Abstract
Context
Hyperglycemic emergencies such as diabetic ketoacidosis (DKA) and hyperosmolar hyperglycemic syndrome (HHS) and new-onset diabetes mellitus (DM) have been reported in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections. Hyperglycemia is a predictor of poor prognosis in COVID-19 disease.
Objectives
The objective of this work is to describe a case series of HHS and/or DKA likely triggered by the COVID-19 vaccine. The aim is to alert physicians of the potential hyperglycemic complications from the COVID-19 vaccination and to provide further insight into the underlying mechanism of the bidirectional relationship between SARS-CoV-2 and DM.
Case Descriptions
All 3 patients developed HHS and/or DKA within 2 to 10 days of the COVID-19 vaccination. PCR testing for SARS-CoV-2 was negative and other clinical precipitating factors were excluded. Two patients had a history of type 2 DM (T2DM) with pre-admission HbA1c levels of 7.0% to 7.5% while 1 patient was newly diagnosed with T2DM during the hospitalization. They were each treated with insulin infusion and were discharged on subcutaneous insulin therapy. Due to the rapid resolution of the hyperglycemia, insulin was discontinued in all patients within 8 weeks and they remain well-controlled on oral DM medications.
Conclusion
Severe hyperglycemia including HHS and DKA may be triggered by COVID-19 vaccination. Early evaluation and screening of patients with hyperglycemic symptoms after COVID-19 vaccination is recommended. The vaccine-induced hyperglycemia may provide further insight into the underlying pathogenesis caused by the SARS-CoV-2 infection itself. The underlying robust inflammatory response and “cytokine storm” may be the primary precipitant.
Keywords: diabetes, COVID-19, SARS-CoV-2, vaccine, hyperglycemia
Since the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was declared a pandemic in March 2020 by the World Health Organization, more than 205 million cases have been detected with over 4.3 million deaths globally [1]. Severe manifestations of the SARS-CoV-2 disease (COVID-19) including acute respiratory distress syndrome (ARDS), septic shock, and about 3-fold greater risk of in-hospital mortality have been associated with pre-existing diabetes mellitus (DM) [2, 3]. More pronounced alterations of inflammatory markers and poor prognosticators such as C-reactive protein (CRP), procalcitonin, D-dimer, and lymphopenia have been demonstrated in patients with pre-existing type 2 DM (T2DM) and especially in uncontrolled DM with hyperglycemia [4]. Prior studies have estimated that 16.2% of patients with severe disease and 22% of COVID-19 nonsurvivors had DM [5]. Moreover, severe hyperglycemia itself, regardless of a concurrent or a pre-existing diagnosis of DM may be an independent predictor of COVID-19 morbidity and mortality [6].
The association between COVID-19 and diabetes is bidirectional. In addition to diabetes and hyperglycemia being associated with worse outcomes in COVID-19 patients, COVID-19 itself may trigger new-onset diabetes or worsen existing diabetes. Indeed, severe hyperglycemia and acute hyperglycemic emergencies such as diabetic ketoacidosis (DKA) and/or hyperosmolar hyperglycemic syndrome (HHS) have been reported in patients presenting with COVID-19 disease, many of whom did not have a prior history of DM [6, 7]. The pathogenesis underlying the association between COVID-19 infection and hyperglycemia is not completely understood but may involve direct infection of the pancreatic islets by SARS-CoV-2 or other mechanisms relating to the inflammatory or stress responses to the virus [5, 7-9].
In this case series, we present patient cases of hyperglycemic emergencies triggered not by COVID-19 disease but likely by COVID-19 vaccination. All 3 patients experienced symptoms within 48 hours of vaccination and ultimately presented with HHS and/or DKA after the first of 2 vaccinations. Each patient tested negative, by polymerase chain reaction (PCR), for active SARS-CoV-2 infection. Within 2 months after hospital discharge, they have all been completely titrated off of insulin and required oral DM medications only. Our data suggest that immune dysregulation caused by the vaccine may worsen diabetes and supports the hypothesis that a similar mechanism underlies the association between COVID-19 infection and diabetes.
Case Presentations
Case presentations are described below and summarized in Table 1.
Table 1.
