Abstract
A history of concussion has been linked to long-term cognitive deficits; however, the neural underpinnings of these abnormalities are poorly understood. This study recruited 26 asymptomatic male Australian footballers with a remote history of concussion (i.e. at least six months since last concussion), and 23 non-collision sport athlete controls with no history of concussion. Participants completed three ocular motor tasks (prosaccade, antisaccade and a cognitively complex switch task) to assess processing speed, inhibitory control and cognitive flexibility, respectively. Diffusion tensor imaging data were acquired using a 3 T MRI scanner, and analysed using tract-based spatial statistics, to investigate white matter abnormalities and how they relate to ocular motor performance. Australian footballers had significantly slower adjusted antisaccade latencies compared to controls (P = 0.035). A significant switch cost (i.e. switch trial error > repeat trial error) was also found on the switch task, with Australian footballers performing increased magnitude of errors on prosaccade switch trials relative to prosaccade repeat trials (P = 0.023). Diffusion tensor imaging analysis found decreased fractional anisotropy, a marker of white matter damage, in major white matter tracts (i.e. corpus callosum, corticospinal tract) in Australian footballers relative to controls. Notably, a larger prosaccade switch cost was significantly related to reduced fractional anisotropy in anterior white matter regions found to connect to the prefrontal cortex (i.e. a key cortical ocular motor centre involved in executive functioning and task switching). Taken together, Australian footballers with a history of concussion have ocular motor deficits indicative of poorer cognitive processing speed and cognitive flexibility, which are related to reduce white matter integrity in regions projecting to important cognitive ocular motor structures. These findings provide novel insights into the neural mechanisms that may underly chronic cognitive impairments in individuals with a history of concussion.
Keywords: MRI, diffuse axonal injury, mild traumatic brain injury, cognition, biomarker
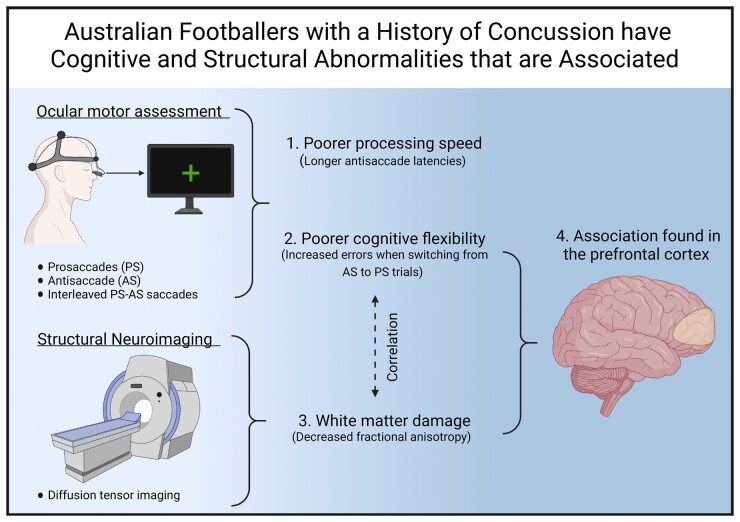
Symons et al. report that Australian footballers with a history of concussion had cognitive ocular motor deficits indicative of poorer processing speed and cognitive flexibility. These are linked to white matter damage, as identified with diffusion MRI, in regions projecting to important cognitive ocular motor structures
Graphical Abstract
Graphical Abstract.
Abbreviated summary
Symons et al. report that Australian footballers with a history of concussion had cognitive ocular motor deficits indicative of poorer processing speed and cognitive flexibility. These are linked to white matter damage, as identified with diffusion MRI, in regions projecting to important cognitive ocular motor structures.
Introduction
Collision sport athletes are susceptible to mild brain traumas, such as sports-related concussion (SRC).1 Long-term neurological consequences, including persistent cognitive deficits, are linked to a history of SRC (HoC).2 For example, we previously identified chronic cognitive abnormalities in Australian footballers (i.e. Australia’s most participated collision sport) with a HoC using ocular motor (OM) tasks that interrogate cognitive function.3
The OM system is a highly ramified neural network that facilitates the movement of the eyes. Saccades are the most common type of eye movement generated by this system, representing the rapid movement of the eyes between two points of fixation.4 Broadly, saccades can either be generated reflexively or volitionally.4 Reflexive saccades are most commonly generated in response to a suddenly appearing visual target. The basic network that generates a saccade incorporates subcortical and midbrain saccade generators (i.e. superior colliculus, brainstem OM nuclei, basal ganglia and cerebellum), as well as cortical areas, such as the frontal eye field, supplementary eye fields and intraparietal sulcus.5 In contrast, volitional saccades are generated in accordance with a decision to move the eyes towards a behaviourally relevant object of interest. This decision recruits additional neural regions, in particular, the cognitive control regions, dorsal lateral prefrontal cortex (DLPFC) and anterior cingulate cortex.6
Each saccade type and corresponding network can be interrogated using specially designed OM tasks.4,7 The prosaccade (PS) task elicits reflexive saccades in response to a suddenly appearing visual target, and activates the basic OM network. In contrast, the classic antisaccade (AS) task, which requires the inhibition of a reflexive response to a suddenly appearing visual target in favour of a saccade to the diametrically opposite target, allows assessment of the cognitive OM network.6,8 Cognitive complexity is increased when AS and PS are interleaved (i.e. the switch task), enabling the assessment of task switching or cognitive flexibility.8
Although we have previously found AS and PS changes, both individually and interleaved, in Australian footballers with a HoC,3 their neural underpinnings remain to be determined. HoC has previously been linked with diffuse axonal injury,9 and diffusion tensor imaging (DTI) is a MRI method that can be used to investigate microstructural white matter damage.10–13 For example, a DTI study of retired professional rugby players with a HoC found evidence of widespread white matter injury in major white matter tracts, as indicated by a reduction in fractional anisotropy (FA), and increased axial (AD) and radial diffusivity (RD), three common DTI metrics used to quantify properties of white matter.14 However, how white matter abnormalities in athletes with a HoC relate to cognitive OM deficits is unknown.
Therefore, here we investigated the relationship between cognitive OM performance and DTI metrics in Australian footballers with a HoC. We hypothesized that Australian footballers with a HoC would perform worse on cognitively complex OM tasks, and display DTI markers indicating white matter damage, compared to non-collision sport athlete controls with no HoC. It was further predicted that OM deficits would be associated with DTI measures of white matter integrity.
Materials and methods
Participants
A total of 49 participants were included in this study. Male Australian footballers with a HoC (n = 26) were recruited from the Victorian amateur football league. A control group of athletes (n = 23), with no history of brain trauma or engagement in collision sports, was recruited from local amateur sporting teams (i.e. basketball, tennis, cricket, track). The sample size was estimated a priori based on the effect size observed in our previous study of OM and DTI in Australian footballers with a HoC which reported Cohen's d = 0.906.3 To achieve 80% power for the effect size, a minimum of 44 participants (22 per group) is required in a Mann–Whitney test. We thus recruited 26 footballers and 23 controls in this study, which would provide 80% power for a minimal detectable effect size of Cohen's d = 0.8388.
