Abstract
Objective: To evaluate the diaphragm thickness and excursion in patients with cervical spinal cord injury and reliability of diaphragmatic ultrasonography.
Design: A Pilot Case–Control Study.
Setting: China Rehabilitation Research Center (CRRC) /Beijing BO AI Hospital.
Participants: Sixty participants with cervical spinal cord injury and sixty control participants were eligible for inclusion in this study.
Interventions: Ultrasonographic evaluation of the diaphragm.
Outcome Measures: All demographic data were evaluated. Diaphragm thickness, thickening ratio, and diaphragm excursions were assessed at the end of quiet tidal breathing and maximal inspiration. The reliability of inter- and intra-ultrasonography operators were evaluated.
Results: Diaphragm thickness was significantly higher in patients with cervical spinal cord injury than the control group (P < 0.001). Diaphragmatic excursion of the right hemidiaphragm was significantly greater in patients with cervical spinal cord injury than the control group (P < 0.001) at the end of quiet tidal breathing. No difference was found in diaphragmatic excursion between two groups (P = 0.32) at the end of maximal inspiration. No significant difference was shown between two groups in thickening ratio. Intraclass correlation coefficients of inter-and intra-ultrasonography operators for the thickness and excursions of the diaphragm were greater than 0.93.
Conclusion: Compared with the control group the diaphragm in patients with cervical spinal cord injury is hypertrophied and the diaphragm excursion is greater. Ultrasound is a highly reliable tool for the evaluation of diaphragm thickness and excursion in patients with cervical spinal cord injury.
Trial Registration: This trail was registered in Chinese Clinical Trial Registry (NO. ChiCTR-ROC-17010973).
Keywords: Diaphragm, Ultrasound, Spinal cord injury, Reliability
Introduction
Lesions in the cervical or thoracic segments of the spinal cord dramatically affect the neural activation of respiratory muscles resulting in significant respiratory dysfunction.1,2 The diaphragm is innervated from C3-C5 spinal segments, patients with injuries above C5 often require mechanical ventilation. When the injury is below the phrenic motor neurons, the diaphragm and sternocleidomastoid muscles may operate normally but expiratory muscles are paralyzed. This may affect diaphragm function because of the loss of abdominal fulcrum for diaphragm descent during tidal inspiration. Hence, precise assessment of diaphragmatic excursion is important for efficient clinical management and return to normal breathing function in cervical spinal cord injury (SCI) patients.
With the advantage of being non-invasive and non-ionizing, ultrasonography is a highly sensitive tool and widely applied to assess diaphragmatic function.3,4 Several neuromuscular ultrasound techniques have been utilized to evaluate diaphragm, including B-mode, M-mode, and measurements of changes in diaphragm thickness.5 The M-mode technique shows high correlation coefficients between and within observers.6 Diaphragm ultrasonography is also useful for objective evaluation of pulmonary function. The reason for this was that there was a significant correlation between diaphragm thickness and forced vital capacity in neuromuscular disorders.7 But ultrasound imaging of the diaphragm is an under-used tool in patients with cervical SCI. A previous study used diaphragm ultrasounds in patients with cervical SCI and found that imaging of the diaphragm movement was well tolerated by subjects with cervical SCI. It also found that diaphragm movement was not fully explained according to the level of injury and to the American Spinal Injury Association classification.8 After inspiratory muscle training, the ultrasound showed significantly increased diaphragm thickness in patients with cervical SCI.9 Studies detailing the basic structure and motion of the diaphragm in patients with cervical SCI are limited. We conducted this study to evaluate whether the diaphragm thickness and excursion of patients with cervical SCI were comparably normal to that of able-bodied participants. The secondary purpose of this study was to examine reliability, inter- and intra- operators of the diaphragm ultrasonography in patients with cervical SCI.