Clinical characteristics at initial presentation
| Patient 1 | Patient 2 | Patient 3 | |
|---|---|---|---|
| Age | 52 | 60 | 87 |
| Gender | Female | Male | Male |
| Ethnicity | Black | Black | Hispanic |
| Covid vaccination | First dose Pfizer | First dose Moderna | First dose Moderna |
| Hyperglycemic presentation | HHS | HHS | HHS-DKA |
| Serum blood glucose (mg/dL) | 1062 | 847 | 923 |
| pH | 7.33 | 7.39 | 7.2 |
| Beta-hydroxybutyrate (mmol/L) | <0.2 | >4 | >4 |
| Anion gap | 20 | 30 | 30 |
| Serum osmolarity (mOsm/Kg) | 381 | 345 | 364 |
| Hemoglobin A1c (%) | 12% | 13.2% | Not available |
Case 1
A 52-year-old woman with a history of hypertension presented with polyuria, polydipsia, lightheadedness, and dysgeusia of 2 days’ duration. She reported no fever, myalgias, cough, dysuria, nausea, diarrhea, decreased appetite, or chest pain. She denied prior positive testing for SARS-CoV-2 but stated that she had received the first of 2 Pfizer COVID-19 vaccination doses 3 days prior to admission. The onset of symptoms began within 48 hours after the vaccination. Upon arrival to the emergency room, her heart rate was 102 beats/min with a respiratory rate of 20/min; blood pressure was 137/88 mmHg, her oxygen saturation was 100% on room air, and the patient remained afebrile. The physical exam was notable for an alert mental status, dry oral mucosa, and body mass index (BMI) of 32.5 kg/m2. The nasopharyngeal swab combined test (by the Cepheid Xpet Xpress real-time PCR assay) for SARS-CoV-2, Influenza A and B, and the respiratory syncytial virus (RSV) was negative. The initial labs were remarkable for a blood glucose of 1062 mg/dL, sodium of 154 mEq/L [reference range (RR) 135-145], bicarbonate of 27 mEq/L, blood urea nitrogen (BUN) of 39 mg/dL, and a creatinine of 2.87 mg/dL. The serum osmolality was elevated at 381 mOsm/Kg, the anion gap was 20 mEq/L (RR, 7-16 mEq/L) with an undetectable beta-hydroxybutyrate, and the pH was 7.33. The hemoglobin A1c (HbA1c) was 12.0%. The white blood cell (WBC) count was 13.1 K/uL (RR, 4.8-10.8 K/uL) with a normal differential, the lactic acid dehydrogenase (LDH) was 230 U/L (RR, <240 U/L), and the D-dimer was 0.42 ug/mL (RR, 0.27-0.5 ug/mL). Inflammatory markers including ferritin and C-reactive protein (CRP) were elevated at 752.5 ng/mL (RR, 10-150 ng/mL) and 1.3 mg/dL (RR, <0.8 mg/dL), respectively. There were no signs of an active infection nor evidence of an acute ischemic event.
The patient was diagnosed with T2DM and nonketotic HHS without coma possibly triggered by the COVID-19 vaccination. Although family medical history was notable for T2DM in the mother, brother, and grandmother, she reported no prior history of diabetes despite regular visits to her primary care physician pre-pandemic. Previous HbA1c values, last checked in 2019, ranged 5.5% to 6.2%. Intravenous hydration and an insulin infusion were initiated. The polyuria, polydipsia, and dizziness resolved, and the patient was then transitioned to a subcutaneous basal-bolus insulin regimen. She was subsequently discharged on insulin glargine 18 units once daily, insulin lispro 12 units with each meal, and metformin 500 mg twice daily. At an outpatient 7-week follow-up visit, her HbA1c had improved significantly to 6.7% and a C-peptide level was 1.45 ng/mL, indicating recovery from the prior acute hyperglycemic emergency and insulin deficiency. Insulins glargine and lispro were discontinued and the regimen was modified to metformin 1000 mg twice daily and weekly dulaglutide 0.75 mg was prescribed. The patient has since continued her weight loss efforts and has reported controlled self-monitored blood glucoses within a range of 80 to 140 mg/dL throughout the day.