Individuals with a history of neurosurgery, major psychiatric or neurological disturbances (e.g. epilepsy, multiple sclerosis, stroke, schizophrenia, bipolar disorder), or any medical contraindications to MRI were excluded. Australian footballers who had sustained a concussion within the past six months were not enrolled into the study and testing was performed during preseason to minimize any confounding effects of recent concussive and sub-concussive injuries. The Melbourne Health Human Ethics committee approved study procedures (#2015.012), and these were in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans. All participants provided written informed consent prior to the study.
Demographics and concussion history
A questionnaire was administered to each participant concerning demographics, HoC, sporting history and education history. The Beck Depression Inventory was used to measure self-reported depression,15 and The National Adult Reading Task was used to measure premorbid intelligence.16
OM recording
The horizontal displacement of both eyes (i.e. saccades) was recorded using an Eyelink II dark pupil, video-oculography system (SR-Research Ltd, Mississauga, Ontario, Canada). This is a high-resolution (noise limited at <0.01 degree) and high-acquisition-rate (500 Hz) system. Participants were seated in a darkened room, 840 mm in front of a 75-Hz CRT monitor (resolution, 1024·768). Task stimuli were presented on a black background and comprised crosses (visual angle, 1 degree) all of equal luminance generated using Experiment Builder (version 1.6.121). A five-point calibration sequence was performed before each task, with in vivo task calibrations performed to confirm the accuracy of the initial calibration.
OM tasks
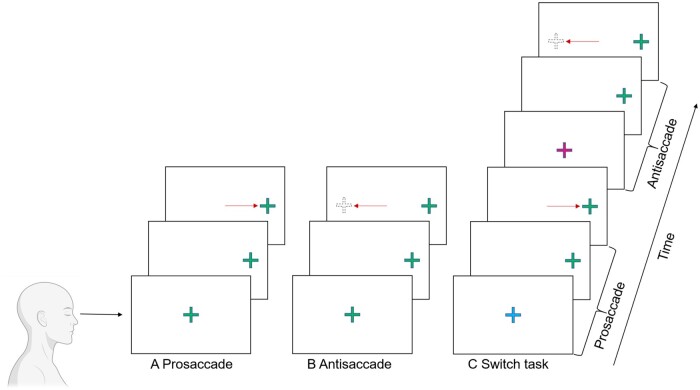
OM assessment consisted of three discrete tasks: (i) PS block task (only PS performed); (ii) AS block task (only AS performed); and (iii) switch task (interleaved PS and AS). Figure 1 represents a schematic of these three tasks. The PS block task assesses the ability to make visually guided saccades, an eye movement to a suddenly appearing visual target. Participants fixated on a central green cross for 1250–1750 ms and performed saccades to a suddenly appearing peripheral target (1250–1750 ms), as it stepped horizontally and pseudo-randomly 5° or 10°, to the left or right of centre.3 A total of 24 trials were completed over one block.
Figure 1.
Schematic representation of the three pertinent ocular motor (OM) tasks. (A) Prosaccade: An eye movement to a suddenly appearing target as a general measure of OM performance and processing speed. (B) Antisaccade: An eye movement away from a diametrically opposite target as a measure of inhibitory control and cognitive processing speed. (C) Switch task: Interleaves prosaccades and antisaccades as a measure of task switching and cognitive flexibility.
The AS block task measures the ability to inhibit a prepotent/reflexive response generated by a suddenly appearing target, and generate a response in the equal and opposite direction.17 Participants fixated on a central green cross for 1250–1750 ms before the central green cross disappeared concomitantly with the appearance of a green target cross at either 5° or 10°, left or right of centre (1250–1750 ms). Participants performed a saccade to the diametrically opposite position, without looking at the green target.3 A total of 48 trials were completed over two blocks.
The switch task interleaves both PS and AS trials, and assess the ability to switch between these tasks.3 Participants fixated a central cross that was either blue or purple. After 1250, 1500 or 1750 ms, a green target cross appeared in one of four peripheral locations 5° or 10°, left or right of centre. The colour of the central cross indicated how a participant was to respond to the appearance of a peripheral green target cross (i.e. blue = PS; magenta = AS). After 1500 ms, a central fixation square appeared, reorienting the participants gaze centrally in preparation for the next trial. All participants were familiarized with the task’s rules, by way of a guided example, followed by a practice block of 12 trials (six AS trials and six PS trials). A total of 96 trials were completed over three test blocks. Each test block consisted of 32 PS and AS trials presented in a pseudo-random order. Trials were classified as a repeat trial where two consecutive trials required the same response (repeat: AS-AS or PS-PS) or a switch trial where a different response was required on two consecutive trials (switch: AS-PS or PS-AS). Repeat trials are usually simpler relative to switch trials (switch cost): the greater the disparity (switch cost) the poorer the cognitive flexibility.3 Across the three test blocks, 48 PS and 48 AS trials were presented with even numbers of switch and repeat trials. The first trial of every block was excluded from switch/repeat trial analyses because they are neither a switch nor repeat trial.3
OM analysis
Monocular analyses were performed for OM output using a customized MATLAB programme. We have previously demonstrated OM differences in latency and error measures, and therefore these measures were included in this analysis.3
For all OM tasks, saccade latency was calculated as the temporal difference between trial onset and saccade onset using a velocity criterion of 30° per second. Trials were removed from the analysis of saccade latency where: (i) the task was not completed in accordance with task rules (error as explained below); (ii) fixation was not maintained within 1.5° of the central target; (iii) a blink occurred around trial onset that was thought to affect saccade onset; (iv) no response was made within the trial period; and (v) a saccade made within 100 ms of target onset. For the AS block task, both unadjusted and adjusted latencies were calculated. Adjusted AS latencies were calculated as a measure of volitional latency (cognitive processing speed) by subtracting PS from the unadjusted AS latencies.18 Error rate was calculated for all OM tasks as a proportion of total trials. PS block task: saccades made in the opposite direction to the peripheral target. AS block task: saccades made towards the peripheral green target cross. Switch task: performance of a PS during an AS trial or vice versa.
For the switch task, switch cost was calculated for both latency and error and was defined as the relative difference between a switch trial and a repeat trial (i.e. switch trial performance/repeat trial performance). Previous studies have shown that only PS trials elicit a switch cost (unidirectional switch cost, PS switch/PS repeat) for this task design.19,20 As such, switch cost related to PS trials was of interest to this study. A larger switch cost indicates increased difficulty with switching and poorer cognitive control. For the analysis of latency, only trials not preceded by an error were included given that evidence suggests that switch costs are only evident when the previous trial is correct.20
MRI acquisition and pre-processing
MRI acquisition was performed on a 3-T scanner (Magnetom Prisma; Siemens, Erlangen, Germany). DWI was acquired at 2 mm isotropic resolution over 64 directions at b = 3000 s/mm2. Imaging was performed with a repetition time = 3400 ms; echo time = 79 ms; field of view = 25.6 × 25.6 mm2; and acquisition matrix = 128 × 128. A pair of non-diffusion weighted images with opposite phase encoding (i.e. anterior to posterior and posterior to anterior) was also acquired to correct for susceptibility-induced B0 inhomogeneities.
