Abstract
Context: Syphilitic meningomyelitis is a rare manifestation of neurosyphilis, not well described in the literature.
Methods: We reported a rare case of a 29-year-old female with syphilitic meningomyelitis. Her clinical manifestations and imaging findings were discussed with the related literatures reviewed.
Results: The patient presented with progressive bilateral lower extremities numbness and weakness for months. Laboratory tests revealed positive serum Treponema pallidum Hemagglutinin Test (TPHA) and rapid plasma reagin test (RPR). The cerebral spinal fluid (CSF) was positive with TPHA but negative for RPR with lymphocytic pleocytosis and elevated protein. Spinal MRI showed swelling and high-signal intensity of thoracic spinal cord except T6-7 level with associated gadolinium enhancement (“flip-flop sign”) and peripheral strip-like enhancement on T1WI (“candle guttering appearance”). She was initially diagnosed as spinal cord tumor due to the chronic clinical onset and cord swelling with central enhancement found on thoracic MRI. After dramatic clinical and radiographic improvement with dexamethosone and serological tests of syphilis, she was diagnosed as probable syphilitic meningomyelitis. Till now, there are 12 cases of syphilitic myelitis reported with spinal cord MR images. Thoracic cord is the predominant involved segment (10/12), “candle guttering appearance” is the most common enhancing characteristics of the lesion (7/12), “flip-flop sign” may be seen in the stage with significant inflammation (3/12).
Conclusion: Syphilitic meningomyelitis can occur at early or late stage of syphilis, the onset may be acute, subacute or chronic. The imaging findings suggested focal inflammation of the spinal cord. Prognosis is relatively good after proper treatment.
Keywords: Neurosyphilis, Myelopathy, Magnetic resonance
Introduction
Neurosyphilis (NS) is defined as Treponema pallidum infection of the central nervous system during any stage of the disease.1 Unlike general paresis and tabes dorsalis, syphilitic meningomyelitis is considered a rare manifestation of the NS.2 And sometimes it can cause significant diagnostic and therapeutic challenges by mimicking other acute, subacute or chronic myelopathy, or space-occupying myelopathy. Here, we report the case of a 29-year-old woman suffering from syphilitic meningomyelitis with chronic clinical course and unusual imaging findings with the related literatures reviewed.
Case presentation
A 29-year-old female was admitted to our hospital with a history of intermittent fever for 9 months, associated with numbness of two lower limbs after 6 months from the beginning of the symptoms, and later progressing to right lower limb weakness for over 2 months period on April 2015. The peak of temperature presented by the patient was 39.1°C. The fever subsided several days after antipyretic treatment without antibiotics. This situation recurred several times for 3 months. Six months prior to the admission, she felt numbness on both feet, which ascended gradually to upper abdomen two months later. Two and half months prior to admission, she developed proximal weakness of the right leg and needed crutches to walk. She reported no autonomic dysfunction. Due to the cord swelling with central enhancement found on thoracic MRI ordered by a neurologist in local hospital, she was initially diagnosed as spinal cord tumor and admitted to neurosurgery department and received dexamethosone 10 mg per day and mannitol 250 ml every 6 h per day for three days. She had subsequent significant clinical and radiographic improvement. She was able to walk without assistance and the cord lesion diminished significantly. Subsequently, she was referred to neurology department. Upon further questioning, the patient revealed red pimples of the perineal region 2 years prior to the beginning of the symptoms that were relieved by Ofloxacin ointment. She denied venereal disease exposure and extramarital sex. She worked as a cashier in a bath center.
Her physical examination on admission showed that she was alert and oriented. The cranial-nerve functions were normal. Argyll Robertson pupils, which fail to react to light but constrict during near vision, were not observed. Muscle tones were normal. The muscle strength of the right leg was grade 4/5 (Oxford scale), other muscles strength was normal. Pinprick sensation below T7 level was decreased. Vibration sensation below T12 level was reduced. Bilateral finger-nose tests were normal. Bilateral heel-knee-shin tests showed slight dysmetria. Romberg’s sign (A sign which is swaying of the body that occurs when the eyes are closed.) was positive. She was unable to perform tandem gait well. She had brisk deep tendon reflexes in the right lower limb and right flexor plantar response.
