heart failure with preserved ejection fraction. Eur Heart J. 2018;39:840–849. DOI: 10.1093/eurheartj/ehx721. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S2


