Abstract
Aims
The aim of this research is to conduct green synthesis of silver nanoparticles in an eco-friendly, economical and more effective approach using Acacia cyanophylla plant extract as well as to study the effects of the preparation conditions on the size of synthesized nanoparticles and its antibacterial activity.
Methodology
In this study, silver nanoparticles have been synthesized by reduction method using aqueous silver nitrate solution and aqueous extract of Acacia cyanophylla. Then, their characterization has been studied by several methods, such as visual inspection, UV–Vis spectroscopy, dynamic light scattering and scanning electron microscope. In addition, the effects of (silver nitrate: extract) ratio, type extract, temperature and reaction time have been studied on the size of prepared silver nanoparticles. Furthermore, the antibacterial effect of these nanoparticles was studied on Escherichia coli using micro-dilution method and determination the Minimum Inhibitory Concentration (MIC).
Results
The results showed that the silver nanoparticles prepared using Acacia cyanophylla extract have reported visible yellowish brown color formation and the absorption peak at 460 nm indicated the biosynthesis of silver nanoparticles. Moreover, they have average diameter (88.11) nm and the polydispersity index (PdI) was suitable. The optimal conditions for synthesis silver nanoparticles were using aqueous extract in 9:1 ratio (silver nitrate: extract) at 35 °C for 48 h. These silver nanoparticles were stable in the in the fridge at 5 °C for a maximum period of 15 days. On the other hand, the antibacterial tests showed that these nanoparticles have high antibacterial activity where the MIC value ranged between (3.125–12.5) μg/ml on E. coli isolates.
Conclusion
We conclude that Acacia cyanophylla extract is considered effective as a reducing agent for the preparation of stable silver nanoparticles in certain conditions and this silver nanoparticle has a high antibacterial activity.
Keywords: Silver nanoparticles, Green synthesis, Acacia cyanophylla
Silver nanoparticles, Green synthesis, Acacia cyanophylla.
1. Introduction
Nanotechnology is an important field in modern sciences, which deal with synthesis particles with dimensions ranging from (1–100) nm [1]. The unique properties of the nanoparticles give it an important role in biomedicine and energy field, optics and other healthcare applications [2]. Metal nanoparticles possess specific features due to their excellent physicochemical properties, such as strong optoelectronic, thermal, catalytic properties, high surface volume ratio and easy to synthase in controllable morphology and distinct crystallinity. Among the metallic nanoparticles, noble metal nanoparticles are known such as gold, silver and platinum [3]. Silver nanoparticles have gained increasing interest, and they have been extensively studied due to their chemical stability and other distinctive properties. They have wide applications as antibacterial agents, catalyst agents, or biosensors. In addition to their anti-inflammatory effectiveness and in biomedicine, they have applications in wound dressings, topical creams and antiseptic sprays. They are being successfully used in the cancer diagnosis and treatment [1, 2]. There are two approaches for the synthesis of nanoparticles: “top-down” and “bottom-up” [4]. The first approach starts with a solid mass and turns it into a smaller nanoscale particle by applying of any mechanical method (such as mechanical grinding); then, the particles are fixed to the required size. However, the expected narrow size is difficult to achieve in this way. The second approach starts with an atomic size material, which is manufactured to the nanoscale required by applying of chemical methods; for example: hydrothermal method, sol-gel method, gas phase method, and hydrolysis. Moreover, it is difficult to do control on the surface chemistry, size and structure of the nanoparticle with these methods. In general, the bottom-up approach to producing nanoparticles is preferred because it starts with particles that are simpler to clusters and then proceed to nanoparticles. It grants greater control over the shape and size of the particles formed [5]. There are a lot of methods for the synthesis of silver nanoparticles such as physical, chemical and biological methods. The physical and chemical approaches for the synthesis of silver nanoparticles have some disadvantages (Ag-NPs) such as long duration for preparation, not economical, the need to use high temperature, pressure, energy and not environmentally friendly [6, 7]. The physical method uses technique such as gas phase deposition, laser burning and mechanical grinding to synthesize nanoparticles with the advantages of simpler principles and higher purity, but the particle size is larger than the synthesized one by chemical and biological methods [6]. The chemical method requires external stabilizer agent to protect nanoparticles from aggregation and reducing agents to reduce Ag+ to Ag0 to synthesize silver nanoparticles such as sodium citrate and sodium borohydrate [7]. The biological approaches overcome most of these drawbacks through the synthesis of silver nanoparticles by using of various biological agents, such as yeasts, enzymes, bacteria, polysaccharides, algae, oligosaccharides, fungi, DNA and human cell lines [8]. The methods based on bacteria are not industrially viable because they require highly sterile conditions and their maintenance [7]. In addition, the synthesis rate is slow and only a limited number of sizes and shapes are malleable as compared to methods involving plant materials [5]. While synthesis of nanoparticles by fungi is a popular method due to the ease of handling biomass and economic ability, it may lead to more contamination because it may create genetic manipulation of the organism. Moreover, the synthesis rate is slow to deal with the entire productive biomass [5]. So, the use of plant extracts is more useful than using microorganisms due to the lower cost as we do not need any special preparation for culture and isolation techniques and it is easily scaled up for large scale syntheses of nanoparticles [2, 7]. In addition, the green synthesis has the advantages that it is environment-friendly, economical as well as that it uses biocompatible agents for the synthesis of silver nanoparticles compared with chemical and physical methods which require more chemicals and larger amounts of energy which leads to the formation of dangerous by-products [2]. The advantage of the use of plants and their extracts in the synthesis of metal nanoparticles is because they are widely available, safely handled and possess a wide variety of metabolites which may help in reduction process [2]. where plant extracts can act as both reducing and stabilizing agents for metal nanoparticles [3]. The Reducing and stabilizing process by combining biomolecules such as proteins, amino acids, and enzymes, Polysaccharides, alkaloids, tannins, phenols, saponins, terpenoids and vitamins [7]. However, the size, shape and antibacterial activities of nanoparticles produced via plants depend on the type and concentration of phytochemicals present in the plant, the synthesis temperature, and the reaction time [3]. The synthesis of mineral nanoparticles using plant extracts have already been reported using several plants such as Matricaria Chamomile, Sphaeranthus indicus, Salvia officinalis, Arabica coffee and Acalypha indica [3]. For the extraction of biomolecules, solvents such as water and ethanol are used but water is preferred [4], because it is the most polar and easy-to-use for the extraction of active substances and it is non-toxic [8].