Pre-processing was performed using MRtrix321 and FSL22 and included denoising followed by motion and distortion correction. B1 inhomogeneity was corrected using the N4 algorithm (included as part of ANTS).23 Global intensity normalization was performed using a white matter mask and DW images upsampled to 1.3 mm isotropic resolution. A total of eight participants (Footballers = 3 and Controls = 5) did not have a reverse phase encoding due to an error in the acquisition. Therefore, no inhomogeneity field estimation correction was performed on this group and a covariate was included in general linear statistical models to account for this discrepancy.
DTI metrics
DTI was used to calculate FA, AD, RD and mean diffusivity (MD). These were calculated using Mrtrix3 and nonlinearly registered to a study-specific template space for voxel-wise statistical analysis using Tract-Based Spatial Statistics (TBSS),24 part of the FSL analysis package.22 Non-parametric permutation testing was performed with 5000 permutations and threshold-free cluster enhancement corrected for multiple comparisons (P < 0.05).
Tractography
Tractography was used to investigate the connectivity of seed regions identified in correlation analysis. A whole-brain tractogram was generated using a probabilistic streamline approach in MRtrix3 using the iFOD2 algorithm seeded from all brain voxels randomly (20 000 000 seeds). The whole-brain tractogram was filtered using SIFT2 to normalise the tract density and then again filtered to include only tracts passing through the voxel cluster identified when increased magnitude of PS switch cost (error) was correlated with reduced FA in TBSS. A tract density image for the resulting tracts was generated using the tckmap command using the tdi option.
Statistical analysis
SPSS software (version 22.0; IBM Corp., Armonk, NY) was used for all statistical analyses with the exception of the TBSS analysis described above. Demographic variables were compared between footballers and controls using Mann–Whitney U-tests. OM performance on the PS and AS task was compared using unpaired t-tests or Mann–Whitney tests where appropriate. Performance on the switch task was assessed using a repeated-measures ANOVA using a trial-type (PS, AS) and previous trial (repeat, switch) as within-subject factors and group (controls, footballers) as the between-subject factor. Where appropriate, post hoc analysis was performed using Bonferroni or Games–Howell correction for equal or unequal variances, respectively. Results were presented as mean difference (Mdiff). Statistical significance was set at P < 0.05 for analysis.
Group differences in DTI metrics were assessed using non-parametric permutation testing (general linear model) and threshold-free cluster enhancement with ‘presence of a reverse phase encode’ included as a nuisance variable. Significantly different OM variables [i.e. AS latency (adjusted) and magnitude of switch cost (error)] were correlated to assess whether there was a relationship between white matter damage and OM performance. This correlation was performed using a general linear model in FSL within areas that had significantly decreased FA identified in the group analysis. The ‘presence of a reverse phase encode’ was included as nuisance variable. One participant did not perform the switch task therefore was allocated the group mean for the purpose of this correlation within TBSS. The number of previous concussions was correlated with white matter and OM changes to investigate the relationship with the postulated underlying mechanism of change. The correlation with reduced FA was performed using a general linear model in FSL with ‘presence of a reverse phase encode’ included as a nuisance variable. The correlation with key OM variables was performed using a two-tailed Pearson correlation in SPSS. Statistical significance was set at P < 0.05 for analysis in SPSS and a family-wise error–corrected P < 0.05 was considered significant in all TBSS analysis.
Data availability
Data will be made available upon request to the corresponding author.
Results
Demographics
There were no significant differences between controls and Australian footballers on any demographic variable (Table 1).
Table 1.
Demographic information
| Controls | Footballers | P-value | |
|---|---|---|---|
| N | 23 | 26 | |
| Age | 23.00 (2.00) | 24.00 (5.25) | 0.467 |
| Years of Education | 16.00 (2.00) | 16.00 (2.00) | 0.435 |
| N = 22 | |||
| NART | 116.00 (6.00) | 116.00 (7.00) | 0.377 |
| N = 25 | |||
| BDI | 3.00 (6.00) | 2.00 (4.00) | 0.363 |
| Total No. previous concussion | 2.00 (1.00) |
Medians and IQR and Mann–Whitney U-test where appropriate.
BDI, Beck depression inventory; NART, National Adult Reading Task.
Australian footballers have cognitive OM deficits
OM findings are presented in Table 2. No significant differences were found on the PS task. For the AS task, when AS latency was normalized to PS latency (i.e. adjusted AS latency), latencies were significantly prolonged in footballers compared to controls (P = 0.035). There were no significant differences in the proportion of errors between controls and footballers on the AS task.
Table 2.
Results of ocular motor tasks
| Task | Measure |
Controls |
Footballers |
P-value | |
|---|---|---|---|---|---|
| N | 23 | 26 | |||
| Prosaccade | Latency (ms) | 186.50 (27.84) | 184.81 (31.20) | 0.841 | |
| Antisaccade | Latency (ms) | 292.74 (57.99) | 307.50 (73.21) | 0.065 | |
| Latency—adjusted (ms)#* | 93.40 (42.66) | 106.64 (61.61) | 0.035* | ||
| Error (%) | 4.17 (10.42) | 7.29 (14.58) | 0.229 | ||
| N | 23 | 25 | |||
| Switch task | Prosaccade | Repeat latency (ms) | 188.45 (32.38) | 176.200 (47.21) | 0.433 |
| Switch latency (ms) | 198.74 (42.04) | 192.00 (43.95) | 0.584 | ||
| Switch cost latency (ms) | 10.94 (14.27) | 11.23 (26.32) | 0.861 | ||
| Repeat error (%) | 0.00 (0.00) | 0.00 (0.00) | 0.601 | ||
| Switch error (%) | 0.00 (4.00) | 4.00 (8.00) | 0.097 | ||
| Switch cost error (%)* | 0.00 (4.00) | 4.00 (8.00) | 0.023* | ||
| Antisaccade | Repeat latency (ms) | 283.58 (47.87) | 286.19 (70.33) | 0.570 | |
| Switch latency (ms) | 275.22 (55.11) | 284.67 (51.47) | 0.081 | ||
| Switch cost latency (ms) | −10.75 (17.45) | −3.71 (29.22) | 0.176 | ||
| Repeat error (%) | 13.04 (17.39) | 13.04 (32.60) | 0.597 | ||
| Switch error (%) | 8.00 (8.00) | 4.00 (12.00) | 0.370 | ||
| Switch cost error (%) | −4.70 (18.78) | −4.70 (11.57) | 0.601 | ||
Medians and IQR and Mann–Whitney U-test or t-test (#) where appropriate.