Case 2
A 59-year-old man with history of T2DM of 8 years duration, hypertension, and COVID-19 infection 10 months earlier presented with fatigue, myalgia, and subjective fevers for 2 days after receiving his first Moderna vaccination for COVID-19 2 days prior to admission. He also reported blurry vision, dry mouth, and polyuria. He denied cough, dyspnea, rhinorrhea, abdominal pain, diarrhea, nausea, or dysuria. At the time of admission and throughout the hospitalization, his vital signs were stable and the patient remained afebrile, normotensive without tachycardia, and with >96% oxygen saturation on room air. The physical exam was significant for a fully alert and oriented mental status, dry oral mucosa, clear lungs, a non-tender and non-distended abdomen, and a BMI of 27.7 kg/m2. Initial laboratory results were remarkable for a blood glucose of 847 mg/dL, a bicarbonate of 23 mEq/L, and a pH of 7.39. The sodium was 137 mEq/L, BUN was 67 mg/dL, the calculated serum osmolality was 345 mOsm/Kg, the urine ketones were mildly elevated at 15 mg/dL, and the creatinine was 3.32 mg/dL, consistent with acute kidney injury (AKI). The troponins were negative. Due to a mild leukocytosis of 16.7 k/uL (RR, 4.8-10.8 k/uL), a neutrophilia of 13 k/uL (RR, 1.8-7.7 K/uL), a normal lymphocyte count, and a chest x-ray that showed a questionable right basal density, the patient was empirically treated for a community-acquired bacterial pneumonia. The coagulation studies were within normal limits but the inflammatory markers CRP and LDH were both elevated at 2109 mg/dL and 616 U/L, respectively; other pro-inflammatory markers were not checked. There were no other signs of an infection, including a negative urinalysis and negative PCR swab tests for SARS-CoV-2, Influenza A and B, and RSV. A recent HbA1c from 8 weeks pre-admission was 7.5%, for which the patient was not taking any T2DM medications. The repeat HbA1c during hospitalization was 13.2%.
The patient was diagnosed with HHS possibly triggered by the COVID-19 vaccination. He received 3 liters of normal saline boluses and was started on a continuous insulin infusion. The infusion was later transitioned to a subcutaneous basal-bolus regimen of glargine 12 units once daily and lispro 6 units before meals. The symptoms of myalgias, fatigue, and polyuria, as well as the AKI, resolved. The patient was discharged home on a simplified regimen of glargine 12 units once daily, metformin 1000 mg twice daily, and sitagliptin 100 mg daily. The patient’s glycemic control improved at home with glucoses consistently ranging <140 mg/dL and the regimen has since been gradually downtitrated to merely metformin 1000 mg twice daily. A repeat HbA1c of 5.9% and a C-peptide of 6.02 ng/mL 2 months after discharge indicated complete resolution of the prior hyperglycemic emergency.
Case 3
An 87-year-old man with history of well-controlled T2DM, hypertension, hyperlipidemia, ischemic stroke, and congestive heart failure presented to the emergency room with weakness and altered mental status. According to the son, the patient developed fatigue and myalgias within 48 hours of having received the first Moderna vaccination against COVID-19 approximately 10 days prior, without any other atypical recent events. There were no complaints of abdominal pain, nausea, vomiting, diarrhea, cough, dyspnea, or dysuria. The patient was noted to be thirsty despite increased fluid intake; he was confused and had poor appetite. Vital signs were afebrile at 36.9 °C; the blood pressure was 111/80, heart rate was 83 beats/min, respiratory rate was 18/min, and the oxygen saturation was 100% on room air. The physical exam revealed a lethargic man, oriented only to self, with dry oral mucous membranes, absent lower extremity edema, lungs clear to auscultation, and a BMI of 26 kg/m2. Initial lab tests were remarkable for hyperglycemia, dehydration, and metabolic acidosis. The blood glucose was 923 mg/dL, the anion gap was 30 mEq/L, the bicarbonate was 12 mEq/L, the pH was 7.2, the lactic acid was 3.6 mmol/L (RR, <2.5 mmol/L), and the beta-hydroxybutyrate was severely increased to >4.0 mmol/L. The basic metabolic panel was notable for a sodium of 144 mEq/L, potassium of 6.2 mEq/L, BUN of 69 mg/dL, and a reduced estimated glomerular filtration rate (eGFR) of 18 mg/min/BSA from a baseline eGFR >50 mg/min/BSA; the calculated serum osmolality was 364 mOsm/Kg. The LDH was slightly increased to 263 U/L; other acute inflammatory markers were not checked. Head computerized tomography (CT) was negative for acute intracranial ischemia or hemorrhage. There was no evidence of an acute coronary syndrome nor were there signs of infection by urinalysis, urine culture, blood culture, or chest x-ray. Additionally, the PCR tests for SARS-CoV-2, Influenza A and B, and RSV were all negative. Interestingly, a SARS-CoV-2 IgG antibody test was positive from 6 months earlier. Upon further questioning, the patient did have a history of COVID-19 disease the prior year in 2020 and had since fully recovered. A recent HbA1c, checked 8 weeks before the current hospitalization, was 7.0% and the son confirmed that the patient was adherent with the home regimen of metformin 500 mg twice daily.