Materials and methods
This study was conducted at the department of spinal and neural functional reconstruction of China Rehabilitation Research Center (CRRC) /Beijing BO AI Hospital for adults between March 2017 and October 2017. Ethical clearance was provided through the Center of Human Research ethics committee (NO.2017-015-1). All the participants provided written informed consent. This trail was registered in Chinese Clinical Trial Registry (NO. ChiCTR-ROC-17010973). Patients were included if they: (1) were between 18 and 60 years of age; (2) suffered from motor-complete cervical SCI (Neurological level C4-C8; American Spinal Injuries Association Impairment Scale A or B); (3) suffered for less than one year since injury and had no history of mechanical ventilation. Patients were excluded if: (1) they had abnormal chest structures, which affected diaphragmatic function; (2) had any history of dyspnea or generalized neuromuscular disease, such as peripheral neuropathy, myopathy, motor neuron disease, or central nervous system disease; (3) ever received respiratory rehabilitation or mobilization; (4) or refuse to participate in the study. In healthy control participants matched on sex and BMI were recruited. Participants were excluded if (1) they had abnormal chest structures, which affected diaphragmatic function; (2) had any history of dyspnea or generalized neuromuscular diseases, such as peripheral neuropathy, myopathy, motor neuron disease, or central nervous system disease.
All participants were examined during spontaneous respiration to help investigate the motion of the diaphragm. The patients were positioned in the supine position as it results in less overall variability, less side-to-side variability, and greater reproducibility.10 Ultrasonography was performed by two experienced operators using “Samsung Medison” Ultrasonic Imaging System (type. SONOACE R3).
Diaphragm thickness was assessed following the procedures described by earlier authors.11,12 A two-dimensional B-mode ultrasound was used to measure the diaphragm thickness at the zone of apposition using a higher frequency linear array transducer (7.5 MHz). The thickening ratio of the diaphragm (TR) was calculated. TR was standardized using the following formula: TR = thickness at maximal inspiration/thickness at the end of quiet tidal breathing.
The anterior subcostal view was used to evaluate diaphragm excursion. A lower frequency curvilinear transducer (3.5 MHz) was placed between the mid-clavicular and anterior axillary lines, in the anterior subcostal region. The two-dimensional model was initially used to visualize the diaphragm, the M-mode was then used to display the excursion of the diaphragm. Ultrasonographic measurements were performed at the end of quiet tidal breathing and maximal inspiration. All Variables were the average of three different breathing cycles.
To assess inter-operator reliability, 10 separate patients with cervical SCI were randomly selected for the study, who were examined by 2 different operators. To test intra-operator reliability, 10 patients with cervical SCI were examined twice in two separate days by the same operator.
Statistical analysis
Continuous variables were expressed as Mean+/–Standard Deviation. For normally distribution variables unpaired Student’s t-test was used. For non-normally distributed variables the Mann–Whitney U test was used. Interrater reliability was evaluated for all operators by using intraclass correlation coefficients (ICCs, 95% confidence interval) statistics. Statistical analysis was performed by using SPSS v20.0 for Windows (SPSS Inc. Chicago, Illinois, USA). Differences were considered significant if P < 0.05.
Results
Ultrasonography of diaphragm thickness and excursion
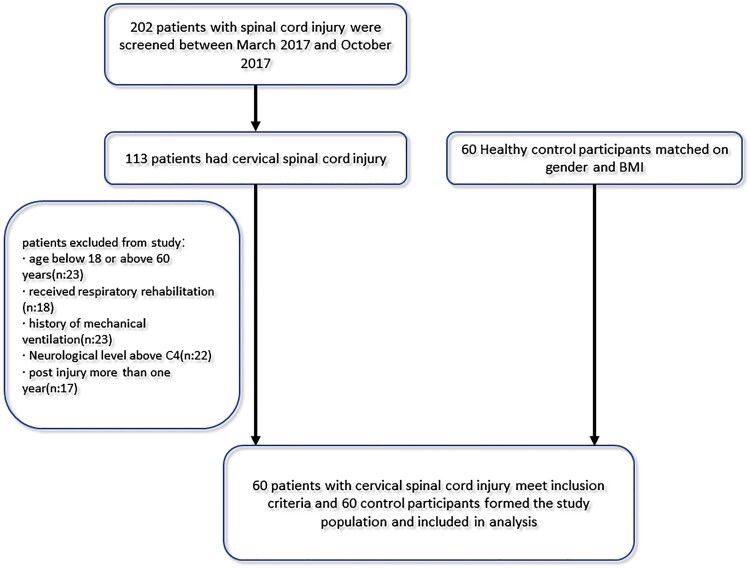
In total, 60 patients with cervical SCI, who met the criteria, and 60 able-bodied participants were recruited (Fig. 1). Table 1 summarizes the demographic data relating to patients with cervical SCI and able-bodied participants. Table 2 shows the diaphragm thickness, thickening ratio at the end of quiet tidal breathing and maximal inspiration in patients with cervical SCI and able-bodied participants. Compared with the able-bodied participant’s diaphragm thickness on both sides were significantly increased in patients with cervical SCI (P < 0.001). No significant differences in thickening ratio were seen between the two groups. Table 3 shows the right diaphragmatic excursions in able-bodied participants and patients with cervical SCI. At the end of quite tidal breathing the diaphragmatic excursion of the right hemidiaphragm was significantly greater in patients with cervical SCI (2.17 ± 0.56, cm) than in able-bodied participants (1.48 ± 0.26, cm, P < 0.001). At the end of maximal inspiration, no difference was found in diaphragmatic excursion between patients with cervical SCI (4.60 ± 0.82, cm) and able-bodied participants (4.79 ± 1.23, cm, P = 0.32).