Significant differences between controls and footballers.
For the switch task, a significant main effect of current trial-type confirmed the stereotypic response of longer latencies [Mdiff = 91.693 ms, F (1, 46) = 704.763, P < 0.001 ŋp2 = 0.939] and increased error rate [Mdiff = 11.894%, F (1, 46) = 46.821, P < 0.001 ŋp2 = 0.504) on AS trials compared to PS trials. In addition, as expected with a switch task, a significant main effect of previous trial-type [F (1, 46) = 5.039, P = 0.030 ŋp2 =0.099] identified a greater proportion of errors performed when the previous trial was a PS compared to an AS [Mdiff = 2.044%, P > 0.03); this was consistent between Australia footballers and controls, with no interaction found (P > 0.05). In contrast, no main effect of previous trial-type or interactions were found for latency (P > 0.05). Again, in line with expectations of this task, we identified a significant previous trial by current trial interaction, that indicated that error [F (1, 46) = 29.339, P < 0.001 ŋp2 = 0.389] and latency [F (1, 46) = 45.636, P < 0.001 ŋp2 = 0.498] were differentially affected when switching from an AS to a PS (PS switch trial) compared to switching from a PS to an AS (AS switch). Specifically, longer latencies (Mdiff = 14.09 ms, P < 0.001) and more errors (Mdiff = 2.77%, P < 0.001) were performed on PS switch trials compared to PS repeat trials; switch cost. In contrast, for AS trials, the opposite relationship was found, with shorter latencies (Mdiff = 8.916 ms, P < 0.001) and fewer errors (Mdiff = 6.90%, P < 0.001) performed on AS switch trials compared to AS repeat trials: switch benefit.
The between-subject factor, group, was used to determine whether results above were different in footballers than controls. Notably, a significant interaction between trial-type and group [F (1, 46) = 5.366, P = 0.025 ŋp2 = 0.104) found that footballers performed longer latencies than controls for both PS trials (Mdiff = 1.66 ms) and AS trials (Mdiff = 17.66 ms). However, post hoc analyses determined that neither PS or AS latencies were individually significant between groups (P = 0.874 and P = 0.175, respectively). In contrast, no group by trial-type interaction was found for error (P > 0.05). Furthermore, a significant three-way interaction between previous trial-type by current trial-type by group was found for the measure of error [F (1, 46) = 4.228, P = 0.045 ŋp2 = 0.084), with footballers alone performing significantly more errors (Mdiff = 4.04%, P < 0.001) on PS switch trials than PS repeat (i.e. switch cost); controls demonstrated comparable performance (Mdiff = 1.39%, P = 0.086). When the magnitude (PS switch—PS repeat) of this switch cost was compared, footballers were found to have a significantly greater magnitude of PS switch cost (error only) than controls (P = 0.023) (Table 2). Furthermore, footballers also performed significantly fewer errors on AS switch trials than AS repeat trials (Mdiff = 9.09%, P < 0.001); again, controls had comparable performance irrespective of switch or repeat trials (Mdiff = 4.51%, P = 0.104). No three-way interaction was seen for the measure of latency (P > 0.05).
Australian footballers have DTI abnormalities
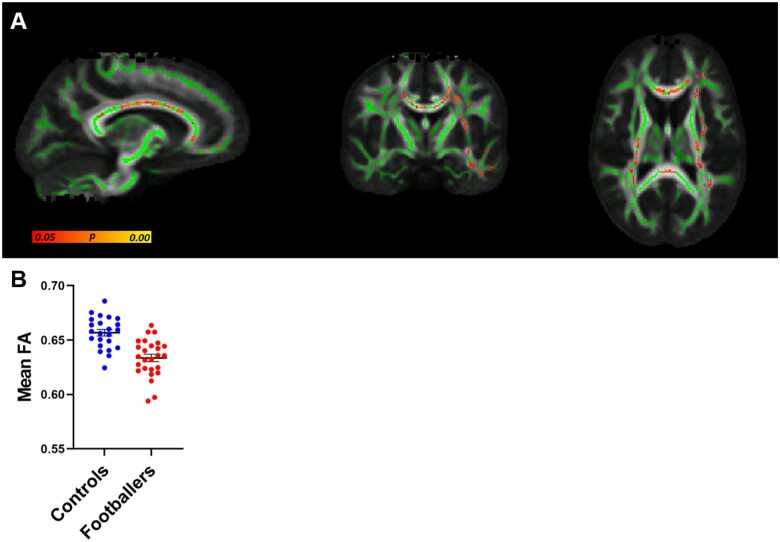
TBSS analysis of DTI data revealed significantly decreased FA in footballers compared to controls (Fig. 2A). These changes were evident in the corpus callosum and the corticospinal tracts. An average FA from each participant was generated from the region identified as significantly different in Fig. 2A. Figure 2B depicts these values graphically demonstrating the distribution of average FA between controls and footballers. No significant differences were found on the measures of RD, AD and MD.
Figure 2.
Decreased fractional anisotropy (FA) in Australian footballers compared to controls. (A) Whole-brain TBSS analysis of FA (green = tracts analysed; yellow/orange/red colour scale = significantly decreased FA in Australian footballers, P < 0.05, corrected for multiple comparisons). (B) For each subject, an average FA was derived from the region of significant group difference shown in A to demonstrate the distribution of average FA between groups. This is a visual representation of the data/analysis shown in A; therefore, no additional statistics were performed in B. Controls are depicted in blue and footballers in red (Mean ± SEM).
