Abstract
Patient: Female, 68-year-old
Final Diagnosis: Sarcoidosis
Symptoms: Dysphagia • epigastric pain • nausea • vomiting
Medication: —
Clinical Procedure: —
Specialty: Gastroenterology and Hepatology
Objective:
Rare disease
Background:
Sarcoidosis is a multisystem granulomatous disease with predominant pulmonary involvement and rare gastrointestinal (GI) involvement. The stomach is the most common site when there is GI involvement. Symptomatic gastric sarcoidosis with biopsy-proven disease has rarely been reported and much of the knowledge is from case reports involving white patients.
Case Report:
Our unique case involves a flare of gastric sarcoid in an African American patient with biopsy-proven disease and we highlight our unique broad, multidisciplinary treatment approach that has not been described previously. A 68-year-old woman with pulmonary sarcoidosis presented with epigastric pain, nausea, vomiting, and dysphagia. The diagnosis of gastric sarcoid was made several years prior based on an upper endoscopy biopsy showing non-caseating granulomas in the antrum. She had previously experienced minimal relief of gastric symptoms with corticosteroids. In addition to a steroid taper, the patient experienced improvement in symptoms with a PPI (proton pump inhibitor), bowel regimen, and speech therapy techniques.
Conclusions:
Gastric symptoms can be a presenting sign for a sarcoid flare in a patient with pulmonary sarcoidosis, which is important for both pulmonologists and gastroenterologists to recognize. In addition to traditional therapy with corticosteroids, our unique broader, multidisciplinary approach with PPI, bowel regimen, and speech therapy techniques such as a liquid wash are important components of treatment for gastric sarcoid that have not been described in previous case reports.
Keywords: Gastrointestinal Diseases, Granuloma, Sarcoidosis, Deglutition Disorders, Treatment Outcome
Background
Sarcoidosis is a multisystem granulomatous disease characterized histologically by non-caseating granulomas, with nearly 90% of patients having involvement of the lungs. Worldwide, patients of northern European descent are more often affected; while in the United States, African Americans are more commonly affected and have a more persistent and severe form of the disease [1]. Gastrointestinal involvement (GI) is rare, with reported incidence of less than 1% of patients with sarcoidosis, but the true incidence is unknown as many cases are subclinical [1]. The stomach is the most common site of GI involvement. Symptomatic gastric sarcoidosis with biopsy-proven disease has rarely been reported, and much of our knowledge is from case reports involving white patients, who are the racial group predominantly affected by this disease. In the literature, there are few cases of African American patients with gastrointestinal sarcoidosis. Here, we present a rare case involving a flare of gastric sarcoidosis in an African American patient with biopsy-proven disease and we highlight our unique broad, multidisciplinary treatment approach that has not been described previously.
Case Report
A 68-year-old African American woman with pulmonary and known GI sarcoidosis presented with epigastric pain, nausea, vomiting, and dysphagia for 2 months. Pulmonary sarcoidosis was diagnosed 10 years prior and she was on methotrexate for maintenance therapy as well as intermittent steroid tapers due to frequent pulmonary sarcoid flare-ups. The diagnosis of GI sarcoidosis was made 3 years ago when she presented to a GI clinic with epigastric pain, bloating, and emesis. Upper endoscopy showed a 2-cm hiatal hernia with a normal-appearing Z-line, stomach, and esophagus; a biopsy showed non-case-ating granulomas in the antrum (see Figure 1). She was continued on treatment with methotrexate and a PPI was added to her medication regimen, which she infrequently took since it provided only partial relief.
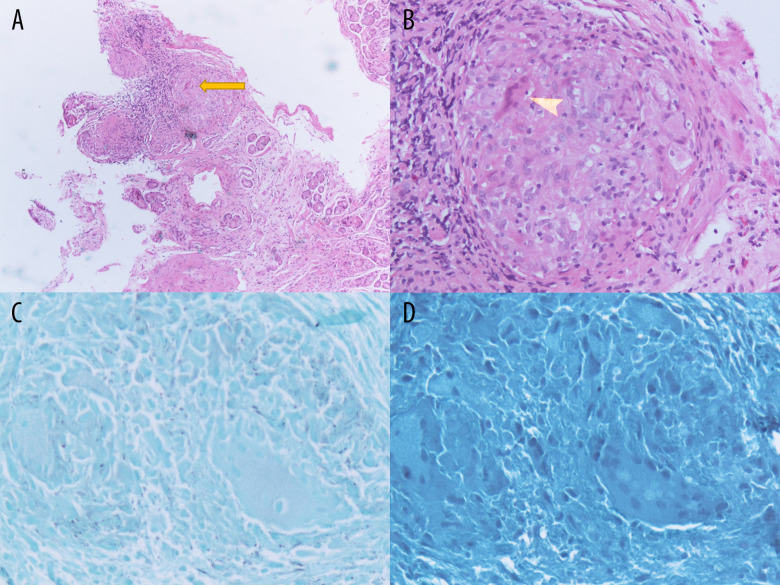
Figure 1.