Figure 1.
Flow chart of study participants.
Table 1. Demographics of patients with cervical SCI and control group.
| Demographic | Patients With CSCI (n = 60) | control group (n = 60) | P value |
|---|---|---|---|
| Age, year | |||
| Mean ± SD | 39.6 ± 15.6 | 43.1 ± 11.2 | 0.16 |
| 95% CI | 35.6–43.8 | 40.3–46.1 | |
| Sex, No. | |||
| Male | 45 | 41 | 0.42 |
| Female | 15 | 19 | |
| Height (in meters) | 1.68 ± 0.09 | 1.70 ± 0.08 | 0.16 |
| Weight (in Kg) | 64.3 ± 11.4 | 64.8 ± 12.6 | 0.83 |
| BMI, kg/m2 | |||
| Mean ± SD | 23.8 ± 3.5 | 23.2 ± 3.6 | 0.32 |
| 95% CI | 22.9–24.7 | 22.3–24.1 | |
| Smoking (%) | 20(30%) | 16(26.7%) | 0.43 |
| ASIA classification | … | … | |
| A | 39 | … | … |
| B | 21 | … | … |
| Neurological level (n) | … | … | |
| Above C4 | 0 | … | … |
| C4 | 24 | ||
| C5 | 22 | ||
| C6 | 8 | ||
| C7 | 4 | ||
| C8 | 2 | … | … |
| Motor level (n) | |||
| Above C4 | 0 | ||
| C4 | 21 | ||
| C5 | 23 | ||
| C6 | 9 | ||
| C7 | 4 | ||
| C8 | 3 | ||
| Time since injury, month | … | … | |
| Mean ± SD | 6.1 ± 2.7 | … | … |
| 95% CI | 5.5–6.9 | … | … |
Table 2. Diaphragm muscle thickness (in cm), thickening ratio in patients with cervical SCI and control group.
| Diaphragm thickness | CSCI patients (mean+/–SD) (5th–95th) | control group (mean+/–SD) (5th–95th) | P value |
|---|---|---|---|
| Right- tidal breathing | 0.20 ± 0.026* (0.19–0.21) |
0.16 ± 0.04 (0.15–0.18) |
0.000 |
| Right-max | 0.37 ± 0.04* (0.36–0.38) |
0.31 ± 0.08 (0.30–0.33) |
0.000 |
| Left- tidal breathing | 0.21 ± 0.03* (0.20–0.21) |
0.17 ± 0.04 (0.16–0.18) |
0.000 |
| Left-max | 0.37 ± 0.05* (0.36–0.38) |
0.32 ± 0.08 (0.30–0.34) |
0.000 |
| Right TR | 1.87 ± 0.24 (1.78–1.91) |
1.99 ± 0.48 (1.81–2.04) |
.081 |
| Left TR | 1.84 ± 0.26 (1.81–1.93) |
1.93 ± 0.47 (1.86–2.11) |
0.228 |
tidal breathing = diaphragm thickness at the end of tidal breathing; max = diaphragm thickness at maximum inspiration; TR = thickening ratio.
*Statistically significant difference from “control group”.