Laboratory tests revealed normal hepatic, renal, thyroid function. She had negative angiotensin-converting enzyme, antinuclear antibodies, antineutrophil cytoplasmic antibodies, anticardiolipin antibodies and rheumatic factor. Serum immunoelectrophoresis and immunoglobulin studies showed no abnormalities. Serum lyme and brucella antibodies were negative. She had positive serum Treponema pallidum Hemagglutinin Test (TPHA) and rapid plasma reagin test (RPR, 1:4). CSF examination revealed 20 × 106 cells/l, 54 mg/dl protein, 37 mg/dl glucose and 112 mg/dl chloride. The cerebral spinal fluid (CSF) was positive with TPHA but negative for RPR. CSF revealed positive oligoband (OB), which indicated intrathecal IgG synthesis. CSF India ink stains were negative for cryptococcus. Bacterial growth was negative both on the blood and on the chocolate culture media Serum and CSF AQP-4 antibody was both negative.
Spinal MRI revealed swelling and diffuse high-signal intensity of the thoracic spinal cord parenchyma except T6-7 level with associated focal gadolinium at T6-7 level (“flip-flop sign”, Figs. 1 and 2). In T10 level, peripheral strip-like enhancement at the surface the spinal cord was shown on T1WI (“candle guttering appearance”, Fig. 2). Brain MRI was normal. The thoracic spinal cord lesion improved significantly after three-day dexamathosone treatment. She was diagnosed as probable syphilitic meningomyelitis based on clinical and imaging features and laboratory examinations. Patient received treatment with intravenous penicillin G 4 million units every 4 h for 14 days and dexamathosone 5 mg/d for 3 days before penicillin to prevent Jarisch-Herxheimer reaction (2015-4-29). Symptoms improved by day seven. By day 12 after treatment, her neurological examination showed normal muscle strength, right flexor plantar response and positive Romberg test. And the follow-up MRI showed limited cord lesion of T6-7 with a slight enhancement. Six months after treatment, she had complete clinical recovery.
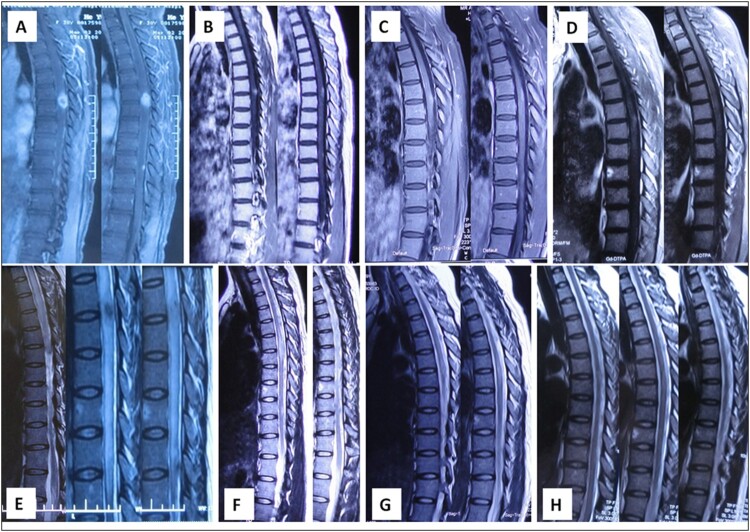
Figure 1.
The spinal cord MRI evolution of syphilic meningomyelitis. Spinal MRI revealed swelling and diffuse high-signal intensity of the whole thoracic spinal cord parenchyma except T6-7 level (E), and focal gadolinium enhancement at this T2WI-low-signal area (A) (“flip-flop sign”). The thoracic spinal cord lesion subsided after three-day dexamathosone treatment (B, F). Gadolinium-enhanced TIWI and T2WI before treatment (C, G, about one month after BF) and 12 days after treatment (D, H) showed further diminished lesion in spinal cord.