Gastrointestinal sarcoidosis. Histopathology of the upper-endoscopic gastric antrum biopsies with H&E staining revealing non-caseating epithelioid cell granulomas (arrow heads) without dysplasia or metaplasia at low power (A) and high power (B). GMS (C) and AFB (D) stains negative to rule out fungi and mycobacteria.
During this presentation to the hospital, she had 2 months of worsening epigastric pain, dysphagia, and non-bloody, non-bilious emesis. She denied association with food intake, blood in stool, constipation, weight changes, and odynophagia. Vitals were unremarkable and her oxygen saturation was >95% on her home oxygen requirement of 4 L by nasal cannula. Her pulmonary status and oxygen requirement were at her baseline. Her abdomen was soft, non-tender, and non-distended, with normoactive bowel sounds and no rebound tenderness or guarding. Laboratory values demonstrated a baseline, stable leukocytosis of 14 K/mm3 and serum potassium level of 2.8 mmol/L. Liver-associated enzymes were normal (AST 21 U/L, ALT 34 U/L, alkaline phosphatase 115 U/L, total bilirubin 0.3 mg/dL), and stool H. pylori was negative.
With her symptoms of epigastric pain and vomiting, similar to her symptoms when gastric sarcoidosis was initially diagnosed, recurrence of gastric sarcoidosis was diagnosed without performing a repeat upper endoscopy. She was started on corticosteroid therapy with prednisone 30 mg daily with a planned 6-month slow taper. She was also placed on a PPI and H2 blocker and given a bowel regimen. Her symptoms persisted without significant change, so Speech Therapy was consulted on hospital day 4. A modified barium swallow (MBS) during this presentation showed esophageal dysmotility with stasis of contrast in the upper esophagus and tertiary contractions in the mid-esophagus. Speech Therapy recommended a liquid wash during solid meals to aid in bolus transit. This Speech Therapy strategy instructs patients to have alternating liquids and solids to aid in esophageal clearance of the food bolus. The Gastroenterology consultation service was following her case and determined on hospital day 7 (after 2 days of multi-disciplinary intervention) that she had objective improvements in her ability to advance her diet, more meals being tolerated, decreased use of antiemetics, and decreased quantitative reports of number of abdominal pain episodes. An inpatient repeat endoscopy was not done because her symptoms improved with our multidisciplinary approach. Post-hospitalization follow-up 5 months later found she had intermittent right-sided pain and mild bloating without dysphagia, epigastric pain, or emesis described during her hospitalization. No repeat endoscopic procedure was planned. Her clinical symptoms, prior interventions, current interventions described in this case, and outcomes are summarized in Table 1.
Table 1.
Symptoms, interventions, and outcomes for a patient with gastric sarcoidosis.
| Symptom and duration | Prior interventions | Present interventions | Outcomes at follow-up |
|---|---|---|---|
| Two months epigastric pain | PPI therapy, taken intermittently | Steroids, PPI and H2RA, bowel regimen | Reported pain 0/10 epigastric pain |
| Two months dysphagia | PPI therapy, taken intermittently | Steroids PPI, Speech therapy consultation with education on liquid wash during solid meals | Resolution of symptoms on follow-up |
| Two months nausea with emesis | PPI therapy, taken intermittently | Steroids, PPI | No antiemetic needed on discharge or at follow-up |
PPI – proton pump inhibitor; H2RA – histamine receptor 2 antagonist.
Discussion
The disease course and clinical manifestations of sarcoidosis are highly variable and dependent on the organ systems involved and the severity of the disease. Sarcoidosis has predominant pulmonary involvement in more than 90% of patients. Roughly 7% of patients have extrapulmonary involvement at onset, which increases as the disease course progresses [2]. Among patients with GI tract involvement, the stomach is the most frequent site compared to esophagus, intestines, gallbladder, liver, and pancreas [3]. Notably, GI sarcoidosis in the literature is described mainly in white patients, while our case highlights this entity in an African American patient. Granulomatous inflammation and fibrosis of the gastric wall can cause peptic ulcerations, narrowing of the gastric lumen, pyloric obstruction, and diminished peristalsis. Sarcoidosis can be concurrently active in one organ system and latent in another, making diagnosis and treatment difficult [4]. In fact, there are no published reports examining changes in lung lesions when gastric lesions recur; there does not appear to be a correlation in disease severity in one organ system (lung) based on expression of disease in another organ system (GI tract). Despite this patient’s multiple pulmonary sarcoid flares, the only confirmed gastric sarcoidosis flare was on diagnosis and was managed with weekly methotrexate and PPI. At that time, symptoms of emesis and heart burn improved on follow-up, without complete resolution. Only after this admission with quiescent pulmonary symptoms and active GI complaints were her physicians able to recognize this as GI sarcoidosis and employ multimodal therapy including steroids.