Table 3. Right diaphragmatic excursions and limit values in patients with cervical SCI and control group (in cm).
| Breath pattern | CSCI patients (mean +/– SD) (5t–95th) | control group (mean +/– SD) (5t–95th) | P value |
|---|---|---|---|
| Quiet breathing | 2.17 ± 0.56* (2.02–2.31) |
1.48 ± 0.26 (1.42–1.55) |
0.000 |
| Deep breathing | 4.60 ± 0.82 (4.39–4.80) |
4.79 ± 1.23 (4.47–5.10) |
0.318 |
*Statistically significant difference from “control group”.
Reliability of ultrasound measurements
Inter-operator reliability and intra-operator reliability were evaluated in 10 patients, respectively, and it was found to be very high. Inter-operator reliability ICCs were 0.95 (95% confidence interval 0.83, 0.99) for thickness at the end of normal expiration and 0.98 (95% CI 0.91,0.99) for thickness at the end of maximal inspiration. Inter-operator reliability ICCs were 0.96 (95% CI 0.86,0.99) for quiet tidal breathing diaphragm excursion and 0.94 (95% CI 0.78,0.99) for maximal inspiration diaphragm excursion.
Intra-operator reliability ICCs were 0.93 (95% CI 0.75,0.98) for thickness at the end of normal expiration and 0.94 (95% CI 0.78,0.99) for thickness at end of maximal inspiration. Intra-operator reliability ICCs were 0.99 (95% CI 0.96,1.0) for quiet tidal breathing diaphragm excursion and 0.93 (95% CI 0.74,0.98) for maximal inspiration diaphragm excursion.
Discussion
Ultrasound imaging of the diaphragm is an accurate and reproducible tool for diagnosis of neuromuscular diaphragm dysfunction.13 This study finds diaphragmatic hypertrophy in patients with low-level cervical SCI. In patients with low-level cervical SCI, the diaphragm thickness of both the left and the right side is statistically thicker than able-bodied participants, but the thickening ratio is not significantly different. Diaphragm atrophy is common in high-level cervical SCI due to mechanical ventilation (MV). Diaphragm muscle thinning begins within the 48 h after initiation of MV, and the length of MV is associated with the degree of diaphragmatic atrophy.14 This study finds no diaphragm atrophy occurred in low-level cervical SCI patients. This is mainly because paralysis of accessory muscles (the intercostals and scalenes) leads to an abnormal chest wall compliance, and the expansion of the thoracic cavity was limited. The diaphragm becomes hypertrophy to compensate for the denervation of all other respiratory muscles. One study15 showed that participants with cervical SCI exhibited significantly higher EMG activity than non-injured participants in the inspiratory muscles and the diaphragm region. The findings demonstrated the compensatory recruitment of accessory muscles and overactivity of the diaphragm in patients with cervical SCI.
Diaphragm thickening ratio (TR) is used to identify the contractility of the diaphragm16 and normal values have been published.17 A positive correlation was found between increased thickening ratio and interval changes in vital capacity (VC) and maximal inspiratory pressure.16 In patients with amyotrophic lateral sclerosis, the TR was highly correlated with %VC.18 In this study, there were no significant differences in thickening ratio between the two groups. This could be an indirect finding of that, even though the compensatory thickening of the diaphragm occurred, there was no corresponding increase in the contractile function of the diaphragm.
Diaphragm excursion was also seen as an index of diaphragmatic contractile activity19,20 and mainly related to the inspired volume.21 Ultrasound imaging measurement is useful to accurately evaluate diaphragm excursions at the end of tidal breathing.22 In tetraplegia, distortion of the respiratory system causes inefficient ventilation. During spontaneous breathing, the lack of activity in the external intercostal muscles causes distortion where the upper anterior rib cage moves inward during inhalation diminishing the extent of rib-cage expansion that the diaphragm can contribute to.23 This study confirms this: at the end of quiet tidal breathing, diaphragm excursion in patients with cervical SCI was greater than control participants when compensating for insufficient tidal volume. Meanwhile, during maximal inspiration, no diaphragm excursion difference was found.
The findings in this study have vital functional and clinical significance. Ultrasound is a reliable instrument for measuring diaphragm movement in patients with cervical SCI. Evaluating the respiratory function of patients with cervical SCI is important. The diaphragm function is an important determinant of long-term respiratory recovery. Whether the changes in the diaphragm impact clinical outcomes? Further research should aim at clarifying the relation between t pulmonary function test results and thickening of the diaphragm in patients with cervical SCI.