OM performance correlates with FA
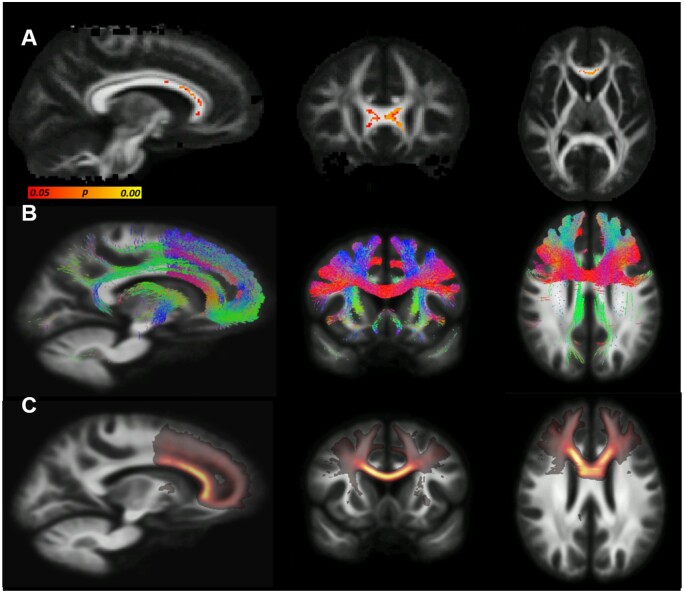
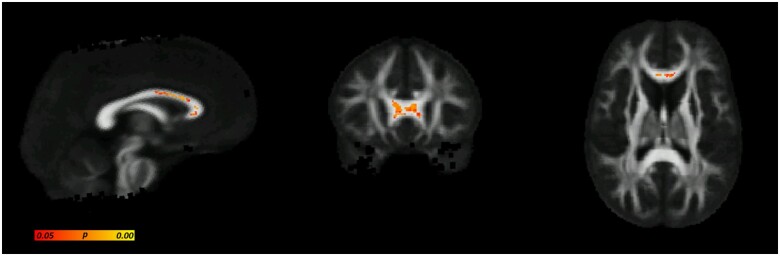
The OM analyses identified two significant differences between Australian footballers and controls [AS latencies (adjusted) and magnitude of PS switch cost]. Therefore, these measures were correlated with regions of significantly decreased FA in TBSS. There was a significant negative correlation between decreased FA and increased magnitude of PS switch cost (error) identified in frontal white matter tracts (Fig. 3A). An average FA from each participant was generated from the region identified as significantly correlated in Fig. 3A. Figure 4A depicts these values graphically demonstrating the distribution of average FA between controls and footballers. Figure 4B also graphically depicts the correlation between average FA and magnitude of PS switch cost (error). Tractography and tract density of this significantly associated ‘seed region’ revealed connectivity of this region to the DLPFC (Fig. 3B and C).
Figure 3.
Relationship between reduced fractional anisotropy (FA) and increased magnitude of prosaccade switch cost (error). (A) Results of TBSS correlation analysis, areas where reduced FA is significantly negatively correlated with increased magnitude of switch cost (error) are indicted in by yellow/orange/red-coloured voxels (P < 0.05, corrected for multiple comparisons). (B) Tractography investigating the connectivity of the significantly correlation seed region identified in A. (C) Tract density image confirming prefrontal connectivity.
Figure 4.
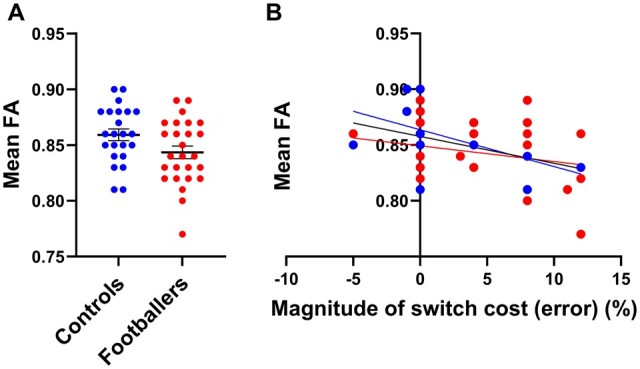
Representative graphs to illustrate significant negative correlation between reduced fractional anisotropy (FA) and increased magnitude of switch cost (error) identified in Fig. 3A. (A) For each subject, an average FA was derived from the region in Fig. 3A where a significant correlation between FA and switch cost (error) to demonstrate the distribution of average FA between groups within this correlated area (Mean ± SEM). (B) Graphical representation of the correlation identified in Fig. 3A. Average subject FA derived from this significantly correlated region graphed against subject’s magnitude of switch cost (error). No additional statistics were performed for the data in Fig. 4 because this analysis was already done for Fig. 3A. Controls are depicted in blue and footballers in red.
HoC correlates with both OM performance and FA
The number of previous concussions was significantly correlated with AS latencies (adjusted) [r(47) = 0.402, P = 0.004], but not magnitude of PS switch cost (error) [r(46) = 0.248, P = 0.09). There was also a significant negative correlation between the number of previous concussions and FA in frontal white matter tracts (Fig. 5).
Figure 5.
Region of significant correlation between fractional anisotropy (FA) and number of previous concussions. Regions where reduced FA is negatively correlated with increased number of concussions are indicted by yellow/orange/red-coloured voxels (P < 0.05, corrected for multiple comparisons).
Discussion
This study aimed to determine whether cognitive OM performance is related to underlying white matter damage in HoC. Consistent with our hypothesis, we found that Australian footballers with a HoC had prolonged adjusted AS latencies, indicating reduced cognitive processing speed. In addition, footballers were less efficient at switching between saccade types than controls as evidenced by a greater magnitude PS switch cost (error), indicative of worse cognitive flexibility. Furthermore, significant white matter damage was found in the Australian footballers compared to controls, as indicated by decreased FA in frontal and long white matter tracts. Importantly, increased PS switch cost correlated with regions of decreased FA in frontal white matter tracts. Tractography revealed that these regions overlap with key OM areas involved in the performance of this tasks, the DLPFC, which is known to mediate executive functions, such as task switching.8 These findings suggest that white matter damage may contribute to some cognitive deficits experienced by individuals with a HoC.
Does white matter damage contribute to cognitive OM abnormalities in individuals with a HoC?
Australian footballers with a HoC had longer AS latencies (adjusted) compared to controls, which is similar to previous findings.25,26 This has been proposed to represent the slowing of cognitive information processing within the networks that subserve executive functioning and inhibitory control.4,6 Interestingly, longer latencies were not found for simpler PSs, supporting the notion that the slower AS latencies is due to the cognitive regions/networks recruited due to the additional cognitive requirement of the task; namely frontal regions (e.g. DLPFC) that help mediate reflexive saccade inhibition.4 Furthermore, and consistent with our previous study,3 Australian footballers with a HoC also had increased magnitude PS switch cost (error), indicating poorer cognitive flexibility compared to controls. The neural network underlying switching/cognitive flexibility in this task similarly implicates frontal regions, particularly the prefrontal cortex, which mediates the maintenance and transition between the different task rules; an error indicates a failure to maintain task rules and/or inhibit a response.8,27,28 These findings collectively suggest that a HoC affects the OM cognitive control network responsible for goal-directed (volitional) saccades, rather than the basic network involved in simple saccade generation (prosaccdes).
In line with previous DTI research in the context of HoC,14,29–31 this study revealed evidence (i.e. decreased FA) of white matter damage in major white matter tracts (i.e. corpus callosum and corticospinal tracts) of Australian footballers compared to controls. These findings are consistent with the proposal that these tracts are particularly vulnerable to the acceleration, deceleration, and rotational forces endured in SRC.32,33 Furthermore, pathophysiological changes, such as oxidative stress and neuroinflammation, can also result in a secondary injury to white matter,34 evidence of which has been seen in cohorts similar to this study.35,36
After confirming the presence of OM and white matter abnormalities in those with a HoC, we next addressed the primary aim of the study, which was to establish whether there was a relationship between the two. Poorer cognitive flexibility [i.e. increased PS switch cost (error)] was significantly related to reduced FA in the genu of the corpus callosum. Tractography from this seed region revealed that the majority of these tracts projected towards the prefrontal cortex, which, as discussed, is pivotal to cognitive saccade control.27 This is supported by previous research that has similarly implicated white matter damage within the DLPFC and the genu of the corpus callosum with poorer executive function post TBI,37–39 as well as young adults40 and healthy aging populations.41 Inferior medial lesions of the prefrontal cortex have also been linked to increased errors on a switch task.42 Taken together, the correlation found here supports the notion that damage to anterior white matter regions, such as the genu of the corpus callosum, may be implicated in poorer executive function and cognitive flexibility in athletes with a HoC.