The patient was diagnosed with HHS and DKA, based on the combined severity of biochemical derangements diagnostic for profound hyperosmolality and anion-gap acidosis with significant ketosis and low bicarbonate levels [10, 11]. Without evidence of another precipitant and symptom onset within 48 hours of vaccination, the HHS and DKA were potentially triggered in the setting of COVID-19 vaccination. There was no prior history of DKA or HHS. Intravenous normal saline hydration and a continuous insulin infusion were immediately initiated and were continued until both the anion gap and serum osmolality normalized, at which point he was transitioned to a subcutaneous basal-bolus insulin regimen. The hospital course was unfortunately complicated by aspiration pneumonia and a lower extremity deep vein thrombosis for which the patient was treated with antibiotics and enoxaparin, respectively. The initial AKI improved to a GFR of 59 mg/min/BSA with return of mental status back to patient’s baseline; he was discharged home on glargine 16 units once daily and metformin 500 mg twice daily. On outpatient follow-up, the regimen has since been further simplified to only metformin.
Discussion
As of the writing of this manuscript, approximately 4.6 billion COVID-19 vaccinations have been given worldwide across 183 countries. In the United States, more than 350 million vaccine doses have been administered and about 55.3% of the population is vaccinated [12]. In the United States and other countries, the infection rates have dramatically decreased as the proportion of vaccinated individuals in the general population has increased. There is no doubt that vaccinating the majority of people around the globe is the only way to stop this pandemic which has devastated the world, infecting close to 205 million people worldwide and causing more than 4.3 million deaths [1].
All COVID-19 vaccines are remarkably safe and effective and serious side effects are extremely rare [13-15]. We describe a potential new complication of the COVID-19 vaccine, HHS and DKA. Previously, there has been only 1 case report of a hyperglycemic emergency, HHS, and another case series describing moderate exacerbation of hyperglycemia associated with the COVID-19 vaccination [16, 17]. While hyperglycemic emergencies are likely extremely rare in patients receiving COVID-19 vaccinations, it is important for clinicians to be aware of these complications and suspect severe hyperglycemia in those who develop postvaccination polyuria, polydipsia, blurry vision, weakness, and even confusion, among other symptoms.
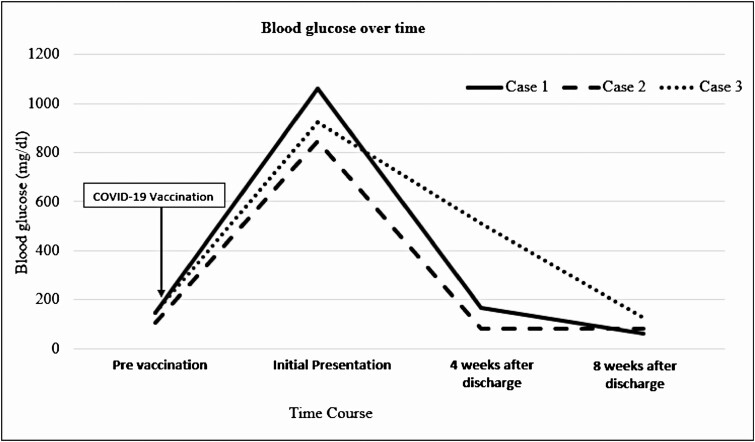
Such symptoms may be seen in patients with pre-existing DM and even in controlled DM. All 3 cases presented with acute onset of classic hyperglycemic symptoms and were diagnosed with HHS and/or DKA within 2 to 10 days of their first COVID-19 vaccination, albeit we cannot absolutely rule out that Case 3 had only HHS, without DKA, and another cause for the anion gap metabolic acidosis. Although there were moderate elevations in pro-inflammatory acute phase reactants, there were no overt biochemical or clinical signs of an active infection at presentation. Other than the recent vaccination, no other triggering events were identified. The resolution of the severe hyperglycemia was equally as rapid as its onset (Fig. 1). In fact, within 8 weeks, none of the 3 patients remained on insulin therapy. The temporal onset of symptoms within 48 hours of vaccination and the brisk, clinical course strongly suggests that the hyperglycemic emergencies were triggered by the COVID-19 vaccination.