The synthesis of silver nanoparticles consists of several stages:
-
1
The preparation of silver salt solution and plant extracts (after collecting, cleaning, drying and grinding the plant). Then, extracting it in the appropriate solution at a certain temperature, finally filtering it to obtain the extract [4].
-
2
The preparation of silver nanoparticles by mixing these two solutions in different proportions, at certain pH and temperature values in different times. In the presence of the plant compounds, Ag+ is reduced to Ag0, then oligomeric aggregates are formed which lead to the formation of silver nanoparticles.
-
3
The purification of the silver nanoparticles by centrifugation to remove the unreacted plant extract, followed by resuspension in distilled water and then centrifugation again to remove unwanted substances.
-
4
The confirmation of silver nanoparticles formation by various analytical methods [8].
There are many factors affecting the reaction rate and shape of the nanoparticles, including: extract concentration, contact time, pH, temperature [9] and silver salt concentration [8].
The silver nanoparticles have antibacterial properties and this activity can be explained by attachment between the silver nanoparticles and the cell wall of bacteria. Accordingly, this binding leads to the accumulation of pro-envelope protein that it causes protein denaturation and loss of proton stimulating power and eventually cell death [3]. The size of nanoparticles plays an important role in antibacterial activity. Smaller nanoparticles are more easily taken up by the cell bacteria membranes. because the smaller particles in size give more space to interact with microorganisms; thus Ag+ is released by oxidation. This speeds up the generation of the reaction oxidative species that cause more damage to the cellular constitution. Ultimately, it leads to cell death [3]. Another study was suggested that silver atoms bind to thiol groups of enzymes forming S–Ag stable bonds with compounds containing thiols and it then deactivates enzymes in the cell membrane. As a result, bacterial cell lysis and this may be one of the reasons for its antibacterial properties [1].
Acacia cyanophylla was selected for study in this research. The genus Acacia belongs to the Fabaceae family and contains a large number of species (about 1500), making it the largest genus within the previous family. It is widespread in Australia, Asia, Africa and the Americas [10]. It has been reported that Acacia species contain many secondary metabolites, including amines and alkaloids, cyanogen glycosides, fatty acids and seed oils, terpenes (including essential oils, diterpene, phytosterol, triterpene and saponins), hydrolyzed tannins, flavonoids and condensed tannins [11].
This research provides eco-friendly, economical and more effective approach to the synthesis of silver nanoparticles via green synthesis method using Acacia cyanophylla plants extract. It also studies the effect of preparation conditions such as temperature, time of reaction, type of extract and the ratio of (silver nitrate: extract) on the size of silver nanoparticles and the antibacterial activity of prepared silver nanoparticles.
2. Materials and methods
2.1. Materials
Chemical materials: Silver nitrates (99.99%) were purchased from sigma Aldrich, Ethanol (Eurolab, UK). Distilled deionized water (dd. H2O).
Plant material: Fresh aerial parts (leaves, flowers and stems) of Acacia cyanophylla were collected between March∖April 2020 from different regions from the north of Syria and the plant materials were authenticated by an expert at the Faculty of Agriculture - University of Aleppo, Syria. The plant materials were cleaned with distilled water and dried at normal room temperature for 15 days and were grinded with the domestic blender until it became powder and were kept in airtight glass container until use [12].
Bacterial Strains: Bacterial strains, which include Escherichia coli, was isolated from patients with urinary tract infections from the bacteriological laboratory at University Hospital in Aleppo, Syria 2020. Isolates were defined by their growth in the selective and differential media and studying the resulting colonies form (Maconkey medium, EMB medium) in addition to examining it microscopically after staining with a Gram stain. Isolations are kept in the liquid nutrient medium with 30% glycerol, at -20 C0 temperature, until use.
2.2. Methods
-
1
Extraction Procedure for the synthesis of silver nanoparticles:
The fine powder of Acacia cyanophylla (5g) was mixed well in distilled water (100 mL) in conical flask and this mixture was boiled at 60 °C for 15–20 min with constant shaking on hot magnetic stirrer. Afterwards, the mixture was refrigerated and filtrated. The obtained aqueous extract deposited at 4 °C and was utilized for the synthesis of silver nanoparticles [12, 13].
-
2
Synthesis of silver nanoparticles:
10 mL plant extract was dropped into Erlenmeyer flask having 90 mL of AgNO3 (1 mM) solution. The mixture was stirred continuously at 35 °C for 24 h for Acacia cyanophylla. The reaction progression for silver nanoparticles synthesis was examined based on the color alteration of the reaction mixture from light yellow to intense reddish brown that confirms the formation of the silver nanoparticles in the solution. The mixture was examined by using UV–vis spectrophotometer within 300–600 nm at different incubation time and the absorption peak was observed [12, 13, 14].
-
3
Studying synthesis parameters effects on silver nanoparticles size:
Several parameters affecting the synthesis of silver nanoparticles have been studied, including:
-
3-1
The plant extract: silver nitrate solution ratio:
The effect of the added extract volume on the size of the synthesized nanoparticles has been studied at several ratios of (plant extract: silver nitrate) which are (9:1) (8:2) (5:5) [8,15].
-
3-2
The type of extract:
The effect of the extract type (aqueous or ethanolic) on the size of the synthesized nanoparticles was studied [8, 15].