We found no statistically significant association between adjusted AS latency and FA. The OM cognitive control network, responsible for generating volitional saccades, involves a highly ramified set of cortical and subcortical regions. This network engages basic OM circulatory (required to generate simple reflexive PSs) and additional cortical engagement reflecting the tasks higher-order requirements.8 Therefore, it may be that the AS latency (adjusted) changes are linked to a more generalised damage within the broader OM network, rather than the more regional specific association identified with increased magnitude of PS switch cost (error). The white matter damage identified in this study was evident across large white matter tracts (i.e. corpus callosum and corticospinal tracts) that are known to overlap with the OM network. Specifically, these tracts are involved in integrating and transmitting ‘top down’ information from key cortical OM regions with ‘bottom up’ saccade machinery located in subcortical, midbrain and brainstem regions for saccade generation.43,44 Therefore, the diffuse damage observed in this study may have a generalised impact on the OM network but the implications of this are only demonstrable when cognitive load is increased.
The present findings bear resemblance to those from previous SRC studies involving athletes from other sports that similarly found chronic OM and structural changes associated with a HoC.14,45–47 While this study provides emerging insight to the long-term neurological effects of engaging in Australian football, a presently understudied cohort, the results of this study may be relevant to footballers and collision athletes more broadly. Specifically, the association identified between magnitude of PS switch cost and reduced FA in frontal white matter tracts, may demonstrate a novel structural basis to cognitive OM deficits and cognitive flexibility in athletes with a HoC.
Future studies are needed to determine the causal nature of the association found here, particularly given the relative lack of studies related to the structural underpinnings of cognitive saccades. Notably, footballers and controls demonstrated a similar trajectory suggesting there may be a structural association related to normal variation in OM performance. Alternatively, structural white matter damage may not modulate OM function in individuals with HoC. For example, other brain abnormalities induced by SRC, including functional changes, grey matter atrophy, cerebral flow and metabolomics may impact OM function.48–50 For example, functional neural correlates have been identified with OM performance acutely after mild traumatic brain injury (mTBI), and in patients with persisting post-concussion symptoms.25,51–53 This highlights the need for further research into the mechanisms that underpin cognitive deficits after mTBI. Such studies could incorporate additional neuroimaging methodologies, such as arterial spin labelling, functional MRI, or magnetic resonance spectroscopy to do so.
Limitations and future directions
Several methodological limitations should be considered when interpreting the data presented here. The HoC data were based on self-report of the number of diagnosed concussions, which may be as participants could be susceptible to poor recall. Prospective longitudinal studies that monitor changes after an acute concussion would help to address this problem.54 Similar to previous findings,13,14,29 white matter damage and worse processing speed were correlated with the number of previous concussions. While our findings provide evidence of the long-term neurological damages associated with an HOC, we could not account for the potential effect of subconcussive impacts within our groups which may also be associated with neurological changes.55,56 Future studies could implement emerging accelerometer technology to better delineate the effects of subconcussive injury.55,57 Furthermore, we did not to include a metric of time since last concussion. This should be included in future studies to investigate whether this mediates chronic cognitive and structural changes. There is also growing evidence that biological sex may contribute to the outcomes following mTBI.58 While we did not include females in this study, in order to replicate our previous male-only OM study,3 future studies should aim to recruit females and determine whether the findings are consistent across the sexes. Notably, Australian footballers provide a unique cohort to study such questions as the rules allowing full-body contact are consistent for both males and females.1
Conclusion
This study found evidence of cognitive OM abnormalities and white matter damage in athletes with a HoC compared to a control group of athletes with no HoC or participation in collision sport. These findings are concerning, particularly considering the relatively young age of the cohort studied. OM and DTI measures were also found to correlate with a HoC. This provides convergent evidence of long-term neurological damages associated with SRC that can be captured through sensitive measures, such as DTI and OM assessment. Importantly, we found a relationship between magnitude of PS switch cost and FA in the genu of the corpus callosum. This region tracts to a key cortical OM centre, the DLPFC, known to be involved in executive functioning and task switching. Although a relationship was not found between AS latency and DTI findings, given the overlap between the OM network and the identified damaged tracts (corpus callosum and corticospinal tracts), it is possible that general destabilisation of these networks contributed to the AS findings. Taken together, the result of this study suggest that structural white matter damage may contribute to cognitive changes in athletes with a HoC.
Acknowledgements
Graphical abstract and Fig. 1 created in BioRender.com.
Funding
This study was funded by a grant and fellowship from the Australian National Health and Medical Research Council (NHMRC) to S.R.S. R.M., T.J.O. and D.K.W. receive salary support from NHMRC.
Competing interests
The authors report no competing interests.
Glossary
- AD =
axial diffusivity
- AS =
antisaccade
- DLPFC =
dorsal lateral prefrontal cortex
- DTI =
diffusion tensor imaging
- FA =
fractional anisotropy
- HoC =
history of concussion
- MD =
mean diffusivity
- mTBI =
mild traumatic brain injury
- OM =
ocular motor
- PS =
prosaccade
- RD =
radial diffusivity
- TBSS =
tract-based spatial statistics
Contributor Information
Georgia F Symons, Department of Neuroscience, Central Clinical School, Monash University, Melbourne, Australia.
Meaghan Clough, Department of Neuroscience, Central Clinical School, Monash University, Melbourne, Australia.
Steven Mutimer, Department of Medicine, Royal Melbourne Hospital, University of Melbourne, Parkville, Australia.
Brendan P Major, Department of Neuroscience, Central Clinical School, Monash University, Melbourne, Australia.
William T O’Brien, Department of Neuroscience, Central Clinical School, Monash University, Melbourne, Australia.
Daniel Costello, Department of Medicine, Royal Melbourne Hospital, University of Melbourne, Parkville, Australia.
Stuart J McDonald, Department of Neuroscience, Central Clinical School, Monash University, Melbourne, Australia.
Zhibin Chen, Department of Neuroscience, Central Clinical School, Monash University, Melbourne, Australia.
Owen White, Department of Neuroscience, Central Clinical School, Monash University, Melbourne, Australia; Department of Medicine, Royal Melbourne Hospital, University of Melbourne, Parkville, Australia.