Study limitations
This study has some limitations. First, this study only assessed, the right hemidiaphragm excursions because the small window of the spleens left hemidiaphragm on imaging is often impeded by gastric and intestinal gas interposition; at the end of maximal inspiration, the lung frequently masked the dome. Second, this study did not correlate diaphragm thickening ratio and baseline diaphragm thickness with pulmonary function. Third, this study involved a small number of participants; this study selected patients according to a strict criterion. Hence, there may be a limitation when generalizing these results to all patients with cervical SCI.
Conclusions
Overall, compared with able-bodied participants, the diaphragms in patients with cervical SCI tends to be hypertrophic and the diaphragm excursion is greater at the end of quite tidal breathing. However, whether this compensatory strategy is enough needs to be investigated. Ultrasound is a highly reliable tool for the evaluation of diaphragm thickness and excursion in patients with cervical spinal cord injury.
Acknowledgment
We also like to thank all other members from the department of Spinal and Neural Function Reconstruction, Beijing Bo Ai Hospital, China Rehabilitation Research Center for their help that they offered.
Funding Statement
This work was supported by the Basic Scientific Research Foundation of China Rehabilitation Research Center [grant number 2017ZX-21].
Disclaimer statements
Ethical statements The study was approved by the Medical Ethics Committee of the Hospital, and all subjects provided written informed consent.
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Branco F, Cardenas DD, Svircev JN.. Spinal cord injury: a comprehensive review. Phys Med Rehabil Clin N Am 2007;18(4):651–79. doi: 10.1016/j.pmr.2007.07.010 [DOI] [PubMed] [Google Scholar]
- 2.Postma K, Post MWM, Haisma JA, Stam HJ, Bergen MP, Bussmann JBJ.. Impaired respiratory function and associations with health-related quality of life in people with spinal cord injury. Spinal Cord. 2016;54(10):866–71. doi: 10.1038/sc.2016.18 [DOI] [PubMed] [Google Scholar]
- 3.Sferrazza PG, Pellegrino GM, Di Marco F, Imeri G, Brochard L, Goligher E, et al. . A review of the ultrasound assessment of diaphragmatic function in clinical practice. Respiration. 2016;91:403–11. doi: 10.1159/000446518 [DOI] [PubMed] [Google Scholar]
- 4.Boon AJ, O’Gorman C.. Ultrasound in the assessment of respiration. J Clin Neurophysiol 2016;33(2):112–9. doi: 10.1097/WNP.0000000000000240 [DOI] [PubMed] [Google Scholar]
- 5.Sarwal A, Walker FO, Cartwright MS.. Neuromuscular ultrasound for evaluation of the diaphragm. Muscle Nerve. 2013;47(3):319–29. doi: 10.1002/mus.23671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boussuges A, Gole Y, Blanc P.. Diaphragmatic motion studied by m-mode ultrasonography: methods, reproducibility, and normal values. Chest. 2009;135(2):391–400. doi: 10.1378/chest.08-1541 [DOI] [PubMed] [Google Scholar]
- 7.Noda Y, Sekiguchi K, Kohara N, Kanda F, Toda T.. Ultrasonographic diaphragm thickness correlates with compound muscle action potential amplitude and forced vital capacity. Muscle Nerve. 2016;53(4):522–7. doi: 10.1002/mus.24902 [DOI] [PubMed] [Google Scholar]
- 8.Hardy F, Walker J, Sawyer T.. Sonographic measurement of diaphragm movement in patients with tetraplegia. Spinal Cord. 2009;47(11):832–4. doi: 10.1038/sc.2009.45 [DOI] [PubMed] [Google Scholar]
- 9.West CR, Taylor BJ, Campbell IG, Romer LM.. Effects of inspiratory muscle training on exercise responses in Paralympic athletes with cervical spinal cord injury. Scand J Med Sci Sports 2014;24(5):764–72. doi: 10.1111/sms.12070 [DOI] [PubMed] [Google Scholar]