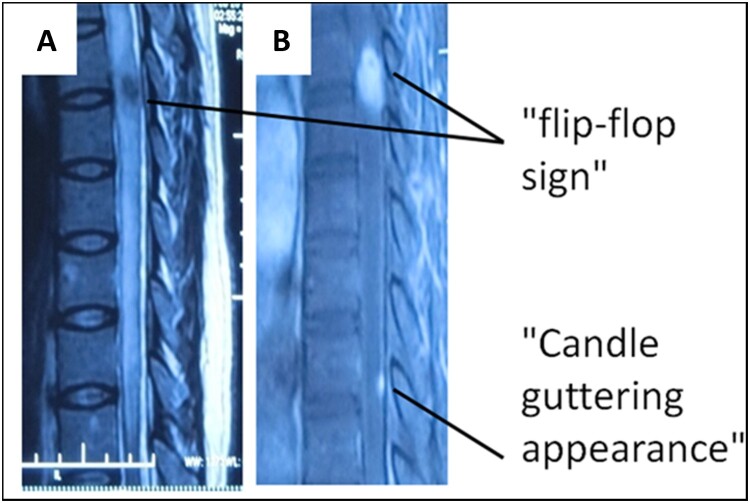
Figure 2.
“Flip-flop sign” and “Candle guttering appearance” of syphilic meningomyelitis “Flip-flop sign”: high-signal intensity of the thoracic spinal cord parenchyma except T6-7 level (A), and abnormally enhanced parenchyma were observed at this T2WI-low-signal area (B). “Candle guttering appearance”: peripheral strip-like enhancement from the surface of the spinal cord in T10 level.
Discussion
Syphilis is a sexually transmitted infectious disease caused by spirochete Treponema pallidum. The incidence of syphilis has decreased significantly with the discovery of penicillin. However, its prevalence has risen due to unprotected sex, mostly of men who have sex with men, and worldwide increased prevalence of acquired immune-deficiency syndrome.3,4 The incidence of NS is estimated to be about 4–10% in untreated syphilis.5 Syphilis can affect any part of the central nervous system at any stage of infection, and NS may be involved in the early course of the disease.1 There are several types of syphilitic myelopathy,2 some of them can happen in both early and late stages, including syphilitic meningomyelitis, spinal vascular syphilis, syphilitic poliomyelitis, and syphilitic spinal pachymeningitis (the last one including spinal cord gumma and syphilitic hypertrophic pachymeningitis). They can develop several weeks to several years after primary infection and affects meninges, vessels and spinal cord respectively or in different combinations. The late-stage syphilitic myelopathy mainly refers to as parenchymatous NS (tabes dorsalis/TD), the commonest type, which occurs 10–20 years after the primary infection and is the result of irreversible neuronal degeneration. The different types of syphilitic myelopathy may coexist and the pathogenesis may be diverse.
Our patient was misdiagnosed initially as a spinal cord tumor due to its chronic onset and MR findings of cord swelling with significant associated enhancement and edema. However, symptoms improved clinically and radiographically with dexamathosone. The diagnosis of syphilis requires use of two serological tests: a nontreponemal test (i.e. Venereal Disease Research Laboratory [VDRL] or Rapid Plasma Reagin [RPR]) and a treponemal test (i.e. fluorescent treponemal antibody absorbed [FTA-ABS] tests, the T. pallidum passive particle agglutination [TPPA] assay or the T. pallidum hemagglutination assay [TPHA]). In our patient, serum TPHA and RPR tests were positive, but CSF PRP test was negative. Patient's CSF showed mononuclear pleocytosis and elevated protein, in combination with a positive response to penicillin treatment. Based on those findings, the patient was diagnosed as probable NS following the diagnostic criteria.6 The subtype of NS could be syphilitic meningomyelitis based on her symptoms, perineal pimples prior history, spinal cord imaging characteristics and response to steroid and antibiotic.