Richelle Mychasiuk, Department of Neuroscience, Central Clinical School, Monash University, Melbourne, Australia.
Meng Law, Department of Neuroscience, Central Clinical School, Monash University, Melbourne, Australia.
David K Wright, Department of Neuroscience, Central Clinical School, Monash University, Melbourne, Australia.
Terence J O’Brien, Department of Neuroscience, Central Clinical School, Monash University, Melbourne, Australia; Department of Medicine, Royal Melbourne Hospital, University of Melbourne, Parkville, Australia.
Joanne Fielding, Department of Neuroscience, Central Clinical School, Monash University, Melbourne, Australia; Department of Medicine, Royal Melbourne Hospital, University of Melbourne, Parkville, Australia.
Scott C Kolbe, Department of Neuroscience, Central Clinical School, Monash University, Melbourne, Australia.
Sandy R Shultz, Department of Neuroscience, Central Clinical School, Monash University, Melbourne, Australia; Department of Medicine, Royal Melbourne Hospital, University of Melbourne, Parkville, Australia.
References
- 1. Symons GF, Clough M, Fielding J, et al. The neurological consequences of engaging in Australian collision sports. J Neurotrauma. 2020;37(5):792–809. [DOI] [PubMed] [Google Scholar]
- 2. Manley GT, Gardner AJ, Schneider KJ, et al. A systematic review of potential long-term effects of sport-related concussion. Br J Sports Med. 2017;51(12):969–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Clough M, Mutimer S, Wright DK, et al. Oculomotor cognitive control abnormalities in Australian rules football players with a history of concussion. J Neurotrauma. 2018;35(5):730–738. [DOI] [PubMed] [Google Scholar]
- 4. Hutton SB. Cognitive control of saccadic eye movements. Brain Cogn. 2008;68(3):327–340. [DOI] [PubMed] [Google Scholar]
- 5. Leigh JR, Zee DS, Leigh RJ, Zee DS. The neurology of eye movements. New York: Oxford University Press; 2015. doi: 10.1093/med/9780199969289.001.0001. [DOI] [Google Scholar]
- 6. McDowell JE, Dyckman KA, Austin BP, Clementz BA. Neurophysiology and neuroanatomy of reflexive and volitional saccades: Evidence from studies of humans. Brain Cogn. 2008;68(3):255–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ventura RE, Balcer LJ, Galetta SL, Rucker JC. Ocular motor assessment in concussion: Current status and future directions. J Neurol Sci. 2016;361:79–86. [DOI] [PubMed] [Google Scholar]
- 8. Jamadar SD, Fielding J, Egan GF. Quantitative meta-analysis of fMRI and PET studies reveals consistent activation in fronto-striatal-parietal regions and cerebellum during antisaccades and prosaccades. Front Psychol. 2013;4:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barkhoudarian G, Hovda DA, Giza CC. The molecular pathophysiology of concussive brain injury - An update. Phys Med Rehabil Clin N Am. 2016;27(2):373–393. [DOI] [PubMed] [Google Scholar]
- 10. Gardner A, Kay-Lambkin F, Stanwell P, et al. A systematic review of diffusion tensor imaging findings in sports-related concussion. J Neurotrauma. 2012;29(16):2521–2538. [DOI] [PubMed] [Google Scholar]
- 11. Suri AK, Lipton ML. Neuroimaging of brain trauma in sports. In: Handbook of clinical neurology, Vol. 158. Elsevier; 2018. 205–216. [DOI] [PubMed] [Google Scholar]
- 12. Wu Y, Harezlak J, Elsaid NMH, et al. Longitudinal white-matter abnormalities in sports-related concussion. Neurology. 2020;95(7):e781–e792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tremblay S, Henry LC, Bedetti C, et al. Diffuse white matter tract abnormalities in clinically normal ageing retired athletes with a history of sports-related concussions. Brain. 2014;137(Pt 11):2997–3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wright DK, Gardner AJ, Wojtowicz M, et al. White matter abnormalities in retired professional rugby league players with a history of concussion. J Neurotrauma. 2021;38(8):983–986. [DOI] [PubMed] [Google Scholar]
- 15. Beck AT, Steer RA. Manual for the revised Beck depression inventory. San Antonio: TX Psychol Corp; 1987. [Google Scholar]
- 16. Nelson HE, Willison J. National Adult Reading Test (NART). Windsor: Nfer-Nelson; 1991. [Google Scholar]
- 17. Munoz DP, Everling S. Look away: The anti-saccade task and the voluntary control of eye movement. Nat Rev Neurosci. 2004;5(3):218–228. [DOI] [PubMed] [Google Scholar]
- 18. Chen YF, Chen T, Tsai TT. Analysis of volition latency on antisaccadic eye movements. Med Eng Phys. 1999;21(8):555–562. [DOI] [PubMed] [Google Scholar]
- 19. Weiler J, Heath M. Task-switching in oculomotor control: Unidirectional switch-cost when alternating between pro- and antisaccades. Neurosci Lett. 2012;530(2):150–154. [DOI] [PubMed] [Google Scholar]
- 20. DeSimone JC, Weiler J, Aber GS, Heath M. The unidirectional prosaccade switch-cost: Correct and error antisaccades differentially influence the planning times for subsequent prosaccades. Vision Res. 2014;96:17–24. [DOI] [PubMed] [Google Scholar]
- 21. Tournier J-DD, Smith R, Raffelt D, et al. MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. Neuroimage. 2019;202:116137. [DOI] [PubMed] [Google Scholar]
- 22. Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23 (Suppl 1):S208–S219. [DOI] [PubMed] [Google Scholar]
- 23. Tustison NJ, Avants BB, Cook PA, et al. N4ITK: Improved N3 bias correction. IEEE Trans Med Imaging. 2010;29(6):1310–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487–1505. [DOI] [PubMed] [Google Scholar]
- 25. Ting WKC, Schweizer TA, Topolovec-Vranic J, Cusimano MD. Antisaccadic eye movements are correlated with corpus callosum white matter mean diffusivity, stroop performance, and symptom burden in mild traumatic brain injury and concussion. Front Neurol. 2015;6:271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kraus MF, Little DM, Donnell AJ, Reilly JL, Simonian N, Sweeney JA. Oculomotor function in chronic traumatic brain injury. Cogn Behav Neurol. 2007;20(3):170–178. [DOI] [PubMed] [Google Scholar]
- 27. Miller BT, D'Esposito M. Searching for “the top” in top-down control. Neuron. 2005;48(4):535–538. [DOI] [PubMed] [Google Scholar]
- 28. Jamadar S, Hughes M, Fulham WR, Michie PT, Karayanidis F. The spatial and temporal dynamics of anticipatory preparation and response inhibition in task-switching. Neuroimage. 2010;51(1):432–449. [DOI] [PubMed] [Google Scholar]
- 29. Multani N, Goswami R, Khodadadi M, et al. The association between white-matter tract abnormalities, and neuropsychiatric and cognitive symptoms in retired professional football players with multiple concussions. J Neurol. 2016;263(7):1332–1341. [DOI] [PubMed] [Google Scholar]