- 10.Gierada DS, Curtin JJ, Erickson SJ, Prost RW, Strandt JA, Goodman LR.. Diaphragmatic motion: fast gradient-recalled-echo MR imaging in healthy subjects. Radiology. 1995;194(3):879–84. doi: 10.1148/radiology.194.3.7862995 [DOI] [PubMed] [Google Scholar]
- 11.Boon AJ, Harper CJ, Ghahfarokhi LS, Strommen JA, Watson JC, Sorenson EJ.. Two-dimensional ultrasound imaging of the diaphragm: quantitative values in normal subjects. Muscle Nerve. 2013;47(6):884–9. doi: 10.1002/mus.23702 [DOI] [PubMed] [Google Scholar]
- 12.Francis CA, Hoffer JA, Reynolds S.. Ultrasonographic evaluation of diaphragm thickness during mechanical ventilation in intensive care patients. Am J Crit Care. 2016;25(1):e1–8. doi: 10.4037/ajcc2016563 [DOI] [PubMed] [Google Scholar]
- 13.Boon AJ, Sekiguchi H, Harper CJ, Strommen JA, Ghahfarokhi LS, Watson JC, et al. . Sensitivity and specificity of diagnostic ultrasound in the diagnosis of phrenic neuropathy. Neurology. 2014;83(14):1264–70. doi: 10.1212/WNL.0000000000000841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grosu HB, Lee YI, Lee J, Eden E, Eikermann M, Rose KM.. Diaphragm muscle thinning in patients who are mechanically ventilated. Chest. 2012;142(6):1455–60. doi: 10.1378/chest.11-1638 [DOI] [PubMed] [Google Scholar]
- 15.Terson de Paleville D, Lorenz D.. Compensatory muscle activation during forced respiratory tasks in individuals with chronic spinal cord injury. Respir Physiol Neurobiol 2015;217:54–62. doi: 10.1016/j.resp.2015.07.001 [DOI] [PubMed] [Google Scholar]
- 16.Summerhill EM, El-Sameed YA, Glidden TJ, McCool FD.. Monitoring recovery from diaphragm paralysis with ultrasound. Chest. 2008;133(3):737–43. doi: 10.1378/chest.07-2200 [DOI] [PubMed] [Google Scholar]
- 17.Mertens L.Diaphragmatic paralysis after cardiac surgery: how to look at it? Pediatr Crit Care Med 2006;7:491–2. doi: 10.1097/01.PCC.0000225029.88916.2D [DOI] [PubMed] [Google Scholar]
- 18.Hiwatani Y, Sakata M, Miwa H.. Ultrasonography of the diaphragm in amyotrophic lateral sclerosis: clinical significance in assessment of respiratory functions. Amyotroph Lateral Scler Frontotemporal Degener 2013;14(2):127–31. doi: 10.3109/17482968.2012.729595 [DOI] [PubMed] [Google Scholar]
- 19.Testa A, Soldati G, Giannuzzi R, Berardi S, Portale G, Gentiloni Silveri N.. Ultrasound M-mode assessment of diaphragmatic kinetics by anterior transverse scanning in healthy subjects. Ultrasound Med Biol 2011;37(1):44–52. doi: 10.1016/j.ultrasmedbio.2010.10.004 [DOI] [PubMed] [Google Scholar]
- 20.Soilemezi E, Tsagourias M, Talias MA, Soteriades ES, Makrakis V, Zakynthinos E, et al. . Sonographic assessment of changes in diaphragmatic kinetics induced by inspiratory resistive loading. Respirology. 2013;18(3):468–73. doi: 10.1111/resp.12011 [DOI] [PubMed] [Google Scholar]
- 21.Houston JG, Angus RM, Cowan MD, McMillan NC, Thomson NC.. Ultrasound assessment of normal hemi-diaphragmatic movement: relation to inspiratory volume. Thorax. 1994;49(5):500–3. doi: 10.1136/thx.49.5.500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dong KN, Jae JL, Joshua HY.. Diaphragm breathing movement measurement using ultrasound and radiographic imaging: a concurrent validity. Bio Med Mater Eng 2014;24(1):947–52. doi: 10.3233/BME-130889 [DOI] [PubMed] [Google Scholar]
- 23.Brown R, DiMarco AF, Hoit JD, Garshick E.. Respiratory dysfunction and management in spinal cord injury. Respir Care. 2006;51(8):853–70. [PMC free article] [PubMed] [Google Scholar]