Syphilitic meningomyelitis is an extremely rare manifestation of syphilis in the modern antibiotic era. It was first reported in 1944.7 Meningomyelitis develops 6 years (1–30) on average after initial infection.2 Thoracic spinal cord is the most commonly affected anatomical location. Patients presented with variable degrees of sensory levels, limb weakness, pyramidal signs, bladder and bowel dysfunction. Onset may be subacute or chronic, and occasionally it can be acute. The pathogenesis of meningomyelitis is diverse. It may be caused by direct involvement of the spinal cord parenchyma by the spirochetes infection, or may be the indirect result of inflammation effect on the meninges and blood vessels, or post-infectious immune-mediated demyelination on the spinal cord. Immune-mediated course may have a subacute duration, while meningo-vascular involvement usually leads to acute onset, versus gummatous NS often has subacute or chronic onset. In our patient, chronic progressive clinical presentation, spinal cord swelling, and the rapid recovery of symptoms and dramatic improvement of image findings after three days dexamathosone suggested meningomyelitis, which mainly resulted from immune-mediated process, rather than ischemia (spinal meningo-vascular syphilis). The diagnosis of NS is based on a CSF WBC count greater than or equal to 20 × 106 cells/l and/or a reactive CSF VDRL results. Without serological studies, the diagnosis of syphilitic meningomyelitis is difficult as it can mimic idiopathic transverse myelitis, acute disseminated encephalomyelitis (ADEM), spinal cord infarction, spinal arteriovenous malformation, spinal cord tumors, other subacute or chronic infectious myelopathy (such as tuberculosis or cryptococcosis).8–10
Tashiro et al. first described the MR imaging findings in syphilitic meningomyelitis,11 and only a few cases have been documented in the international literatures. Besides long extensive high-signal lesion on T2-weighted images of the spinal cord parenchyma, focal nodular enhancement and swelling spinal cord, there are two specific imaging features of syphilitic meningomyelitis mentioned,12 one is “flip-flop sign” which has gadolinium-enhanced T1-weighted images and reversed low-signal intensities on T2-weighted images (Fig. 1), the other is “candle guttering appearance” which refers to abnormal peripheral enhancement in the spinal cord parenchyma (Fig. 2). Our patient's imaging findings had these two signs. “Flip-flop sign” reflects severe parenchymatous inflammation with blood–spinal cord barrier breach. The low-signal intensity of T2-weighted image (T2WI) may be due to rich proteins products during the infectious and inflammatory process after Treponema invasion.13 It may be an indicative of different forms of granuloma. Similar characteristics could be seen occasionally in the MR image of tuberculoma and cryptococcoma as well.9,10 The “candle guttering appearance” on MRI suggests that the pathological process of NS invasion of the spinal cord is from its surface. For the past 30 years, since MR became available in clinical practice, there are nine cases of syphilitic myelitis reported with spinal cord MR images. We conclude that among the documented cases with spinal cord MRI in Table 1,11,12,14–22 the thoracic cord is the predominant part of involvement (10/12), whole spinal cord involvement for the other two cases, candle guttering appearance is the most common enhancing characteristics of the lesion (7/12), flip-flop sign may be seen occasionally in the stage probably with significant inflammation (3/12) . But these findings may be nonspecific and can be seen among other infectious myelopathies,9,10 occasionally primary spinal cord tumor and intramedullary metastases,23,24 which may need further differential diagnosis just like our case's situation. Unlike tabes dorsalis, most of the syphilitic meningomyelitis usually improve with penicillin treatment (complete 10/12, partial 2/12).
Table 1. Cases of syphilitic myelitis reported previously and current case.