-
3-3
Temperature:
Preparation was done at different temperatures which are (100, 35, 20) ° C and the effect of this on size and preparation time was studied [8, 15].
-
3-4
Reaction time:
The reaction time influencing on the production of the silver nanoparticles was studied at (24, 48, 72) hours [8, 15].
-
4
Characterization of silver nanoparticles:
-
4-1
Visual inspection:
The confirmation of silver nanoparticles formation was made visually by the color change from pale yellow to yellowish brown [15].
-
4-2
UV-vis spectroscopy:
The absorption spectra of samples were observed at 300–600 nm wavelengths using UV-visible spectrophotometer [UV-1800 spectrophotometer (Shimadzu, Japan)] and the absorption peak was observed and this confirms the production of silver nanoparticles [14].
-
4-3
Dynamic light scattering (DLS):
The particle size is determined by dynamic light scattering (DLS) technique which was performed on a Zetasizer instrument (DLS; Zetasize Nano-ZS; Malvern Instruments, UK) at 25 °C. DLS analyzes the velocity distribution of particle movement by measuring dynamic fluctuations of light scattering intensity caused by the Brownian motion of the particle. The mean particle diameter is calculated by the software from the particle distributions measured, and the polydispersity index (PdI) given is a measure of the size ranges present in the solution. The PdI scale ranges from 0 to 1, with 0 being monodisperse and 1 being polydisperse [16].
-
4-4
Scanning electron microscope [SEM]:
SEM can be used to determine the surface morphology and size of the synthesized silver nanoparticle [5, 8]. Samples were prepared by placing a drop of colloidal on a slide and drying it at room temperature and then they were examined at (SEM MAG = 50.00, 40.00 kx) and (SEM HV = 30.00 kv) using a scanning electron microscope (Tescan vega 2 xmu, European).
-
4-5
Determination of the concentration of the prepared silver nanoparticles:
Ultraviolet-visible spectrophotometer [Double Beam Spectrophotometer Cecil Aquarius CE 7200 (Cecil -UK)] was used to determine the concentrations of silver nanoparticles after synthesis at 460 nm where it was seen that absorbance difference varies well linearly with Ag+ concentration in specific concentration range of the solution of silver nanoparticles (0–120 μg∖ml).
After drawing the standard curve between absorbance difference and concentration of standard solution of silver nanoparticles, the concentration of silver nanoparticles, which was calculated from the standard curve, was in μg/ml [6].
-
5
Stability of the prepared silver nanoparticles after 15 days:
The size of the synthesized silver nanoparticles was measured using a zetasizer apparatus. To test stability of the synthesized silver nanoparticles, the size was obtained from the silver nanoparticles solution at two different time points (first time point immediately after being synthesized and second time point after being stored in solution for 15 days) [17].
-
6
Determination of antibacterial activity using micro-dilution method
The antimicrobial activities of silver nanoparticles were determined by establishing the minimum inhibitory concentration (MIC) within microtiter plates using micro-dilution method. For this purpose, stock solutions of silver nanoparticles and Mueller-Hinton broth were prepared. First, adding 50 μL of Muellur Hington broth (MHB) and 50 μL of bacterial suspension at a concentration of 0.5 McFarland to the first well as a negative control. Then, adding 50 μL of the stock silver nanoparticles to the second well and 50 μL of bacterial suspension as a positive control. Second, 50 μL of the silver nanoparticles at the highest concentration to the third well and it is diluted by adding 50 μL of MHB, then 50 μL are taken from the mixture for the fourth well and so on until the end. Thus, decreasing concentrations were obtained from the silver nanoparticles in the remaining ten wells. In this study, the range of tested concentrations of extracts for the MIC between (0.0488–50) μg/ml was adopted. Then 50 μL of bacterial suspension are added to each well. The microtiter plate was sealed with parafilm and incubated at 37 °C for 24 h for bacterial. After 24 h of incubation, the MIC of each test organism was detected by adding 20 μL of 0.2 mg/mL indicator dye of 2, 3, 5-triphenyltetrazolium chloride (TTC) into the wells of the microtiter plate. Then, the microtiter plate was incubated for 30 min at 37 °C and any color change was observed [18, 19]. The dehydrogenase of living bacteria reduces tetrazolium salt into intensely pinkish-red formazan. When solutions in the wells of microtiter plate remained clear (without color change), we inferred that the bacterial were inhibited [20, 21].
3. Results and discussion
-
1
Study of synthesis parameters effects on silver nanoparticles size:
-
1-1
The silver nitrate: plant extract ratio:
The effect of adding extract volume on the resulting particle size of silver nanoparticles was studied as shown in Table 1.
Table 1.
Effect of varying concentration of plant extract on silver nanoparticles size.
| Extract type | silver nitrates volume | Extract volume | silver nitrate: extract ratio | Preparation time | temperature | Solution color | Z-Average size (nm) | PDI |
|---|---|---|---|---|---|---|---|---|
| Aqueous | 50 ml | 50 ml | 5:5 | 24 h | 35 °C | dark yellow | 231.5 | 0.338 |
| 48 h | brown | 347.2 | 0.412 | |||||
| Aqueous | 80 ml | 20 ml | 8:2 | 24 h | 35 °C | dark yellow | 139.3 | 0.293 |
| 48 h | brown | 132.1 | 0.247 | |||||
| Aqueous | 90 ml | 10 ml | 9:1 | 24 h | 35 °C | dark yellow | 62.41 | 0.280 |
| 48 h | brown | 86.98 | 0.281 |
From the table we conclude that when we increased the extract concentration, the particle size of the sample increased. The sizes were (132.1) nm when using the ratio of 9: 2 (extract solution: silver nitrate), while the sizes increased significantly when using the ratio of 5: 5 as it reported (347.2) nm and the PDI was very high (0.412), which indicates that the sample is highly dispersed. While the ratio of 9: 1 reported the smallest size with a value of (86.98) nm which indicates that it is the optimal concentration, it also indicates that at higher concentration, it might increase the possibility of particles agglomeration [15]. Whereas, the ratio of 9: 1 allows providing a sufficient amount of antioxidants to synthesize silver nanoparticles with acceptable size and in the presence of an excess amount of the extract, it may cause the reaction to continue and increase the size.