Figure 1.
Time course depicting blood glucoses before and after COVID-19 vaccination.
As mentioned, COVID-19 disease is associated with worsening of existing DM or new-onset DM but the mechanism by which SARS-CoV-2 infection triggers hyperglycemia is not known [6, 7]. This case series may shed light on the relationship between COVID-19 infection and DM by demonstrating that the COVID-19 vaccination itself can exacerbate DM and precipitate hyperglycemia. Several hypotheses have been proposed to explain how SARS-CoV-2 may trigger new-onset or exacerbate known DM including: (1) binding of SARS-CoV-2 to pancreatic angiotensin-converting enzyme 2 (ACE2) receptors, thereby causing direct or bystander destruction of pancreatic beta cells or direct infection of pancreatic beta cells by SARS-CoV-2 [5, 7-9, 18, 19]; (2) the effects of acute stress and release of stress hormones such as cortisol and catecholamines; (3) disruption of the renin-angiotensin-aldosterone and angiotensin 1-7 balance, favoring upregulation of the pro-inflammatory angiotensin II pathway with downregulation of the vasodilatory, protective angiotensin 1-7 pathway with local, detrimental effects at the pancreas [6, 9]; or (4) immunological dysregulation triggered by the infection that leads to a systemic inflammatory response and release of cytokines, a “cytokine storm” [20, 21]. Our case series demonstrating that the COVID-19 vaccine may trigger hyperglycemia supports the fourth hypothesis, namely that DM is potentiated by the systemic immune dysregulation and inflammatory response. Since both COVID-19 vaccine and COVID-19 infection trigger a robust systemic immune-inflammatory response, it is likely that this is the common mechanism that explains both phenomena. All 3 patients promptly experienced symptoms of malaise and weakness within 48 hours of the COVID-19 vaccination. And 2 patients developed acute onset of overt hyperglycemic symptoms, including polyuria, polydipsia, and blurry vision within 48 hours. Furthermore, patient cases 2 and 3, interestingly, had prior COVID-19 infections suggesting that the vaccination may have triggered an even more robust immune and cytokine response. It is possible that specific cytokines such as IL-1, IFNγ, TNFα, and IL-6 that have been previously shown to be toxic to beta cells [22] may cause the hyperglycemia seen in patients that are infected with or vaccinated for COVID-19. Future studies will help determine the specific inflammatory mediator causing the hyperglycemia. Although cytokine-triggered hyperglycemia seems a likely mechanism, it is possible that postvaccination general malaise associated with anorexia and dehydration can contribute to the acute hyperglycemia.
In conclusion, we describe here 3 rare cases of hyperglycemic emergencies, HHS and DKA, developing after COVID-19 vaccinations. Clinicians should be aware of this potential rare complication of COVID-19 vaccinations. Future studies will hopefully pinpoint the underlying mechanisms.
Glossary
Abbreviations
- AKI
acute kidney injury
- BMI
body mass index
- BUN
blood urea nitrogen
- COVID-19
coronavirus disease 2019
- CRP
C-reactive protein
- DKA
diabetic ketoacidosis
- DM
diabetes mellitus
- eGFR
estimated glomerular filtration rate
- HbA1c
glycated hemoglobin A1c
- HHS
hyperosmolar hyperglycemic syndrome
- LDH
lactate dehydrogenase
- PCR
polymerase chain reaction
- RR
reference range
- RSV
respiratory syncytial virus
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- T2DM
type 2 diabetes mellitus
- WBC
white blood cell
Additional Information
Disclosures: Dr. Tomer declares that he has submitted 4 patent disclosures that are not related to the content of this manuscript. All other authors have no potential conflict of interest to declare.