The diagnosis of GI sarcoidosis is particularly difficult because its symptoms can mimic many other GI diseases such as irritable bowel syndrome (IBS), gastroesophageal reflux disease (GERD), gastroparesis, and even gastric cancer. In the stomach, sarcoidosis is often clinically silent but can present with epigastric pain, reflux, nausea, vomiting, and weight loss. Epigastric pain and emesis are the 2 most common symptoms, at 70% and 33% of cases, respectively [5]. Given the non-specific nature of symptoms, it is important to obtain histological evidence to make the diagnosis and guide treatment.
The optimal treatment approach to GI sarcoidosis is not definitively known. Most case reports demonstrate benefit with corticosteroids, typically starting at prednisone 20–40 mg/day and then tapered over time, although the timeline of the taper is varied [5,6]. Disease-modifying immunosuppressive drugs such as methotrexate may play a role in steroid-refractory cases. For severe gastric sarcoidosis, gastric resection may be needed to control life-threatening bleeding or bowel stricture causing obstruction [3]. For most patients, including ours, clinical symptoms are followed to determine the impact of treatment. To be as objective as possible, one can follow time to tolerating diet, percentage of meal tolerated, use of antiemetic therapy, pain scale for abdominal pain, and frequency of abdominal pain episodes. The patient discussed in this case had improvement following initiation of Speech Therapy’s recommendations, suggesting this had a large impact in her improvement. At present, there are no standardized criteria to monitor GI sarcoidosis symptoms and response to therapy. At follow-up, our patient’s symptoms and their impact on quality of life were assessed to determine the next steps for management.
Our patient’s symptoms of abdominal pain and vomiting were non-specific and could reflect (in addition to gastric sarcoidosis) GERD, IBS, and esophageal dysmotility. Thus, we opted to pursue a broader treatment approach. We initiated prednisone 30 mg daily dose with a slow taper over a period of weeks. We also treated her with a PPI, H2 blocker, and a bowel regimen. MBS showed esophageal dysmotility with stasis of contrast in the upper esophagus and tertiary contractions in the mid-esophagus. Speech Therapy initiated liquid washes with meals to aid in bolus transit, which aided in symptomatic relief.
Conclusions
Our case report highlights the importance of using prior histo-logical evidence of gastric sarcoidosis to help guide management of GI symptom flare. Gastric symptoms such as epigastric pain, nausea, vomiting, and early satiety can be presenting signs for a sarcoidosis flare in a patient with pulmonary sarcoidosis, which is important for both pulmonologists and gastroenterologists to be cognizant of. GI sarcoidosis can mimic other diseases such as achalasia, GERD, IBS, or malignancy, so biopsy evidence of non-caseating granulomas on histology is needed for diagnosis. In addition to steroids for gastric sarcoid, a PPI, bowel regimen, and speech therapy techniques are important components of treatment. Our broader, multi-disciplinary approach has not been demonstrated in previous reports of GI sarcoidosis flares and provided the patient with significant relief of symptoms.
Footnotes
Declaration of Figures Authenticity
All figures submitted have been created by the authors, who confirm that the images are original with no duplication and have not been previously published in whole or in part.
References:
- 1.Statement on sarcoidosis Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med. 1999;160(2):736–55. doi: 10.1164/ajrccm.160.2.ats4-99. [DOI] [PubMed] [Google Scholar]
- 2.Takada K, Ina Y, Noda M, et al. The clinical course and prognosis of patients with severe, moderate or mild sarcoidosis. J Clin Epidemiol. 1993;46(4):359–66. doi: 10.1016/0895-4356(93)90150-y. [DOI] [PubMed] [Google Scholar]
- 3.Brito-Zerón P, Bari K, Baughman RP, Ramos-Casals M. Sarcoidosis involving the gastrointestinal tract: diagnostic and therapeutic management. Am J Gastroenterol. 2019;114(8):1238–47. doi: 10.14309/ajg.0000000000000171. [DOI] [PubMed] [Google Scholar]
- 4.Shkolnik LE, Shin RD, Brabeck DM, Rothman RD. Symptomatic gastric sarcoidosis in a patient with pulmonary sarcoidosis in remission. BMJ Case Rep. 2012;2012:bcr2012006559. doi: 10.1136/bcr-2012-006559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Afshar K, BoydKing A, Sharma OP, Shigemitsu H. Gastric sarcoidosis and review of the literature. J Natl Med Assoc. 2010;102(5):419–22. doi: 10.1016/s0027-9684(15)30577-0. [DOI] [PubMed] [Google Scholar]
- 6.Chinitz MA, Brandt LJ, Frank MS, et al. Symptomatic sarcoidosis of the stomach. Dig Dis Sci. 1985;30(7):682–88. doi: 10.1007/BF01308419. [DOI] [PubMed] [Google Scholar]