-
1-2
The type of extract:
The effect of the extract type (aqueous or ethanolic) on the resulting particle size of silver nanoparticles was studied as shown in Table 2.
Table 2.
Effect of type extract on silver nanoparticles sizes.
| Extract type | Extract volume | silver nitrates volume | silver nitrate: extract ratio | Preparation time | temperature | Solution color | Z-Average size (nm) | PDI |
|---|---|---|---|---|---|---|---|---|
| Aqueous | 10 ml | 90 ml | 9:1 | 24 h | 35 °C | dark yellow | 62.41 | 0.280 |
| 48 h | brown | 86.98 | 0.281 | |||||
| Ethanolic | 10 ml | 90 ml | 9:1 | 24 h | 35 °C | dark yellow | 121.5 | 0.251 |
| 48 h | brown | 198.6 | 0.453 |
From the table we conclude that when we used the ethanolic extract, the particle size of the sample increased. The sizes were (121.5) nm after 24 hours, while the sizes increased significantly after 48 hours as it reported (198.6) nm and the PDI was very high (0.453), which indicates that the sample is highly dispersed. While the aqueous extract reported the smallest size with a value of (62.41) nm after 24 hours and 86.98 after 48 hours and the PDI was suitable (0.281) which indicates that it is the optimal extract in this study.
-
1-3
Temperature
The preparation of silver nanoparticles was done at different temperatures, and the effect of that on the size and preparation time was studied, as shown in Table 3.
Table 3.
Effect of Temperature on silver nanoparticles size.
| Extract type | silver nitrates volume | Extract volume | silver nitrate: extract ratio | temperature | Z-Average size (nm) | PDI | Preparation time | solution color |
|---|---|---|---|---|---|---|---|---|
| Aqueous | 90 ml | 10 ml | 9:1 | 35 °C | 104.2 | 0.206 | 24 h | brown |
| Aqueous | 90 ml | 10 ml | 9:1 | 20 °C | No forming of silver nanoparticles | No forming of silver nanoparticles | 48 h | light yellow |
| Aqueous | 90 ml | 10 ml | 9:1 | 100 °C | 182.2 | 0.197 | immediately | brown |
From the table we conclude that the temperature of preparation has a great influence on the speed of formation of the silver nanoparticles and on the size also due to the increase in the reduction of silver ions and thus the increase in the production of silver nanoparticles [8, 15]. The brown color appeared immediately upon conducting the reaction at high temperatures but the size was relatively large (182.2) nm while the brown color did not appear at all and the silver nanoparticles did not form at low temperatures (20 °C). While the brown color appeared after 24 hours at 35 °C and the size was relatively small (104.2) nm and the PDI was adequate (0.206), which indicates that it is the optimal temperature.
-
1-4
Reaction time:
The effect of reaction time on the size of resulting silver nanoparticles was studied as shown in Table 4.
Table 4.
Effect of Time on silver nanoparticles size.
| Extract type | Extract volume | silver nitrates volume | silver nitrate: extract ratio | temperature | Preparation time | Z-Average size (nm) | PDI |
|---|---|---|---|---|---|---|---|
| Aqueous | 10 ml | 90 ml | 9:1 | 35 °C | 24 h | 62.41 | 0.280 |
| 48 h | 86.98 | 0.281 | |||||
| 72 h | 107.7 | 0.240 | |||||
| Aqueous | 50 ml | 50 ml | 5:5 | 35 °C | 24 h | 231.5 | 0.338 |
| 48 h | 347.2 | 0.412 | |||||
| 72 h | 788.3 | 0.417 | |||||
| Aqueous | 20 ml | 80 ml | 8:2 | 35 °C | 24 h | 139.3 | 0.293 |
| 48 h | 132.1 | 0.247 | |||||
| 72 h | 829.3 | 0.442 |
We notice from the table that the size increased with the increase in the reaction time, as the highest size was reported at a time of 72 hours using the 5: 5 ratio with a value of (788.3) nm and the PDI was very high (0.417) while the size increased at a time of 72 hours using the ratio (8: 1) with a value of (829.3) nm and the PDI was also very high (0.442) while the size increased slightly at a time of 72 hours using the ratio (8: 1) as it reached 107.7 nm compared to 86.98 nm at 48 hours which indicates that it is the optimal reaction time. This can be explained by the increase in the formation of silver nanoparticles with the increase in the reaction time, but after an optimal period the particles started to agglomerate and the particle size increased [8]. Therefore, we stored the prepared silver nanoparticles after 48 hours in the fridge at 5 °C to stop the reaction.
The optimal conditions for synthesis of silver nanoparticles are shown in Table 5:
Table 5.
Optimal condition for synthesis silver nanoparticles.
| The selected plant | Acacia cyanophylla |
| Extract type | Aqueous extract |
| silver nitrate: extract ratio | 9:1 |
| Temperature | 35 °C |
| Preparation time | 48 h |
We conclude from the table that the optimal conditions for the synthesis of silver nanoparticles were using aqueous extract in 9:1 ratio (silver nitrate: extract) at 35 °C for 24 h and on these conditions the stability study was conducted.
-
3
Characterization of silver nanoparticles:
-
3-1
Visual inspection
10 ml extracts of Acacia cyanophylla were prepared to reduce 90ml of 1mM aqueous AgNO3 solution to prepare colloidal silver nanoparticles and color change of solution from yellow to dark brown was observed due to the production of silver nanoparticles.