Data Availability
Data sharing is not applicable as no datasets were generated or analyzed for this clinical case series.
References
- 1. Johns Hopkins University & Medicine. COVID-19 Dashboard - Johns Hopkins Coronavirus Resource Center. Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). Accessed August 13, 2021. https://coronavirus.jhu.edu/map.html
- 2. Mantovani A, Byrne CD, Zheng MH, Targher G. Diabetes as a risk factor for greater COVID-19 severity and in-hospital death: A meta-analysis of observational studies. Nutr Metab Cardiovasc Dis. 2020;30(8):1236-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Agarwal S, Schechter C, Southern W, Crandall JP, Tomer Y. Preadmission diabetes-specific risk factors for mortality in hospitalized patients with diabetes and coronavirus disease 2019. Diabetes Care. 2020;43(10):2339-2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhu L, She ZG, Cheng X, et al. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020;31(6):1068-1077.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liu SP, Zhang Q, Wang W, et al. Hyperglycemia is a strong predictor of poor prognosis in COVID-19. Diabetes Res Clin Pract. 2020;167:108338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eskandarani RM, Sawan S. Diabetic ketoacidosis on hospitalization with COVID-19 in a previously nondiabetic patient: a review of pathophysiology. Clin Med Insights Endocrinol Diabetes. 2020;13:1179551420984125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pal R, Banerjee M, Yadav U, Bhattacharjee S. Clinical profile and outcomes in COVID-19 patients with diabetic ketoacidosis: a systematic review of literature. Diabetes Metab Syndr. 2020;14(6):1563-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yang JK, Lin SS, Ji XJ, Guo LM. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010;47(3):193-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boddu SK, Aurangabadkar G, Kuchay MS. New onset diabetes, type 1 diabetes and COVID-19. Diabetes Metab Syndr. 2020;14(6):2211-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kiabchi AE, Umpierrez GD, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. 2009;32(7):1335-1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pasquel FJ, Umpierrez GE. Hyperosmolar hyperglycemic state: a historic review of the clinical presentation, diagnosis, and treatment. Diabetes Care. 2014;37(11):3124-3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bloomberg L.P. COVID-19 Vaccine Tracker. Accessed August 13, 2021. https://www.bloomberg.com/graphics/covid-vaccine-tracker-global-distribution/
- 13. Polack FP, Thomas SJ, Kitchin N, et al. ; C4591001 Clinical Trial Group. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383(27):2603-2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Baden LR, El Sahly HM, Essink B, et al. ; COVE Study Group. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384(5):403-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Frenck RW Jr, Klein NP, Kitchin N, et al. ; C4591001 Clinical Trial Group. Safety, Immunogenicity, and Efficacy of the BNT162b2 Covid-19 Vaccine in Adolescents. N Engl J Med. 2021;385(3):239-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Abu-Rumaileh MA, Gharaibeh AM, Gharaibeh NE. COVID-19 vaccine and hyperosmolar hyperglycemic state. Cureus. 2021;13(3):314125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mishra A, Ghosh A, Dutta K, Tyagi K, Misra A. Exacerbation of hyperglycemia in patients with type 2 diabetes after vaccination for COVID19: Report of three cases. Diabetes Metab Syndr. 2021;15(4):102151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li H, Tian S, Chen T, et al. Newly diagnosed diabetes is associated with a higher risk of mortality than known diabetes in hospitalized patients with COVID-19. Diabetes Obes Metab. 2020;22(10):1897-1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yang L, Han Y, Nilsson-Payant BE, et al. A Human Pluripotent Stem Cell-based Platform to Study SARS-CoV-2 Tropism and Model Virus Infection in Human Cells and Organoids. Cell Stem Cell. 2020;27(1):125-136.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu B, Li M, Zhou Z, Guan X, Xiang Y. Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? J Autoimmun. 2020;111:102452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fajgenbaum DC, June CH. Cytokine Storm. N Engl J Med. 2020;383(23):2255-2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wilcox NS, Rui J, Hebrok M, Herold KC. Life and death of β cells in Type 1 diabetes: A comprehensive review. J Autoimmun. 2016;71:51-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable as no datasets were generated or analyzed for this clinical case series.