| Case | Age | Sex | Time to the initial infection of syphilis | Time between onset to nadir | MRI findings of spinal cord | Treatment | Recovery |
|---|---|---|---|---|---|---|---|
| Tashiro-198711 | 31 | M | 7 months | 10 days | Thoracic spinal cord, enhancement at the level of T3/4 | Penicillin G with prednisolone | Yes(about 1 month later) |
| Strom-199114 | 28 | M | NA | 6 months | Hyperintense on T2 from T6-8 | Penicillin G with dexamethasone | Partial relief |
| Nabatame-199215 | 46 | M | NA | NA | Thoracic spinal cord, Candle guttering appearance and abnormal enhancement in the central part of spinal cord | Antibiotic treatment | Yes |
| Bulundwe-200016 | 53 | M | NA | 11 months | Hyperintense with swelling on T2 from T3-6 | Penicillin G | Yes |
| Tsui-200212 | 52 | F | 2 years | 5 days | Whole spinal cord, Candle guttering appearance and abnormal enhancement in the central part of spinal cord | Penicillin G with dexamethasone | wheelchair |
| Kikuchi-200317 | 36 | M | NA | 3 months | Abnormal signals involving the whole spinal cord, Candle guttering appearance and flip-flop sign | Penicillin G | Yes(about 1 month later) |
| Matijošaitis 200618 | 38 | M | About 1.5 years | 4 months | Hyperintense on T2 from T6-8, ring-like enhancement | Penicillin G with prednisone | Partial relief |
| Chilver-200919 | 46 | M | NA | 7 days | Abnormal signals below T6, Candle guttering appearance and abnormal enhancement in the central part of spinal cord | Penicillin G with methylprednisolone | Yes(about 3 months later) |
| Dongmei He-201420 | 63 | M | NA | 12 days | T6-11, Candle guttering appearance and abnormal enhancement in the central part of spinal cord | Ceftriaxone with methylprednisolone | Yes(about 3 months later) |
| Siu-201721 | 41 | M | NA | NA | spinal cord edema at C3 through T1 levels, with focal spinal cord enhancement at C6 | Penicillin G | Yes(about1 week later) |
| Sun-201822 | 50 | F | NA | 2.5 months | Hyperintense with swelling on T2 from T1 to T10, Candle guttering appearance with flip-flop sign | Penicillin G, Ceftriaxone with methylprednisolone | Yes(about1 week later) |
| This case | 29 | F | 2 years | 6 months | Whole thoracic spinal cord, Candle guttering appearance with flip-flop sign | Penicillin G with dexamethasone | Yes(about 6 months later) |
In conclusion, syphilitic myelitis is a very rare type of NS. Because of the nonspecific clinical and imaging findings, this treatable and potentially curable disease should be included in the differential diagnosis of unclear myelopathy. Serum and CSF serologic testing for syphilis are definitive in the diagnosis of these patients.
Disclaimer statements
Contributors None.
Conflict of interest The authors declare no conflict of interests.
Funding Statement
This work was supported by the National Key R&D Program of China, Precision Medicine Program, Cohort study on nervous system diseases [grant number 2017YFC0907700]; Beijing health system clinicians training plan [grant number 20143054]; the National Natural Science Foundation of China [grant number 81571633].
References
- 1.Bhai S, Lyons JL.. Neurosyphilis update: atypical is the new typical. Curr Infect Dis Rep. 2015;17(5):481–486. doi: 10.1007/s11908-015-0481-x [DOI] [PubMed] [Google Scholar]
- 2.Berger JR.Neurosyphilis and the spinal cord: then and now. J Nerv Ment Dis. 2011;199(12):912–913. doi: 10.1097/NMD.0b013e31823928e8 [DOI] [PubMed] [Google Scholar]
- 3.Kent ME, Romanelli F.. Reexamining syphilis: an update on epidemiology, clinical manifestations, and management. Ann Pharmacother. 2008;42(2):226–223. doi: 10.1345/aph.1K086 [DOI] [PubMed] [Google Scholar]
- 4.Cohen SE, Klausner JD, Engelman J, Philip S.. Syphilis in the modern era: an update for physicians. Infect. Dis. Clin. North Am. 2013;27(4):705–722. doi: 10.1016/j.idc.2013.08.005 [DOI] [PubMed] [Google Scholar]
- 5.Conde-Sendın MA, Amela-Peris R, Aladro-Benito Y, Maroto AA.. Current clinical spectrum of neurosyphilis in immunocompetent patients. Eur Neurol. 2004;52(1):29–35. doi: 10.1159/000079391 [DOI] [PubMed] [Google Scholar]