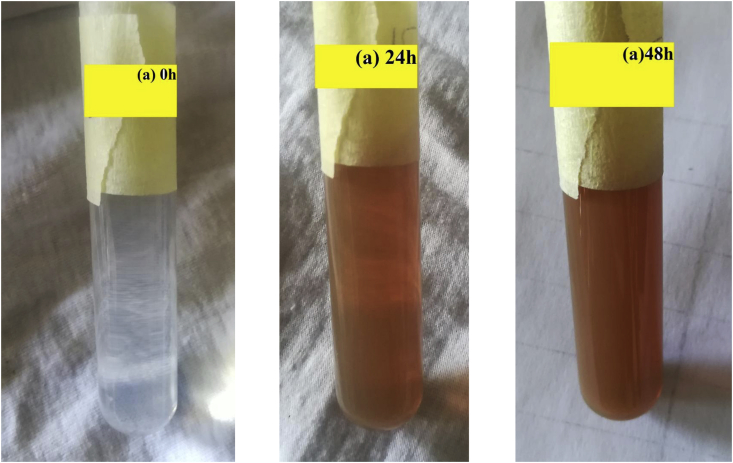
Figure 1 represents the visual appearance of the colloids prepared using Acacia cyanophylla plant extract. The color of the silver nanoparticles prepared using Acacia cyanophylla appeared dark yellowish brown which indicates high concentration of silver nanoparticles in the suspension.
Figure 1.
Visual appearance of the silver nanoparticles based colloids prepared using (a) Acacia cyanophylla. (0h) zero hours, (24h) 24 hours and (48h) 48 hours
Where we notice at the zero moment, the solution was colorless and after 24 hours a deep yellowish brown color appeared which confirms the formation of the silver nanoparticles and the intensity of the color increased after 48 hours indicating an increase in its concentration.
Comparing the works of literature, our results were similar to other studies. For instance, Acacia concinna fruit extract was used to synthesize silver nanoparticles with a similar protocol [14].
-
3-2
UV-vis spectroscopy
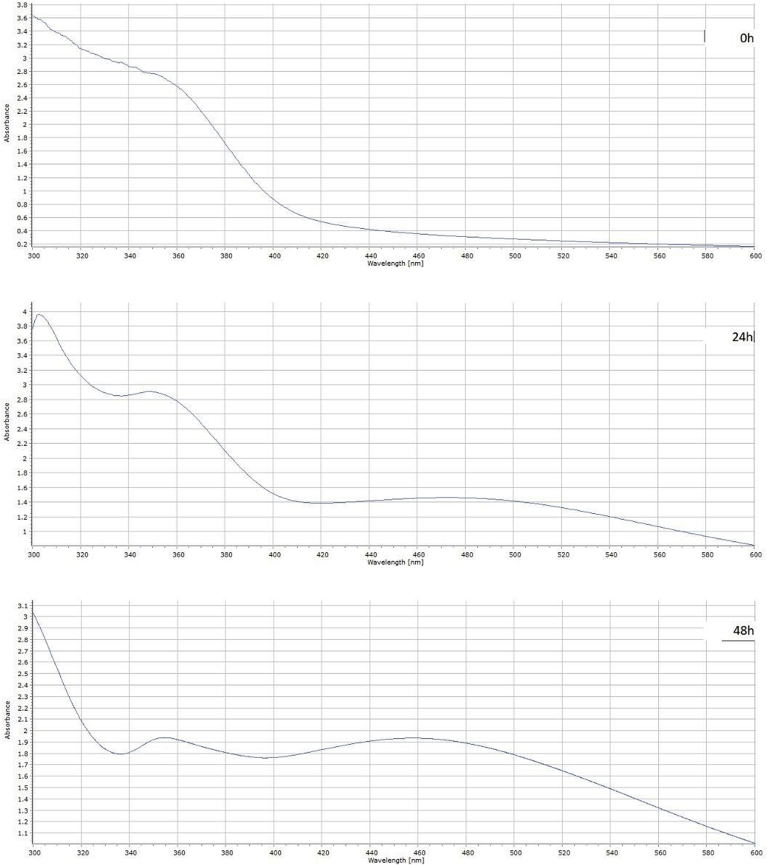
In our study, the synthesis process was studied periodically using UV-visible Spectra. The synthesized silver nanoparticles were characterized by UV-Vis spectroscopy and the absorption peak observed. Figure 2 shows the UV-vis absorbance spectra of colloidal silver nanoparticles prepared using Acacia cyanophylla as reducing agents. The spectra consist of absorption peaks in the visible region of the electromagnetic spectrum it shows absorption peak at 460 nm.
Figure 2.
UV-vis absorbance spectra of the colloidal silver nanoparticles prepared using (a) Acacia cyanophylla. (0h) zero hours, (24h) 24 hours and (48h) 48 hour
It could be concluded that 24 hours were sufficient to obtain the silver nanoparticles using Acacia cyanophylla extract. This is evidence that a peak appeared at 460 nm and the absorbance was 0.987. After 48 hours, the peak intensity slightly increased and the absorbance was 1.0084, indicating the formation of an additional amount of silver nanoparticles.
Compared with works of literature, our results differ from other studies. For instance, when using Acacia concinna fruit extract to synthesis silver nanoparticles, the peak of absorption appeared around at 430 nm [14]. In another study, an Acacia leucophloea extract was used to synthesize silver nanoparticles and the peak of absorption appeared around at 433 nm [22].
-
3-3
Dynamic light scattering technique (DLS):
After synthesis process, the distribution size of the dispersed particles was measured using a dynamic light scattering (DLS) technique.
Table 6 shows the particle size distribution analysis of the silver nanoparticles that we obtained depending on Acacia cyanophylla plant extract.
Table 6.