- 30. Kraus MF, Susmaras T, Caughlin BP, Walker CJ, Sweeney JA, Little DM. White matter integrity and cognition in chronic traumatic brain injury: A diffusion tensor imaging study. Brain. 2007;130(Pt 10):2508–2519. [DOI] [PubMed] [Google Scholar]
- 31. Eierud C, Craddock RC, Fletcher S, et al. Neuroimaging after mild traumatic brain injury: Review and meta-analysis. Neuroimage Clin. 2014;4:283–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shenton ME, Hamoda HM, Schneiderman JS, et al. A review of MRI and DTI findings in mild traumatic brain injury. Brain Imaging Behav. 2012;6(2):137–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bayly PV, Cohen TS, Leister EP, Ajo D, Leuthardt EC, Genin GM. Deformation of the human brain induced by mild acceleration. J Neurotrauma. 2005;22(8):845–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Webster KM, Wright DK, Sun M, et al. Progesterone treatment reduces neuroinflammation, oxidative stress and brain damage and improves long-term outcomes in a rat model of repeated mild traumatic brain injury. J Neuroinflammation. 2015;12(1):238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Major BP, McDonald SJ, O'Brien WT, et al. Serum protein biomarker findings reflective of oxidative stress and vascular abnormalities in male, but not female, collision sport athletes. Front Neurol. 2020;11:549624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Symons GF, Clough M, O’Brien WT, et al. Shortened telomeres and serum protein biomarker abnormalities in collision sport athletes regardless of concussion history and sex. J Concussion. 2020;4:205970022097560. [Google Scholar]
- 37. Lipton ML, Gulko E, Zimmerman ME, et al. Diffusion-tensor imaging implicates prefrontal axonal injury in executive function impairment following very mild traumatic brain injury. Radiology. 2009;252(3):816–824. [DOI] [PubMed] [Google Scholar]
- 38. Kinnunen KM, Greenwood R, Powell JH, et al. White matter damage and cognitive impairment after traumatic brain injury. Brain. 2011;134(Pt 2):449–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ware AL, Wilde EA, Newsome MR, et al. A preliminary investigation of corpus callosum subregion white matter vulnerability and relation to chronic outcome in boxers. Brain Imaging Behav. 2020;14(3):772–786. [DOI] [PubMed] [Google Scholar]
- 40. Vallesi A, Mastrorilli E, Causin F, D’Avella D, Bertoldo A. White matter and task-switching in young adults: A diffusion tensor imaging study. Neuroscience. 2016;329:349–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Perry ME, McDonald CR, Hagler DJ Jr, et al. White matter tracts associated with set-shifting in healthy aging. Neuropsychologia. 2009;47(13):2835–2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shallice T, Stuss DT, Picton TW, Alexander MP, Gillingham S. Multiple effects of prefrontal lesions on task-switching. Front Hum Neurosci. 2007;1:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Goldstein A, Covington BP, Mahabadi N, Mesfin FB. Neuroanatomy, Corpus Callosum. In: StatPearls. Treasure Island (FL): StatPearls Publishing; July 31, 2021. https://pubmed.ncbi.nlm.nih.gov/28846239/ Accessed 20 May 2021. [PubMed] [Google Scholar]
- 44. Natali AL, Reddy V, Bordoni B. Neuroanatomy, Corticospinal Cord Tract. In: StatPearls. Treasure Island (FL): StatPearls Publishing; July 31, 2021. https://pubmed.ncbi.nlm.nih.gov/30571044/ Accessed 20 May 2021. [PubMed] [Google Scholar]
- 45. Taghdiri F, Chung J, Irwin S, et al. Decreased number of self-paced saccades in post-concussion syndrome associated with higher symptom burden and reduced white matter integrity. J Neurotrauma. 2018;35(5):719–729. [DOI] [PubMed] [Google Scholar]
- 46. Ledwidge SP, Patterson NJ, Molfese LD, et al. Clinical utility of oculomotor and electrophysiological measures in identifying concussion history. Clin J Sport Med. 2019;29(4):292–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Adler CM, Delbello MP, Weber W, et al. MRI evidence of neuropathic changes in former college football players. Clin J Sport Med. 2018;28(2):100–105. [DOI] [PubMed] [Google Scholar]
- 48. Jackson GD, Makdissi M, Pedersen M, et al. Functional brain effects of acute concussion in Australian rules football players. J Concussion. 2019;3:2059700219861200. [Google Scholar]
- 49. Churchill N, Hutchison M, Richards D, Leung G, Graham S, Schweizer TA. Brain structure and function associated with a history of sport concussion: A multi-modal magnetic resonance imaging study. J Neurotrauma. 2017;34(4):765–771. [DOI] [PubMed] [Google Scholar]
- 50. Vagnozzi R, Signoretti S, Tavazzi B, et al. Temporal window of metabolic brain vulnerability to concussion: A pilot 1H-magnetic resonance spectroscopic study in concussed athletes – Part III. Neurosurgery. 2008;62(6):1286–1296. [DOI] [PubMed] [Google Scholar]
- 51. Johnson B, Zhang K, Hallett M, Slobounov S. Functional neuroimaging of acute oculomotor deficits in concussed athletes. Brain Imaging Behav. 2015;9(3):564–573. [DOI] [PubMed] [Google Scholar]
- 52. Johnson B, Hallett M, Slobounov S. Follow-up evaluation of oculomotor performance with fMRI in the subacute phase of concussion. Neurology. 2015;85(13):1163–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rockswold SB, Burton PC, Chang A, et al. Functional magnetic resonance imaging and oculomotor dysfunction in mild traumatic brain injury. J Neurotrauma. 2019;36(7):1099–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wright DK, Symons GF, Brien WTO, et al. Diffusion imaging reveals sex differences in the white matter following sports-related concussion. Cereb Cortex. 2021;1–9. [DOI] [PubMed] [Google Scholar]
- 55. Hirad AA, Bazarian JJ, Merchant-Borna K, et al. A common neural signature of brain injury in concussion and subconcussion. Sci Adv. 2019;5(8):eaau3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zonner SW, Ejima K, Fulgar CC, et al. Oculomotor response to cumulative subconcussive head impacts in US high school football players: A pilot longitudinal study. JAMA Ophthalmol. 2019;137(3):265–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rubin LH, Tierney R, Kawata K, et al. NFL blood levels are moderated by subconcussive impacts in a cohort of college football players. Brain Inj. 2019;33(4):456–462. [DOI] [PubMed] [Google Scholar]
- 58. Giza C, Greco T, Prins ML. Concussion: Pathophysiology and clinical translation. Handb Clin Neurol. 2018;158:51-61. doi: 10.1016/B978-0-444-63954-7.00006-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available upon request to the corresponding author.