- 6.Berger JR, Dean D.. Neurosyphilis. Handb Clin Neurol. 2014;121:1461–1472. doi: 10.1016/B978-0-7020-4088-7.00098-5 [DOI] [PubMed] [Google Scholar]
- 7.Adams RD, Merritt HH.. Meningeal and vascular syphilis of the spinal cord. Medicine (Baltimore). 1944;23:181–214. doi: 10.1097/00005792-194405000-00003 [DOI] [Google Scholar]
- 8.Kitley JL, Leite MI, George JS, Palace JA.. The differential diagnosis of longitudinally extensive transverse myelitis. Multi Scler. 2012;18(3):271–285. doi: 10.1177/1352458511406165 [DOI] [PubMed] [Google Scholar]
- 9.Muthukumar N, Venkatesh G, Senthilbabu S, Rajbaskar R.. Surgery for intramedullary tuberculoma of the spinal cord: report of 2 cases. Surg Neurol. 2006;66(1):69–74. doi: 10.1016/j.surneu.2005.10.024 [DOI] [PubMed] [Google Scholar]
- 10.Gültaşli NZ, Ercan K, Orhun S, Albayrak S.. MRI findings of intramedullary spinal cryptococcoma. Diagn Interv Radiol. 2007;13(2):64–67. [PubMed] [Google Scholar]
- 11.Tashiro K, Moriwaka F, Sudo K, Akino M, Abe H.. Syphilitic myelitis with its magnetic resonance imaging (MRI) verification and successful treatment. Jpn J Psychiatry Neurol. 1987;41(2):269–271. [DOI] [PubMed] [Google Scholar]
- 12.Tsui EY, Ng SH, Chow L, Lai KF, Fong D, Chan JH.. Syphilitic myelitis with diffuse spinal cord abnormality on MR imaging. Eur Radiol. 2002;12(12):2973–2976. doi: 10.1007/s00330-001-1244-7 [DOI] [PubMed] [Google Scholar]
- 13.Zimny A, Neska-Matuszewska M, Bladowska J, Sasiadek MJ.. Intracranial lesions with Low signal intensity on T2-weighted MR images – review of Pathologies. Pol J Radiol. 2015;80:40–50. doi: 10.12659/PJR.892146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Storm T, Schneck SA.. Syphilitic meningomyelitis. Neurology. 1991;41(2(Pt1)):325–326. doi: 10.1212/WNL.41.2_Part_1.325 [DOI] [PubMed] [Google Scholar]
- 15.Nabatame H, Nakamura K, Matuda M, Fujimoto N, Dodo Y, Imura T.. MRI syphilitic myelitis. Neuroradiology. 1992;34(2):105–106. doi: 10.1007/BF00588152 [DOI] [PubMed] [Google Scholar]
- 16.Bulundwe KK, Myburgh CJ, Gledhill RF.. Syringomyelia complicating syphilitic spinal meningitis: a case report. Eur J Neurol. 2000;7(2):231–236. doi: 10.1046/j.1468-1331.2000.00047.x [DOI] [PubMed] [Google Scholar]
- 17.Kikuchi S, Shinpo K, Niino M, Tashiro K.. Subacute syphilitic meningomyelitis with characteristic spinal MRI findings. J Neurol. 2003;250(1):106–107. doi: 10.1007/s00415-003-0921-7 [DOI] [PubMed] [Google Scholar]
- 18.Matijošaitis V, Vaitkus A, Pauza V, Valiukeviciene S, Gleizniene R.. Neurosyphilis manifesting as spinal transverse myelitis. Medicina (Kaunas). 2006;42(5):401–406. [PubMed] [Google Scholar]
- 19.Chilver-Stainer L, Fischer U, Hauf M, Fux CA, Sturzenegger M.. Syphilitic myelitis: rare, nonspecific, but treatable. Neurology. 2009;72(7):673–675. doi: 10.1212/01.wnl.0000342460.07764.5c [DOI] [PubMed] [Google Scholar]
- 20.He D, Jiang B.. Syphilitic myelitis: magnetic resonance imaging features. Neurol India. 2014;62(1):89–91. doi: 10.4103/0028-3886.128347 [DOI] [PubMed] [Google Scholar]
- 21.Siu G.Syphilitic meningomyelitis. J Amer Osteo Assoc. 2017;117(10):670–671. [DOI] [PubMed] [Google Scholar]
- 22.Sun L, Zheng N, Yang Y, Zhang HN.. Syphilitic meningomyelitis presenting with visceral crisis: a case report. Medicine (Baltimore). 2018;97(30):e11661. doi: 10.1097/MD.0000000000011661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farrokh D, Fransen P, Faverly D.. MR findings of a primary intramedullary Malignant Melanoma: case report and literature review. Am J Neuroradiol. 2001;22(10):1864–1866. [PMC free article] [PubMed] [Google Scholar]
- 24.Diehn FE, Rykken JB, Wald JT, Wood CP, Eckel LJ, Hunt CH, Schwartz KM, Lingineni RK, Carter RE, Kaufmann TJ.. Intramedullary spinal cord metastases: prognostic value of MRI and clinical features from a 13-year institutional case series. Am J Neuroradiol. 2015;36(3):587–593. doi: 10.3174/ajnr.A4160 [DOI] [PMC free article] [PubMed] [Google Scholar]