Particle size distribution analysis of silver nanoparticles was determined by zetasizer.
| Plant extracts | Plant,s part | Time preparation | AgNO3 concentration | Temperature ° C | Z-Average size (nm) | PDI | absorbance |
|---|---|---|---|---|---|---|---|
| Acacia cyanophylla | aerial parts | 24h | 1mM | 35 | 62.41 | 0.280 | 0.9952 |
| 48h | 1mM | 35 | 86.98 | 0.281 | 1.0962 |
From the table we conclude that:
The size of the prepared silver nanoparticles using Acacia cyanophylla extract was (62.41 nm) and PDI was suitable (0.280) after 24h and the concentration was high while after 48h the size was (86.98 nm) and PDI was suitable (0.281) and the concentration was higher.
Comparing the works of literature, our results differ from other studies. For instance, Acacia concinna fruit extract was used to synthesize silver nanoparticles with particle size between 2-20 nm [14]. In another study, an Acacia leucophloea extract was used to synthesize silver nanoparticles with a size range of 17–29 nm [22].
-
3-4
Scanning electron microscope (SEM):
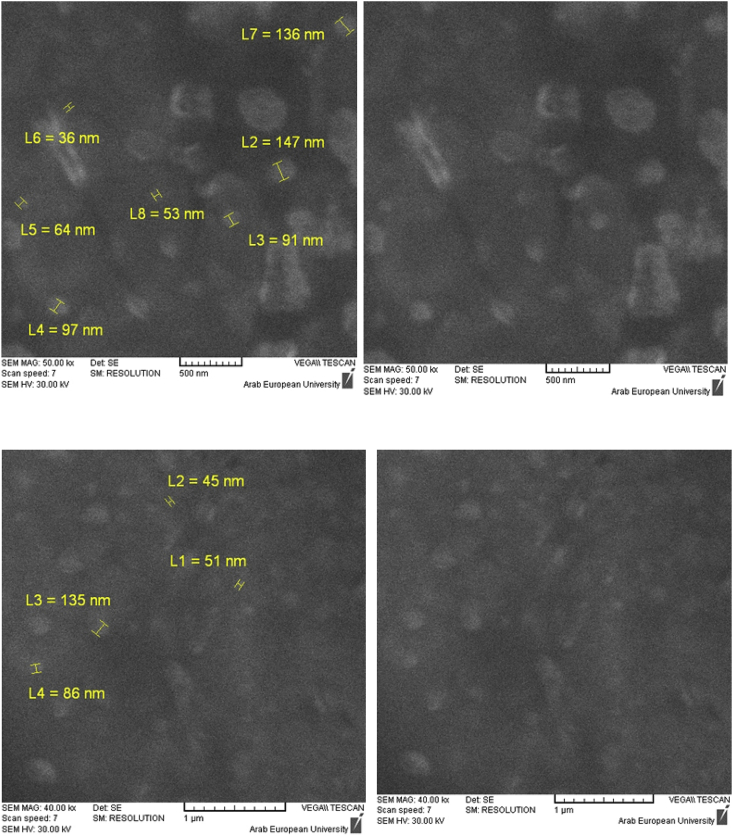
The particle size of the prepared silver nanoparticles using Acacia cyanophylla plant extract was measured using a scanning electron microscope as shown in Figure 3.
Figure 3.
Images of a scanning electron microscope of silver nanoparticles prepared with Acacia cyanophylla extract.
From the figure, the appearance of bright spherical particles is noticed, which confirms the formation of silver nanoparticles and that the size measured using a scanning electron microscope (89.14, 88.11) nm corresponded relatively to the size measured using the zeta sizer (88.11) nm which are relatively small sizes, which indicates the effectiveness of the Acacia cyanophylla extract in the synthesis of silver nanoparticles.
-
3-5
Determination of the concentration of the prepared silver nanoparticles:
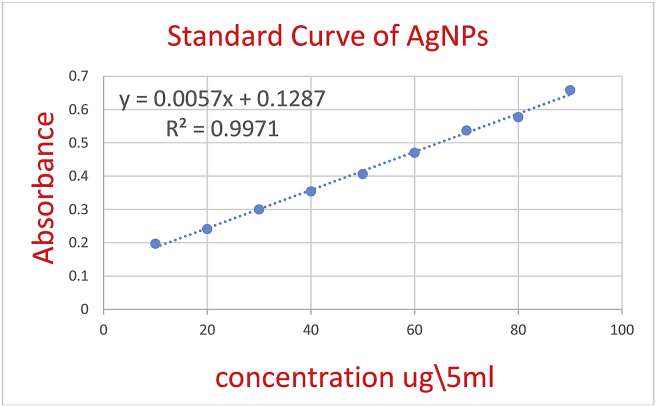
The spectrophotometer is used to determine the concentration of the prepared silver nanoparticles and the results were derived from the calibration curve based on the linear standard curve equation of standard silver nanoparticles (y = 0.0057x + 0.0711 R2 = 0.9979).
Figure 4 shows the linear standard curve for the silver nanoparticle standard series, which is adopted to calculate the concentration of the prepared nanoparticles.
Figure 4.
Standard curve for silver nanoparticle standard series.
The concentration of the prepared silver nanoparticles using Acacia cyanophylla aqueous extract in a ratio (9: 1) was 50.71 μg∖ ml at absorbance 1.574.
-
4.
Stability of the prepared silver nanoparticles after 15 days:
The prepared silver nanoparticles were stored at different temperatures and the effect of this on the size was studied as shown in Table 7.
Table 7.
Effect of the storage temperature on silver nanoparticles size.
| Extract type | extract: silver nitrate ratio | temperature | Duration | Z-Average size (nm) | PDI | R = SF∖SI |
|---|---|---|---|---|---|---|
| Aqueous | 9:1 | 5 °C | Zero point | 70.39 | 0.272 | 1 |
| 3 days | 97.4 | 0.226 | 1.38 | |||
| 6 days | 173.4 | 0.421 | 2.46 | |||
| 9 days | 156.4 | 0.413 | 2.22 | |||
| 15 days | 134.5 | 0.394 | 1.91 | |||
| 15 days after Filtering using 0.45 μm filters | 107.7 | 0.291 | 1.53 | |||
| Aqueous | 9:1 | 25 °C | Zero point | 76.72 | 0.276 | 13.76 |
| 5 days | 1056 | 0.321 |
Storage in the fridge for a short period (15 days) increased the size slightly from (69.38) to (134.5) nm and the PDI increased slightly (0.394) so we filtered the suspension using (0.45) μm filters and the size became (107.7) and the PDI was (0.291). The R value was relatively small (1.53), while storage at room temperature (25 °C) greatly increased the size from (76.72) to (1056) nm, thus destroying the sample as the R value increased very significantly (13.76) compared with the sample stored in the fridge 5 °C for 6 days (2.46). We conclude that the fixing agents in the sample are sufficient to stabilize the suspension for 15 days in the fridge. Therefore, we suggest lyophilizing the sample to maintain its stability.
-
5
Determination of antibacterial activity using micro-dilution method
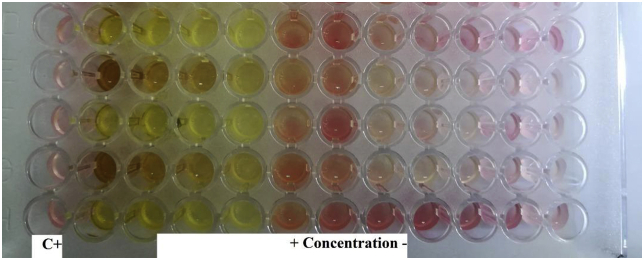
The MIC value was taken as the lowest concentration of the silver nanoparticles which inhibits any visible bacterial growth, and this was detected after adding tetrazolium salts and observing color change as shown in Figure 5, where serial dilutions were performed for the silver nanoparticles solution and the concentrations were adjusted in the range of (0.0488–50) μg/ml. Table 8 shows the MIC values for the silver nanoparticles against Escherichia coli.
Figure 5.
The minimal inhibitory concentrations (MICs) of silver nanoparticles against Escherichia Coli according to the micro-dilution method.
Table 8.
The MIC values for the silver nanoparticles prepared using Acacia cyanophylla.
| Isolate number | MIC |
|---|---|
| 1 | 12.5 |
| 2 | 12.5 |
| 3 | 6.25 |
| 4 | 6.25 |
| 5 | 3.125 |
| 6 | 3.125 |
| 7 | 6.25 |
| 8 | 3.125 |
| 9 | 6.25 |
| 10 | 6.25 |
| 11 | 3.125 |
| 12 | 6.25 |
| 13 | 6.25 |
| 14 | 6.25 |
| 15 | 12.5 |
The results in Table 8 showed that the silver nanoparticles prepared from the aqueous extract of Acacia cyanophylla strong antibacterial activity on E. coli isolates, where the activity ranged between (3.125–12.5) μg/ml for the studied E. coli isolates, where the greater concentration of silver nanoparticles showed the highest antibacterial activity was 3.125 μg/ml.
This is in agreement with a 2019 study in which silver nanoparticles prepared from the leaves of Acacia nilotica were used to study the anti-bacterial activity of E. coli where MIC values were of 18.5 μg/ml [12]. While these results differ from a 2018 study in which silver nanoparticles prepared from Acacia rigidula extract were used to study the antibacterial activity of E. coli where MIC values were (62.5) ppm [17]. The antibacterial activity of silver nanoparticles can explain that in Escherichia coli bacteria, silver nanoparticles inhibit phosphate uptake and phosphate release from phosphates, mannitol, succinate, Proline and glutamine cells of these bacteria. The high tendency of silver particles to sulfur and phosphorous are the main reason for its antibacterial properties. Sulfur and phosphorous are found abundantly throughout the membrane of bacterial cells. Silver nanoparticles interact with the proteins that contain sulfur inside or outside the cell membrane, which affects survival of cells [18].
4. Conclusion
Colloidal silver nanoparticles have been prepared successfully by using Acacia cyanophylla which are characterized as eco-friendly, economical and more effective approach than physical and chemical approach. The plant extract not only functions as a reducing agent but also coats the produced nanoparticles, providing them with stability. Acacia cyanophylla has been considered good reducing agent for the preparation of stable colloidal silver nanoparticles. The silver nanoparticles prepared using its has intense absorption peak in the visible region with the peak at 460 nm. Moreover, they have average diameter (88.11) nm and the PDI was suitable. The optimal conditions for the synthesis of silver nanoparticles were using aqueous extract in 9:1 ratio (silver nitrate: extract) at 35 °C for 48 h. These silver nanoparticles were stable in the in the fridge at 5 °C for a maximum period of 15 days. On the other hand, the antibacterial tests showed that these nanoparticles have high antibacterial activity where the MIC value ranged between (3.125–12.5) μg/ml on E. coli isolates.
Declarations
Author contribution statement
Joud Jalab: Conceived and designed the experiments; Performed the experiments; Wrote the paper.
Wassim Abdelwahed; Adawia Kitaz; Rawaa Al-Kayali: Conceived and designed the experiments; Analyzing and interpreting the date.
Funding statement
This work was supported by the research laboratory, Department of Pharmacognocy- Aleppo University Syria.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Ahmed S., Ahmad M., Swami B.L., Ikram S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: a green expertise. J. Adv. Res. 2016;7(1):17–28. doi: 10.1016/j.jare.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gavade S.J.M., Nikam G.H., Sabale S.R., Dhabbe R.S., Mulik G.N., Tamhankar B.V. Green synthesis of silver nanoparticles by using Acacia concinna fruit extract and their antibacterial activity. Nanosci. Nanotechnol. 2015;9(3):89–94. [Google Scholar]
- 3.Jemilugba O.T., Sakho E.M., Parani S., Mavumengwana V., Oluwafemi O.S. Green synthesis of silver nanoparticles using Combretum erythrophyllum leaves and its antibacterial activities. Colloid Interfac. Sci. Commun. 2019;31:100191. [Google Scholar]
- 4.Ana-Alexandra S., Nuta A., Ion R.M., Bunghez R. Green synthesis of silver nanoparticles using plant extracts. The 4th International Virtual Conference on Advanced Scientific Results. 2016;1:188–193. [Google Scholar]
- 5.Devatha C.P., Thalla A.K. 2018. Green Synthesis of Nanomaterials. Chapter 7- Synthesis of Inorganic Nanomaterials; pp. 169–184. [Google Scholar]
- 6.Ren Y.y., Yang H., Wang T., Wang C. Bio-synthesis of silver nanoparticles with antibacterial activity. Mater. Chem. Phys. 2019;235:121746. [Google Scholar]
- 7.Pand S.K., Sen S., Roy S., Moyez A. Synthesis of colloidal silver nanoparticles by reducing aqueous AgNO3 using green reducing agent. Mater. Today: Proceedings. 2018;5(3):10054–10061. [Google Scholar]
- 8.Corciova A., Ivanescu B. Biosynthesis, characterization and therapeutic applications of plant-mediated silver nanoparticles. J. Serb. Chem. Soc. 2018;83(5):515–538. [Google Scholar]
- 9.Bajpai K., Kumari M. A green approach to prepare silver nanoparticles loaded gumacacia/poly(acrylate) hydrogels. Int. J. Biol. Macromol. 2015;80:177–188. doi: 10.1016/j.ijbiomac.2015.06.048. [DOI] [PubMed] [Google Scholar]
- 10.Maroyi A. Acacia karroo Hayne: ethnomedicinal uses, phytochemistry and pharmacology of an important medicinal plant in southern Africa. Asian Pac. J. Trop. Med. 2017;10(4):351–360. doi: 10.1016/j.apjtm.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 11.bbasian K.A., Asgarpanah J., Ziarati P. Chemical composition profile of Acacia nilotica seed growing wild in South of Iran. Orient. J. Chem. 2015;31(2):1027–1033. [Google Scholar]
- 12.Saratale R.G., Saratale G.D., Cho S.K., Ghodake G., Kadam A., Kumar S. Phyto-fabrication of silver nanoparticles by Acacia nilotica leaves: investigating their antineoplastic, free radical scavenging potential and application in H 2 O 2 sensing. J. Taiwan Inst. Chem. Eng. 2019;99:239–249. [Google Scholar]
- 13.Jalab J., Kitaz A., Abdelwahed W., Kayali R. Green synthesis of silver nanoparticles using some medicinal plants. Int. Res. J. Pure Appl. Chem. 2020;21(24):13–26. [Google Scholar]
- 14.Meena R.K., Ansari K., Kishor N., Chouhan N. Green synthesis of silver nanoparticles using acacia concinna plant extract and their antibacterial activity. Res. J. Recent Sci. 2018;7(3):1–6. [Google Scholar]
- 15.Kumar R., Ghoshal G., Jain A., Goyal M. Rapid green synthesis of silver nanoparticles (Ag NPs) using (Prunus persica) plants extract: exploring its antimicrobial and catalytic activities. J. Nanomed. Nanotechnol. 2017;8(4) [Google Scholar]
- 16.Murdock R.C., B-Stolle L., Schrand A.M., Schlager J.J., Hussain S.M. Characterization of nanomaterial dispersion in solution prior to in vitro exposure using dynamic light scattering technique. Toxicol. Sci. 2008;101(2):239–253. doi: 10.1093/toxsci/kfm240. [DOI] [PubMed] [Google Scholar]
- 17.González C.E.E., Cervantes J., Rodríguez V., Peralta Z.M., González T., Castro E.B., Salazar S., Morales C., Soto R., González T., Rosales C., Cruz R.V., Ramírez J.R.M. In vivo antimicrobial activity of silver nanoparticles produced via a green chemistry synthesis using Acacia rigidula as a reducing and capping agent. Int. J. Nanomed. 2018;13:2349–2363. doi: 10.2147/IJN.S160605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Behravan M., Panahi A.H., Naghizadeh A., Ziaee M., Mahdavi R., Mirzapour A. Facile green synthesis of silver nanoparticles using Berberis vulgaris leaf and root aqueous extract and its antibacterial activity. Int. J. Biol. Macromol. 2019;124:148–154. doi: 10.1016/j.ijbiomac.2018.11.101. [DOI] [PubMed] [Google Scholar]
- 19.Adamczak A., Ożarowski M., Karpiński T.M. Antibacterial activity of some flavonoids and organic acids widely distributed in plants. J. Clin. Med. 2020 Jan;9(1):109. doi: 10.3390/jcm9010109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gebreyohannes G., Nyerere A., Bii C., Sbhatu D.B. Determination of antimicrobial activity of extracts of indigenous wild mushrooms against pathogenic organisms. Evid. base Compl. Alternative Med. 2019;2019 doi: 10.1155/2019/6212673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choma I.M., Grzelak E.M. Bioautography detection in thin-layer chromatography. J. Chromatogr. A. 2011;1218:2684–2691. doi: 10.1016/j.chroma.2010.12.069. [DOI] [PubMed] [Google Scholar]
- 22.Murugan K., Senthilkumar B., Senbagam D., Al-Sohaibani S. Biosynthesis of silver nanoparticles using Acacia leucophloea extract and their antibacterial activity. Int. J. Nanomed. 2014;3(9):2431–2438. doi: 10.2147/IJN.S61779. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.